Abstract
Lung ultrasonography (LUS), an imaging modality quickly performed, interpreted, and integrated by the treating physician at the bedside, is a particularly useful tool for acutely ill patients. In the evaluation of a patient with respiratory failure in the ICU or ED, LUS is superior to chest radiograph and generally comparable with CT imaging and reduces the need for patient transport and radiation exposure. This article will provide a concise review of LUS as it pertains to respiratory failure in general and will include examples of relevant ultrasound images and video clips from critically ill patients.
Key Words: acute lung injury, critical care, ultrasound
Abbreviations: COVID-19, coronavirus disease 2019; CXR, chest radiograph; LUS, lung ultrasonography
Because of its favorable diagnostic performance characteristics and overall ease of use, lung ultrasonography (LUS) is a useful imaging modality for the treatment of critically ill patients with respiratory failure. It is superior to physical examination and chest radiograph (CXR) for the diagnosis and evaluation of many respiratory conditions that are encountered commonly in the ICU or ED,1, 2, 3 and it generally performs comparably with CT imaging.4, 5, 6 As a test performed by the clinician at the point of care, it is fast, safe, and efficient because modern ultrasound machines are portable, widely available, inexpensive, and easy to use. With the onset of the COVID-19 pandemic, erstwhile resource-rich health-care systems have been stressed by a major surge in operational requirements, which has highlighted further the utility of ultrasound imaging in reducing the use of CXR and CT resources.7
This review will provide an update on the utility of LUS for the evaluation of respiratory failure in acutely ill patients. Clinicians with limited experience in performing LUS should consult the companion “Better with Ultrasound” review, published in this issue of CHEST,8 which serves as an introductory primer. Providers particularly interested in the specific applications of LUS for patients with coronavirus disease 2019 (COVID-19) should consult the companion article on that subject, also published in this issue of CHEST.9 Although the focus here is on lung imaging, the complimentary nature of point-of-care ultrasound imaging should not be forgotten; in many instances cardiac, abdominal, and deep vein studies will increase the diagnostic yield of LUS.10
LUS Technique
Transducer Selection
There is significant debate within the ultrasound community regarding the optimal transducer for lung imaging. Most providers recommend a lower-frequency transducer as their primary tool, choosing between a convex transducer (commonly used in abdominal imaging and offering the ability to visualize a large portion of the lung surface and offering a rapid examination of the whole chest) and a phased-array transducer (commonly used in cardiac imagining and offering a smaller footprint, enabling easier access to the intercostal spaces). Probe selection is determined primarily by availability and operator preference. Interestingly, some pathologic findings such as B-lines (discussed later) appear slightly different depending on which lower-frequency transducer is selected, due to differences in lateral resolution. Many of the classic LUS findings were originally described with the use of a microconvex transducer, but this type of transducer is used less commonly by most ultrasound providers. A higher frequency linear probe (commonly used for guidance of venous access and detection of DVT) has potential applications for detailed examinations of pleural line morphology.
Patient Positioning
Patients, especially those who are critically ill, are examined generally in the supine position, either flat or with the head-of-bed slightly elevated. The patient’s arms are positioned such that access to the lateral thorax is unimpeded; under ideal circumstances, they can be turned to the left and right lateral decubitus positions during the examination to facilitate examination of the posterior lung aspects.
Machine Setup
Most modern ultrasound machines now possess a specific “lung” setting, which should be used regardless of which transducer is deployed. For older machines, the “abdominal” setting is a generally acceptable alternative. In all cases, but especially considering the current COVID-19 situation, great attention must be paid to infection-control practices. Deference should be paid to established local protocols for sheathing and cleaning machines and transducers.
Image Capture
Scanning depth will vary based on patient body habitus; however, a starting depth of 8 to 10 cm is reasonable for either lower-frequency transducer. Gain should be adjusted such that it is uniform throughout the entire scanning depth. Too much gain, a common error for beginners, can emphasize certain findings falsely, such as B-lines. Study archiving is essential for quality assurance, safety, and later scientific study; the recording of video clips (or still images where applicable) is strongly recommended.
Transducer Orientation
Some providers, which is typical for those who favor the large convex transducer, advocate for a longitudinal orientation where the transducer is parallel to the ribs. Those providers more accustomed to using the phased-array transducer may prefer an oblique orientation with the probe perpendicular to the ribs, yielding the familiar image of two ribs casting acoustic shadows and the pleural line in between.
Scanning Protocol
There is disagreement within the ultrasound community with respect to specific scanning protocols. Some providers favor a simple 3-point-per-hemithorax sequence, essentially copying the seminal bedside lung ultrasound in emergency protocol11 intended for critically ill patients with severe dyspnea. Others prefer a more comprehensive scanning protocol such as that described in the 2012 international evidence-based recommendations for point-of-care LUS,12 arguably more applicable to a broader group of patients with less-severe symptoms in clinical environments outside the ICU. Regardless of the scanning protocol selected, an examination that is both flexible and systematic is recommended, especially for conditions like COVID-related lung disease that may present with heterogenous findings.
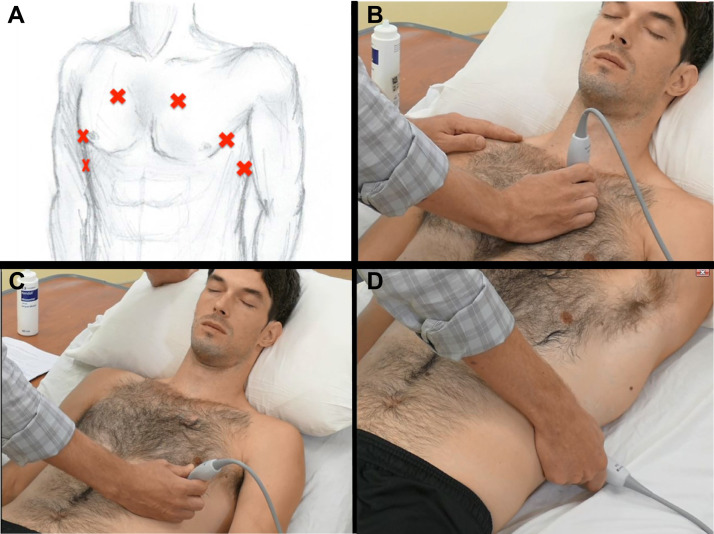
A basic 3-point scanning sequence11 would include (Fig 1 ): (1) At the 2nd intercostal space in the mid-clavicular line, the operator examines the anterior pleural line for lung sliding. Some providers use the high-frequency linear transducer here to examine pleural line morphology in detail. Switching to a lower-frequency probe for the remainder of the examination, the operator examines this same location to identify common LUS findings, such as A-lines and B-lines. (2) At the 5th or 6th intercostal space in the lateral-clavicular line, the lower-frequency probe is applied to identify common LUS findings, such as A-lines and B-lines. (3) At the posterior-axillary line just above the level of the diaphragm, the lower-frequency probe is again applied. Here the particular focus is determining the presence or absence of lung consolidation or pleural effusion.
Figure 1.
A-D, Thoracic ultrasound technical aspects. A, Diagram illustrates three basic transducer positions. B, Upper BLUE point. C, Lower BLUE point. D, Posterolateral alveolar and/or pleural syndrome point.
Providers should be prepared to deviate from any chosen protocol in response to pathologic evidence detected in a given view. More elaborate protocols range from four points (per hemithorax)12 to six points13 and beyond. A specific protocol for COVID-related disease is proposed in the accompanying article.9
LUS for the Evaluation of Respiratory Failure in the ICU
Most imaging modalities, including LUS, CXR, and CT imaging, share a common weakness: the resulting images have no utility until they are integrated with clinical information to generate a diagnosis and management plan. With LUS, the bedside clinician is responsible for all aspects from image acquisition to image interpretation and from clinical integration to action at the bedside.
Chest CT imaging has the advantage of excellent image quality but is burdened by major disadvantages that include the need for patient transport, radiation exposure, and the inability to perform frequent repeat assessments; this leaves CXR and LUS as the two practical alternatives for most routine ICU imaging, particularly for patients with an unstable condition. These two modalities have been compared in evaluating a variety of causes of respiratory failure common to critically ill patients; LUS has been shown to be more sensitive than CXR for the assessment of acute dyspnea, heart failure, ARDS, pneumonia, and pneumothorax.14, 15, 16, 17, 18, 19, 20, 21
As such, the intensivist can forgo CXR confidently in favor of LUS for many ICU applications. CXR maintains an important role in confirming the placement of central lines and airway devices, in helping confirm or refute LUS findings where doubt exists, in investigating pathologic condition at the level of the mediastinum and ribs, and in adding diagnostic confidence for providers who are less experienced in LUS. Chest CT imaging also retains a significant role but can usually be reserved for situations in which LUS fails to answer the question at hand. LUS has added advantages as a dynamic imaging modality that can be repeated frequently to assess the effect of therapeutic interventions; using serial LUS examinations during weaning from mechanical ventilation, lung recruitment maneuvers, positive end expiratory pressure titration, or identification of the patient who may respond to prone positioning are common examples.13 , 22, 23, 24, 25, 26, 27, 28 Limitations associated with LUS relate primarily to operator factors (as an operator-dependent skill where training and experience are important) and patient factors (where elements such as obesity and immobility can make performing examinations difficult).
Common Clinical Questions
Can I Quickly Rule Out a Pneumothorax?
Ultrasound imaging of the normally aerated lung will show the pleura line moving with the respiratory or cardiac cycle (lung sliding and lung pulse, respectively), except when the pathologic presence of air drives the two pleural layers apart and impedes ultrasound transmission. The presence of lung sliding, a lung pulse, or B-lines achieves the goal of ruling out a pneumothorax at the point of transducer contact by establishing the absence of intervening air (Video 1). For lung sliding in particular, a steady hand is important to avoid a false-positive result.
How this information is integrated will depend on the specific clinical scenario at hand. For a patient in shock, the presence of lung sliding, a lung pulse, or B-lines at the most anterior point bilaterally (often the 2nd intercostal space in the mid-clavicular line is used) would be enough to rule out a large or tension pneumothorax as a cause of hemodynamic instability. When searching for a smaller, more localized pneumothorax, a more thorough examination will be required; in such cases, detection of a lung “point”29 confirms with high specificity the diagnosis of pneumothorax. The specific location of the lung point can also facilitate the estimation of the degree of lung collapse.30
Is There Evidence of an Interstitial Syndrome?
Integrating the number, pattern, and density of B-lines (Video 2) into the overall clinical presentation can aid in determining both cause and severity, remaining mindful that conditions including ARDS, pneumonia, cardiogenic pulmonary edema, lung contusion, lung infarction, chronic interstitial lung disease, and lymphangitic carcinomatosis (among others) can all manifest similar LUS findings.31 Closer examination of the pleura line morphologic evidence, in the presence of B-lines, may be useful in distinguishing cardiogenic from noncardiogenic pulmonary edema.32
The distribution and intensity of B-lines, combined with relevant clinical information, can be very helpful in the development of a differential diagnosis. In cardiogenic pulmonary edema, B-lines are expected to be bilateral, diffuse, homogeneous, and more severe in dependent areas. In ARDS (and COVID-19 pneumonia), the distribution is typically patchy and heterogeneous, with abrupt alternations between intense B-lines and spared areas. Anecdotally, with chronic conditions such as interstitial lung diseases or lymphangitic carcinomatosis, there is typically a mismatch between the severity of B-line distribution and relatively milder symptoms. In bacterial infection, lung contusion, and lung infarction, the distribution is typically focal, and the severity of the clinical condition may be disproportionate in comparison with the relatively unimpressive B-line pattern.
Is There Alveolar Consolidation, and If So, What Is Its Distribution?
As with B-lines, there are many potential causes when consolidation is detected with LUS. The degree and location of consolidated lung, as well as the presence or absence of air bronchograms, may suggest a particular diagnosis such as pneumonia or atelectasis (Video 3).
In particular, the relationship between the area of consolidated lung and an adjacent pleural effusion may be crucial to establishing a diagnosis. Compressive atelectasis (as opposed to atelectasis caused by an obstructed airway, for example) typically is accompanied (and caused) by a large pleural effusion, therefore the absence of such an effusion makes this diagnosis unlikely. The amount of atelectasis can also be seen to lessen with inspiration, although such dynamic respiratory recruitment is usually absent in pulmonary infection or malignancy. Air bronchograms, which typically move with the respiratory cycle and therefore demonstrate the patency of the bronchial tree, are common in pneumonia but absent in atelectasis, particularly obstructive atelectasis.
Finally, extensive consolidated areas in ARDS may respond well to recruitment maneuvers, and a pattern of inferoposterior consolidation in particular may predict a positive response to prone positioning.24 The effect of recruitment maneuvers can be followed in real time with the use of LUS (Video 3).
Is There a Pleural Effusion?
LUS can be used to determine the size of a pleural effusion (Video 4) in addition to its distribution, complexity, and echogenicity,33 being careful to distinguish rib shadows from pleural fluid. Thoracentesis with ultrasound guidance can be performed with low complication rates, especially in patients who are mechanically ventilated.34 Pleural effusion can be difficult to distinguish from consolidation on CXR, and LUS is the ideal tool to make this distinction.
At this point, the answers to the aforementioned four questions can be integrated, and any abnormal findings may have already led to a presumptive diagnosis. A perfectly normal LUS examination, however, is also very useful. Ruling out pneumothorax, pulmonary edema, consolidation, and pleural effusion in a patient with significant dyspnea or hypoxemia can lead to the consideration of conditions such as pulmonary embolism, asthma exacerbation, or COPD exacerbation.
Can I Monitor Changes in Lung Aeration?
Although the routine application of recruitment maneuvers in ARDS remains controversial, LUS is effective in monitoring lung recruitment in critically ill patients who are mechanically ventilated; this potential has been demonstrated in both ventilator-associated pneumonia and in ARDS.13 , 35 A LUS score of both aeration and recruitment (based on the evaluation of three basic patterns in 12 anterior, lateral, and posterior chest areas) has been validated in comparison with the pressure-volume curve in patients who receive ventilation therapy. Dynamic changes can be followed over time in response to drugs, ventilatory strategies, and recruitment maneuvers by assigning a recruitment score (positive or negative) to each lung region.
LUS is also useful in the quantification of extravascular lung water and compares well to more invasive estimates.36, 37, 38 This technique can be helpful in decision-making around fluid management in patients with a complex condition.
Conclusion
LUS, an imaging modality quickly performed, interpreted, and integrated by the treating physician at the bedside, is a particularly useful tool for acutely ill patients. Commonly encountered conditions such as pneumonia, pulmonary edema, and pneumothorax can be efficiently evaluated, and the skill set is generally straightforward to learn.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. K. has received honoraria for educational lectures for Fuji SonoSite and Cook Medical. None declared (P. M., G. V., S. M.).
Additional information: The Videos can be found in the Multimedia section of the online article.
Supplementary Data
References
- 1.Alrajab S., Youssef A.M., Akkus N.I., Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. doi: 10.1186/cc13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein D., Goldstein I., Mourgeon E., Cluzel P., Grenier P., Rouby J.J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Xirouchaki N., Magkanas E., Vaporidi K. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 2011;37(9):1488–1493. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 4.Tierney D.M., Huelster J.S., Overgaard J.D. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48(2):151–157. doi: 10.1097/CCM.0000000000004124. [DOI] [PubMed] [Google Scholar]
- 5.Nazerian P., Volpicelli G., Vanni S. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33(5):620–625. doi: 10.1016/j.ajem.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. vol. 4, no. 1, 2014. [DOI] [PMC free article] [PubMed]
- 7.Brogi E., Bignami E., Sidoti A. Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound. 2017;15(1):23. doi: 10.1186/s12947-017-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendin A., Koenig S., Millington S.J. Better with ultrasound: thoracic ultrasound. Chest. 2020;158(5):2082–2089. doi: 10.1016/j.chest.2020.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Koenig S, Volpicelli G, Millington S, Mayo P. How I do it: lung ultrasound for patients with COVID-19 pulmonary disease [published online ahead of print August 21, 2020]. 10.1016/j.chest.2020.08.2054. [DOI]
- 10.Narasimhan M. A whole-body approach to point of care ultrasound. Chest. 2016;150(4):772–776. doi: 10.1016/j.chest.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein D., Mezière G. The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit Ultrasound J. 2011;3(5):109–110. [Google Scholar]
- 12.Volpicelli G., Elbarbary M., Blaivas M. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 13.Bouhemad B., Brisson H., Le-Guen M. Bedside ultrasound assessment of positive end-expiratory pressure–induced lung recruitment. Am J Respir Crit Care Med. 2011;183(1):341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 14.Pivetta E., Goffi A., Nazerian P., Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial.,”. Eur J Heart Fail. 2019;21(6):754–766. doi: 10.1002/ejhf.1379. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein D., Mezière G. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134(5):117–122. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanobetti M., Scorpiniti M., Gigli C. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017;151(6):1295–1301. doi: 10.1016/j.chest.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Pesenti A., Musch G., Lichtenstein D. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016;42(5):686–698. doi: 10.1007/s00134-016-4328-1. [DOI] [PubMed] [Google Scholar]
- 18.Laursen C.B., Sloth E., Lassen A.T. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, randomised controlled trial. Lancet Respir Med. 2014;2(8):638–646. doi: 10.1016/S2213-2600(14)70135-3. [DOI] [PubMed] [Google Scholar]
- 19.Al Deeb M., Barbic S., Featherstone R., Dankoff J., Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843–885. doi: 10.1111/acem.12435. [DOI] [PubMed] [Google Scholar]
- 20.Ye X., Xiao H., Chen B., Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia. PLoS One. 2015;10(6):e0130066. doi: 10.1371/journal.pone.0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staub L.J., Biscaro R.R., Maurici R. Accuracy and applications of lung ultrasound to diagnose ventilator-associated pneumonia: a systematic review. J Intensive Care Med. 2018;33(8):447–455. doi: 10.1177/0885066617737756. [DOI] [PubMed] [Google Scholar]
- 22.Constantin J.M., Grasso S., Chanques G. Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38(4):1108–1117. doi: 10.1097/CCM.0b013e3181d451ec. [DOI] [PubMed] [Google Scholar]
- 23.Prat G., Guinard S., Bizien N. Can lung ultrasonography predict prone positioning response in acute respiratory distress syndrome patients? J Crit Care. 2016;32(8):36–41. doi: 10.1016/j.jcrc.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Haddam M., Zieleskiewicz L., Perbet S. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42(8):1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang X.T., Ding X., Zhang H.M., Chen H., Su L., Liu D.W. Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care. 2016;20(1):385. doi: 10.1186/s13054-016-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tenza-Lozano E., Llamas-Alvarez A., Jaimez-Navarro E., Fernàndez-Sanchez J. Lung and diaphragm ultrasound as predictors of success in weaning from mechanical ventilation. Crit Ultrasound J. 2018;10(1):12. doi: 10.1186/s13089-018-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayo P., Volpicelli G., Lerolle N., Schreiber A., Doelken P., Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42(7):1107–1117. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
- 28.Nazerian N., Volpicelli G., Gigli C. Diagnostic performance of wells score combined with point-of-care lung and venous ultrasonography in suspected PE. Acad Emerg Med. 2017;24(3):270–280. doi: 10.1111/acem.13130. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein D., Mezière G., Biderman P., Gepner A. The “Lung Point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 30.Volpicelli G., Boero E., Sverzellati N. Semi-quantification of Pneumothorax Volume by Lung Ultrasound. Intensive Care Med. 2014;40(10):1460–1467. doi: 10.1007/s00134-014-3402-9. [DOI] [PubMed] [Google Scholar]
- 31.Volpicelli G., Mussa A., Garofalo G. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24(6):689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Copetti R., Soldati G., Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovascular Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattison L.E., Coppage L., Alderman D.F., Herlong J.O., Sahn S.A. Pleural effusions in the medical ICU: prevalence, causes, and clinical implications. Chest. 1997;111(4):1018–1023. doi: 10.1378/chest.111.4.1018. [DOI] [PubMed] [Google Scholar]
- 34.Mayo P.H., Goltz H.R., Tafreshi M., Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059–1062. doi: 10.1378/chest.125.3.1059. [DOI] [PubMed] [Google Scholar]
- 35.Bouhemad B., Liu Z., Arbelot C. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38(1):84–92. doi: 10.1097/CCM.0b013e3181b08cdb. [DOI] [PubMed] [Google Scholar]
- 36.Agricola E., Bove T., Oppizzi M. “Ultrasound Comet-Tail Images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 37.Volpicelli G., Skurzak S., Boero E. Lung Ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121(2):320–327. doi: 10.1097/ALN.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 38.Anile A., Russo J., Castiglione G., Volpicelli G. A simplified lung ultrasound approach to detect increased extravascular lung water in critically ill patients. Crit Ultrasound J. 2017;9(1):13. doi: 10.1186/s13089-017-0068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.