The motor cortex sculpts complex spatiotemporal patterns of output by actively turning off muscles, as well as by turning them on.
Abstract
Even the simplest movements are generated by a remarkably complex pattern of muscle activity. Fast, accurate movements at a single joint are produced by a stereotyped pattern that includes a decrease in any preexisting activity in antagonist muscles. This premovement suppression is necessary to prevent the antagonist muscle from opposing movement generated by the agonist muscle. Here, we provide evidence that the primary motor cortex (M1) sends a command signal that generates this premovement suppression. Thus, output neurons in M1 sculpt complex spatiotemporal patterns of motor output not only by actively turning on muscles but also by actively turning them off.
INTRODUCTION
Simple movements at single joints are produced by a remarkably complex pattern of muscle activity, which was first described by Wachholder and Altenburger (1) [for references and review, see Sternad (2)]. This pattern includes a striking premovement suppression of activity in antagonist muscles (Fig. 1A). This decrease in activity precedes the onset of activity in agonist muscles by 40 to 50 ms (Fig. 1B). The antagonist suppression was rediscovered by Hufschmidt and Hufschmidt (3) and is often referred to as the “Hufschmidt phenomenon” (4, 5). The premovement suppression of antagonist muscle activity is essential for normal motor control; its impairment in Huntington’s disease and cerebral palsy is associated with striking motor disability (6, 7).
Fig. 1. The Hufschmidt phenomenon associated with rapid wrist movements.
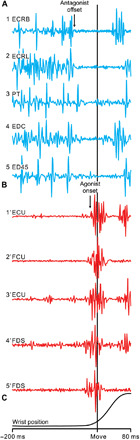
(A) Early suppression of muscle activity in antagonist muscles before the onset of activity in agonist muscles (i.e., the Hufschmidt phenomenon). (B) Later bursts of activity in agonist muscles before movement onset. (C) Wrist position measured by potentiometers coupled to a manipulandum. The muscle activity in (A) and (B) is aligned on movement onset (vertical line). These patterns of muscle activity are associated with different directions of wrist movement made with the wrist in different postures. Extensor carpi radialis brevis (ECRB) acts as an antagonist (blue), and extensor carpi ulnaris (ECU) acts as an agonist (red) for a movement to the 135° target (flexion plus ulnar deviation) with the wrist pronated. Extensor carpi radialis longus (ECRL) acts as an antagonist (blue), and flexor carpi ulnaris (FCU) acts as an agonist (red) for a movement to the 225° target (flexion plus ulnar deviation) with the wrist in the middle posture. Pronator teres (PT) acts as an antagonist (blue), and ECU acts as an agonist (red) for a movement to the 180° target (ulnar deviation) with the wrist in the middle posture. Extensor digitorum communis (EDC) acts as an antagonist (blue), and flexor digitorum sublimis (FDS) acts as an agonist (red) for a movement to the 225° target with the wrist in the middle posture. Extensor digiti quarti and quinti proprius (ED45) acts as an antagonist (blue), and FDS acts as an agonist (red) for movements to the 225° target with the wrist in the middle posture.
There is a general consensus that the Hufschmidt phenomenon is centrally generated. This conclusion is based on two key observations: (i) the onset of the antagonist inhibition is too early to be generated by peripheral feedback, and (ii) the Hufschmidt phenomenon is present even in subjects who have neuropathies that abolish peripheral feedback (8). Here, we provide further evidence for the central origin of the Hufschmidt phenomenon. We show that it can be generated by a specific set of output neurons in primary motor cortex (M1) that disynaptically inhibit the activity of motoneurons, which innervate antagonist muscles.
RESULTS
We recorded neuron activity in M1 and the activity of 13 different forearm muscles while a monkey performed a center-out wrist task that required 24 rapid movements in three different wrist postures (9, 10). In addition, we used spike-triggered averaging (SpTA) to identify M1 output neurons that produced postspike effects (n = 50) in the electromyographic (EMG) activity of at least one of the 13 recorded muscles (11–13).
Fifty of the M1 neurons recorded during the wrist task displayed postspike effects. Sixteen of these neurons (32% of the sample) produced postspike suppression in at least one of the recorded muscles [termed “target” muscles; Figs. 2 (B and C), 3 (B and C), and 4B]. The average latency between the neuron spikes and the onset of the postspike suppressions was 9.9 ms (range, 7 to 13.6 ms). In contrast, the average latency between neuron spikes and the onset of postspike facilitations in our sample was 9.6 (range, 5.6 to 14.4 ms). These latency measures are consistent with those observed by others for postspike effects in wrist and digit muscles (14) and, specifically, for postspike suppressions observed by others [minimum, 7.3 ms (15); maximum, 14.7 ms; (16)]. Cheney et al., (17) first described the presence of postspike suppression triggered by recordings from M1 neurons, and they argued that the suppression was the result of a disynaptic corticospinal pathway that inhibits motoneurons (Figs. 2A and 3A). The timing of the postspike suppressions we and others have observed is consistent with this linkage (18–22).
Fig. 2. M1 neuron with a postspike suppression effect: Generation of the Hufschmidt phenomenon.
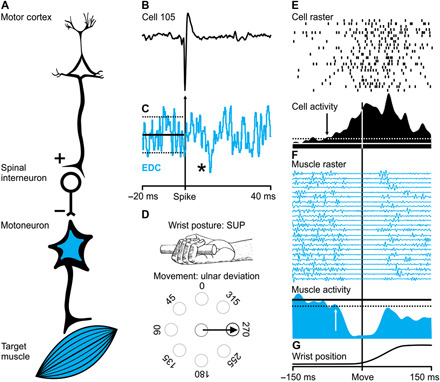
(A) Schematic representation of the putative neural circuitry that links an M1 neuron (cell 105) with a target muscle (EDC) to cause a postspike suppression. (B) An action potential of cell 105 (the trigger for averaging). (C) Postspike suppression in the SpTA (n = 7136 spikes) of EDC muscle activity. The asterisk indicates the size of the effect: *2 to 3.9 SD. (D) Premovement suppression for target muscle EDC occurs during a wrist movement to the 270° target (ulnar deviation) when the wrist is in the supinated position. (E) Raster and average of neural activity for cell 105. The arrow in the average indicates the onset time for the change in neural activity. (F) Raster and average of muscle activity (EDC) for the same trials as in (E). The arrow in the average indicates the offset time for the change in muscle activity. (G) Wrist position. All trials (n = 23) in (D) and (E) are aligned on movement onset for the condition depicted in (D). Note that the average change in neural activity precedes both the onset of the suppression of muscle activity and the onset of movement.
Fig. 3. M1 neuron with a postspike suppression in one muscle and a postspike facilitation in two other muscles.
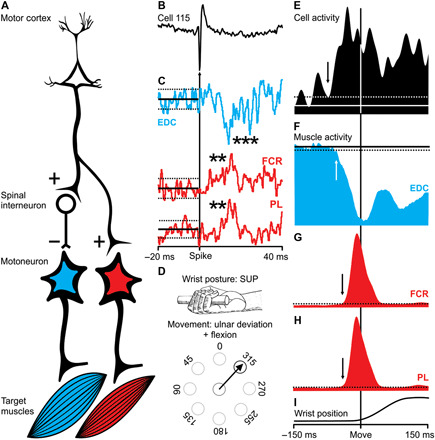
(A) Schematic representation of the putative neural circuitry that links an M1 neuron (cell 115) with target muscles to cause postspike suppression and facilitation. (B) An action potential of cell 115 (the trigger for averaging). (C) Postspike suppression (EDC) and facilitation [flexor carpi radialis (FCR) and palmaris longus (PL)] in the SpTAs (n = 7739, n = 5118, and n = 6155 spikes). The asterisks indicate the size of the effects: EDC = ***6 to 8.9 SD; FCR and PL = **4 to 5.9 SD. (D) Premovement suppression for target muscle EDC occurs during a wrist movement to the 315° target (flexion + ulnar deviation) when the wrist is in the supinated position. (E) Raster and average of neural activity for cell 115. The arrow in the average indicates the onset time for the change in neural activity. (F) Raster and average of muscle activity (EDC) for the same trials as in (E). The arrow in the average indicates the offset time for the change in muscle activity. (G and H) Averages of muscle activity (FCR and PL) for the same trials as in (E). (I) Wrist position. All trials (n = 11) in (E) to (H) are aligned on movement onset for the condition depicted in (D).
Fig. 4. M1 neurons producing postspike suppression in antagonist target muscles reflect functional tuning.
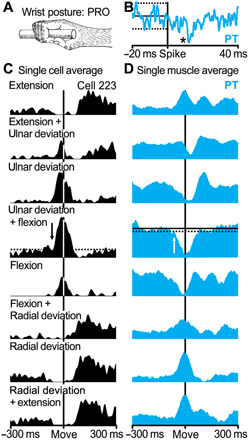
(A) Movement-related activity for cell 223 and suppressed target muscle PT when the wrist was in the pronated (PRO) position. (B) Postspike suppression in the SpTA (n = 16,406 spikes) of PT muscle activity. The asterisk indicates the size of the effect: *2 to 3.9 SD. (C) Average of neural activity for cell 223. M1 activity is aligned on movement onset for eight different movement conditions (n = 25 trials for each condition). The movement condition most closely associated with the cell’s preferred direction of movement also produced the largest premovement inhibition in the cell’s suppressed target muscle (PT). (D) Average of target muscle activity (PT) for the same movement conditions as in (C).
Eight of the 16 neurons that produced postspike suppression were “functionally tuned” to the Hufschmidt phenomenon (Figs. 2 to 4). In other words, the activity for these neurons was greatest when the target muscle served as an antagonist, and the target muscle showed the greatest premovement suppression (Fig. 4). SpTAs of four neurons in our sample produced postspike suppression in one muscle and postspike facilitation in another (Fig. 3C). This pattern of postspike effects is consistent with a cortical neuron that branches (17) (Fig. 3A). One branch provides monosynaptic input to a set of motoneurons (leading to postspike facilitation); another branch provides monosynaptic input to spinal interneurons that inhibit a different set of motoneurons (leading to postspike suppression). Movements made in the preferred direction of the neurons producing these combined postspike effects were associated with a Hufschmidt phenomenon in the target muscle displaying a postspike suppression (the antagonist; Fig. 3F), along with a later increase in the activity of the target muscles displaying a postspike facilitation (the agonists; Fig. 3, G and H).
An analysis of the timing of neuron and muscle activity associated with movement onset provides clear support for the causal link between M1 neurons that produce postspike suppression and the Hufschmidt phenomenon. The mean change in the movement-related activity for the population of neurons that produce postspike suppression and the mean change in the suppression of their target muscles occurred well before movement onset (Fig. 5A). Furthermore, the mean change in neuron activity preceded the mean onset of movement-related suppression in their target antagonist muscles by 12.3 ms. Similarly, the onset of the change in activity related to movement for neurons that produced postspike suppression occurred early enough to generate the onset of the Hufschmidt phenomenon in their target muscles (Fig. 5B). The median time for the onset of a change in the activity for individual neurons that produced postspike suppression was 70.9 ms before movement onset. The median time for the onset of the Hufschmidt phenomenon in their target muscles was 54.3 ms before movement onset. The difference of 16.6 ms between these measures is clearly sufficient to allow the change in neuron activity to contribute to, if not cause, the change in muscle activity.
Fig. 5. M1 neurons producing postspike suppression in antagonist target muscles reflect timing relationships consistent with their contribution to the Hufschmidt phenomenon.
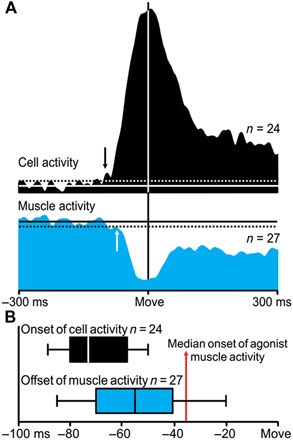
(A) Sample population of eight output neurons in M1 and their nine suppressed target muscles. Average activity for output neurons in M1 and their suppressed target muscle for the movement condition associated with the cell’s preferred direction in each of three wrist postures (n = 324 trials). The arrows in the averages show that the onset of cell activity consistently led the offset of target muscle activity. (B) Black box and whisker plot represents the individual measurements of the onset of cortical output neurons from the same movement conditions averaged in (A). Blue box and whisker plot represent the individual measurements of the offset of the suppressed target muscle’s activity from the same movement conditions averaged in (A). The center line represents the individual median values, the box limits represent the lower and upper quartiles, and the whiskers mark the smallest and largest sample values. The red vertical line marks the median onset of agonist muscle activity measured in the same movement conditions averaged in (A).
The concept that neurons producing postspike suppression are involved in the generation of the Hufschmidt phenomenon is further supported by the effects of M1 lesions on step-tracking movements of the wrist (23). Large lesions of M1 that included regions of cortex in the anterior bank of the central sulcus and the adjacent cortical surface caused marked changes in the performance of step-tracking movements. Even after considerable retraining, movement kinematics and the patterns of activity in agonist, synergist, and antagonist muscles were markedly altered. Specifically, the Hufschmidt phenomenon was abolished or greatly reduced in antagonist muscles. These results, along with those from the present study, support the conclusion that M1 is a source of a descending command signal that can turn off muscle activity.
As a population, the neurons that produced postspike suppression in antagonist muscles all displayed changes in movement-related activity that began well before movement onset (mean, −60.7 ms). These neurons displayed some of the earliest changes in the sample of movement-related neurons with postspike effects (P < 0.001; Fig. 6, A and B). The onset and peak activity of these neurons preceded that of neurons that displayed postspike facilitation in the same antagonist muscles (Fig. 6, B and C). Typically, one associates early increases in the activity of neurons in the motor cortex with the activation of agonist muscles that are necessary to initiate movement (Fig. 6D). However, the neurons that produce postspike suppression provide a clear example of early activity in M1 that results in the suppression of muscle activity. As a population, the time course of this activity is no different than that of neurons displaying postspike facilitation of an agonist (Fig. 6, B and D). This observation provides an alternative perspective about the significance of the earliest changes in the activity of neurons the motor cortex.
Fig. 6. Cortical output neurons in M1 that contribute to the Hufschmidt phenomenon are among the first to turn on.
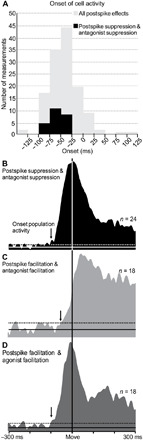
(A) Onset of the activity for M1 neurons that produced a postspike effect in averages of EMG activity (n = 50) for all wrist postures that were recorded (n = 136). Gray bars represent the entire sample of cortical output neurons in M1 that produced postspike facilitation or suppression in one or more target muscles. Black bars represent the subset of cortical output neurons that produced postspike suppression and were active to turn off their target muscle’s antagonist activity. (B) Neuron activity for the population of eight neurons that displayed postspike suppression of a target muscle and had a preferred direction that matched when the muscle functioned as an antagonist (same as Fig. 5A). (C) Neuron activity for the population of six neurons that displayed postspike facilitation of a target muscle and had a preferred direction that matched when the muscle functioned as an antagonist. (D) Neuron activity for the population of six neurons that displayed postspike facilitation of a target muscle and had a preferred direction that matched when the muscle functioned as an agonist. The arrows in the averages in (B) to (D) indicate the onset of the population’s activity.
DISCUSSION
The current trend in the field of motor control is to record from a large population of unidentified cortical neurons during naturalistic or trained arm movements. This approach has led to many valuable insights and new concepts about the cortical processing that is necessary to generate and control movement (24–28). Our results highlight the importance of using a different experimental approach. We recorded from “identified neurons” and their “target muscles” and used a motor task that produced recognizable phases of muscle activity. This enabled us to identify unique sets of cortical output neurons and draw specific conclusions about their function. We feel that it is important to emphasize the utility of this approach. Although it is time-consuming, low yield in terms of neuron numbers, and labor intensive, this is the only way one can unequivocally identify the functional contribution of individual output neurons in M1. In this case, it allowed us to recognize a unique set of output neurons that suppress muscle activity.
One of the new concepts that has emerged from this and our prior publication (10) is that output neurons in M1 are functionally tuned. For example, output neurons that have monosynaptic connections with a set of motoneurons are preferentially active when the innervated muscle is used for a specific function (e.g., as an agonist). Other output neurons that influence the same muscle are preferentially active for different functions (e.g., when the muscle is used as a synergist, fixator, or antagonist). The present results add to this concept by demonstrating a unique set of output neurons that are specifically active to suppress the activity of their target muscle when that muscle would oppose movement onset. These neurons provide a critical tool for generating dexterous movements. They enable control of decreases in muscle activity with high spatiotemporal precision. Just as a sculptor reveals the “angel in the marble” by carving away the superfluous stone (29), neurons that produce postspike suppression can “remove” excitation to sculpt the peaks and valleys of muscle activity with the precision necessary for dexterity. There are examples where we and others (30, 31) have failed to discover a fit between the pattern of postspike effects and the relationship between cell and target muscle activity. At this point, it is unclear whether this failure is because no fit exists or because the functional significance of a fit is not as readily apparent.
MATERIALS AND METHODS
All experimental procedures were conducted according to National Institutes of Health and the U.S. Department of Health and Human Services guidelines as reported in the Association for Assessment and Accreditation of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals. All procedures were approved by the appropriate institutional animal care and use committees. We trained a 17-year-old, wild-type, purpose-bred, male rhesus macaque (Macaca mulatta) to perform step-tracking wrist movements for fluid reward (32). During task performance, the monkey sat in a primate chair with his elbow bent at a 90° angle and his forearm supported. The monkey gripped the handle of a lightweight, low-friction manipulandum with his right hand. The manipulandum rotated freely along the axes of flexion-extension and radial-ulnar deviation. Two rotational potentiometers detected movements of the manipulandum. A computer digitized the potentiometer signals at 1 kHz. The manipulandum position was represented as a small circular “cursor” on a computer screen in front of the animal. To initiate a trial, the monkey rotated the handle of the manipulandum to place the cursor into an open circle located in the center of the screen (“center target”). After successful completion of an initial hold period (0.8 to 1.2 s), a second target appeared at one of eight peripheral locations on the screen. Each peripheral target was evenly spaced at 45° intervals around the center target. The monkey was required to hold the cursor in the center target for another 1 to 1.8 s (“hold center target”). Then, this target disappeared as the “go” cue for the animal to move the cursor into the peripheral target. Movement from the center target to each of the eight peripheral targets required a 20° change in wrist angle. For initial training purposes, if the cursor was placed in the peripheral target in less than 500 ms, the animal was given a fluid reward. Eventually, the monkey was trained to move much faster (120 to 375 ms; median, 152 ms). This sequence of events was repeated for movements from a peripheral target (“target hold”) back to the center target. Peripheral targets were presented in a pseudorandom fashion. The internal diameter of the center and peripheral targets was limited to 5° of wrist movement. The monkey was trained on this task for more than 15 years.
All surgical procedures were performed in an aseptic environment with the animal under isoflurane anesthesia. In the first surgery, we installed a recording chamber over the hand area of M1 and implanted a device for stabilizing the animal’s head during recording sessions. In a later surgery, we implanted EMG electrodes into 13 forearm muscles. Each pair of EMG wires was tunneled subcutaneously to the muscle belly from a connector, which was embedded in acrylic near the recording chamber. We stimulated through the implanted electrodes to confirm the location of the wires at the time of implantation and at regular intervals during neuron recording sessions. Initially, we recorded from the following 13 muscles for a period of 4 months: extensor carpi radialis brevis (ECRB), extensor carpi radialis longus (ECRL), extensor digitorum communis (EDC), extensor digiti quarti and quinti proprius (ED45), extensor carpi ulnaris (ECU), extensor digiti secondi and tertii proprius (ED23), abductor pollicis longus (APL), flexor carpi radialis (FCR), pronator teres (PT), flexor digitorum profundus (FDP), flexor digitorum sublimis (FDS), flexor carpi ulnaris (FCU), and palmaris longus (PL). Then, recordings in FDS were lost, and we recorded from the remaining set of 12 muscles for another 12 months. Last, the electrodes in PT migrated to brachioradialis, and we recorded from this set of 12 muscles for 15 months.
Before extensive neuron recording, we used intracortical microstimulation (11 biphasic stimulus pulses at 333 Hz, 30-ms train duration) to locate the hand region of M1. We considered the hand region of M1 to be the region where intracortical microstimulation evoked movements of the wrist or digits with stimulus intensities of 15 μA or lower. Then, in over 350 daily experimental sessions, we recorded the activity of single neurons in M1 using standard single microelectrode recording techniques. Initially, microelectrode penetrations explored the anterior bank of the central sulcus and the adjacent cortical surface. Anatomical findings from our laboratory suggested that most corticomotoneuronal (CM) cells are concentrated in the anterior bank of the sulcus (33, 34). In addition, we had the greatest success finding CM cells and neurons displaying postspike suppression when recording in the bank of the sulcus. Therefore, as recording sessions progressed, our electrode penetrations were increasingly directed at the bank of the sulcus. We focused on task-modulated neurons and stable spike recordings. Neuron activity was high-pass filtered and then digitized online at 20 kHz. EMG activity was filtered from 30 Hz to 1 kHz and digitized at 4 kHz for SpTA. Action potentials from single units in M1 were isolated online and offline using a custom spike sorting software package. To generate SpTAs of muscle activity, we aligned averages of EMG activity on single-action potentials (11). If a neuron did not display a postspike effect online in 2000 triggers, we moved on to record from another cell.
We used established procedures for processing EMG signals. This included measuring SpTAs to determine statistical significance, overall strength, and onset and offset of the effect and to excluded synchrony effects (14, 20, 35–37). We compiled averages of activity of each muscle triggered from individual spikes of single neurons (SpTAs), both online and offline, over a 60-ms epoch that included 20 ms before and 40 ms after each spike. The SpTAs included all action potentials from an individual neuron across all task conditions (pronated, middle, and supinated wrist postures). We excluded epochs with EMG activity below a noise level of 5% of the full analog-to-digital scale input (14). We required at least 2000 trigger events for each average (35). To determine statistical significance for each SpTA, we first calculated the baseline mean of each SpTA from the first 19 ms of each average. Significant postspike effects were those with a ±2 SD change (increase or decrease) in peak activity compared with baseline mean activity (10, 36). To limit our analysis to pure postspike effects, we excluded SpTAs that showed synchrony components (n = 18). Synchrony effects appear in SpTAs as a consequence of neurons that have activity synchronized with other output neurons. Previous studies have shown that SpTAs with synchrony effects could be largely excluded by requiring a minimum onset latency of 5 ms for a postspike facilitation effect or postspike suppression effect and a maximum peak width at half maximum of 9 ms (20, 37, 38). We recorded a total of 305 neurons. Fifty neurons produced significant postspike facilitation or suppression in one or more recorded muscle. This significance was verified independently by both authors.
We processed kinematic signals by first calculating a single displacement signal from the X and Y potentiometer signals. Then, we calculated the first derivative of displacement to determine a single velocity signal for each movement. We defined movement onset as the time when the velocity signal crossed a threshold of 15°/s (average maximum velocity was ~125°/s). We aligned M1 neuron activity and EMG activity on movement onset to generate movement-related trial activity and averages. We displayed movement-aligned activity from individual trials without processing (e.g., single-trial raster; Figs. 1, A and B, and 2, E and F). We also displayed movement-aligned averages after smoothing with a Gaussian-weighted sliding box car filter that had a 20-ms kernel [e.g., cell and muscle activity; Figs. 2 (E and F), 3 (E to H), 4 (C and D), 5A, and 6 (B to D)].
We calculated preferred directions of EMG activity (20 ms before to 20 ms after move onset) and cell activity (60 ms before to 60 ms after move onset) for each forearm posture using established methods (39). We used the Rayleigh test (40) to determine whether EMGs and cells had significant unimodal directional tuning. We also used a bootstrapping method (41) to determine the degree of directional bias in each cell’s movement-related activity. We required a minimum of P < 0.05 for statistical significance of directional tuning.
We measured the onset of M1 cell activity by first calculating a baseline level of activity present before movement onset. This included measuring the average firing rate of cell activity present from 300 to 100 ms before movement onset (e.g., solid white lines in Figs. 2E, 3E, 4C, 5A, and 6, B to D). We then calculated 2 SD above the average firing rate before movement onset (e.g., dashed white lines in Figs. 2E, 3E, 4C, 5A, and 6, B to D). The onset of cell activity was defined as the time when the activity rose above 2 SD of average baseline activity (e.g., black arrows in Figs. 2E, 3E, 4C, 5A, and 6, B to D). We measured the offset of target muscle activity by first calculating a baseline level of activity present before movement onset. This included measuring the average of EMG activity present from 300 to 100 ms before movement onset (e.g., solid black lines in Figs. 2F, 3F, 4D, and 5A). We then calculated 2 SD below the average in EMG activity before movement onset (e.g., dashed black lines in Figs. 2F, 3F, 4D, 5A, and 6, B to D). The offset of target muscle activity was defined as the time when the activity fell below 2 SD of average baseline activity (e.g., white arrows in Figs. 2F, 3F, 4D, and 5A).
The difference in M1 cell onset and target muscle offset was compared in two analyses. First, we calculated the overall population averages of M1 cell and target muscle activity (Fig. 5A). We summed the M1 neural activity and target muscle activity present in the movement condition associated with the individual cells’ preferred direction in each of three wrist postures. Muscle activity was weighted by the strength of postspike suppression effect. This yielded a single value for average onset of cell activity and average offset of target muscle activity. Second, we measured the onset of M1 cell activity and offset of target muscle activity for each of the movement conditions associated with the cell’s preferred direction in each of three recorded postures. This yielded three values for each M1 neuron (onset of cell activity) and target muscle (offset of target muscle activity). We plotted this range of onsets and offsets as bar and whisker plots (Fig. 5B). For these plots, the center line represents the individual median values, the box limits represent the lower and upper quartiles, and the whiskers mark the smallest and largest sample values. These values are also plotted as a histogram for comparison against the larger M1 dataset (Fig. 6A). A Student’s t test was used to compare the mean of the onset of cell activity from the two populations: (i) the neurons showing postspike suppression and antagonist suppression (n = 24) and (ii) all other output neurons in M1 (n = 136).
Acknowledgments
We thank R. Dum for surgical assistance, K. Thiel for technical assistance, and M. Page (Great Island Software, Chatham, MA) and S. Hoffman (Reflective Computing, Olympia, WA) for development of custom computer programs. We also extend thanks to N. Picard and D. Hoffman for thoughtful discussions regarding early drafts of the manuscript. Funding: This work was supported by NIH grants: R01NS24328 (P.L.S.), P30NS076405 (P.L.S.), and FNS070366A (D.M.G.). Author contributions: D.M.G. conducted experiments. D.M.G. and P.L.S. analyzed the data. D.M.G. and P.L.S. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. All data, code, and experimental protocols as well as additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Wachholder K., Altenburger H., Beiträge zur Physiologie der willkürlichen Bewegung. Pflugers Arch. Gesamte Physiol. Menschen Tiere 212, 666–675 (1926). [Google Scholar]
- 2.Sternad D., Wachholder and Altenberger 1927: Foundational experiments for current hypotheses on equilibrium-point control in voluntary movements. Motor Control 6, 299–302 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Hufschmidt H. J., Hufschmidt T., Antagonist inhibition as the earliest sign of a sensory-motor reaction. Nature 174, 607 (1954). [DOI] [PubMed] [Google Scholar]
- 4.Hallett M., Shahani B. T., Young R. R., EMG analysis of patients with cerebellar deficits. J. Neurol. Neurosurg. Psychiatry 38, 1163–1169 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berardelli A., Hallett M., Rothwell J. C., Agostino R., Manfredi M., Thompson P. D., Marsden C. D., Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain 119 (Pt. 2), 661–674 (1996). [DOI] [PubMed] [Google Scholar]
- 6.van Vugt J. P., Stijl M., Roos R. A., van Dijk J. G., Impaired antagonist inhibition may contribute to akinesia and bradykinesia in Huntington’s disease. Clin. Neurophysiol. 114, 295–305 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Geertsen S. S., Kirk H., Nielsen J. B., Impaired ability to suppress excitability of antagonist motoneurons at onset of dorsiflexion in adults with cerebral palsy. Neural Plast. 2018, 1265143 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forget R., Lamarre Y., Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum. Neurobiol. 6, 27–37 (1987). [PubMed] [Google Scholar]
- 9.Kakei S., Hoffman D. S., Strick P. L., Muscle and movement representations in the primary motor cortex. Science 285, 2136–2139 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Griffin D. M., Hoffman D. S., Strick P. L., Corticomotoneuronal cells are “functionally tuned”. Science 350, 667–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fetz E. E., Cheney P. D., Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J. Neurophysiol. 44, 751–772 (1980). [DOI] [PubMed] [Google Scholar]
- 12.Muir R. B., Lemon R. N., Corticospinal neurons with a special role in precision grip. Brain Res. 261, 312–316 (1983). [DOI] [PubMed] [Google Scholar]
- 13.Davidson A. G., O'Dell R., Chan V., Schieber M. H., Comparing effects in spike-triggered averages of rectified EMG across different behaviors. J. Neurosci. Methods 163, 283–294 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKiernan B. J., Marcario J. K., Karrer J. H., Cheney P. D., Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J. Neurophysiol. 80, 1961–1980 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Kasser R. J., Cheney P. D., Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J. Neurophysiol. 53, 959–978 (1985). [DOI] [PubMed] [Google Scholar]
- 16.Lemon R. N., Muir R. B., Mantel G. W., The effects upon the activity of hand and forearm muscles of intracortical stimulation in the vicinity of corticomotor neurones in the conscious monkey. Exp. Brain Res. 66, 621–637 (1987). [DOI] [PubMed] [Google Scholar]
- 17.Cheney P. D., Fetz E. E., Palmer S. S., Patterns of facilitation and suppression of antagonist forelimb muscles from motor cortex sites in the awake monkey. J. Neurophysiol. 53, 805–820 (1985). [DOI] [PubMed] [Google Scholar]
- 18.Humphrey D. R., Corrie W. S., Properties of pyramidal tract neuron system within a functionally defined subregion of primate motor cortex. J. Neurophysiol. 41, 216–243 (1978). [DOI] [PubMed] [Google Scholar]
- 19.Maier M. A., Perlmutter S. I., Fetz E. E., Response patterns and force relations of monkey spinal interneurons during active wrist movement. J. Neurophysiol. 80, 2495–2513 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Schieber M. H., Rivlis G., A spectrum from pure post-spike effects to synchrony effects in spike-triggered averages of electromyographic activity during skilled finger movements. J. Neurophysiol. 94, 3325–3341 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Takei T., Seki K., Spinal interneurons facilitate coactivation of hand muscles during a precision grip task in monkeys. J. Neurosci. 30, 17041–17050 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankowska E., Padel Y., Tanaka R., Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J. Physiol. 258, 467–487 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman D. S., Strick P. L., Effects of a primary motor cortex lesion on step-tracking movements of the wrist. J. Neurophysiol. 73, 891–895 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Russo A. A., Bittner S. R., Perkins S. M., Seely J. S., London B. M., Lara A. H., Miri A., Marshall N. J., Kohn A., Jessell T. M., Abbott L. F., Cunningham J. P., Churchland M. M., Motor cortex embeds muscle-like commands in an untangled population response. Neuron 97, 953–966.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham J. P., Yu B. M., Dimensionality reduction for large-scale neural recordings. Nat. Neurosci. 17, 1500–1509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallego J. A., Perich M. G., Naufel S. N., Ethier C., Solla S. A., Miller L. E., Cortical population activity within a preserved neural manifold underlies multiple motor behaviors. Nat. Commun. 9, 4233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson I. H., London B. M., Oby E. R., Sachs N. A., Reimer J., Englitz B., David S. V., Shamma S. A., Blanche T. J., Mizuseki K., Zandvakili A., Hatsopoulos N. G., Miller L. E., Kording K. P., Functional connectivity and tuning curves in populations of simultaneously recorded neurons. PLOS Comput. Biol. 8, e1002775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suway S. B., Orellana J., Morland A. J. C. M., Fraser G. W., Liu Z., Velliste M., Chase S. M., Kass R. E., Schwartz A. B., Temporally segmented directionality in the motor cortex. Cereb. Cortex 28, 2326–2339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.I. Stone, The Agony and the Ecstasy: A Biographical Novel of Michelangelo (Penguin Books, 1961). [Google Scholar]
- 30.Bennett K. M., Lemon R. N., Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J. Neurophysiol. 75, 1826–1842 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Schieber M. H., Dissociating motor cortex from the motor. J. Physiol. 589, 5613–5624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman D. S., Strick P. L., Activity of wrist muscles during step-tracking movements in different directions. Brain Res. 367, 287–291 (1986). [DOI] [PubMed] [Google Scholar]
- 33.Rathelot J.-A., Strick P. L., Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl. Acad. Sci. U.S.A. 106, 918–923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathelot J.-A., Strick P. L., Muscle representation in the macaque motor cortex: An anatomical perspective. Proc. Natl. Acad. Sci. U.S.A. 103, 8257–8262 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheney P. D., Fetz E. E., Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J. Physiol. 349, 249–272 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin D. M., Hudson H. M., Belhaj-Saïf A., McKiernan B. J., Cheney P. D., Do corticomotoneuronal cells predict target muscle EMG activity? J. Neurophysiol. 99, 1169–1986 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Baker S. N., Lemon R. N., Computer simulation of post-spike facilitation in spike-triggered averages of rectified EMG. J. Neurophysiol. 80, 1391–1406 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Flament D., Fortier P. A., Fetz E. E., Response patterns and postspike effects of peripheral afferents in dorsal root ganglia of behaving monkeys. J. Neurophysiol. 67, 875–889 (1992). [DOI] [PubMed] [Google Scholar]
- 39.Georgopoulos A. P., Kalaska J. F., Caminiti R., Massey J. T., On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J. Neurosci. 2, 1527–1537 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crammond D. J., Kalaska J. F., Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp. Brain Res. 108, 45–61 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Schwartz A. B., Kettner R. E., Georgopoulos A. P., Primate motor cortex and free arm movements to visual targets in three-dimensional space. I. Relations between single cell discharge and direction of movement. J. Neurosci. 8, 2913–2927 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]


