Abstract
Purpose:
“FLASH” radiotherapy (RT) is a potential paradigm-changing RT technology with marked tumor killing and normal tissue sparing. However, the mechanism of the FLASH effect is not well understood. We hypothesize that the ultra-high dose rate FLASH-RT significantly reduces the killing of circulating immune cells which may partially contribute to the reported FLASH effect.
Methods:
This computation study directly models the effect of radiation dose rate on the killing of circulating immune cells. The model considers an irradiated volume that takes up A% of cardiac output and contains B% of total blood. The irradiated blood volume and dose were calculated for various A%, B%, blood circulation time, and irradiation time (which depends on the dose rate). The linear-quadratic model was used to calculate the extent of killing of circulating immune cells at ultra-high vs. conventional dose rates.
Results:
A strong sparing effect on circulating blood cells by FLASH-RT was noticed; i.e., killing of circulating immune cells reduced from 90-100% at conventional dose rates to 5-10% at ultra-high dose rates. The threshold FLASH dose rate was determined to be ~40 Gy/second for mice in an average situation (A% = 50%), consistent with the reported FLASH dose rate in animal studies, and it was approximately one order of magnitude lower for humans than for mice. The magnitude of this sparing effect increased with the dose/fraction, reached a plateau at 30-50 Gy/fraction, and almost completely vanished at 2 Gy/fraction.
Conclusion:
We have calculated a strong sparing effect on circulating immune cells by FLASH-RT, which may contribute to the reported FLASH effects in animal studies.
Keywords: FLASH Radiotherapy, Circulating immune cells, Radiation induced immune toxicity, Normal tissue toxicity, Tumor control
1. INTRODUCTION
FLASH radiotherapy (RT) delivers ultra-fast radiation treatment with a dose rate several orders of magnitude higher than conventional dose rates [1-7]. It has been reported that FLASH-RT can significantly spare normal tissues in comparison to RT at conventional dose rates, whereas tumor responses are the same as or better than those resulting from conventional dose rate RT [1-7]. This FLASH effect has been demonstrated in various experimental animal models (mice, zebrafish, pigs, cats), various organs (lung, gut, brain, skin) and by various research groups [1-7]. Therefore, FLASH-RT is considered a potential paradigm-changing RT technology [7]. However, the mechanism of the FLASH effect is not well understood.
Oxygen depletion has been proposed to be one mechanism responsible for the FLASH-RT-related normal tissue sparing [8]. Under the extremely high dose rates of FLASH-RT, tissue oxygen is consumed much faster than the transport of oxygen into tissues, resulting in a transient hypoxia and radiation protection. However, one study suggested that a 10-Gy single fraction of radiation would reduce tissue oxygen tension by only 4.2 mm Hg, which is not sufficient to significantly change the oxygen content and induce significant radiation protection [8]. Furthermore, FLASH-RT could deplete oxygen in tumors as well. Therefore, the oxygen depletion theory may not fully explain the reported therapeutic gain with FLASH-RT.
In this study, we hypothesized that FLASH-RT significantly reduces the killing of circulating immune cells and thus may contribute to the reported FLASH effect [9-11]. At an ultra-high FLASH dose rate, a large radiation dose is delivered in a very short time (<0.1 second), so that only those circulating immune cells within the treatment volume are irradiated and killed. On the other hand, at a conventional dose rate, circulating immune cells continuously flow into the irradiated volume with the blood, so that more immune cells are irradiated and killed. Therefore, it is reasonable to consider that FLASH-RT may spare a substantial portion of the circulating immune cells in comparison to a conventional dose rate. In addition, it has been reported that the immune system plays a role in both normal tissue toxicities [12-14] and tumor control [15-17], and radiation-induced lymphopenia is a negative prognostic factor for overall survival of patients treated with RT for various solid tumors [11, 18-21]. Thus, if FLASH-RT could spare circulating immune cells, it would result in normal tissue sparing and therapeutic gain enhancement similar to the reported FLASH effect in animal studies.
2. METHODS AND MATERIALS
2.1. A dose rate-dependent dose model for circulating blood
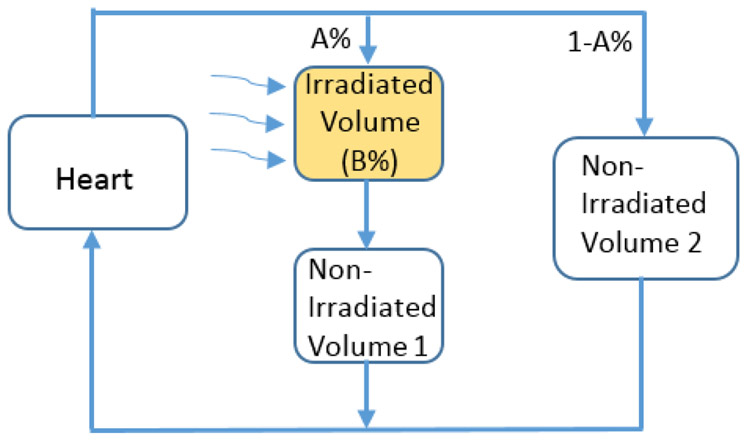
We used a previously described model to compute the dose to circulating immune cells in blood [22-23], which was modified from another blood dose computation model first reported by Yovino et al. [11]. The blood circulation system is composed of the heart and numerous blood-containing organs with complex parallel and/or series connections. As shown in Fig. 1, for a partial irradiation, we assumed that the irradiated volume can be simplified as a single blood-containing organ, and the rest of the non-irradiated organs be simplified as a heart, a series-connection organ (non-irradiated volume 1), and a parallel-connection organ (non-irradiated volume 2). We considered that the cardiac output, which is the amount of blood pumped out from the heart at a unit time, is A0 liters/second, and the total amount of blood in the system is B0 liters. In addition, the percentage cardiac output that flow into the irradiated organ is A%, and the percentage of total amount of blood in the irradiated organ is B%.
Figure 1.
Modeling the dose to the circulating immune cells. A% of cardiac output branches into the path through the irradiated volume and the non-irradiated volume 1, while the remainder (100-A%) of cardiac output flows through the non-irradiated volume 2. B% of total blood is contained in the irradiated volume, while the remainder (100-B%) of blood is contained in the combination of heart, non-irradiated volume 1 and non-irradiated volume 2. The dose received by and volume of the circulating blood can be determined according to the continuality principle. They depend on A%, B%, the dose delivered to the irradiated volume (D0), the delivery time (t), and the blood circulation time for one cycle (T).
Considering a D0 dose delivered to the irradiated organ in one fraction, the delivery time t
| (1) |
We further considered that the blood circulation time for one cycle is T = B0/A0. When t << T, there is almost no blood flow during irradiation so that only B% of blood is irradiated, and the dose to the cells within this volume of blood is D0. When t = T, all the A% of cardiac output in one cycle flows through the volume, and is irradiated with a dose of D = D0*(B%/A%). Therefore, generally, when t ≤ T, the irradiated blood volume is
| (2a) |
and the dose to the irradiated blood is
| (2b) |
When t > T, the irradiated blood completes one cycle of circulation and flows through the irradiated volume again. We assume that the irradiated blood mixes with unirradiated blood completely before flowing into the irradiated volume. Therefore, for the scenario of t = 2*T, the blood is irradiated with 2 cycles. The radiation dose to the blood in each cycle is . The total blood can then be divided into 3 portions: unirradiated, exposed to one cycle of radiation, and exposed to 2 cycles of radiation. The dose and volume of the 3 portions can be calculated as:
| (3a) |
| (3b) |
| (3c), |
where the number “2” in di,2 and vi,2 stands for the number of irradiation cycles.
More generally, when t = n*T, the total blood is irradiated with n cycles. The radiation dose to the blood in each cycle is . Similarly, after j (j = 1,2…n) cycles of irradiation, the blood can be divided into j+1 portions with different doses. We assume that di,j and vi,j(i = 0,1,2…j) are the dose and volume of the ith portion after j cycles of irradiation. Then we have:
| (4a) |
| (4b) |
| (4c) |
| (4n) |
Using equations (2a-2b) and (4a-4n), the blood dose volume distribution under various conditions can be accurately determined. The blood dose volume distribution depends on the dose rate through t in equations (2a-2b), and through n in equations (4a-4n). We have used a program in Excel to calculate the complicated dose volume distribution for equations (4a-4n) for n up to 30 cycles.
2.2. Modeling radiation killing of circulating immune cells
The linear-quadratic model was used to calculate the percentage of killed immune cells in circulating blood. When t ≤ T, the percentage of killed immune cells was calculated as
| (5) |
where α and β are parameters describing the immune cell’s radiosensitivity, and V% is determined from equation (1a).
When t > T, the immune cells move multiple times through the irradiated volume so that various portions of immune cells would receive various doses. The percentage of killed immune cells would be the summation of killed cells in each portion. Thus,
| (6) |
where di,n and vi,n are determined from equations (4a-4n).
2.3. Study of ultra-high dose rate effect for various parameters
Using equations (1-6), we first studied the radiation dose rate effect on killing of circulating immune cells to determine whether there is a significant difference between different dose rates, and whether there is a threshold FLASH dose rate, at which the killing of circulating immune cells is much less than that at a conventional dose rate, and further increasing the dose rate would not significantly reduce the killing. We then studied how various physiological or dosimetric parameters alter the dose rate effect.
The dose rate ranged from 0.1 to 20000 Gy/minute (or 0.0017 to 333 Gy/second) in this study. As indicated in equation (1), D0 affects t and may also be a parameter that affects the dose rate effect. D0 was typically 10-40 Gy in those animal studies that reported FLASH effects [1-7]. Here we studied the effect of D0 values ranging from 2 to 50 Gy. The blood circulation time (T) in one cycle is a physiological parameter. The effect of T on the dose rate effect was studied. The typical value of T for an adult human is 60 seconds [24] and 5-10 seconds for adult mice [24-26].
The parameters of A% and B% reflect the volume and location of the irradiated organ and may vary substantially. We studied the dose rate effect for wide ranges of A% and B%. Typically, we would assign A%=100%, B%~8% if the heart is considered as the irradiated organ; A%=100%, B%~12% for total lung; A%=50%, B%~6% for half lung; A%~20%, B%~10% for whole brain; and A%~5-10%, B%~1-5% for partial skin. For an irradiation involving tumor and multiple organs, B%=10% corresponds to an irradiated volume of 7000 cc for a typical adult human, if blood distribution in the irradiated volume is average. The volume may be much less in blood-rich regions and vice versa. A% would typically be 20-50% depending on the location.
We used α=0.4 Gy−1 and α/β=2 Gy (or β=0.2 Gy−2) for most of the simulations in this study. These data were based on a study that directly measured the radiosensitivity of cultured lymphocytes from blood samples [27], and our own study that models radiation-induced lymphocyte loss during radiotherapy [23]. However, the actual α and β values for immune cells have large uncertainties. The radiosensitivity of the immune cells may vary greatly for different species (humans, mice, rats), for different individuals within the same species, and for different types of immune cells. Therefore, the variation of α and β values on the dose rate effect was also studied.
3. RESULTS
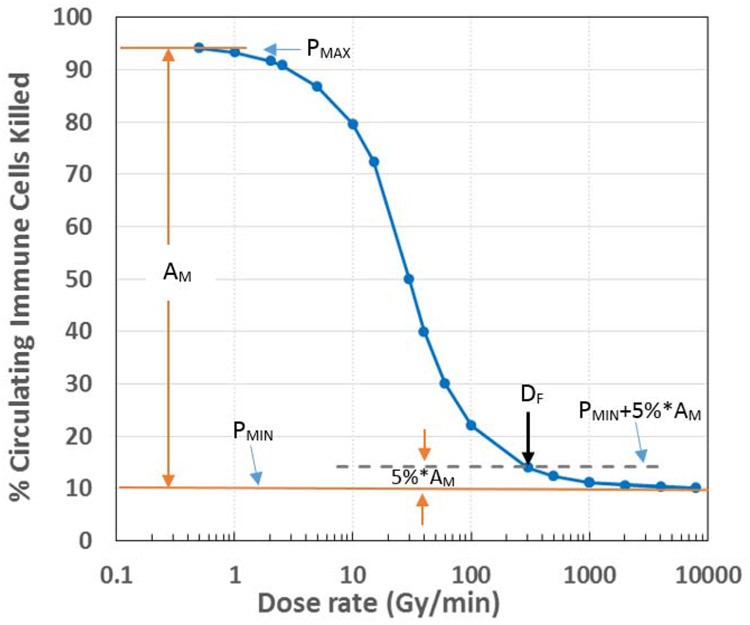
Sparing of circulating immune cells by ultra-high dose rate FLASH-RT is clearly shown in Figure 2 for a typical scenario of A%=50%, B%=10%, D0=30 Gy and T=60 seconds. The percentage of killed circulating immune cells varies with the dose rate, following a sigmoid-shaped curve. At conventional dose rates (<5 Gy/minute), the killing reaches nearly 95%, whereas it is only 10% at extremely high dose rates (>200 Gy/minute). Considering the maximal killing at low dose rate as PMAX, and the minimal killing at the extremely high dose rate as PMIN, the amplitude of this sparing effect is AM = PMAX - PMIN, and the sparing index (SIN), which reflects the effectiveness of the spring effect, is SIN = AM/PMIN. Here we define a threshold FLASH dose rate (DF) as the dose rate at which further increase of the dose rate would reduce the killing by only 5% of AM. That is, the percentage killing at DF is P(DF) = PMIN + 5%*AM, as illustrated in Fig. 2. Thus, at the condition in Fig. 2, AM = 85%, SIN = 8.5, and DF = 280 Gy/minute = 4.7 Gy/second.
Figure 2.
Change of circulating immune cell killing with RT dose rate in a typical situation (A% = 50%, B% = 10%, D0 = 30 Gy, T = 60 seconds). The plot is shown as a sigmoid-shaped curve. We define the maximal percentage of killing at the very low dose rate as PMAX , the minimal percentage killing at the extremely high dose rate as PMIN, and AM = PMAX – PMIN as the amplitude of the sparing effect. We further define the threshold dose rate (DF) as the dose rate at which further increase of dose rate would only reduce killing of immune cells by 5% of the amplitude, or P(DF) = PMIN + 5%*AM. DF ~ 280 Gy/minute in the figure.
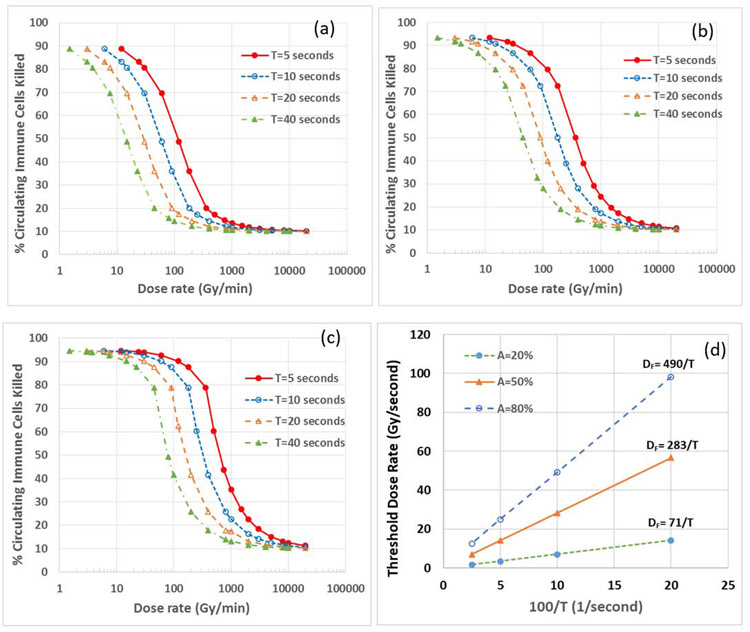
The threshold FLASH dose rate DF is inversely proportional to T, the blood circulation time of one cycle, as shown in Figs. 3a-3c, which show the variation of immune cell killing with dose rate for different values of T(5, 10, 20 and 40 seconds) under 3 different A% values: a) A%=20%, b) A%=50%, and c) A%=80%. The parameter B% was kept constant as B%=10% (as shown later, B% had relatively small effect on DF). The sigmoid curves shift to the left with increasing Tfor all three A% values. Using the definition of DF in Fig. 2, we determined the DF value for each T and A%, and plotted the variation of DF with (1/T), as shown in Fig. 3d. We found that DF can be expressed as DF = k/T, where k is 71, 283 and 490 for A%=20%, 50% and 80%, respectively. Considering that T is ~7 seconds in mice, DF is 10, 40 and 70 Gy/second, for A% = 20%, 50% and 80%, respectively, with DF = 40 Gy/second for the average A% = 50%. Considering that A% is approximately 10% for irradiation volume in skin, 20% for whole brain, 50% for abdomen or half lung, and 100% for heart and total lung, the corresponding DF for these irradiation volumes would be estimated to be 5, 10, 40 and 90 Gy/second, respectively. According to a review article that summarized all the published FLASH effect data [10], the minimal dose rate for FLASH effect was 2.5-17 Gy/second for skin, 30-37 Gy/second for whole brain, 40 Gy/second for lung, and 70 Gy/second for abdomen, suggesting that our estimated DF is on the same order of magnitude as the reported data in the animal studies. It should be noted that for humans, T ~ 60 seconds, the corresponding DF is approximately one order of magnitude lower than that for mice.
Figure 3.
Dependence of the threshold dose rate on the blood circulation time in one cycle (T). Panels a-c show the variation of immune cell killing with the dose rate for different values of T (5, 10, 20 and 40 seconds) under the condition of three different A% values: a) A%=20%, b) A%=50%, and c) A%=80%. Panel d shows the dependence of DF on T. DF = k/T, where k is 71, 283 and 490 for A%=20%, 50% and 80%, respectively.
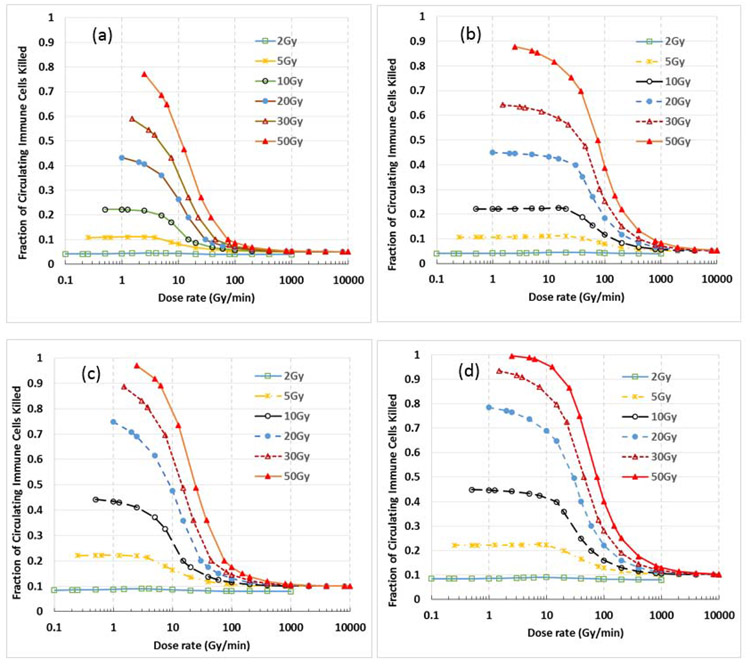
The sparing effect on immune cells by ultra-high dose rate FLASH-RT depends strongly on D0, the delivered dose in one fraction, as shown in Fig. 4, which shows the variation of immune cell killing with dose rate for different D0 values (2, 5, 10, 20, 30 and 50 Gy) under 4 different scenarios: (a) A%=10%, B%=5%; (b) A% =50%, B%=5%; (c) A%=20%, B%=10%; and (d) A%=50%, B%=10%. When D0 ≥30 Gy, the killing of circulating immune cells at conventional dose rates reaches 90-100%, while the killing is only 5-10% for FLASH-RT, depending on the B% value. The Sparing index (SIN is about 8.5 for D0=30 Gy with B%=10%, and it achieves 17 for B%=5%. SIN rapidly reduces with decreasing D0. When D0=5 Gy, SIN is only about 1.2 for both B%=5% and 10%. SIN reduces to 0 when D0=2Gy, indicating no sparing effect when conventional fractionation is used.
Figure 4.
Dependence of sparing effect of immune cells on the dose/fraction (D0). The figure shows the sparing effect in single fraction RT for different D0 values (2, 5, 10, 20, 30 and 50 Gy) under 4 different scenarios: (a) A%=10%, B%=5%; (b) A% =50%, B%=5%; (c) A%=20%, B%=10%; and (d) A%=50%, B%=10%. The magnitude of this sparing effect increases with the dose/fraction, reaches a maximum by 40-50 Gy/fraction, and almost completely vanishes at 2 Gy/fraction.
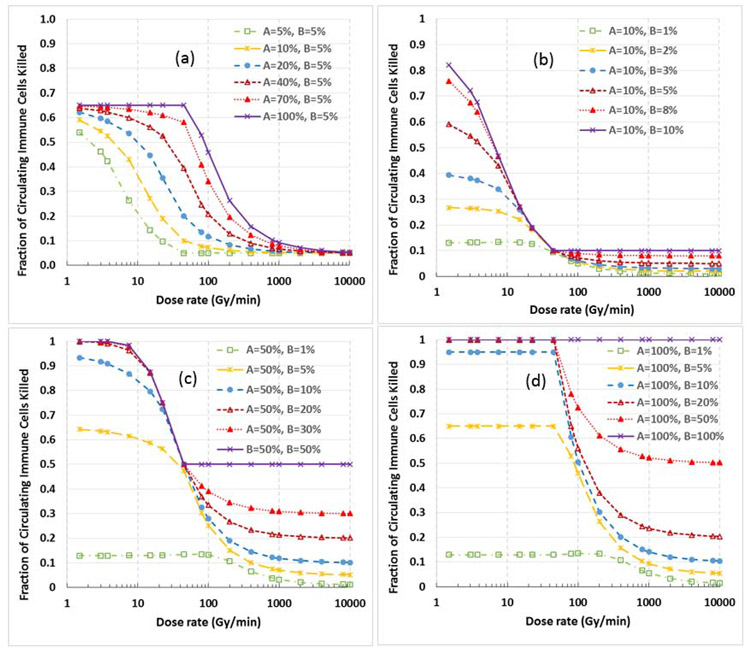
The impact of the parameters A% and B% on the sparing effect are shown in Fig. 5. Specifically, Fig. 5a shows the variation of sparing effect for various A% values (5% to 100%) with parameter B% fixed at 5%. Figs. 5b, 5c, and 5d show the variations of sparing effect for various B% values with parameter A% fixed at 10%, 50% and 100%, respectively. We note from these figures: 1) Parameter A% affects the threshold FLASH dose rate (the larger the A%, the higher the threshold FLASH dose rate). 2) Parameter B% has a relatively small effect on the threshold FLASH dose rate and the sparing index when B% is relatively small (B%<20%); however, the absolute killing of immune cells at all dose rate levels increases with increasing B%. 3) The sparing effect varies less with increasing B% when B% is large (B%>20%), and the sparing effect vanishes when B%=100%.
Figure 5.
Impact of parameters A% and B% on the immune sparing effect. (a) Variation of the sparing effect for various A% values (5% to 100%), with B% fixed at 5%. It shows that the threshold dose rate increases with increasing A% value, while the amplitude of the sparing effect changes little. (b-d) Variations of sparing effect for various B% values with A% fixed at 10% (b); 50% (c) or 100% (d). The parameter B% has a relatively small effect on the threshold dose rate and the sparing effect when B% is relatively small (B%<20%); however, the absolute killing of immune cells at all dose rate levels increases with increasing B%. The sparing effect varies less with increasing B% when B% is large (B%>20%), and the sparing effect completely vanishes when B%=100%.
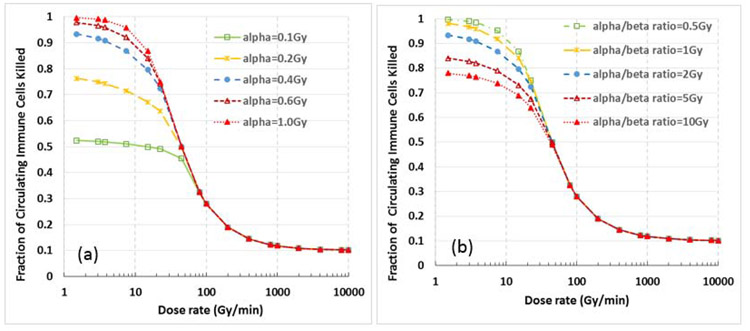
The impact of the parameters α and β (or the radiosensitivity of immune cells) on the sparing effect is illustrated in Fig. 6. Specifically, Fig. 6a shows the killing of circulating immune cells vs. dose rate for various α values with fixed α/β ratio = 2 Gy, and Fig. 6b shows the killing of circulating immune cells vs. dose rate for various α/β ratios with a fixed α = 0.4 Gy−1. These data indicate that 1) different α and β values have a relatively small effect on the threshold FLASH dose rate; 2) the more radiosensitive immune cells, which are represented by larger α values or smaller α/β ratios, would have larger sparing effect (higher sparing index) than the less radiosensitive immune cells.
Figure 6.
Effect of α and β values (or the radiosensitivity of immune cells) on the immune sparing effect, assuming A% = 50%, B=10%, T=40 seconds, and D=30 Gy. (a) for various α values with a fixed α/β ratio = 2 Gy, and (b) for various α/β ratios with a fixed α = 0.4 Gy−1. These data indicate that 1) α and β values have a relatively small effect on the threshold dose rate; and 2) the more radiosensitive immune cells (larger α values or smaller α/β ratios) would have a greater sparing effect than the less radiosensitive immune cells.
4. DISCUSSION
We showed a FLASH dose rate effect on the killing of circulating immune cells by directly modeling the dose to the circulating blood. Specifically, we showed: 1) irradiation with a dose of ≥30 Gy in a single fraction may kill 90-100% of the circulating immune cells at conventional dose rates, but only 5-10% at the extremely high dose rates of FLASH-RT; 2) the calculated threshold FLASH dose rates are in the same order of magnitude as the reported FLASH dose rates in animal studies; 3) the threshold FLASH dose rate for humans is about one order of magnitude lower than that for mice due to the longer blood circulation time in humans, suggesting it may be less challenging to achieve photon-based FLASH RT in humans than previously expected; 4) the sparing effect on circulating immune cells decreases with decreasing dose per fraction, and almost vanishes when the dose is reduced to 2 Gy/fraction; 5) the sparing effect gradually decreases with increasing irradiation volume, and completely vanishes when the total body is irradiated.
This is the first computation study that illustrates a very strong sparing effect in killing of circulating immune cells by ultra-high dose rate FLASH-RT. This strong sparing of immune cells may have the potential to reduce radiation-induced normal tissue toxicities. Elements of the immune system, including lymphocytes and cytokines such as TGF-β and IL-8, have been reported to be associated with radiation-induced pneumonitis and lung fibrosis [12-14]. Others have speculated that the immune system is recruited for the repair of radiation-induced damage to the various cells and tissues that compose an organ of concern [12]. An injured immune system may not be able to repair damage in these cells and tissues and consequently may cause more final functional damage of the organ. Therefore, radiation-induced organ toxicity may be the combined effect of both radiation damage to the immune system and damage directly to the cells and tissues of the organ. Further studies are needed to quantify both effects to better understand radiation-induced organ toxicities.
Additionally, this immune sparing effect may improve tumor control. It has been widely reported that radiation-induced lymphopenia inversely correlates with patient survival for a variety of cancers treated with RT [18-21]. Patients with absolute lymphocyte counts <500 have significantly poorer survival than patients with lymphocytes count >500 [21], suggesting that damage to the immune system by radiation affects patient survival or tumor control. Mice that received a single radiation dose of 30 Gy to their tumor achieved good tumor control, while mice receiving additional radiation of 30 Gy to the tumor had poorer tumor control, more lung metastases [28], and fewer immune cells [28]. We have observed that the baseline level of IDO (a primary immune modulator) inversely correlated with the survival of lung cancer patients treated with chemotherapy and RT [29-30]. The IDO level significantly decreased during RT and increased post RT. A lower level of IDO post RT significantly correlated with better overall survival, progression free survival and less metastasis. A similar result was also observed in lung cancer patients treated with stereotactic body RT, but with a different IDO change pattern during RT [30].
Interestingly, the calculated threshold FLASH dose rates for various conditions are in the same order of magnitude as the FLASH dose rates observed in animal studies. This result and the potential that immune sparing may reduce normal tissue toxicities support the hypothesis that our calculated immune sparing effect by the FLASH-RT may be an important factor contributing to the reported FLASH effect in animal studies. However, FLASH-RT was shown to increase cell survival compared to conventional RT in an in vitro experiment with appropriate physiological oxygen levels [31], and of course there was no immune system present. In addition, an oxygen depletion model by FLASH-RT appeared to well describe the dose-rate dependent novel object recognition rate for mice with 10-Gy of whole brain radiation [32]. Therefore, it might be the combination of the immune sparing and the oxygen depletion that contributes mainly to the FLASH effect. The immune sparing and oxygen depletion effect both have the positive contribution to the normal tissue toxicity, so that their combined effect is positive. However, the immune sparing effect is positive, while the oxygen depletion effect is negative for tumor control, so that the combination may be positive, neutral or negative, depending on situations. It should be noted that all the reported mice studies on normal tissue toxicities were conducted in immunogenic mice [10], while those on tumor controls were conducted either in immunogenic or immune compromised nude mice [10, 33].
It is also interesting that the threshold FLASH dose rate for humans is about one order of magnitude less than that for mice. This suggests that developing a clinical photon-based FLASH-RT technology could be much less challenging than what has been previously anticipated. When the tumor is in locations other than the thorax, or not close to the aorta or vena cava, A% could be less than 20%. Considering that humans have a T~60 s, the corresponding threshold FLASH dose rate for A=20% is 1.2 Gy/second, or 7200 cGy/minute. Since the maximum dose rate for current commercially available linacs is 2400 cGy/minute, FLASH-RT conditions could be realized for treating those tumors with A% ≤ 20% by reducing the source to isocenter distance from 100 cm to 50 cm, or by simultaneously using 3 linacs, or by a combination of both methods.
The immune sparing effect by FLASH-RT depends on the total dose delivered in a single fraction. This effect increases with increasing dose/fraction, and gradually achieves maximum when the dose/fraction reaches 30-50 Gy/fraction. It is relatively weak at 5 Gy/fraction, and almost completely vanishes at 2 Gy/fraction. This result suggests that the FLASH-RT technology might only work for RT fractionation schemes similar to stereotactic radiosurgery (SRS) or stereotactic body radiotherapy (SBRT). However, current clinical experience indicates that SRS/SBRT only works for relatively small tumors. For large tumors, there is a concern of normal tissue toxicities with a large dose/fraction. While the FLASH-RT technology has the potential to reduce the normal tissue toxicities, as discussed above, a quantitative model that is able to estimate the amount of normal tissue sparing for various conditions is required to determine whether a hypo-fractioned FLASH-RT is better than conventional RT. Only with the availability of such model can optimized-utilization of FLASH technology be achieved.
It should be pointed out that immune cells not only present in circulating blood, but also in the lymphatic system including lymph nodes and ducts, lymphatic organs such as spleen, thymus, and bone marrow, and lymphatic tissues in non-lymphatic organs such as lung, liver, and bowel. A comprehensive consideration of dose to these structures is required to model radiation damage to the entire immune system [23]. The sparing effect for the entire immune system may be substantially less than the calculated sparing effect of immune cells in circulating blood. In addition, the amplitude of sparing effect may be reduced when the immune sparing is translated into the normal tissue sparing. Therefore, further studies are required to directly connect the calculated immune sparing effect to the reported FLASH effect and consequently build a model to predict the FLASH effect for various conditions.
This computation study indicated a strong sparing effect on circulating immune cells induced by ultra-fast FLASH-RT. This extraordinary sparing of circulating immune cells may be a major factor contributing to the reported FLASH effect in animal studies, because the calculated threshold FLASH dose rate is in the same order of magnitude as that reported in the literature. Whether it is or is not a mechanism of the reported FLASH effect, the sparing of immune cells may be beneficial to radiation treatment of cancers due to the importance of the immune system in tumor control and in normal tissue toxicities.
FLASH-radiotherapy is a hot topic and potential paradigm-changing technology
Present a strong FLASH effect in sparing of the circulating immune cells using computer simulation
Calculated threshold FLASH dose rate for mice is almost the same as the reported FLASH dose rate in animal studies
Threshold FLASH dose rate for humans is about one order of magnitude less than that for mice, suggesting that it may be much less challenging to develop a clinical FLASH-RT system than previously anticipated.
Acknowledgments
【Grant Support】
This work was partially supported by the National Cancer Institute, National Institutes of Health, R01 CA142840 (Kong).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
【Conflict of Interest】
The authors declare no actual or potential conflicts of interest.
REFERENCES
- 1.Vozenin MC, De Fornel P, Petersson K et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019. January 1;25(1):35–42. [DOI] [PubMed] [Google Scholar]
- 2.Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a FLASH: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017. September;124(3):365–369. [DOI] [PubMed] [Google Scholar]
- 3.Schüler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, Praxel AJ, Loo BW Jr, Maxim PG. Experimental Platform for Ultra-high Dose Rate FLASH Irradiation of Small Animals Using a Clinical Linear Accelerator. Int J Radiat Oncol Biol Phys. 2017. January 1;97(1):195–203. [DOI] [PubMed] [Google Scholar]
- 4.Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014. July 16;6(245):245ra93. [DOI] [PubMed] [Google Scholar]
- 5.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, Moeckli R, Vozenin MC. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019. October;139:18–22. [DOI] [PubMed] [Google Scholar]
- 6.Montay-Gruel P, Acharya MM, Petersson K et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. 2019. May 28;116(22):10943–10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vozenin MC, Hendry JH, Limoli CL. Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin Oncol (R Coll Radiol). 2019. July;31(7):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pratx Guillem and Daniel S Kapp, A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio, https://arxiv.org/ftp/arxiv/papers/1905/1905.06992.pdf [DOI] [PubMed]
- 9.Durante M, Bräuer-Krisch E, Hill M. Faster and safer? FLASH ultra-high dose rate in radiotherapy. Br J Radiol. 2018;91(1082):20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson JD, Hammond EM, Higgins GS, Petersson K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool's Gold? Front Oncol. 2020;9:1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. (2013) 31:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirsdörfer F, Jendrossek V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front Immunol. 2016. December 14;7:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong FM, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015. April;25(2):100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenmark MH, Cai XW, Shedden K, Hayman JA, Yuan S, Ritter T, Ten Haken RK, Lawrence TS, Kong FM. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2012. October 1;84(2):e217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012. March 22;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonia SJ, Villegas A, Daniel D, et al. PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017. November 16;377(20):1919–1929. [DOI] [PubMed] [Google Scholar]
- 18.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011;17:5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013; 31:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, Welsh J, Cox JD, Crane CH, Hsu CC, Lin SH. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys. 2017. September1;99(1):128–135. [DOI] [PubMed] [Google Scholar]
- 21.Wild AT, Herman JM, Dholakia AS et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2016. March 1;94(3):571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin JY, Chen H, and Ying X et al. Higher Radiation Dose to Immune System Correlates with Poorer Survival in Patients with Stage III Non-small Cell Lung Cancer: A Secondary Analysis of a Randomized Phase III Cooperative Group Trial (NRG Oncology RTOG0617), Int J Radiat Oncol Biol Phys. October1; 99(2) 2017. Supplement, S151–S152 (Oral presentation in ASTRO 2017), Submitted for publication [Google Scholar]
- 23.Jin JY, Mereniuk T, Yalamanchali A, Wang W, Machtay M, Spring Kong FM, Ellsworth S. A framework for modeling radiation induced lymphopenia in radiotherapy. Radiother Oncol. 2020. March;144:105–113. [DOI] [PubMed] [Google Scholar]
- 24.Janssen BJ, Smits JF. Autonomic control of blood pressure in mice: basic physiology and effects of genetic modification. Am J Physiol Regul Integr Comp Physiol. 2002. June;282(6):R1545–64. Review. [DOI] [PubMed] [Google Scholar]
- 25.Vogel J Measurement of cardiac output in small laboratory animals using recordings of blood conductivity. Am J Physiol. 1997. November;273(5):H2520–7. [DOI] [PubMed] [Google Scholar]
- 26.Riches AC, Sharp JG, Thomas DB, Smith SV. Blood volume determination in the mouse. J Physiol. 1973. January;228(2):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geara FB, Peters LJ, Ang KK, Wike JL, Sivon SS, Guttenberger R, Callender DL, Malaise EP, Brock WA. Intrinsic radiosensitivity of normal human fibroblasts and lymphocytes after high- and low-dose-rate irradiation. Cancer Res. 1992. November 15;52(22):6348–52. [PubMed] [Google Scholar]
- 28.Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. 2015. August 15;21(16):3727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Huang L, Jin JY, Jolly S, Zang Y, Wu H, Yan L, Pi W, Li L, Mellor AL, Kong FS. IDO Immune Status after Chemoradiation May Predict Survival in Lung Cancer Patients. Cancer Res. 2018. February 1;78(3):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Huang L, Jin JY, Pi W, Ellsworth SG, Jolly S, Mellor AL, Machtay M, Kong FS. A Validation Study on IDO Immune Biomarkers for Survival Prediction in Non-small Cell Lung Cancer: Radiation Dose Fractionation Effect in Early Stage Disease. Clin Cancer Res. 2020;26(1):282–289. [DOI] [PubMed] [Google Scholar]
- 31.Adrian G, Konradsson E, Lempart M, Bäck S, Ceberg C, Petersson K. The FLASH effect depends on oxygen concentration. Br J Radiol. 2020;93(1106):20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersson K, Adrian G, Butterworth K, McMahon SJ. A Quantitative Analysis of the Role of Oxygen Tension in FLASH Radiation Therapy, Int J Radiat Oncol Biol Phys. 2020;S0360– 3016(20)30884–1. [DOI] [PubMed] [Google Scholar]
- 33.Bourhis J, Montay-Gruel P, Gonçalves Jorge P, et al. Clinical translation of FLASH radiotherapy: Why and how?. Radiother Oncol. 2019;139:11. [DOI] [PubMed] [Google Scholar]