Abstract
Background and Purpose:
To identify a PSA threshold value at an intermediate follow-up time after low dose rate (LDR) prostate brachytherapy associated with cure, defined as long-term (10 to 15 year) freedom from prostate cancer.
Materials and Methods:
Data from 7 institutions for 14,220 patients with localized prostate cancer treated with LDR brachytherapy, either alone (8,552) or with external beam radiotherapy (n=1,175), androgen deprivation (n=3,165), or both (n=1,328), were analyzed. Risk distribution was 42.4% favorable, 49.2% intermediate, and 8.4% high-risk. Patients with clinical failure before 3.5 years were excluded. Kaplan-Meier analysis was used with clinical failure (local, distant, regional or biochemical triggering salvage) as an endpoint for each of four PSA categories: PSA ≤0.2, >0.2 to ≤0.5, >0.5 to ≤1.0, and >1.0 ng/mL. PSA levels at 4 years (±6 months) in 8,746 patients without clinical failure were correlated with disease status at 10–15 years.
Results:
For the 77.1% of patients with 4-year PSA ≤0.2, the freedom-from-recurrence (FFR) rates were 98.7% (95% CI 98.3–99.0) at 10 years and 96.1% (95% CI 94.8–97.2) at 15 years. Three independent validation cohorts confirmed 97%–99% 10-year FFR rates with 4-year PSA ≤0.2. Successive PSA categories were associated with diminished disease-free rates at 10 and 15 years. PSA category was strongly associated with treatment success (p<0.0005).
Conclusions:
Since 98.7% of patients with PSA ≤0.2 ng/mL at 4 years after LDR prostate brachytherapy were disease-free beyond 10 years, we suggest adopting this biochemical definition of cure for patients with ≥4 years’ follow-up after LDR brachytherapy.
Keywords: adenocarcinoma of prostate, brachytherapy, low dose rate brachytherapy, prostate specific antigen, PSA definition of cure
INTRODUCTION
Since post-treatment PSA values were first introduced into clinical practice in the 1980s, clinicians have grappled with interpretation of these values. Two consensus conferences, ASTRO in 1996 (1) and Phoenix in 2005 (2), defined biochemical failure after external beam radiotherapy (EBRT), but neither included data from patients treated with androgen deprivation therapy (ADT) or brachytherapy (3). Neither consensus conference attempted to define cure and neither definition is readily comparable to a surgical definition of failure.
Having observed over the past two decades that the incorporation of brachytherapy into the radiation management of prostate cancer is associated with extremely low and stable PSA values, we extracted, combined, and analyzed mature, prospectively collected brachytherapy data sets from 7 institutions to identify a PSA threshold value associated with cure, as defined by long-term (10–15 year) freedom from prostate cancer.
MATERIALS AND METHODS
A total of 14,220 patients with localized prostate cancer from 7 participating international institutions (Table 1) were treated with low-dose-rate (LDR) brachytherapy, either alone (n=8,552) or combined with ADT (n=3,165), EBRT (n=1,175), or both (n=1,328). Clinical failures and dates of failure were defined by the institutions and were considered to be local, nodal, distant or biochemical failure triggering salvage ADT. All analyses were approved by the Institutional Review Board at University of Texas MD Anderson Cancer Center in compliance with each participating institution’s policies, and consents were waived given the retrospective nature of the data.
Table 1 —
Contribution of each institution and reasons for exclusion from 3.5–4 .5 year interval follow-up.
| Institution | Total No. of Patients | Excluded 1st for lack of PSA follow-up | Excluded 2nd for no PSA value at 3.5–4.5 years | Excluded 3rd for failure <3.5 years | Total remaining |
|---|---|---|---|---|---|
| BC Cancer | 3,656 | 771 | 328 | 43 | 2,514 |
| Cleveland | 4,094 | 1,968 | 249 | 2 | 1,875 |
| Galway | 380 | 262 | 16 | 1 | 101 |
| MD Anderson | 717 | 294 | 80 | 3 | 340 |
| Mt Sinai NY | 1,842 | 100 | 437 | 16 | 1,289 |
| Toronto | 604 | 50 | 54 | 2 | 498 |
| Wheeling | 2,927 | 484 | 309 | 5 | 2,129 |
| Total | 14,220 | 3,929 | 1,473 | 72 | 8,746 |
After either 3.5 or 4.5 years of failure-free follow-up, a particular PSA value was used to predict long-term success or failure as follows. For patients with at least 3.5 years of follow-up, the “4-year” PSA measurement was selected that was closest to, but still less than 4.5 years and grouped into one of four categories: PSA ≤0.2 ng/mL, >0.2 to ≤0.5 ng/mL, >0.5 to ≤1.0 ng/mL, and >1.0 ng/mL. The most favorable of these categories (≤0.2 ng/mL) is comparable to a surgical PSA threshold of ≤0.2 ng/mL, and the least favorable (PSA >1.0 ng/ml) still encompasses 5% of the readings. Similarly, for patients with at least 4.5 years’ follow-up, the PSA measurement that was closest to, but still less than 5.5 years was selected as the “5-year PSA.”
Patients with insufficient PSA follow-up data, within 3.5 years after treatment, were excluded (n=3,929), as were patients without a PSA value in the interval of interest (n=1,473) and those with clinical failure before 3.5 years (n=72), leaving 8,746 patients for analysis based on a 4-year PSA level. By a similar exclusion process, 7,615 patients were analysed based on a 5-year PSA. The analyses were performed using the last-measured (most recent) PSA during the interval of interest and then repeated using the first-measured PSA value in the interval to determine if there was obvious time dependency.
Statistics
Kaplan-Meier (KM) analysis was carried out for each interval (to 10 or 15 years) using clinical failure (local, distant, regional or biochemical triggering ADT) as the endpoint for each of the four PSA categories. The starting date for the KM analysis was the date of the brachytherapy implant, and the final date was that of the clinical failure or the last PSA measurement. Equality of failure rates was tested using the log-rank test (which assumes proportional hazards across the PSA categories) and checked by using the Wilcoxon-Breslow-Gehan test (which does not assume proportional hazards). The KM results are presented as percentage of patients in each PSA category who were free of disease at 10 and 15 years after treatment.
Results were validated by analyses of three independent, prospectively collected data sets. The first was prospectively collected LDR brachytherapy data from Melbourne Australia (n=377) for patients with favorable- (75%) or intermediate-risk (25%) prostate cancer with >10 years of follow-up (6). This cohort was treated largely with monotherapy, although short-term ADT for prostate downsizing was used in 12.6% of patients and 2% had supplemental EBRT. The second validation cohort was from a 300-patient prospective phase II study of LDR brachytherapy alone for intermediate-risk prostate cancer conducted at the University of Texas MD Anderson Cancer Center (4) with a median follow-up time of 6 years. The final validation cohort was the brachytherapy arm of the Canadian 398-patient phase III randomized trial (Ascende-RT) comparing 12 months of ADT combined with either dose-escalated EBRT or EBRT plus an LDR brachytherapy prostate boost (5) for patients with high-risk (70%) or upper-tier intermediate-risk (30%) prostate cancer with >10 years follow up.
RESULTS
The clinical characteristics of the patients and tumors are shown in Table 2. In the index cohort the distribution by risk category was 42.4% favorable, 49.2% intermediate, and 8.4% high-risk. Median follow-up time was 8.0 years (range 3.5–21.3), with more than 2800 patients followed beyond 10 years and more than 400 beyond 15 years. The median number of PSA readings per patient was 13. Clinical failures occurred in 660 patients, 245 before 3.5 years (patients excluded from analysis) and 351 afterward.
Table 2 —
Patient and tumor characteristics.
| Characteristic | Cohort Patients (%) | Validation Grp 1 (Australia) | Validation Grp 2 (MD Anderson) | Validation Grp 3 (Ascende-RT) |
|---|---|---|---|---|
| No. of Patients | 8,746 | 366 | 221 | 160 |
| Baseline PSA Level, ng/mL, median | 6.2 | 5.5 | 5.1 | 10.0 |
| <4 | 1184 (13.5) | 76 (20.8) | 56 (25.4) | 7 (4.4) |
| ≥4–<10 | 6154 (70.4) | 279 (76.2) | 157 (71.0) | 68 (42.5) |
| ≥10–<20 | 1202 (13.7) | 11 (3.0) | 8 (3.6) | 60 (37.5) |
| ≥20–<30 | 119 (1.4) | 0 (0.0) | 0 (0.0) | 17 (10.6) |
| ≥30 | 87 (1.0) | 0 (0.0) | 0 (0.0) | 8 (5.0) |
| Not reported | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gleason Score | ||||
| ≤6 | 4739 (54.2) | 314 (85.8) | 10 (4.5) | 10 (6.3) |
| 7 | 3379 (38.6) | 52 (14.2) | 211 (95.5) | 81 (50.6) |
| 8 | 362 (4.1) | 0 (0.0) | 0 (0.0) | 37 (23.1) |
| 9 or 10 | 232 (2.7) | 0 (0.0) | 0 (0.0) | 32 (20.0) |
| Not reported | 34 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| T Category | ||||
| T1a-T2a | 7376 (84.3) | 325 (88.8) | 217 (98.2) | 67 (41.9) |
| T2b-T2c | 1093 (12.5) | 41 (11.2) | 4 (1.8) | 49 (30.6) |
| T3a-T3b | 61 (0.7) | 0 (0.0) | 0 (0.0) | 44 (27.5) |
| Not reported | 216 (2.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age, years, median | 66.0 | 61.0 | 64.9 | 67 |
| <50 | 156 (1.8) | 5 (1.4) | 9 (4.1) | 0 (0.0) |
| ≥50–<60 | 1803 (20.6) | 136 (37.1) | 48 (21.7) | 21 (13.1) |
| ≥60–<70 | 4162 (47.6) | 176 (48.1) | 114 (51.6) | 64 (40.0) |
| ≥70 | 2624 (30.0) | 49 (13.4) | 50 (22.6) | 75 (46.9) |
| Not reported | 1 (0.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| No. of PSA measurements | ||||
| <10 | 2443 (27.9) | 0 (0.0) | 42 (19.0) | 4 (2.5) |
| ≥10–<20 | 4777 (54.6) | 254 (69.4) | 172 (77.8) | 105 (65.6) |
| ≥20 | 1526 (17.5) | 112 (30.6) | 7 (3.2) | 51 (31.9) |
| PSA follow-up, years, median, maximum | 7.8 (19.7) | 12.1 (18.9) | 6.0 (12.2) | 9.7 (15.5) |
Nearly 80% of patients were in PSA category 1 (Table 3) with a PSA ≤0.2 ng/mL at 3.5–4.5 years. For those patients, the probability of being free of clinical failure at 10 years was 98.7% and at 15 years, 96.1%. Analysis was repeated in a similar fashion using the 5-year PSA level, at which time 85.4% of patients had a PSA of ≤0.2 ng/mL associated with a 99.1% chance of being disease-free at 10 years and 96.6% at 15 years. The results of KM analysis with the log-rank test (with 95% confidence intervals) for the four PSA categories are shown in Table 3. The association of treatment success with PSA range was highly significant (p<0.0005); results were also significant with the Wilcoxon-Breslow-Gehan test (p<0.01).
Table 3 —
Kaplan-Meier estimated free-of-clinical failure rates by PSA category at 3.5–4.5 years for combined cohort and for validation cohorts.
| PSA category | % in category (no.) | Combined Cohort PSA @ 4 years | Validation Cohort 1 (Australia) 75% low-risk/25% intermediate-risk | Validation Cohort 2 (MD Anderson) intermediate-risk | Validation Cohort 3 (Ascende-RT) 70% high-risk/30% upper-tier intermediate-risk | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % NED 10 years | % NED 15 years | % pts in category (no.) | % NED 10 years | % NED 15 years | % in category (no.) | % NED 10 years | % NED 15 years | % pts in category (no.) | % NED 10 years | % NED 15 years | ||
| ≤0.2 | 77.1% (6746) | 98.7 (98. 3–99.0) | 96.1 (94. 8–97.2) | 54.6% (200) | 99.0 (96. 1–99.8) | 99.0 (96.1–99.8) | 73.5% (164) | 99.4 (95. 8–99.9) | ------ | 85.7% (137) | 96.7 (89. 9–99.0) | 96.7 (89.9–99.0) |
| >0.2 to ≤0.5 | 12.6% (1100) | 93.5 (91. 0–95.3) | 86.8 (81. 4–90.7) | 25.7% (94) | 98.9 (92. 7–99.9) | 96.2 (88. 4–98.8) | 14.8% (33) | 92.7 (53. 9–98.8) | ------ | 8.1% (13) | 88.9 (43.3–98.4) | ------ |
| >0.5 to ≤1.0 | 4.1% (444) | 85.9 (80. 6–89.8) | 78.2 (68. 6–85.2) | 9.6 (35) | 94.3 (79. 0–98.5) | 91.0 (74. 6–97.0) | 6.3% (14) | 90.9 (50. 8–98.7) | ------ | 0.6% (1) | ------ | ------ |
| > 1.0 | 5.1% (456) | 48.0 (41. 8–53.8) | 33.2 (24. 9–41.6) | 10.1 (37) | 73.0 (55. 6–84.4) | 54.6 (32. 9–71.9) | 5.4% (12) | ------ | ------ | 5.6% (9) | 33.3 (7.8–62.3) | ------ |
%: percentage of patients with readings in that category
NED 10 years / 15 years: % of patients with no evidence of recurrence at 10 years or 15 years (followed by 95% confidence intervals)
For patients in PSA category 2 (4-year PSA >0.2 ng/mL but ≤0.5 ng/mL), the probability of being disease-free at 10 and 15 years decreased but remained high at 93.5% at 10 years and 86.8% at 15 years. PSA categories 3 and 4 did less well, but many patients still had not experienced failure by 15 years. Based on 4,864 PSA readings between 9.5 and 10.5 years; the median 10-year PSA level was 0.01 (interquartile range [IQR] 0–0.05 ng/mL). At 15 years, based on 1,137 readings between 14.5 and 15.5 years, the median PSA level was 0.0 (IQR 0.0–0.02 ng/mL).
Because no statistically significant difference was found in disease-free survival (data not shown) when the analyses were repeated using either the first PSA in the 3.5–4.5-year interval for all patients, or the last PSA in the interval, the results presented are for the first PSA in the interval.
Table 4 shows the distribution of PSA categories within each risk group. A significantly higher portion of high-risk patients had a 4-year PSA level in category 1 (≤0.2 ng/mL) compared with favorable-risk (82% vs. 75%, p<0.00001: 2-sided binomial test), but also a significantly higher proportion in the >1.0 ng/ml category (8% vs. 4%, p<0.00001). However, these risk groups were originally defined for patients receiving EBRT and may differ for those treated with brachytherapy.
Table 4 —
Distribution of 4-year PSA levels in primary cohort according to risk grouping.
| PSA Level, ng/mL | Favorable Risk | Intermediate Risk | High Risk | P Value |
|---|---|---|---|---|
| ≤0.2 | 2738 (75%) | 3383 (77%) | 578 (82%) | <0.00001 |
| >0.2 – ≤0.5 | 573 (16%) | 516 (12%) | 39 (6%) | <0.00001 |
| >0.5 – ≤1.0 | 194 (5%) | 232 (5%) | 29 (4%) | 0.000126 |
| >1.0 | 156 (4%) | 248 (6%) | 60 (9%) | <0.00001 |
Validation cohorts
Characteristics of the validation cohorts are shown in Table 2. For the three validation sets, largely favorable-risk, intermediate-risk, and higher-risk, a PSA level of ≤0.2 ng/mL at 4 years was associated with 10-year freedom from failure rates of 99.0%, 99.4%, and 96.7%, respectively (Table 3).
In the multi-institutional international cohort, 77.1% patients had a 4 year PSA ≤0.2 ng/mL. In the high-risk Ascende-RT validation cohort, this was achieved in 85.7%, and in the intermediate-risk MD Anderson cohort, 73.5%. In the largely favorable-risk Australian cohort, only 54.6% had a 4-year PSA level of ≤0.2 ng/mL; however, by 5 years this had increased to 72%.
DISCUSSION
In more than 3 decades of clinical use, no serum marker has caused more controversy than PSA, a serum protease produced in the prostate. PSA elevation is associated with many conditions, diminishing its utility in prostate cancer screening. However, as a marker of response to treatment, PSA is much more reliable. The absence of circulating PSA is an indicator of success after radical prostatectomy and a PSA level of >0.2 ng/mL is an accepted definition for biochemical failure (7) (8). After radiation therapy for prostate cancer, the PSA level cannot be expected to be zero, but the actual target level associated with cure has been much debated.
The 1996 ASTRO Consensus Panel (1) considered clinical outcomes for men treated with EBRT without ADT from several institutions in an effort to standardize an approach to PSA monitoring and reporting after radiation treatment. Although PSA nadir was recognized as a strong prognostic factor, no threshold nadir to determine successful treatment was identified. Three consecutive rises, at a minimum of 3- to 4-month intervals, was chosen as an appropriate definition of biochemical failure for reporting of results; but importantly this was not to be mistaken as a surrogate for clinical progression or survival, or to be a justification for salvage intervention. Further, the definition was not derived from patients whose treatment included either ADT or brachytherapy, both of which subsequently came into more frequent clinical use.
A second consensus conference took place in Phoenix, Arizona (2) in 2005. It was recognized that the definition of biochemical cure is very different from that for biochemical failure. Because biochemical failure correlates with clinical endpoints of local failure, distant metastasis, and cause-specific mortality, it often triggers intervention intended to prevent or delay these consequences. Clearly, specificity in the definition is a priority, and the false-positive rate with the Phoenix “nadir+2” definition ranges from <3% to 5%. However, many patients who do not meet this definition of failure are not cured. A definition of successful treatment and cure remains to be determined. If the absence of failure is considered to be cure, then a highly specific definition of failure will overestimate cure. Similarly, a rigorous definition of cure would risk misidentifying patients who have not met the strict definition of cure as therefore having experienced failure. There must be widespread acceptance that depending on the dose and method of delivery of radiation, there will always be a proportion of patients who cannot be declared as “cured” or as “failed.” Such patients need continued follow-up and PSA monitoring.
Brachytherapy has long been recognized to result in lower post-treatment PSA nadirs, with a PSA level of ≤0.2 ng/mL at 3–5 years predicting sustained disease-free status (9) (10) (11), with some even suggesting that monitoring for such patients can be ceased (11). Lo et al demonstrated that a PSA of < 0.2 at 4 years after brachytherapy predicted for long-term biochemical control with no biochemical failures at 8 years (9). Tetreault-Laflamme et al. reported on more than 2,000 patients treated with LDR brachytherapy (12). At a median follow-up time of 7 years, 86% demonstrated very low and stable PSA levels, with the median PSA nadir being 0.03 ng/mL and the 5-year PSA level 0.04 ng/mL.
Morris et al. (13) suggested using the surgical definition of biochemical failure (PSA >0.2 ng/mL) for the 398 patients with intermediate- or high-risk prostate cancer treated in the Ascende-RT randomized trial (12 months ADT, 46 Gy in 23 fractions of pelvic EBRT followed by either a further 32 Gy EBRT prostate boost or LDR brachytherapy). When a failure definition of PSA >0.2 ng/mL was applied, relapse-free survival rates in the 78-Gy arm decreased from 76% (nadir+2 definition) to 38%, whereas in the BT arm, 85% of patients maintained a PSA ≤0.2 ng/mL at 7 years.
In the current analysis of more than 14,000 patients treated with brachytherapy, either alone or combination with EBRT, ADT, or both, we chose a relatively early but intermediate time point as being potentially predictive of long-term clinical outcome. To avoid PSA perturbations from the benign PSA bounce phenomenon (14) (15) (16), we chose 3.5–4.5 years as the earliest suitable time point to assess the predictive capacity of the post-brachytherapy PSA value. We found that a 4-year PSA level of ≤0.2 ng/mL was associated with a 98.7% chance of freedom from prostate cancer at 10 years and a 96.3% chance at 15 years. Almost 80% of the patients in this analysis achieved this PSA threshold at 4 years, and 85.4% had done so by 5 years. Further, the median PSA at 10 and 15 years was maintained at 0.01 ng/mL.
The definition of ‘cure’ seems to be equally valid across risk groups, as shown by the three validation cohorts. Clearly the percentage of patients in any risk group achieving a PSA level of ≤0.2 ng/mL will depend on the intensity of treatment. Nadir is known to be dose-dependent (17) (18), but one weakness of our study is that we have not yet assessed the correlation of nadir with implant quality in our cohort. In addition, PSA testing was not standardized among the contributing centers.
Although some controversy remains over which PSA level best predicts subsequent failure-free survival, we recommend using the 0.2 ng/mL threshold at 4 years after brachytherapy to define cure. This threshold applies to almost 80% of patients who have received LDR brachytherapy, either alone or in combination with EBRT, ADT, or both. It has high specificity, and facilitates direct comparisons with surgical series. This threshold can be adopted as an intermediate biochemical endpoint in future clinical trials involving brachytherapy. Notably, however, failure to achieve this strict threshold does not imply failure and should not trigger investigation or intervention. The established nadir + 2ng/ml definition of failure should still be followed and remains unchallenged. Those patients who do not achieve a PSA ≤ 0.2 ng/ml by 4 or 5 years should continue to be monitored. Although many will remain free of clinical recurrence, advanced imaging or biopsy can be considered, especially for those in PSA category 4, with a PSA level of >1.0 ng/mL, for whom more than 50% are destined to experience clinical recurrence.
Validation from the three independent prospective cohorts in our analysis supports the value of 4-year PSA ≤0.2 ng/mL as the biochemical definition of cure after LDR brachytherapy across all risk groups, with predicted rates of freedom from prostate recurrence of 97% to 99% at 10–15 years. However, the significance of reaching this PSA threshold still requires validation for patients treated with other types of radiation, specifically EBRT or stereotactic body radiotherapy (19).
Use of this definition of cure for comparison with surgical series may seem to put brachytherapy outcomes at a disadvantage because not all of the patients who do not meet this threshold will ultimately fail; however, the same is true of surgical patients with a PSA level of >0.2 ng/mL. Not all will experience clinical failure or require salvage therapy. Although most patients who have a PSA level of >0.1 ng/mL after radical prostatectomy will progress to >0.2 ng/mL (7), only about 50% will develop metastases by 10 years (20). Factors such as PSA doubling time can help to define the risk of clinical progression (21).
Over 20 years ago Critz et al called for the adoption of a post-treatment PSA ≤ 0.2 ng/ml to define disease freedom after radiotherapy for prostate cancer based on patients treated with combined transperineal implant and external radiotherapy(22). He sought a universal PSA nadir goal, equally applicable after prostatectomy or radiotherapy. It’s time we followed his lead.
Conclusion:
By 4 years after LDR brachytherapy, most patients will achieve a PSA level of ≤0.2 ng/mL. Regardless of risk group, this is associated with rates of freedom from prostate cancer recurrence of 97% to 99% at 10 years. Adoption of PSA ≤0.2 ng/mL as a common definition of cure should facilitate comparison with outcomes from surgical series. Many patients who do not achieve a PSA ≤ 0.2 ng/ml may also remain free of prostate cancer recurrence, but the risk of recurrence is sufficient that they require continued monitoring.
Fig. 1 —
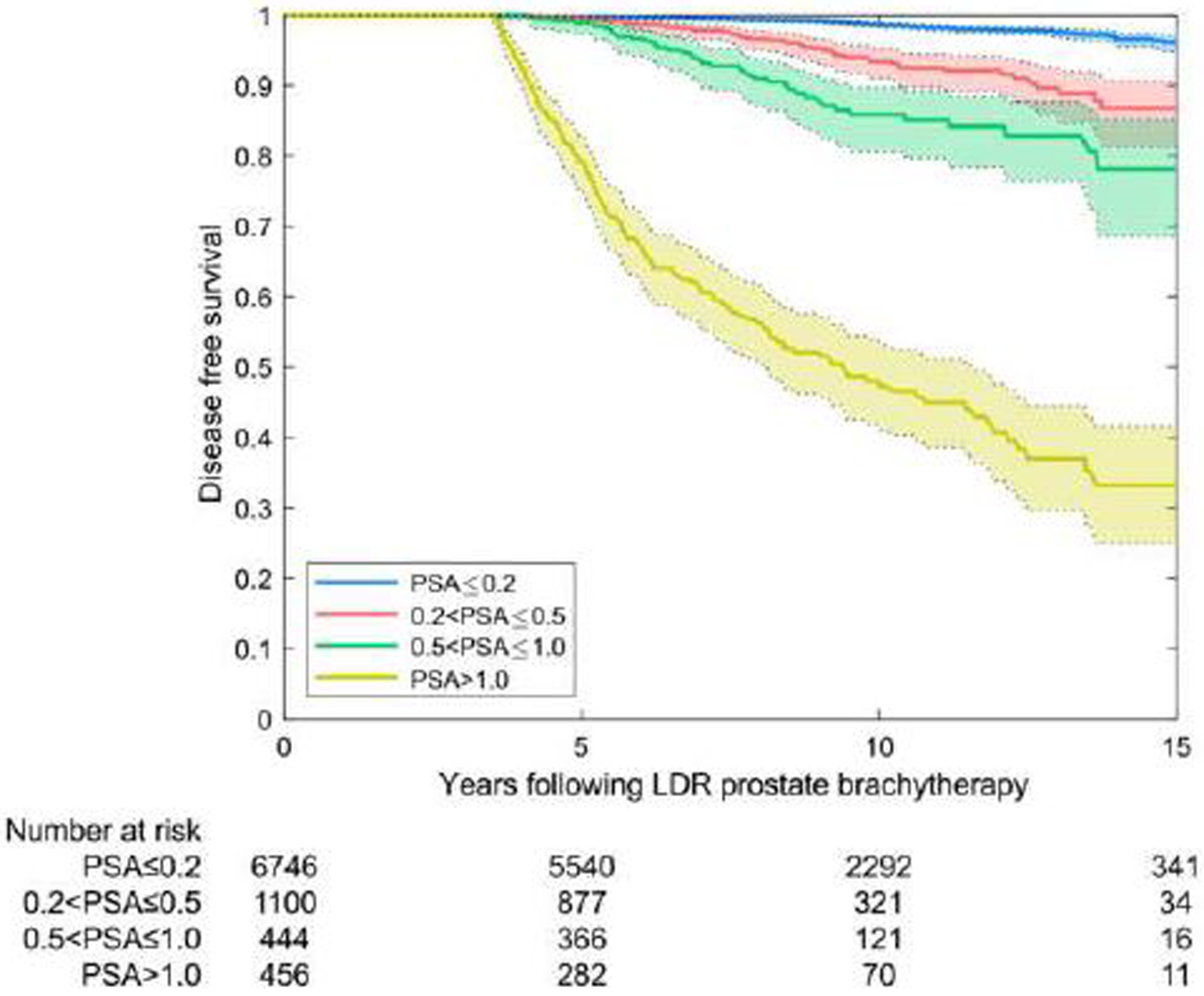
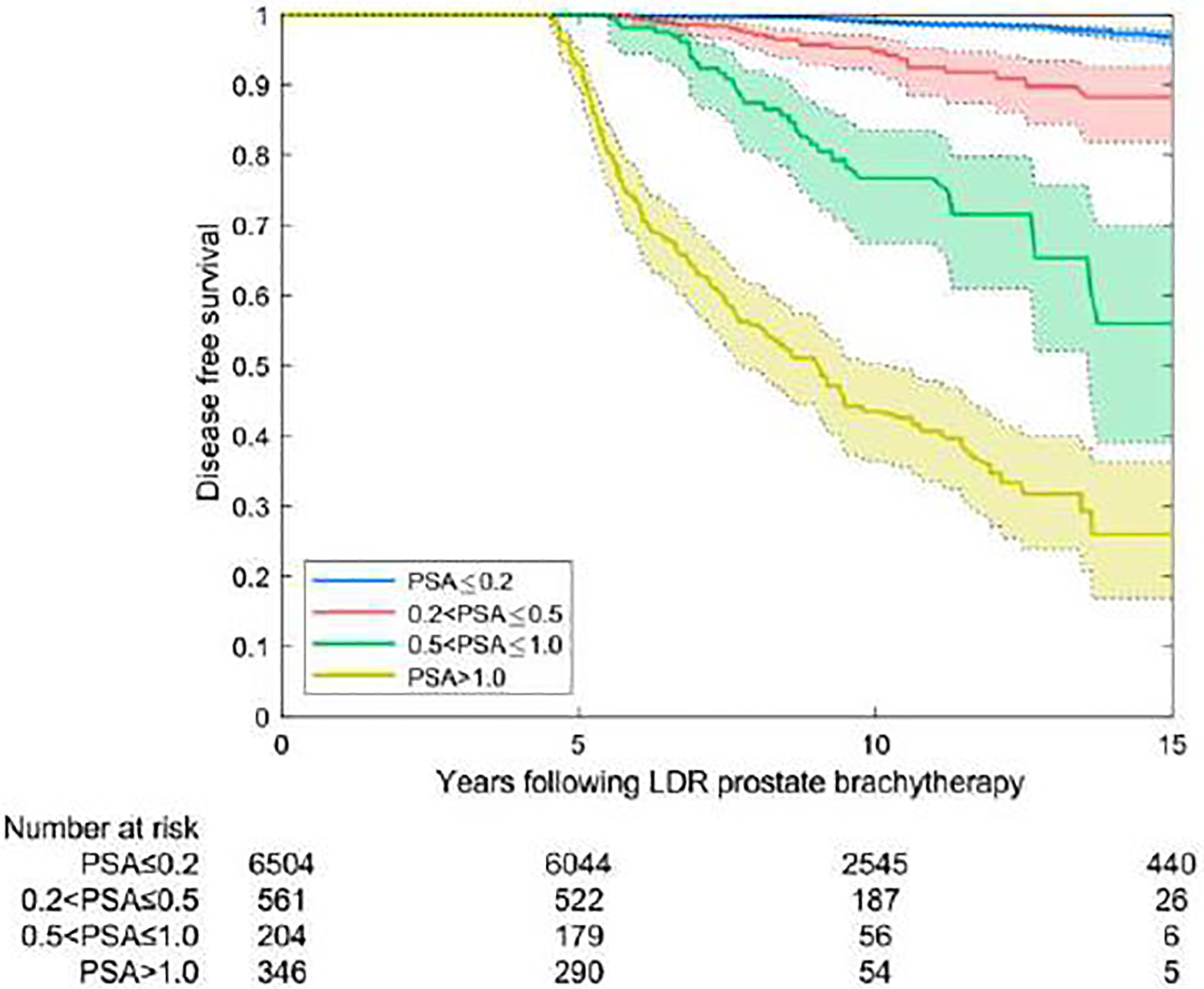
Disease-free survival rates by prostate-specific antigen (PSA) category at 4 years (A) and 5 years (B). PSA measurements before the interval of interest were excluded.
Fig 2 —
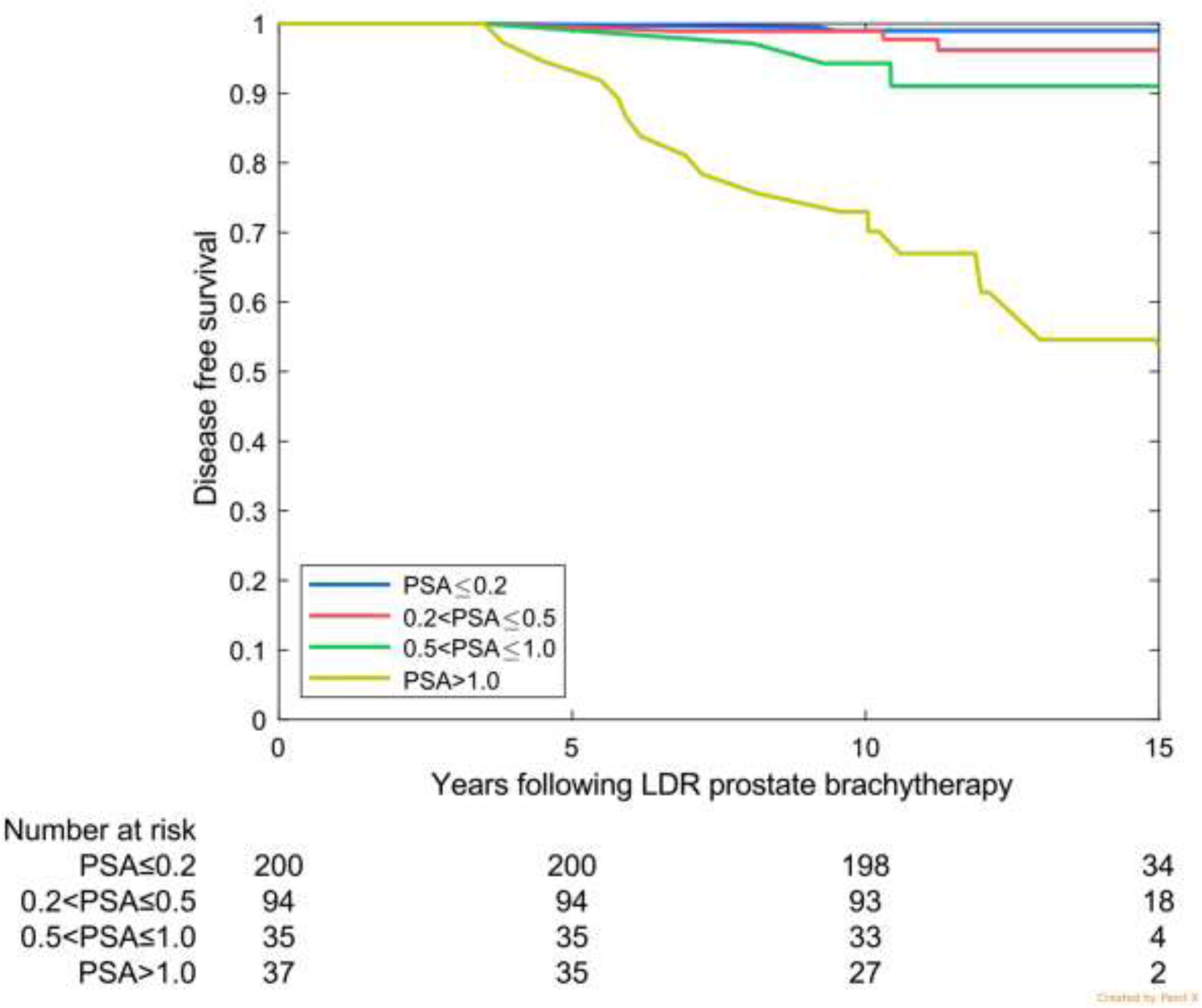
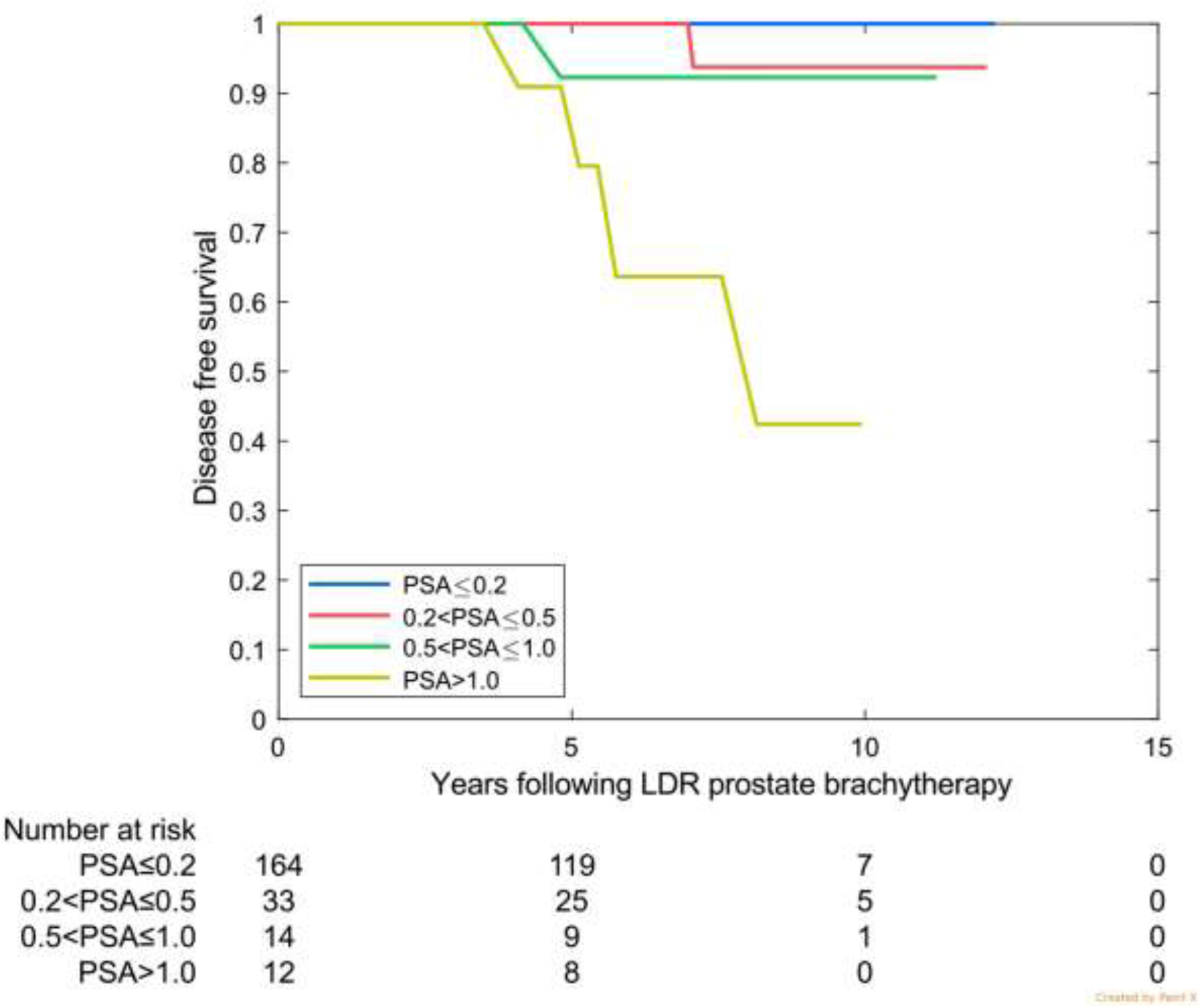
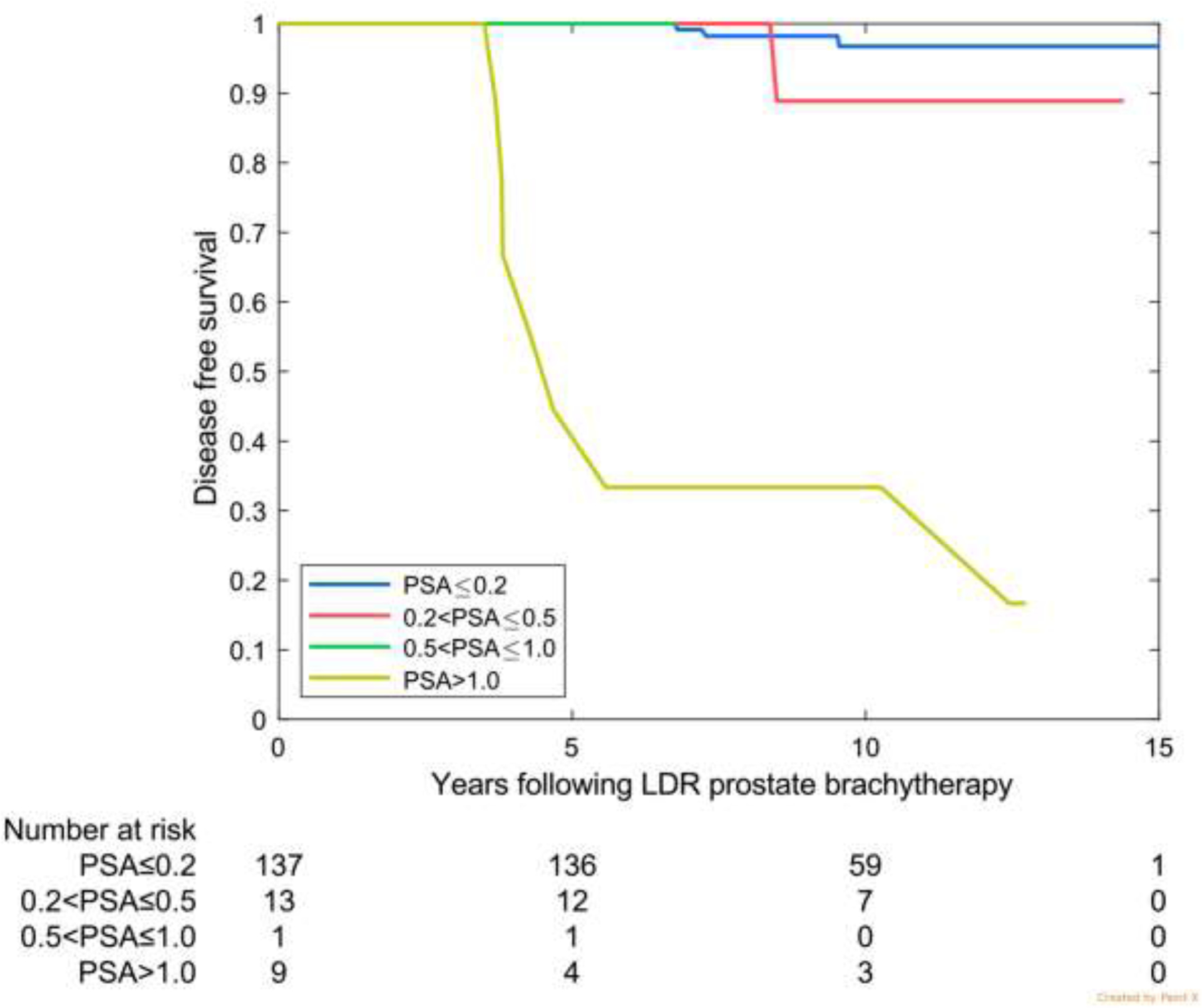
Disease-free survival by prostate-specific antigen (PSA) category at 4 years for the 3 validation cohorts. (A) Favorable risk (Australia: 75% favourable risk and 25% intermediate risk). (B) Intermediate risk (MD Anderson). (c) High risk (Ascende RT: 70% high risk and 30% upper tier intermediate risk).
Highlights.
Close to 80% of men 4 –5 years after LDR prostate brachytherapy will achieve a PSA of 0.2 ng/ml or less.
PSA ≤ 0.2 ng/ml is associated with 97–99% freedom from prostate cancer recurrence at 10–15 years
This applies across all risk groups and to LDR brachytherapy alone or combined with ADT and/or EBRT
Funding:
Funded in part by Cancer Center Support (Core) Grant P30 CA016672 from the National Cancer Institute, National Institutes of Health to the University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys 1997. March 15;37(5):1035–41. [PubMed] [Google Scholar]
- (2).Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006. July 15;65(4):965–974. [DOI] [PubMed] [Google Scholar]
- (3).Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys 2003. November 15;57(4):929–43. [DOI] [PubMed] [Google Scholar]
- (4).Frank SJ, Pugh TJ, Blanchard P, et al. Prospective phase 2 trial of permanent seed implantation prostate brachytherapy for intermediate-risk localized prostate cancer: efficacy, toxicity, and quality of life outcomes. Int J Radiat Oncol Biol Phys 2018. February 1;100(2):374–382. [DOI] [PubMed] [Google Scholar]
- (5).Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017. June 1;98(2):275–285. [DOI] [PubMed] [Google Scholar]
- (6).Ong WL, Matheson B, Millar J. Decline in acute urinary toxicities with increased institutional experience: 15-year experience of permanent seed prostate brachytherapy in a single Australasian institution. Brachytherapy 2017. Mar-Apr;16(2):313–322. [DOI] [PubMed] [Google Scholar]
- (7).Budaus L, Schiffmann J, Graefen M, et al. Defining biochemical recurrence after radical prostatectomy and timing of early salvage radiotherapy : Informing the debate. Strahlenther Onkol 2017. September;193(9):692–699. [DOI] [PubMed] [Google Scholar]
- (8).Malik RD, Goldberg JD, Hochman T, Lepor H. Three-year postoperative ultrasensitive prostate-specific antigen following open radical retropubic prostatectomy is a predictor for delayed biochemical recurrence. Eur Urol 2011. September;60(3):548–553. [DOI] [PubMed] [Google Scholar]
- (9).Lo AC, Morris WJ, Lapointe V, et al. Prostate-specific antigen at 4 to 5 years after low-dose-rate prostate brachytherapy is a strong predictor of disease-free survival. Int J Radiat Oncol Biol Phys 2014. January 1;88(1):87–93. [DOI] [PubMed] [Google Scholar]
- (10).Helou J, D’Alimonte L, Loblaw A, et al. High dose-rate brachytherapy boost for intermediate risk prostate cancer: Long-term outcomes of two different treatment schedules and early biochemical predictors of success. Radiother Oncol 2015. March 11. [DOI] [PubMed] [Google Scholar]
- (11).Niwa N, Matsumoto K, Nishiyama T, et al. Selection of patients who would not require long-term prostate-specific antigen monitoring after low-dose-rate brachytherapy. Brachytherapy 2018. Nov-Dec;17(6):899–905. [DOI] [PubMed] [Google Scholar]
- (12).Tetreault-Laflamme A, Crook J, Hamm J, et al. Long-Term Prostate Specific Antigen Stability and Predictive Factors of Failure after Permanent Seed Prostate Brachytherapy. J Urol 2018. January;199(1):120–125. [DOI] [PubMed] [Google Scholar]
- (13).Morris WJ, Pickles T, Keyes M. Using a surgical prostate-specific antigen threshold of >0.2 ng/mL to define biochemical failure for intermediate- and high-risk prostate cancer patients treated with definitive radiation therapy in the ASCENDE-RT randomized control trial. Brachytherapy 2018. Nov-Dec;17(6):837–844. [DOI] [PubMed] [Google Scholar]
- (14).Ciezki JP, Reddy CA, Garcia J, et al. PSA kinetics after prostate brachytherapy: PSA bounce phenomenon and its implications for PSA doubling time. Int J Radiat Oncol Biol Phys 2006. February 1;64(2):512–517. [DOI] [PubMed] [Google Scholar]
- (15).Crook J, Gillan C, Yeung I, Austen L, McLean M, Lockwood G. PSA kinetics and PSA bounce following permanent seed prostate brachytherapy. Int J Radiat Oncol Biol Phys 2007. October 1;69(2):426–433. [DOI] [PubMed] [Google Scholar]
- (16).Frank SJ, Levy LB, Crook J, et al. Outcomes following prostate brachytherapy are even better than predicted. Cancer 2009. February 1; 118(3): 839–847. [DOI] [PubMed] [Google Scholar]
- (17).Ray ME, Thames HD, Levy LB, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int J Radiat Oncol Biol Phys 2006. March 15;64(4):1140–1150. [DOI] [PubMed] [Google Scholar]
- (18).McLaren DB, Kerr G, Law AB, et al. The Importance of Prostate-specific Antigen (PSA) Nadir and Early Identification of PSA Relapse after 10 Years of Prostate Iodine 125 Seed Brachytherapy in Edinburgh. Clin Oncol (R Coll Radiol) 2015. September;27(9):519–526. [DOI] [PubMed] [Google Scholar]
- (19).Jiang NY, Dang AT, Yuan Y, et al. Multi-Institutional Analysis of Prostate-Specific Antigen Kinetics After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2019. November 1;105(3):628–636. [DOI] [PubMed] [Google Scholar]
- (20).Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int 2011. August;108(3):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Van den Broeck T, van den Bergh RCN, Briers E, et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur Urol Focus 2019. June 24. [DOI] [PubMed] [Google Scholar]
- (22).Critz FA, Williams WH, Holladay CT, Levinson AK, Benton JB, Holladay DA, et al. Post-treatment PSA < or = 0.2 ng/mL defines disease freedom after radiotherapy for prostate cancer using modern techniques. Urology 1999. December;54(6):968–71. [DOI] [PubMed] [Google Scholar]


