Summary
Katon J, Reiber G, Williams MA, Yanez D, Miller E. Antenatal haemoglobin A1c and risk of large-for-gestational-age infants in a multi-ethnic cohort of women with gestational diabetes. Paediatric and Perinatal Epidemiology 2012; 26: 208–217.
Gestational diabetes mellitus (GDM) is a risk factor for delivering a large-for-gestational-age (LGA) infant. Haemoglobin A1c (A1C) is an indicator of glycaemic control. The objective of this study was to test whether higher A1C quartile at the time of diagnosis of GDM is associated with increased risk of delivering a LGA or macrosomic infant. Women with singleton pregnancies treated for GDM at a large diabetes and pregnancy programme located in Charlotte, North Carolina, were eligible for inclusion in this retrospective cohort study. Clinical information, including A1C at diagnosis, treatment, prior medical and obstetric history, and birth data were abstracted from medical records. LGA was defined as birthweight >90th percentile for gestational age and sex and macrosomia as birthweight >4000 g. Logistic regression was used to analyse the association of A1C at GDM diagnosis with risk of delivering LGA or macrosomic infants. This study included 502 women. Prevalences of LGA and macrosomia were 4% and 6% respectively. After adjustment there was no detectable trend of increased risk for LGA (P for trend = 0.12) or macrosomia (P for trend = 0.20) across increasing quartiles of A1C at GDM diagnosis. A1C at GDM diagnosis may not be linearly associated with LGA or macrosomia, possibly because of the mediating effect of strict glycaemic control in this clinical setting.
Keywords: gestational diabetes, Hb A1c, glycaemic control, birthweight
Introduction
Gestational diabetes mellitus (GDM), defined by the American College of Obstetricians and Gynecologists as glucose intolerance that is first evident during pregnancy,1 is a recognised risk factor for delivery of a large-for-gestational-age (LGA) infant. Women with GDM have 3.4 times higher odds of delivering a LGA infant compared with women without GDM.2 Delivery of an LGA infant is associated with increased risk of neonatal morbidity and complications including infant hypoglycaemia, shoulder dystocia, perinatal asphyxia, and perinatal hypoxia and its sequelae.3,4 Compared with appropriate-for-gestational-age infants of mothers with and without GDM, LGA offspring of mothers with GDM are also at increased risk of obesity,5 metabolic syndrome6 and insulin resistance beginning in childhood and adolescence.6 Recently, two randomised clinical trials demonstrated that management of glucose levels among pregnant women with mild gestational glucose intolerance can reduce the incidence of LGA by 38–51%,7,8 indicating that appropriate identification and treatment of women at highest risk of LGA can lower the risk of LGA and its related sequelae.
Haemoglobin A1c (A1C), a haemoglobin variant, is an accepted and standardised measure of glycated haemoglobin, and a marker of glycaemic control over the preceding 120 days.9 Recently an international committee of experts at the American Diabetes Association (ADA) recommended the use of A1C for the diagnosis of type 2 diabetes.10 Pregnant women were excluded from the ADA recommendation because of metabolic11–13 and haematological changes associated with pregnancy,14–16 which are essential for meeting the changing energy needs of the mother and fetus17 and result in decreased A1C during pregnancy.18,19 However, there is increasing interest in the use of A1C for the clinical management of GDM.20
Although there is evidence of a positive linear association between A1C in the second or third trimester and risk of LGA among women with established diabetes,21–23 in the context of GDM there are conflicting findings.24–26 Results from the Hyperglycemia and Pregnancy Outcomes study indicate that among pregnant women with impaired glucose below the conventional thresholds for diagnosing GDM, maternal glucose levels at GDM screening have a linear relationship with birthweight.27 A1C indicates higher average daily glucose levels, and women with higher A1C at GDM diagnosis may have more severe GDM or be less likely to achieve optimal glycaemic control,28,29 and therefore be at increased risk of LGA or macrosomia. Additionally, women with higher A1C at GDM diagnosis may have hyperglycaemia earlier in their pregnancy, and among women with pre-gestational diabetes there is evidence of a postponed effect of poor glycaemic control in the first trimester leading to excess fetal growth in the third trimester.30 The objective of this study was to test, in a multi-ethnic cohort of women with GDM, whether higher A1C quartile at the time of GDM diagnosis is associated with an increased risk of LGA or macrosomia, which are markers of excess fetal growth.
Methods
Study setting
This study took place in a large diabetes and pregnancy programme, which is a consortium of two health care clinics located in Charlotte, North Carolina. Clinic A, housed within the main medical centre, provides comprehensive education and care for women with type 1 diabetes, type 2 diabetes and GDM and serves a primarily White, privately insured population. Clinic B provides obstetric care for women with and without diabetes in pregnancy. The majority of patients cared for at Clinic B are racial/ethnic minorities and Medicaid recipients. These two clinics, located within a mile of each other, use a common GDM clinical management protocol developed by the Medical Director, a Board Certified Endocrinologist (E.M.). The programme serves all of Mecklenburg County, the nine surrounding counties and manages the majority of GDM patients diagnosed within the greater Charlotte area. Screening for GDM is universal among women attending prenatal care in the US with <2% of pregnant women attending less than three prenatal visits.31
Typically women are referred to one of the two diabetes and pregnancy programme clinics at 24–28 weeks gestation following a positive 50-g oral glucose challenge test (GCT) and a positive 3-h 100-g oral glucose tolerance test (OGTT).32 A1C and other laboratory tests are performed at or near the first appointment at Clinic A and at enrolment in the diabetes and pregnancy programme at Clinic B. At both clinics women considered at particularly high risk for GDM may be referred to the diabetes and pregnancy programme earlier in their pregnancy with or without GDM screening. Additionally, women may be diagnosed with GDM and referred to the diabetes and pregnancy programme at any point in their pregnancy following an extremely elevated GCT or random blood glucose (RBG).
Goals for treatment in both clinics are: fasting glucose <90 mg/dL (<5 mmol/L); once medication is prescribed, a goal of 70–80 mg/dL (3.89–4.44 mmol/L) is used; and 1-h postprandial glucose <120 mg/dL (<6.66 mmol/L). Insulin or oral hypoglycaemic agents typically are initiated if after a week with diet and exercise, the glucose goals are not met. This trial of diet and exercise may be shortened for women with extremely elevated 1-h GCT, 3-h OGTT values, or presence of other risk factors.
Study participants
Women managed for GDM with a live singleton delivery between 15 November 2000 and 15 April 2010 were eligible for inclusion in the study. Additional inclusion criteria were: diagnosed with GDM at ≥24 weeks gestation by either a 3-h 100-g OGTT, a GCT ≥200 mg/dL (≥11.10 mmol/L) or a RBG ≥160 mg/dL (≥8.88 mmol/L); and complete birth data (date of birth, infant’s sex and birthweight). Study exclusion criteria were: established type 1 or type 2 diabetes; untreated endocrinopathies (hyperadrenalism, hypoadrenalism, hyperthyroidism, hypothyroidism and acromegaly); haemoglobin variants (HbS, HbC, HbF and HbE) or conditions (uraemia, thalassaemia) that impair interpretation of A1C; and first A1C measurement >4 weeks after initial visit to the diabetes and pregnancy programme. The University of Washington and Carolinas HealthCare System institutional review boards approved the protocol for this study.
Laboratory measures
The two clinics measured A1C using different analytical methods. Using a point-of-care instrument, Clinic A measured A1C with a DCA 2000. Clinic B sends all samples to the main laboratory for analysis; at the main laboratory A1C is measured via high-performance liquid chromatography using a Diabetes Control and Complications Trial aligned method (Bio-Rad VARIANT™II TURBO). The laboratory method and point-of-care method for A1C measurement are highly correlated with a reported Pearson correlation of >0 9 33–35
Study variables
The primary exposure was the first A1C, measured and recorded at a diabetes and pregnancy programme within 4 weeks of GDM diagnosis. Key covariates included variables related to maternal demographics, medical and obstetric history, pregnancy and delivery. Demographic variables included: clinic (Clinic A, Clinic B), maternal age at GDM diagnosis (years), parity [primipara (parity = 0), multipara (parity ≥ 1)], insurance (private, uninsured or Medicaid), maternal race/ethnicity (White, non-White) and pre-pregnancy body mass index (BMI) as calculated from height and pre-pregnancy weight (kg/m2). Medical and obstetric history variables included: prior history of GDM, prior history of pre-eclampsia/eclampsia, history of polycystic ovary syndrome, history of miscarriage and history of intrauterine fetal demise. Pregnancy and delivery variables included: gestational week at the first A1C as recorded on the medical chart based either on ultrasound or on last menstrual period, GDM diagnostic method (3-h OGTT; or GCT or RBG), treatment modality (diet only; or glyburide, insulin, metformin or other combination), preterm delivery (delivery at <37 weeks gestation) and gestational week at delivery.
Study outcomes
The primary outcomes were LGA defined as birthweight >90th percentile for gestational age and sex based on US vital statistics36 and macrosomia defined as birthweight ≥4000 g regardless of gestational age.37 Both LGA and macrosomia are considered indicators of excess fetal growth.37 Gestational week at delivery was calculated from the date of the last visit to the diabetes and pregnancy programme, the date of delivery and the gestational week at the last clinic visit. If the gestational week at the last clinic visit was not available, then the gestational week at delivery was calculated from the date of the first clinic visit, the date of delivery and the gestational week at the first clinic visit (n = 10).
Statistical analysis
Logistic regression was used to examine the association across quartiles of A1C and risk of delivering an LGA infant. The primary test was a test for trend of risk of LGA or macrosomia across increasing quartiles of A1C. The test for trend was conducted by assigning A1C quartile values in ascending order from 1 to 4 and entering A1C quartile into the regression model as a continuous variable.38 Similarly, logistic regression was used to examine the association across quartiles of A1C and the risk of macrosomia. Additionally, we dichotomised A1C (<5.7%, ≥5.7%), based on the new ADA definition of increased risk for diabetes10 and analysed the association between A1C ≥5.7% at GDM diagnosis and LGA and macrosomia, respectively, using logistic regression. Finally, we examined A1C as a continuous predictor of LGA and macrosomia using logistic regression.
Recognising that an association between A1C and LGA or macrosomia might be strongest among women with more severe disease or poor glycaemic control, we conducted a sensitivity analysis that included only women who required insulin or oral hypoglycaemic agents. Additionally, because of concern regarding the use of definitions for LGA that might not be appropriate for Asian Indian women among whom the 90th percentile of birthweight for gestational age may be substantially lower,39 we conducted a sensitivity analysis excluding women with Asian Indian or other race/ethnicity. Finally, we also examined the prevalence of LGA and macrosomia by change in A1C (increased vs. decreased or unchanged) among the subset of women who had multiple antenatal A1C measurements during their pregnancy.
Potential confounders were retained in the final model if their inclusion resulted in a >10% change in the estimate or if they were a priori considered important (non-White race/ethnicity, pre-pregnancy BMI and gestational week at first A1C). Unadjusted and adjusted odds ratios (ORs) are reported with 95% confidence intervals [CI]. All analyses used robust standard error estimates and statistical significance was defined at the two-sided alpha level of 0.05. All analyses were completed using STATA 9 software.40
Results
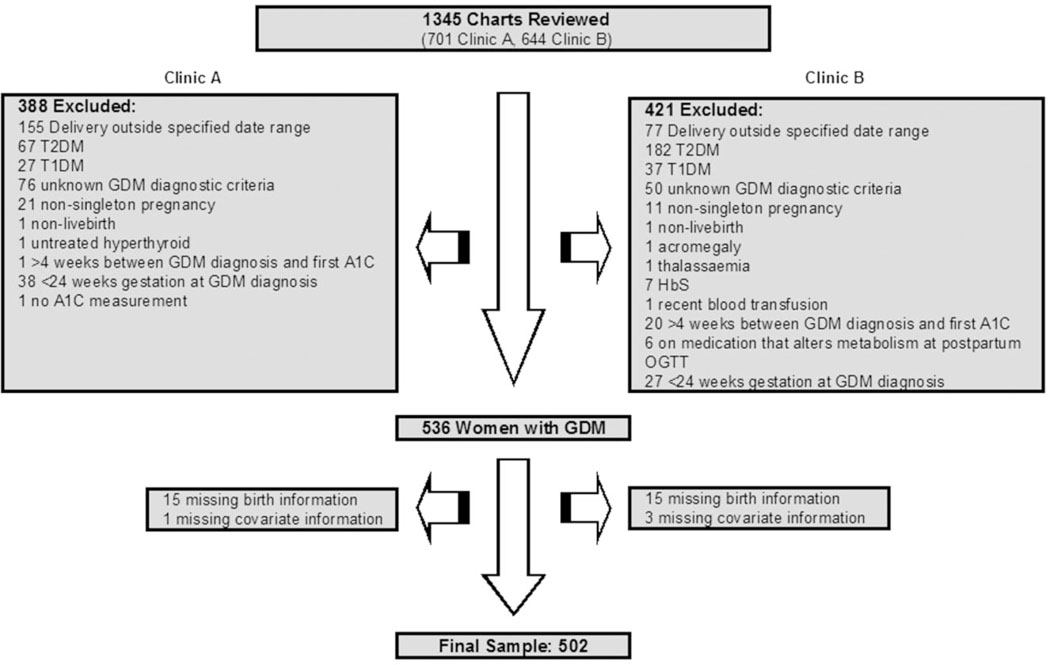
We reviewed 1345 charts (701 from Clinic A, 644 from Clinic B) resulting in a final sample of 502 women (Figure 1). Out of the 536 women deemed to be eligible for our study, 506 (94%) had complete birth data including birthweight, date of delivery and infant sex. Those with missing birth data did not differ from those with complete information (Table S1). An additional four women were missing pre-pregnancy BMI and were excluded from the analysis. Overall, 22 (4%) LGA infants and 28 (6%) macrosomic infants were delivered.
Figure 1.
Exclusions based on maternal and pregnancy characteristics. T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus; GDM, gestational diabetes mellitus; A1C, haemoglobin A1c; HbS, sickle cell haemoglobin; OGTT, oral glucose tolerance test.
Demographic, medical and obstetric history, and pregnancy and delivery characteristics of the study population are described in Table 1 by clinic and by A1C quartile at diagnosis of GDM. Study participants had an average age of 31 (SD 5.4), 41% were primiparae, 64% were of non-White race/ethnicity, 68% were obese or overweight prior to pregnancy, and 75% were diagnosed with GDM using a 3-h OGTT. Mean gestational age at first A1C was 30 weeks (SD 5.4), and 71% of the study participants required insulin or oral hypoglycaemic agents. Compared with women managed at Clinic A women managed at Clinic B were less likely to be primiparae, and more likely to be enrolled in Medicaid or uninsured, to be of non-White race/ethnicity, to be overweight or obese prior to pregnancy, to have a prior history of GDM or pre-eclampsia, to have higher A1C at diagnosis of GDM and to require insulin or hypoglycaemic agents. Similarly, compared with women in the first A1C quartile those in successively higher quartiles were less likely to be primiparae, and more likely to be enrolled in Medicaid or uninsured, to be of non-White race/ethnicity, to be overweight or obese prior to pregnancy, to have a prior history of GDM and to require insulin or hypoglycaemic agents.
Table 1.
Demographic, medical and obstetric history, pregnancy and delivery characteristics of the study population by clinic and by A1C quartile at GDM diagnosis
| Clinic |
A1C quartile at GDM diagnosis (range) |
||||||
|---|---|---|---|---|---|---|---|
| Total | Clinic A | Clinic B | First (4.1–5.3) | Second (5.4–5.6) | Third (5.7–6.1) | Fourth (6.2–10.9) | |
| n | 502 | 297 | 205 | 148 | 109 | 144 | 101 |
| Demographic characteristics of mother | |||||||
| Mean (SD) age at diagnosis (years) | 31 (5.4) | 32 (5.0) | 30 (5.7) | 31 (5.0) | 31 (5.3) | 31 (5.6) | 31 (5.5) |
| Age at diagnosis (years), n (%) | |||||||
| <25 | 55 (11) | 20 (7) | 35 (17) | 11 (8) | 10 (10) | 20 (14) | 14 (14) |
| 25–<30 | 138 (28) | 86 (29) | 52 (25) | 45 (30) | 32 (29) | 36 (25) | 25 (25) |
| 30–<35 | 168 (33) | 94 (31) | 74 (36) | 50 (34) | 32 (29) | 51 (35) | 35 (34) |
| ≥35 | 141 (28) | 97 (33) | 44 (22) | 42 (28) | 35 (32) | 37 (26) | 27 (27) |
| Primiparae, n (%) | 206 (41) | 151 (51) | 55 (27) | 72 (49) | 49 (45) | 46 (32) | 39 (39) |
| Medicaid or uninsured, n (%) | 203 (40) | 10 (3) | 193 (94) | 26 (18) | 25 (23) | 87 (60) | 65 (64) |
| Race/ethnicity, n (%) | |||||||
| White | 182 (36) | 168 (57) | 14 (7) | 98 (66) | 49 (45) | 24 (16) | 11 (11) |
| African American | 107 (21) | 74 (25) | 33 (16) | 15 (10) | 22 (20) | 40 (28) | 30 (30) |
| Hispanic | 163 (33) | 11 (4) | 152 (74) | 22 (15) | 21 (19) | 65 (45) | 55 (54) |
| Asian Indian | 42 (8) | 39 (13) | 3 (1.5) | 10 (7) | 16 (15) | 11 (8) | 5 (5) |
| Other | 8 (2) | 5 (1.7) | 3 (1.5) | 3 (2) | 1 (1) | 4 (3) | 0 |
| Pre-pregnancy BMI, n (%) | |||||||
| <25 kg/m2 | 162 (32) | 121 (41) | 41 (20) | 76 (51) | 43 (40) | 30 (21) | 13 (13) |
| 25–29.9 kg/m2 | 147 (29) | 78 (26) | 69 (34) | 37 (25) | 38 (35) | 48 (43) | 24 (24) |
| ≥30 kg/m2 | 193 (39) | 98 (33) | 95 (46) | 35 (24) | 28 (26) | 66 (46) | 64 (63) |
| Prior pregnancy history | |||||||
| Prior GDM, n (%) | 66 (13) | 31 (10) | 35 (17) | 16 (11) | 15 (14) | 15 (10) | 20 (20) |
| Prior pre-eclampsia/eclampsia, n (%) | 25 (5) | 13 (4) | 12 (6) | 7 (5) | 3 (3) | 11 (7) | 4 (4) |
| PCOS, n (%) | 15 (3) | 14 (5) | 1 (1) | 5 (3) | 5 (5) | 2 (1) | 3 (3) |
| Miscarriage, n (%) | 98 (20) | 56 (19) | 42 (21) | 25 (17) | 23 (21) | 34 (24) | 16 (16) |
| Intrauterine fetal death, n (%) | 7 (1) | 2 (1) | 5 (2) | 1 (1) | 1 (1) | 4 (3) | 1 (1) |
| Pregnancy and delivery | |||||||
| Mean (SD) gestation at first A1C (weeks) | 30 (2.4) | 30 (1.8) | 31 (2.9) | 30 (2.0) | 31 (2.4) | 30 (2.2) | 31 (3.0) |
| GDM diagnostic method, n (%) | |||||||
| 3-h OGTT | 376 (75) | 247 (83) | 129 (63) | 125 (85) | 92 (84) | 112 (78) | 47 (46) |
| GCT | 102 (20) | 47 (16) | 55 (27) | 21 (14) | 13 (12) | 29 (20) | 39 (39) |
| RBG | 24 (5) | 3 (1) | 21 (10) | 2 (1) | 4 (4) | 3 (2) | 15 (15) |
| A1C quartile (min–max) at GDM diagnosis, n (%) | |||||||
| First (4.1–5.3) | 148 (29) | 123 (41) | 25 (12) | 148 (100) | |||
| Second (5.4–5.6) | 109 (22) | 85 (29) | 24 (12) | 109 (100) | |||
| Third (5.7–6.1) | 144 (29) | 60 (20) | 84 (41) | 144 (100) | |||
| Fourth (6.2–10.9) | 101 (20) | 29 (10) | 72 (35) | 101 (100) | |||
| Treatment, n (%) | |||||||
| Diet only | 147 (29) | 128 (43) | 19 (9) | 77 (52) | 41 (37) | 20 (14) | 9 (9) |
| Glyburide | 68 (14) | 8 (3) | 60 (29) | 12 (8) | 15 (14) | 26 (18) | 15 (15) |
| Insulin | 260 (52) | 160 (54) | 100 (49) | 56 (38) | 49 (45) | 87 (60) | 68 (67) |
| Metformin | 5 (1) | 0 | 5 (3) | 1 (1) | 0 | 1 (1) | 3 (3) |
| Other | 22 (4) | 1 (0.3) | 21 (10) | 2 (1) | 4 (4) | 10 (7) | 6 (6) |
| Median (IQR) gestation at delivery (weeks) | 39 (38–40) | 39 (38–40) | 39 (38–39) | 39 (39–40) | 39 (38–40) | 39 (38–39) | 39 (38–39) |
| Preterm birth (<37 weeks gestation), n (%) | 44 (9) | 25 (8) | 19 (9) | 12 (8) | 8 (7) | 15 (10) | 9 (9) |
A1C, haemoglobin A1c; GDM, gestational diabetes mellitus; SD, standard deviation; BMI, body mass index (kg/m2); PCOS, polycystic ovary syndrome; OGTT, oral glucose tolerance test; GCT, glucose challenge test; RBG, random blood glucose; IQR, interquartile range.
After adjustment for clinic, maternal age at GDM diagnosis, parity, non-White race/ethnicity, pre-pregnancy BMI (kg/m2) and gestational week at first A1C, there was no detectable trend of increased risk for LGA (P for trend = 0.12) or macrosomia (P for trend = 0.20) across increasing quartiles of A1C (Table 2). Notably, compared with women in the lowest A1C quartile (4.1–5.3) those in the highest A1C quartile (6.2–10.9) had a nearly fourfold increased odds of LGA (OR: 3.60 [95% CI 0.73,17.38]) and an almost threefold increased odds of macrosomia (OR: 2.68 [95% CI 0.75, 9.88]), although these associations did not reach statistical significance. After adjustment, A1C ≥5.7% was associated with 2.78 times higher odds of LGA [95% CI 0.82, 9.41] and 1.90 times higher odds of macrosomia [95% CI 0.72, 5.02], but these associations did not reach statistical significance (Table 3). After adjustment a 1% increase in A1C at diagnosis was associated with 1.40 times higher odds of LGA [95% CI 0.81,2.42] and with 1.34 times higher odds of macrosomia [95% CI 0.81, but these associations did not reach statistical significance.
Table 2.
Odds ratios for the association between quartile of A1C at diagnosis of GDM and LGA and macrosomia among women with GDM (n = 502)
| Outcome | A1C quartile | n | n (%)a | UOR [95% CI] | Pb | AORc [95% CI] | Pb |
|---|---|---|---|---|---|---|---|
| LGA | First | 148 | 4 (3) | 1.00 Reference | 0.06 | 1.00 Reference | 0.12 |
| Second | 109 | 3 (3) | 1.02 [0.22, 4.66] | 1.03 [0.23, 4.51] | |||
| Third | 144 | 7 (5) | 1.84 [0.53, 6.43] | 2.11 [0.59, 7.59] | |||
| Fourth | 101 | 8 (8) | 3.10 [0.91, 10.59] | 3.60 [0.73, 17.83] | |||
| Macrosomia | First | 148 | 8 (6) | 1.00 Reference | 0.23 | 1.00 Reference | 0.20 |
| Second | 109 | 4 (4) | 0.67 [0.20, 2.28] | 0.71 [0.21, 2.40] | |||
| Third | 144 | 5 (4) | 0.63 [0.20, 1.97] | 0.77 [0.26, 2.32] | |||
| Fourth | 101 | 11 (11) | 2.14 [0.83, 5.53] | 2.68 [0.73, 9.88] |
n (%) with outcome.
P for trend.
Adjusted for: maternal age at GDM diagnosis, parity (0, ≥1), clinic, non-White race/ethnicity, pre-pregnancy body mass index (kg/m2) and gestation at first A1C.
A1C, haemoglobin A1c; GDM, gestational diabetes mellitus; LGA, large for gestational age; UOR, unadjusted odds ratio; CI, confidence interval; AOR, adjusted odds ratio.
Table 3.
Odds ratios for the association between level of A1C at diagnosis of GDM and LGA and macrosomia among women with GDM (n = 502)
| Outcome | A1C | n | n (%)a | UOR [95% CI] | P | AORb [95% CI] | P |
|---|---|---|---|---|---|---|---|
| LGA | <5.7 | 292 | 8 (3) | 1.00 Reference | 0.04 | 1.00 Reference | 0.10 |
| ≥5.7 | 210 | 14 (7) | 2.54 [1.04, 6.17] | 2.78 [0.82, 9.41] | |||
| Macrosomia | <5.7 | 292 | 13 (5) | 1.00 Reference | 0.20 | 1.00 Reference | 0.20 |
| ≥5.7 | 210 | 15 (7) | 1.65 [0.77, 3.55] | 1.90 [0.72, 5.02] | |||
| LGA | Continuous | 1.40 [0.97, 2.03] | 0.07 | 1.40 [0.81, 2.42] | 0.23 | ||
| Macrosomia | Continuous | 1.25 [0.83, 1.89] | 0.29 | 1.34 [0.81, 2.23] | 0.26 |
n (%) with outcome.
Adjusted for: maternal age at GDM diagnosis, parity (0, ≥1), clinic, non-White race/ethnicity, pre-pregnancy body mass index (kg/m2) and gestation at first A1C.
A1C, haemoglobin A1c; GDM, gestational diabetes mellitus; LGA, large for gestational age; UOR, unadjusted odds ratio; CI, confidence interval; AOR, adjusted odds ratio.
When we restricted our analysis to women managed with either insulin or oral hypoglycaemics after adjustment, we found a slightly stronger association of A1C >5.7 at GDM with odds of LGA (OR: 3.90 [95% CI 0.80, 19.02]) and odds of macrosomia (OR: 2.06 [95% CI 0.69, 6.10]). This was less apparent when A1C was entered into the model as a continuous measure (LGA OR: 1.41 [95% CI 0.81, 2.44]; macrosomia OR: 1.37 [95% CI 0.85, 2.24]).
Excluding women of Asian Indian or other race/ethnicity we found a statistically significant trend of increased risk of LGA across increasing A1C quartiles (P for trend = 0.01) and a similar trend of increased risk of macrosomia (P for trend = 0.05). After adjusting for clinic, maternal age at GDM diagnosis, parity, non-White race/ethnicity, pre-pregnancy BMI (kg/m2) and gestational week at first A1C, we found that those in the second quartile had a 1.04 times higher risk of LGA compared with women in the first A1C quartile [95% CI 0.18, 6.00], those in the third quartile had a 4.28 times higher risk of LGA compared with women in the first A1C quartile [95% CI 1.22,15.04], and those in the fourth quartiles had a 7.38 times higher risk of LGA compared with women in the first A1C quartile [95% CI 1.45, 37.51]. After adjustment, those in the second quartile had a 0.66 times lower risk of macrosomia compared with women in the first A1C quartile [95% 0.17, 2.66], those in the third quartile had a 1.21 times higher risk of macrosomia compared with women in the first A1C quartile [95% CI 0.40, 3.59], and those in the fourth quartile had a 4.29 times higher risk of macrosomia compared with women in the first A1C quartile [95% CI 1.13, 16.33].
Among the women in our population who had multiple antenatal A1C measurements after GDM diagnosis (n = 340), the prevalences of LGA and macrosomia were higher among those whose A1C increased (n = 169) compared with those whose A1C decreased or remained unchanged (n = 171) (LGA 6% vs. 3%; macrosomia 7% vs. 5%).
Discussion
This study found no detectable trend of increased odds of LGA or macrosomia across increasing quartiles of A1C at diagnosis of GDM, nor was there an association between A1C ≥5.7% at diagnosis of GDM and LGA or macrosomia. However, there was limited evidence of a two- to fourfold increased odds of LGA and macrosomia among women in the highest quartile of A1C at diagnosis [range 6.2, 10.9] compared with women in the lowest A1C quartile [range 4.1, 5.3], and a trend between increased risk of LGA and macrosomia associated with increasing A1C quartile at diagnosis of GDM was detected when women of Asian Indian or other race/ethnicity were excluded from the study population.
The prevalences of LGA (4%) and macrosomia (6%) in our study population were lower than the 13% prevalence of LGA and 10% prevalence of macrosomia reported in the treatment arm of the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS).7 Notably, ACHOIS included women with lesser degrees of glucose intolerance in pregnancy than those in our study. However, the treatment goals in our study population were more aggressive than those used in ACHOIS. Specifically, in our study population, prior to initiation of insulin or oral hypoglycaemic agents the target values for fasting glucose and 1-h postprandial glucose were <90mg/dL (<5 mmol/L) and <120mg/dL (<6.66 mmol/L) respectively. In the ACHOIS trial glucose targets were fasting glucose 63–99 mg/dL (3.50–5.50 mmol/L) and 2-h postprandial glucose <126 mg/dL (<6.99 mmol/L).7 The aggressive clinical management of hyperglycaemia in our study population may explain the lower than expected prevalence of LGA and macrosomia.
Any association between A1C at GDM diagnosis and LGA may be mediated or modified by glycaemic control during the third trimester, which may explain our null findings. Previous studies indicate that the association between A1C and birthweight may be strongest when A1C is measured after management of GDM has commenced.25,41–43 We found indirect evidence of this in our study population. Women who require insulin or oral hypoglycaemic agents may constitute a population with poor glycaemic control, and when we included only women who required insulin or oral hypoglycaemic agents in our analysis we found a slightly stronger association between A1C ≥5.7% and LGA and macrosomia that approached statistical significance. A subset of women in our population had multiple A1C measurements after GDM diagnosis, and among those whose A1C increased between diagnosis and delivery, the prevalence of LGA and macrosomia was higher than among those whose A1C decreased or remained unchanged.
Our inability to detect an association between A1C at GDM diagnosis and risk of LGA or macrosomia may also be due to the definition of outcome. LGA and macrosomia are markers of excessive fetal growth and adiposity, which may be better measured by infant body composition.44 Other anthropometric measures, which were not available in the medical records, including accurate measures of lean body mass and fat mass, might have a more reliable association with maternal A1C at GDM diagnosis. For example, Catalano et al. reported in a study of 95 women with GDM and 220 without GDM that while mean infant birthweights were comparable between the two groups (3398 g vs. 3337 g, P = 0.26), the infants of women with GDM had greater fat mass compared with those without GDM (436 g vs. 362 g, P < 0.001).45 Additionally, we did not use race/ethnicity-specific growth curves for defining LGA, and therefore it is possible that there may have been misclassification of LGA particularly among women of Asian Indian descent.39 When we limited our analysis to White, African American and Hispanic women, we began to see statistically significant trends of increased risk for LGA and macrosomia across increasing quartiles of A1C.
Finally, there is increasing evidence that obesity24 and fuels other than glucose, including triglycerides,46 may be important risk factors for the development of LGA and macrosomia.47 Therefore, particularly in a patient population with very tight glycaemic control, other factors including maternal pre-pregnancy obesity and high triglycerides may be more strongly associated with the risk of LGA or macrosomia than A1C at diagnosis of GDM.
This study had several important strengths including the diversity of the population and application of strict inclusion criteria. Over 60% of women in this study were of non-White race/ethnicity. Additionally, we restricted our analysis to women diagnosed with GDM at ≥24 weeks gestation. Women diagnosed earlier in pregnancy are managed for hyperglycaemia for a greater proportion of their pregnancy and therefore, even if they have higher A1C at diagnosis, may achieve greater glycaemic control and be at lower risk of LGA compared with women diagnosed at 24 weeks gestation,48 the time at which most women are screened for GDM.
A key limitation to the current study was the lower than expected incidence of LGA and macrosomic infants. This may be due to the aggressive management of hyperglycaemia within the two clinics or the use of a non-race/ethnicity-specific definition of LGA. Our study was also conducted in a single diabetes and pregnancy programme and, despite the diversity of our sample, our results may not be generalisable outside this population, particularly given the aggressive clinical management of GDM in our study.
A1C at GDM diagnosis may not be associated with risk of LGA or macrosomia; however, this may be dependent on the growth curves used for defining LGA. Particularly in a clinical setting where aggressive goals for maternal glycaemic control are implemented, the impact of the severity of hyperglycaemia at GDM diagnosis on fetal growth may be mediated by glycaemic control in the third trimester. These findings highlight the importance of glycaemic control in lowering the risk of LGA or macrosomia among women with GDM. Future studies using larger populations and repeated measures of maternal glycaemia and fetal growth are needed to replicate these findings and explore possible mechanisms, which might explain some of the observed differences in subgroups.
Supplementary Material
Acknowledgements
Jodie Katon was supported by grant number T32 HD052462 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH; Achievement Rewards for College Scientists (ARCS); and the Samuel and Althea Stroum Foundation. This study was also funded by a grant from the University of Washington Department of Epidemiology. The authors would like to thank Misty Morris, RN for data collection.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Please note:Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. number 30, September 2001 (replaces technical bulletin number 200, December 1994). Gestational diabetes. Obstetrics and Gynecology 2001; 98:525–538. [PubMed] [Google Scholar]
- 2.Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabetic Medicine 2010; 27:436–441. [DOI] [PubMed] [Google Scholar]
- 3.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. American Journal of Obstetrics and Gynecology 2003; 188:1372–1378. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence EJ. A matter of size: Part 2. Evaluating the large-for-gestational-age neonate. Advances in Neonatal Care 2007; 7:187–197. [DOI] [PubMed] [Google Scholar]
- 5.Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care 1999; 22:1284–1291. [DOI] [PubMed] [Google Scholar]
- 6.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005; 115:e290–e296. [DOI] [PubMed] [Google Scholar]
- 7.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005; 352:2477–2486. [DOI] [PubMed] [Google Scholar]
- 8.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009; 361:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein DE, Nathan D, Little RR, Peterson CM, Lorenz RA, Sacks DB, et al. Tests of glycemia in diabetes. Diabetes Care 2004; 27:1761–1773. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleicher SJ, O’Sullivan JB, Freinkel N. Carbohydrate metabolism in pregnancy. V. The interrelations of glucose, insulin and free fatty acids in late pregnancy and post partum. New England Journal of Medicine 1964; 271:866–872. [DOI] [PubMed] [Google Scholar]
- 12.Lind T Metabolic changes in pregnancy relevant to diabetes mellitus. Postgraduate Medical Journal 1979; 55:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind T, Billewicz WZ, Brown G. A serial study of changes occurring in the oral glucose tolerance test during pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973; 80:1033–1039. [DOI] [PubMed] [Google Scholar]
- 14.Berlin NI, Goetsch C, Hyde GM, Parsons RJ. The blood volume in pregnancy as determined by P32 labeled red blood cells. Surgery, Gynecology & Obstetrics 1953; 97:173–176. [PubMed] [Google Scholar]
- 15.Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. Journal of Obstetrics and Gynaecology of the British Empire 1963; 70:402–407. [DOI] [PubMed] [Google Scholar]
- 16.Lurie S, Danon D. Life span of erythrocytes in late pregnancy. Obstetrics and Gynecology 1992; 80:123–126. [PubMed] [Google Scholar]
- 17.Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Seminars in Fetal & Neonatal Medicine 2009; 14:66–71. [DOI] [PubMed] [Google Scholar]
- 18.Herranz L, Saez-de-Ibarra L, Grande C, Pallardo F. Non-glycemic-dependent reduction of late pregnancy A1C levels in women with type 1 diabetes. Diabetes Care 2007; 30:1579–1580. [DOI] [PubMed] [Google Scholar]
- 19.Mosca A, Paleari R, Dalfra MG, Di Cianni G, Cuccuru I, Pellegrini G, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clinical Chemistry 2006; 52:1138–1143. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovic L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1C during pregnancy as a treatment tool to guide therapy. Diabetes Care 2010; 34:53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evers IM, de Valk HW, Mol BW, ter Braak EW, Visser GH. Macrosomia despite good glycaemic control in type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia 2002; 45:1484–1489. [DOI] [PubMed] [Google Scholar]
- 22.Penney GC, Mair G, Pearson DW. The relationship between birth weight and maternal glycated haemoglobin (HbA1c) concentration in pregnancies complicated by Type 1 diabetes. Diabetic Medicine 2003; 20:162–166. [DOI] [PubMed] [Google Scholar]
- 23.Kerssen A, de Valk HW, Visser GH. Increased second trimester maternal glucose levels are related to extremely large-for-gestational-age infants in women with type 1 diabetes. Diabetes Care 2007; 30:1069–1074. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer-Graf UM, Heuer R, Kilavuz O, Pandura A, Henrich W, Vetter K. Maternal obesity not maternal glucose values correlates best with high rates of fetal macrosomia in pregnancies complicated by gestational diabetes. Journal of Perinatal Medicine 2002; 30:313–321. [DOI] [PubMed] [Google Scholar]
- 25.Zawiejska A, Wender-Ozegowska E, Brazert J, Sodowski K. Components of metabolic syndrome and their impact on fetal growth in women with gestational diabetes mellitus. Journal of Physiology and Pharmacology 2008; 59 (Suppl. 4):5–18. [PubMed] [Google Scholar]
- 26.Lapolla A, Dalfra MG, Bonomo M, Castiglione MT, Di Cianni G, Masin M, et al. Can plasma glucose and HbAlc predict fetal growth in mothers with different glucose tolerance levels? Diabetes Research and Clinical Practice 2007; 77:465–470. [DOI] [PubMed] [Google Scholar]
- 27.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008; 358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Quintero VH, Istwan NB, Rhea DJ, Tudela CM, Flick AA, de la Torre L, et al. Antenatal factors predicting subsequent need for insulin treatment in women with gestational diabetes. Journal of Women’s Health 2008; 17:1183–1187. [DOI] [PubMed] [Google Scholar]
- 29.Sapienza AD, Francisco RP, Trindade TC, Zugaib M. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Research and Clinical Practice 2010; 88:81–86. [DOI] [PubMed] [Google Scholar]
- 30.Mulder EJ, Koopman CM, Vermunt JK, de Valk HW, Visser GH. Fetal growth trajectories in Type-1 diabetic pregnancy. Ultrasound in Obstetrics & Gynecology 2010; 36:735–742. [DOI] [PubMed] [Google Scholar]
- 31.Buekens P, Kotelchuck M, Blondel B, Kristensen FB, Chen JH, Masuy-Stroobant G. A comparison of prenatal care use in the United States and Europe. American Journal of Public Health 1993; 83:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20:1183–1197.
- 33.Arsie MP, Marchioro L, Lapolla A, Giacchetto GF, Bordin MR, Rizzotti P, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetologica 2000; 37:1–7. [DOI] [PubMed] [Google Scholar]
- 34.John WG, Edwards R, Price CP. Laboratory evaluation of the DCA 2000 clinic HbA1c immunoassay analyser. Annals of Clinical Biochemistry 1994; 31 (Pt 4):367–370. [DOI] [PubMed] [Google Scholar]
- 35.Guerci B, Durain D, Leblanc H, Rouland JC, Passa P, Godeau T, et al. Multicentre evaluation of the DCA 2000 system for measuring glycated haemoglobin. DCA 2000 Study Group. Diabetes & Metabolism 1997; 23:195–201. [PubMed] [Google Scholar]
- 36.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatrics 2003; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriksen T The macrosomic fetus: a challenge in current obstetrics. Acta Obstetricia et Gynecologica Scandinavica 2008; 87:134–145. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ, Greenland S. Modern Epidemiology, 2nd edn Philadelphia: Lippincott Williams & Wilkins, 1998. [Google Scholar]
- 39.Kandraju H, Agrawal S, Geetha K, Sujatha L, Subramanian S, Murki S. Gestational age-specific centile charts for anthropometry at birth for South Indian infants. Indian Pediatrics 2011; published online 15.8.2011. [DOI] [PubMed] [Google Scholar]
- 40.StataCorp. Stata Statistical Software: Release 9. College Station, TX: Statacorp LP, 2005. [Google Scholar]
- 41.Djelmis J, Blajic J, Bukovic D, Pfeifer D, Ivanisevic M, Kendic S, et al. Glycosylated hemoglobin and fetal growth in normal, gestational and insulin dependent diabetes mellitus pregnancies. Collegium Antropologicum 1997; 21:621–629. [PubMed] [Google Scholar]
- 42.Gandhi RA, Brown J, Simm A, Page RC, Idris I. Hb A1c during pregnancy: its relationship to meal related glycaemia and neonatal birth weight in patients with diabetes. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008; 138:45–48. [DOI] [PubMed] [Google Scholar]
- 43.Miller JM, Crenshaw MC Jr, Welt SI. Hemoglobin A1c in normal and diabetic pregnancy. JAMA 1979; 242: 2785–2787. [PubMed] [Google Scholar]
- 44.Durnwald C, Huston-Presley L, Amini S, Catalano P. Evaluation of body composition of large-for-gestational-age infants of women with gestational diabetes mellitus compared with women with normal glucose tolerance levels. American Journal of Obstetrics and Gynecology 2004; 191:804–808. [DOI] [PubMed] [Google Scholar]
- 45.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology 2003; 189:1698–1704. [DOI] [PubMed] [Google Scholar]
- 46.Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstetricia et Gynecologica Scandinavica 2010; 89: 700–704. [DOI] [PubMed] [Google Scholar]
- 47.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? American Journal of Obstetrics and Gynecology 2011; 204: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, et al. Detection and care of women with gestational diabetes mellitus from early weeks of pregnancy results in birth weight of newborn babies appropriate for gestational age. Diabetes Research and Clinical Practice 2008; 80:199–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.