Abstract
The repositioning of therapeutic agents already approved by the regulatory agencies for the use of drugs is very interesting due to the immediacy of their use; similarly, the possibility of using molecules derived from foods, whether nutrients or not, is of great importance, also because of their immediate therapeutic applicability. Candidates for these natural therapies against COVID-19 should show certain effects, such as restoring mitochondrial function and cellular redox balance. This would allow reducing the susceptibility of risk groups and the cascade of events after SARS-CoV-2 infection, responsible for the clinical picture, triggered by the imbalance towards oxidation, inflammation, and cytokine storm. Possible strategies to follow through the use of substances of food origin would include: a) the promotion of mitophagy to remove dysfunctional mitochondria originating from free radicals, proton imbalance and virus evasion of the immune system; b) the administration of transition metals whose redox activity would lead to their own oxidation and the consequent generation of a reduced environment, which would normalize the oxidative state and the intracellular pH; c) the administration of molecules with demonstrated antioxidant capacity; d) the administration of compounds with anti-inflammatory and vasodilatory activity; e) the administration of immunomodulatory compounds.
Keywords: Curcumin, Polyphenols, Vitamin C, Vitamin D, Zinc
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was described late in 2019 after a multiple number of pneumonia cases in Wuhan, China (Zhu et al., 2020). The disease caused by SARS-CoV-2 is named COVID-19 (coronavirus disease 2019) (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, 2020). The most common clinical features of the disease are fever, dry cough, anorexia, myalgia, dyspnea, anosmia, dysgeusia and dermal alterations (Zabetakis et al., 2020). On July 7th, 2020, over thirteen million confirmed cases of COVID-19 and more than 500,000 deaths were found globally (“COVID-19 Map,” n. d.), and the number of cases is rapidly increasing. The SARS-CoV-2 pandemic leads to an extreme emergency situation that makes it essential to find new therapies and actions aimed at reducing the spread of the virus and finding new therapeutic applications for existing and approved drugs that allow its rapid application for treatment of this new disease. In the same way, based on the increasingly better knowledge of the mechanisms of action of the virus in human cells, it is essential to search for new molecules and combined therapies that may be useful in the treatment of COVID-19.
Food could be a good source of these molecules, some of them are nutrients, such as zinc or vitamins C and D, whereas others are biologically active non-nutrient molecules, such as certain compounds of polyphenolic nature. One of the great advantages of using food-derived molecules is the fact that they are natural, usually with low or no toxicity, and their approval process would be fast in case of some usefulness is reported, for example as nutraceuticals (Santini et al., 2018). In fact, some substances of this type are already being used as therapies against various diseases (Newman and Cragg, 2016). Meanwhile the world is waiting for vaccines or safe and effective treatments, the use of natural therapies, alone or as a combinatory therapy should be fully considered.
2. Pathogenesis of SARS-CoV-2
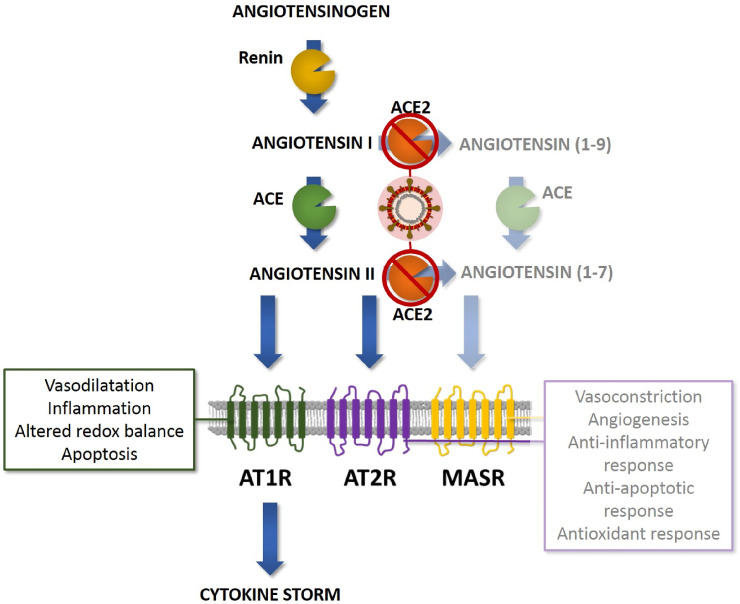
COVID-19 has raised the importance of the renin-angiotensin system (RAS) (Fig. 1 ). RAS activation increases in patients with different conditions, such as diabetes, cardiovascular diseases and others (Ribeiro-Oliveira et al., 2008). The angiotensin converting enzyme (ACE) converts angiotensin I to angiotensin II, the latter is a vasoconstrictor able to produce oxidative stress because many signaling pathways activated in response to angiotensin II are mediated by reactive oxygen species (ROS) generated by NAD(P)H oxidase activity. In turn, oxidative stress is deeply associated with the progression of different cardiovascular disorders. When angiotensin II level is high, it causes insulin resistance, endothelial dysfunction, proteinuria, and high blood pressure. ACE2 receptors produce angiotensin 1-7 by using angiotensin II as substrate (Lelis et al., 2019; Nehme et al., 2019; Ribeiro-Oliveira et al., 2008).
Fig. 1.
SARS-CoV-2 and its interaction with the Renin Angiotensin Aldosterone System (RAS). ACE2 degrades angiotensin I to angiotensin (1–9), which is a ligand for angiotensin II receptor type 2 (AT2R). ACE2 also converts angiotensin II to angiotensin (1–7) that binds to the Mas receptor (MASR). Angiotensin (1–9) has regenerative and anti-inflammatory effects, angiotensin (1–7) mediates anti-inflammatory and vasodilatory effects and reduces reactive oxygen species (ROS) through its binding to AT2R. Thus, angiotensin (1–7) and angiotensin (1–9) counteract the vasoconstriction and pro-inflammatory effects of angiotensin II preventing tissue injuries. SARS-CoV-2 infection would reduce ACE2 expression dysregulating RAS protective pathways.
ACE inhibitors (ACEI) prevent the formation of angiotensin II from angiotensin I, the latter will be converted to angiotensin 1-9 by ACE2 (Lelis et al., 2019; Nehme et al., 2019; Ribeiro-Oliveira et al., 2008). In turn, angiotensin 1-9 can also be transformed by ACE2 to angiotensin 1-7 (Nehme et al., 2019; Ribeiro-Oliveira et al., 2008). Angiotensin receptor antagonists (ARBs) prevent angiotensin II from binding to the receptor. ARBs increase ACE2 (Cure and Cumhur Cure, 2020a). Angiotensin II, which cannot bind to the receptor, is rapidly converted to angiotensin 1-7 by increasing ACE2 (Cure and Cumhur Cure, 2020b). ACE and ACE2 share homology in their catalytic domain proving different key functions in the RAS.
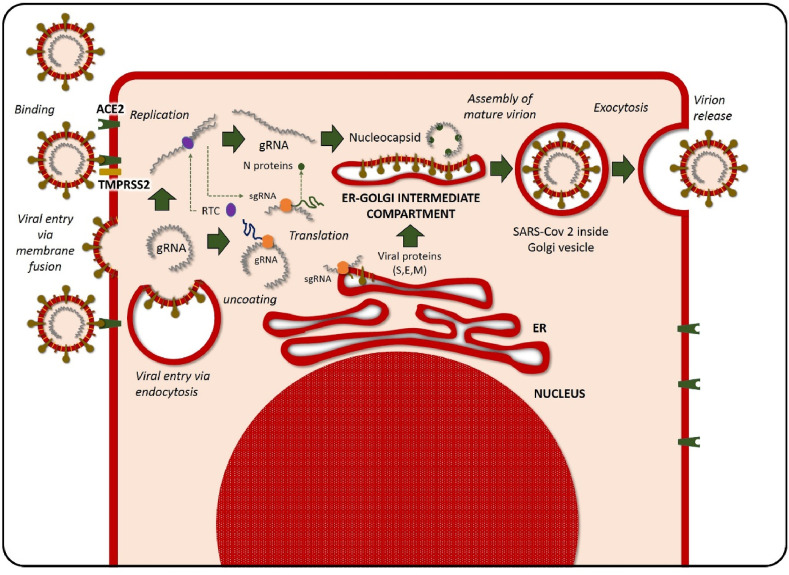
The SARS-CoV-2 virus enters the human body through ACE2 receptors, present in lungs but also in the kidneys, heart, gastrointestinal tract, and other sites. The process of entry of the virus into the cell need the binding of glycoprotein S to ACE2, which acts as a receptor (Tai et al., 2020). After binding and entry, the fusion of the viral membrane and the host cell membrane happens. Type II transmembrane serine protease (TMPRSS2), present on the surface of the host cell, will remove ACE2 after fusion and activate receptor-like spike S proteins (Rabi et al., 2020) (Fig. 2 ). Activation of S proteins induces changes allowing the virus to enter the cell (Simmons et al., 2013). TMPRSS2 and ACE2 are responsible then for the entry of this virus. At the respiratory tract, nasal epithelial cells have the highest level of ACE2 expression (Sungnak et al., 2020). Once inside the cell, SARS-CoV-2 will translate its genetic material into the nucleus after liberation into the cytoplasm (Resnick et al., 1987).
Fig. 2.
Internalization and viral replication. The process of entry of SARS-CoV-2 into the cell needs the binding of glycoprotein S to angiotensin-converting enzyme 2 (ACE2) (present in lungs but also in the kidneys, heart, gastrointestinal tract, and other sites), which act as a receptor. Type II transmembrane serine protease (TMPRSS2) present on the surface of the host cell would remove ACE2-activated receptor-like spike S proteins inducing changes that allows the virus to enter the cell. Once inside the cell, SARS-CoV-2 will translate its genetic material into the nucleus after liberation into the cytoplasm.
However, for the pathogenesis of SARS-CoV-2, ACE2 has been shown not only to be the input receptor for the virus, but also to protect against lung injury. Therefore, in contrast to most other coronaviruses, SARS-CoV-2 has a higher lethality because the virus deregulates a lung protection pathway (Kuba et al., 2005; Yang et al., 2007).
As previously described, ACE2 is expressed in the intestinal tract. According to that, it has been proposed that intestine could also be an important entry site for the SARS-CoV-2. This is also important when considering the chance of a fecal-oral transmission. The distribution of ACE2 receptors in different tissues and organs might be in the basis for the described multiorgan failure found in some patients (Hashimoto et al., 2012). However, the advice from the Center for Disease Control and Prevention that states that no evidence has been found about the chance of getting COVID-19 by touching contaminated surfaces or objects and then touching mucous membranes should also be considered. (Zhang et al., 2020).
The binding of SARS-CoV-2 to ACE2 is produced under conditions of low cytosolic pH (Cure and Cumhur Cure, 2020b). Some conditions, such as hypertension or diabetes, and also aging, are associated with low cytosolic pH, which increases the opportunity for the virus to enter cells (Resnick et al., 1987). Angiotensin II increases pH, this alkalinizing effect is maintained even after a heavy acid load (Costa-Pessoa et al., 2013). ACEI and ARBs reduce angiotensin II, then acidize cytosolic pH. Angiotensin 1-7 causes no changes in the pH and changes in its concentration have no effect on the viral load. ARS activity and angiotensin II levels decrease with aging, particularly in hypertense or diabetic patients; therefore, COVID-19 may be more severe because of a lower cytosolic pH. It has been proposed that angiotensin 1–7 may protect against acute respiratory distress syndrome in COVID-19 because its vasodilatory effect (Cure and Cumhur Cure, 2020c). Although there are doubts about if an increase in angiotensin 1-7 would has a preventive effect, and it has been seen useful only in an experimental model (Komukai et al., 2010).
2.1. Role of mitochondria, redox imbalance, and mitophagy in SARS-CoV-2 infection
Certain risk groups, such as the elderly, diabetics, and cancer patients share a condition: the deterioration of mitochondrial function, which leads to pro-oxidant redox imbalance at cellular level. This is associated with a cellular pH imbalance favorable to the presence of protons that would facilitate the interaction between the virus and the ACE2 receptor and, thus, its route of entry into the cell (Haas, 2019). Additionally, aging and age-related diseases are worsened by the subsequent blockade of these ACE2 receptors and their corresponding activation pathway, canceling its physiological function and its vasodilatory, antioxidant, alkalizing and anti-inflammatory effects, amplifying the cycle of oxidation, inflammation, and vasoconstriction (Gheblawi et al., 2020). Mitochondria are essential organelles responsible for energy production, but also involved in the biosynthesis of metabolites, apoptosis, several aspects of the immune response, etc. Energy metabolism plays a key role in cells involved in the innate immunity (Rambold and Pearce, 2018). For this reason, maintaining integrity and activity of the mitochondrial network is a key aspect for the immune system function. Selective mitochondrial autophagy (mitophagy) is involved in the control of mitochondrial number in the cell by removing damaged organelles, which helps the cell to survive and to respond to aggressions, including infections (Giampieri et al., 2019). A deficient function of autophagy machinery will lead to an exacerbated inflammatory response (Michaličková et al., 2020). A reduction in the expression of genes associated with mitochondria has recently been shown in ACE2-positive Leydig and Sertoli cells, a finding indicative of increased impairment of mitochondrial function in these patients (Wang and Xu, 2020). Furthermore, it has been proposed that the mechanism by which SARS-CoV-2 escape from the innate immune surveillance is based on mitochondrial alterations, placing senescent mitochondria as a factor in increasing susceptibility to the virus and as a key agent in immune evasion of the virus simultaneously (Malavolta et al., 2020). In this way, therapy with drugs contributing to adequate mitochondrial function will exert a protective effect reducing the susceptibility to the virus and therapeutic, reducing the effects derived from the infection associated with the production of free radicals and the activation of the inflammatory response and cytokine storm. In this sense, we point to the compounds inducing mitochondrial autophagy as candidates for the treatment of COVID-19 disease (Michaličková et al., 2020; Yan and Li, 2018).
3. Substances from foods potentially useful in treating COVID-19
There are different nutrients and other molecules from food sources that could be useful in the treatment and/or prevention of COVID-19. In this section we will review these substances. Table 1 shows the foods that are the richest in these molecules.
Table 1.
Main sources of food-derived compounds potentially useful in treating COVID-19.
| Compound | Main sources |
|---|---|
| Zinc | Whole grains and whole grain products |
| Dairy products | |
| Oysters | |
| Red meat | |
| Poultry | |
| Resveratrol | Grapes |
| Red wine | |
| Nuts | |
| Berries | |
| Chocolate | |
| Hydroxytyrosol | Virgin olive oil |
| Leaves of the olive tree (Olea europaea) | |
| Curcumin | Rhizome of turmeric (Curcuma longa) |
| Quercetin | Apples |
| Berries | |
| Cilantro (coriander) | |
| Onions | |
| Capers | |
| Lovage | |
| Dill | |
| Vitamin C | Green and red peppers |
| Tomatoes | |
| Broccoli, Brussels sprouts, and cauliflower | |
| Leafy greens (Spinach, cabbage, turnip greens) | |
| Sweet and white potatoes | |
| Winter squash | |
| Vitamin D | Dairy products |
| Eggs | |
| Fish |
3.1. Zinc
Zinc is a mineral nutrient that acts as a cofactor for many key cell reactions, playing an important role in the growth of cells. Zinc also plays a role in the development and it has functions associated with metabolism and the immune system (Hoang et al., 2020). It has been described in vitro that increased intracellular Zn2+ levels are able to disturb the replication of several RNA viruses, including influenza virus, polio virus, and SARS-CoV (te Velthuis et al., 2010). These authors suggested that intracellular Zn2+ levels affect a common step in cell replication cycles. Enzymatic studies using recombinant RNA-dependent RNA polymerases (RdRPs) (SARS-CoV nsp 12) purified from Escherichia coli revealed that Zn2+ directly inhibited SARS-CoV RdRp elongation and reduced template binding (te Velthuis et al., 2010).
According to estimations, approximately 20% of the population in the world has low levels of zinc in the blood, and the numbers become more relevant in older adults. The deficiency of zinc leads to a diminished production of antibodies. This situation also alters the innate immune system, for example by reducing the activity of natural killer cells. In the same way, zinc deficiency is responsible for a lower production of cytokines by mononuclear cells. Finally, zinc deficiency also reduces chemotaxis response and the respiratory burst of neutrophils (Ibs and Rink, 2003). The difficulty of inorganic Zn salts to access cells can be solved with the administration of organozinc compounds. Currently, in this sense, chloroquine plays a major role, acting as an ionophore, allowing zinc to enter the infected cell (Xue et al., 2014). Furthermore, zinc has beneficial immunomodulatory effects against respiratory infections, which improve the immune response, including the response against SARS-CoV (Jayawardena et al., 2020; Shankar and Prasad, 1998), and it is a transition metal whose intracellular redox activity (Quiles et al., 2020) contributes to the antioxidant defense during the powerful oxidative response inherent in COVID-19 (Cure and Cumhur Cure, 2020a). Zinc acetate, a compound approved by the FDA (Galzin NDA: 020,458), is a zinc-based drug already in use, this drug meets the described characteristics to which we attribute the indicated effects against COVID-19.
3.2. Resveratrol
Resveratrol belongs to the family of polyphenols present in plant foods, such as grape, nuts, red wine, berries, chocolate, and others. Resveratrol belongs to the stilbene family, which is classified as phytoalexins because stilbenes are synthesized by plants in response to ultraviolet rays, bacterial and fungal lesions or toxins (Wahedi et al., 2020). It is well known that resveratrol protects from a series of diseases including malignancies, cardiovascular and respiratory diseases, and others (Horne and Vohl, 2020). At the cellular level, resveratrol acts as an antioxidant, cytostatic, antiviral, anti-inflammatory and it extends the life span of the cells (Wahedi et al., 2020). Resveratrol is also an agonist for sirtuin deacetylase SIRT1. Sirtuins are master regulators of metabolism with multiple objectives. SIRT1 deacetylates Trp 53, destabilizing it and leading the cell to activate the cell cycle and inhibit apoptosis (Navarro et al., 2017).
In neuronal cultures, resveratrol treatment (40 μM, after excitotoxicity) decreases the production of superoxide anion, prevents the overload of intracellular Ca2+ associated with mitochondrial failure, decreases the release of the lactate dehydrogenase enzyme, and decreases death. It also promotes mitophagy (increasing Beclin 1 level, favoring the recruitment of LC3-II, reducing LAMP1, and decreasing the levels of the mitochondrial matrix protein HSP60). Resveratrol (1.8 mg/kg; i. v.; administered at the beginning of reperfusion) increased the levels of phosphorylated AMPK in the cerebral cortex of rats subjected to middle cerebral artery occlusion. A similar effect was found in primary cultured neurons exposed to glutamate-induced excitotoxicity. Therefore, resveratrol acted as an autophagy-inducing agent, and it has shown an important role in mitochondrial function in the mentioned neuronal models (Pineda-Ramírez et al., 2020). In both models, inhibition of AMPK activation with Compound C obstructed the effect of resveratrol, showing that its protective effect depends, partially, on the activation of the AMPK/autophagy pathway. An increase in the autophagic process might increase intracellular pH and thus it might be a way to reduce SARS-CoV-2 infection.
We have found three studies analyzing the role of resveratrol in relation to ACE2 receptors. A study performed in rats fed with 50 mg kg−1 · day of resveratrol showed an increase in the level ofACE2 protein (Tiao et al., 2018). Another study performed in mice fed a high-fat diet compared to mice fed a high-fat diet supplemented with resveratrol showed a significant increase in ACE2 gene expression in mice supplemented with resveratrol. Therefore, resveratrol added to the diet can help reduce the deleterious consequences of diets rich in fat on the ACE2 gene expression (Oliveira Andrade et al., 2014). Lastly, an in vitro study performed in smooth muscle cells from human aorta showed that the expression of the ACE2 gene and protein were significantly enhanced in the cells after 24 h incubation with resveratrol (Moran et al., 2017).
Navarro et al. analyzed if the application of inhaled resveratrol can protect mice with a lung condition from accelerated aging (Navarro et al., 2017). These researchers found that resveratrol treatment delayed loss of lung function, maintained lung structure, and blocked DNA damage in parenchymal cells. As above-mentioned, resveratrol is a known SIRT1 deacetylase agonist and the authors suggested that it acts at pulmonary level promoting the destabilization of p53 and decreasing the expression of Bax (proapoptototic proteins) and, consequently, decreasing apoptosis and increasing survival of alveolar epithelial type 2 cells. Furthermore, it maintains the levels of PGC1α, a stimulator of mitochondrial biogenesis. All these effects led the study authors to suggest that inhalation resveratrol prophylaxis is a potential approach to curb the deterioration of lung function and structure associated with aging while maintaining the integrity of ACE2.
3.3. Hydroxytyrosol
Hydroxytyrosol, or 3,4-dihydroxhyphenyl ethanol, is a polyphenolic compound with amphipathic properties that has a molecular weight of 154.16 g/mol. One of the most important characteristics is its phenylethyl alcohol structure, which is thought to be the basis of its biological functionality. Hydroxytyrosol can be found as a member of the minor components of extra virgin olive oil. Olive tree (Olea europaea) leaves are one of the main sources of hydroxytyrosol. This molecule has an extensive range of well-documented biological activities (Robles-Almazan et al., 2018). It has great antioxidant, anti-inflammatory and antiatherogenic capacities (Granados-Principal et al., 2010). Additionally, its powerful antimicrobial activity has also been documented (Zoric et al., 2013).
Removability of reactive species is among the most important properties of hydroxytyrosol. These ROS scavenger properties have been demonstrated at the extracellular level, where hydroxytyrosol eliminates reactive species generated by UV rays (Zwane et al., 2012). In addition, it has a scavenger capacity at the intracellular level, particularly against superoxide anion, hydrogen peroxide and hypochlorous acid. Moreover, it can act as a metal chelator (Granados-Principal et al., 2010). Several studies (Killeen et al., 2014) showed that hydroxytyrosol is able to modulate the proinflammatory transcription factor NF-κB. As it is well known, NF-κB controls the expression of approximately 150 genes, many of them involved in inflammatory responses, such as cell adhesion molecules, chemokines, but also cytokines, such as interleukins 1, 6, 17 and the tumor necrosis factor alpha. Regarding respiratory diseases, Visioli et al. have reported that hydroxytyrosol reduces oxidative stress related to the respiratory burst of neutrophils (Visioli et al., 1998). It has also been reported that hydroxytyrosol has the ability to reduce the production of superoxide anion, both in vivo and in vitro experimental models (Braga et al., 1997). In the same way, it has been observed that this polyphenol eliminates hydrogen peroxide after respiratory burst in stimulated human neutrophils. This effect has been shown to be dose dependent (O'Dowd et al., 2004). Furthermore, concerning the antibacterial activity of hydroxytyrosol, it has been reported that the growth of different Gram-positive and Gram-negative bacteria typical from respiratory tract infections is inhibited (Bisignano et al., 1999). The above-mentioned suggests that hydroxytyrosol may be used in the treatment of infections produced by several bacterial sources in the context of respiratory tract infections. Hydroxytyrosol has also been found to be effective in the treatment of some viral diseases, such as influenza virus (Yamada et al., 2009) and HIV (Bedoya et al., 2016). Finally, Liu et al. have shown that hydroxytyrosol is effective against pulmonary fibrosis in rats (Liu et al., 2015). All the results described above suggest that hydroxytyrosol is a natural molecule of potential interest in treating COVID-19, reducing the oxidative and inflammatory response, and it may be a good adjuvant therapy in combination with other treatments, such as antivirals.
3.4. Curcumin
Curcumin [1,7-bis (4-hydroxy-3-methoxyphenyl) −1,6-heptadiene-3,5-dione] is a polyphenol extracted from the rhizome of turmeric (Curcuma longa). It is a yellow pigment of polyphenolic nature that is found in tropical and subtropical regions around the world. Curcumin is a spice widely used in gastronomy, mainly in Asian countries. In addition to its culinary use, curcumin is a traditional herbal medicine in different countries since ancient times. The main properties attributed to curcumin are based on its great antioxidant capacity, but also on its role as an anti-inflammatory agent. It has also been shown that curcumin possesses antimutagenic and antimicrobial properties. It has been also demonstrated to be useful in the fight against several types of cancer. Antiviral properties have also been attributed to curcumin. In this sense, it has been described how curcumin is able to inhibit human immunodeficiency virus (HIV) replication (Prasad and Tyagi, 2015). Antiviral activity of curcumin has also been described against Chikungunya and Zika virus (Mounce et al., 2017). However, despite the large number of studies conducted with this compound, many of the intracellular changes causing the known effects are still unknown (Bielak-Zmijewska et al., 2019; Nelson et al., 2017; Vera-Ramirez et al., 2013). Curcumin use was found to increase ACE2 receptor expression in rat tissue (Pang et al., 2015). In this study, authors reported that curcumin administration to rats to which angiotensin II was applied as an intravenous infusion partially prevented fibrosis at the myocardial muscle. This effect was related to the observed increase in the protein expression of ACE2 in this organ. In a study using molecular docking with target receptors that might be associated with the viral infection, such as SARS-CoV-2 protease, spike glycoprotein-RBD and PD-ACE2 and that were compared as references with the known ligand or drugs, it was been reported that curcumin might be useful because it has the ability to bind to the above-mentioned receptors (Utomo et al., 2020). The wide variety of beneficial health effects shown by curcumin, including its high antioxidant and anti-inflammatory capacities, as well as its antifibrotic effects on the lung (Punithavathi et al., 2000) makes curcumin a molecule with promising effects in treating COVID-19.
3.5. Quercetin
Quercetin is a flavonol, which is one of the six subfamilies of flavonoids. It represents the most abundant flavonoid molecule found in different fruits and vegetables, including apples, berries, onions, dill, lovage, cilantro (coriander) or capers (Anand David et al., 2016). It is a yellow compound soluble in lipids and alcohol. Many pharmacological activities of quercetin have been reported. Among these, the anticancer capacity and the ability to fight against viruses can be highlighted. Moreover, it is useful treating allergic diseases and, from the point of view of cardiovascular disorders, metabolic diseases and different conditions in which inflammation is a key factor (Batiha et al., 2020).
Quercetin has also been associated with autophagy induction. It seems that quercetin could activate protective autophagy in ovarian cancer cell lines and in primary ovarian cancer cells concomitantly by activating the intrinsic apoptosis p-STAT3/Bcl-2 axis in this process (Liu et al., 2017). Likewise, a quercetin (Qu) modified polysorbate 80 (P-80)-coated AuPd core-shell structure (Qu@P-80@AuPd) can activate autophagy of SH-SY5Y cells and promote the fusion of autophagosomes and lysosomes, (Liu et al., 2019).
The antiviral activity of quercetin has been described for example for the Japanese encephalitis virus (JEV), the human T-lymphotropic virus 1, the mosquito-borne disease (Johari et al., 2012), the dengue virus type-2 and hepatitis C virus (Bachmetov et al., 2012). Other quercetin derivatives, such as quercetin-3-O-D-glucuronide, quercetin-enriched lecithin formulations, and quercetin 7-rhamnoside have been found to be useful against the treatment of the porcine epidemic diarrhea virus and influenza-A virus, respectively (Fan et al., 2011; Song et al., 2011). Quercetin has been also investigated in the context of respiratory diseases. In this sense, Henson et al. (2008) in a double-blind parallel randomized controlled trial supplemented 18 adults with quercetin for 21 days before the Western States Endurance Run; but no effect on illness rates were found after the race. However, other authors have found that quercetin reduced illness after intensive exercise (Nieman et al., 2007).
Concerning the potential usefulness of quercetin for COVID-19, a recent study identified quercetin as a molecule of interest, probably with capacity to reduce the interaction between the virus and the receptor ACE-2 using supercomputer-based in silico drug-docking to the COVID-19 viral spike protein (Sargiacomo et al., 2020). Although this is a in silico approach that needs to be tested, it suggests that quercetin may be useful from this point of view. Another issue about the putative effect of quercetin against COVID-19 comes from a quercetin derivative, quercetin-3-b-galactoside. This compound has been identified through molecular docking, SPR and FRET bioassays, and mutagenesis studies as a new class of inhibitors against SARS-CoV 3CLpro (Chen et al., 2006). SARS-CoV 3CLpro shares some features with SARS-CoV2; therefore, quercetin might exert some protective or curative role against COVID-19, particularly considering its antioxidant (Xu et al., 2019) and anti-inflammatory (Li et al., 2016) properties, as well as the effects of quercetin observed against other viruses described above.
3.6. Vitamin C
The functions of the water-soluble vitamin C (ascorbic acid) in biological systems are associated with the capacity of this molecule to change between the two redox states. This ability leads vitamin C to act as a cofactor in several human enzymes and as a powerful antioxidant and co-antioxidant (Padayatty and Levine, 2000). Scurvy, a condition that can be mortal, is the main result of a continued lack of vitamin C intake (Granger and Eck, 2018). Concerning its role as antioxidant, ascorbate is able to scavenge several types of free radicals, although under certain circumstances vitamin C may become prooxidant (Frei et al., 1989). Ascorbic acid also participates in different biological processes being some of them related to the immune system (Carr and Maggini, 2017). Concerning the antiviral properties of vitamin C, it has been described that this molecule may be useful for patients affected by different herpes viruses or influenza virus (Colunga Biancatelli et al., 2020). These antiviral effects might be due to at least two aspects. The first is that usually low levels of vitamin C are found in the blood of patients affected by different acute infectious diseases and, secondly, because ascorbate has the capacity to increase the production of interferons and to downregulate the production of different cytokines, it has demonstrated positive effects from the point of view of immunomodulation in patients affected with different viral infections (Colunga Biancatelli et al., 2020). Concerning the participation of vitamin C in the protection against upper respiratory tract infections (URTIs), a meta-analysis of 29 controlled trials with 11,306 participants has shown no prevention of URTIs after a regular vitamin C intake of around 1 g/day. However, the same trials found that vitamin C shortened and alleviated URTIs that occurred during the period of vitamin C administration (Hemilä and Chalker, 2013). In the present COVID-19 pandemic, several attempts to use vitamin C against SARS-CoV2 have been already initiated, including a phase II clinical trial (NCT04264533) to evaluate high-dose IV vitamin C in ICU patients with severe COVID-19-associated pneumonia (Peng, 2020). Those responsible for this clinical trial are assessing the need for mechanical ventilation and the use of vasopressor drugs. The risk of failure in organs other than the lung, the total length of ICU stays as well as mortality based on periods of 28 days will also be analyzed. Another study (Cheng, 2020) has shown that a single dose of intravenously administered vitamin C is successful in treating 50 patients in China who had moderate to severe symptoms of COVID-19. Specifically, the dose used was 10 or 20 g a day administered over a period of between 8 and 10 h. It is thought that in the case of critically ill patients, a bolus dose of vitamin C might also be required. For this study, the researchers reported positive results regarding the oxygenation index in real time. In addition, it has been reported that all patients achieved cure and discharge from hospital. While waiting for the results of the clinical trial described above and others that have been initiated, one thing seems clear, the ability of vitamin C to defend the body against oxidative stress and its ability to modulate the immune system, including the inflammatory aspects, make it pertinent to consider the possibility that vitamin C could be useful in the management of COVID-19.
3.7. Vitamin D
Vitamin D belongs to the group of fat-soluble vitamins. It can be incorporated into the body through the diet by the dietary intake of dairy products, eggs, fish, etc., in the form of vitamin D2 (ergocalciferol) or transformed into the skin by the effect of the sun to vitamin D3 (cholecalciferol). The active form of vitamin D is 1,25-dihydroxicholecalciferol (calcitriol). Vitamin D is involved in the absorption of calcium, magnesium and phosphate, but it is also related to multiple actions on the body, including immune system regulation (Aranow, 2011). Furthermore, the deficiency in vitamin D has been associated with several disorders, such as diabetes, alterations in the regulation of the immune system, cancer, inflammation, hypertension, cognitive alterations, cardiovascular diseases and osteoporosis, among others (Holick, 2017).
Concerning COVID-19 and vitamin D, in a retrospective study including 780 confirmed patients, authors found that elder men with pre-existing conditions and with levels of vitamin D below normality were associated with increasing risk of suffering from COVID-19 (Raharusun et al., 2020). In a recent cross-sectional analysis performed with data from 20 European countries (Ilie et al., 2020), authors observed a negative correlation between the circulating levels of vitamin D and the number of cases of COVID-19 per million people for each country. A similar negative correlation was also found between vitamin D and deaths caused by COVID-19 per million people. Moreover, in a retrospective investigation in a cohort of 107 patients from Switzerland from which 27 were positive for SARS-CoV-2, authors found significantly lower levels of 25-hydroxyvitamin D in patients with positive PCR for SARS-CoV-2 compared with patients with negative PCR (D'Avolio et al., 2020). These studies, together with other subjective observations in addition to that older people tend to have low vitamin D levels, make that this population group becomes the most affected by COVID-19, leading to many scientists and health professionals to suggest that vitamin D supplementation could be useful in managing the disease. However, is there a logic in recommending this beyond the correlations and associations mentioned?
Vitamin D supports immune function by maintaining cell physical barrier integrity. It is also responsible for this role by increasing the capacity of the cell to produce antimicrobial proteins. Additionally, vitamin D increases the response of cells associated with innate (mainly monocytes and macrophages) and adaptive immunity (dendritic cells and T-cells) leading to a more anti-inflammatory state (Adams et al., 2020). The role that vitamin D deficiency plays in the onset and severity of respiratory infections with viral origin and severe lung damage has been revealed by epidemiological studies (Hansdottir and Monick, 2011). Furthermore, calcitriol has been shown to protect against acute lung damage. This role at the lung level seems to be exerted by calcitriol through the expression of ACE2 and other members of the renin-angiotensin system. (Xu et al., 2017), this represents a clue about the importance of vitamin D deficiency as a pathogenic conditioner for COVID-19. Therefore, based on the existing evidence, it might make sense to start studies in COVID-19 patients undergoing vitamin D supplementation to really assess the usefulness of vitamin D. Furthermore, and in any case, given the prevalence of people, especially the elders, with low levels of vitamin D, precisely the population group that is being hardest hit by COVID-19, it would be desirable to launch campaigns aimed at correcting such deficiencies.
Table 2 shows a summary of the potential targets against which the substances analyzed in this section would exert their action.
Table 2.
Potential targets for the analyzed molecules.
| Compound | Potential target pathways |
|---|---|
| Zinc | Replication inhibition |
| Immunomodulatory effects | |
| Intracellular redox activity | |
| Antibodies production | |
| NK cells activity | |
| Cytokines production by mononuclear cells | |
| Chemotaxis response reduction | |
| Neutrophil respiratory burst reduction | |
| Resveratrol | Sirtuin deacetylase SIRT1 agonist |
| AMPK activation | |
| PGC1alpha levels maintenance | |
| Mitochondrial biogenesis activation | |
| Mitophagy promotion | |
| ACE2 protein level increase | |
| DNA damage reduced | |
| Destabilization of p53 promoted | |
| Bax expression decreased | |
| Hydroxytyrosol | Neutrophil respiratory burst effective-caused oxidative stress reduction |
| Pulmonary fibrosis decrease | |
| Curcumin | ACE2 level increase |
| Antiviral activities | |
| SARS-CoV-2 protease, spike glycoprotein-RBD and PD-ACE2 binding | |
| Virus-ACE2 interaction reduction | |
| Antioxidant capacity | |
| Anti-inflammatory | |
| Quercetin | Antiviral activity |
| Autophagy promotion | |
| Virus-ACE2 interaction reduction | |
| Antioxidant capacity | |
| Anti-inflammatory | |
| Vitamin C | Antioxidant capacity |
| Biological processes related to the immune system | |
| Interferon production | |
| Cytokines production downregulated | |
| Vitamin D | Cell physical barrier integrity maintenance |
| Increased antimicrobial protein production | |
| Anti-inflammatory state | |
| ACE2 and other members of the RAS expression |
3.8. Combined therapy
The combined use of effective therapeutic agents having different mechanisms of action could provide a synergistic response and greater therapeutic potency. In this sense, in the context of the present review, various proposals have been made. For example, based on a gene enrichment analysis, triple therapy with quercetin, vitamin D and estradiol has been suggested (Glinsky, 2020). This study investigated genes that SARS-CoV-2 need in its way to enter the cell, namely ACE2 and FURIN. The authors used these genes to build molecular maps. Once panels of repressors and activators were found, they were used to analyze pharmacological compounds, synthetic or natural, already recognized, known to exert their action on any of the genes that appeared in the genetic maps constructed so that they could serve to treat COVID-19. In addition, following a systems biology analysis approach, the combined use of vitamin C, curcumin and glycyrrhizic acid (an active compound derived from licorice root, which is considered an ingredient in traditional Chinese medicine) has been suggested. In this study (Chen et al., 2020), the authors suggested the use of this combination based on the capacity of this mixture to regulate the immune system to fight against the infections associated with COVID-19. The aim was also to reduce, at least in part, the exacerbated inflammation and consequently to prevent the onset of the cytokine storm. Roy et al. (2020) suggested the combined use of curcumin and zinc. This proposal was based on the well-known antiviral capacity of curcumin (for example, by the inhibition of the entry of the virus into the cell) and in the ability of zinc to inhibit the RNA polymerase. These authors speculate that zinc plus curcumin might lead to the formation of ionophore complexes resulting in a stronger and synchronized antiviral action. It has also been suggested a combinatory therapy of nutrients/food-derived bioactive molecules with antivirals. In this sense, the study of a synergistic use of vitamin D and remdesivir has been suggested (Arya and Dwivedi, 2020). Zinc has also been proposed to be combined with chloroquine since the latter increases zinc uptake into the lysosomes. Moreover, this is also based on the observed induction of apoptosis in malignant cells when zinc and chloroquine are administered together (Xue et al., 2014). However, as stated, except the study about chloroquine and zinc (Xue et al., 2014), the rest of all above-mentioned proposals are hypothetical suggestions based on computer studies or separate evidence, although, to our knowledge, no experimental studies have yet been conducted to test these or other combined therapy proposals. Necessarily, any of these proposals must go through the promotion of studies at the preclinical level followed by the subsequent randomized controlled clinical trials before being able to assert the actual usefulness of these therapies.
4. Conclusions
Waiting for the generation of vaccines and for proven, safe, and effective treatments, any therapy showing to be safe and capable of mitigating the effects of the disease on the body should be welcomed. Foods are the basis for the maintenance of living beings, and alterations of eating patterns can lead to the appearance of diseases. Moreover, the possibility of using nutrients or bioactive compounds from the diet for the prevention of diseases, and even at a therapeutic level, is well known. The use of the molecules analyzed in the present review, all tested for safety and non-toxicity, could be a therapeutic tool to be assayed against COVID-19, either alone or in combination with other nutritional substances, antivirals or other drugs. Science is awaiting this possibility, and although the available trials are currently very scarce, they must be performed with the hope that these molecules will serve to treat patients and to reduce the severity of COVID-19 and its high mortality.
Credit author statement
José L. Quiles: Conceptualization, Writing-original draft. Lorenzo Rivas-García: Writing -review & editing. Alfonso Varela-López: Writing -review & editing, Visualization, preparation of figures. Juan Llopis: Conceptualization, Writing -review & editing. Maurizio Battino: Conceptualization, Writing -review & editing. Cristina Sánchez-González: Conceptualization, Writing-original draft.
Funding sources
This research received no external funding. The authors acknowledge Nutraceutical Translations for English language editing of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams K.K., Baker W.L., Sobieraj D.M. 2020. Myth Busters: Dietary Supplements and COVID-19. Ann Pharmacother 1060028020928052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharm. Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranow C. Vitamin D and the immune system. J. Invest. Med. 2011;59:881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya A., Dwivedi V.D. Synergistic effect of vitamin D and remdesivir can fight COVID-19. J. Biomol. Struct. Dyn. 2020;1–2 doi: 10.1080/07391102.2020.1773929. [DOI] [PubMed] [Google Scholar]
- Bachmetov L., Gal-Tanamy M., Shapira A., Vorobeychik M., Giterman-Galam T., Sathiyamoorthy P., Golan-Goldhirsh A., Benhar I., Tur-Kaspa R., Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012;19:e81–88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- Batiha G.E.-S., Beshbishy A.M., Ikram M., Mulla Z.S., El-Hack M.E.A., Taha A.E., Algammal A.M., Elewa Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods 9. 2020 doi: 10.3390/foods9030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya L.M., Beltrán M., Obregón-Calderón P., García-Pérez J., de la Torre H.E., González N., Pérez-Olmeda M., Auñón D., Capa L., Gómez-Acebo E., Alcamí J. Hydroxytyrosol: a new class of microbicide displaying broad anti-HIV-1 activity. AIDS. 2016;30:2767–2776. doi: 10.1097/QAD.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A., Grabowska W., Ciolko A., Bojko A., Mosieniak G., Bijoch L., Sikora E. The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 2019;20:1239. doi: 10.3390/ijms20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisignano G., Tomaino A., Lo Cascio R., Crisafi G., Uccella N., Saija A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999;51:971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- Braga P.C., Mancini L., Guffanti E.E., Dal Sasso M., Sala M., Reggio S. Effects of nedocromil sodium on the oxidative burst of polymorphonuclear leukocytes: comparison with salbutamol. Drugs Exp. Clin. Res. 1997;23:33–38. [PubMed] [Google Scholar]
- Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Hu C., Hood M., Zhang X., Zhang L., Kan J., Du J. A novel combination of vitamin C, curcumin and glycyrrhizic acid potentially regulates immune and inflammatory response associated with coronavirus infections: a perspective from system biology analysis. Nutrients. 2020;12 doi: 10.3390/nu12041193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li J., Luo C., Liu H., Xu W., Chen G., Liew O.W., Zhu W., Puah C.M., Shen X., Jiang H. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): structure-activity relationship studies reveal salient pharmacophore features. Bioorg. Med. Chem. 2006;14:8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev. Anti Infect. Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pessoa J.M. da, Figueiredo C.F.D.S.R., Thieme K., Oliveira-Souza M. The regulation of NHE₁ and NHE₃ activity by angiotensin II is mediated by the activation of the angiotensin II type I receptor/phospholipase C/calcium/calmodulin pathway in distal nephron cells. Eur. J. Pharmacol. 2013;721:322–331. doi: 10.1016/j.ejphar.2013.08.043. [DOI] [PubMed] [Google Scholar]
- COVID-19 map WWW document], n.d. . Johns hopkins coronavirus resource center. URL. https://coronavirus.jhu.edu/map.html accessed 7.7.20.
- Cure E., Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr. 2020;14:349–350. doi: 10.1016/j.dsx.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure E., Cumhur Cure M. Comment on “Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure E., Cumhur Cure M. Comment on “should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144:251–252. doi: 10.1159/000507786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., Keller F., Cantù M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12 doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D., Zhou X., Zhao C., Chen H., Zhao Y., Gong X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia. 2011;82:805–810. doi: 10.1016/j.fitote.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri F., Afrin S., Forbes-Hernandez T.Y., Gasparrini M., Cianciosi D., Reboredo-Rodriguez P., Varela-Lopez A., Quiles J.L., Battino M. Autophagy in human health and disease: novel therapeutic opportunities. Antioxidants Redox Signal. 2019;30:577–634. doi: 10.1089/ars.2017.7234. [DOI] [PubMed] [Google Scholar]
- Glinsky G.V. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Principal S., Quiles J.L., Ramirez-Tortosa C.L., Sanchez-Rovira P., Ramirez-Tortosa M.C. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr. Rev. 2010;68:191–206. doi: 10.1111/j.1753-4887.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- Granger M., Eck P. Dietary vitamin C in human health. Adv. Food Nutr. Res. 2018;83:281–310. doi: 10.1016/bs.afnr.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Haas R.H. 2019. Mitochondrial Dysfunction in Aging and Diseases of Aging. Biology (Basel) p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S., Monick M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 2011;86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M.R., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev CD000980. 2013 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson D., Nieman D., Davis J.M., Dumke C., Gross S., Murphy A., Carmichael M., Jenkins D.P., Quindry J., McAnulty S., McAnulty L., Utter A., Mayer E. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int. J. Sports Med. 2008;29:856–863. doi: 10.1055/s-2007-989424. [DOI] [PubMed] [Google Scholar]
- Hoang B.X., Hoang H.Q., Han B. Zinc Iodide in combination with Dimethyl Sulfoxide for treatment of SARS-CoV-2 and other viral infections. Med. Hypotheses. 2020;143:109866. doi: 10.1016/j.mehy.2020.109866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- Horne J.R., Vohl M.-C. Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am. J. Physiol. Endocrinol. Metab. 2020;318:E830–E833. doi: 10.1152/ajpendo.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibs K.-H., Rink L. Zinc-altered immune function. J. Nutr. 2003;133 doi: 10.1093/jn/133.5.1452S. 1452S–6S. [DOI] [PubMed] [Google Scholar]
- Ilie P.C., Stefanescu S., Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020 doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari J., Kianmehr A., Mustafa M.R., Abubakar S., Zandi K. Antiviral activity of baicalein and quercetin against the Japanese encephalitis virus. Int. J. Mol. Sci. 2012;13:16785–16795. doi: 10.3390/ijms131216785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen M.J., Linder M., Pontoniere P., Crea R. NF-κβ signaling and chronic inflammatory diseases: exploring the potential of natural products to drive new therapeutic opportunities. Drug Discov. Today. 2014;19:373–378. doi: 10.1016/j.drudis.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Komukai K., Mochizuki S., Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010;24:687–698. doi: 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelis D. de F., Freitas D.F. de, Machado A.S., Crespo T.S., Santos S.H.S. Angiotensin-(1-7), adipokines and inflammation. Metab. Clin. Exp. 2019;95:36–45. doi: 10.1016/j.metabol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gong W., Yang Z.Y., Zhou X.S., Gong C., Zhang T.R., Wei X., Ma D., Ye F., Gao Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 2017;22:544–557. doi: 10.1007/s10495-016-1334-2. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhou H., Yin T., Gong Y., Yuan G., Chen L., Liu J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer's disease. J. Colloid Interface Sci. 2019;552:388–400. doi: 10.1016/j.jcis.2019.05.066. [DOI] [PubMed] [Google Scholar]
- Liu Z.-H., Fan W., Chen R.-C. 3,4-dihydroxyphenylethanol suppresses irradiation-induced pulmonary fibrosis in adult rats. Int. J. Clin. Exp. Pathol. 2015;8:3441–3450. [PMC free article] [PubMed] [Google Scholar]
- Malavolta M., Giacconi R., Brunetti D., Provinciali M., Maggi F. Exploring the relevance of senotherapeutics for the current SARS-CoV-2 emergency and similar future global health threats. Cells 9. 2020 doi: 10.3390/cells9040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaličková D., Hrnčíř T., Canová N.K., Slanař O. Targeting Keap 1/Nrf 2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 2020;873:172973. doi: 10.1016/j.ejphar.2020.172973. [DOI] [PubMed] [Google Scholar]
- Moran C.S., Biros E., Krishna S.M., Wang Y., Tikellis C., Morton S.K., Moxon J.V., Cooper M.E., Norman P.E., Burrell L.M., Thomas M.C., Golledge J. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017;37:2195–2203. doi: 10.1161/ATVBAHA.117.310129. [DOI] [PubMed] [Google Scholar]
- Mounce B.C., Cesaro T., Carrau L., Vallet T., Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017;142:148–157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Navarro S., Reddy R., Lee J., Warburton D., Driscoll B. Inhaled resveratrol treatments slow ageing-related degenerative changes in mouse lung. Thorax. 2017;72:451–459. doi: 10.1136/thoraxjnl-2016-208964. [DOI] [PubMed] [Google Scholar]
- Nehme A., Zouein F.A., Zayeri Z.D., Zibara K. An update on the tissue renin angiotensin system and its role in physiology and pathology. J Cardiovasc Dev Dis. 2019;6 doi: 10.3390/jcdd6020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. The essential medicinal chemistry of curcumin: miniperspective. J. Med. Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Gross S.J., Jenkins D.P., Davis J.M., Murphy E.A., Carmichael M.D., Dumke C.L., Utter A.C., McAnulty S.R., McAnulty L.S., Mayer E.P. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007;39:1561–1569. doi: 10.1249/mss.0b013e318076b566. [DOI] [PubMed] [Google Scholar]
- O'Dowd Y., Driss F., Dang P.M.-C., Elbim C., Gougerot-Pocidalo M.-A., Pasquier C., El-Benna J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004;68 doi: 10.1016/j.bcp.2004.06.023. 2003–2008. [DOI] [PubMed] [Google Scholar]
- Oliveira Andrade J.M., Paraíso A.F., Garcia Z.M., Ferreira A.V.M., Sinisterra R.D.M., Sousa F.B., Guimarães A.L.S., de Paula A.M.B., Campagnole-Santos M.J., dos Santos R.A., Santos S.H.S. Cross talk between angiotensin-(1-7)/Mas axis and sirtuins in adipose tissue and metabolism of high-fat feed mice. Peptides. 2014;55:158–165. doi: 10.1016/j.peptides.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Padayatty S.J., Levine M. Vitamin C and myocardial infarction: the heart of the matter. Am. J. Clin. Nutr. 2000;71:1027–1028. doi: 10.1093/ajcn/71.5.1027. [DOI] [PubMed] [Google Scholar]
- Pang X.-F., Zhang L.-H., Bai F., Wang N.-P., Garner R.E., McKallip R.J., Zhao Z.-Q. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Dev. Ther. 2015;9:6043–6054. doi: 10.2147/DDDT.S95333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. clinicaltrials.gov; 2020. Vitamin C Infusion for the Treatment of Severe 2019-nCoV Infected Pneumonia: a Prospective Randomized Clinical Trial (Clinical Trial Registration No. NCT04264533) [Google Scholar]
- Pineda-Ramírez N., Alquisiras-Burgos I., Ortiz-Plata A., Ruiz-Tachiquín M.-E., Espinoza-Rojo M., Aguilera P. Resveratrol activates neuronal autophagy through AMPK in the ischemic brain. Mol. Neurobiol. 2020;57:1055–1069. doi: 10.1007/s12035-019-01803-6. [DOI] [PubMed] [Google Scholar]
- Prasad S., Tyagi A.K. Curcumin and its analogues: a potential natural compound against HIV infection and AIDS. Food Funct. 2015;6:3412–3419. doi: 10.1039/c5fo00485c. [DOI] [PubMed] [Google Scholar]
- Punithavathi D., Venkatesan N., Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiles J.L., Sánchez-González C., Vera-Ramírez L., Giampieri F., Navarro-Hortal M.D., Xiao J., Llopis J., Battino M., Varela-López A. Reductive stress, bioactive compounds, redox-active metals, and dormant tumor cell biology to develop redox-based tools for the treatment of cancer. Antioxidants Redox Signal. 2020 doi: 10.1089/ars.2020.8051. [DOI] [PubMed] [Google Scholar]
- Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9 doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raharusun P., Priambada S., Budiarti C., Agung E., Budi C. SSRN; 2020. Patterns of COVID-19 Mortality and Vitamin D: an Indonesian Study. [DOI] [Google Scholar]
- Rambold A.S., Pearce E.L. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 2018;39:6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Resnick L.M., Gupta R.K., Sosa R.E., Corbett M.L., Laragh J.H. Intracellular pH in human and experimental hypertension. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7663–7667. doi: 10.1073/pnas.84.21.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Oliveira A., Nogueira A.I., Pereira R.M., Boas W.W.V., Dos Santos R.A.S., Simões e Silva A.C. The renin-angiotensin system and diabetes: an update. Vasc. Health Risk Manag. 2008;4:787–803. [PMC free article] [PubMed] [Google Scholar]
- Robles-Almazan M., Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Rodriguez-Garcia C., Quiles J.L., Ramirez-Tortosa Mc. Hydroxytyrosol: bioavailability, toxicity, and clinical applications. Food Res. Int. 2018;105:654–667. doi: 10.1016/j.foodres.2017.11.053. [DOI] [PubMed] [Google Scholar]
- Roy A., Sarkar B., Celik C., Ghosh A., Basu U., Jana M., Jana A., Gencay A., Sezgin G.C., Ildiz N., Dam P., Mandal A.K., Ocsoy I. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother Res. 2020. [DOI] [PMC free article] [PubMed]
- Santini A., Cammarata S.M., Capone G., Ianaro A., Tenore G.C., Pani L., Novellino E. Nutraceuticals: opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018;84:659–672. doi: 10.1111/bcp.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12:6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Simmons G., Zmora P., Gierer S., Heurich A., Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Shim J.K., Choi H.J. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol. J. 2011;8:460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., Hca Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiao M.-M., Lin Y.-J., Yu H.-R., Sheen J.-M., Lin I.-C., Lai Y.-J., Tain Y.-L., Huang L.-T., Tsai C.-C. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018;17:178. doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo R.Y., Ikawati M., Meiyanto E. 2020. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. [DOI] [Google Scholar]
- Vera-Ramirez L., Pérez-Lopez P., Varela-Lopez A., Ramirez-Tortosa M., Battino M., Quiles J.L. Curcumin and liver disease. Biofactors. 2013;39:88–100. doi: 10.1002/biof.1057. [DOI] [PubMed] [Google Scholar]
- Visioli F., Bellomo G., Galli C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998;247:60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, A target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 9. 2020 doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hu M.-J., Wang Y.-Q., Cui Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24 doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.-Q. Chloroquine is a zinc ionophore. PloS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Ogawa H., Hara A., Yoshida Y., Yonezawa Y., Karibe K., Nghia V.B., Yoshimura H., Yamamoto Y., Yamada M., Nakamura K., Imai K. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antivir. Res. 2009;83:35–44. doi: 10.1016/j.antiviral.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Yan C., Li T.-S. Dual role of mitophagy in cancer drug resistance. Anticancer Res. 2018;38:617–621. doi: 10.21873/anticanres.12266. [DOI] [PubMed] [Google Scholar]
- Yang X.-H., Deng W., Tong Z., Liu Y.-X., Zhang L.-F., Zhu H., Gao H., Huang L., Liu Y.-L., Ma C.-M., Xu Y.-F., Ding M.-X., Deng H.-K., Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007;57:450–459. [PubMed] [Google Scholar]
- Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12 doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoric N., Horvat I., Kopjar N., Vucemilovic A., Kremer D., Tomic S., Kosalec I. Hydroxytyrosol expresses antifungal activity in vitro. Curr. Drug Targets. 2013;14:992–998. doi: 10.2174/13894501113149990167. [DOI] [PubMed] [Google Scholar]
- Zwane R.E., Parker A., Kudanga T., Davids L.M., Burton S.G. Novel, biocatalytically produced hydroxytyrosol dimer protects against ultraviolet-induced cell death in human immortalized keratinocytes. J. Agric. Food Chem. 2012;60:11509–11517. doi: 10.1021/jf300883h. [DOI] [PubMed] [Google Scholar]