Abstract
NFκB signaling and protein trafficking network play important roles in various biological and pathological processes. NIK-and-IKK2-binding protein (NIBP), also known as trafficking protein particle complex 9 (TRAPPC9), is a prototype member of a novel protein family, and has been shown to regulate both NFκB signaling pathway and protein transport/trafficking. NIBP is extensively expressed in the nervous system and plays an important role in regulating neurogenesis and neuronal differentiation. NIBP/TRAPPC9 mutations have been linked to an autosomal recessive intellectual disability (ARID) syndrome, called NIBP Syndrome, which is characterized by non-syndromic ARID along with other symptoms such as obesity, microcephaly, and facial dysmorphia. As more cases of NIBP Syndrome are identified, new light is being shed on the role of NIBP/TRAPPC9 in the central nervous system developments and diseases. NIBP is also involved in the enteric nervous system. This review will highlight the importance of NIBP/TRAPPC9 in central and enteric nervous system diseases, and the established possible mechanisms for developing a potential therapeutic.
Keywords: NIBP, TRAPPC9, NFκB, Neurogenesis, Intellectual Disability, NIBP Syndrome
Introduction
The NIK-and-IKK2-Binding Protein (NIBP) is a protein first discovered in 2005 through a yeast two-hybrid screen that was used to identify molecules involved in the NFκB signaling pathway in the central nervous system (CNS) 1. The C-terminal portion of NIBP interacts with NFκB-inducing kinase (NIK), and NIBP preferentially binds to IKK2 (IKKβ), not IKK1 (IKKα) or NEMO (IKKγ) 1. According to the PANTHER classification system (http://www.pantherdb.org), NIBP is classified as a novel family of proteins and is highly conserved amongst species, especially between humans and mice 2.
Trafficking protein particle II (TRAPPII) is one of three TRAPP complexes (TRAPPI, TRAPPII and TRAPPIII) that act as multimeric guanine nucleotide exchange factors (GEFs) to activate certain GTPases, helping regulate vesicular trafficking between organelles. The yeast Trs120 protein was discovered in 2000 3, and plays an important role in TRAPPII. To identify the subunit of mammalian TRAPPII, Blast analyses showed that NIBP was the closest mammalian homologues of yeast Trs120 4. Further experimental evidence proved that NIBP is a Trs120 ortholog of mammalian TRAPPII 5, thus also called trafficking protein particle complex 9 (TRAPPC9) 6–8.
NIBP/TRAPPC9 Gene Characteristics and Expression Patterns
Human NIBP/TRAPPC9 covers 726.10 kb on the reverse strand of chromosome 8q24.3 9. Full length NIBP/TRAPPC9 consists of 23 exons (Table S1); however, there are 35 distinct introns (34 gt-ag, 1 at-ac) present in the genomic sequence. A total of 17 transcript variants (16 alternatively spliced, 1 unspliced) have been reported in the GenBank database, potentially encoding 13 different protein isoforms (Table S1). NIBP/TRAPPC9 primarily encodes three isoforms of protein in humans: isoform a (1246 amino acids, aa), b (1148 aa), and c (1139 aa). Isoform a represents the most complete transcript, utilizing a start codon in exon 1, and is normally used when referencing coordinates. However, isoform b is actually found to be more highly expressed, which utilizes a downstream start codon in exon 2 during protein translation. According to the GenBank entry 10 for NIBP/TRAPPC9 (NM_031466.8), the extended 5’ coding sequence is not conserved, thus making isoform b the primary isoform expressed in humans. Isoform c uses the same start codon as isoform b but has an alternate splicing pattern resulting in the skipping of exon 5. Exon 5 is the shortest exon of NIBP/TRAPPC9, encompassing only 27 nucleotides; the function of this exon-skipping is unknown. These transcripts are highly conserved, as mouse Nibp/Trappc9 (NM_180662.2) has a complete 22 exon isoform 2 (encodes a 1139 aa protein; exon 5 is skipped), an isoform 1 that uses a downstream start codon in exon 2 (encodes a 960 aa protein), and an isoform 3 that uses the same start codon as isoform 1 and has a truncated C-terminus (encodes a 940 aa protein). Different isoforms in other species can be found in Table S1. Northern blot analysis and other experimental evidence suggest that shorter isoforms of NIBP/TRAPPC9 may also be expressed in human tissues such as the kidney and muscles, but the function of these isoforms is unknown 1. To date, only the larger transcripts have been detected in the human brain 1, 7, 11, 12.
AceView analysis 9 identifies a high level of NIBP/TRAPPC9 mRNA expression, 2.1 times higher than the average gene. Northern blot analysis of human multiple tissues reveals that the mRNA of NIBP/TRAPPC9 is abundant in organs such as the heart, kidney, brain, and skeletal muscle, with lower levels in immune tissues like the spleen and thymus 1. The extensive expression of NIBP/TRAPPC9 mRNA in brain is also confirmed by in situ hybridization of human embryonic brain sections 6. From the developmental transcriptome data for human brains of BrainSpan (http://www.brainspan.org/), we found that the NIBP/TRAPPC9 mRNA shows in the top level in cortical plate, no matter the age of the brain. From the microarray data of the Allen Human Brain Atlas (https://human.brain-map.org/), we also found that NIBP/TRAPPC9 shows strong expression in the cortex and subcortex in 6 donor patients. Immunohistochemical studies demonstrated extensive expression of NIBP protein in brain postmitotic neurons 1, 6, spinal cord motor neurons 1, as well as enteric neurons and enteric neural stem cells 13, all of which suggest specialized functions of NIBP in the nervous system.
The expression of NIBP/TRAPPC9 in the mouse brain was examined by in situ hybridization and immunofluorescent analysis. The level of Nibp/Trappc9 RNA is low in E14 mouse brain, slightly higher in P0 mouse brain, and strong in adult mouse brain 6, indicating accumulation with growth. Confocal microscopy of mouse cortical sections showed that NIBP protein localizes in the cytoplasm, with no localization in the nucleus or any organelles 6. In situ hybridization using Nibp/Trappc9 RNA probe for mouse brain sagittal sections (male C57BL/6J mouse aged 56 days) from the Allen Mouse Brain Atlas data (https://mouse.brain-map.org/) showed that its expression value (0–5) is highest at 1.12 in hippocampal formation region, second highest at 0.87 in cortical subplate region, 0.4–0.5 in isocortex, thalamus and olfactory areas, and less than 0.2 in other regions.
The RNA-seq gene expression profile data across 16 selected tissues shown in NCBI AceView (https://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/), which comes from the Non-Human Primates Reference Transcriptome Resource (http://nhprtr.org/index.html), shows that the expression pattern of NIBP/TRAPPC9 in non-human primates has some differences compared to that in humans. For example, NIBP/TRAPPC9 is abundant in human skeletal muscle but scarce in non-human primate skeletal muscle. Moreover, NIBP/TRAPPC9 is poorly expressed in the spleen, lymph nodes, and thymus of humans, however is abundant in these organs of non-human primates. In other organs and tissues, NIBP/TRAPPC9 showed similar expression between human and non-human primates.
Experimental evidence using anti-NIBP/TRAPPC9 antibody for Western blot demonstrated the presence of single isoform in human fibroblasts 8 and lymphoblasts 6. However, several bands of different sizes of NIBP protein were reported in gut tissues 13 and other cells 14–17. Different sizes of NIBP protein, indicating different isoforms, were also reported in the brain 13. The mechanisms underlying the formation of different NIBP isoforms remain elusive, and better antibodies against different NIBP isoforms are still needed. Additionally, CRISPR-mediated tagging of endogenous NIBP at the N-terminal or C-terminal of different isoforms may provide solid experimental evidence for the existence and function of NIBP isoforms18. To address this in humans, the induced pluripotent stem cell (iPSC)-derived organoids via CRISPR tagging might be an excellent model19, 20.
Important Role of NIBP/TRAPPC9 in NFκB Signaling and Protein Trafficking
Regulation of NFκB signaling pathways
The NFκB protein family are transcription factors that are involved in the regulation of many different cellular pathways such as immune responses, cell proliferation and survival, and even memory, neurogenesis, and synaptic plasticity 13, 21. When NFκB is not activated, it remains in the cytoplasm sequestered by its inhibitor IκB. NFκB is activated if the cell is stimulated, which could be caused by cytokines, antigens, or viruses.
NFκB is activated via three different pathways: classical (canonical), non-classical (non-canonical or alternative), and atypical. The classical pathway can be activated by molecules such as TNFα, IL-1, CD40 ligand, or lymphotoxin-β. In the classical pathway, IKK (composed of 3 subunits: IKK1, IKK2, and regulatory subunit NEMO) is recruited and phosphorylates IκB proteins, leading to the ubiquitination and degradation of IκB via proteasome. With IκB degraded, the NFκB subunit, which classically is a p65 (RelA)/p50 heterodimer, becomes activated and moves from the cytosol to the nucleus to activate transcription. In the non-classical pathway, which can be activated by lymphotoxin-β, CD40 ligand, or B-cell activating factor, NIK is recruited and phosphorylates the IKK complex, which consists of only two IKK1 subunits. The phosphorylated IKK1 then phosphorylates p100 (p52 precursor), causing the transformation of p100 into p52, which forms activated NFκB complex RelB/p52 and moves into the nucleus. Atypical pathways of NFκB activation can be initiated by such causes as UV radiation and oxidative stress. This pathway is atypical because it does not rely on IKK, but the outcome of IκB degradation and NFκB translocation still occurs.
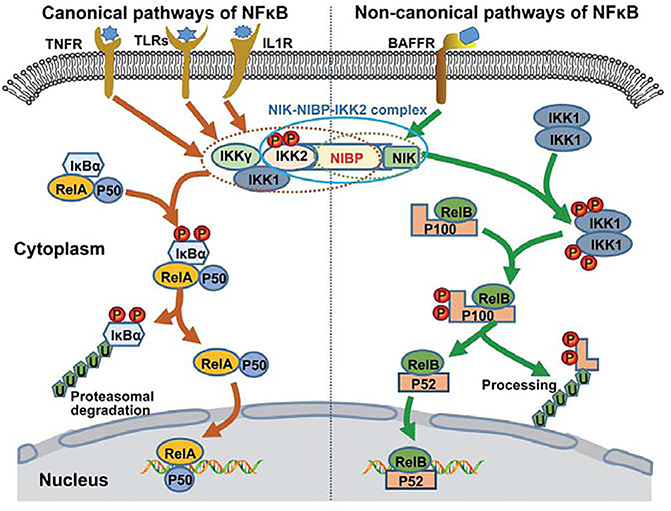
NIBP is involved in both the canonical and non-canonical NFκB activation pathways 1. NIBP directly interacts with IKK2 of the canonical NFκB pathway and NIK of the non-canonical pathway 1, and enhances cytokine-induced IKK2 kinase activity 1, 2. These interactions result in the formation of a unique NIK-NIBP-IKK2 complex, which may bridge the canonical and non-canonical NFκB activation pathways (Figure 1). How NIBP balances the canonical and non-canonical NFκB signaling in various physiological and pathological conditions remains largely unknown. When stimulating NIBP-deficient human fibroblast cells with TNFα (a cytokine that strongly activates the canonical NFκB pathway), there was a defect in the degradation of IκB, leading to less activation of NFκB 8. This indicates that NIBP is required for cytokine-induced canonical NFκB activation in human fibroblast cells 8. The insufficient NFκB activation induced by human NIBP loss-of-function mutation was confirmed by Mochida et al. 2009 and Zahoor et al. 2010 6, 14. These clinical studies validated the role of NIBP as an enhancer for cytokine-stimulated activation of NFκB signaling1, 2, 13, 17, 22, 23.
Figure 1. NIBP/TRAPPC9 function within the NFκB activation pathway.
NFκB can be activated via three main pathways: the classical/canonical, alternative/non-canonical, and the atypical pathways. The classical pathway involves a three subunit IKK complex composed of IKK1, IKK2, and regulatory subunit IKKγ, IκB proteins, and the NFκB p65/p50 heterodimer. The alternative pathway involves NIK, a two-subunit IKK complex composed of two IKK1 subunits, p100, and the NFκB RelB/p52 heterodimer. NIBP binds directly to IKK2 and NIK and may bridge both classical and alternative activation pathways together. NIBP may be essential for NFκB activation and its dysregulation may be involved in the pathogenesis of various NFκB-associated disorders.
Notes. IL1R, interleukin-1 receptor; TLR, toll-like receptor; TNFR, tumor necrosis factor receptor; BAFFR, B-cell activating factor receptor; NIK, NFκB-inducing kinase; IκB, Inhibitor of NFκB; IKK, IκB kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Regulation of protein trafficking
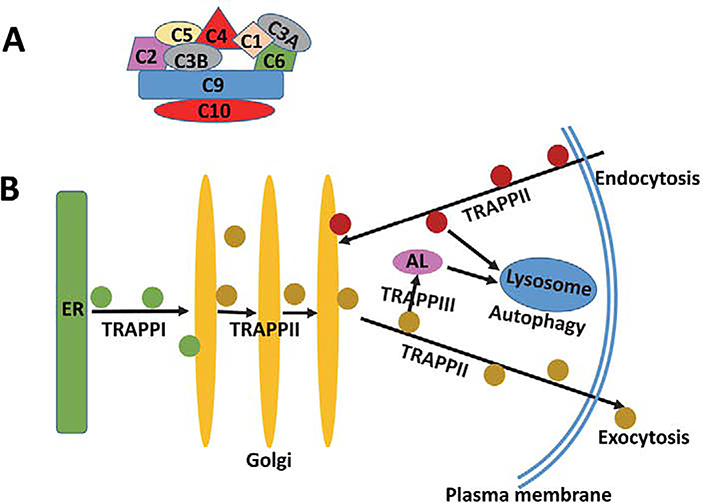
Intracellular protein/membrane trafficking plays a critical role in normal cellular functions and various pathological processes. The proteins and lipids are assembled into vesicles and delivered to their target sites through budding, movement, tethering, docking, and fusion at each organelle. The vesicle or cargo trafficking is mediated mainly by the anterograde secretory/exocytic and the retrograde endocytic transport pathways. The intracellular destinations of the endocytic and exocytic vesicles are determined by the specific coat protein complex (COPI and COPII) that recruit different cargoes. COPI complex coats vesicles transporting proteins from cis-Golgi back to the rough endoplasmic reticulum (ER) and between Golgi compartments. COPII complex transports proteins from ER to Golgi. The retromer complex regulates retrograde endocytic trafficking 24, 25, while TRAPP complex generally regulates the anterograde exocytic/secretory trafficking. However, TRAPPI tethers the ER-derived COPII vesicles 26–29, while TRAPPII binds to COPI coatomer and participates in the anterograde transport from trans-Golgi network (TGN) to membrane 30, 31 and the retrograde trafficking from endosomes to TGN (Figure 2) 32, 33. TRAPPIII regulates the trafficking from endosome to TGN and formation of autophagy 34–38.
Figure 2. Location and function of NIBP/TRAPPC9 during protein trafficking.
A. Schematic of mammalian TRAPPII complex. TRAPPC9 subunit interacts directly with TRAPPC10, TRAPPC2, and TRAPPC6. B. Function of TRAPP complexes. TRAPPI is involved in vesicular transport from ER to Golgi, TRAPPII is involved in trans-Golgi, Golgi-to-plasma membrane, and endosome-to-Golgi transport, and TRAPPIII is involved in formation of autolysosome for autophagy.
Notes. ER, endoplasmic reticulum; AL, autolysosome; TRAPP, trafficking protein particle.
TRAPPII acts as a COPII-vesicle tether by activating Rab1 GTPase and is needed for docking and fusion of the vesicle with the target membrane 39, 40. In addition to Rab1 activation, TRAPPII activates Rab18 17, a key player in regulating the intracellular trafficking and/or dynamics of the cytosolic organelle lipid droplet 41, 42. NIBP/TRAPPC9 is a key player of TRAPPII 4, 5, 40. NIBP/TRAPPC9 is essential for lipid droplet homeostasis via interacting with Rab18 in HEK293T cells and hepatocytes 17. NIBP/TRAPPC9 inactivation by various methods including siRNA depletion and CRISPR/Cas9-mediated deletion induces defective recruitment of Rab18 onto lipid droplet surface, reduces lipolysis and results in aberrantly large lipid droplets 17. The role of NIBP/TRAPPC9 in neuronal lipid droplets remains to be determined 43, 44.
NIBP/TRAPPC9 protein competitively binds to the dynactin subunit, P150Glued, preventing it from binding to Sec23/24, which is needed for the vesicle to move from the ER to the Golgi 15. This binding allows the uncoupling of the COPII-vesicle from dynactin, allowing for the vesicle to start the docking and fusion process. TRAPPC9 protein contains a Tetratricopeptide Repeat (TPR) domain for protein-protein interactions and 2 ASH (ASPM, SPD-2, Hydin) domains to help localize proteins to the Golgi and help with the interaction of proteins to microtubules 12, 45. Recently, the TRAPPII complex has been identified as an important regulator of stress granule maturation during the integrated stress response, recruiting COPII to stress granules, which leads to impaired ER export and Golgi fragmentation, thereby halting secretary functions 46. However, this mechanism is protective to cells, as TRAPPII-depleted cells produce smaller stress granules that cannot properly recruit the signaling proteins RACK1 and Raptor, ultimately making cells less resistant to stress and more prone to apoptosis. Intriguingly, TRAPPII and COPII seem to be recruited only to stress granules in actively proliferating cells in a CDK1/2-dependent manner 46.
NIBP/TRAPPC9 directly binds to TRAPPC2, the mammalian ortholog of Trs20 and part of the core TRAPP complex, and then binds to TRAPPC10, the mammalian ortholog of Trs130 47 to help form the complete TRAPPII complex. NIBP/TRAPPC9 was found to interact with several additional members of TRAPPII in yeast and Cos-7 cells including Bet3 (TRAPPC3) 5, Trs65 (TRAPPC7), etc 48. One difference between yeast and mammalian TRAPPII is that there is no mammalian ortholog of Trs65, so TRAPPC6A and TRAPPC6B are required for proper assembly. The STRING interaction network (http://string-db.org/newstring_cgi/show_network_section.pl) identified 14 proteins interacting with NIBP/TRAPPC9, including TRAPPC2, 3, 4, 8, 10, 12. These interactions suggest that NIBP/TRAPPC9 is a critical member of TRAPPII and essentially regulates TGN function.
The molecular mechanisms underlying protein trafficking regulation by NIBP/TRAPPC9 remain largely unclear. NIBP/TRAPPC9 interacts with Rabin8, which is recruited by Rab11 through TRAPPII to the pericentrasomal vesicles and activates Rab8 to promote ciliogenesis 45, 49, 50. Mutations in the gene brunelleschi (bru), which encodes the Drosophila ortholog of NIBP/TRAPPC9, cause defects in both actomyosin ring constriction and cleavage furrow ingression, indicating the important role of Bru/NIBP/TRAPPC9 in regulating the efficiency of membrane addition to the cleavage furrow, thus promoting cytokinesis in Drosophila male meiotic cells 51. This cytokinetic role results from the genetic interaction of Bru with Rab11 and PI4Kβ in dividing spermatocytes 51–53. Knockdown of bru in circadian neurons resulted in increased locomotor activity and reduced sleep, suggesting the neuronal function of Bru/NIBP/TRAPPC9 in regulating circadian rhythm 54. Our preliminary studies identified Myo5B as a new partner for NIBP (unpublished data). Myo5B associates with recycling endosomes and triggers rapid spine recruitment of endosomes and local exocytosis in spines upon NMDA receptor-mediated Ca2+ influx 55. Disruption of Myo5B or its interaction with the adaptor Rab11-FIP2 abolishes exocytosis from recycling endosomes and prevents both AMPAR insertion and spine growth 56, 57. Altogether, NIBP/TRAPPC9 may play important role in meiotic cytokinesis, neuronal polarization and synaptic plasticity via regulating intracellular protein trafficking.
NIBP/TRAPPC9 Loss-of-function Causes Central Nervous System Diseases
NIBP syndrome
A recent review by Mbimba et al. has briefly summarized the basic information of NIBP/TRAPPC9 and its association with various human diseases 58. Here, we focus primarily on the correlation between NIBP/TRAPPC9 mutations and nervous system diseases. Clinically, genetic recessive mutations leading to function-loss of NIBP/TRAPPC9 are associated with non-syndromic autosomal recessive intellectual disability (NS-ARID). The common phenotypes seen within these patients have led clinicians to designate NIBP syndrome. The human disease database MalaCards (https://www.malacards.org/) assigned it as “Intellectual Disability-Obesity-Brain Malformations-Facial Dysmorphism Syndrome”. It is also referred to as “Mental Retardation, autosomal recessive 13” (MIM #613192) by the Mendelian Inheritance in Man online database 59.
ID affects about 1–3% of the population worldwide and is clinically assessed through the patient’s IQ score below 70 and at least two behavioral issues such as delayed language, impaired social skills, or impaired self-help skills 7, 60, 61. About 25% of ID genetic causes are due to autosomal recessive mutations, making it a more common cause than X-linked mutations 8, 47, 62. The actual number of autosomal genes linked to ID is estimated to be over 2500, with a majority of these being autosomal recessive 47, 54, 63, 64. This estimate has been further validated by recent large-scale studies identifying 903 ARID candidate genes 65. It is important to note that genetic heterogeneity makes it difficult to identify causative genes as patients have multiple mutations of unknown effects. With the rise of next generation sequencing, the number of patients diagnosed will likely increase.
Of these reported genes linked to NS-ARID, NIBP/TRAPPC9 is one of the most prevalent. To date (04/2020), there are at least 26 families worldwide, with a total of 55 patients (including 5 cases in the DECIPHER database 66 that have not been included in any published clinical reports to date) (Table 1, Table S2), which have reported homozygous or compound heterozygous mutations in the NIBP/TRAPPC9 gene. Most of the patients with NIBP/TRAPPC9 mutations are geographically located in countries where consanguineous families are common, such as Middle Eastern countries, making any autosomal recessive mutations within these families more visible. There are also reports of 4 non-consanguineous families with a total of 6 patients 62, 67.
Table 1.
Summary of patients with genetic mutations affecting TRAPPC9 and exhibiting NS-ARID.
| Mutation (GRCh37/hg19) | Mutation Type | Affected Families | Cases, Sex | Consanguineous | Major symptoms |
|---|---|---|---|---|---|
| p.Leu178Pro (c.533T>C) | Nonsense homozygous mutation in exon 2 | -Moroccan | 1 M, 1 F 82 | Likely | Severe ID, congenital microcephaly, severe language and motor delays, MRI findings (white matter abnormalities), hyperkinesia |
| p.Trp190Argfs*95 (c.568_574delTGGCCAC) | Homozygous 7 nucleotide deletion in exon 2 | -Maltese | 1 M 75 | No | ID, microcephaly, absence of speech, facial dysmorphia, MRI findings, ASD, stereotypical movements |
| Splice site defect (c.1024+1G>T) | Homozygous skip of exon 3 or exons 3 and 4 | -Pakistani | 3 F 129 | Yes | Moderate ID, microcephaly, speech and motor delay |
| p.Arg475* (c.1423C>T) | Nonsense homozygous mutation in exon 7 | -Pakistani -Pakistani -Syrian -Israeli Arab -Egyptian |
3 M 12 1 M, 6 F 7 3 M, 3 F 130 3 F 6 1 F 131 (Decipher ID 296553) |
Yes Yes Yes Yes Yes |
ID, microcephaly, speech delay Microcephaly, speech and motor delays, mild obesity, MRI findings Severe ID, moderate-severe microcephaly, severe motor and speech delay, hypotonia, severe growth retardation, stereotypic movements Severe ID, microcephaly, speech delay, MRI findings Moderate ID, microcephaly, speech and motor delay, MRI findings, facial dysmorphia, stereotypical movements |
| p.Arg500Cys (c.1498C>T) | Missense homozygous mutation in exon 8 | n/a | 1 M (unpublished; Decipher ID 262147) | n/a | Global developmental delay, brachycephaly, delayed speech developmental, mild facial dysmorphia |
| p.Arg570* (c.1708C>T) | Nonsense homozygous mutation in exon 9 | -Tunisian -Tunisian |
3 M 8 1 M, 2 F 67 |
Yes Yes |
Moderate-Severe ID, speech delay, mild microcephaly, truncal obesity, mild facial dysmorphia, MRI findings Severe ID, microcephaly, obesity, facial dysmorphia |
| p.Glu689* (c.2065G>T) | Nonsense homozygous mutation in exon 11 | -Pakistani | 2 M, 1 F 12 | Yes | Severe ID, microcephaly, speech and motor delay |
| p.Gly753Glufs*5 (c.2258del) | Nonsense homozygous mutation in exon 13 | n/a | 2 F (unpublished; Decipher ID 284294 and 284295) | n/a | Severe ID, congenital microcephaly, absence of speech, mild facial dysmorphia, stereotypic movements |
| p.Leu772Trpfs*7 (c.2311-2314delTGTT) | Homozygous out of frame 4 nucleotide deletion in exon 14 | -Iranian | 3 M 7 | Yes | ID, microcephaly, absence of speech, obesity |
| p.Arg929* (c.2785C>T) | Nonsense homozygous mutation in exon 19 | n/a | 1 M 132 | Yes | ID, microcephaly, global developmental delay, abnormal gait, facial dysmorphia, MRI findings (including reduced myelination) |
| p.Thr951Tyrfs*17 (c.2851-2A>C) | Homozygous splice variant causing skip of exon 18 leading to frameshift | -Italian | 2 F 62 | No | Severe ID, hypotonia, seizures, microcephaly, facial dysmorphia, speech and motor delay, MRI findings (white matter abnormalities), obesity |
| p.Arg1072* (c.3214C>T) | Nonsense homozygous mutation in exon 20 | -Indian | 1 F 133 | Yes | ID, primary microcephaly, Moderate-Severe global developmental delay, truncal obesity, MRI findings, stereotypic movements |
| 8q24.3 microdeletion | Homozygous 141 kb deletion in subtelomeric region of 8q24.3 deleting only TRAPPC9 | -Filipino | 1 F 11 | Yes | Severe ID, microcephaly, MRI findings, facial dysmorphia, severe motor and speech delay, hypotonia, obesity |
| Intragenic tandem duplication | Homozygous 115 kb intragenic tandem duplication | -Algerian | 1 F 67 (Decipher ID 349431) | Yes | Moderate-Severe ID, microcephaly, MRI findings, facial dysmorphia, obesity, stereotypic movements |
| p.Trp190Argfs*95 (c.568_574delTGGCCAC) and duplication | Maternally inherited 7 nucleotide deletion in exon 2 and paternally inherited 119 kb in-frame intragenic duplication | -Italian | 1 F 67 (Decipher ID 314942) | No | Moderate-Severe ID, microcephaly, MRI findings, facial dysmorphia, obesity, stereotypic movements |
| p.Arg712* (c.2134C>T) and deletion | Paternally inherited heterozygous nonsense variant in exon 12 and maternally inherited 189 kb intragenic deletion | -French | 1 F 67 | No | Moderate-Severe ID, microcephaly, MRI findings, facial dysmorphia, obesity, stereotypic movements |
| p.His806Profs*9 and splice site defect (c.2415_2416insC, c.3349+1G>A) | Compound heterozygous mutations in exon 15 (nonsense) and intron 21 (splice variant) | -Thai | 1 M 74 1 F 74 |
No No |
ID, developmental delay, microcephaly autism spectrum disorder (ASD), cleft lip |
| p.Arg475* and splice site defect (c.1423C>T, c.3350-2A>G) | Compound heterozygous mutations in exon 7 (nonsense, maternal) and intron 21 (splice variant, paternal) | -European | 1 M 134 | Yes | ID, global developmental delay, speech and motor delays, microcephaly, MRI findings including myelination defects, facial dysmorphia |
| Compound splice site defects (c.2148+1G>A, c.2851-1G>C) | Compound heterozygous mutations in intron 12 (maternal) and intron 17 (paternal) | n/a | 1 F (unpublished; Decipher ID 271665) | n/a | Severe global developmental delay, microcephaly childhood-onset truncal obesity, facial dysmorphia, stereotypic movements |
| p.Arg570* and p.Arg500Cys (c.1708C>T, c.1498C>T) | Compound heterozygous mutations in exon 9 (nonsense, maternal) and exon 8 (missense, paternal) | n/a | 1 M (unpublished; Decipher ID 277516) | n/a | Global developmental delay, absence of speech, facial dysmorphia |
| p.Ser569Pro, p.Arg570Profs*80; p.Asn758Thrfs*22 (c.1705C>T, c.1708dupC, c.2273delA) | Compound heterozygous nonsense mutations in exon 9 (maternal) and exon 13 (paternal) | -Chinese | 1 M 135 | n/a | ID, language delay, abnormal MRI findings including dysplasia of corpus callosum |
Note: To date, 26 families with a total of 55 patients have been identified, with most being from consanguineous families. Clinical symptoms seen in all patients include moderate to severe intellectual disability, speech disorder, postnatal microcephaly, dysmorphic facial features, obesity, hypotonia, and brain white matter abnormalities.
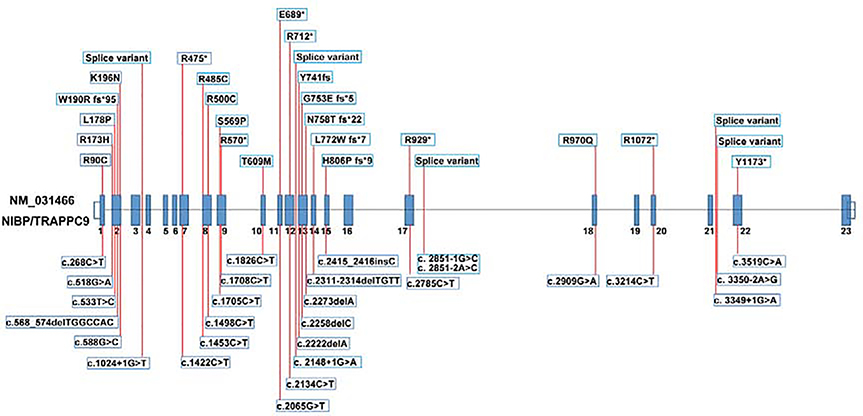
There are various types of mutations that occur within the NIBP/TRAPPC9 gene (Table 1 and Table S2). A large majority of these are the truncating nonsense or frameshift mutations, though deleterious missense and splice site mutations have also been reported. In all instances, these recessive mutations lead to the translation of non-functional NIBP/TRAPPC9 proteins (Figure 3). As both alleles are affected either via homozygous or compound heterozygous mutations, little to no functional protein is produced, resulting in NIBP/TRAPPC9 deficiency. Patients with NIBP syndrome exhibit some, if not all, of the following common phenotypical features: moderate to severe ID, speech disorder, postnatal microcephaly, dysmorphic facial features (round face, straight eyebrows, deep set eyes, short philtrum, and thin upper lips), obesity, hypotonia, and brain abnormalities (thin corpus collosum, reduced white matter) 12, 47, 62.
Figure 3. NIBP/TRAPPC9 gene mutations result in the translation of a truncated, non-functional protein.
Various genetic mutations within the NIBP/TRAPPC9 gene have been discovered and affect different exons within the gene. Most genetic mutations reported in this gene lead to a premature stop codon, which results in a truncated NIBP/TRAPPC9 protein. This mutated protein is non-functional and has decreased expression within NIBP Syndrome patients.
Other NIBP/TRAPPC9-Associated CNS Disorders
While homozygous or compound heterozygous mutations are correlated with the phenotypes associated with NIBP syndrome, a number of heterozygous mutations or copy number variants have been linked to various neurodevelopmental disorders (Table S3). The Simons Foundation Autism Research Initiative (SFARI) Gene database classifies TRAPPC9 as a strong candidate autism spectrum disorder (ASD) risk gene 68. A number of studies have identified heterozygous NIBP/TRAPPC9 mutations in patients with ASD that are considered likely damaging 69–73. In agreement with this, many NIBP syndrome patients have been reported to have ASD-related features, such as stereotyped/repetitive movements or other behavioral disorders (Table 1). It is also of note that at least two NIBP syndrome patients were originally diagnosed with ASD prior to genomic analysis 74, 75. One recent report on a patient with a frameshift mutation (c.2415_2416insC, p.His806Profs*9) and a splice site mutation (c.3349C1G>A) showed autistic features and cleft lip, broadening the spectrum of clinical manifestations of TRAPPC9 74. It is possible that deficiency of NIBP/TRAPPC9 may be a risk factor for ASD, which would be further exacerbated in cases with homozygous or compound heterozygous mutations. However, as some healthy individuals in these families have heterozygous mutations and no apparent ASD phenotype, the role of NIBP/TRAPPC9 is likely much more complex.
NIBP/TRAPPC9 mutations and copy number variants have also been linked to schizophrenia 76, 77. These findings are significant as there is a surprisingly high overlap in risk genes for ASD and schizophrenia 77, 78, as well as overlap for genes related to ID 79. Recently NIBP/TRAPPC9 was identified as a potential attention deficit hyperactivity disorder (ADHD) risk gene based on data from large gene studies, which was corroborated using a knockdown Drosophila melanogaster model 54. A probably damaging heterozygous missense mutation of NIBP/TRAPPC9 was discovered in a patient with normosmic hypogonadotropic hypogonadism (nHH)/Kallmann syndrome (KS) 80. While features related to this disorder have not been reported in any NIBP syndrome patients, it does present a potential role for NIBP/TRAPPC9 in normal hypothalamus function, which may explain the obesity phenotype seen in >60% patients with NIBP syndrome. However, this patient also had a second mutation on another gene (PDE3A) of unknown significance that may have contributed to the observed phenotype 80. NIBP/TRAPPC9 mutations are significantly associated with the prevalence of intracerebral hemorrhage 81. A study has reported 2 patients that demonstrated prenatal microcephaly 82, however these patients also had a homozygous mutations in MCPH1 (Microcephaly Primary Hereditary 1) gene that may play a role in this phenotype.
NIBP-NFκB Pathway Involved in CNS Disorders
The causal correlation of NIBP/NFκB recessive mutations with intellectual dysfunction in NIBP syndrome remains elusive. NFκB signaling plays a double-edged sword role after CNS injuries or diseases 83–87. In most cases, activation of NFκB signaling in immune cells, microglia/macrophage, and astrocytes produces overwhelming inflammatory mediators and neurotoxic molecules, leading to neurodestruction 86, 87. For example, excess neuroinflammatory responses induced by NFκB overactivation is involved in the development of Alzheimer’s disease, Parkinson’s disease, and AIDS dementia 8, 88–90. The expression and function of NIBP in these chronic neurodegenerative diseases have not yet been investigated, although some microarray data was reported in GEO profiles (www.ncbi.nlm.nih.gov/geoprofiles/?term=NIBP). Considering NIBP is an enhancer of cytokine-induced NFκB activation, we speculate that NIBP may be a potential therapeutic target for these neurodegenerative diseases. However, NFκB signaling in neural stem cells, neuron and oligodendrocytes is crucial in regulating neurogenesis, neuronal survival, synaptogenesis, neural plasticity, axonal myelination, learning and memory 84, 86, 87, 91–97. Thus, disruption of NFκB signaling may contribute to the development of neurodevelopmental disorders. NIBP might be a critical regulator of NFκB signaling during the normal neurodevelopment.
NFκB activation is involved in synaptic plasticity, such as via long-term potentiation (LTP) 1, 13. Impaired NFκB activation inhibited LTP in the hippocampus and amygdala 98, 99 as well as reduced synapse and dendritic spine number and altered spine morphology 12, 94. Deletion of NFκB in the forebrain demonstrates impaired spatial memory formation, synaptic transmission, and plasticity 100. Since NIBP bridges the canonical and non-canonical NFκB pathways, decreased NIBP expression significantly impairs NFκB activation within neurons. NIBP is highly expressed in neurons of the human brain, and patients with NIBP Syndrome have been shown to have axon connectivity defects 6, 12, 80. We speculate that loss of NIBP resulting in diminished NFκB activation may lead to decreased gene expression needed for synaptic activity, which could result in the dendritic spine morphological and quantitative changes and lead to impairments in LTP, affecting learning and memory, and contribute to the ID phenotype seen in NIBP Syndrome.
NFκB activation leads to anti-apoptotic gene expression, allowing neuronal survival 1, 86, 101. With the loss of NIBP, NFκB activation in neurons is decreased, which leads to lowered expression of anti-apoptotic genes and, as a result, increased neuronal death. NIBP is expressed in postmitotic neurons 6, therefore, a loss of NIBP may lead to increased postmitotic neuronal death, which may explain why microcephaly in patients with NIBP syndrome is not present at birth, but is developed postnatally, which is called secondary microcephaly 12.
Dysregulation of neurogenesis during the embryonic or neonatal stages has been shown to contribute to ID, epilepsy, autism, and some genetic disorders such as Fragile X and Down’s Syndrome 102–104. NFκB is shown to be involved in regulation of both embryonic and adult neurogenesis, which involves the proliferation, migration, and differentiation of neural stem cells (NSCs) 11, 62, 102, 105, 106. In order to initiate the early differentiation of NSC, TNFα-induced NFκB activation is required, which downregulates CCAAT/enhancer binding protein beta (C/EBPβ) 95, 102 required for NSC self-renewal. When NFκB activation is blocked, asymmetric cell division of NSC is inhibited and NSC self-renewal is maintained, with the NSC remaining undifferentiated 105. Impaired neurogenesis has been implicated in the pathogenesis of a wide range of disorders, and therefore is hypothesized to be associated with intellectual disability that is seen in NIBP syndrome.
NFκB regulation of neurogenesis can also be seen as a double-edged sword, with transient NFκB activation regarded as beneficial in neural repair and daily maintenance of neurons while chronic, overactivation of NFκB could lead to a depleted NSC pool and insufficient neurogenesis 102, 107. In this regard, inhibiting NIBP may be a key to help attenuate NFκB overactivation and help to slow or even treat the implicated neurological deficits. However, caution must be taken since a reduced level of NFκB activation could also lead to insufficient neurogenesis, as seen in NIBP knockdowns, which prevents nerve growth factor (NGF)-induced neuronal differentiation via decreased activation of NFκB and less suppression of C/EBPβ 1, 100, 102, 106. NIBP may regulate white matter development 100, 106, 108. Knockdown of NIBP prevents NFκB-regulated axon and dendritic growth, branching, and cell differentiation 1. In patients with NIBP syndrome, little NIBP expression 6, 8 may result in reduced NFκB-mediated axon growth and less CNS myelination91, leading to the characteristic white matter hypoplasia seen in these patients.
NIBP/TRAPPC9-Mediated Protein Trafficking in CNS Disorders
Mutations in TRAPP subunits are thought to lead to a build-up of non-functional proteins, which may impair vesicular trafficking, a common biologic defect seen in neurologic disorders 48, 109. Mutations in other genes involved in vesicle trafficking, such as Vacuolar Protein Sorting 13 Homolog B (VPS13B), GDP Dissociation Inhibitor 1 (GDI1), and Tuberous Sclerosis Complex 2 (TSC2) have been associated with NS-ARID 12, 110. Mutations of TRAPP proteins have previously been reported to result in neurologic disorders, which recently have been collectively termed “TRAPPopathies” 111. For example, mutation of TRAPPC2 causes X-linked spondyloepiphyseal dysplasia tarda (X-linked SEDT) 16, 112, 113. Homozygous truncating mutations on TRAPPC6B are associated with microcephaly, epilepsy, and NS-ARID 114–116. A homozygous mutation in TRAPPC6A is linked to ID 114, and a separate TRAPPC6A mutation is associated with Alzheimer’s disease 117. Mutations in TRAPPC11 result in neuromuscular and developmental phenotypes 118–120. A TRAPPC12 mutation is identified in progressive childhood encephalopathy 121. Very recently, a homozygous splice variant of TRAPPC4 is linked to a novel neurodevelopmental disorder marked by severe ID, microcephaly, early-onset epilepsy, and spastic quadriparesis 122. Since NIBP/TRAPPC9 is a part of a TRAPPII complex, mutations or impairments in expression lead to impairments in TRAPPII complex formation and trafficking 16, 123. This altered trafficking seen in NIBP/TRAPPC9 mutations may be associated with TRAPPC9-associated intellectual disability and neural development 16, 47, suggesting that the trafficking complex TRAPPII may be compromised in NIBP syndrome patients.
NIBP/TRAPPC9 Contribution in Enteric Nervous System Diseases
NIBP may also have a role within the enteric nervous system (ENS), which consists of intrinsic neurons and glial cells and is involved in regulating gut motility, secretion, absorption, and mucosal secretion. In mice, NIBP is expressed in enteric neurons and may be expressed in enteric neural stem cells 13, but is not present in glial cells, colonic smooth muscle cells, or interstitial cells of Cajal 13. The highest NIBP expression within the ENS is within the myenteric plexus, with different levels of expression in different subpopulations of enteric neurons 13. This may indicate that NIBP is being trafficked within these neurons or possibly indicates posttranslational modification of NIBP. Future research is needed to get a better understanding of why the different levels of expression exist within these subpopulations. One hypothesized role of NIBP in the ENS is that it regulates the plasticity of the enteric neurons 13. ENS plasticity, like CNS plasticity, is the strengthening of neuronal connectivity and is believed to be essential for adapting to changes within the environment the nerves are located in. ENS structural and functional changes are a consequence of gut inflammation and can lead to dysfunctional ENS plasticity, which is associated with inflammatory bowel diseases 13, 124. Experiments using an enteric neural cell line show that a knockdown of NIBP leads to inhibited TNFα-induced NFκB activation and decreased enteric neuronal differentiation, whereas NIBP overexpression promoted NFκB activation and ENS differentiation 13. Similar to its role in the CNS, NIBP and NFκB may also be involved in neurogenesis within the ENS125, 126, and decreased expression of NIBP and subsequent inhibition of NFκB activation within these neuronal cells may be associated with inflammation-mediated gastrointestinal disorders. Future research into the function and mechanisms of NIBP within enteric neurons and enteric NSCs is needed for further understanding and treatment of inflammatory bowel diseases and possibly other ENS-related disorders such as Hirschsprung’s Disease, in which NSC transplant is currently being investigated as a treatment option 127, 128.
Conclusion and Future Directions
The current understanding of NIBP/TRAPPC9 demonstrates that this protein has both physiologic and pathologic roles and is involved in CNS and ENS. NIBP can potentiate NFκB activation within cells, being beneficial within neuronal cells by inducing neurogenesis, neuronal cell growth, branching, and differentiation, as well as promoting synaptic plasticity, which is associated with learning and memory in the CNS and maintaining gut homeostasis and enteric neuron differentiation within the ENS. NIBP/TRAPPC9 is also a subunit in mammalian TRAPPII complex that is essential for trans-Golgi vesicular trafficking important in proper neuronal development.
Decreased expression or functional loss of NIBP/TRAPPC9 in the CNS leads to intellectual disability-obesity-brain malformations-facial dysmorphism syndrome (NIBP syndrome). Mutations of NIBP/TRAPPC9 should be screened in patients exhibiting similar phenotypic characteristics as seen in NIBP syndrome, especially if they come from a consanguineous family background or if an autosomal recessive mutation is suspected.
Nevertheless, several outstanding questions regarding NIBP/TRAPPC9 and nervous system diseases remain. Whether and how NIBP bridges the classical and non-classical NFκB pathways needs solid experimental evidence. The molecular mechanisms underlying various phenotypic changes such as obesity in NIBP syndrome or those seen in NIBP deficient animal models needs to be explored for potential therapeutic development. How NIBP/TRAPPC9 regulates protein trafficking and whether cell type-dependent difference exists in these regulations also needs more investigation. This protein trafficking is critical for the pathogenesis of diseases like Alzheimer’s disease (AD), where trafficking of amyloid precursor protein (APP) and related secretases, in particular the β-site APP cleaving enzyme 1 (BACE1), is essential. As part of TRAPPII, whether and how NIBP/TRAPPC9 regulates APP trafficking and influences AD pathogenesis remains to be discovered. Another area of investigation is whether there are any changes in neurocytological behavior, such as cell structure, axon and cell connection, in NIBP/TRAPPC9 related diseases. Future research is needed to understand the roles and mechanism of NIBP/TRAPPC9 in central and enteric neuropathy diseases.
Supplementary Material
Acknowledgement
This work was supported by the National Institute of Health R01-MH101041, R01-DK075964 and R21RR032123 (WH). The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. All authors declare that they have no conflict of interest. No English writing service was sought for this manuscript.
Abbreviation
- AD
Alzheimer’s disease
- ADHD
attention deficit hyperactivity disorder
- AL
autolysosome
- APP
amyloid precursor protein
- ASD
autism spectrum disorder
- BACE1
β-site APP cleaving enzyme 1
- BAFFR
B-cell activating factor receptor
- C/EBPβ
CCAAT/enhancer binding protein beta
- CNS
central nervous system
- COP
coat protein complex
- ENS
enteric nervous system
- ER
endoplasmic reticulum
- GDI1
GDP dissociation inhibitor 11
- GEFs
guanine nucleotide exchange factors
- ID
intellectual disability
- IκB
inhibitor of NFκB
- IKK2
IκB kinase 2
- IL1R
interleukin-1 receptor
- KS
Kallmann syndrome
- LTP
long-term potentiation
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NGF
nerve growth factor
- nHH
normosmic hypogonadotropic hypogonadism
- NIK
NFκB-inducing kinase
- NIBP
NIK-and-IKK2-binding protein
- NS-ARID
non-syndromic autosomal recessive intellectual disability
- NSCs
neural stem cells
- SFARI
Simons Foundation Autism Research Initiative
- TGN
trans-Golgi network
- TLR
toll-like receptor
- TNFR
tumor necrosis factor receptor
- TPR
Tetratricopeptide Repeat
- TRAPP
trafficking protein particle
- TRAPPC9
trafficking protein particle complex 9
- TSC2
tuberous sclerosis complex 2
- VPS13B
vacuolar protein sorting 13 homolog B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hu WH, Pendergast JS, Mo XM, et al. NIBP, a novel NIK and IKK(beta)-binding protein that enhances NF-(kappa)B activation. The Journal of biological chemistry. 2005;280:29233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang Y, Liu S, Wang H, et al. Elevated NIBP/TRAPPC9 mediates tumorigenesis of cancer cells through NFkappaB signaling. Oncotarget. 2015;6:6160–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sacher M, Barrowman J, Schieltz D, Yates JR, Ferro-Novick S. Identification and characterization of five new subunits of TRAPP. Eur J Cell Biol. 2000;79:71–80. [DOI] [PubMed] [Google Scholar]
- [4].Cox R, Chen SH, Yoo E, Segev N. Conservation of the TRAPPII-specific subunits of a Ypt/Rab exchanger complex. BMC Evol Biol. 2007;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kümmel D, Oeckinghaus A, Wang C, Krappmann D, Heinemann U. Distinct isocomplexes of the TRAPP trafficking factor coexist inside human cells. FEBS Lett. 2008;582:3729–33. [DOI] [PubMed] [Google Scholar]
- [6].Mochida GH, Mahajnah M, Hill AD, et al. A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly. Am J Hum Genet. 2009;85:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mir A, Kaufman L, Noor A, et al. Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-beta-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am J Hum Genet. 2009;85:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Philippe O, Rio M, Carioux A, et al. Combination of linkage mapping and microarray-expression analysis identifies NF-kappaB signaling defect as a cause of autosomal-recessive mental retardation. Am J Hum Genet. 2009;85:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7 Suppl 1:S12 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Benson DA, Cavanaugh M, Clark K, et al. GenBank. Nucleic Acids Res. 2017;45:D37–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koifman A, Feigenbaum A, Bi W, et al. A homozygous deletion of 8q24.3 including the NIBP gene associated with severe developmental delay, dysgenesis of the corpus callosum, and dysmorphic facial features. Am J Med Genet A. 2010;152A:1268–72. [DOI] [PubMed] [Google Scholar]
- [12].Abbasi AA, Blaesius K, Hu H, et al. Identification of a novel homozygous TRAPPC9 gene mutation causing non-syndromic intellectual disability, speech disorder, and secondary microcephaly. Am J Med Genet B Neuropsychiatr Genet. 2017;174:839–45. [DOI] [PubMed] [Google Scholar]
- [13].Zhang Y, Bitner D, Pontes Filho AA, et al. Expression and function of NIK- and IKK2-binding protein (NIBP) in mouse enteric nervous system. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zahoor MA, Yamane D, Mohamed YM, et al. Bovine viral diarrhea virus non-structural protein 5A interacts with NIK- and IKKbeta-binding protein. J Gen Virol. 2010;91:1939–48. [DOI] [PubMed] [Google Scholar]
- [15].Zong M, Satoh A, Yu MK, et al. TRAPPC9 mediates the interaction between p150 and COPII vesicles at the target membrane. PLoS One. 2012;7:e29995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zong M, Wu XG, Chan CW, et al. The adaptor function of TRAPPC2 in mammalian TRAPPs explains TRAPPC2-associated SEDT and TRAPPC9-associated congenital intellectual disability. PLoS One. 2011;6:e23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qin M, Zhang J, Xu C, et al. Knockdown of NIK and IKKbeta-Binding Protein (NIBP) Reduces Colorectal Cancer Metastasis through Down-Regulation of the Canonical NF-kappaBeta Signaling Pathway and Suppression of MAPK Signaling Mediated through ERK and JNK. PLoS One. 2017;12:e0170595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao Y, Hisey E, Bradshaw TWA, et al. Plug-and-Play Protein Modification Using Homology-Independent Universal Genome Engineering. Neuron. 2019;103:583–97 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Latour YL, Yoon R, Thomas SE, et al. Human GLB1 knockout cerebral organoids: A model system for testing AAV9-mediated GLB1 gene therapy for reducing GM1 ganglioside storage in GM1 gangliosidosis. Mol Genet Metab Rep. 2019;21:100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Allende ML, Cook EK, Larman BC, et al. Cerebral organoids derived from Sandhoff disease-induced pluripotent stem cells exhibit impaired neurodifferentiation. J Lipid Res. 2018;59:550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–8. [DOI] [PubMed] [Google Scholar]
- [22].Fu ZH, Liu SQ, Qin MB, et al. NIK and IKKbetabinding protein contributes to gastric cancer chemoresistance by promoting epithelialmesenchymal transition through the NFkappaB signaling pathway. Oncology reports. 2018;39:2721–30. [DOI] [PubMed] [Google Scholar]
- [23].Qin M, Liu S, Li A, et al. NIK- and IKKbeta-binding protein promotes colon cancer metastasis by activating the classical NF-kappaB pathway and MMPs. Tumour Biol. 2016;37:5979–90. [DOI] [PubMed] [Google Scholar]
- [24].Reitz C The role of the retromer complex in aging-related neurodegeneration: a molecular and genomic review. Mol Genet Genomics. 2015;290:413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vardarajan BN, Bruesegem SY, Harbour ME, et al. Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiology of aging. 2012;33:2231 e15–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu S, Liang Y. A trapper keeper for TRAPP, its structures and functions. Cell Mol Life Sci. 2012;69:3933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lord C, Bhandari D, Menon S, et al. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature. 2011;473:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haas AK, Barr FA. COP sets TRAPP for vesicles. Dev Cell. 2007;12:326–7. [DOI] [PubMed] [Google Scholar]
- [29].Sacher M, Barrowman J, Wang W, et al. TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Molecular cell. 2001;7:433–42. [DOI] [PubMed] [Google Scholar]
- [30].Kim JJ, Lipatova Z, Segev N. TRAPP Complexes in Secretion and Autophagy. Front Cell Dev Biol. 2016;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pinar M, Arst HN, Jr, Pantazopoulou A, et al. TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11. Proc Natl Acad Sci U S A. 2015;112:4346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cai H, Zhang Y, Pypaert M, Walker L, Ferro-Novick S. Mutants in trs120 disrupt traffic from the early endosome to the late Golgi. The Journal of cell biology. 2005;171:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lipatova Z, Segev N. Ypt/Rab GTPases and their TRAPP GEFs at the Golgi. FEBS Lett. 2019;593:2488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lamb CA, Nuhlen S, Judith D, et al. TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J. 2016;35:281–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shirahama-Noda K, Kira S, Yoshimori T, Noda T. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci. 2013;126:4963–73. [DOI] [PubMed] [Google Scholar]
- [36].Thomas LL, Joiner AMN, Fromme JC. The TRAPPIII complex activates the GTPase Ypt1 (Rab1) in the secretory pathway. The Journal of cell biology. 2018;217:283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramirez-Peinado S, Ignashkova TI, van Raam BJ, et al. TRAPPC13 modulates autophagy and the response to Golgi stress. J Cell Sci. 2017;130:2251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Riedel F, Galindo A, Muschalik N, Munro S. The two TRAPP complexes of metazoans have distinct roles and act on different Rab GTPases. The Journal of cell biology. 2018;217:601–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yamasaki A, Menon S, Yu S, et al. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol. 2010;11:759–63. [DOI] [PubMed] [Google Scholar]
- [41].Kiss RS, Nilsson T. Rab proteins implicated in lipid storage and mobilization. J Biomed Res. 2014;28:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu D, Li Y, Wu L, et al. Rab18 promotes lipid droplet (LD) growth by tethering the ER to LDs through SNARE and NRZ interactions. The Journal of cell biology. 2018;217:975–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Welte MA. Expanding roles for lipid droplets. Curr Biol. 2015;25:R470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pennetta G, Welte MA. Emerging Links between Lipid Droplets and Motor Neuron Diseases. Dev Cell. 2018;45:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schou KB, Morthorst SK, Christensen ST, Pedersen LB. Identification of conserved, centrosome-targeting ASH domains in TRAPPII complex subunits and TRAPPC8. Cilia. 2014;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zappa F, Wilson C, Di Tullio G, et al. The TRAPP complex mediates secretion arrest induced by stress granule assembly. EMBO J. 2019;38:e101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kakar N, Goebel I, Daud S, et al. A homozygous splice site mutation in TRAPPC9 causes intellectual disability and microcephaly. Eur J Med Genet. 2012;55:727–31. [DOI] [PubMed] [Google Scholar]
- [48].Tokarev AA, Taussig D, Sundaram G, et al. TRAPP II complex assembly requires Trs33 or Trs65. Traffic (Copenhagen, Denmark). 2009;10:1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Knodler A, Feng S, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Westlake CJ, Baye LM, Nachury MV, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Robinett CC, Giansanti MG, Gatti M, Fuller MT. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J Cell Sci. 2009;122:4526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Polevoy G, Wei HC, Wong R, et al. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. The Journal of cell biology. 2009;187:847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Giansanti MG, Belloni G, Gatti M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol Biol Cell. 2007;18:5034–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Klein M, Singgih EL, van Rens A, et al. Contribution of Intellectual Disability-Related Genes to ADHD Risk and to Locomotor Activity in Drosophila. Am J Psychiatry. 2020:appiajp201918050599. [DOI] [PubMed] [Google Scholar]
- [55].Lise MF, Wong TP, Trinh A, et al. Involvement of myosin Vb in glutamate receptor trafficking. The Journal of biological chemistry. 2006;281:3669–78. [DOI] [PubMed] [Google Scholar]
- [56].Wang Z, Edwards JG, Riley N, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schafer JC, Baetz NW, Lapierre LA, et al. Rab11-FIP2 interaction with MYO5B regulates movement of Rab11a-containing recycling vesicles. Traffic (Copenhagen, Denmark). 2014;15:292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mbimba T, Hussein NJ, Najeed A, Safadi FF. TRAPPC9: Novel insights into its trafficking and signaling pathways in health and disease (Review). Int J Mol Med. 2018;42:2991–7. [DOI] [PubMed] [Google Scholar]
- [59].Amberger JS, Bocchini CA, Scott AF, Hamosh A. OMIM.org: leveraging knowledge across phenotype-gene relationships. Nucleic Acids Res. 2019;47:D1038–D43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zablotsky B, Black LI, Maenner MJ, et al. Prevalence and Trends of Developmental Disabilities among Children in the United States: 2009–2017. Pediatrics. 2019;144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Harripaul R, Noor A, Ayub M, Vincent JB. The Use of Next-Generation Sequencing for Research and Diagnostics for Intellectual Disability. Cold Spring Harb Perspect Med 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Marangi G, Leuzzi V, Manti F, et al. TRAPPC9-related autosomal recessive intellectual disability: report of a new mutation and clinical phenotype. Eur J Hum Genet. 2013;21:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mir YR, Kuchay RAH. Advances in identification of genes involved in autosomal recessive intellectual disability: a brief review. Journal of medical genetics. 2019;56:567–73. [DOI] [PubMed] [Google Scholar]
- [64].Musante L, Ropers HH. Genetics of recessive cognitive disorders. Trends Genet. 2014;30:32–9. [DOI] [PubMed] [Google Scholar]
- [65].Martin HC, Jones WD, McIntyre R, et al. Quantifying the contribution of recessive coding variation to developmental disorders. Science. 2018;362:1161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Firth HV, Richards SM, Bevan AP, et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet. 2009;84:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mortreux J, Busa T, Germain DP, et al. The role of CNVs in the etiology of rare autosomal recessive disorders: the example of TRAPPC9-associated intellectual disability. Eur J Hum Genet. 2018;26:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Abrahams BS, Arking DE, Campbell DB, et al. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol Autism 2013;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li J, Wang L, Guo H, et al. Targeted sequencing and functional analysis reveal brain-size-related genes and their networks in autism spectrum disorders. Mol Psychiatry. 2017;22:1282–90. [DOI] [PubMed] [Google Scholar]
- [70].Krumm N, Turner TN, Baker C, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Iossifov I, O’Roak BJ, Sanders SJ, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kalsner L, Twachtman-Bassett J, Tokarski K, et al. Genetic testing including targeted gene panel in a diverse clinical population of children with autism spectrum disorder: Findings and implications. Mol Genet Genomic Med. 2018;6:171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].De Rubeis S, He X, Goldberg AP, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hnoonual A, Graidist P, Kritsaneepaiboon S, Limprasert P. Novel Compound Heterozygous Mutations in the TRAPPC9 Gene in Two Siblings With Autism and Intellectual Disability. Front Genet. 2019;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wilton KM, Gunderson LB, Hasadsri L, Wood CP, Schimmenti LA. Profound intellectual disability caused by homozygous TRAPPC9 pathogenic variant in a man from Malta. Mol Genet Genomic Med. 2020:e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McCarthy SE, Gillis J, Kramer M, et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry. 2014;19:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kushima I, Aleksic B, Nakatochi M, et al. Comparative Analyses of Copy-Number Variation in Autism Spectrum Disorder and Schizophrenia Reveal Etiological Overlap and Biological Insights. Cell Rep. 2018;24:2838–56. [DOI] [PubMed] [Google Scholar]
- [78].Brainstorm C, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vissers LE, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nat Rev Genet. 2016;17:9–18. [DOI] [PubMed] [Google Scholar]
- [80].Quaynor SD, Bosley ME, Duckworth CG, et al. Targeted next generation sequencing approach identifies eighteen new candidate genes in normosmic hypogonadotropic hypogonadism and Kallmann syndrome. Molecular and cellular endocrinology. 2016;437:86–96. [DOI] [PubMed] [Google Scholar]
- [81].Yoshida T, Kato K, Yokoi K, et al. Association of genetic variants with hemorrhagic stroke in Japanese individuals. Int J Mol Med. 2010;25:649–56. [DOI] [PubMed] [Google Scholar]
- [82].Duerinckx S, Meuwissen M, Perazzolo C, et al. Phenotypes in siblings with homozygous mutations of TRAPPC9 and/or MCPH1 support a bifunctional model of MCPH1. Molecular genetics & genomic medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Imielski Y, Schwamborn JC, Luningschror P, et al. Regrowing the adult brain: NF-kappaB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS One. 2012;7:e30838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-kappaB. Trends Neurosci. 2011;34:316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–60. [DOI] [PubMed] [Google Scholar]
- [87].Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets 2007;11:123–32. [DOI] [PubMed] [Google Scholar]
- [88].Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer’s disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiology of aging. 2000;21:427–32; discussion 51–3. [DOI] [PubMed] [Google Scholar]
- [89].Rostasy K, Monti L, Yiannoutsos C, et al. NFkappaB activation, TNF-alpha expression, and apoptosis in the AIDS-Dementia-Complex. J Neurovirol. 2000;6:537–43. [DOI] [PubMed] [Google Scholar]
- [90].Hunot S, Brugg B, Ricard D, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Philippe O, Rio M, Malan V, et al. NF-kappaB signalling requirement for brain myelin formation is shown by genotype/MRI phenotype correlations in patients with Xq28 duplications. Eur J Hum Genet. 2013;21:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–7. [DOI] [PubMed] [Google Scholar]
- [93].Fridmacher V, Kaltschmidt B, Goudeau B, et al. Forebrain-specific neuronal inhibition of nuclear factor-kappaB activity leads to loss of neuroprotection. J Neurosci. 2003;23:9403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Boersma MC, Dresselhaus EC, De Biase LM, et al. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31:5414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang Y, Liu J, Yao S, et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells. 2012;30:510–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I kappa B alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37:832–44. [DOI] [PubMed] [Google Scholar]
- [97].Schultz C, Konig HG, Del Turco D, et al. Coincident enrichment of phosphorylated IkappaBalpha, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol Cell Neurosci. 2006;33:68–80. [DOI] [PubMed] [Google Scholar]
- [98].Mémet S NF-kappaB functions in the nervous system: from development to disease. Biochem Pharmacol. 2006;72:1180–95. [DOI] [PubMed] [Google Scholar]
- [99].Romano A, Freudenthal R, Merlo E, Routtenberg A. Evolutionarily-conserved role of the NF-kappaB transcription factor in neural plasticity and memory. Eur J Neurosci. 2006;24:1507–16. [DOI] [PubMed] [Google Scholar]
- [100].Kaltschmidt B, Ndiaye D, Korte M, et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006;26:2936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Teng FY, Tang BL. NF-kappaB signaling in neurite growth and neuronal survival. Rev Neurosci. 2010;21:299–313. [DOI] [PubMed] [Google Scholar]
- [102].Zhang Y, Hu W. NFkB signaling regulates embryonic and adult neurogenesis. Frontiers in Biology. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Callan MA, Zarnescu DC. Heads-up: new roles for the fragile X mental retardation protein in neural stem and progenitor cells. Genesis. 2011;49:424–40. [DOI] [PubMed] [Google Scholar]
- [104].Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nature reviews Neuroscience. 2007;8:466–79. [DOI] [PubMed] [Google Scholar]
- [105].Zhang Y, Liu J, Yao S, et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells. 2012;30:510–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Denis-Donini S, Dellarole A, Crociara P, et al. Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci. 2008;28:3911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang Y, Kim MS, Jia B, et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 2017;548:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Gutierrez H, Hale VA, Dolcet X, Davies A. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development (Cambridge, England). 2005;132:1713–26. [DOI] [PubMed] [Google Scholar]
- [109].Novarino G, Fenstermaker AG, Zaki MS, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science. 2014;343:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Khattak NA, Mir A. Computational analysis of TRAPPC9: candidate gene for autosomal recessive non-syndromic mental retardation. CNS Neurol Disord Drug Targets. 2014;13:699–711. [DOI] [PubMed] [Google Scholar]
- [111].Sacher M, Shahrzad N, Kamel H, Milev MP. TRAPPopathies: An emerging set of disorders linked to variations in the genes encoding transport protein particle (TRAPP)-associated proteins. Traffic (Copenhagen, Denmark). 2019;20:5–26. [DOI] [PubMed] [Google Scholar]
- [112].Davis EE, Savage JH, Willer JR, et al. Whole exome sequencing and functional studies identify an intronic mutation in TRAPPC2 that causes SEDT. Clin Genet. 2014;85:359–64. [DOI] [PubMed] [Google Scholar]
- [113].Adachi H, Takahashi I, Takahashi T. Novel TRAPPC2 mutation in a boy with X-linked spondylo-epiphyseal dysplasia tarda. Pediatr Int. 2014;56:925–8. [DOI] [PubMed] [Google Scholar]
- [114].Mohamoud HS, Ahmed S, Jelani M, et al. A missense mutation in TRAPPC6A leads to build-up of the protein, in patients with a neurodevelopmental syndrome and dysmorphic features. Scientific reports. 2018;8:2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Marin-Valencia I, Novarino G, Johansen A, et al. A homozygous founder mutation in TRAPPC6B associates with a neurodevelopmental disorder characterised by microcephaly, epilepsy and autistic features. Journal of medical genetics. 2018;55:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Harripaul R, Vasli N, Mikhailov A, et al. Mapping autosomal recessive intellectual disability: combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families. Mol Psychiatry. 2018;23:973–84. [DOI] [PubMed] [Google Scholar]
- [117].Chang JY, Lee MH, Lin SR, et al. Trafficking protein particle complex 6A delta (TRAPPC6ADelta) is an extracellular plaque-forming protein in the brain. Oncotarget. 2015;6:3578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Stanga D, Zhao Q, Milev MP, et al. TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic (Copenhagen, Denmark). 2019. [DOI] [PubMed] [Google Scholar]
- [119].Larson AA, Baker PR, 2nd, Milev MP, et al. TRAPPC11 and GOSR2 mutations associate with hypoglycosylation of alpha-dystroglycan and muscular dystrophy. Skelet Muscle. 2018;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Fee DB, Harmelink M, Monrad P, Pyzik E. Siblings With Mutations in TRAPPC11 Presenting With Limb-Girdle Muscular Dystrophy 2S. J Clin Neuromuscul Dis 2017;19:27–30. [DOI] [PubMed] [Google Scholar]
- [121].Milev MP, Grout ME, Saint-Dic D, et al. Mutations in TRAPPC12 Manifest in Progressive Childhood Encephalopathy and Golgi Dysfunction. Am J Hum Genet. 2017;101:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Van Bergen NJ, Guo Y, Al-Deri N, et al. Deficiencies in vesicular transport mediated by TRAPPC4 are associated with severe syndromic intellectual disability. Brain. 2020;143:112–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yu S, Satoh A, Pypaert M, et al. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. The Journal of cell biology. 2006;174:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2005;17:4–15. [DOI] [PubMed] [Google Scholar]
- [125].Chng SH, Pachnis V. Enteric Nervous System: lessons from neurogenesis for reverse engineering and disease modelling and treatment. Curr Opin Pharmacol. 2020;50:100–6. [DOI] [PubMed] [Google Scholar]
- [126].Jonscher R, Belkind-Gerson J. Concise Review: Cellular and Molecular Mechanisms of Postnatal Injury-Induced Enteric Neurogenesis. Stem Cells. 2019;37:1136–43. [DOI] [PubMed] [Google Scholar]
- [127].Goto K, Kawahara I, Inada H, et al. Activation of 5-HT4 receptors facilitates neurogenesis from transplanted neural stem cells in the anastomotic ileum. J Physiol Sci. 2016;66:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu W, Zhang L, Wu R. Enteric Neural Stem Cells Expressing Insulin-Like Growth Factor 1: A Novel Cellular Therapy for Hirschsprung’s Disease in Mouse Model. DNA and cell biology. 2018;37:642–8. [DOI] [PubMed] [Google Scholar]
- [129].Kakar N, Goebel I, Daud S, et al. A homozygous splice site mutation in TRAPPC9 causes intellectual disability and microcephaly. Eur J Med Genet. 2012;55:727–31. [DOI] [PubMed] [Google Scholar]
- [130].Abou Jamra R, Wohlfart S, Zweier M, et al. Homozygosity mapping in 64 Syrian consanguineous families with non-specific intellectual disability reveals 11 novel loci and high heterogeneity. Eur J Hum Genet. 2011;19:1161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Giorgio E, Ciolfi A, Biamino E, et al. Whole exome sequencing is necessary to clarify ID/DD cases with de novo copy number variants of uncertain significance: Two proof-of-concept examples. Am J Med Genet A. 2016;170:1772–9. [DOI] [PubMed] [Google Scholar]
- [132].Anazi S, Maddirevula S, Salpietro V, et al. Expanding the genetic heterogeneity of intellectual disability. Hum Genet. 2017;136:1419–29. [DOI] [PubMed] [Google Scholar]
- [133].Boonsawat P, Joset P, Steindl K, et al. Elucidation of the phenotypic spectrum and genetic landscape in primary and secondary microcephaly. Genet Med. 2019;21:2043–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Trujillano D, Bertoli-Avella AM, Kumar Kandaswamy K, et al. Clinical exome sequencing: results from 2819 samples reflecting 1000 families. Eur J Hum Genet. 2017;25:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Bai Z, Kong X. Diagnosis of a case with mental retardation due to novel compound heterozygous variants of TRAPPC9 gene. Chinese Journal of Medical Genetics. 2019;36:1115–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.