Abstract
Background:
Growing evidence suggests that under-diagnosis of acute myocardial infarction (AMI) may be common in sub-Saharan Africa. Prospective studies of routine AMI screening among patients presenting to emergency departments in sub-Saharan Africa are lacking. Our objective was to determine the prevalence of AMI among patients in a Tanzanian emergency department.
Methods:
In a prospective observational study, consecutive adult patients presenting with chest pain or shortness of breath to a referral hospital emergency department in northern Tanzania were enrolled. Electrocardiogram (ECG) and troponin testing were performed for all participants to diagnose AMI types according to the Fourth Universal Definition. All ECGs were interpreted by two independent physician judges. ECGs suggesting ST-elevation myocardial infarction (STEMI) were further reviewed by additional judges. Mortality was assessed 30 days following enrollment.
Results:
Of 681 enrolled participants, 152 (22.3%) had AMI, including 61 STEMIs and 91 non-STEMIS (NSTEMIs). Of AMI patients, 91 (59.9%) were male, mean (sd) age was 61.2 (18.5) years, and mean (sd) duration of symptoms prior to presentation was 6.6 (12.2) days. In the emergency department, 35 (23.0%) AMI patients received aspirin and none received thrombolytics. Of 150 (98.7%) AMI patients completing 30-day follow-up, 65 (43.3%) had died.
Conclusions:
In a northern Tanzanian emergency department, AMI is common, rarely treated with evidence-based therapies, and associated with high mortality. Interventions are needed to improve AMI diagnosis, care, and outcomes.
Keywords: Myocardial infarction, Tanzania, sub-Saharan Africa, emergency department
Introduction
Acute myocardial infarction (AMI) is a life-threatening emergency and a leading cause of death worldwide.1 Although AMI has historically been considered to be rare in sub-Saharan Africa (SSA), the burden of AMI risk factors has grown across the region in recent decades.2 Despite the rise of these risk factors, existing data suggest that AMI is rarely diagnosed in the region.3–6 For example, a retrospective study conducted in Tanzania in 2017–2018 found that the ratio of AMI admissions to acute stroke admissions was 1:23, compared to a ratio of 1:1 in the United States in 2014.5 Similarly, recent retrospective studies from elsewhere in SSA identified very few AMI admissions, especially when compared to admissions for other cardiovascular diseases like stroke and heart failure.4, 7
The apparent scarcity of AMI in SSA remains poorly understood. One potential explanation is that AMI is under-diagnosed across the region.6, 8 Qualitative studies from SSA have identified several factors that may contribute to AMI under-diagnosis, including inadequate provider training regarding AMI, lack of diagnostic equipment, and delayed patient care-seeking.9, 10 Additionally, two recent observational studies in Tanzanian referral hospitals suggested that under-testing may be contributing to AMI under-diagnosis.6, 11 In the Tanzanian emergency department (ED) where the present study was conducted, for example, only half of adults presenting with acute chest pain or shortness of breath underwent electrocardiogram (ECG) testing and less than 3% underwent cardiac biomarker testing.6 The reasons for such low rates of testing are likely myriad, but in-depth interviews among Tanzanian physicians found that physician failure to consider the diagnosis of AMI, concerns about cost or availability of testing, and lack of confidence in interpreting ECGs contributed to hesitancy to pursue diagnostic workups for AMI.9
Despite growing evidence that AMI under-diagnosis may be common in SSA, there is little data regarding the true burden of AMI among patients presenting for emergency care in the region. A handful of retrospective studies have quantified AMI cases among hospitalized patients in whom clinicians decided to pursue AMI testing, thus not allowing for identification of missed AMI cases.3–5, 11, 12 To date, no high-quality prospective studies from SSA report objective AMI testing performed systematically among patients presenting with possible AMI symptoms. Moreover, little is known about AMI outcomes in SSA. The few existing studies of AMI outcomes have been performed in advanced facilities with cardiac catheterization capacity and have relied on clinician discretion to determine who should be tested for AMI.12–15 Given that most adults in SSA do not have access to hospitals with cardiac catheterization and that AMI may be frequently missed, AMI outcomes have yet to be described in the prevailing SSA context, where cardiac catheterization is not possible and misdiagnosis may be common.
We aimed to quantify the prevalence of AMI among patients presenting to the emergency department in northern Tanzania and to determine 30-day mortality among AMI patients. To do so, we conducted a prospective observational study in northern Tanzania. As this study was conducted in an ED where recent historical data regarding rates of AMI diagnosis are available, a secondary aim of this study was to compare our findings to the reported prevalence of AMI when AMI testing was directed by physician discretion.
Methods
Study setting
This study was conducted at Kilimanjaro Christian Medical Centre (KCMC), a regional tertiary care center in Moshi, Tanzania. In 2014, the community prevalence of hypertension among adults in Moshi was 28%,16 and the prevalence of diabetes was 6%.17 The KCMC ED receives all patients who present for unscheduled care; no referral is necessary to be seen. A prospective observational study of adults presenting to the KCMC ED with chest pain or shortness of breath in 2018 found that 1.8% of such patients were diagnosed with AMI, although diagnostic workups for AMI were rare.6 KCMC does not presently have capacity for cardiac catheterization, but thrombolytics and a laboratory-based troponin-I assay are available. The KCMC ED is staffed by a mix of physicians and clinical officers, most of whom do not have formal residency training in emergency medicine.
Participant selection
All patients aged ≥18 years presenting to the KCMC ED were screened by trained research staff. Patients with chest pain or shortness of breath as a primary or secondary complaint were eligible for enrollment. Exclusion criteria were chest pain secondary to trauma, self-reported fever, or inability to provide informed consent. All patients meeting inclusion criteria were enrolled. Enrollment was conducted from 8AM to 11PM, five days per week, from 9 January 2019 through 12 October 2019. In order to allow for comparisons to historical patterns of AMI diagnosis at KCMC, the eligibility and recruitment protocols in this study were identical to those of the previously mentioned observational study conducted in the KCMC ED in 2018 in which AMI testing was dictated by physician discretion.6
Study procedures
Research assistants administered standardized tablet-based questionnaires based on the World Health Organization (WHO) STEPS survey for non-communicable disease to enrolled participants.18 Participant height and weight were measured and recorded. An ECG was obtained for all participants at time of enrollment using the tablet-based PADECG (Edan Instruments, Shenzhen, China). Point-of-care troponin I assays (Abott iSTAT cTnI assay, Abbott Point of Care, Princeton, New Jersey, United States) were performed for all enrolled participants at time of enrollment or at least six hours after symptom onset, whichever came later.19, 20 The manufacturer-recommended cutoff for this troponin assay is at the 99th percentile of the reference range (0.08 ng/ml, coefficient of variation 16.5%). Because of supply limitations and the fact that participants typically presented several days after symptom onset, only a single troponin assay was performed for each patient rather than obtaining serial troponins. Results of all study investigations were shared with the ED clinical team during the index visit. Renal function testing was not performed by the study team, but creatinine levels were collected from the electronic medical record when obtained by the ED clinician. For the duration of each participant’s ED stay, all treatments were observed and recorded; inpatient testing and treatments were not observed. Follow-up care was determined by the inpatient clinical team and was not observed by research staff. Thirty days after enrollment, participants were contacted via telephone to assess mortality. When participants were unreachable by phone, a research assistant visited the participant’s home to assess vital status.
Study definitions
Participants were classified as having presumptive AMI if they met criteria for ST-elevation myocardial infarction (STEMI) or non-STEMI (NSTEMI), consistent with the Fourth Universal Definition of Myocardial Infarction,21 as detailed below. STEMI was defined as pathologic elevation of the ST segment in ≥2 contiguous leads; age-and sex-specific cutoffs were used for anterior leads as per universal guidelines. In cases of left bundle branch block, the modified Sgarbossa criteria were used to define pathologic ST elevation.22 NSTEMI was defined as a troponin value above the 99th percentile of the manufacturer-defined normal range in the absence of a STEMI. Although universal guidelines acknowledge causes of troponin elevation other than NSTEMI, given limited diagnostic capacity at the study site, any patient presenting with acute chest pain or shortness of breath without fever was classified as having an NSTEMI if troponin I was above the cutoff value. The term “presumptive AMI” was selected to acknowledge (1) that NSTEMI was defined based on a single elevated troponin value rather than serial cardiac biomarkers and (2) that confirmatory testing with coronary angiography was not possible. Cases of acute ischemia were defined by the presence of pathologic T wave inversions or ST depressions in contiguous leads in the absence of STEMI or NSTEMI, in accordance with universal guidelines. Cases of prior infarct were defined by the presence of pathologic Q waves in contiguous leads.21 History of hypertension, diabetes, hyperlipidemia, chronic kidney disease, heart failure, tobacco use, and HIV infection were defined by patient self-report. Personal and family history of cardiovascular disease were defined by self-reported history of AMI or stroke in self and in a first-degree relative, respectively. Anginal severity was classified according to the Canadian Cardiovascular Society grading scale.23 Body mass index (BMI) was calculated directly from each patient’s measured weight and height. Estimated glomerular filtration rate (eGFR) was calculated via the CKD-EPI Creatinine Equation.24
ECG interpretation
All ECGs were interpreted by two independent physician judges with residency training in either emergency medicine or cardiology. Physician judges were blinded to all clinical data other than patient age and sex. Physician judges assigned one or more of the following diagnoses to ECGs: STEMI, acute ischemia, prior infarct, or none of these. In cases of disagreement, a third physician judge served as the tiebreaker. Agreement among judges regarding presence of STEMI was excellent (94.6% agreement, κ = 0.67). ECGs deemed by two judges to be STEMIs underwent additional review before final adjudication. An independent cardiologist not involved in the initial ECG interpretation reviewed all STEMI ECGs and identified any ECGs that were equivocal or possibly representative of other pathology, such as pericarditis. These ECGs were arbitrated by an external interventional cardiologist with experience treating AMI in multiple countries, who was not a member of the research team. Any ECGs judged by the external reviewer to be equivocal or inconsistent with STEMI were not categorized as STEMIs.
Statistical analyses
All statistical analyses were performed in the R suite (version 3.6.1). Characteristics of patients with and without AMI were compared using Welch’s t-test (for continuous variables) or Pearson’s chi-squared (for categorical variables). Odds ratios were calculated from 2×2 contingency tables. A threshold of 5% was used for statistical significance, using two-sided p- values. There were no missing data in this study. Sample size calculations were based on a prior study at KCMC, in which 1.8% of patients presenting with chest pain or shortness of breath were diagnosed with AMI.6 Assuming the true prevalence of AMI was 1.8%, 675 patients would need to be enrolled to determine this proportion with 95% confidence and a 1% margin of error.
Sensitivity analyses
As patients with advanced renal disease may have elevated serum troponin levels in the absence of AMI, sensitivity analyses were performed by excluding patients with eGFR<15 ml/min/1.73m2 from the NSTEMI study definition.
Ethical approval
This study received ethics approval from institutional review boards at Duke Health, KCMC, and the Tanzania National Institute for Medical Research. All participants provided written informed consent before enrollment.
Patient and public involvement
The perspectives of patients regarding barriers to AMI diagnosis and care were obtained in preliminary studies in Tanzania25–27; these perspectives informed the design of this study.
Funding
This study received support from the US National Institutes of Health Fogarty International Center (grant number D43TW009337) and the Duke Hubert-Yeargan Center for Global Health. Abbott Point of Care donated the troponin assays used in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
During study recruitment hours, 6,038 adult patients were screened, of whom 681 (11.3%) were eligible for participation (Figure 1). All eligible patients consented to participation and were enrolled. The mean (sd) age of participants was 59.6 (19.7) years and 364 (53.5%) were females.
Figure 1.
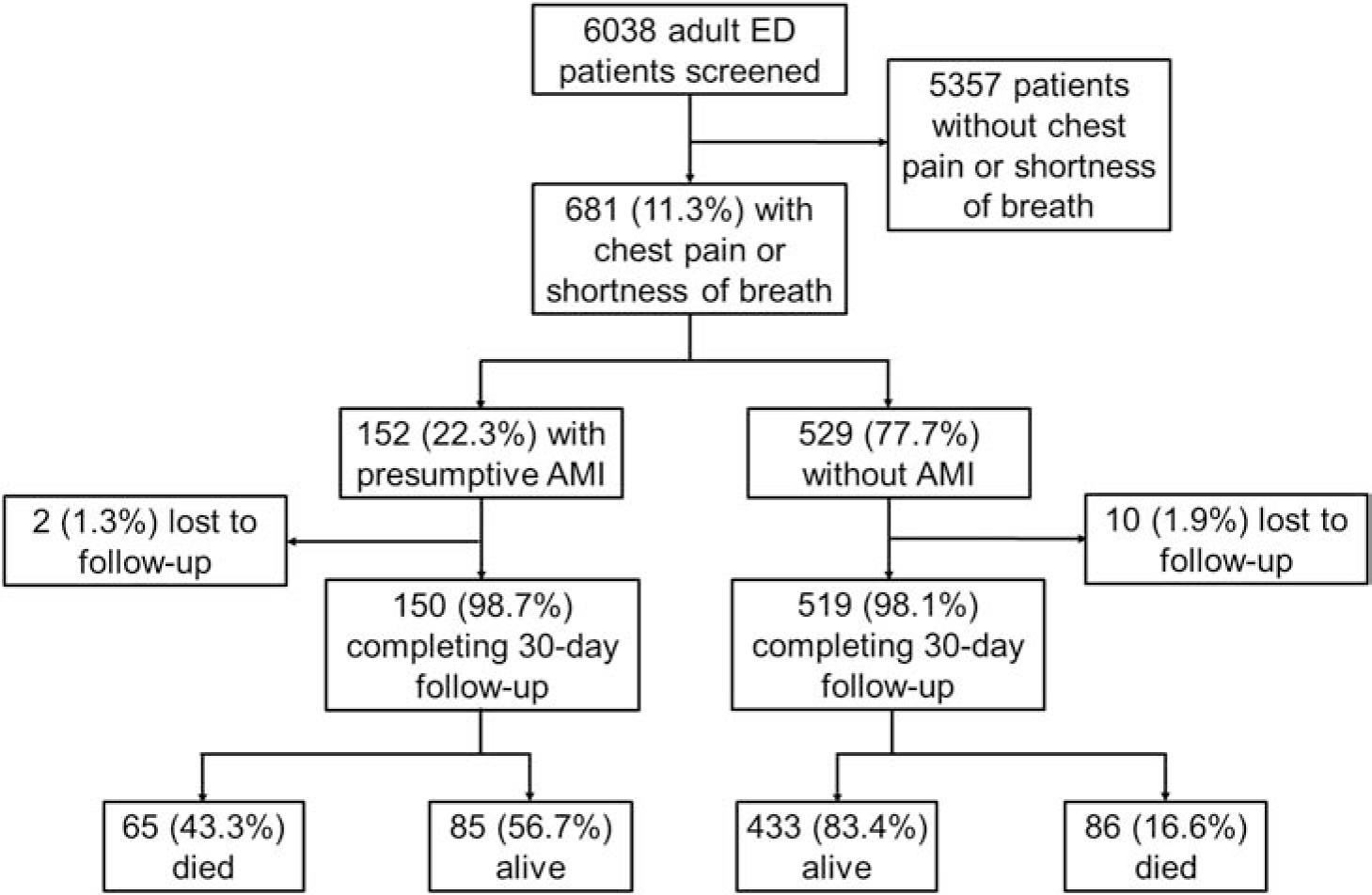
Flow of study participants from screening through 30-day follow-up.
Of participants, 152 (22.3%) had presumptive AMI, including 61 (40.1%) STEMIs and 91 (59.9%) NSTEMIs. Of 529 participants without AMI, 175 (33.1%) had ECGs demonstrating acute ischemia and 74 (14.0%) had ECGs demonstrating prior infarct. Figure 2 compares the observed burden of presumptive AMI to the proportion of adults presenting with chest pain or shortness of breath who were diagnosed with AMI in 2018, when AMI testing was directed by physician discretion.6
Figure 2.
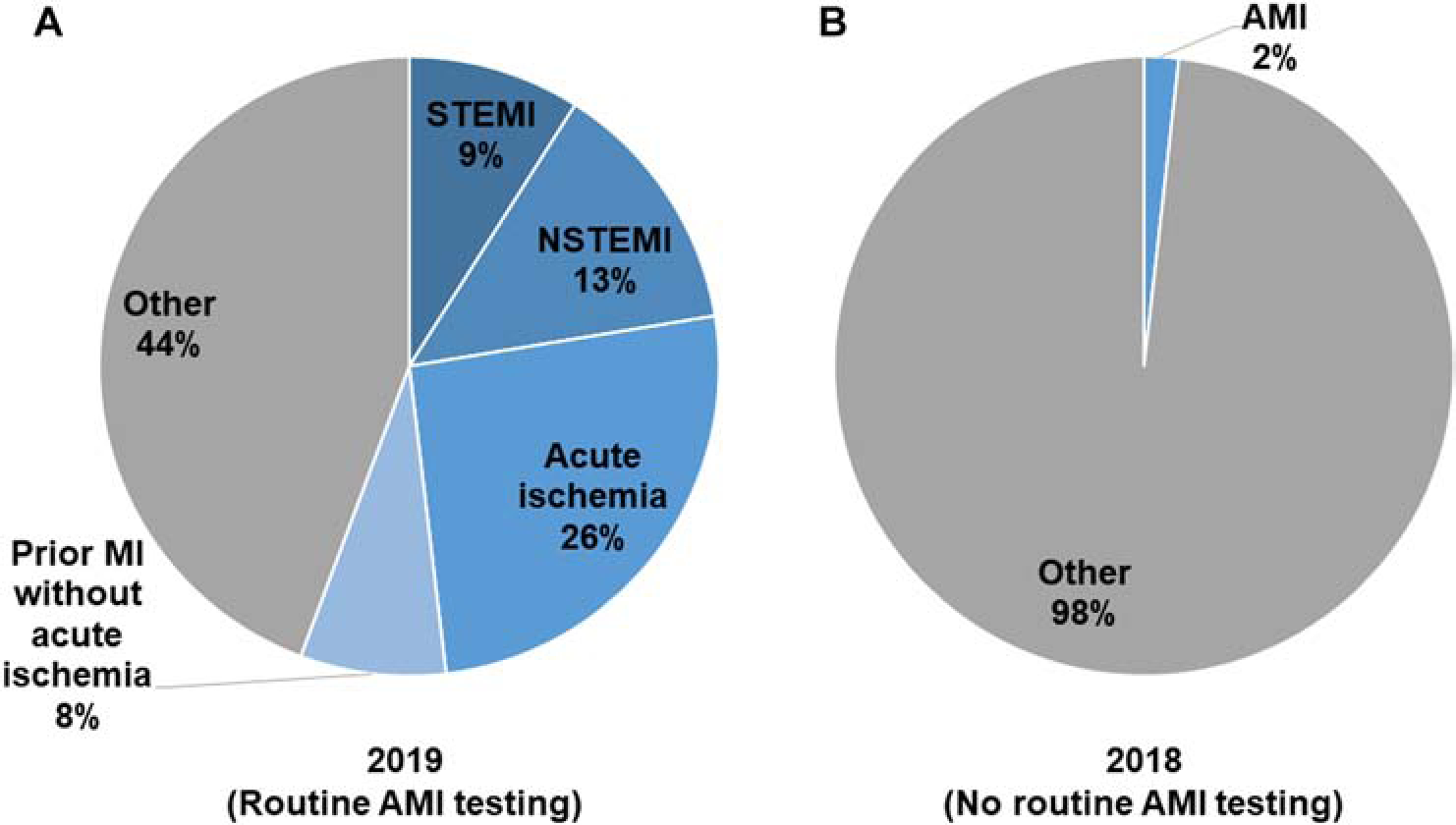
Diagnoses of adult patients presenting to the KCMC emergency department with chest pain or shortness of breath, 2018–2019. Panel A: The proportion of patients found to have STEMI, NSTEMI, acute ischemic ECG findings, and ECG evidence of prior infarct when all patients underwent AMI testing in 2019 (N=681). Panel B: The proportion of patients who were diagnosed with AMI in 2018 when AMI testing was driven by physician discretion, as previously reported (N=339).6
Of participants with presumptive AMI, 94 (61.8%) reported a history of hypertension, and 54 (35.5%) reported a history of tobacco use (Table 1). Compared to other participants, patients with presumptive AMI were more likely to be male (59.9% vs 42.7%, OR 2.00, 95% CI 1.39–2.89, p<0.001). There were otherwise no significant differences in age or known medical comorbidities between patients with and without presumptive AMI. The mean (sd) duration of symptoms among patients with presumptive AMI prior to hospital presentation was 6.6 (12.2) days, compared to 7.2 (11.3) days among those without AMI (p = 0.561).
Table 1.
Characteristics of emergency department patients presenting with chest pain or shortness of breath, northern Tanzania, 2019 (N=681)
| Patient characteristic | Patients with presumptive AMI (N = 152) mean (sd) | Patients without AMI (N = 529) mean (sd) | pa | |
|---|---|---|---|---|
| Age, years | 61.2 (18.5) | 59.2 (20.0) | .. | 0.244 |
| Body mass index, kg/m2 | 23.9 (4.4) | 25.2 (5.8) | .. | 0.003* |
| Serum troponin I, ng/mlb | 0.97 (3.49) | 0.01 (0.02) | <0.001* | |
| Duration of symptoms prior to presentation, days | 6.6 (12.2) | 7.2 (11.3) | 0.561 | |
| Patients with AMI (N = 152) n (%) | Patients without AMI (N = 529) n (%) | Odds ratio (95% CI) | pa | |
| Male | 91 (59.9%) | 226 (42.7%) | 2.00 (1.39–2.89) | <0.001* |
| History of hypertension | 94 (61.8%) | 321 (60.7%) | 1.05 (0.73–1.53) | 0.796 |
| History of diabetes | 27 (17.8%) | 123 (23.3%) | 0.72 (0.44–1.12) | 0.150 |
| History of hyperlipidemia | 8 (5.3%) | 39 (7.4%) | 0.71 (0.30–1.48) | 0.366 |
| History of chronic kidney disease | 16 (10.5%) | 40 (7.5%) | 1.45 (0.76–2.62) | 0.241 |
| History of heart failure | 48 (31.6%) | 179 (33.8%) | 0.90 (0.61–1.33) | 0.603 |
| History of tobacco use | 54 (35.5%) | 157 (29.7%) | 1.31 (0.89–1.91) | 0.169 |
| Personal history of cardiovascular disease | 9 (5.9%) | 32 (6.0%) | 0.99 (0.43–2.05) | 0.953 |
| Family history of cardiovascular disease | 27 (17.8%) | 97 (18.3%) | 0.97 (0.59–1.53) | 0.872 |
| Self-reported history of HIV | 5 (3.3%) | 11 (2.1%) | 1.63 (0.49–4.65) | 0.385 |
| Chagga tribe | 92 (60.5%) | 316 (59.7%) | 1.03 (0.72–1.50) | 0.861 |
p<0.05
AMI: Acute myocardial infarction
HIV: Human immunodeficiency virus
Univariate comparisons between continuous and categorical variables were assessed via Welch’s t-test; univariate comparisons between categorical variables were assessed via Pearson’s chi-squared.
Manufacturer-recommended cutoff is 0.08 ng/ml, the 99th percentile of the reference range
The most common primary symptom among presumptive AMI patients was shortness of breath (n=78, 51.0%) (Table 2). Seventeen (11.2%) presumptive AMI patients had eGFR <15 ml/min/1.73m2. In the ED, 35 (22.8%) presumptive AMI patients received aspirin and no patient received thrombolytics.
Table 2.
Features of present illness and treatments of emergency department patients with presumptive acute myocardial infarction, northern Tanzania, 2018 (N=152)
| Feature of present illness | n | (%) |
|---|---|---|
| Other facility visited prior to ED presentation for current illness episode | ||
| Other hospital | 79 | (52.0) |
| Health center | 26 | (17.1) |
| Dispensary | 2 | (1.3) |
| Self-treatment at home | 16 | (10.5) |
| Pharmacy | 1 | (0.7) |
| None | 32 | (21.1) |
| Primary symptom | ||
| Shortness of breath | 77 | (50.7) |
| Chest pain | 39 | (25.7) |
| Other | 36 | (23.7) |
| Secondary symptoms | ||
| Chest pain | 75 | (49.3) |
| Shortness of breath | 64 | (42.1) |
| Leg swelling | 42 | (27.6) |
| Palpitations | 35 | (23.0) |
| Back pain | 26 | (17.1) |
| Epigastric pain | 24 | (15.8) |
| Generalized weakness | 23 | (15.1) |
| Symptoms began within the past 12 hours | 14 | (9.2) |
| CCS grading for anginal severity | ||
| Class I | 2 | (1.3) |
| Class II | 10 | (6.6) |
| Class III | 64 | (42.1) |
| Class IV | 76 | (50.0) |
| Estimated glomerular filtration rate (ml/min/1.73m2) | ||
| ≥60 | 81 | (53.3) |
| 45–59 | 15 | (9.9) |
| 30–44 | 13 | (8.6) |
| 15–29 | 14 | (9.2) |
| <15 | 17 | (11.2) |
| Not obtained | 15 | (9.9) |
| Home medications | ||
| Anti-platelet | 17 | (11.2) |
| Anti-hypertensive | 43 | (28.3) |
| Anti-hyperlipidemic | 2 | (1.3) |
| Anti-hyperglycemic | 11 | (7.2) |
| Treatment administered in the ED | ||
| Aspirin | 35 | (23.0) |
| Clopidogrel or other antiplatelet | 13 | (8.6) |
| Heparin | 3 | (2.0) |
| Thrombolytic | 0 | (0) |
CCS: Canadian Cardiovascular Society grading scale for anginal severity23
Thirty-day follow-up was achieved for 669 (98.2%) participants, including 150 (98.7%) presumptive AMI patients. At thirty days, 65 (43.3%) presumptive AMI patients had died, compared to 86 (16.6%) patients without AMI (OR 3.84, 95% CI: 2.58–5.72, p <0.001). Of participants without AMI, 51 of 223 (22.9%) participants with ECGs demonstrating ischemia or prior infarction died within 30 days, compared to 35 of 296 (11.8%) participants with ECGs demonstrating neither ischemia nor prior infarction (Figure 3). Of the 65 MI patients who died, 58 (89.2%) died in-hospital.
Figure 3.
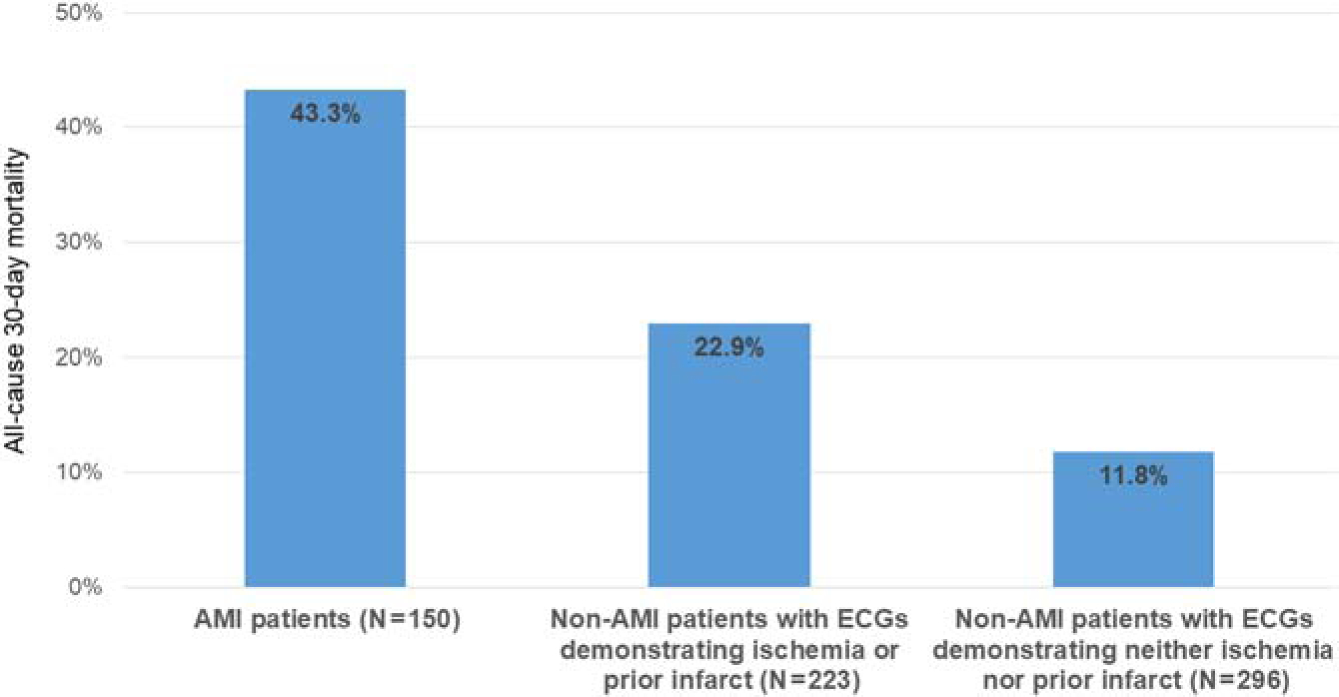
All-cause thirty-day mortality among adults presenting to the emergency department with chest pain or shortness of breath, northern Tanzania, 2019 (N=669)
Sensitivity analyses
Excluding patients with eGFR < 15 ml/min/1.73m2 from the NSTEMI definition resulted in 137 participants meeting the study definition for presumptive AMI (20.1% of all enrolled participants). Of these presumptive AMI patients, 31 (22.6%) received aspirin, and 57 (41.6%) died within thirty days.
Discussion
To our knowledge, this is the first prospective study of routine AMI testing with ECGs and cardiac biomarkers among ED patients in SSA. We found that AMI was surprisingly common, not consistently treated with evidence-based therapies, and associated with high 30-day mortality. When compared to data from the same hospital in 2018,6 our findings suggest that the vast majority of AMI cases were likely missed when testing was driven by physician discretion. These findings call attention to the urgent need to improve AMI care in Tanzania.
Presumptive AMI was remarkably common among patients presenting to the ED with chest pain or shortness of breath. Despite our broad inclusion criteria, 22% of such patients had presumptive AMI, compared to approximately 5% of such patients in the United States.28, 29 Moreover, substantial proportions of participants without AMI had ECG findings of acute ischemia or prior infarct, suggesting that coronary ischemia may have been contributing to their presenting symptoms. Furthermore, a recent community survey in northern Tanzania found that few adults would present to a hospital for chest pain, suggesting that the AMI cases detected in this study represent only a fraction of cases in the community.25
The large number of presumptive AMI cases identified with objective testing stands in contrast to historical rates of AMI diagnosis at KCMC. In a 2018 study conducted in the same ED, less than 3% of patients with chest pain or shortness of breath underwent troponin testing, and only 1.8% of such patients were diagnosed with AMI.6 However, in the present study when routine AMI testing was performed on all patients using identical inclusion criteria, over ten-fold more presumptive AMI cases were found. Assuming the burden of AMI among this patient population was relatively stable from 2018 to 2019, these findings suggest that approximately 90% of AMI cases were missed in 2018. Given low AMI testing rates in SSA,6, 9, 11 some epidemiologists have speculated that low reported rates of AMI in the region may be due to widespread under-diagnosis.8 Our results suggest that, in northern Tanzania, AMI under-diagnosis is common, and retrospective hospital-based data may grossly underestimate disease burden.
AMI patients in our study typically presented to the ED after long delays, excluding opportunities to administer time-sensitive treatments such as thrombolytics in the vast majority of cases. Prior research in SSA found that patients with AMI often do not recognize the need to present promptly to a hospital for their symptoms,9, 10, 25, 26 and our results support the need for educational interventions to improve community awareness of AMI.
Few AMI patients in our study received evidence-based therapies such as anti-platelet agents in the ED, and the infrequent use of these therapies warrants further understanding. Prior qualitative work in SSA found that inadequate provider training, medication stockouts, and lack of treatment protocols were perceived by providers as barriers to high-quality AMI care.9, 10 In-depth interviews with ED physicians in Tanzania found that a lack of provider awareness of AMI diagnosis and treatment was a particularly salient problem; participants in these interviews cited a need to increase education regarding ECG interpretation and AMI care in all phases of medical training.9 Interventions to address these and other barriers to evidence-based treatment of AMI in Tanzania are urgently needed.
The all-cause thirty-day mortality rate we observed among AMI patients is among the highest described globally, and to our knowledge is the highest all-cause thirty-day mortality rate following AMI reported in the last decade.30 Moreover, our study only captured AMI patients who survived to hospital presentation; thus the true AMI mortality rate in the community is likely higher than we observed. Possible explanations for high AMI-associated mortality in Tanzania include poor uptake of evidence-based therapies, delay in care-seeking, and limited access to advanced cardiac care. The alarmingly high mortality we observed highlights the pressing need to develop interventions to improve AMI care across SSA.
Our study had several strengths including broad inclusion criteria and excellent follow-up rates. This study also had several limitations. First, only patients with chest pain or shortness of breath were eligible for study inclusion; thus excluding patients with atypical AMI presentations. Secondly, although we used ECG criteria from the Fourth Universal Definition of MI for defining STEMIs and acute ischemia,21 patients with other conditions causing ST changes such as pericarditis or left ventricular hypertrophy may have been included in our STEMI or acute ischemia groups. To minimize this possibility, we required independent ECG review by multiple physician judges to adjudicate STEMIs and acute ischemia. Thirdly, NSTEMI was defined as an elevated troponin value but there are other conditions that may result in an elevated troponin, such as renal failure or myocarditis. We attempted to minimize the inclusion of such patients by only enrolling patients with acute symptoms suggestive of AMI and by excluding patients with self-reported fever. Furthermore, advanced renal dysfunction was uncommon among participants with presumptive AMI and excluding such patients did not substantially affect our findings. Cardiac catheterization, echocardiography, and serial troponins would have improved our ability to determine when elevated troponins were due to acute coronary heart disease, but was not possible in this study due to resource limitations. Fourthly, delayed presentations of AMI patients may have led to failure to capture some AMI cases, as ST-segment elevations and elevated troponin levels persist only temporarily following AMI. Additionally, we only observed ED treatments; observation of inpatient care may have revealed more use of evidence-based therapies on a delayed basis. We also did not collect detailed data about what care participants received at other hospitals prior to presenting to KCMC; such data would have given a fuller picture of the degree of uptake of evidence-based diagnosis and treatment for AMI in the wider community. Moreover, the specific causes of deaths of participants are unknown, and some observed deaths may be unrelated to AMI. Finally, this was a single-center study, limiting the generalizability of our findings.
In conclusion, in a Tanzania ED, AMI was common, rarely treated with evidence-based therapies, and associated with very high thirty-day mortality. AMI patients typically present to hospital after prolonged delays, and AMI misdiagnosis was likely common when AMI testing was directed by physician discretion. Interventions are urgently needed to reduce AMI-associated mortality in Tanzania. Further study is needed to describe the burden of AMI and the pervasiveness of AMI misdiagnosis across SSA.
Highlights.
AMI is common in an emergency department in northern Tanzania
22% of adults presenting with chest pain or shortness of breath had AMI
>90% of AMI cases were likely missed in this emergency department in 2018
Only 23% of AMI patients received aspirin in the emergency department
All-cause 30-day mortality among AMI patients was 43%
Acknowledgements:
We gratefully acknowledge the collaboration of the KCMC ED staff. We thank Oscar Kamanga for assistance with patient enrollment and follow-up. We thank Dr. Mohammed Akhter for providing external ECG review. Finally, we gratefully acknowledge Abbott Point of Care for donating the troponin assays used in this study.
Funding: This study received support from the US National Institutes of Health Fogarty International Center (grant number D43TW009337) and the Duke Hubert-Yeargan Center for Global Health. Abbott Point of Care donated the troponin assays used in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No author received any payment for writing this article. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: ATL’s institution received research support from Roche Diagnostics, Abbott Laboratories, and Siemens Diagnostics for studies in which he was a co-investigator. JTH’s institution received research support form Roche Diagnostics for a study in which he is an investigator.
References
- 1.Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 2017;390(10100):1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell NR, Lemogoum D. Hypertension in sub-Saharan Africa: a massive and increasing health disaster awaiting solution. Cardiovascular journal of Africa 2015;26(4):152–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Kolo PM, Fasae AJ, Aigbe IF, Ogunmodede JA, Omotosho AB. Changing trend in the incidence of myocardial infarction among medical admissions in Ilorin, north-central Nigeria. The Nigerian postgraduate medical journal 2013;20(1):5–8. [PubMed] [Google Scholar]
- 4.Appiah LT, Sarfo FS, Agyemang C, Tweneboah HO, Appiah N, Bedu-Addo G, et al. Current trends in admissions and outcomes of cardiac diseases in Ghana. Clinical cardiology 2017;40(10):783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertz JT, Sakita FM, Limkakeng AT, Mmbaga BT, Appiah LT, Bartlett JA, et al. The burden of acute coronary syndrome, heart failure, and stroke among emergency department admissions in Tanzania: a retrospective observational study. Afr J Emergency Medicine 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz JT, Kweka GL, Bloomfield G, Limkakeng AT, Loring Z, Temu G, et al. Patterns of emergency care for possible acute coronary syndrome among patients with chest pain or shortness of breath at a Tanzanian referral hospital. Global heart 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ukpabi OJ, Uwanurochi K. Comparing indications for cardiovascular admissions into a Nigerian and an Israeli Hospital. Annals of African medicine 2017;16(2):70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nkoke C, Luchuo EB. Coronary heart disease in sub-Saharan Africa: still rare, misdiagnosed or underdiagnosed? Cardiovascular diagnosis and therapy 2016;6(1):64–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz JT, Kweka GL, Manavalan P, Watt MH, Sakita FM. Provider-perceived barriers to diagnosis and treatment of acute coronary syndrome in Tanzania: a qualitative study. Int Health 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahiru E, Temu T, Mwanga J, Ndede K, Vusha S, Gitura B, et al. Facilitators, context of and barriers to acute coronary syndrome care at Kenyatta National Hospital, Nairobi, Kenya: a qualitative analysis. Cardiovascular journal of Africa 2018;29(3):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed AS, Sawe HR, Muhanuzi B, Marombwa NR, Mjema K, Weber EJ. Non-traumatic chest pain in patients presenting to an urban emergency Department in sub Saharan Africa: a prospective cohort study in Tanzania. BMC cardiovascular disorders 2019;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahiru E, Temu T, Gitura B, Farquhar C, Huffman MD, Bukachi F. Presentation, management and outcomes of acute coronary syndrome: a registry study from Kenyatta National Hospital in Nairobi, Kenya. Cardiovascular journal of Africa 2018;29(4):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varwani MH, Jeilan M, Ngunga M, Barasa A. Outcomes in patients with acute coronary syndrome in a referral hospital in sub-Saharan Africa. Cardiovascular journal of Africa 2019;30(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shavadia J, Yonga G, Otieno H. A prospective review of acute coronary syndromes in an urban hospital in sub-Saharan Africa. Cardiovascular journal of Africa 2012;23(6):318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao H, Ekou A, Hadeou A, N’Djessan JJ, Kouame I, N’Guetta R. Medium and long-term follow-up after ST-segment elevation myocardial infarction in a sub-Saharan Africa population: a prospective cohort study. BMC cardiovascular disorders 2019;19(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galson SW, Staton CA, Karia F, Kilonzo K, Lunyera J, Patel UD, et al. Epidemiology of hypertension in Northern Tanzania: a community-based mixed-methods study. BMJ open 2017;7(11):e018829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanifer JW, Cleland CR, Makuka GJ, Egger JR, Maro V, Maro H, et al. Prevalence, Risk Factors, and Complications of Diabetes in the Kilimanjaro Region: A Population- Based Study from Tanzania. PloS one 2016;11(10):e0164428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. WHO STEPS Instrument: The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). Geneva: World Health Organization; 2003. [Google Scholar]
- 19.Huggon AM, Chambers J, Nayeem N, Tutt P, Crook M, Swaminathan S. Biochemical markers in the management of suspected acute myocardial infarction in the emergency department. Emergency medicine journal : EMJ 2001;18(1):15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. The New England journal of medicine 1997;337(23):1648–53. [DOI] [PubMed] [Google Scholar]
- 21.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Global heart 2018;13(4):305–338. [DOI] [PubMed] [Google Scholar]
- 22.Meyers HP, Limkakeng AT Jr., Jaffa EJ, Patel A, Theiling BJ, Rezaie SR, et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: A retrospective case-control study. American heart journal 2015;170(6):1255–64. [DOI] [PubMed] [Google Scholar]
- 23.Campeau L Letter: Grading of angina pectoris. Circulation 1976;54(3):522–3. [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertz JT, Madut DB, Tesha RA, William G, Simmons RA, Galson SW, et al. Perceptions of chest pain and healthcare seeking behavior for chest pain in northern Tanzania: A community-based survey. PloS one 2019;14(2):e0212139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hertz JT, Madut DB, Tesha RA, William G, Simmons RA, Galson SW, et al. Knowledge of myocardial infarction symptoms and perceptions of self-risk in Tanzania. American heart journal 2019;210:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertz JT, Sakita FM, Kweka GL, Loring Z, Thielman NM, Temu G, et al. Healthcare- seeking behaviour, barriers to care and predictors of symptom improvement among patients with cardiovascular disease in northern Tanzania. Int Health 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahler SA, Lenoir KM, Wells BJ, Burke GL, Duncan PW, Case LD, et al. Safely Identifying Emergency Department Patients With Acute Chest Pain for Early Discharge. Circulation 2018;138(22):2456–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohn MA, Kwan E, Gupta M, Tabas JA. Prevalence of acute myocardial infarction and other serious diagnoses in patients presenting to an urban emergency department with chest pain. The Journal of emergency medicine 2005;29(4):383–90. [DOI] [PubMed] [Google Scholar]
- 30.OECD. OECD Health Statistics 2018: Health Care Quality Indicators. In. Paris: Organisation for Economic Co-operation and Development; 2018. [Google Scholar]


