Abstract
Background:
Patients with coarctation of the aorta (CoA) have a high prevalence of intracranial aneurysms (IA) and suffer subarachnoid hemorrhage (SAH) at younger ages than the general population. AHA/ACC Guidelines recommend IA screening, but appropriate age and interval of screening, and its effectiveness remain a critical knowledge gap.
Methods and Results:
To evaluate the benefits and cost-effectiveness of magnetic resonance angiography screening for IA in patients with CoA, we developed and calibrated a Markov model to match published IA prevalence estimates. The primary outcome was the incremental cost-effectiveness ratio (ICER). Secondary outcomes included lifetime cumulative incidence of prophylactic IA treatment and mortality, and SAH deaths prevented. Using a payer perspective, a lifetime horizon, and a willingness-to-pay of $150,000 per quality-adjusted life year (QALY) gained, we applied a 3% annual discounting rate to costs and effects and performed 1-way, 2-way, and probabilistic sensitivity analyses. In a simulated cohort of 10,000 patients, no screening resulted in a 10.1% lifetime incidence of SAH and 183 SAH-related deaths. Screening at ages 10, 20 and 30 years led to 978 prophylactic treatments for unruptured aneurysms, 19 procedure-related deaths, and 65 SAH-related deaths. Screening at ages 10, 20 and 30 years was cost-effective compared to screening at ages 10 and 20 years (ICER $106,841/QALY). Uncertainty in the outcome after aneurysm treatment and quality of life after SAH influenced the preferred screening strategy. In probabilistic sensitivity analysis, screening at ages 10, 20 and 30 years was cost-effective in 41% of simulations and at ages 10 and 20 in 59% of simulations.
Conclusions:
Our model supports the AHA/ACC recommendation to screen patients with CoA for IA and suggests screening at ages 10 and 20 or at 10, 20 and 30 years would extend life and be cost-effective.
Coarctation of the aorta (CoA) is a common congenital cardiac defect occurring in 4 per 10,000 live births.1 Despite adequate relief of aortic arch obstruction, patients with CoA suffer increased morbidity and reduced long-term survival.2,3 One of the etiologies for premature morbidity and death is subarachnoid hemorrhage (SAH) secondary to ruptured intracranial aneurysms (IA), which occurs at a significantly younger age than in the general population. By middle age, 10–13% of patients with CoA, compared with 3–7% of the general population, have an IA on screening magnetic resonance angiography (MRA).4–9 Prophylactic treatment (endovascular or surgical) of IAs identified by screening may prevent SAH.
Routine MRA screening of other populations at increased risk for IAs, such as those with a first-degree relative with history of SAH, has been found to increase quality-adjusted life expectancy and be cost-effective.10,11 Given the high prevalence of IAs in patients with CoA, the 2018 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Management of Adult Congenital Heart Disease and the 2015 AHA/American Stroke Association Guidelines for the Management of Patients with Unruptured Intracranial Aneurysms recommended screening patients with history of CoA for IA. However, the timing and frequency of screening patients with CoA and its effectiveness remain a critical knowledge gap.
Decision modeling is a valuable tool to inform clinical decision making in the setting of tradeoffs and uncertainty through the integration of disparate sources of evidence. Tradeoffs of different screening strategies include risks of false positives from screening that lead to unnecessary further diagnostic tests and psychological distress, and morbidity and mortality from preventative treatment of aneurysms that may never rupture. These risks vary with timing and frequency of screening. Electing to not screen also carries risk of failing to detect an aneurysm that may rupture with resultant high mortality. Patient preferences for different outcomes need to be incorporated when alternative options are being assessed. A decision model explicitly incorporates these potential tradeoffs and individual preferences for an outcome.12 To inform clinical decision making, we developed a Markov model to evaluate the clinical benefits and cost-effectiveness of screening strategies for IA in patients with CoA.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Markov Model
We developed a Markov state transition model to simulate the natural history of aneurysm development and rupture in patients with CoA to assess the benefits and cost-effectiveness of magnetic resonance angiography (MRA) screening for IA compared with no screening. In a Markov simulation, the natural history of CoA is modeled by following a cohort of hypothetical patients over time as they move among predefined states of health over time until all have died (lifetime horizon). Figure 1 is a representation of the Markov health states and events that may occur to patients in each health state. All patients enter the model at birth in the state ‘well with CoA and without aneurysm.’ In the no screening strategy, a patient without aneurysm has an annual risk of developing an IA, remaining well, or dying due to reasons other than ruptured IA. Background mortality is determined by the age- and sex-matched general population Centers for Disease Control and Prevention (CDC) life tables and the standardized mortality ratio (SMR) for CoA.13,14 Once a patient develops an IA, the aneurysm has an annual risk of growth or rupture. If it ruptures, those individuals may survive, become disabled, or die. Those surviving carry an increased annual mortality and if disabled, both impaired quality of life and even higher annual mortality.
Figure 1.
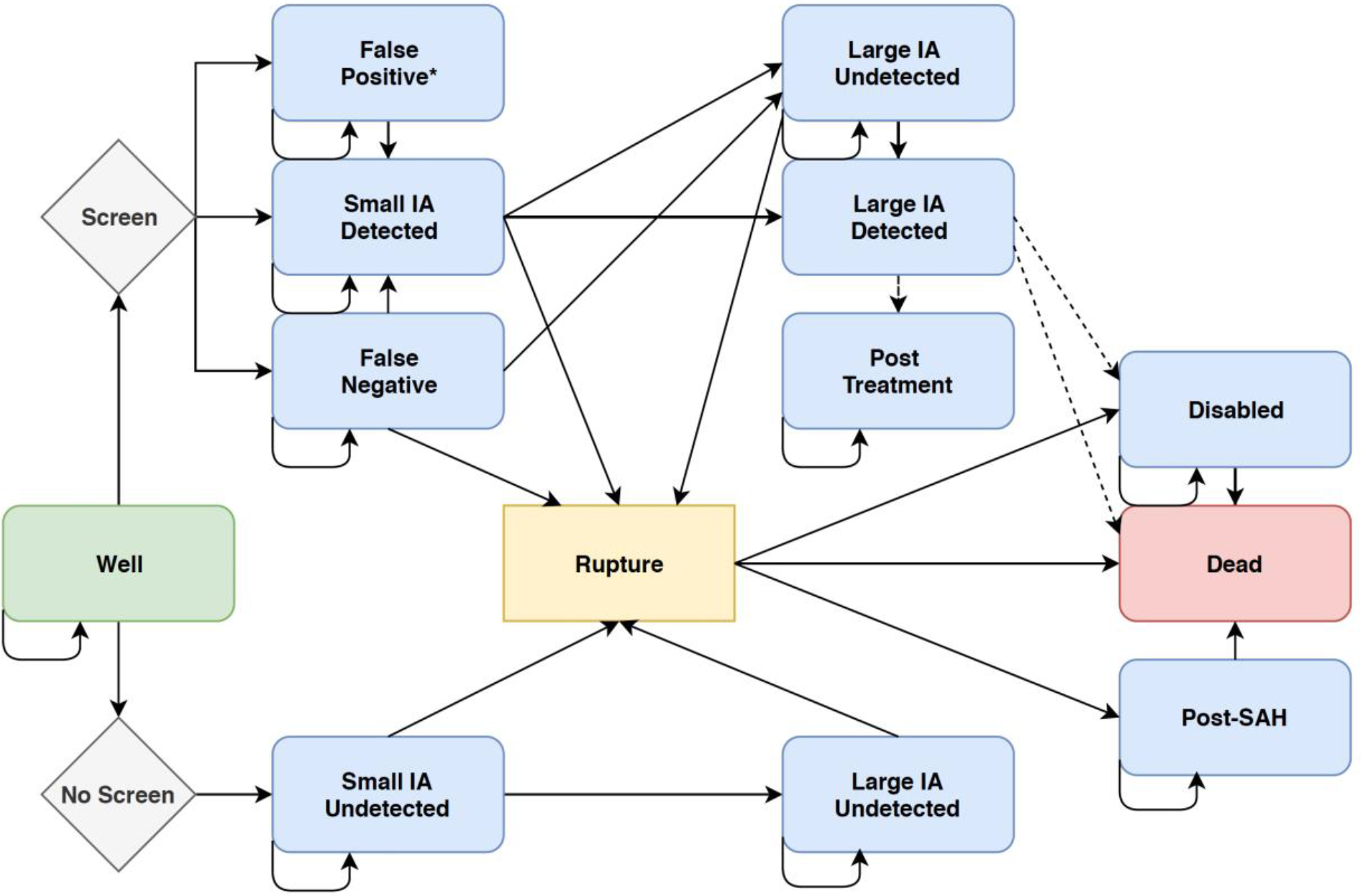
A simplified Markov state transition diagram. All patients with coarctation enter the model at birth in the state ‘well’ without aneurysm. Arrows show how patients can transition among states at each cycle. Dashed lines represent prophylactic treatment. The health states for retreatment of aneurysm recurrence have been excluded for simplicity. All states can transition to ‘dead’ even if not explicitly drawn.
In the screening strategy, patients are initially screened depending on the specific start age for that strategy and thereafter depending on the screening strategy’s frequency and stop age. If an IA is detected by screening MRA, it may be a true or false positive, according to the sensitivity and specificity of MRA. Patients with false negatives enter the state of ‘well with unknown aneurysm’ and have an annual risk of IA rupture and growth. Patients with a true positive are surveilled annually by MRA. Patients with a false positive enter the state of ‘false positive’ and undergo surveillance until a negative test obtained. They continue to have a risk of developing an actual IA with a true positive according to the test sensitivity and specificity.15 Screen detected IA ≥ 5 mm or an IA <5 mm with interval growth are prophylactically treated with clipping or endovascular coil, as determined by published rates of endovascular treatment in the US.16 Preventative clipping and coiling have associated risks of disability and death. Patients in the screening strategy may suffer hemorrhagic stroke secondary to 1) rupture of an aneurysm below the prophylactic treatment threshold (<5 mm), 2) rupture of an aneurysm that developed or grew in the interval between screenings, or 3) a false negative screen. Following clipping or coiling, patients are surveilled annually by CTA or MRA, respectively, and have a risk of rupture and of aneurysm recurrence according to the treatment modality received. Aneurysms that recur and necessitate re-intervention receive endovascular therapy consistent with national rates and undergo surveillance with CTA if they ever received a clip. As in the no screening strategy, patients in any health state may die from competing causes unrelated to an IA. Supplemental Figure 1 demonstrates a simplified version of the model for one health state. All analyses were performed using TreeAge Pro (2018), Microsoft Excel (2017), and Parameter Solver (Version 3.0, University of Texas MD Anderson Cancer Center). The study was exempt from Institutional Review Board approval due to the use of previously published data.
Strategies
We compared the natural history without screening to various screening strategies. Initial age, final age, and interval of screening were varied to identify optimal strategies. Initial screening age was assessed from ages 10–25 years. Our model does not consider screening patients younger than age 10 because of the risks of anesthesia necessary for performing an MRA. Final age and interval of screening were assessed from ages 20–80 years and every 1–10 years, respectively. Screening is assumed to be performed by non-contrast 3D time-of-flight MRA without additional MRI (base-case).
Data
Transition Probabilities
We assigned transition probabilities using data available from the literature. Distributions and ranges were defined for each model input to reflect parameter uncertainty (Tables 1 and 2).
Table 1.
Base-case model values and ranges for sensitivity analyses
| Variables | Event | Value | Cases per event/population (follow-up years) | 95% CI | Type of Distribution | Source |
|---|---|---|---|---|---|---|
| Initial Health State | ||||||
| Well | Small IA | 0.00439 | 0.002–0.007 | β | 5,6,17 | |
| Small IA <5 mm | Growth | 0.057 | 156/1484 (1.9) | β | 18 | |
| Small IA <5 mm | Rupture | 0.0033 | 0.0021–0.0051 | β | 19 | |
| Large IA ≥5 mm | Rupture (Hazard Rate Ratio for Large vs. Small IA) |
12.24 | 7.15–20.93 | Lognormal | 19 | |
| Post endovascular procedure | Retreatment* | 0.058 | 572/5582 (1.7) | β | 20 | |
| Post clipping | Retreatment* | 0.0082 | 2/245 | β | 21 | |
| Post SAH without disability | Death (Relative Survival Ratio for post SAH vs. general population) | 0.83 | 0.80–0.85 | Lognormal | 22 | |
| Post SAH with permanent disability | Death | 0.16 | 56/92 (3.7) | β | 23 | |
| Probability of | ||||||
| Male sex | 0.72 | 0.68–0.76 | β | 24 | ||
| Endovascular treatment for IA | 0.63 | 7488/11829 | β | 25 | ||
| Endovascular Treatment Complications | ||||||
| Permanent disability | 0.059 | 0.038–0.081 | β | 26 | ||
| Mortality | 0.0076 | 0.0046–0.011 | β | 26 | ||
| Surgical Clipping Complications | ||||||
| Permanent disability | 0.080 | 0.020–0.14 | β | 26 | ||
| Mortality | 0.011 | 0.0046–0.017 | β | 26 | ||
| Standardized Mortality Ratio for CoA vs. general population |
4.3 | 3.7–5.0 | β | 13 | ||
| Screening Test Characteristics | ||||||
| MRA sensitivity | 0.96 | 0.85–0.99 | β | 27 | ||
| MRA specificity | 0.93 | 0.85–0.97 | β | 27 | ||
| Subarachnoid Hemorrhage | ||||||
| Probability of death | Age dependent | β | 28 | |||
| Probability of permanent disability | 0.11 | 213/1969 | β | 29 | ||
Excluding cases that were retreated as part of initial treatment episode
Table 2.
Base-case utilities, costs, and ranges for sensitivity analyses
| Variable | Value | 95% CI | Type of Distribution | Source |
|---|---|---|---|---|
| Preference-based Quality of Life Scores for Model Health States (Utilities) | ||||
| Health States | ||||
| Well | 0.92 | 0.89–0.94 | β | 30 |
| Well after SAH | 0.80 | 0.62–0.93 | β | 31 |
| Disabled | 0.22 | 0.18–0.26 | β | 31 |
| Short-term Utility Reductions (days)* | ||||
| Positive MRA | −9.1 | −20.1, −2.6 | β | 32 |
| Endovascular Treatment | −2.2 | 33 | ||
| Clipping | −3.7 | 33 | ||
| Costs | ||||
| MRA | $283 | $245–$322 | γ | 34 |
| CTA | $300 | $262–$339 | γ | 34 |
| SAH | $72,177 | $36,643–$141,709 | γ | 34 |
| Endovascular Treatment | $30,688 | $22,252–$40,459 | γ | 35 |
| Endovascular Treatment: Disabled | $48,298 | $30,322–$70,391† | γ | 35 |
| Endovascular Treatment: Death | $73,300 | $54,580–$94,738 | γ | 35 |
| Clipping | $28,112 | $15,648–$44,166 | γ | 35 |
| Clipping: Disabled | $43,154 | $21,145–$72,881 | γ | 35 |
| Clipping: Death | $81,826 | $23,072–$177,091 | γ | 35 |
| Disabled (per year in nursing home) | $9,829 | $0-$41,477 | γ | 36 |
Number of lost days of perfect health
Assuming standard deviation of “Endovascular Treatment: Disabled”
Development of IA
The rate of IA development in patients with CoA remains unknown. Prior studies found a prevalence of IA in CoA patients of 10.3%, 11.0%, and 12.9% at mean ages of 29, 33, and 41 years, respectively.4–6 Therefore, we calibrated the probability of IA formation to minimize the least square difference between model prediction of IA prevalence and observed estimates. All patients were considered aneurysm-free at birth. Calibration resulted in a probability of IA development of 0.439% per year (Supplemental Figure 2). The rate of aneurysm development is assumed to be constant throughout life. The 95% confidence interval for IA development was derived from calibrating the rate to the 95% CI for prevalence estimates.
Growth, Rupture, Treatment of IA
In the absence of CoA-specific data, IAs in patients with CoA were assumed to grow at the same rate as the general population. Growth was defined as the probability of IA <5 mm increasing in size to ≥1.0 mm or undisputable change in aneurysm shape (i.e. change from regular shape to irregular shape) as per Backes D., et al.18 All aneurysms were assumed to start small and then either stay small or grow. Similarly, the risk of rupture was determined by size < or ≥ 5 mm19 and was considered to be equivalent to that of aneurysms occurring in the general population in the base-case. In sensitivity analysis, the hazard rate ratio for risk of rupture in CoA relative to the general population was varied from 0.75 to 2.0 to determine the effect on model results. Risk of rupture of large aneurysms was modeled as a hazard rate ratio of risk of rupture for small aneurysms to facilitate sensitivity analyses. Probability of death following SAH was age-dependent.28 Probabilities of disability or death following IA clipping or endovascular treatment were determined by a secondary analysis of data from a pragmatic meta-analysis of clipping versus coiling for IA including only studies that reported mortality and disability (Supplemental Table 1).26 Random effects method was used to account for between study variance. Following survival of SAH without disability, annual mortality was determined by age- and sex-matched general population CDC life tables, SMR for CoA, and relative survival ratio for SAH compared to general population.13,14,22 Annual mortality due to disability secondary to SAH or prophylactic treatment was determined from a study of aneurysmal SAH survivors in nursing homes.23
Utilities
Health-related quality of life values (utilities) were assigned to all health states from the literature with Death given a utility of 0 by convention (Table 2)30–33. The negative effect of a true or false positive screen was assumed to persist for three months.10,32 The positive effect of a negative screen was not included in the base-case to avoid biasing the results in favor of screening. As data regarding the transient utility decrement for prophylactic treatment were lacking, patients received a short-term utility deduction equivalent to the average length of stay of prophylactic clipping (4.1 days) or endovascular treatment (2.2 days).33 Quality of life data were derived from adults and assumed to be equivalent in children and adolescents due to absence of validated utility measures in the pediatric population with CoA.
Costs
The model applies the payer perspective. Costs for MRA, CTA, aneurysm treatment, SAH, and long-term disability were derived from the Centers for Medicare and Medicaid Services (CMS) and previously published estimates.34–36 In the base-case, the non-contrast MRA excluded the additional cost of a brain MRI, which may occasionally be obtained in some cases. As cost of prophylactic treatment varies according to the outcome, individual costs were assigned for clipping or endovascular treatment complicated by disability or death. The 95% confidence intervals for cost of MRA and CTA were derived from the CMS geographic ranges and calculated as per Hozo et al.37 Both costs and effects were discounted at an annual rate of 3% per recommendations from the Second Panel on Cost-Effectiveness in Health and Medicine.38 A half-cycle correction was applied. Costs were standardized to 2019 US dollars to account for inflation.
Cohort Analysis
Simulated cohort analyses for 10,000 individuals were performed to determine clinical outcomes, including the life expectancy, quality-adjusted life expectancy, lifetime cumulative incidence of prophylactic IA treatment and resulting mortality, and SAH deaths prevented. To estimate the life expectancy, the software tracked the number of patients in each health state at the end of each simulated year with each alive patient contributing 1 person-year to the total survival of the cohort. Quality-adjusted life expectancy reduces life expectancy by a quality-adjustment factor, e.g., if a disabling stroke has a quality of life of 0.2, these individuals only receive credit for 0.2 quality-adjusted life years. The computer simulation tracked the proportion of the cohort developing an aneurysm (any size, known or unknown) and similarly tracked the cumulative probability of aneurysm rupture (status post rupture, and dead secondary to aneurysm rupture) or prophylactic treatment (surgical or endovascular treatment).
Cost-effectiveness Analysis
The primary outcome of the cost-effectiveness analysis is the incremental cost-effectiveness ratio (ICER), which is the additional cost per additional benefit, as measured in QALYs, when comparing strategies. Strategies are ranked from lowest to highest costs, and then the effectiveness measures are compared. Strategies with higher costs but lower effectiveness are eliminated from consideration. The remaining strategies are compared with the next cheaper one based on the absolute difference in costs divided by the difference in effectiveness, yielding the ICER. Less efficient strategies (those with higher ICER than a more expensive strategy) are excluded. As recommended by the AHA/ACC panel on economic evaluation in cardiovascular disease, we considered cost-effective strategies to be those falling below a willingness-to-pay threshold of $150,000 per QALY gained.39
Sensitivity Analyses
One-way and two-way sensitivity analyses were performed to determine the impact of parameter uncertainty on the primary outcome. Upper and lower bounds used in sensitivity analyses were based on 95% CIs unless otherwise noted. Probabilistic sensitivity analyses with Monte Carlo simulation were performed to explore stochastic uncertainty (random variability in outcomes between identical patients). These analyses examine the effect of varying all parameters by sampling the value of each model parameter from mathematical probability distributions (Tables 1 and 2). We used a hypothetical cohort of 10,000 persons with 10,000 simulations.
Results
Cohort Analysis
Screening for IA in patients with CoA reduced the risk of SAH and increased life expectancy compared with no screening. In a lifetime simulation of 10,000 CoA patients, no screening resulted in a 10.1% lifetime risk of SAH at a median age of 44 years with 183 SAH-related deaths and 89 cases of permanent disability (Figure 2, Table 3). Screening at ages 10, 20 and 30 years led to 978 prophylactic treatments for unruptured IAs at a median age of 28 years with 19 procedure-related deaths (Table 3). Treatment, however, prevented 118 deaths from SAH and increased the median rupture age to 53 years (Figure 2, Table 3).
Figure 2.
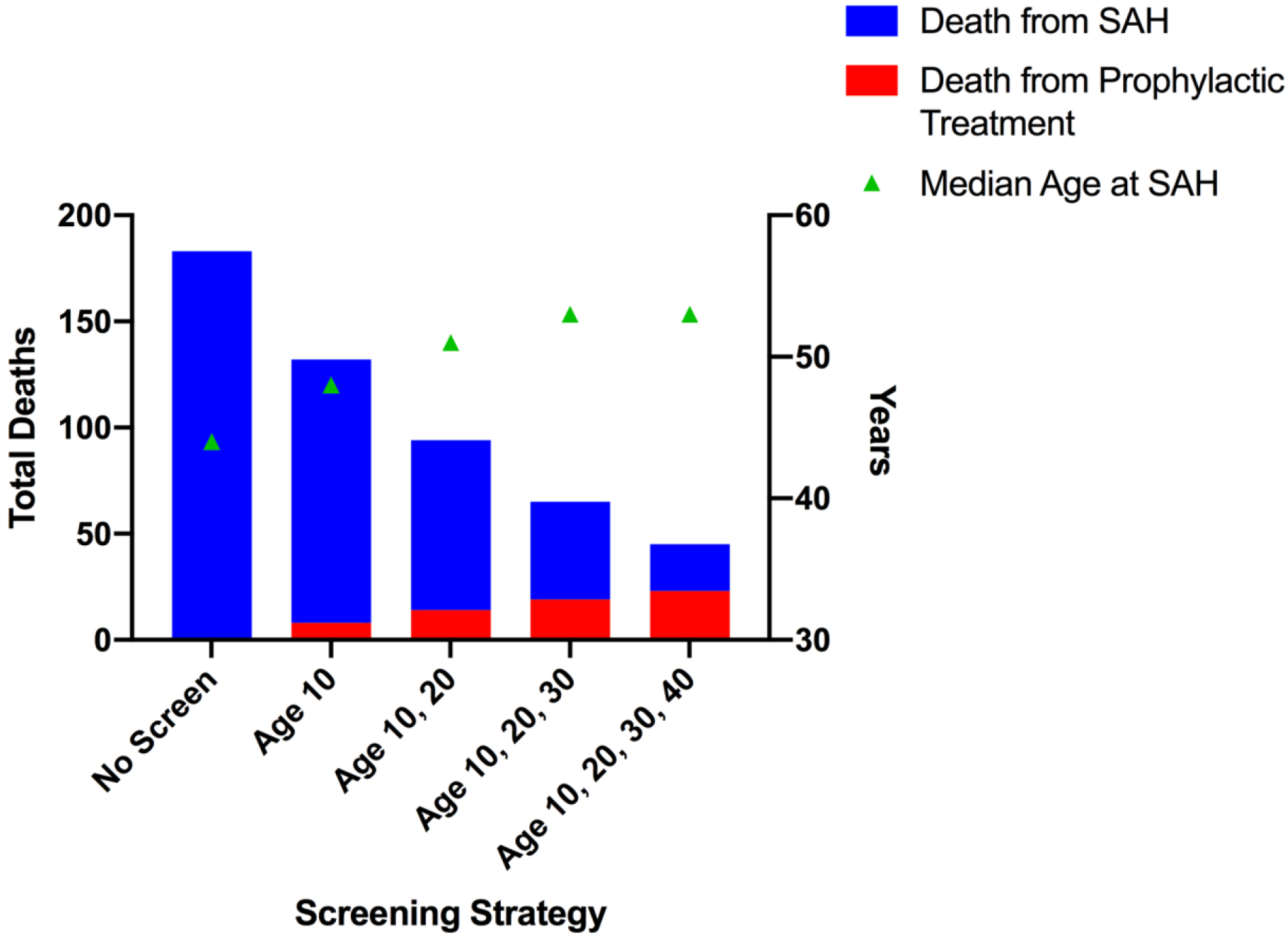
Each bar represents a different screening strategy with increased screening episodes to the right. The left y-axis is total deaths due to subarachnoid hemorrhage (blue; SAH) and prophylactic treatment of an unruptured intracranial aneurysm (red) and the right y-axis is median age at SAH (years) according to screening strategy.
Table 3.
Clinical Outcomes, Costs, and Incremental Cost-Effectiveness Ratios for Screening Strategies for 10,000 Patients with CoA
| Screening Strategy | Prophylactic Treatment*† | SAH* | LE | QALY‡ | Cost‡ | ICER | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Death | MC | All | Death | MC | (yr) | (yr) | ($) | ($/QALY) | |
| No Screen | 0 | 0 | 0 | 1013 | 183 | 272 | 57.86 | 24.212 | 90 | Reference |
| Age 10 | 381 | 8 | 61 | 729 | 132 | 197 | 57.99 | 24.246 | 1,617 | 45,921 |
| Age 10, 20 | 701 | 14 | 107 | 515 | 94 | 140 | 58.06 | 24.260 | 2,562 | 65,243 |
| Age 10, 20, 30 | 978 | 19 | 144 | 353 | 65 | 96 | 58.08 | 24.266 | 3,157 | 106,841 |
| Age 10, 20, 30, 40 | 1203 | 23 | 171 | 244 | 45 | 67 | 58.10 | 24.267 | 3,513 | 265,764 |
MC = major complication (death or permanent disability); SAH = subarachnoid hemorrhage; LE = life expectancy; yr = year; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life expectancy
Number experiencing the outcome in a population of 10,000 patients with CoA
Only initial treatment, excludes re-treatment
QALYs and costs discounted at 3% per year
Cost-effectiveness Analysis
Screening at ages 10, 20 and 30 years was cost-effective versus screening at ages 10 and 20 (ICER $106,841/QALY) at a willingness-to-pay threshold of $150,000 per QALY gained (Table 3). Strategies screening more frequently than every 10 years were not cost-effective because they were more costly with fewer QALYs or less efficient (higher ICER than a more expensive option) (Supplemental Table 2). An additional screen at the age of 40, beyond ages 10, 20, and 30 years, was not cost-effective (ICER $265,764) and screening beyond age 40 reduced QALYs (Supplemental Table 2).
Sensitivity Analyses
The tornado diagram in Figure 3 illustrates results from one-way sensitivity analyses examining the impact of uncertainty with respect to each input parameter on the ICER estimate comparing screening at ages 10, 20 and 30 years to screening at only 10 and 20 years. Screening at ages 10 and 20 becomes preferred when risk of death or disability following prophylactic clipping exceeds 10.4% or quality of life (utility) post SAH exceeds 0.84. Variables that accounted for less than 0.1% of total uncertainty were excluded. Additional tornado diagrams comparing alternative strategies are available in Supplemental Figure 3, and a one-way sensitivity analysis of probability of a small aneurysm growing ≥ 5 mm is available in Supplemental Figure 4.
Figure 3.
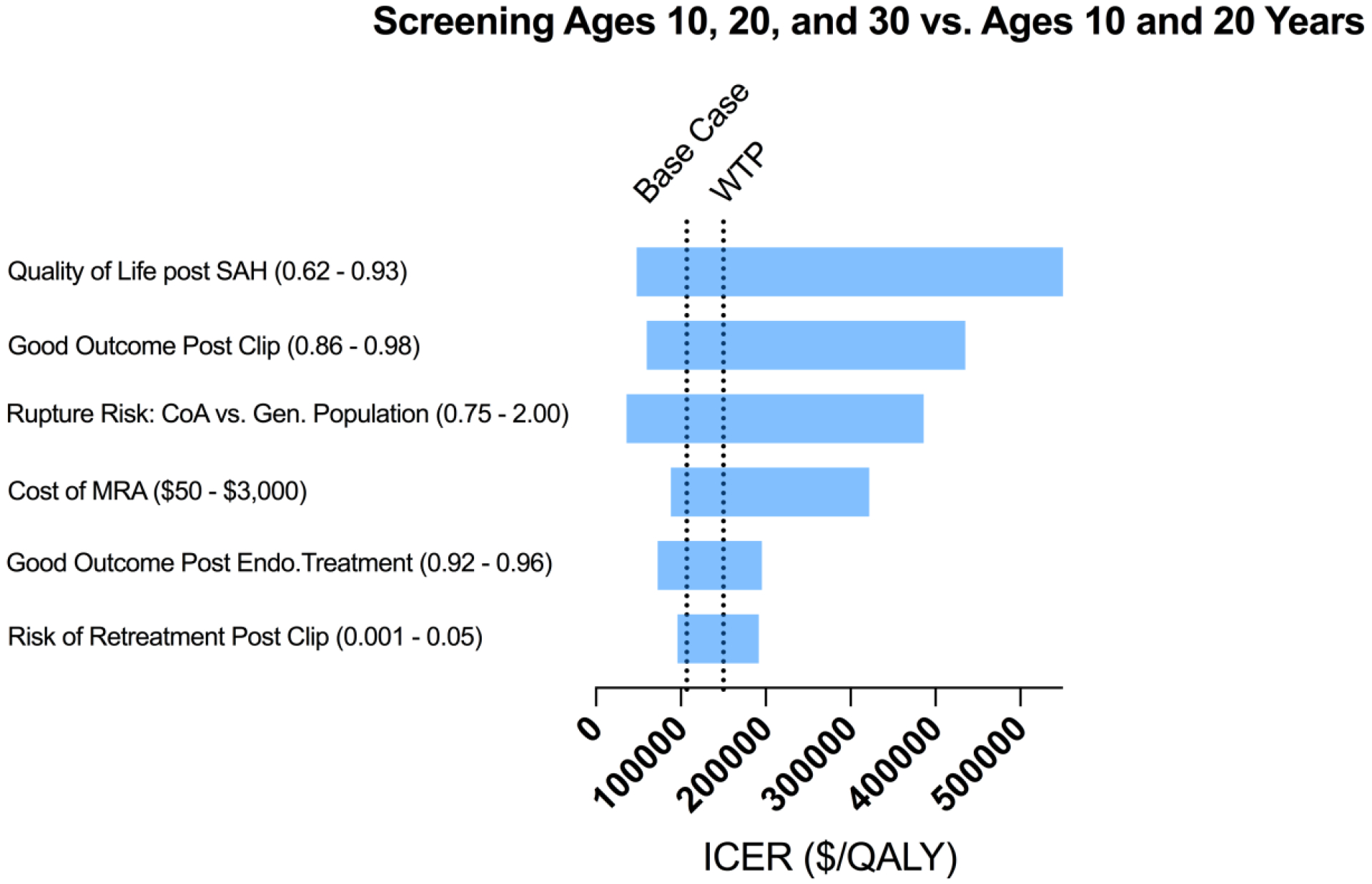
A tornado diagram of one-way sensitivity analyses to demonstrate the effects of varying parameters on the incremental cost-effectiveness ratio (ICER) for screening at ages 10, 20 and 30 years versus at ages 10 and 20 years. The wider bars at the top have the greatest effect on the ICER [quality of life post subarachnoid hemorrhage (SAH)], while variations in inputs at the bottom have small effects. The willingness-to-pay (WTP) line is at an ICER of $150,000. Variables that accounted for less than 0.1% of total uncertainty were excluded from the diagram. Numbers in parentheses after the variables are the parameter ranges.
When considering all strategies, screening at ages 10, 20, and 30 remained cost-effective through the entire plausible range of IA development rates. If the hazard rate of IA rupture in patients with CoA exceeded 1.2 times the general population then screening at ages 10, 20, 30, and 40 years became cost-effective. We selected the base-case SMR of 4.3 (95% CI 3.7–5.0)) from a US-based multicenter registry as it best represented the target screening population and sensitivity analysis over the 95% CI did not alter the preferred strategy. In extended sensitivity analysis for countries such as the UK with reported lower SMR, the model supports an additional screen at age 40 if SMR is less than 2.8.13,40 If the cost of MRA surpassed $828, which is nearly three times greater than the average CMS reimbursement, screening at ages 10, 20 and 30 years would no longer be cost-effective and screening only at ages 10 and 20 would be the optimal strategy.
In probabilistic sensitivity analysis, screening at age 10, 20 and 30 years, vs. 10 and 20 years only, was cost-effective in 41% of simulations; screening at ages 10 and 20 years vs. 10 years only was cost-effective in 59% of simulations. No screening was not cost-effective in any simulations when willing to spend $150,000 per QALY gained. Figure 4 displays these results as a cost-effectiveness acceptability curve, showing the probabilities at which each screening strategy would be considered cost-effective for various willingness-to-pay cost-effectiveness thresholds.
Figure 4.
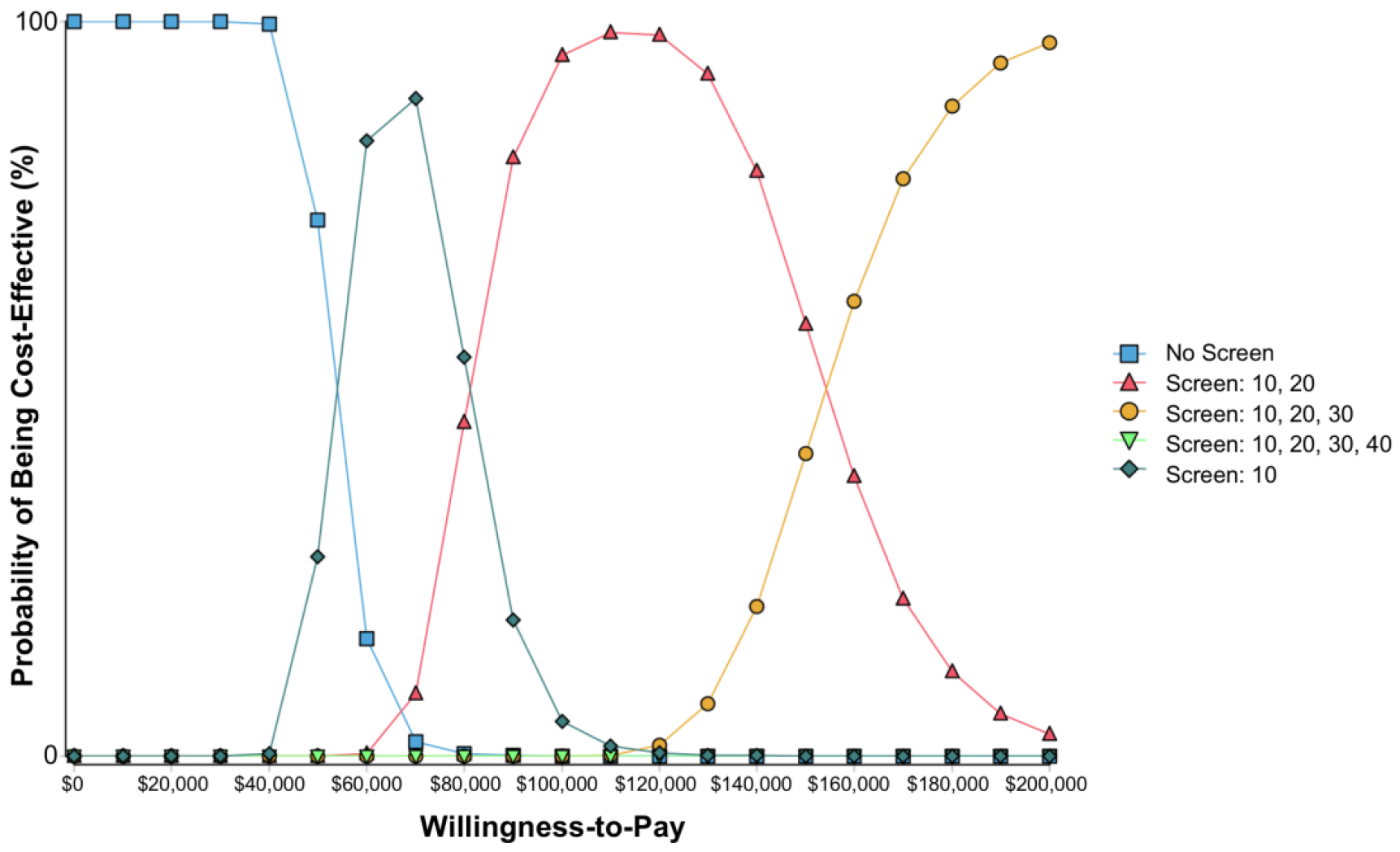
Cost-effectiveness acceptability curve. The y-axis shows the likelihood that each screening strategy would be cost-effective for a given willingness-to-pay cost-effectiveness threshold (x-axis). As the willingness-to-pay increases, increasing additional screening is recommended. Note that Screen at age 10, 20, 30 and 40 is not cost-effective at any of these willingness-to-pay thresholds. Each color represents a different screening strategy.
Discussion
Our calibrated CoA Markov model to evaluate the clinical benefits and cost-effectiveness of screening strategies for IA in patients with CoA supports the AHA/ACC recommendation to screen patients with CoA for IA and suggests that screening at ages 10 and 20 (per the probabilistic sensitivity analysis) or at 10, 20 and 30 years (per the base-case deterministic analysis) would prevent SAH, extend life, and be cost-effective.
The deterministic analysis applies our best point estimates (fixed numbers) of all parameters. It predicts the overall average outcome when accounting for the uncertainties of having good or bad outcomes. In this case, the strategy to screen at ages 10, 20, and 30 is preferred because it yields the highest life expectancy gain at an ICER below the $150,000 willingness-to-pay threshold. This is the optimal choice for patients and clinicians if the goal is to maximize life expectancy at an acceptable societal willingness to pay threshold. Although parameters must be estimated, there remains some uncertainty surrounding each parameter value. Results from our probabilistic sensitivity analyses showed that screening at ages 10 and 20 years vs. screening at 10 years only had higher likelihood of an ICER below the willingness-to-pay threshold (59%) than screening at ages 10, 20 and 30 years vs. 10 and 20 years only (41% of estimates < $150,000/QALY gained). This would be the optimal choice for a policymaker who wants to have the highest likelihood that the strategy will be “cost-effective.”
In the base-case analysis, screening beyond age 40 did not increase QALYs due to the morbidity associated with IA screening and treatment relative to the time necessary for an aneurysm to develop, grow, and potentially rupture. However, if the life-expectancy of patients with CoA increases (e.g., the SMR falls) or if patients with CoA have a higher risk of rupture than the general population (base-case analysis assumes risk equivalent to general population), continued screening later in life may be beneficial and cost-effective. For comparison, a prior model of screening strategies for people with a first-degree relative with SAH, who are also at increased risk of IA, found that screening at ages 40 and 55 was cost-effective with a willingness to pay threshold of €20,000/QALY.10 Our model supports screening at an earlier age in CoA than in those with a family history of SAH in part due to the higher prevalence of IAs at younger ages and the increased mortality from other causes in patients with CoA.10,11
Our model predicted a lifetime risk of SAH in the absence of screening of 10.1% (median age 44 years). Lanz et al. estimated a 9.5% cumulative risk of stroke (ischemic or hemorrhagic) in patients with left-sided congenital heart disease, which may be an underestimate due to considering only ages 18 to 65. A Swedish registry study found only a 1% probability of SAH in patients with CoA up to the age of 40; however, given our model’s estimated median age of SAH of 44 years, over half of SAHs may have been missed in their cohort.41 The model estimated median age of SAH is older than previously reported (44 vs. 23 years).7 One possible explanation is that our model is calibrated to published prevalence estimates at different ages and extends the time horizon to a lifetime. The risk of IA rupture in patients with CoA may exceed that in the general population and thus the model may overestimate the median age of SAH. The relatively rarity of the condition limits accurate risk assessment. Or alternatively, younger patients may have been preferentially included in the prior administrative database study resulting in selection bias and underestimation of the median age of SAH. Frequent screening could theoretically eliminate the risk of SAH in CoA if it were not for the associated morbidity and mortality of prophylactic treatment. As IA treatment strategies improve through advances in endovascular therapy, more frequent screening may become clinically beneficial.
Aneurysm development rates were derived from calibrating the model to cross-sectional prevalence estimates. The patients included in these cross-sectional studies were sampled from tertiary centers and repaired during an earlier operative era when patients were often older.5,6,17,42 It is unknown if repair at a younger age or aggressive management of hypertension prevent IA formation in CoA. Therefore, the development rate may be an overestimate in younger patients with CoA. Sensitivity analyses, however, reveal that even if the model is overestimating risk of IA development and rupture, screening remains the preferred strategy with regards to life expectancy and cost. In none of the probabilistic sensitivity analysis simulations was “no screening” the preferred strategy. Centers should continue to report IA outcomes as they care for CoA patients to inform these estimates and further research should be done to determine if treatment of risk factors reduces IA formation in CoA.
Besides the above assumptions, additional limitations merit consideration. First, the correlation between rupture risk and aneurysm growth is tenuous, especially for small aneurysms. While we selected best available evidence on rupture risk for small IAs, there remains significant patient-level heterogeneity and variability in clinical practice.43,44 As more data emerge on risk features of small IAs, these should be incorporated in the model. Second, quality of life parameters were derived from adult patients and assumed to apply to children and adolescents due to the relative rarity of SAH and absence of these data in the pediatric population. It is unknown if the reduction in quality of life following an event differs in children compared with adults. Third, anatomic distribution of IAs in patients with CoA remains unknown, so we stratified rupture risk only by size and not location. If future research determines that the distribution pattern of IAs in CoA differs from that in the general population, this information should be incorporated into the model. Fourth, we accounted for the negative impact of a positive screen on quality of life, but did not account for the positive impact of a negative screen or the potential negative impact of forgoing screening and its associated anxiety and possible regret if a SAH occurs. There is some evidence that a negative screen in the setting of a known risk factor improves quality of life; however, we elected to exclude this potential to avoid biasing the results in favor of frequent screening.45 Fifth, costs included in the model are nationally representative and may underestimate cost for younger individuals who are privately or commercially insured per Congressional Budget Office analyses.46,47 Finally, we do not consider the ionizing radiation risks associated with endovascular treatment and lifelong surveillance by CTA for those who have undergone surgical clipping. A prior simulation model estimates an excess lifetime risk of cerebral malignancy of 0.115% for a 30-year-old man undergoing yearly surveillance to age 81.48 Patients with congenital heart disease have an increased incidence of malignancy, so consideration should be given to minimizing unnecessary radiation in this population.49,50
In conclusion, our model supports the AHA/ACC recommendation to screen patients with CoA for IA and suggests screening at ages 10 and 20 or at 10, 20 and 30 years would extend life and be cost-effective.
Supplementary Material
What is Known
Patients with coarctation of the aorta have a high prevalence of intracranial aneurysms and suffer subarachnoid hemorrhage at younger ages than the general population.
What the Study Adds
Our model supports the American Heart Association and American College of Cardiology recommendation to screen patients with coarctation of the aorta for intracranial aneurysms.
Screening magnetic resonance angiography at ages 10 and 20 or at 10, 20 and 30 years would extend life and be cost-effective at a willingness-to-pay threshold of $150,000 per quality-adjusted life year.
Funding:
Financial support was provided by the Pediatric Heart Network Scholars Grant (SSP), NIH/NHLBI T32 HL007572-32 (SSP), and the Tupper Research Fund (JBW).
Footnotes
Disclosures: None
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhary P, Canniffe C, Jackson DJ, Tanous D, Walsh K, Celermajer DS. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–1195. doi: 10.1136/heartjnl-2014-307035 [DOI] [PubMed] [Google Scholar]
- 3.Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. doi: 10.1016/j.jacc.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 4.Curtis SL, Bradley M, Wilde P, Aw J, Chakrabarti S, Hamilton M, Martin R, Turner M, Stuart AG. Results of Screening for Intracranial Aneurysms in Patients with Coarctation of the Aorta. AJNR Am J Neuroradiol. 2012;33:1182–1186. doi: 10.3174/ajnr.A2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SC, Hickey J, Maul TM, Zumberge N, Krieger EV., Valente AM, Zaidi AN, Daniels CJ. Assessment of the Cerebral Circulation in Adults with Coarctation of the Aorta. Congenit Heart Dis. 2013;8:289–295. doi: 10.1111/chd.12024 [DOI] [PubMed] [Google Scholar]
- 6.Egbe AC, Padang R, Brown RD, Khan AR, Luis SA, Huston J, Akintoye E, Connolly HM. Prevalence and predictors of intracranial aneurysms in patients with bicuspid aortic valve. Heart. 2017;103:1508–1514. doi: 10.1136/heartjnl-2016-311076 [DOI] [PubMed] [Google Scholar]
- 7.Pickard SS, Gauvreau K, Gurvitz M, Gagne JJ, Opotowsky AR, Jenkins KJ, Prakash A. Stroke in Adults With Coarctation of the Aorta: A National Population‐Based Study. J Am Heart Assoc. 2018;7:e009072. doi: 10.1161/JAHA.118.009072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 9.Li M-H, Chen S-W, Li Y-D, Chen Y-C, Cheng Y-S, Hu D-J, Tan H-Q, Wu Q, Wang W, Sun Z-K, Wei X-E, Zhang J-Y, Qiao R-H, Zong W-H, Zhang Y, Lou W, Chen Z-Y, Zhu Y, Peng D-R, Ding S-X, Xu X-F, Hou X-H, Jia W-P. Prevalence of unruptured cerebral aneurysms in Chinese adults aged 35 to 75 years: a cross-sectional study. Ann Intern Med. 2013;159:514–521. doi: 10.7326/0003-4819-159-8-201310150-00004 [DOI] [PubMed] [Google Scholar]
- 10.Hopmans EM, Ruigrok YM, Bor AS, Rinkel GJ, Koffijberg H. A cost-effectiveness analysis of screening for intracranial aneurysms in persons with one first-degree relative with subarachnoid haemorrhage. Eur Stroke J. 2016;1:320–329. doi: 10.1177/2396987316674862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bor ASE, Koffijberg H, Wermer MJH, Rinkel GJE. Optimal screening strategy for familial intracranial aneurysms: a cost-effectiveness analysis. Neurology. 2010;74:1671–1679. doi: 10.1212/WNL.0b013e3181e04297 [DOI] [PubMed] [Google Scholar]
- 12.Dahabreh IJ, Trikalinos TA, Balk EM, Wong JB. Recommendations for the Conduct and Reporting of Modeling and Simulation Studies in Health Technology Assessment. Ann Intern Med. 2016;165:575. doi: 10.7326/M16-0161 [DOI] [PubMed] [Google Scholar]
- 13.Spector LG, Menk JS, Knight JH, McCracken C, Thomas AS, Vinocur JM, Oster ME, St Louis JD, Moller JH, Kochilas L. Trends in Long-Term Mortality After Congenital Heart Surgery. J Am Coll Cardiol. 2018;71:2434–2446. doi: 10.1016/j.jacc.2018.03.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). US Department of Health and Human Services. No Title. National Health and Nutrition Examination Survey Data
- 15.Sailer AMH, Wagemans BAJM, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing Intracranial Aneurysms With MR Angiography. Stroke. 2014. January;45(1):119–26. doi: 10.1161/STROKEAHA.113.003133 [DOI] [PubMed] [Google Scholar]
- 16.Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better Outcomes with Treatment by Coiling Relative to Clipping of Unruptured Intracranial Aneurysms in the United States, 2001–2008. Am J Neuroradiol. 2011;32:1071–1075. doi: 10.3174/ajnr.A2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis SL, Bradley M, Wilde P, Aw J, Chakrabarti S, Hamilton M, Martin R, Turner M, Stuart AG. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol. 2012;33:1182–1186. doi: 10.3174/ajnr.A2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backes D, Rinkel GJE, Greving JP, Velthuis BK, Murayama Y, Takao H, Ishibashi T, Igase M, terBrugge KG, Agid R, Jääskeläinen JE, Lindgren AE, Koivisto T, von Und Zu Fraunberg M, Matsubara S, Moroi J, Wong GKC, Abrigo JM, Igase K, et al. ELAPSS score for prediction of risk of growth of unruptured intracranial aneurysms. Neurology. 2017;88:1600–1606. doi: 10.1212/WNL.0000000000003865 [DOI] [PubMed] [Google Scholar]
- 19.Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I, Arakawa H, Irie K, Urashima M, Molyneux AJ. Risk Analysis of Unruptured Intracranial Aneurysms. Stroke. 2016;47:365–371. doi: 10.1161/STROKEAHA.115.010698 [DOI] [PubMed] [Google Scholar]
- 20.Ferns SP, Sprengers MES, van Rooij WJ, Rinkel GJE, van Rijn JC, Bipat S, Sluzewski M, Majoie CBLM. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40:e523–9. doi: 10.1161/STROKEAHA.109.553099 [DOI] [PubMed] [Google Scholar]
- 21.McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, Albuquerque FC. The Barrow Ruptured Aneurysm Trial. J Neurosurg. 2012;116:135–144. doi: 10.3171/2011.8.JNS101767 [DOI] [PubMed] [Google Scholar]
- 22.Huhtakangas J, Lehto H, Seppä K, Kivisaari R, Niemelä M, Hernesniemi J, Lehecka M. Long-Term Excess Mortality After Aneurysmal Subarachnoid Hemorrhage. Stroke. 2015;46:1813–1818. doi: 10.1161/STROKEAHA.115.009288 [DOI] [PubMed] [Google Scholar]
- 23.Greebe P, Rinkel GJ, Algra A. Long-Term Outcome of Patients Discharged to a Nursing Home After Aneurysmal Subarachnoid Hemorrhage. Arch Phys Med Rehabil. 2010;91:247–51. doi: 10.1016/j.apmr.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 24.Michalski AM, Richardson SD, Browne ML, Carmichael SL, Canfield MA, Vanzutphen AR, Anderka MT, Marshall EG, Druschel CM. Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997–2009. Am J Med Genet Part A. 2015;167:1071–1081. doi: 10.1002/ajmg.a.36865 [DOI] [PubMed] [Google Scholar]
- 25.Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol. 2011;32:1071–1075. doi: 10.3174/ajnr.A2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk Delgado A, Andersson T, Falk Delgado A. Clinical outcome after surgical clipping or endovascular coiling for cerebral aneurysms: a pragmatic meta-analysis of randomized and non-randomized trials with short- and long-term follow-up. J Neurointerv Surg. 2017;9:264–277. doi: 10.1136/neurintsurg-2016-012292 [DOI] [PubMed] [Google Scholar]
- 27.Sailer AMH, Wagemans BAJM, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing Intracranial Aneurysms With MR Angiography: Systematic Review and Meta-Analysis. Stroke. 2014;45:119–126. doi: 10.1161/STROKEAHA.113.003133 [DOI] [PubMed] [Google Scholar]
- 28.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 29.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet (London, England). 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6 [DOI] [PubMed] [Google Scholar]
- 30.Berghammer M, Karlsson J, Ekman I, Eriksson P, Dellborg M. Self-reported health status (EQ-5D) in adults with congenital heart disease. Int J Cardiol. 2013;165:537–543. doi: 10.1016/j.ijcard.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 31.Post PN, Stiggelbout AM, Wakker PP. The Utility of Health States After Stroke A Systematic Review of the Literature. Stroke. 2001;32:1425–9. doi: 10.1161/01.str.32.6.1425. [DOI] [PubMed] [Google Scholar]
- 32.Wermer MJH, van der Schaaf IC, Van Nunen P, Bossuyt PMM, Anderson CS, Rinkel GJE. Psychosocial Impact of Screening for Intracranial Aneurysms in Relatives With Familial Subarachnoid Hemorrhage. Stroke. 2005;36:836–840. doi: 10.1161/01.STR.0000158906.79898.3a [DOI] [PubMed] [Google Scholar]
- 33.Duan Y, Blackham K, Nelson J, Selman W, Bambakidis N. Analysis of short-term total hospital costs and current primary cost drivers of coiling versus clipping for unruptured intracranial aneurysms. J Neurointerv Surg. 2015;7:614–618. doi: 10.1136/neurintsurg-2014-011249 [DOI] [PubMed] [Google Scholar]
- 34.Physician Fee Schedule. Centers for Medicaid and Medicare Services. Published 2019. https://www.cms.gov/apps/physician-fee-schedule/
- 35.Brinjikji W, Kallmes DF, Lanzino G, Cloft HJ. Hospitalization costs for endovascular and surgical treatment of unruptured cerebral aneurysms in the United States are substantially higher than medicare payments. AJNR Am J Neuroradiol. 2012;33:49–51. doi: 10.3174/ajnr.A2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfeld KS, Stevenson DG, Hamel MB, Mitchell SL. Medicare Expenditures Among Nursing Home Residents With Advanced Dementia. Arch Intern Med. 2011;171:824–830. doi: 10.1001/archinternmed.2010.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses. JAMA. 2016;316:1093. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 39.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures. J Am Coll Cardiol. 2014;63:2304–2322. doi: 10.1016/j.jacc.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 40.Diller G-P, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA, Ph DPD. Survival Prospects and Circumstances of Death in Contemporary Adult Congenital Heart Disease Patients Under Follow-Up at a Large Tertiary Centre Running title: Diller et al.; Survival Prospects in Contemporary ACHD Patients. Circulation. 2015;132:2118–2125. doi: 10.1161/CIRCULATIONAHA.115.017202 [DOI] [PubMed] [Google Scholar]
- 41.Giang KW, Mandalenakis Z, Dellborg M, Lappas G, Eriksson P, Hansson P-O, Rosengren A. Long-Term Risk of Hemorrhagic Stroke in Young Patients With Congenital Heart Disease. Stroke. 2018;49:1155–1162. doi: 10.1161/STROKEAHA.117.020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raissadati A, Nieminen H, Jokinen E, Sairanen H. Progress in Late Results Among Pediatric Cardiac Surgery Patients. Circulation. 2015;131:347–353. doi: 10.1161/CIRCULATIONAHA.114.011190 [DOI] [PubMed] [Google Scholar]
- 43.Malhotra A, Wu X, Forman HP, Nardini HKG, Matouk CC, Gandhi D, Moore C, Sanelli P. Growth and rupture risk of small unruptured intracranial aneurysms a systematic review. Ann Intern Med. 2017;167:26–33. doi: 10.7326/M17-0246 [DOI] [PubMed] [Google Scholar]
- 44.Malhotra A, Wu X, Geng B, Hersey D, Gandhi D, Sanelli P. Management of Small Unruptured Intracranial Aneurysms: A Survey of Neuroradiologists. AJNR Am J Neuroradiol. 2018;39:875–880. doi: 10.3174/ajnr.A5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buijs JE, Greebe P, Rinkel GJE. Quality of Life, Anxiety, and Depression in Patients With an Unruptured Intracranial Aneurysm With or Without Aneurysm Occlusion. Neurosurgery. 2012;70:868–872. doi: 10.1227/NEU.0b013e3182367295 [DOI] [PubMed] [Google Scholar]
- 46.Pelech D An Analysis of Private-Sector Prices for Physician Services In: Academy Health Annual Research Meeting. Congressional Budget Office; 2017. [Google Scholar]
- 47.Maeda JL, Nelson L. An Analysis of Hospital Prices for Commercial and Medicare Advantage Plans In: Academy Health Annual Research Meeting. Congressional Budget Office; 2017. [Google Scholar]
- 48.Malhotra A, Wu X, Chugh A, Mustafa A, Matouk CC, Gandhi D, Sanelli P. Risk of Radiation-Induced Cancer From Computed Tomography Angiography Use in Imaging Surveillance for Unruptured Cerebral Aneurysms. Stroke. 2019;50:76–82. doi: 10.1161/STROKEAHA.118.022454 [DOI] [PubMed] [Google Scholar]
- 49.Gurvitz M Increasing Evidence for and a Word of Caution About an Association Between Cancer and Congenital Heart Disease. JAMA Netw Open. 2019;2:e196756. doi: 10.1001/jamanetworkopen.2019.6756 [DOI] [PubMed] [Google Scholar]
- 50.Gurvitz M, Ionescu-Ittu R, Guo L, Eisenberg MJ, Abrahamowicz M, Pilote L, Marelli AJ. Prevalence of Cancer in Adults With Congenital Heart Disease Compared With the General Population. Am J Cardiol. 2016;118:1742–1750. doi: 10.1016/j.amjcard.2016.08.057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


