We read with interest the paper by Montopoli et al. about the association between androgen deprivation therapies (ADT) in prostate cancer patients and protection against coronavirus disease 2019 (COVID-19). The TMPRSS2 regulated expression by the androgen receptor (AR) in non-prostatic tissues might explain the increased susceptibility of men to develop severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. ADT, based on luteinizing hormone-releasing hormone (LHRH) agonist/antagonists or AR inhibitors, might reduce SARS-CoV-2 infections or complications in high-risk male populations.1 The outbreak of the pandemic shows a marked geographical variation in the prevalence and mortality of COVID-19. Some Western European regions (e.g. Bergamo in Italy, Noord-Brabant in the Netherlands, Limburg in Belgium) were severely affected. As a potentially protective role of the double X chromosome in females was suggested,2 we postulated that Y-chromosome polymorphisms might also partly explain the variable prevalence and mortality.
The phylogenetic resolution of the Y-chromosome haplogroup (YHG) tree is now sufficiently high to be able to detect geographic patterns on a micro-regional scale.3 , 4 A critical role for genetic variation in chromosome Y in regulating susceptibility to influenza A virus infection and in augmenting pathogenic immune responses in the lung has been demonstrated in a murine model.5 We have compared the prevalence of Y-chromosome haplotypes in the Netherlands and Dutch-speaking Belgium (Flanders) with the prevalence and mortality of COVID-19 using individualized data of 12 Dutch and 5 Flemish provinces. In parallel, prevalence and mortality of COVID-19 were compared with epidemiological data on Y-chromosome haplotypes and several polymorphisms [angiotensin-converting enzyme 1 (ACE1), human homeostatic iron regulator protein (HFE), and complement component C3] in 28 (mainly European) countries. Infection-related data reported on 30 April 2020 by Belgian and Dutch health authorities, as well as Johns Hopkins, were analyzed. COVID-19 prevalence (Figure 1 A) and mortality frequency in the Dutch and Flemish provinces strongly correlated with the R1b-S116 haplotype frequency (r 2 = 0.601 and 0.453, respectively). Similarly, in European countries, a marked correlation was noted: COVID-19 prevalence (Figure 1B) and mortality showed a strong correlation with the R1b-S116 haplotype frequency (r 2 = 0.390 and 0.493, respectively). Even in separate multivariate regression models for COVID-19 prevalence and mortality frequency including the listed candidate markers, R1b-S116 remained a significant factor (next to ACE1 polymorphism for COVID-19 prevalence). Remarkably, among Italians, the heavily affected Bergamo area is characterized by a very high R1b-S116 haplotype frequency (0.179). Among European countries, a linear positive correlation was found between R1b-S116 allele frequency and basic reproduction numbers [calculated from a susceptible-infectious-recovered COVID-19 model (r 2 = 0.281)].
Figure 1.
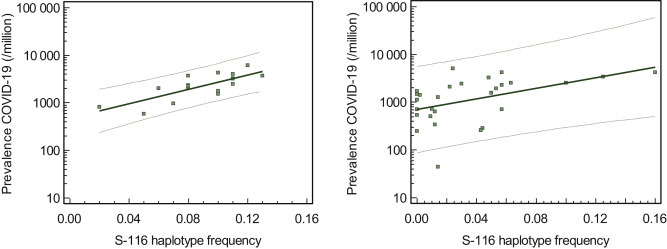
R1b-S116 haplotype frequency versus COVID-19 prevalence (30 April 2020) in the Netherlands and Flanders (A) [log (prevalence) = 2.8684 + 7.543 X (R1b-S116 haplotype frequency), r2 = 0.601] and in a group of 28 countries (B) [log (prevalence) = 2.848 + 5.561 X (R1b-S116 haplotype frequency), r2 = 0.390].
On the one hand, the Y-chromosome influences immune and inflammatory responses, resulting in a genetically programmed susceptibility to diseases with a strong immune component. On the other hand, the R1b-S116 haplotype frequency might also be regarded as a population marker, which stands for cosegregated genes and associated epigenetic control. Further research should focus on the interaction between the AR, TMPRSS2, and the pattern of Y-chromosome haplotype distribution in different COVID-19 patient population groups.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Montopoli M., Zumerle S., Vettor R., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci. 2020;21:3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altena E., Smeding R., van der Gaag K.J., et al. The Dutch Y-chromosomal landscape. Eur J Hum Genet. 2020;28:287–299. doi: 10.1038/s41431-019-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myres N.M., Rootsi S., Lin A.A., et al. A major Y-chromosome haplogroup R1b Holocene era founder effect in Central and Western Europe. Eur J Hum Genet. 2011;19:95–101. doi: 10.1038/ejhg.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krementsov D.N., Case L.K., Dienz O., et al. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc Natl Acad Sci U S A. 2017;114:3491–3496. doi: 10.1073/pnas.1620889114. [DOI] [PMC free article] [PubMed] [Google Scholar]


