Abstract
Purpose:
Extent of resection remains a paramount prognostic factor for long-term outcomes for glioblastoma. As such, supramaximal resection or anatomic lobectomy have been offered for non-eloquent glioblastoma in an attempt to improve overall survival. Here, we conduct a propensity-matched analysis of patients with non-eloquent glioblastoma who underwent either lobectomy or gross total resection of lesion to investigate the efficacy of supramaximal resection of glioblastoma.
Methods:
Patients who underwent initial surgery for gross total resection or lobectomy for non-eloquent glioblastoma at our tertiary care referral center from 2010-2019 were included for this propensity-matched survival analysis. Propensity scores were generated with the following covariates: age, location, preoperative KPS, product of perpendicular maximal tumor diameters, and product of perpendicular FLAIR signal diameters. Inverse probability of treatment weighting (IPTW) with generated propensity scores was used to compare progression-free survival and overall survival.
Results:
Sixty-nine patients were identified who underwent initial resection of glioblastoma for non-eloquent glioblastoma from 2010-2019 (GTR=37, lobectomy=32). Using IPTW, overall survival (30.7 vs. 14.1 months) and progression-free survival (17.2 vs. 8.1 months were significantly higher in the lobectomy cohort compared to the GTR group (p<.001). There was no significant difference in pre-op or post-op KPS or complication rates between the two groups.
Conclusion:
Our propensity-matched study suggests that lobectomy for non-eloquent glioblastoma confers an added survival benefit compared to GTR alone. For patients with non-eloquent glioblastoma, a supramaximal resection by means of an anatomic lobectomy should be considered as a primary surgical treatment in select patients if feasible.
Keywords: lobectomy, gross total resection, brain tumor, glioma, outcomes
INTRODUCTION
Overall survival for newly diagnosed glioblastoma remains dismal (approximately 14 months) despite maximal safe resection and chemoradiation1. In the current molecular era of glioma research, favorable molecular phenotypes (methylguanine methyltransferase promoter methylation and isocitrate dehydrogenase mutations) have been identified as the most important prognostic factor for high-grade gliomas2-4. Aside from these molecular profiles, it is well known that the extent of resection for glioblastoma remains an important prognostic factor for long-term outcomes. 5-8 With surmounting evidence supporting a maximal safe resection, there has been a recent push advocating for a supramaximal resection of gliomas to extend a survival benefit for these patients. Supramaximal resection can be attained by either resecting a generous margin of FLAIR changes (1-2 cm.) around the contrast-enhancing lesion or by means of a formal lobectomy. For lesions confined to one lobe, anatomic lobectomy typically provides wide margins of surgical resection with direct landmarks that can facilitate a supramaximal resection. Despite a survival benefit reported in a few series, supramaximal resection by means of lobectomy has not been rigorously assessed against lesionectomy/gross total resection (GTR). At our institution, we offer both lesionectomy and lobectomy to patients with non-eloquent region glioblastoma, depending on surgeon-preference. Due to the difficulty in conducting a prospective randomized study comparing GTR and supramaximal resection, we offer an alternative approach utilizing a propensity matched cohort analysis to compare institutional outcomes between patients offered a GTR or lobectomy for non-eloquent newly diagnosed GBM.
Here, we present the largest series of anatomic lobectomy for glioblastoma, assessing its safety and efficacy compared to standard lesionectomy. In this paper, we will also discuss the indications, outcomes, disadvantages and pitfalls associated with lobectomy for glioblastoma.
METHODS
This study was conducted and reported in accordance with STROBE guidelines.9 After Institutional Review Board approval, a retrospective patient study was conducted for all patients with newly diagnosed glioblastoma from 2010-2019 at our tertiary referral center. Patient consent was waived as all data was de-identified. All patients included in our analysis must have met the following inclusion criteria:
newly diagnosed glioblastoma (no previous resection/radiation/chemotherapy)
located in non-eloquent cortex (right frontal/temporal/occipital or left occipital)
underwent lesionectomy (GTR) or lobectomy
Left and right occipital lesions were considered non-eloquent as patients already suffered from pre-operative homonymous hemianopsia and thus there was no risk of post-operative loss of function that would dramatically alter work or activities of daily living. 69 cases were identified from our tertiary care referral center who had met these inclusion criteria. All patient undergoing GTR also met criteria for lobectomy. Relevant patient information including demographics, tumor histology, molecular markers, tumor location, adjuvant treatments, extent of resection, functional status (Karnofsky Performance Status) and follow-up were included. The molecular markers obtained include indication of the presence of methylguanine methyltransferase promoter methylation (MGMT+) and/or isocitrate dehydrogenase R132H mutation (IDH+), determined through immunohistochemical analysis of biopsy samples and/or genetic testing. In our institution, a dichotomy exists between several surgeons who prefer lesionectomy (GTR) of nGBM and those who prefer lobectomy when possible. Pooled data was collected from each of these surgeons’ case series to determine extent of resection and outcomes. All patients were referred for standard postoperative chemoradiation with temozolomide. 1
Pre-operative tumor burden was measured as the product of orthogonal diameters of T1 contrast-enhancing lesions based on previously established criteria by the RANO group10. Measurement of FLAIR hyperintensity burden was conducted in a similar manner. Two independent reviewers evaluated the postoperative MRIs to ensure lesionectomy as evidenced by removal of T1 contrast-enhancing lesion. Our primary outcome of overall survival (OS) and progression-free survival (PFS), measured from date of surgery, was collected as well as perioperative patient morbidity. Progression free survival was identified as clinical symptomatic progression in the absence of steroid taper. Radiographic recurrence (local control) was identified by board-certified neuroradiologists and detailed on patient charts.
Surgical Technique
Frontal lobectomy was performed as described previously.11,12 Briefly, using a small paramedian craniotomy (4-6 cm.), a frontal corticectomy is conducted to the anterior skull-base (preserving the olfactory tracts). A subpial dissection is then carried down laterally to the level of sylvian fissure and falx with special attention to stay rostral to the corpus callosum. In most cases, an attempt was made to avoid entering the frontal horn of the lateral ventricle. Similarly, temporal lobectomies were carried out as previously described with the superior and posterior aspect of the dissection guided by the tumor boundaries.13 An amygdalohippocampectomy was performed in all cases and portions of the anterior superior temporal gyrus were removed depending on the lesion location. Occipital lobectomy was performed based on procedures previously described by Conner et al14. All patients in our cohort were treated with postoperative dexamethasone tapers over 10-14 days to reduce postoperative edema.
Statistical Analysis
Propensity scores were generated using the following covariates: age at surgery, location, product of perpendicular maximal tumor diameters, product of perpendicular FLAIR signal diameters, and pre-operative KPS. KM curves were then created and weighted based on generated propensity scores through IPTW. Molecular testing was done after surgery or biopsy and thus was not included in the generation of propensity scores but factored into multivariate Cox regression. Multiple imputation with eight iterations was used to simulate molecular marker data for patients that lacked immunohistochemical or genetic analysis of their tumor sample. PFS, OS, and local control was compared between patients receiving lobectomy and GTR using inverse probability of treatment weighting (IPTW). A Gehan-Breslow-Wilcoxon test was used as the ideal test to compare weighted Kaplan-Meier (KM) curves as multiple patients were lost to follow up at later time points with no clear patterns, therefore the assumption of proportional hazards necessary for the logrank test was not fulfilled. To compare multiple KM curves, the logrank test for trend was used. Other continuous parameters were compared using independent samples t-test while categorical variables were compared using Chi-square analysis or Fisher’s Exact Test. Ordinal variables (KPS) was compared using a Mann-Whitney U test. P values less than 0.05 were considered significant. All statistics analyses were performed using SPSS Statistics v24.0 (IBM, Armonk, NY) and PRISM (GraphPad Software, San Diego, CA) software.
RESULTS
Patient Characteristics
Sixty-nine patients underwent either lobectomy or gross total resection who met our aforementioned inclusion criteria (Fig. 1). Patient characteristics are given in Table 1. The median age was 64 years with a male predominance (57.9%). The majority of patients received lobectomy underwent temporal (n=19, 59.4%) and occipital (n=8, 25%) lobectomies. For the GTR group, the majority of patients underwent temporal (n=20, 54.1%) and frontal craniotomies (n=15, 40.5%). Preoperative KPS was 90 and 80 for the GTR and lobectomy group respectively (p=.388). Mean product of maximum orthogonal lesion diameters was 15.4±9.5 and 18.7±7.8 for the GTR and lobectomy groups respectively. Mean product of FLAIR hyperintensity diameters was 21.1±10.6 and 31.5±12.2 for GTR and lobectomy groups respectively. There was no difference in the mean product of maximum perpendicular diameters of the contrast-enhancing lesion for the GTR group or lobectomy group, however FLAIR hyperintensity diameters was significantly higher for the lobectomy group (p=.127; p<001). There was no difference between the groups in terms of MGMT hypermethylation status (p=.551); however, there was significantly more IDH-wild type phenotypes in the lobectomy groups (n=23, 71.9% vs. n=16, 43.2%, p = .001). Finally, 61 patients in total completed post-operative chemo- and radiotherapy (ChemoRT); completion of adjuvant ChemoRT was not significantly different between patients receiving lobectomy and GTR (p=.627). Clinical outcomes for both groups are presented in Table 2.
Figure 1: Side by side comparisons of T1-weighted, contrast-enhanced MRI radiographs comparing lesionectomy (left) and lobectomy (right).
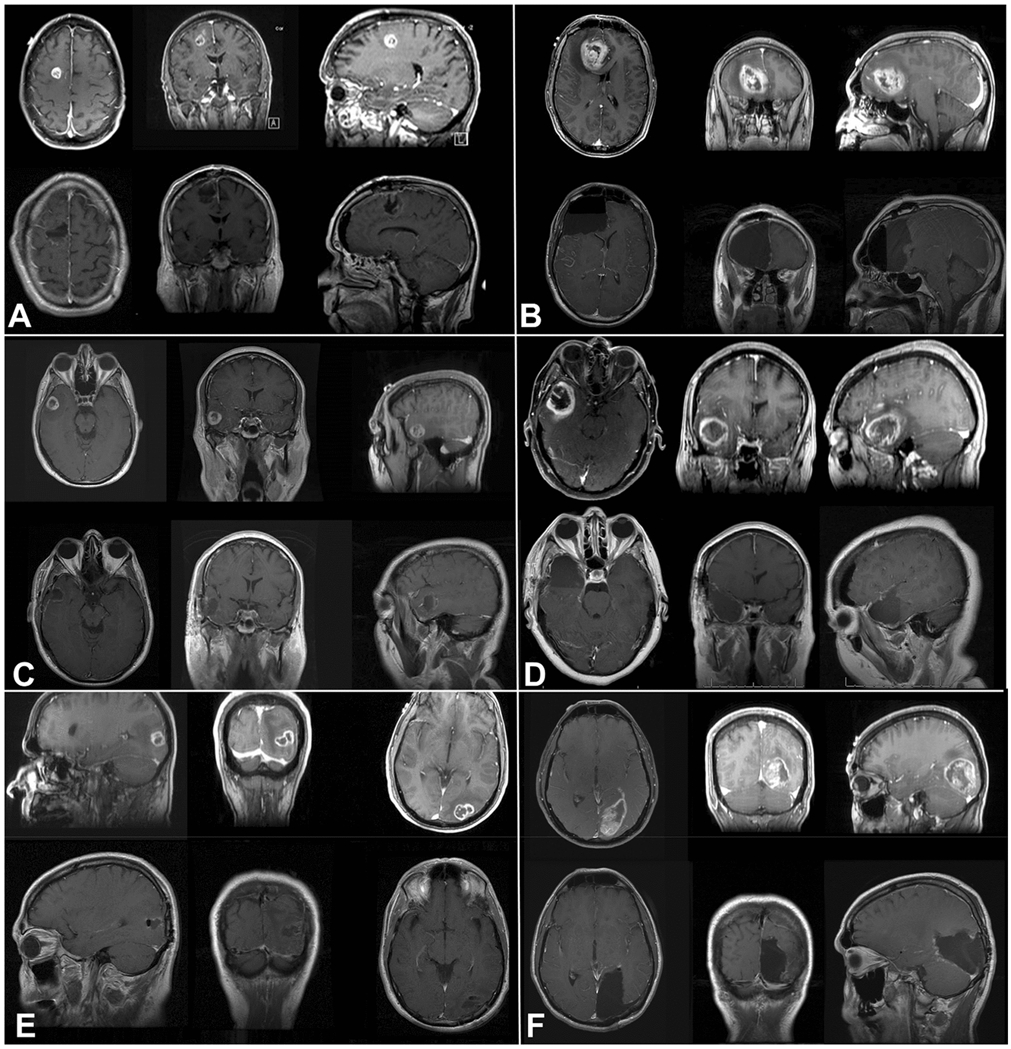
a, b: Frontal lesionectomy versus lobectomy showing pre-operative lesion (top) and post-operative changes (bottom). c, d: Post-operative changes after temporal lesionectomy versus lobectomy. e, f: Similar comparison for occipital lesionectomy versus lobectomy.
Table 1:
Baseline characteristics for patient cohort
| Characteristic | Total | GTR | Lobectomy | p-value |
|---|---|---|---|---|
| Median age (IQR) | 64 (54, 73) | 65 (54, 71) | 60 (54, 75) | .662 |
| Sex (%) | ||||
| Female | 29 (43.1) | 22 (59.5) | 7 (21.9) | .002 |
| Male | 40 (57.9) | 15 (40.5) | 25 (78.1) | |
| Product of diameters (cm^2, mean ± S.D.) | 16.92±8.7 | 15.4±9.5 | 18.7±7.8 | .127 |
| FLAIR Product | 25.9±12.5 | 21.1±10.6 | 31.5±12.2 | <.001 |
| Median Preoperative KPS (IQR) | 80 (80, 90) | 90 (80, 90) | 80 (70, 90) | .388 |
| IDH1 | ||||
| Wild type | 39 (56.5) | 16 (43.2) | 23 (71.9) | .010 |
| Positive | 1 (1.5) | 0 (0) | 1 (3.1) | |
| No Data | 29 (42) | 21 (56.8) | 8 (25) | |
| MGMT promoter status | ||||
| Unmethylated | 28 (40.6) | 13 (35.1) | 15 (46.9) | .551 |
| Hypermethylated | 8 (11.6) | 4 (10.8) | 4 (12.5) | |
| No Data | 33 (47.8) | 20 (54.1) | 13 (40.6) | |
| Location | ||||
| Frontal | 20 (29) | 15 (40.5) | 5 (15.6) | .009 |
| Temporal | 39 (56.5) | 20 (54.1) | 19 (59.4) | |
| Parietal | 1 (1.4) | 1 (2.7) | 0 (0) | |
| Occipital | 9 (13) | 1 (2.7) | 8 (25) | |
| ChemoRT | ||||
| Yes | 61 (88.4) | 32 (86.5) | 29 (90.6) | .627 |
| No Data | 8 (11.6) | 6 (13.5) | 3 (9.4) |
Table 2.
Clinical outcomes of GTR versus lobectomy (unweighted)
| Group | Median length of follow-up (months), (IQR) |
Postoperative KPS (IQR) |
Progression Free Survival (months), (95% CI) |
Local Control (months), (95% CI) |
Overall Survival (months), (95% CI) |
Death, n (%) |
Complications, n (%) |
|---|---|---|---|---|---|---|---|
| GTR | 6 (2.9, 13.8) | 80 (70, 90) | 8.1 (6.3, 9.9) | 11 (5.8, 16.1) | 14.1 (6.9, 21.3) | 23 (62.2) | 2 (5.4) |
| Lobectomy | 12.4 (5.1, 18.15) | 80 (60, 90) | 17.2 (6.1, 28.2) | 14.4 (2.5, 26.3) | 30.7 (10.7, 50.7) | 10 (31.2) | 3 (9.4) |
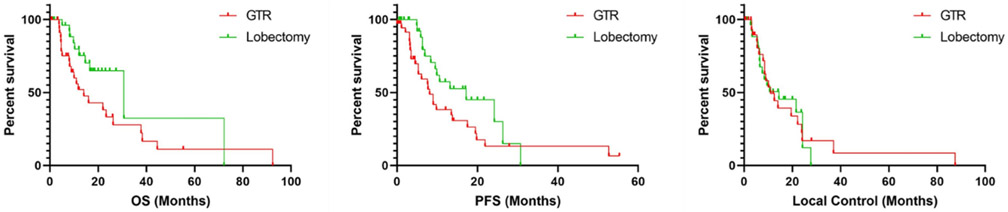
For the entire cohort, median overall survival and progression free survival was 22 months (mo) and 9.9 mo respectively. On non-weighted KM analysis OS (30.7, 95% CI 10.7-50.7, vs 14.1, 6.9-21.3, mo, p=.019) and PFS (17.2, 6.1-28.2, vs 8.1, 6.3-9.9, mo, p=.015) but not local control (14.4, 2.5-26.3, vs 11, 5.8-16.1, mo, p=.991) were significantly higher in patients receiving lobectomy compared to GTR. Using IPTW, overall survival (30.7, 20.5-40.9, vs. 14.1, 9.4-18.8, mo),progression free survival (17.2, 11.5-22.9, vs. 8.1, 5.7-10.5, mo), but not local control (14.4, 9.3-19.5, vs. 11.1, 7.7-14.3, mo) were significantly greater in the lobectomy cohort with a mean follow up of 13.7 months (Fig. 2, p<0.001, p=.382). Cox regression with multiple imputations showed that differences in OS, PFS, and local control persisted when accounting for MGMT hypermethylation and IDH1 mutational status (p=.022, p=.008, p=.366).
Figure 2. Kaplan Meier analysis of OS, PFS, and local control with and without IPTW.
Top: OS (p=.019) and PFS (p=.015) but not local control (p=.992) was statistically significantly greater in patients receiving lobectomy compared to GTR.
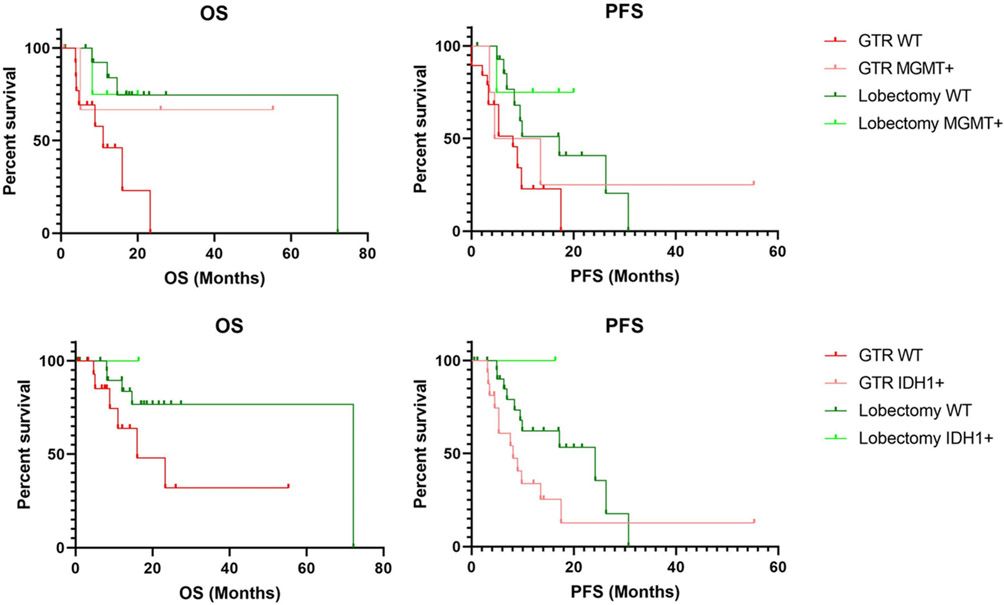
Additionally, a subgroup analysis was conducted for patients with MGMT hypermethylation and IDH1 mutational status. Patients with MGMT hypermethylation receiving lobectomy exhibited significantly increased OS and PFS compared to patients receiving lesionectomy; similar results were seen for IDH1 mutations for PFS although trends of increased OS were not significant (p<.05, p=.0569, Fig. 3). At last follow-up, postoperative KPS was not significantly different between the lobectomy and GTR group (80 vs 80, p=.829). In the immediate post-operative time, two complications occurred in the GTR group (5.4%) including (1) instance of post-operative seizure and (1) instance of DVT. Three complications occurred in the lobectomy group (9.4%) including (1) instance of meningitis, (1) post-operative seizure, and (1) CSF leak. Complication rates did not differ between groups (p=.657). Rate of post-operative seizures did not significantly differ between patients receiving lobectomy or GTR (2.7% vs 3.1%, p=1.00).
Figure 3: Subgroup survival analysis of patients with MGMT hypermethylation and IDH1 mutations.
MGMT WT patients receiving lobectomy displayed higher median OS (72.2 vs 11) and PFS (17.2 vs 8.1) compared to GTR. IDH1 WT patients receiving lobectomy also displayed higher OS (72.2 vs 16) and PFS (24.2 vs 8.1). Logrank test for trend was statistically significant for all comparisons (p<.05) other than OS for IDH1 mutations which was not significant at p=.0569.
DISCUSSION
The concept of supramaximal resection for glioblastoma dates back to the early 20th century in an effort to reduce recurrence and white matter spread. 15,16 However, with the lack of reliable neuroimaging or adjuvant modalities, survival for these patients remained undeniably poor with significant complications. Due to the early high morbidity of lobectomy and presence of tumor-infiltrating cells in normal parenchyma, the concept of lobectomy was disregarded over the ensuing decades in favor of a tailored resection (lesionectomy). 17,18 In addition, with the advent of microsurgical techniques over thirty years ago, gross total resection offered similar overall survival compared to lobectomy (12.6 vs. 12.9 respectively). 19 Furthermore, over the last two decades, several studies have suggested and validated a threshold for extent of resection for glioblastomas (~78-98%) to confer a survival benefit. 5,20-22 However, recently, it has been suggested that supramaximal resection should be offered for patients with nGBM when feasible. 23,24 Initially proposed for low-grade gliomas, the primary aim of these initial studies was to maximize resection of peritumoral FLAIR signal (tumor infiltrated parenchyma) to prevent disease recurrence. [19, 20]25-28 To date, few studies have demonstrated that resection of FLAIR signal around the GBM (~50%) would confer an survival benefit; however this advantage has not been replicated in other smaller series. 23,24,29 As such, the benefit of supramaximal resection of nGBM has yet to be established.
Anatomic lobectomy may serve as a surrogate to optimize supramaximal resection for non-eloquent glioblastoma. In previous smaller series, lobectomy has conferred a survival benefit for nGBM without significant additional morbidity. 11,30 Here, we present the largest comparative series of lobectomy versus oncologic resection (lesionectomy) for newly diagnosed glioblastoma using a propensity matched survival analysis. Patients in the lobectomy cohort demonstrated a significantly improved OS and PFS (symptomatic progression) compared to GTR alone after adjusting for age, location and size. Overall, our data suggests that supramaximal resection via lobectomy may confer a survival benefit for non-eloquent glioblastoma. We do acknowledge however that our results are limited only to non-eloquent regions as we have not yet explored the utility of supramaximal resection in eloquent regions.
Aside from establishing tumor margins, lobectomy may confer an added benefit with adjuvant chemoradiation through cytoreduction and extended radiotherapy limits. Standard adjuvant radiation is delivered at least 2 centimeters beyond the ring-enhancing tumor; in the case of lobectomy, the margin is widened to cover a greater amount of normal parenchyma, potentially eliminating viable tumor infiltrating cells. Therefore, we argue that a patient’s response to adjuvant treatment may be inversely proportional on the burden of residual disease; supramaximal resection minimizes residual microscopic disease, thereby optimizing adjuvant treatments.
Our results seem to compliment the findings from another recent investigation published by Molinaro et al31. In a retrospective, multicenter study of 761 patients with newly diagnosed GBM, significantly improved OS was identified with resection of non-contrast enhancing (NCE) tumor volume. However, this effect was only identified in patients younger than 65 years of age but not in elderly patients. This raises the question as to whether older patients may tolerate and benefit from aggressive supramaximal resection. Our results seem to suggest so with a median cohort age of 64 years, highly representative of the median age of diagnosis for GBM32. Previously, we have shown that GTR of brain tumors to be safe and tolerable in a subset of octogenarians and nonagerians33. Future studies may seek to evaluate specifically the impact of supramaximal resection for elderly patients with nGBM.
Complications
Overall morbidity in our study was relatively low and complication rates were not significantly different between groups. Although theoretically lobectomy may increase the risk of post-operative hydrocephalus due to opening the ventricle, this was not seen in our study. Ultimately, patient outcome in terms of KPS at final follow-up was not significantly different between groups while median OS and PFS was increased in patients receiving lobectomy.
Future studies
In the future, we plan to assess whether supramaximal resection of nGBM can be safely employed for eloquent region tumors. Primarily, using surgical adjuncts such as awake mapping techniques, motor evoked potentials, and diffuse tensor imaging, we hope to evaluate outcomes for patients with eloquent region lesions. Currently, we are employing these techniques for eloquent region lesions to offer supramaximal resection in optimal settings. In essence, we offer a tailored lobectomy for left frontal and temporal lesions to optimize extent of resection while preserving functional cortical networks. An important factor of our study is that our lobectomy cohort maintained similar functional status (KPS) to our lesionectomy group; however, we did not formally assess neuropsychological outcomes following resection. Our institution is currently running a study to compare neurocognitive function after lesionectomy versus lobectomy. In the future, we would like to include formal preoperative and postoperative neuropsychological testing after resection since this has not been systematically performed for patients with glioblastoma. Lastly, since our study is a retrospective case-control study, we hope that we can conduct a prospective trial in the future to definitely assess outcomes between GTR and lobectomy.
Limitations
Our study is prone to all biases related to studies of retrospective, non-randomized design including selection and reporting biases. For this, we attempted to control for possible biases through the generation of propensity scores and an IPTW analysis to help control for the potential selection biases. Though our sample size was small, this is a relatively large study compared to previous single-center reports on the survival benefit of supramaximal resection. Additionally, our study was of sufficient statistical power to detect a significant survival benefit with anatomic lobectomy compared to GTR. Still, additional investigation is warranted in the form of larger, multi-center trials.
Given the importance of molecular profiling in neuro-oncology, we aimed to control for prognostic mutational phenotypes; while rates of IDH1 mutations and MGMT hypermethylation were not significantly different between GTR and lobectomy groups, multiple patients did not have genetic data available, thus limiting the value of the Cox regression analysis. Despite this, KM analysis show increased survival in patients receiving lobectomy in nearly all cases; nevertheless, future prospective studies with more thorough molecular analysis may provide more information on the impact of lobectomy versus GTR in genetic variants of GBM.
Finally, all patients with occipital tumors included in our series presented with pre-operative homonymous hemianopsia and thus preservation of function did not considerably influence resection margins. However, for future studies, the possibility of selection bias in occipital tumors may exist. Patients with smaller lesions and thus preserved visual function may likely undergo a more conservative resection in hopes of retaining the majority of the occipital visual apparatus. Alternatively, patients with larger tumors may present with fixed, permanent visual field deficits and so preservation of function may not be a consideration and thus a larger resection may be achieved. In these cases, rigorous neurocognitive assessment must be performed as the technique of anatomic lobectomy is further investigated which, as mentioned prior, we are currently pursuing.
CONCLUSION
Recent literature has endorsed the use of supramaximal resection to extend survival benefit in select patients. Here, we present the largest series comparing survival outcomes in patients receiving lobectomy versus lesionectomy. Our propensity-matched study suggests that lobectomy for non-eloquent glioblastoma confers an added survival benefit compared to lesionectomy alone. For patients with non-eloquent glioblastoma, a supramaximal resection by means of an anatomic lobectomy should be considered as a primary surgical treatment in select patients if feasible. Future studies should aim to assess and compare neurologic outcome in patients undergoing lobectomy with rigorous neuropsychiatric testing.
Acknowledgments
Funding
National Institute of Neurological Disorders and Stroke (R25NS108937-01)
ABBREVIATIONS
- GTR
gross total resection
- KPS
Karnofsky performance status
- KM
Kaplan-Meier
- OS
overall survival
- PFS
progression-free survival
- LC
local control
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest/Competing Interests
The authors have no financial or personal conflicts of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Millward CP, Brodbelt AR, Haylock B, et al. The impact of MGMT methylation and IDH-1 mutation on long-term outcome for glioblastoma treated with chemoradiotherapy. Acta Neurochir (Wien). 2016;158(10):1943–1953. [DOI] [PubMed] [Google Scholar]
- 3.Combs SE, Rieken S, Wick W, et al. Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: one step forward, and one step back? Radiat Oncol. 2011;6:115–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molenaar RJ, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro-Oncology. 2014;16(9):1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival.J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Kim DG, Kim JW, et al. The role of surgical resection in the management of brain metastasis: a 17-year longitudinal study. Acta neurochirurgica. 2013;155(3):389–397. [DOI] [PubMed] [Google Scholar]
- 8.Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2012;14(7):e378–385. [DOI] [PubMed] [Google Scholar]
- 9.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805–835. [DOI] [PubMed] [Google Scholar]
- 10.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 11.Roh TH, Kang SG, Moon JH, et al. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Wen HT, Da Roz LM, Rhoton AL Jr., Castro LH, Teixeira MJ. Frontal Lobe Decortication (Frontal Lobectomy with Ventricular Preservation) in Epilepsy-Part 1: Anatomic Landmarks and Surgical Technique. World Neurosurg. 2017;98:347–364. [DOI] [PubMed] [Google Scholar]
- 13.Conner AK, Burks JD, Baker CM, et al. Method for temporal keyhole lobectomies in resection of low- and high-grade gliomas. J Neurosurg. 2018;128(5):1388–1395. [DOI] [PubMed] [Google Scholar]
- 14.Conner AK, Baker CM, Briggs RG, et al. A Technique for Resecting Occipital Pole Gliomas Using a Keyhole Lobectomy. World Neurosurg. 2017;106:707–714. [DOI] [PubMed] [Google Scholar]
- 15.Dandy W Removal of right cerebral hemisphere for certain tumors with hemiplegia. J Am Med Assoc. 1928;90:823–825. [Google Scholar]
- 16.Phillippides D, Montreuil B, Steimle R. [Considerations on 50 glioblastomas treated by extensive excision (lobectomy)]. Rev Neurol (Paris). 1951;84(5):483–485. [PubMed] [Google Scholar]
- 17.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 18.Pia HW. Microsurgery of gliomas. Acta Neurochir (Wien). 1986;80(1-2):1–11. [DOI] [PubMed] [Google Scholar]
- 19.Hollerhage HG, Zumkeller M, Becker M, Dietz H. Influence of type and extent of surgery on early results and survival time in glioblastoma multiforme. Acta Neurochir (Wien). 1991;113(1-2):31–37. [DOI] [PubMed] [Google Scholar]
- 20.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 22.Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
- 23.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 24.Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017;135(1):129–139. [DOI] [PubMed] [Google Scholar]
- 25.Duffau H Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien). 2012;154(4):575–584; discussion 584. [DOI] [PubMed] [Google Scholar]
- 26.Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. J Neurosurg. 2011;115(2):232–239. [DOI] [PubMed] [Google Scholar]
- 27.Esquenazi Y, Friedman E, Liu Z, Zhu JJ, Hsu S, Tandon N. The Survival Advantage of "Supratotal" Resection of Glioblastoma Using Selective Cortical Mapping and the Subpial Technique. Neurosurgery. 2017;81(2):275–288. [DOI] [PubMed] [Google Scholar]
- 28.Duffau H Is supratotal resection of glioblastoma in noneloquent areas possible? World Neurosurg. 2014;82(1-2):e101–103. [DOI] [PubMed] [Google Scholar]
- 29.Altieri R, Melcarne A, Soffietti R, et al. Supratotal Resection of Glioblastoma: Is Less More? Surg Technol Int. 2019;35:432–440. [PubMed] [Google Scholar]
- 30.Schneider M, Potthoff AL, Keil VC, et al. Surgery for temporal glioblastoma: lobectomy outranks oncosurgical-based gross-total resection. J Neurooncol. 2019;145(1):143–150. [DOI] [PubMed] [Google Scholar]
- 31.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncology. 2020;6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamimi AF, Juweid M. Epidemiology and Outcome of Glioblastoma In: De Vleeschouwer S, ed. Glioblastoma. Brisbane (AU)2017. [PubMed] [Google Scholar]
- 33.Eichberg DG, Di L, Shah AH, et al. Brain Tumor Surgery is Safe in Octogenarians and Nonagenarians: A Single-Surgeon 741 Patient Series. World Neurosurg. 2019;132:e185–e192. [DOI] [PubMed] [Google Scholar]