Abstract
Skin pigmentation is a result of melanin produced by melanocytes in the epidermis. Melanocyte activity, along with the type and distribution of melanins, is the main driver for diversity of skin pigmentation. Dark melanin acts to protect against the deleterious effects of ultraviolet (UV) radiation, including photoaging and skin cancer formation. In turn, UV radiation activates skin melanocytes to induce further pigmentation (i.e., “tanning pathway”). The well-characterized MSH/MC1R-cAMP-MITF pathway regulates UV-induced melanization. Pharmacologic activation of this pathway (“sunless tanning”) represents a potential strategy for skin cancer prevention, particularly in those with light skin or the “red hair” phenotype who tan poorly after UV exposure due to MC1R inactivating polymorphisms. Skin hyperpigmentation can also occur as a result of inflammatory processes and dermatological disorders such as melasma. While primarily of cosmetic concern, these conditions can dramatically impact quality of life of affected patients. Several topical agents are utilized to treat skin pigmentation disorders. Here, we review melanogenesis induced by UV exposure and the agents that target this pathway.
Keywords: Melanocytes, Pigmentation, cAMP, MITF
SKIN PIGMENTATION AND DYSPIGMENATION
UV-induced skin pigmentation: The “tanning pathway”
Ultraviolet (UV) radiation is one of the most well characterized factors governing skin pigmentation. Exposure to UV results in synthesis of melanin pigments within highly specialized cells termed melanocytes [1]. In human skin, a single melanocyte utilizes dendritic structures to contact approximately 36 keratinocytes, thereby comprising the epidermal melanin unit [2]. Within melanocytes, melanin is synthesized in melanosomes, which are specialized membrane-bound organelles that are transferred to the surrounding keratinocytes in order to induce homogenous pigmentation and photoprotection from DNA damage.
Many signaling pathways have been shown to affect melanin production, as reviewed in detail by Lin and Fisher [3]. The key molecules in the UV-dependent tanning pathway are melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH), cleaved from pro-opiomelanocortin (POMC) in keratinocytes, and the corresponding G-protein-coupled melanocortin 1 receptor (MC1R) on melanocytes. Hypomorphic MC1R variants are more commonly found in individuals with lighter skin tones and/or red hair, frequently of European/Caucasian descent, and are associated with poor tanning responses [4–6]. The observation that red-haired individuals have weak tanning responses led to the finding that UV intensely triggers induction of POMC/α-MSH by the p53 tumor suppressor protein within epidermal keratinocytes (>30 fold) [7, 8]. DNA damage after UV exposure in keratinocytes results in upregulation of POMC expression by p53. POMC is subsequently enzymatically cleaved in keratinocytes to produce α-MSH. α-MSH is then released and binds MC1R on melanocytes, triggering an increase in cyclic AMP (cAMP) levels. Upregulation of cAMP in melanocytes activates protein kinase A (PKA), which phosphorylates the cAMP-responsive element-binding protein (CREB). This subsequently results in increased transcription of microphthalmia-associated transcription factor (MITF), which serves as the master regulator of melanocyte survival and pigment production. MITF target genes include regulators of melanin synthesis such as tyrosinase, as well as other melanosomal components and even the melanosome trafficking machinery, ultimately resulting in increased melanin production and export to overlying keratinocytes in the epidermis, where most epidermal melanin resides (Figure 1) [9, 10]. Note that pigmentation of hair follicles involves some distinctive mechanisms and will not be reviewed in this manuscript.
Figure 1: The tanning pathway in response to UV radiation.
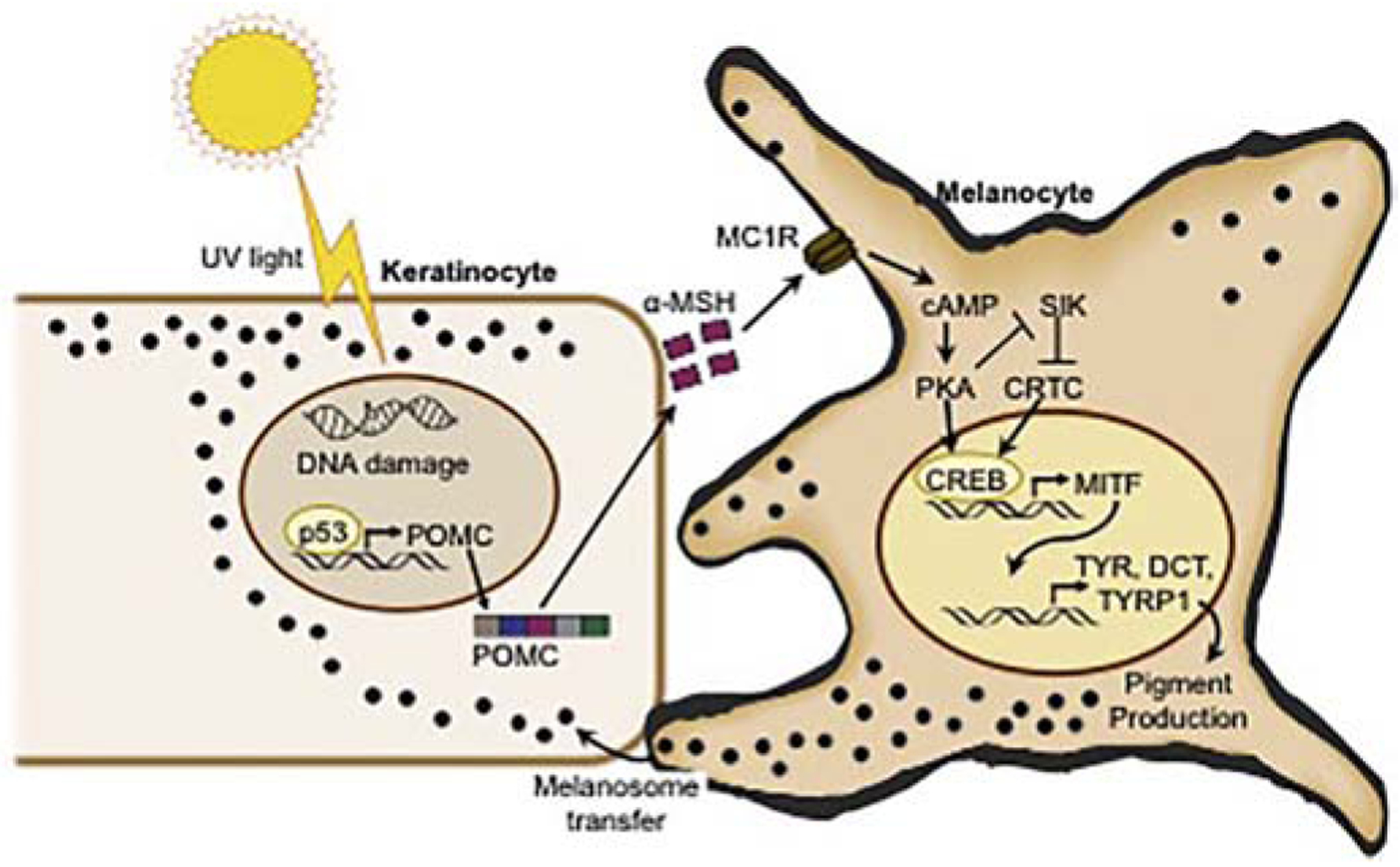
Exposure to UV radiation induces DNA damage, which activates p53 in keratinocytes. This leads to upregulation of the POMC gene and cleavage of POMC to α-MSH. α-MSH binds MC1R on adjacent melanocytes, leading to an increase of cAMP and activation of PKA. PKA phosphorylates CREB, which binds to the MITF promoter resulting in MITF expression, melanin production, and ultimately transfer of melanosomes containing melanin to neighboring keratinocytes. The figure was modified from Nguyen et al., Pigment Cell Melanoma Res (2019)[79].
Agents for “sunless tanning” and skin cancer prevention
The incidence of skin cancers has been increasing over the last few decades, with more than 9,000 new cases diagnosed in the United States every day https://www.aad.org/media/stats/conditions/skin-cancer. UV exposure is strongly implicated in skin cancer carcinogenesis. Eumelanin in the skin provides protection for keratinocytes against UV-induced DNA damage [11]. There are two broad types of melanin that are synthetized in all human melanocytes, the brown/black eumelanin that effectively absorbs the energy of UV radiation, and yellow/red pheomelanin that is less photoprotective and has been shown to induce reactive oxygen species both dependently and independently of UV [12–15]. Individuals who tan easily have significantly lower skin cancer risk relative to fair-skinned individuals who burn easily [16–19]. This is due in large part to the increased synthesis of eumelanin through the α- MSH/cAMP signaling pathway, which may help to block penetration of UV radiation into the deeper layers of the skin [16–19]. Fair-skinned individuals and especially those with the red hair color phenotype are much more likely to be diagnosed with melanoma and other skin cancers, in part due to the reduced levels of protective eumelanin synthesized after UV exposure [20]. Moreover, the deleterious actions of pheomelanin also likely play a role in skin cancer formation in these individuals. Therefore, pharmacological induction of skin darkening independent of UV may be particularly useful as a skin cancer prevention strategy in individuals with MC1R nonsignaling variants. Such strategies to target the tanning pathway are discussed below.
cAMP inducers: Forskolin and phosphodiesterase PDE4D3
Pharmacologic activation of the α-MSH/cAMP/MITF pathway was first attempted through design of a synthetic α-MSH analog, afamelanotide (Melanotan) [21]. Afamelanotide has been shown to diminish measurable phototoxicity in adult patients with erythropoietic protoporphyria [22]. While this compound effectively stimulates the tanning pathway, it requires systemic delivery and is poorly tolerated due to associated side effects. It has become a drug of choice in underground markets for consumers seeking a tan, where it is sold illegally as the “Barbie drug.” In individuals receiving this drug, there have been reports of eruptive melanocytic nevi, atypical nevi, and even melanoma [23–25]. At this time, it is still unclear whether there is a direct causal link between the drug and melanomagenesis, as it is possible that pigmentation changes induced by the drug within pre-existing lesions may have led to their recognition.
Design of topical agents for stimulation of the tanning pathway may allow for more focused side effect profiles of sunless tanning agents for skin cancer prevention.
Forskolin is a diterpene produced by the roots of Plectranthus barbatus (Family: Lamiaceae) (also known as Coleus forskohlii) and has been used extensively in traditional medicine. Its safety profile has also been studied using modern techniques. It has been shown that forskolin can increase cytoplasmic cAMP levels by direct activation of adenylyl cyclase [26, 27]. Since the “switch” between pheomelanin and eumelanin species is strongly induced by cAMP signaling, our group hypothesized that topical treatment with forskolin may bypass the defective cAMP signaling associated with MC1R variants in order to induce the production of protective eumelanin.
Though epidermal melanocytes are not normally found in the inter-follicular furry skin of mice, animals have been genetically engineered to direct epidermal localization of melanocytes. There is significant deposition of dark eumelanin pigment in this “humanized model” with wild type MC1R, which confers UV protection [28, 29]. In contrast, humanized mice with nonfunctional MC1R signaling have red-blond pigmentation and minimal eumelanin in the skin with a susceptibility to burning, similar to that seen in human red-haired individuals [7]. Application of forskolin to the skin of Mc1r-defective mice induced robust eumelanin production and conferred significant protection against subsequent UV-induced squamous cell carcinoma formation. This study suggested that eumelanin deposition can be enhanced by topical agents that bypass defective MC1R signaling by inducing downstream mediators of the tanning pathway to mimic the dark-pigmentation phenotype associated with functional MC1R. This study implicated forskolin as a potential candidate for clinical application in products for UV-independent tanning. It also offered the opportunity for topical small-molecule modulation of pigmentation that might confer protection against UV-induced DNA damage and tumorigenesis [7]. A similar approach used an inhibitor of the phosphodiesterase PDE4D3, which was subsequently identified as a key regulator of cAMP homeostasis in melanocytes. Suppression of PDE4D3 produced UV-independent hyperpigmentation in mice similar to that seen in red-haired mice after forskolin treatment [30].
While these studies have demonstrated impressive melanization of murine skin, attempts to translate the drugs to clinical application in humans have been challenging, often due to poor penetration of small molecules through human skin, which is several fold thicker than mouse skin.
Salt inducible kinase (SIK) inhibitors
A related signaling pathway that potently modulates the tanning pathway includes the salt-inducible kinase (SIK) and the factor called cAMP-regulated transcriptional co-activator (CRTC), of which there are 3 isoforms of each [31]. The CRTCs have been identified as strong co-activators of CREB [32]. Cellular localization of CRTC is tightly regulated by SIK. Activated SIK phosphorylates CRTC resulting in its cytoplasmic retention; however, if SIK is inhibited, CRTC can migrate to the nucleus, bind CREB, and increase its activity [32].
The SIK/CRTC/CREB signaling pathway was initially implicated in melanin synthesis in murine melanoma, in which overexpression of the CRTCs was noted to produce hyperpigmention through induction of CREB, MITF, and tyrosinase [31]. This could be suppressed by overexpression of SIK. The authors also demonstrated that germline knockout of SIK2 in mice led to darkening of the fur in the genetic background of yellow/redhaired agouti mice [31].
SIK has been demonstrated to be a direct target of PKA; phosphorylation of SIK by PKA results in SIK inhibition[33]. A mutated form of SIK2 that cannot be phosphorylated by PKA results in strong inhibition of tyrosinase expression, even in the presence of the cAMP inducer forskolin. Collectively, this data suggests that SIK inhibition by PKA is required for the MC1R/cAMP/MITF tanning pathway and establishes a key role for the SIK-CRTC-CREB/MITF pathway in the tanning response [31] (Figure 1).
Based on these observations, our group recently tested whether a strategy targeting SIK through skin-permeable small-molecule antagonists could be applied to modulate pigmentation [34]. Application of the previously published SIK inhibitor HG 9-91-09 to normal human melanocytes and melanoma cells significantly increased MITF and its target, TRPM1 [35]. Furthermore, application of HG 9-91-09 to the Mc1r-deficient mice (“red-haired” model) resulted in localized skin darkening, indicating that the drug activated the melanin-producing pathway. Modified compounds were derived to optimize human skin permeation, and topical treatment of human skin with the small molecules YKL 06–061 and YKL 06–062 resulted in skin darkening, with histologic changes showing melanization similar to the UV-induced tanning response (Figure 2). Of note, the induced pigmentation was seen not only in epidermal melanocytes but also through highly efficient transfer of melanosomes into adjacent/overlying keratinocytes. There the melanosomes coalesce into supranuclear caps, closely mimicking the pigmentation pattern seen after UV-induced tanning and in constitutively darkly pigmented individuals [34].
Figure 2: Topical treatments of human skin with SIK inhibitors induce pigmentation.

Skin explants from human breast treated with SIK inhibitor YKL 06–061, YKL 06–062, or HG 9-91-01, demonstrating obvious darkening grossly (A) and after Fontana-Masson staining (B).The figure was adapted from Mujahid N et al., Cell Reports (2017)[34].
These studies collectively describe a topically delivered pharmacological approach to the modulation of eumelanin production in a UV-independent manner. Although this approach is potentially translatable to humans, additional studies are needed to generate a comprehensive safety profile for these novel small molecules in clinical settings. For example, while MITF amplifications have been associated with melanoma risk, elevated pigmentation is strongly associated with protection against melanoma (and non-melanoma skin cancers) in humans. As this approach would not be targeting permanent/genetic alteration of MITF or the pigmentation pathway, it might be anticipated that beneficial effects of pigmentation may be exploited without the potential hazard of “fixed” genomic alterations (akin to the use of growth factors to enhance white blood cell counts after chemotherapy administration). Notably, SIK has been reported to demonstrate both tumorigenic and anti-metastatic functions [36, 37]. Additionally, pharmacological sunless skin tanning may not be sufficient alone to prevent skin damage associated with UV exposure. Melanin in darker skinned individual provides a ultraviolet protection factor (UPF) of less than 5, which is weaker than almost all sunscreens commercially available [13]. Therefore, the combination of sunless tanning product with sunscreen together may confer better UV protection than either approach alone, especially for patients at a high risk of skin cancer.
Causes of skin hyperpigmentation
Epidermal hyperpigmentation
Acquired hyperpigmentation of skin is often associated with epidermal hyperpigmentation, which is characterized histologically by a normal or increased number of melanocytes in the basal layer, increased activation of melanocytes, and increased epidermal melanin in all layers. Hyperpigmented lesions may result from a mix of epidermal and dermal processes. Overall, these changes are likely due to pathologic activation of the tanning pathway. This has been best demonstrated in melasma, where within active lesions, α-MSH has been reported to be upregulated [38–40]. However, other changes such as epigenetic changes in keratinocytes or melanocytes might also contribute to increased melanocyte activation. Additional clinical examples include café-au-lait lesions and lentigines [41]. Examination using a Wood’s lamp (320–400 nm) reveals a darker brown color within lesions of epidermal hyperpigmentation compared with processes involving deeper hyperpigmentation. Most skin lightening agents target epidermal pigmentation either at the level of tyrosinase action or at other points of the tanning pathway.
Dermal hyperpigmentation
Conditions such as melasma and post-inflammatory hyperpigmentation are associated with increased epidermal pigmentation, but also have variable involvement of dermal hyperpigmentation (Figure 2) [42]. Dermal hyperpigmentation is associated with the presence of melanophages in the superficial dermis, along with an infiltrate of lymphohistiocytes around blood vessels and in dermal papillae. Melanophages are myeloid-derived macrophages that, unlike other dermal macrophages, upregulate genes related to glutathione metabolism and phagosome maturation [43, 44]. Additional clinical examples include fixed drug eruption and ashy dermatosis. Often, these lesions take on a blueish hue due to the Tyndall effect, as short wavelengths of light on the blue color spectrum are scattered and reflected more than longer wavelengths. Therefore, while more superficial melanin in the epidermis may appear black, deeper pigment takes on a blue-grey color, and pigment in the deep reticular dermis (as seen with blue nevi) appears blue [45].
Other causes of hyperpigmentation and hypopigmentation of skin
This review focuses on the tanning pathway that leads to melanin production in melanocytes and therapeutic strategies that target this pathway. Activating this pathway provides opportunities for sunless tanning and skin cancer prevention, while antagonizing this pathway may offer treatment opportunities for common disorders of pigmentation such as melasma and post-inflammatory hyperpigmentation. In addition to UV-induced pigmentary changes, there are numerous other causes of skin hypopigmentation and hyperpigmentation. Vitiligo is typically caused by autoimmune destruction of melanocytes or chemical toxicity to melanocytes. It is a clinically important entity, but not thought to be a result of tanning pathway alterations. Skin hypopigmentation can also be a result of inflammatory and infectious processes, and while this is not entirely a result of UV exposure, is possible that sunless activation of the tanning pathway could benefit this condition.
Other mechanisms for skin hyperpigmentation include dermal melanocytosis due to localized increased number of dermal melanocytes. Certain drugs may also induce hyperpigmentation through pigment deposition within dermal macrophages. Depending on the offending agent, the pigment may be composed of hemosiderin (venous stasis dermatitis), drug complexes (e.g., minocycline), and lipofuscin (medication-induced, normal aging).
Clinical problems associated with skin hyperpigmentation
Melasma.
Melasma is an acquired, symmetrical hyperpigmentation most often affecting areas of the forehead, cheeks, temples, and upper lip. It is most commonly seen in women and often associated with pregnancy and use of oral contraceptives. It is also significantly exacerbated by sunlight exposure. Epidermal and dermal melasma types have been recognized, although most cases span a combination of both types.
Post-inflammatory hyperpigmentation.
Inflammatory skin conditions can produce clinical hypopigmentation, hyperpigmentation, or both. This is most commonly seen in darker skinned individuals, resulting in marked hyperpigmentation that may be dramatic in relation to the primary lesion (i.e., acne vulgaris, atopic dermatitis). Post-inflammatory hyperpigmentation can also occur in response to outside insults, as seen in scars after trauma or even smaller insults such as a needle injection site. Additionally, it is a risk associated with cosmetic treatments such as laser procedures and chemical peels. Post inflammatory pigmentary changes often have a prominent dermal component and persist long after the primary process has resolved.
Dyspigmentation associated with photoaging
Circumscribed pigmented lesions (freckle, lentigo).
Ephelides or freckles are small hyperpigmented macules 1–2 mm in diameter most commonly distributed on the face, shoulders, and arms of fair-skinned individuals [46]. They often persist after episodes of severe blistering sunburns. Histologically, there is increased pigment deposition in the basal layer with typically sharp demarcation of abnormal areas, while preserving normal numbers of melanocytes. Senile or solar lentigines have been shown to be related to sun exposure and show similar histologic features [46]. Although individuals with many of these lesions may exhibit increased skin cancer risk, these lesions themselves are not thought to harbor premalignant behaviors.
Diffuse hyperpigmentation.
Chronic UV-exposed sites in fair-skinned individuals are often characterized by mottled hyper- and hypo-pigmentation or generalized hyperpigmentation. This hyperpigmentation often persists years after UV exposure has ceased. Histologically, this is associated with an increased number of melanocytes and increased melanocyte activation [47].
AGENTS FOR TREATMENT OF SKIN HYPERPIGMENTATION
Agents that act through inhibition of tyrosinase
Hydroquinone.
Hydroquinone is the one of the most commonly used therapies for skin hyperpigmentation. In the United States, it is available in the form of over-the-counter “bleaching creams” at a strength of 2% and in prescription formulations of 1.5–4%. Hydroquinone is a small (MW 110.1) hydrophilic molecule that penetrates human skin effectively, with up to 43% reaching blood circulation [48]. Hydroquinone acts by inhibiting the enzymatic oxidation of tyrosine to 4-dihydroxyphenylalanine (DOPA) and by suppressing other melanocyte metabolic processes (i.e., cytotoxicity) [49–53]
The first clinical trial demonstrating the efficacy of hydroquinone was published in 1961, with subsequent clinical trials further supporting these findings [54]. Hydroquinone may also be used in combination with other compounds to modulate its effect. Conjugating hydroquinone with azelaic acid enhances skin uptake and enhances dermal delivery of both compounds [55]. A co-drug synthesized through joining hydroquinone and salicylic acid demonstrates improved retention in the skin with tolerable irritation [56]. Additionally, conjugating hydroquinone with tranexamic acids (TXA) results in increased skin deposition of both drugs, resulting in enhanced skin targeting by decreasing transdermal penetration [57].
There are additional compounds structurally related to hydroquinone, including arbutin (hydroquinone β-D-glucopyranoside) and mequinol (hydroquinone monomethyl ether), that have been shown to inhibit tyrosinase through similar mechanisms as hydroquinone, though other pathways have been proposed [58–60]. These products can also commonly be found in over-the-counter skin lightening products in the United States and other countries.
Azelaic acid.
Azelaic acid (C9 dicarboxylic acid, MW 118.22) is a medium-chain-length saturated dicarboxylic acid with skin lightening properties. Interestingly, dicarboxylic acids were found to be produced by cultures of Pityrosporum and postulated to be causative to the hypopigmentation seen in tinea versicolor [61]. While dicarboxylic acids, including azelaic acid, were identified as inhibitors of tyrosinase, they do so at a relatively low affinity and would require cytotoxic doses to demonstrate inhibition in vivo [62]. There is no known in vivo or in vitro melanocidal or depigmenting effect, in contrast to other skin lightening compounds such as hydroquinone [62]. Azelaic acid also has anti-inflammatory activity and has FDA approved indications for treatment of acne vulgaris and rosacea. This anti-inflammatory property of azelaic acid may also play a large role in its efficacy in post inflammatory hyperpigmentation and melasma. Off-label use of azelaic acid for melasma was shown to be more effective than 2% hydroquinone and equivalent to prescription strength 4% hydroquinone [63–65].
Targeting the cAMP pathway: α-viniferin and diacetylcaffeic acid cyclohexyl ester (DACE).
The tanning pathway is central to many pigmentary processes such as melasma and lentigines, although the precise pathogenesis remains to be shown. A novel agent for targeting the MSH/cAMP/MITF pathway has recently been reported by Cheong Yong et al., who show that topical administration of the natural compound α-viniferin can decrease melanin production in vivo and in vitro without disturbing melanocyte viability or proliferation [66]. α-Viniferin is the active oligostilbene component of Caragana sinica, a plant in the Leguminosae family. Treatment of mouse melanoma and melanocyte cell lines with α-viniferin inhibited melanin production without affecting their viability. The authors also conducted a small split-face clinical trial with 23 patients with melasma and ephelides on the face bilaterally. Topical treatment with C. sinica cream to one side of the face for 8 consecutive weeks resulted in a significantly decreased melanin index and increased lightening index compared with vehicle control treatment of the contralateral face. All patients completed the study without complaints of itch or other adverse events. Importantly, the authors also demonstrated that although it had been previously reported to inhibit tyrosinase, α-viniferin primarily acts to accelerate the feedback termination of cAMP/PKA signaling by targeting PKA [66, 67].
In a more recent study, the same authors studied the effects of diacetylcaffeic acid cyclohexyl ester (DACE), a caffeic acid derivative, which had previously been shown to inhibit melanin production in α-MSH-activated murine melanoma cells [68, 69]. Topical treatment of the dorsal skin of guinea pigs with DACE prevented hyperpigmentation in the skin following UV exposure while also demonstrating a decrease in MITF and its target tyrosinase [69]. In vitro, DACE also inhibited production of melanin in α-MSH-stimulated human epidermal melanocyte and melanoma cell lines. To understand the anti-melanogenic mechanism of DACE, the authors demonstrated that DACE directly blocked the nuclear import of CRTC1 in melanocytes.
Collectively, these studies showed that targeting the cAMP pathway either by inhibition of PKA or CRTC nuclear localization might be a valuable treatment for disorders of hyperpigmentation. It should be noted, however, that these molecules likely have additional protein targets, and further studies should be conducted to evaluate safety.
Other Agents
Tranexamic acid.
Tranexamic acid (TA) is an anti-fibrinolytic agent with FDA approved indications for menorrhagia and dental procedures in patients with hemophilia. There are also numerous off-label indications for treatment of hemorrhage and prevention of surgical blood loss. It was first noted in 1979 that patients taking tranexamic acid had improvement of melasma. Subsequent studies, including recent retrospective and randomized control trials, have supported these findings [70–72]. Although TA can be delivered topically and through intradermal injection, the majority of studies have used oral delivery of TA. Doses for melasma are significantly lower than doses used for hematological indications. Oral TA appears to have a very favorable side effect profile with no reported thromboembolic events, though careful screening is needed to exclude those with risk factors such as thromboembolism, oral contraceptive use, stroke, or heart disease before initiating therapy. TA is postulated to act by blocking conversion of plasminogen to plasmin within keratinocytes; this may decrease free arachidonic acid and subsequently diminish production of prostaglandins, thereby decreasing melanogenesis.
Kojic acid.
Kojic acid is a chelating agent first identified as a product of Aspergillus. It acts by chelating copper at the active site of tyrosinase to block the conversion of tyrosine to melanin [73, 74]. Kojic acid has been shown to be effective in the treatment of melasma [75]
Tretinoin and other retinoids.
Retinoids exhibit depigmenting action through increasing keratinocyte turnover and reducing melanosome transfer to keratinocytes. Retinoids are used alone or in combination with other treatments for melasma, post-inflammatory hyperpigmentation, and other disorders of pigmentation. In addition, tretinoin has an FDA approved indication for skin photoaging, and numerous studies have shown that its anti-aging action includes normalization of pigmentation in chronically photodamaged skin [76–78].
Summary and Conclusions
A pharmacological means to control skin pigment has been a goal in both the medical and cosmetic realms and has significant implications for skin cancer prevention, photoaging, and aesthetics. Here we review a number of agents and approaches to stimulate and inhibit pigmentation by targeting the cAMP pathway. It should be noted that the majority of the agents described have been met with minimal success in the cutaneous treatment of patients, either due to the challenge of penetrating the human skin barrier, an adverse side effect profile, minimal clinical efficacy, or insufficient clinical testing. Despite these difficulties, targeting the cAMP pathway remains an approach that holds potential for the treatment of pigmentary disorders, and the development of new specific SIK inhibitors offers another avenue for skin cancer prevention that might help to curtail the growing trend of skin cancer diagnoses [35]. There is an increasing body of evidence implicating the SIK/CRTC pathway in pigmentation and depigmentation, and understanding this pathway in detail could lead to more specific inhibitors to modulate this process [35, 68, 69].
Figure 3:
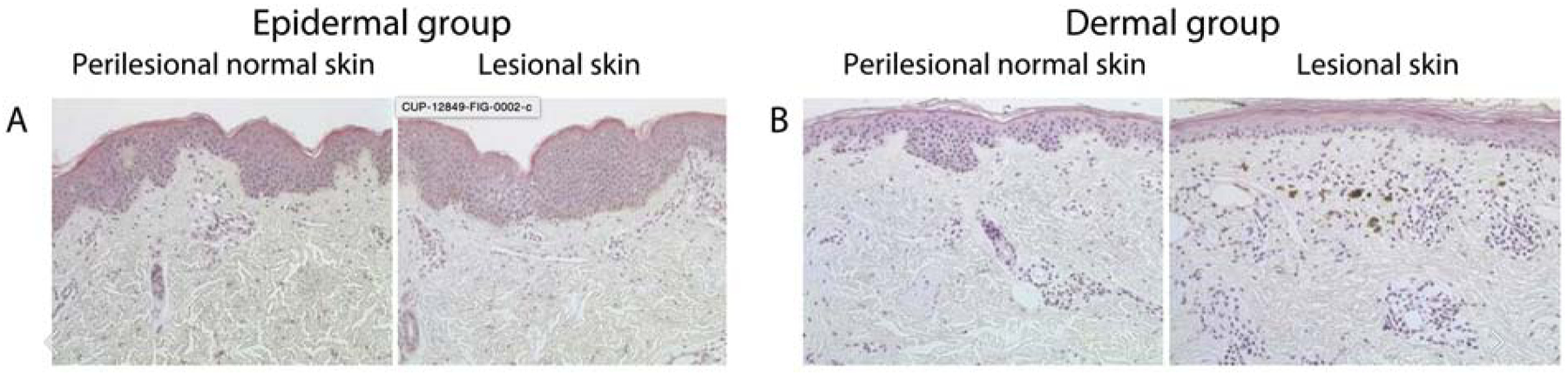
Fontana Masson staining shows increased pigmentation in the epidermis (A) and dermis (B) within lesions of melasma as compared to perilesional skin. There is often an admixture of epidermal and dermal pigmentation within lesions of melasma. Adapted from Park et al., J Cutan. Pathol. (2017)[42].
Acknowledgments
We are grateful to Dr. C. Thomas Powell for his help in preparing this manuscript. We regret that we cannot cite all studies due to space constraints. This work was supported by the NIH grants P01 CA163222, R01 CA222871, R01 AR072304, and R01 AR043369, as well as funding from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and the Melanoma Research Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Statement
Dr. Fisher has a financial interest in Soltego, Inc., a company developing SIK inhibitors for topical skin darkening treatments that might be used for a broad set of human applications. Dr. Fisher’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- [1].Fitzpatrick TB, Breathnach AS, [the Epidermal Melanin Unit System], Dermatol Wochenschr, 147 (1963) 481–489. [PubMed] [Google Scholar]
- [2].Slominski A, Tobin DJ, Shibahara S, Wortsman J, Melanin pigmentation in mammalian skin and its hormonal regulation, Physiol Rev, 84 (2004) 1155–1228. [DOI] [PubMed] [Google Scholar]
- [3].Lin JY, Fisher DE, Melanocyte biology and skin pigmentation, Nature, 445 (2007) 843–850. [DOI] [PubMed] [Google Scholar]
- [4].Valverde P, Healy E, Jackson I, Rees JL, Thody AJ, Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans, Nat Genet, 11 (1995) 328–330. [DOI] [PubMed] [Google Scholar]
- [5].Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD, Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function, Cell, 72 (1993) 827–834. [DOI] [PubMed] [Google Scholar]
- [6].Rana BK, Hewett-Emmett D, Jin L, Chang BH, Sambuughin N, Lin M, Watkins S, Bamshad M, Jorde LB, Ramsay M, Jenkins T, Li WH, High polymorphism at the human melanocortin 1 receptor locus, Genetics, 151 (1999) 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE, Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning, Nature, 443 (2006) 340–344. [DOI] [PubMed] [Google Scholar]
- [8].Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE, Central role of p53 in the suntan response and pathologic hyperpigmentation, Cell, 128 (2007) 853–864. [DOI] [PubMed] [Google Scholar]
- [9].Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R, Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes, J Cell Biol, 142 (1998) 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, Fisher DE, alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome, J Biol Chem, 273 (1998) 33042–33047. [DOI] [PubMed] [Google Scholar]
- [11].Hunt G, Kyne S, Ito S, Wakamatsu K, Todd C, Thody A, Eumelanin and phaeomelanin contents of human epidermis and cultured melanocytes, Pigment Cell Res, 8 (1995) 202–208. [DOI] [PubMed] [Google Scholar]
- [12].Sakai C, Ollmann M, Kobayashi T, Abdel-Malek Z, Muller J, Vieira WD, Imokawa G, Barsh GS, Hearing VJ, Modulation of murine melanocyte function in vitro by agouti signal protein, EMBO J, 16 (1997) 3544–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brenner M, Hearing VJ, The protective role of melanin against UV damage in human skin, Photochem Photobiol, 84 (2008) 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].d’Ischia M, Wakamatsu K, Napolitano A, Briganti S, Garcia-Borron JC, Kovacs D, Meredith P, Pezzella A, Picardo M, Sarna T, Simon JD, Ito S, Melanins and melanogenesis: methods, standards, protocols, Pigment Cell Melanoma Res, 26 (2013) 616–633. [DOI] [PubMed] [Google Scholar]
- [15].Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, Robinson KC, Devi SP, Vanover JC, D’Orazio JA, McMahon M, Bosenberg MW, Haigis KM, Haber DA, Wang Y, Fisher DE, An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background, Nature, 491 (2012) 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ, Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis, FASEB J, 20 (2006) 1486–1488. [DOI] [PubMed] [Google Scholar]
- [17].Halder RM, Bridgeman-Shah S, Skin cancer in African Americans, Cancer, 75 (1995) 667–673. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Borron JC, Abdel-Malek Z, Jimenez-Cervantes C, MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation, Pigment Cell Melanoma Res, 27 (2014) 699–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rees JL, Genetics of hair and skin color, Annu Rev Genet, 37 (2003) 67–90. [DOI] [PubMed] [Google Scholar]
- [20].Hennessy A, Oh C, Diffey B, Wakamatsu K, Ito S, Rees J, Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation, Pigment Cell Res, 18 (2005) 220–223. [DOI] [PubMed] [Google Scholar]
- [21].Hadley ME, Dorr RT, Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization, Peptides, 27 (2006) 921–930. [DOI] [PubMed] [Google Scholar]
- [22].Langendonk JG, Balwani M, Anderson KE, Bonkovsky HL, Anstey AV, Bissell DM, Bloomer J, Edwards C, Neumann NJ, Parker C, Phillips JD, Lim HW, Hamzavi I, Deybach JC, Kauppinen R, Rhodes LE, Frank J, Murphy GM, Karstens FPJ, Sijbrands EJG, de Rooij FWM, Lebwohl M, Naik H, Goding CR, Wilson JHP, Desnick RJ, Afamelanotide for Erythropoietic Protoporphyria, N Engl J Med, 373 (2015) 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cousen P, Colver G, Helbling I, Eruptive melanocytic naevi following melanotan injection, Br J Dermatol, 161 (2009) 707–708. [DOI] [PubMed] [Google Scholar]
- [24].Ong S, Bowling J, Melanotan-associated melanoma in situ, Australas J Dermatol, 53 (2012) 301–302. [DOI] [PubMed] [Google Scholar]
- [25].Langan EA, Nie Z, Rhodes LE, Melanotropic peptides: more than just ‘Barbie drugs’ and ‘sun-tan jabs’?, Br J Dermatol, 163 (2010) 451–455. [DOI] [PubMed] [Google Scholar]
- [26].Seamon KB, Padgett W, Daly JW, Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells, Proc Natl Acad Sci U S A, 78 (1981) 3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seamon KB, Daly JW, Forskolin: a unique diterpene activator of cyclic AMP-generating systems, J Cyclic Nucleotide Res, 7 (1981) 201–224. [PubMed] [Google Scholar]
- [28].Kunisada T, Lu SZ, Yoshida H, Nishikawa S, Nishikawa S, Mizoguchi M, Hayashi S, Tyrrell L, Williams DA, Wang X, Longley BJ, Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor, J Exp Med, 187 (1998) 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yamazaki F, Okamoto H, Miyauchi-Hashimoto H, Matsumura Y, Itoh T, Tanaka K, Kunisada T, Horio T, XPA gene-deficient, SCF-transgenic mice with epidermal melanin are resistant to UV-induced carcinogenesis, J Invest Dermatol, 123 (2004) 220–228. [DOI] [PubMed] [Google Scholar]
- [30].Khaled M, Levy C, Fisher DE, Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit, Genes Dev, 24 (2010) 2276–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Horike N, Kumagai A, Shimono Y, Onishi T, Itoh Y, Sasaki T, Kitagawa K, Hatano O, Takagi H, Susumu T, Teraoka H, Kusano K, Nagaoka Y, Kawahara H, Takemori H, Downregulation of SIK2 expression promotes the melanogenic program in mice, Pigment Cell Melanoma Res, 23 (2010) 809–819. [DOI] [PubMed] [Google Scholar]
- [32].Altarejos JY, Montminy M, CREB and the CRTC co-activators: sensors for hormonal and metabolic signals, Nat Rev Mol Cell Biol, 12 (2011) 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Henriksson E, Jones HA, Patel K, Peggie M, Morrice N, Sakamoto K, Goransson O, The AMPK-related kinase SIK2 is regulated by cAMP via phosphorylation at Ser358 in adipocytes, Biochem J, 444 (2012) 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mujahid N, Liang Y, Murakami R, Choi HG, Dobry AS, Wang J, Suita Y, Weng QY, Allouche J, Kemeny LV, Hermann AL, Roider EM, Gray NS, Fisher DE, A UV-Independent Topical Small-Molecule Approach for Melanin Production in Human Skin, Cell Rep, 19 (2017) 2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patel K, Foretz M, Marion A, Campbell DG, Gourlay R, Boudaba N, Tournier E, Titchenell P, Peggie M, Deak M, Wan M, Kaestner KH, Goransson O, Viollet B, Gray NS, Birnbaum MJ, Sutherland C, Sakamoto K, The LKB1-salt-inducible kinase pathway functions as a key gluconeogenic suppressor in the liver, Nat Commun, 5 (2014) 4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hsiao JJ, Fisher DE, The roles of microphthalmia-associated transcription factor and pigmentation in melanoma, Arch Biochem Biophys, 563 (2014) 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Du WQ, Zheng JN, Pei DS, The diverse oncogenic and tumor suppressor roles of salt-inducible kinase (SIK) in cancer, Expert Opin Ther Targets, 20 (2016) 477–485. [DOI] [PubMed] [Google Scholar]
- [38].Esposito ACC, Brianezi G, de Souza NP, Miot LDB, Marques MEA, Miot HA, Exploring pathways for sustained melanogenesis in facial melasma: an immunofluorescence study, Int J Cosmet Sci, 40 (2018) 420–424. [DOI] [PubMed] [Google Scholar]
- [39].Im S, Kim J, On WY, Kang WH, Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma, Br J Dermatol, 146 (2002) 165–167. [DOI] [PubMed] [Google Scholar]
- [40].Miot LD, Miot HA, Polettini J, Silva MG, Marques ME, Morphologic changes and the expression of alpha-melanocyte stimulating hormone and melanocortin-1 receptor in melasma lesions: a comparative study, Am J Dermatopathol, 32 (2010) 676–682. [DOI] [PubMed] [Google Scholar]
- [41].Ortonne JP, Bissett DL, Latest insights into skin hyperpigmentation, J Investig Dermatol Symp Proc, 13 (2008) 10–14. [DOI] [PubMed] [Google Scholar]
- [42].Park JY, Park JH, Kim SJ, Kwon JE, Kang HY, Lee ES, Kim YC, Two histopathological patterns of postinflammatory hyperpigmentation: epidermal and dermal, J Cutan Pathol, 44 (2017) 118–124. [DOI] [PubMed] [Google Scholar]
- [43].Baranska A, Shawket A, Jouve M, Baratin M, Malosse C, Voluzan O, Vu Manh TP, Fiore F, Bajenoff M, Benaroch P, Dalod M, Malissen M, Henri S, Malissen B, Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal, J Exp Med, 215 (2018) 1115–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weiss JS, James WD, Cooper KD, Melanophages in inflammatory skin disease demonstrate the surface phenotype of OKM5+ antigen-presenting cells and activated macrophages, J Am Acad Dermatol, 19 (1988) 633–641. [DOI] [PubMed] [Google Scholar]
- [45].Weismann K, Lorentzen HF, Dermoscopic color perspective, Arch Dermatol, 142 (2006) 1250. [DOI] [PubMed] [Google Scholar]
- [46].Praetorius C, Sturm RA, Steingrimsson E, Sun-induced freckling: ephelides and solar lentigines, Pigment Cell Melanoma Res, 27 (2014) 339–350. [DOI] [PubMed] [Google Scholar]
- [47].Gilchrest BA, Blog FB, Szabo G, Effects of aging and chronic sun exposure on melanocytes in human skin, J Invest Dermatol, 73 (1979) 141–143. [DOI] [PubMed] [Google Scholar]
- [48].Wester RC, Melendres J, Hui X, Cox R, Serranzana S, Zhai H, Quan D, Maibach HI, Human in vivo and in vitro hydroquinone topical bioavailability, metabolism, and disposition, J Toxicol Environ Health A, 54 (1998) 301–317. [DOI] [PubMed] [Google Scholar]
- [49].Palumbo A, d’Ischia M, Misuraca G, Prota G, Mechanism of inhibition of melanogenesis by hydroquinone, Biochim Biophys Acta, 1073 (1991) 85–90. [DOI] [PubMed] [Google Scholar]
- [50].Sakuma K, Ogawa M, Sugibayashi K, Yamada K, Yamamoto K, Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives, Arch Pharm Res, 22 (1999) 335–339. [DOI] [PubMed] [Google Scholar]
- [51].Stratford MR, Ramsden CA, Riley PA, The influence of hydroquinone on tyrosinase kinetics, Bioorg Med Chem, 20 (2012) 4364–4370. [DOI] [PubMed] [Google Scholar]
- [52].Smith CJ, O’Hare KB, Allen JC, Selective cytotoxicity of hydroquinone for melanocyte-derived cells is mediated by tyrosinase activity but independent of melanin content, Pigment Cell Res, 1 (1988) 386–389. [DOI] [PubMed] [Google Scholar]
- [53].Jimbow K, Obata H, Pathak MA, Fitzpatrick TB, Mechanism of depigmentation by hydroquinone, J Invest Dermatol, 62 (1974) 436–449. [DOI] [PubMed] [Google Scholar]
- [54].Gupta AK, Gover MD, Nouri K, Taylor S, The treatment of melasma: a review of clinical trials, J Am Acad Dermatol, 55 (2006) 1048–1065. [DOI] [PubMed] [Google Scholar]
- [55].Hsieh PW, Al-Suwayeh SA, Fang CL, Lin CF, Chen CC, Fang JY, The co-drug of conjugated hydroquinone and azelaic acid to enhance topical skin targeting and decrease penetration through the skin, Eur J Pharm Biopharm, 81 (2012) 369–378. [DOI] [PubMed] [Google Scholar]
- [56].Hsieh PW, Aljuffali IA, Fang CL, Chang SH, Fang JY, Hydroquinone-salicylic acid conjugates as novel anti-melasma actives show superior skin targeting compared to the parent drugs, J Dermatol Sci, 76 (2014) 120–131. [DOI] [PubMed] [Google Scholar]
- [57].Hsieh PW, Chen WY, Aljuffali IA, Chen CC, Fang JY, Co-drug strategy for promoting skin targeting and minimizing the transdermal diffusion of hydroquinone and tranexamic acid, Curr Med Chem, 20 (2013) 4080–4092. [DOI] [PubMed] [Google Scholar]
- [58].Maeda K, Fukuda M, Arbutin: mechanism of its depigmenting action in human melanocyte culture, J Pharmacol Exp Ther, 276 (1996) 765–769. [PubMed] [Google Scholar]
- [59].Chawla S, deLong MA, Visscher MO, Wickett RR, Manga P, Boissy RE, Mechanism of tyrosinase inhibition by deoxyArbutin and its second-generation derivatives, Br J Dermatol, 159 (2008) 1267–1274. [DOI] [PubMed] [Google Scholar]
- [60].Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H, Arbutin increases the pigmentation of cultured human melanocytes through mechanisms other than the induction of tyrosinase activity, Pigment Cell Res, 11 (1998) 12–17. [DOI] [PubMed] [Google Scholar]
- [61].Breathnach AC, Nazzaro-Porro M, Passi S, Zina G, Azelaic acid therapy in disorders of pigmentation, Clin Dermatol, 7 (1989) 106–119. [DOI] [PubMed] [Google Scholar]
- [62].Schallreuter KU, Wood JW, A possible mechanism of action for azelaic acid in the human epidermis, Arch Dermatol Res, 282 (1990) 168–171. [DOI] [PubMed] [Google Scholar]
- [63].Breathnach AS, Melanin hyperpigmentation of skin: melasma, topical treatment with azelaic acid, and other therapies, Cutis, 57 (1996) 36–45. [PubMed] [Google Scholar]
- [64].Verallo-Rowell VM, Verallo V, Graupe K, Lopez-Villafuerte L, Garcia-Lopez M, Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma, Acta Derm Venereol Suppl (Stockh), 143 (1989) 58–61. [PubMed] [Google Scholar]
- [65].Balina LM, Graupe K, The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream, Int J Dermatol, 30 (1991) 893–895. [DOI] [PubMed] [Google Scholar]
- [66].Yun CY, Mi Ko S, Pyo Choi Y, Kim BJ, Lee J, Mun Kim J, Kim JY, Song JY, Kim SH, Hwang BY, Tae Hong J, Han SB, Kim Y, alpha-Viniferin Improves Facial Hyperpigmentation via Accelerating Feedback Termination of cAMP/PKA-Signaled Phosphorylation Circuit in Facultative Melanogenesis, Theranostics, 8 (2018) 2031–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee KT, Kim BJ, Kim JH, Heo MY, Kim HP, Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation, Int J Cosmet Sci, 19 (1997) 291–298. [DOI] [PubMed] [Google Scholar]
- [68].Jo H, Choi M, Sim J, Viji M, Li S, Lee YH, Kim Y, Seo SY, Zhou Y, Lee K, Kim WJ, Hong JT, Lee H, Jung JK, Synthesis and biological evaluation of caffeic acid derivatives as potent inhibitors of alpha-MSH-stimulated melanogenesis, Bioorg Med Chem Lett, 27 (2017) 3374–3377. [DOI] [PubMed] [Google Scholar]
- [69].Yun CY, Hong SD, Lee YH, Lee J, Jung DE, Kim GH, Kim SH, Jung JK, Kim KH, Lee H, Hong JT, Han SB, Kim Y, Nuclear Entry of CRTC1 as Druggable Target of Acquired Pigmentary Disorder, Theranostics, 9 (2019) 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Del Rosario E, Florez-Pollack S, Zapata L Jr., Hernandez K, Tovar-Garza A, Rodrigues M, Hynan LS, Pandya AG, Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma, J Am Acad Dermatol, 78 (2018) 363–369. [DOI] [PubMed] [Google Scholar]
- [71].Lee HC, Thng TG, Goh CL, Oral tranexamic acid (TA) in the treatment of melasma: A retrospective analysis, J Am Acad Dermatol, 75 (2016) 385–392. [DOI] [PubMed] [Google Scholar]
- [72].Lajevardi V, Ghayoumi A, Abedini R, Hosseini H, Goodarzi A, Akbari Z, Hedayat K, Comparison of the therapeutic efficacy and safety of combined oral tranexamic acid and topical hydroquinone 4% treatment vs. topical hydroquinone 4% alone in melasma: a parallel-group, assessor- and analyst-blinded, randomized controlled trial with a short-term follow-up, J Cosmet Dermatol, 16 (2017) 235–242. [DOI] [PubMed] [Google Scholar]
- [73].Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS, Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes, Nature, 454 (2008) 1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Battaini G, Monzani E, Casella L, Santagostini L, Pagliarin R, Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid, J Biol Inorg Chem, 5 (2000) 262–268. [DOI] [PubMed] [Google Scholar]
- [75].Lim JT, Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid, Dermatol Surg, 25 (1999) 282–284. [DOI] [PubMed] [Google Scholar]
- [76].Ellis CN, Weiss JS, Hamilton TA, Headington JT, Zelickson AS, Voorhees JJ, Sustained improvement with prolonged topical tretinoin (retinoic acid) for photoaged skin, J Am Acad Dermatol, 23 (1990) 629–637. [DOI] [PubMed] [Google Scholar]
- [77].Weiss JS, Ellis CN, Headington JT, Tincoff T, Hamilton TA, Voorhees JJ, Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study, JAMA, 259 (1988) 527–532. [PubMed] [Google Scholar]
- [78].Weinstein GD, Nigra TP, Pochi PE, Savin RC, Allan A, Benik K, Jeffes E, Lufrano L, Thorne EG, Topical tretinoin for treatment of photodamaged skin. A multicenter study, Arch Dermatol, 127 (1991) 659–665. [PubMed] [Google Scholar]
- [79].Nguyen NT, Fisher DE, MITF and UV responses in skin: From pigmentation to addiction, Pigment Cell Melanoma Res, 32 (2019) 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]


