Abstract
Background & Aims:
It is a challenge to make a diagnosis of gastroparesis. There is good agreement in results from wireless motility capsule (WMC) analysis and gastric emptying scintigraphy (GES), but the diagnostic yield of WMC is unclear and the accuracy of this method has not been validated. We compared the performance characteristics of WMC vs GES in assessing gastric emptying in patients with suspected gastroparesis.
Methods:
We performed a prospective study of 167 subjects with gastroparesis (53 with diabetes and 114 without) at 10 centers, from 2013 through 2016. Subjects were assessed simultaneously by GES and with a WMC to measure gastric emptying and regional transit. Delayed gastric emptying by GES was defined as more than 10% meal retention at 4 hrs whereas delayed gastric emptying by WMC was defined as more than 5 hrs for passage of the capsule into the duodenum; a severe delay in gastric emptying was defined as a gastric emptying time of more than 12 hrs by WMC or more than 35% retention at 4 hrs by GES. Rapid gastric emptying was defined as less than 38% meal retention at 1 hr based on by GES or gastric emptying times less than 1:45 hrs by WMC. We compared diagnostic and performance characteristics of GES vs WMC.
Results:
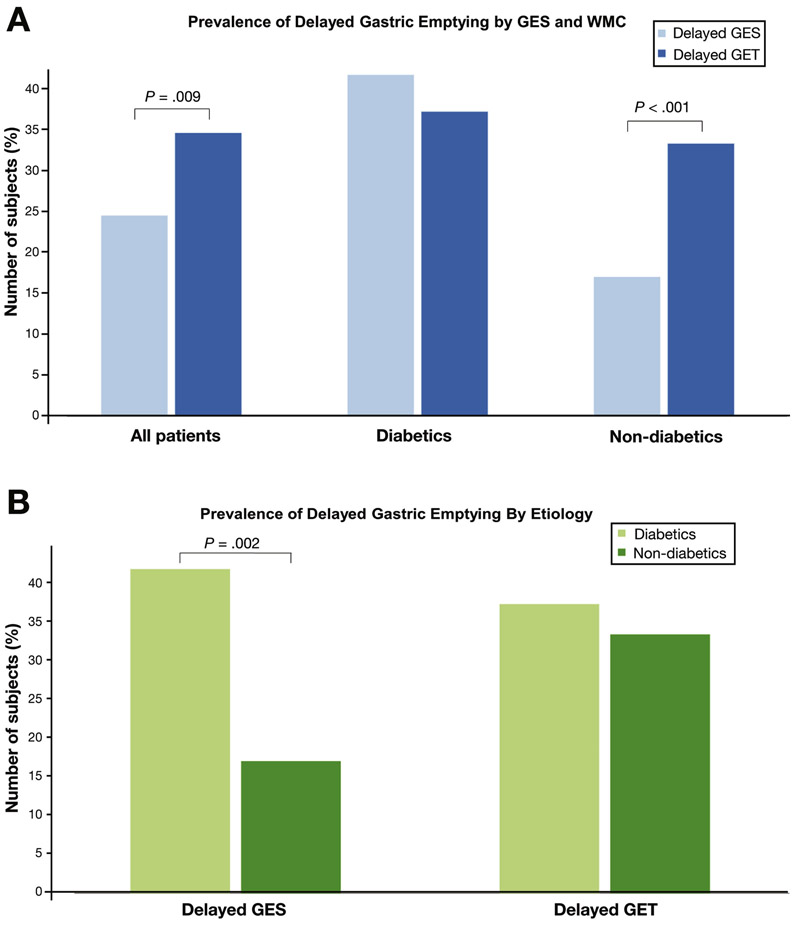
Delayed gastric emptying was detected in a higher proportion of subjects by WMC (34.6%) than by than GES (24.5%) (P=.009). Overall agreement in results between methods was 75.7% (kappa=0.42). In subjects without diabetes, the WMC detected a higher proportion of subjects with delayed gastric emptying (33.3%) than GES (17.1%) (P<.001). A higher proportion of subjects with diabetes had delayed gastric emptying detected by GES (41.7%) than by WMC (17.1%) (P=.002). Severe delays in gastric emptying were observed in a higher proportion of subjects by WMC (13.8%) than by GES (6.9%) (P=.02). Rapid gastric emptying was detected in a higher proportion of subjects by GES (13.8%) than by WMC (3.3%) (P<.001). Regional and generalized transit abnormalities were observed in 61.8% subjects and only detected by WMC.
Conclusion:
Although there is agreement in analysis of gastric emptying by GES vs WMC, WMC provides higher diagnostic yield than GES. WMC detects delayed gastric emptying more frequently than GES and identifies extra-gastric transit abnormalities. Diabetic vs non-diabetic subjects have different results from GES vs WMC. These findings could affect management of patients with suspected gastroparesis. ClinicalTrials.gov no: NCT02022826.
Keywords: idiopathic, gastrointestinal motility, small bowel transit, colonic transit
Introduction:
Gastroparesis is a chronic condition characterized by delayed gastric emptying in the absence of mechanical obstruction. Gastric emptying scintigraphy (GES) is often performed to diagnose gastroparesis but is limited by lack of standardization and radiation exposure.1 Furthermore, co-existing disorders, such as irritable bowel syndrome (IBS) are commonly encountered in gastroparesis.2 These coexisting problems may be a consequence of generalized gastrointestinal dysmotility that may remain uninvestigated if scintigraphy alone is performed.3,4 It is possible that targeting specific physiologic abnormalities in gastroparesis may lead to improved patient outcomes.
Wireless motility capsule (WMC) is a non-invasive ambulatory test that measures transit times and pressure parameters throughout the gastrointestinal tract. Although a previous study demonstrated good agreement in gastric emptying between GES and WMC, this study was enriched with subjects who previously had an abnormal GES.5 The primary aim of this study was to prospectively evaluate the diagnostic and performance characteristics of GES and WMC in patients with unexplained upper GI symptoms suggestive of gastroparesis. Additionally, we examined the prevalence of regional and generalized transit delays as well as gastroduodenal contractile abnormalities with WMC. Finally, as prior studies have suggested physiologic differences between diabetic and idiopathic gastroparesis, we also performed subgroup analyses to examine differences in test characteristics between diabetic and non-diabetic subjects.2,6
Methods:
This was a multicenter, comparative, prospective cohort study (ClinicalTrials.gov no: NCT02022826). Subjects ≥18 years with ≥2 upper GI symptoms for ≥12 weeks suggestive for gastroparesis (nausea, vomiting, upper abdominal pain, early satiation, bloating, postprandial fullness) were enrolled at ten academic and community centers in the US from 2013 to 2016. The protocol was approved by the Institutional Review Board at each center. Informed consent was obtained from each subject before enrollment.
Study Protocol:
Subjects were asked to discontinue all substances (e.g. prokinetic agents, anti-cholinergic medications, opioids, cannabinoids) that may affect gastrointestinal motility at least 72h prior to testing. After an overnight fast, subjects consumed a standardized low-fat meal. Immediately after meal ingestion, subjects swallowed the WMC while simultaneously undergoing image acquisition for scintigraphy as previously described (see Supplement for details).5,7
Assessment of Gastric Emptying:
Delayed GET was defined as >5h for passage of the capsule into the duodenum while abnormal GES was defined as >10% meal retention at 4h.7 Severe delay in gastric emptying was defined as GET >12h by WMC or >35% retention at 4h by GES.1,8 Rapid gastric emptying by GES was defined as <38% meal retention at 1h or GET <1:45h by WMC.9 All WMC studies were interpreted by a centralized, blinded reader (BK) to ensure data consistency.
Regional and Whole Gut Transit:
Delayed small bowel transit time (SBTT) was defined as >6h while delayed colonic transit time (CTT) was defined as >58:45h.9 Generalized transit delays were defined as ≥2 regions with delayed transit while global transit delays were defined as delay in all three regions (gastric, small bowel, and colon).
Pressure Parameters:
Normal values for gastric and small bowel number of contractions (Ct) were defined as >29/h and >36/h, respectively, while normal gastric and small bowel area under the curve (AUC) values were set as ≥1359 and ≥1456 mmHg/min, respectively.8
Statistical Analysis:
The primary outcome was analyzed as a measure of per patient device agreement for the diagnosis of delayed gastric emptying between WMC and GES. Positive agreement is defined as the probability of a positive test by WMC given positive GES while negative agreement is defined as the probability of a negative test by WMC given negative GES. Maximum likelihood estimates of positive and negative percent agreement were computed based on conditional relative frequencies in addition to corresponding 95% CI. Additional measures including estimates of overall percent agreement and Cohen’s kappa were also calculated. Differences between groups were compared using Wilcoxon rank sum test for continuous measures, Chi-square or Fisher’s exact test for unpaired categorical measures, and McNemer’s tests for paired categorical data. Multiple and logistic regression models were utilized to assess the robustness of group comparison results and to statistically adjust for group differences in age, gender, body mass index (BMI), duration of symptoms, marijuana and/or opiate use. P-values <.05 were considered significant. Data were analyzed using R (version 3.4.3) and GraphPad Prism (version 7.05). All authors had access to the study data and reviewed and approved the final manuscript.
Results:
Agreement in Gastric Emptying Between WMC and GES:
A total of 167 subjects (53 diabetic, 114 non-diabetic) were enrolled (Table 1) while data from 154 subjects who underwent simultaneous WMC and GES were available to review (Supplemental Figure 1). GET was delayed in 53 subjects (34.6%) while GES showed delayed gastric emptying in 39 subjects (24.5%, P=.009) (Fig. 1a). Overall device agreement between WMC and GES was noted in 75.7%, 95% CI [68.8, 82.5%] with positive agreement seen in 72.2% (n=26) and negative agreement in 76.7% (n=89). There was moderate agreement in parameters of gastric emptying by WMC and GES (kappa=0.42; 95% CI [0.27, 0.57]).
Table 1.
Demographic characteristics of enrolled subjects.
| Study Demographicsa | ||||
|---|---|---|---|---|
| All Patients (n=167) |
Diabetics (n=53) |
Non- Diabetics (n=114) |
p-valueb | |
| Age, years | 45.1 (13.8) | 51.1 (12.7) | 42.4 (13.4) | <0.01 |
| Female gender | 131 (78.4%) | 36 (67.9%) | 95 (83.3%) | 0.02 |
| BMI | 28.1 (6.9) | 31.0 (6.3) | 26.8 (6.7) | <0.01 |
| Caucasian race | 144 (87.3%) | 43 (82.7%) | 101 (89.4%) | 0.23 |
| Diabetes Subtype Type 1 Type 2 |
18 (44.0%) 35 (66.0%) |
|||
| Duration of symptoms (years) | 6.0 (7.4) | 6.6 (7.9) | 5.8 (7.1) | 0.51 |
| GCSI Total Score at Baseline | 2.9 (1.0) | 2.5 (1.0) | 2.7 (1.0) | 0.23 |
| Active marijuana usec | 14 (8.4%) | 5 (9.4%) | 9 (7.9%) | 0.74 |
| Active opiate usec | 19 (11.4%) | 5 (9.4%) | 14 (12.3%) | 0.59 |
Mean (standard deviation) unless otherwise noted
p-values are comparing diabetic vs. non-diabetic subjects
Marijuana and opiate use were active at time of baseline visit but held at least 72 h prior to motility testing
Figure 1.
a) Overall, there was a significantly higher rate of delayed gastric emptying detected by GET (dark blue) when compared to GES (light blue) (34.6% vs. 24.5%, p=0.009). In non-diabetic subjects, WMC detected significantly more subjects with delayed gastric transit when compared to GES (33.3% vs. 17.1%, p<0.001). In diabetic subjects, there were no differences in rates of delayed gastric emptying by GES and GET (37.2% vs. 41.7%, p=0.48).
b) Diabetic subjects (light green) were more likely to have delayed gastric emptying detected by GES when compared to non-diabetic subjects (dark green) (41.7% vs. 17.1%, p=0.002). However, there were no differences in rates of delayed gastric emptying by WMC between diabetic and non-diabetic subjects.
Agreement in Gastric Emptying Between Diabetic vs Non-Diabetic Etiology:
Subgroup analyses for device agreement in diabetic vs. non-diabetic subjects showed distinct results (Table 2). In diabetic subjects, GET was delayed in 37.2% while delayed GES was noted in 41.7% (P=.48) (Figure 1a). Overall device agreement between WMC and GES in diabetic subjects was 81.0% (n=34) with positive agreement seen in 72.2% (n=13) and negative agreement in 87.5% (n=21) (kappa = 0.61; 95% CI [0.36, 0.85]). In non-diabetic subjects, overall device agreement between WMC and GES was seen in 73.6% (n=81) with positive agreement in 72.2% (n=13) and negative agreement in 73.9% (n=68) (kappa=0.33; 95% CI [0.14, 0.50]). In non-diabetics, there was a significant increase in delayed gastric transit by WMC (33.3%, n=37) compared with GES (17.1%, n=19) (P<.001). Conversely, diabetic subjects were more likely to show delayed gastric emptying with GES vs. non-diabetics (41.7% vs. 17.1%, P=.002) (Figure 1b). However, there were no differences in rates of delayed GET between diabetic and non-diabetic subjects.
Table 2.
Prevalence of Transit Delays and Contractility Abnormalities in Patients with Suspected Gastroparesis by WMC and GES.
| Measure | All Patients (N=167) N (%) or Median (IQR) |
Diabetic Patients (N=53) |
Non-Diabetic Patients (N=114) |
p-valuea | Adjusted p-valueb |
|---|---|---|---|---|---|
| Delayed GES (>10% solid retention at 4 h) | 39/159 (24.5%) | 20/48 (41.7%) | 19/111(17.1%) | 0.002 | 0.01 |
| Severely Delayed GES (>35% solid retention at 4 h) | 11/159 (6.9%) | 7/48 (14.6%) | 4/111 (3.6%) | 0.01 | 0.07 |
| Rapid GES (<38% solid retention at 1 h) | 22/160 (13.8%) | 9/49 (18.4%) | 13/111 (11.7%) | 0.26 | 0.94 |
| Delayed WMC GET (GET > 5 h) | 53/153 (34.6%) | 16/43 (37.2%) | 37/110(33.3%) | 0.67 | 0.19 |
| Severely Delayed WMC GET (GET > 12 h) | 21/153 (13.8%) | 9/43 (20.9%) | 14/110 (12.7%) | 0.21 | 0.19 |
| Rapid WMC GET (GET < 1:45 h) | 5/153 (3.3%) | 4/43 (9.3%) | 1/110 (0.9%) | 0.02 | 0.28 |
| WMC SBTT (h) | 4.6 (3.5, 5.8) | 4.2 (3.1, 5.2) | 4.8 (4.0, 6.1) | 0.01 | 0.21 |
| Delayed WMC SBTTc (SBTT > 6 h) | 34/149 (22.8%) | 5/40 (12.5%) | 29/109 (26.6%) | 0.07 | 0.40 |
| WMC CTT (h) | 39.3 (17.3, 67.9) | 43.7 (20.2, 65.3) | 36.9 (16.8, 67.9) | 0.23 | 0.15 |
| Delayed WMC CTTc (CTT > 58:45 h) | 47/149 (31.5%) | 13/40 (32.5%) | 34/109 (31.2%) | 0.88 | 0.39 |
| Transit Delay in ≥ 2 regions by WMC | 32/152 (21.1%) | 5/40 (12.5%) | 27/109 (24.8%) | 0.11 | 0.62 |
| Contractility Measures | |||||
| Gastric Ct/h | 47.5 (26, 87) | 48 (23, 93) | 47 (29, 87) | 0.84 | 0.91 |
| Reduced gastric Ct (<29/h) | 40/150 (26.7%) | 13/43 (30.2%) | 27/107 (25.2%) | 0.53 | 0.20 |
| Gastric AUC | 3003 (1546, 5104) | 2533 (1390, 5626) | 3088 (1740, 4982) | 0.52 | 0.45 |
| Reduced gastric AUC (<1359) | 28/149 (18.8%) | 10/42 (23.8%) | 18/107 (16.8%) | 0.32 | 0.22 |
| Small bowel Ct/h | 111 (49, 180) | 134 (66, 237) | 110 (43, 166) | 0.17 | 0.61 |
| Reduced small bowel Ct (<36/h) | 27/151 (17.9%) | 6/42 (14.3%) | 21/109 (19.3%) | 0.47 | 0.53 |
| Small Bowel AUC | 3435 (1464, 5689) | 4440 (1533, 8117) | 3297 (1393, 5195) | 0.09 | 0.14 |
| Reduced small bowel AUC (< 1456) | 38/151 (25.2%) | 9/42 (21.4%) | 29/109 (26.6%) | 0.51 | 0.60 |
GES, gastric emptying scintigraphy; WMC, wireless motility capsule; GET, gastric emptying time; SBTT, small bowel transit time; CTT, colonic transit time; Ct, contractions; AUC, area under the curve.
p-value comparing diabetics vs. non-diabetic subjects.
p-value comparing diabetics vs. non-diabetic subjects after adjusting for age, gender, BMI, duration of symptoms, and history of marijuana and/or opioid use.
Small bowel and colonic transit times could not be calculated on a total of 4 subjects due to incomplete data (3 studies due to capsule retention in the stomach and 1 study related to operator error).
Agreement for Severe Delay in Gastric Emptying:
Severely delayed gastric emptying by GES was seen in 6.9% (n=11) vs. 13.8% by GET (n=21, P=.02). Diabetic subjects were more likely to have severe delays in gastric emptying by GES compared with non-diabetic subjects (14.6% vs. 3.6%, P=.01). There were no differences in rates of severe delay by WMC between diabetic and non-diabetic subjects. There was fair agreement between severe gastric emptying delays by WMC and GES (kappa=0.38; 95% CI [0.15, 0.60]).
Rapid Gastric Emptying:
Rapid emptying by GES was seen in 13.8% (n=22) compared with rapid GET in 3.3% (n=5, P<.001) (Figure 2). In diabetics, 18.4% of subjects showed rapid GES compared with 9.3% with rapid GET (P=.16). In non-diabetic subjects, a higher proportion of subjects showed rapid GES (11.7%) compared with WMC (0.9%) (P<.001). There were no differences in the rates of rapid GES when comparing diabetic vs. non-diabetic subjects (18.4% vs. 11.7%, P=.26). However, WMC was more likely to detect rapid gastric emptying in diabetic compared with non-diabetic subjects (9.3% vs. 0.9%, P=.02).
Figure 2.
There were significantly higher rates of rapid gastric emptying with GES (light blue) when compared to GET (dark blue) in all subjects (13.8% vs. 3.3%, p<0.001). In non-diabetic subjects, a higher number of subjects showed rapid gastric emptying with GES compared to WMC (11.7% vs. 0.9%, p<0.001). In diabetic subjects, there were no differences in detection of rapid gastric emptying by GES compared with GET (18.4% vs. 9.3%, p=0.16). WMC was more likely to detect rapid gastric emptying in diabetics compared with non-diabetic subjects (9.3% vs. 0.9%, p=0.02). However, there were no differences in rapid gastric emptying by GES in diabetics vs. non-diabetics.
Extra-gastric Transit Delays by WMC:
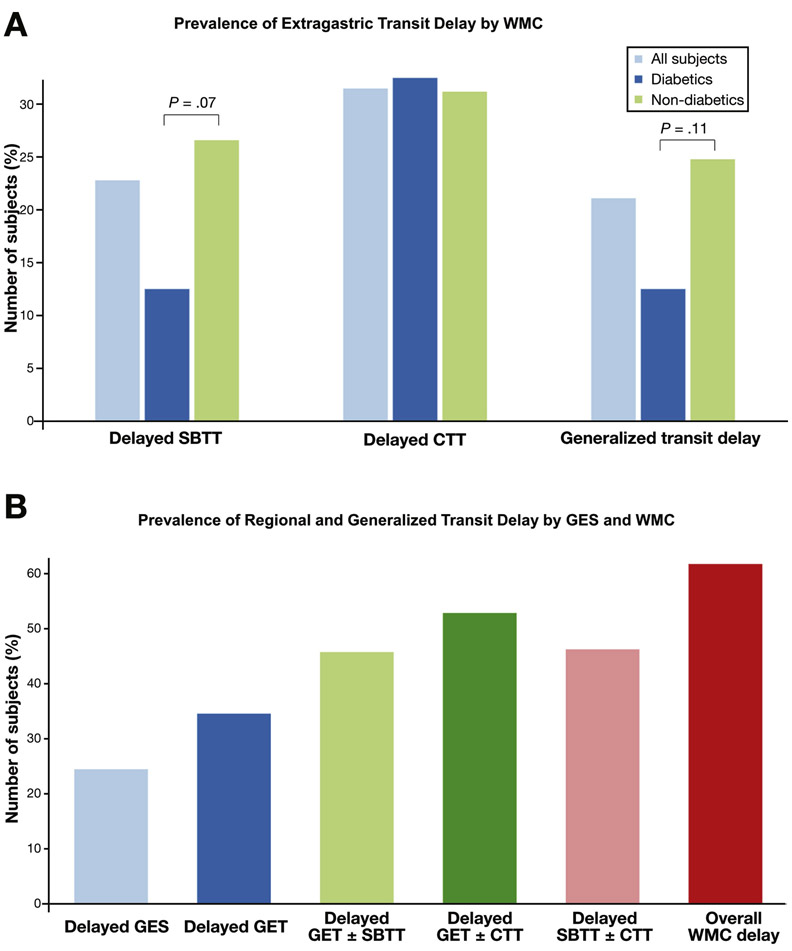
Extra-gastric transit delays were seen in 45.6% (n=68) subjects including delayed SBTT in 22.8% (n=34) and delayed CTT in 31.5% (n=47) (Figure 3a). Generalized transit delays were seen in 21.1% (n=32) while global transit delays were noted in 5.4% (n=8). Overall, 61.8% subjects (n=94) had isolated or generalized transit delays by WMC (Figure 3b). In diabetic subjects, delayed SBTT was seen in 12.5% (n=5), delayed CTT in 32.5% (n=13), and generalized transit delays in 12.5% (n=5). In non-diabetic subjects, delayed SBTT was found in 26.6% (n=29), delayed CTT in 31.2% (n=34) and generalized transit delays in 24.8% (n=27). Non-diabetic subjects displayed a longer small bowel transit time compared with diabetics (5.18 vs. 4.34h, P=.01). Non-diabetic subjects also showed trends toward higher rates of delayed SBTT (P=.07) as well as generalized delays (P=.11) compared with diabetics.
Figure 3.
(A) Delayed SBTT was noted in 22.8% of all subjects (light blue) including 12.5% of diabetic (dark blue) and 26.6% of non-diabetic subjects (green) (p=0.07). Delayed CTT was seen in 31.5% of all subjects including 32.5% of diabetics and 31.2% of non-diabetic subjects. Generalized transit delays were found in 21.1% of subjects including 12.5% of diabetics and 24.8% of non-diabetic subjects (p=0.11). p-values are comparing diabetics with non-diabetics.
(B) Delayed GES (light blue) was seen in 24.5% while delayed GET (dark blue) was detected in 34.6%. Delayed GET±SBTT (light green) was found in 45.8%, GET±CTT (dark green) in 52.9%, SBTT±CTT (pink) in 46.3% and overall any delay by WMC (red) was detected in 61.8%.
Gastric and Small Bowel Contractile Abnormalities by WMC:
Gastric and/or small bowel contractility abnormalities were seen in 40.4% (n=61) of all subjects (Table 2). Abnormal gastric contractility measures were noted in 26.7% (n=40) with reduced antral Ct and 18.8% (n=28) with abnormal AUC. Abnormal small bowel contractility measures were seen in 17.9% (n=27) with reduced Ct and 25.2% (n=38) with abnormal AUC. There were no differences in gastric or small bowel contractility measures between diabetic and non-diabetic subjects.
Association of Gastric Emptying by WMC to Other Measures:
There was a significantly increased rate of delayed GES in those subjects with delayed GET vs. those with normal GET (49.1% vs. 10.1%, P<.001) (Table 3). This association between delayed GET and delayed GES was observed in both diabetic and non-diabetic subjects. In addition, delayed SBTT was more commonly observed in those subjects with delayed GET than with normal GET (34.0% vs. 17.1%, P=.02). This association was observed in non-diabetic (41.7% vs. 19.2%, P=0.01) but not in diabetic subjects (14.3% vs. 11.5%, P=.80). Furthermore, non-diabetic subjects with delayed GET showed higher rates of small bowel contractile abnormalities, including small bowel Ct (36.1% vs. 11.0%, P=.004) and AUC (38.9% vs. 20.5%, P=.06) compared with normal GET. However, these associations were not observed in diabetic subjects.
Table 3.
Association of Normal vs. Abnormal GET to Other Transit and Contractile Measures
| Transit Measures | All Subjects | p-valuea | Adjusted p-valueb |
|
|---|---|---|---|---|
| Delayed GET | Normal GET | |||
| Delayed GES (> 10% solid retention at 4h) | 26/53 (49.1%) | 10/99 (10.1%) | <0.001 | <0.001 |
| Delayed SBTT (> 6h) | 17/50 (34.0%) | 17/99 (17.1%) | 0.02 | 0.11 |
| Delayed CTT (> 58:45 h) | 19/50 (38.0%) | 28/99 (28.3%) | 0.23 | 0.10 |
| Reduced Gastric Ct (< 29/h) | 18/50 (36.0%) | 22/100 (22.0%) | 0.07 | 0.14 |
| Reduced Gastric AUC (< 1359 mmHg/min) | 12/49 (24.5%) | 16/100 (16.0%) | 0.21 | 0.28 |
| Reduced SB Ct (< 36/h) | 16/51 (31.4%) | 11/100 (11.0%) | 0.002 | 0.01 |
| Reduced SB AUC (< 1456 mmHg/min) | 18/51 (35.3%) | 20/100 (20.0%) | 0.04 | 0.32 |
| Transit Measures |
Diabetic Subjects | p- valuea* |
Non-diabetic Subjects | p- valuea |
Adjusted p-valueb |
||
|---|---|---|---|---|---|---|---|
| Delayed GET |
Normal GET |
Delayed GET |
Normal GET |
||||
| Delayed GES (> 10% solid retention 4h) | 13/16 (81.3%) | 5/26 (19.2%) | <0.001 | 13/37 (35.1%) | 5/73 (6.8%) | <0.001 | 0.002 |
| Delayed SBTT (> 6h) | 2/14 (14.3%) | 3/26 (11.5%) | 0.80 | 15/36 (41.7%) | 14/73 (19.2%) | 0.01 | 0.06 |
| Delayed CTT (> 58:45 h) | 5/14 (35.7%) | 8/26 (30.8%) | 0.75 | 14/36 (38.9%) | 20/73 (27.4%) | 0.22 | 0.08 |
| Gastric Ct (< 29/h) | 8/16 (50.0%) | 5/27 (18.5%) | 0.03 | 10/34 (29.4%) | 17/73 (23.3%) | 0.50 | 0.66 |
| Gastric AUC (< 1359) mmHg/min) | 5/15 (33.3%) | 5/27 (18.5%) | 0.28 | 7/34 (20.6%) | 11/73 (15.1%) | 0.48 | 0.40 |
| SB Ct (< 36/h) | 3/15 (20.0%) | 3/27 (11.1%) | 0.43 | 13/36 (36.1%) | 8/73 (11.0%) | 0.004 | 0.005 |
| SB AUC (< 1456 mmHg/min) | 4/15 (26.7%) | 5/27 (18.5%) | 0.54 | 14/36 (38.9%) | 15/73 (20.5%) | 0.06 | 0.27 |
p-value comparing delayed GET vs. normal GET.
p-value comparing delayed GET vs. normal GET after adjusting for age, gender, BMI, duration of symptoms, and history of marijuana and/or prior opioid use.
Multivariate analyses were not performed for diabetic subjects due to the small sample size.
Adverse Events:
A total of 37 adverse events (AEs) were reported, including 22 non-serious and 15 serious AEs (Supplemental Table 1). All serious AEs resolved. One serious AE of capsule retention was noted with WMC which resolved after endoscopic retrieval. No serious AEs were related to GES. There were no AEs related to fasting for 8h after WMC ingestion.
Discussion:
This was a prospective multicenter study designed to critically examine the diagnostic and performance characteristics of WMC compared to the current standard of GES in patients with suspected gastroparesis. We found evidence to validate the use of WMC in evaluating these patients, including good device agreement between GES and WMC. Furthermore, WMC detected a significantly higher proportion of subjects with both delayed as well as severely delayed gastric emptying when compared with GES. Also, subgroup analyses based on the etiology for gastroparesis demonstrated important differences between GES and WMC. Specifically, we found that non-diabetic subjects who accounted for two-thirds of the study population were significantly more likely to demonstrate delayed GET with WMC when compared to GES. In contrast, diabetic subjects were more likely to show delayed gastric emptying with GES when compared to non-diabetic subjects. Finally, WMC detected 61.8% subjects with regional and/or generalized transit delays resulting in an additional diagnostic yield of 37.3% when compared to 24.5% of subjects with delayed GES.
Our results confirm and extend previous findings by Kuo et al. who reported good correlation (r=0.73) between WMC and GES.5 However, there are important differences that merit discussion. First, this current study was performed in a cohort of patients with suspicion of but without formal diagnosis of gastroparesis. In contrast, eligibility requirements in the study by Kuo required a prior history of delayed GES that led to an enriched population of gastroparesis. Also, our study investigated extra-gastric transit and contractile abnormalities. Recently, Hasler et al. also compared performance characteristics of WMC and GES.6 This study showed lower device agreement between WMC and GES (kappa = 0.12). However, a key difference in this previous study was sequential performance of GES followed by WMC up to 6 months later. This is in contrast to the current study where GES and WMC were performed simultaneously. The significant intra-individual variation in gastric emptying rates of up to 24% and the time difference between the two studies may each explain some of the differences seen between these studies.10 Secondly, Hasler also evaluated an enriched population with prior abnormal GES. Our results suggest that the level of agreement between WMC and GES is more in line with data reported by Kuo.
This study demonstrated that WMC was significantly more likely to detect delayed gastric emptying when compared to GES. An additional 23% of subjects with normal GES had delayed GET by WMC which is similar to prior results.5 Although gastric emptying by WMC and GES are highly correlated, they may not be measuring identical parameters. While both tests depend on the rate of meal emptying, WMC is measuring emptying of an indigestible object which is facilitated by return of phase III activity of the migrating motor complex (MMC).11 Thus, WMC may have increased sensitivity for detecting gastroparesis as it measures both gastric emptying time, impaired MMC and dyscoordination of gastric and small bowel motility whereas scintigraphy only measures meal emptying.
Our study further demonstrated important differences when subtyped by etiology. Diabetic subjects were more likely to show delayed gastric emptying by GES compared with non-diabetic subjects. In contrast, non-diabetic subjects were more likely to show delayed GET compared with GES. Our results contrast with data from Hasler who found higher rates of delayed GET but not GES in diabetics compared with idiopathic gastroparesis.6 Since GES was always performed first in this study, it is possible that gastric emptying had normalized in a subset of idiopathic patients by the time WMC was performed. This is supported by preliminary data from the Gastroparesis Consortium that suggests gastric emptying is more likely to normalize over 48 weeks in idiopathics compared with diabetic gastroparetics (41% vs. 22%, P=.07).12 Our results support potential phenotypic differences between diabetic and non-diabetic patients. Antral hypomotility is commonly seen in patients with gastroparesis.13 This is important because emptying of digestible solids depends mainly on antral function whereas the emptying of indigestible solids, such as WMC, depends on the antral component of phase III of MMC.14 It is possible that diabetic gastroparesis preferentially affects processes related to postprandial antral hypomotility while non-diabetic subjects display abnormal interdigestive function which is not assessed by GES.
Severe delays in gastric emptying were more likely to be detected by WMC compared with GES. Few studies have looked at stratifying subjects based on the degree of gastric emptying delays. Parkman et al. demonstrated that severe delays in GES correlated with more severe overall symptoms in idiopathic gastroparesis.15 Meanwhile, Hejazi et al. suggested that patients with severe delays in GES were less likely to respond to medical therapy which often led to placement of gastric electrical stimulators.16 Although these findings are intriguing, data on severe delays in gastric emptying are difficult to interpret as true cut-offs are not clearly defined.
Our results indicate that rapid gastric emptying was more likely to be detected by GES compared with WMC likely because rapid emptying is not dependent on MMC. Accelerated gastric emptying is seen in approximately 20% of patients with diabetes. Recently, hyperglycemia was found to increase the numbers of gastric ICCs leading to rapid gastric emptying in a mouse model of obesity.17 However, the definitions for accelerated gastric emptying are still not clearly defined and the clinical significance for rapid emptying requires further validation.
An intriguing finding in our study was the high rate of small bowel dysmotility, particularly in non-diabetic subjects. We also demonstrated significant associations between delayed GET by WMC and small bowel dysmotility, including delayed SBTT and reduced small bowel contractile parameters. These abnormalities were more commonly observed in non-diabetic subjects, which suggests that altered gastric-small bowel coordination and a more diffuse gastrointestinal dysmotility may be important in this subset of patients. Although the clinical significance of delayed small bowel transit is not clearly understood, there is evidence to suggest its relevance in gastroparesis. One study demonstrated that patients with both IBS and gastroparesis commonly show small bowel dysmotility.18 Another study demonstrated that patients with chronic, unexplained gastrointestinal symptoms and presence of small intestinal bacterial overgrowth showed significant delays in SBTT.19 These results suggest that small bowel dysmotility may play an important but underrecognized pathogenic factor in gastroparesis.
Prior studies have demonstrated considerable overlap between upper and lower GI symptoms in gastroparesis.2,15 Our results confirm that 62% of subjects had transit abnormalities by WMC and nearly one-half showed extragastric transit abnormalities which may explain the wide range of symptoms encountered in gastroparesis. Previous studies have also documented high prevalence of extragastric transit delays, termed diagnostic gain, which influenced patient management.3,4 Similar to our results, Hasler demonstrated extra-gastric transit delays in >40% of subjects.6 Our findings support investigation of whole gut transit and motility in this population to identify important physiologic abnormalities and allow for more objective therapeutic choices.
There are limitations to our study. First, there was a high prevalence of patients with normal gastric emptying. However, this reflects clinical reality where symptoms do not reliably distinguish patients with gastroparesis.20 In addition, there was a predominance of Caucasian female subjects in our cohort which mirrors the clinical population seeking care for gastroparesis.2,15 Secondly, a liquid meal was administered 8h after WMC ingestion to mitigate against concerns for hypoglycemia. This liquid meal could prolong GET and artificially increase the number of subjects with severe delays by WMC. However, only two subjects in this severely delayed cohort had a GET < 18h while the mean GET was >50h. This suggests that these findings are likely related to underlying severe motor abnormalities rather than methodologic issues. Finally, we did not correlate physiologic results with symptoms or outcome data as this was outside of the scope for this specific study. This introduces the potential for bias with our study. Furthermore, our understanding of the clinical significance of detecting transit abnormalities remains incomplete given the poor correlation between gastric emptying and/or contractile measures and symptoms.6 Other pathogenic factors, such as gastric accommodation and gastric hypersensitivity, which cannot be measured with gastric emptying tests may also be important in some patients.20 However, transit measures may inform therapeutic response as suggested by a recent clinical trial showing that gastric motility testing may have important clinical implications that predict outcomes.21 Further transit measures often help pinpoint the location of a motility abnormality more precisely than symptoms alone.22 Information on symptom data in our study has been collected and correlations between physiologic data and symptom outcomes will be analyzed in future reports.
In conclusion, we found that WMC demonstrates comparable performance characteristics as well as important differences with GES in a prospective cohort of patients with suspected gastroparesis. Overall, WMC had significantly higher diagnostic yield for delayed gastric emptying when compared to GES suggesting greater sensitivity for detection of gastroparesis. For diabetics, the yield for delayed gastric emptying was similar between the two devices but in non-diabetics there was a significantly higher yield with WMC. Also, WMC identified delays in small bowel and colonic transit that will be missed if testing solely evaluates gastric emptying. Together, these findings indicate that WMC offers a more comprehensive assessment of patients with suspected gastroparesis, both because of increased sensitivity for detecting delayed gastric emptying as well as higher diagnostic yield. These improved diagnostic findings could impact patient management.
Supplementary Material
Acknowledgements:
The authors thank Dr. Richard Krause for his assistance in the conduct of this study as well as Mathilde Lourde, Senior Biostatistician Medtronic, for her assistance with statistical analyses for this manuscript.
Abbreviations:
- AUC
area under the curve
- Ct
number of contractions
- CTT
colonic transit time
- GES
gastric emptying study
- GET
gastric emptying time
- IBS
irritable bowel syndrome
- ICC
interstitial cells of Cajal
- MMC
migrating motor complex
- SBTT
small bowel transit time
- WMC
wireless motility capsule
Footnotes
Disclosures: Satish Rao, Baharak Moshiree, Richard McCallum, William Hasler, and Braden Kuo received research grant support from Medtronic. Irene Sarosiek and Braden Kuo serve as consultants for Medtronic. All other authors have no disclosures to report.
Declaration of Funding Interests: This study was supported by a grant from Medtronic.
References:
- 1.Abell TL, Camilleri M, Donohoe K, et al. Consensus Recommendations for Gastric Emptying Scintigraphy: A Joint Report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 2008;103:753–763. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol 2011;9:1056–1064-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SSC, Mysore K, Attaluri A, et al. Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol 2011;45:684–690. [DOI] [PubMed] [Google Scholar]
- 4.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci 2011;56:2928–2938. [DOI] [PubMed] [Google Scholar]
- 5.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther 2008;27:186–196. [DOI] [PubMed] [Google Scholar]
- 6.Hasler WL, May KP, Wilson LA, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil 2018;30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol 2000;95:1456–1462. [DOI] [PubMed] [Google Scholar]
- 8.Kloetzer L, Chey WD, McCallum RW, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil 2010;22:527–533, e117. [DOI] [PubMed] [Google Scholar]
- 9.Wang YT, Mohammed SD, Farmer AD, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther 2015;42:761–772. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012;24:1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil 2008;20:311–319. [DOI] [PubMed] [Google Scholar]
- 12.Parkman HP, Yates K, McCallum R, et al. Gastric emptying changes over time in gastroparesis: Comparison of initial and 48 week follow up gastric emptying tests in the Gastroparesis Registry of the Gastroparesis Consortium. Present Dig Dis Week Wash DC 2018. [Google Scholar]
- 13.Stanghellini V, Ghidini C, Maccarini MR, et al. Fasting and postprandial gastrointestinal motility in ulcer and non-ulcer dyspepsia. Gut 1992;33:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minami H, McCallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology 1984;86:1592–1610. [PubMed] [Google Scholar]
- 15.Parkman HP, Yates K, Hasler WL, et al. Clinical Features of Idiopathic Gastroparesis Vary With Sex, Body Mass, Symptom Onset, Delay in Gastric Emptying, and Gastroparesis Severity. Gastroenterology 2011;140:101–115.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hejazi RA, Sarosiek I, Roeser K, et al. Does Grading the Severity of Gastroparesis Based on Scintigraphic Gastric Emptying Predict the Treatment Outcome of Patients with Gastroparesis? Dig Dis Sci 2011;56:1147–1153. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Toyomasu Y, Saravanaperumal SA, et al. Hyperglycemia Increases Interstitial Cells of Cajal via MAPK1 and MAPK3 Signaling to ETV1 and KIT, Leading to Rapid Gastric Emptying. Gastroenterology 2017;153:521–535.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans PR, Bak YT, Shuter B, et al. Gastroparesis and small bowel dysmotility in irritable bowel syndrome. Dig Dis Sci 1997;42:2087–2093. [DOI] [PubMed] [Google Scholar]
- 19.Roland BC, Ciarleglio MM, Clarke JO, et al. Small Intestinal Transit Time Is Delayed in Small Intestinal Bacterial Overgrowth. J Clin Gastroenterol 2015;49:571–576. [DOI] [PubMed] [Google Scholar]
- 20.Karamanolis G, Caenepeel P, Arts J, et al. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut 2007;56:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talley NJ, Locke GR, Saito YA, et al. Effect of Amitriptyline and Escitalopram on Functional Dyspepsia: A Multicenter, Randomized Controlled Study. Gastroenterology 2015;149:340–349.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HC, Prather C, Fisher RS, et al. Measurement of gastrointestinal transit. Dig Dis Sci 2005;50:989–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.