Abstract
Purpose
To ascertain the dose-toxicity relationship for the prevalence of self-reported trismus in long-term survivors after intensity-modulated radiotherapy (IMRT) for oropharyngeal carcinoma (OPC).
Materials and Methods
Self-reported mouth opening was ascertained prospectively via a cross-sectional survey of OPC survivors using the intraoral finger-test. RT dose-volume histograms (DVHs) were generated for the following masticatory regions of interest: medial pterygoid, lateral pterygoid, and masseter muscles which were designated as ipsilateral or contralateral to the primary tumor. Trismus was defined as self-reported mouth opening of <3 finger-widths. Recursive partitioning analysis (RPA) was performed to identify the dose-volume thresholds associated with late trismus.
Results
At a median follow-up time of 72 months (95% CI 68–74), 168 of the 587 (29%) survey respondents reported late trismus. Multivariate analysis demonstrated a significant association between late trismus and the following clinical variables: tonsillar primary site, advanced T stage, or higher total RT dose. RPA showed DVH-derived ipsilateral lateral pterygoid (ILP) mean dose of 61 Gy and volume receiving 27 Gy of at least 98.6% were independently associated with late trismus. The association between the ILP dosimetric parameters and the prevalence of late trismus was maintained after adjustment for clinical variables.
Conclusion
The integral dose of IMRT results in unavoidable low/intermediate dose to non-target masticatory muscles that is associated with increased prevalence of late trismus in OPC survivors. Whenever clinically and technically applicable, applying the proposed dosimetric constraints to the ILP (V27 < 98.6 and Dmean < 61 Gy) may reduce the prevalence of late trismus after IMRT for OPC patients.
Keywords: Oropharyngeal, patient-reported, trismus, survivorship, IMRT, dosimetric, dose-volume
Introduction
Survivors of oropharyngeal carcinoma (OPC) treated with radiotherapy (RT) risk a host of substantial, lifelong functional impairments. Functional toxicity is of particular interest in OPC survivorship as the rise in biologically favorable HPV-associated disease in younger patients has led to unprecedented numbers of long-term survivors facing late effects of RT at relatively young ages (commonly in their 60s). Thus, it is more imperative than ever to develop and implement proactive risk reduction strategies in these patients to minimize or prevent loss of function and improve quality of life (QOL) after RT [1].
The potentially harmful effects of the RT beam path on surrounding non-target normal structures are well established [2, 3]. Bystander effects vary by treatment modality, tumor burden, and disease site. Although with the high conformity index of intensity modulated radiotherapy (IMRT), the characteristic beam feature (i.e., integral dose) delivers low/intermediate doses to uninvolved normal tissues; this low-dose bath is associated with early and late functional morbidities [4].
Trismus is a potentially devastating late oral morbidity after RT with an incidence rate of 5% to 50% in patients with mixed sites of head and neck cancer (HNC) [5, 6]. Patients with trismus experience restricted or painful mouth opening that may limit oral intake, impair speech, and worsen oral hygiene [7]. The mouth opening is driven by the paired muscles of mastication including the medial and lateral pterygoids and masseters [8]. Therefore, institutional programs that instruct patients for a daily range of motion exercises may contribute to lower rates of trismus [9]. Although the adverse impact of trismus is well known, the exact mechanism and best prevention plans are not well defined [10]. Studies have demonstrated that both the site of disease and the volume of irradiated tissue contribute to the development of trismus. For example, patients receiving RT for laryngeal, hypopharyngeal or nasopharyngeal cancer experience minimal trismus rates at 0%, 3%, and 6%, respectively. In comparison, about 14% of patients receiving RT for OPC had chronic trismus at 33 months (range, 6–68) as defined by Common Toxicity Criteria- Adverse Events, Version 4.0 [10]. Therefore, a better understanding of structure-specific radiation doses across different subsites of HNC that predispose patients to long-term trismus is crucial to improve the QOL of those who undergo organ-preserving RT [7, 10, 11], especially young survivors.
To maximize the benefit of IMRT, structures at risk should be segmented, and dose constraints need be identified. During the plan optimization for patients with OPC, muscles of mastication are usually located in high/intermediate dose regions due to the obligate need to irradiate the high/intermediate risk nodal chain. Even in patients at low risk (i.e., with a negative nodal status), the spatial locations of masticatory structures put these structures within the unavoidable low-dose beam path dose. Consequently, identification of patients at risk for RT-induced chronic or late trismus and characterization of optimal dose constraints for the masticatory structures may reduce the risk of trismus in OPC survivors, who can live decades with RT sequelae.
We hypothesize that radiation dose thresholds to uninvolved masticatory muscles predict the risk of trismus in long-term OPC survivors. Although researchers have investigated trismus as an RT-induced toxicity in HNC patients [10–12], to date, investigation of a dose-toxicity relationship and establishment of radiation dose thresholds for masticatory muscles in a sample consisting exclusively of OPC survivors treated with IMRT is lacking. Therefore, in the present study, we aimed to address this gap. Herein, we correlate the patient-reported trismus scores with the radiation doses delivered to masticatory muscle in long-term OPC survivors who underwent IMRT. Specifically, we sought to determine:
The relationship between subjective trismus scores and the RT dose delivered to the masticatory muscles;
The dose-volume thresholds associated with late trismus in OPC survivors after IMRT; and
Clinical and treatment predictors of late trismus that may identify high-risk patients who could benefit most from proactive trismus rehabilitation programs.
Materials and Methods
Participants
This is a secondary dose-toxicity analysis of a cross-sectional survivorship survey study. Patients given IMRT for OPC at The University of Texas MD Anderson Cancer Center between from January 2000 to April 2014 were sampled from 989 survey respondents (58% response rate). Eligible patients for the survey administration had no evidence of distant metastasis, recurrence or second primary at the time of survey. The survey also asked respondents to verify their cancer free status. Inclusion in this analysis mandated completion of the intraoral 3-finger survey for detection of trismus, pathologically confirmed OPC, and receipt of IMRT as a component of definitive treatment of OPC with restorable treatment plans comprising of non-contrast enhanced simulation CTs and corresponding dosimetric data. To focus on late effects of RT, surveys were acquired at least 12-months following completing of the RT. This survey-based study was approved by the MD Anderson Institutional Review Board, and informed consent was obtained from the participants via survey responses. Our clinics follow NCCN guidelines in terms of indications of single versus combined treatment modalities, and institutional consensus for treating patients with HNC. The techniques and indications of RT for HNC are detailed in our team publications [13, 14].
Survey administration
Participants were asked to complete the survey once. The survey was administered using an adapted version of Dillman’s Tailored Design method [15], included 1) a letter of invitation mailed via the US postal service to eligible patients 2–3 weeks prior to the initial contact, 2) delivery of the survey questionnaire to all eligible patients via an online server (Qualtrics, Provo, UT or US postal service); and 3) two reminders mailed to non-responders via US postal service at 2–3 weeks and 4–5 weeks after the initial contact. Participants were contacted using multiple modes of communication, including e-mail (for those with e-mail addresses on file), via Qualtrics online server or myMDAnderson (a secure, personalized patient website), and US postal service via first-class mail with a return envelope.
Patient-reported trismus
The survey constrained of a single item inquiring about the presence and severity of trismus, which was self-reported by participants based on the number of vertically stacked fingers they could fit between the central incisors or gums. The assessment was carried out by asking two questions: “How wide can you open your mouth?” and “How many fingers can you place between your upper and lower teeth or gums like the picture shown?”
Three or more fingers
At least two fingers
At least one finger
Less than one finger (I cannot fit one finger between my teeth or gums)
For analysis, trismus groups were coded as follows: three or more fingers was considered “no trismus,” at least two fingers was considered “mild” trismus, only one finger was considered “moderate” trismus, and inability to fit any fingers was considered “severe” trismus.
Data Collection
Patient, tumor, and treatment characteristics were gathered from electronic medical records. Treatment plans with corresponding dosimetric data for each patient were restored from MDACC archives. The treatment plans were initially created using Pinnacle software (Phillips Medical Systems, Andover, MA). The planning non-contrast computed tomography (CT) scans were then exported to a commercially available deformable image registration and segmentation software program (ADMIRE; Siemens Healthineers, Erlangen, Germany) where regions of interest (ROI) were auto-contoured using an existing atlas dataset [16] and subsequently reviewed by two radiation oncologists (ASRM and MK). Tumor laterality principally was determined according to the tumor epicenter location on treatment plans. The laterality of tumors with significant bulk spanning the midline was assigned based on the side containing the majority of the tumor volume as determined via volumetric analysis. In comparison, the laterality of small or apparently midline tumors was assigned based on the mean dose (Dmean) delivered to the parotid glands with the assumption that a higher parotid dose indicates closer proximity to the tumor and therefore small-scale lateralization. Using this information, the medial and lateral pterygoid and masseter muscles were defined as either ipsilateral or contralateral to the primary tumor. The resultant six ROIs (ipsilateral medial pterygoid [IMP], ipsilateral lateral pterygoid [ILP], ipsilateral masseter [IM], contralateral medial pterygoid [CMP], contralateral lateral pterygoid [CLP], and contralateral masseter [CM] muscles) were investigated. ROI specific dose-volume histograms (DVHs) were then generated using CT DICOM files and corresponding dosimetric data (Velocity AI 3.0.1 software, Velocity Medical Solutions, Atlanta, GA), and used in the final analysis, Figure 1.
Fig. 1.
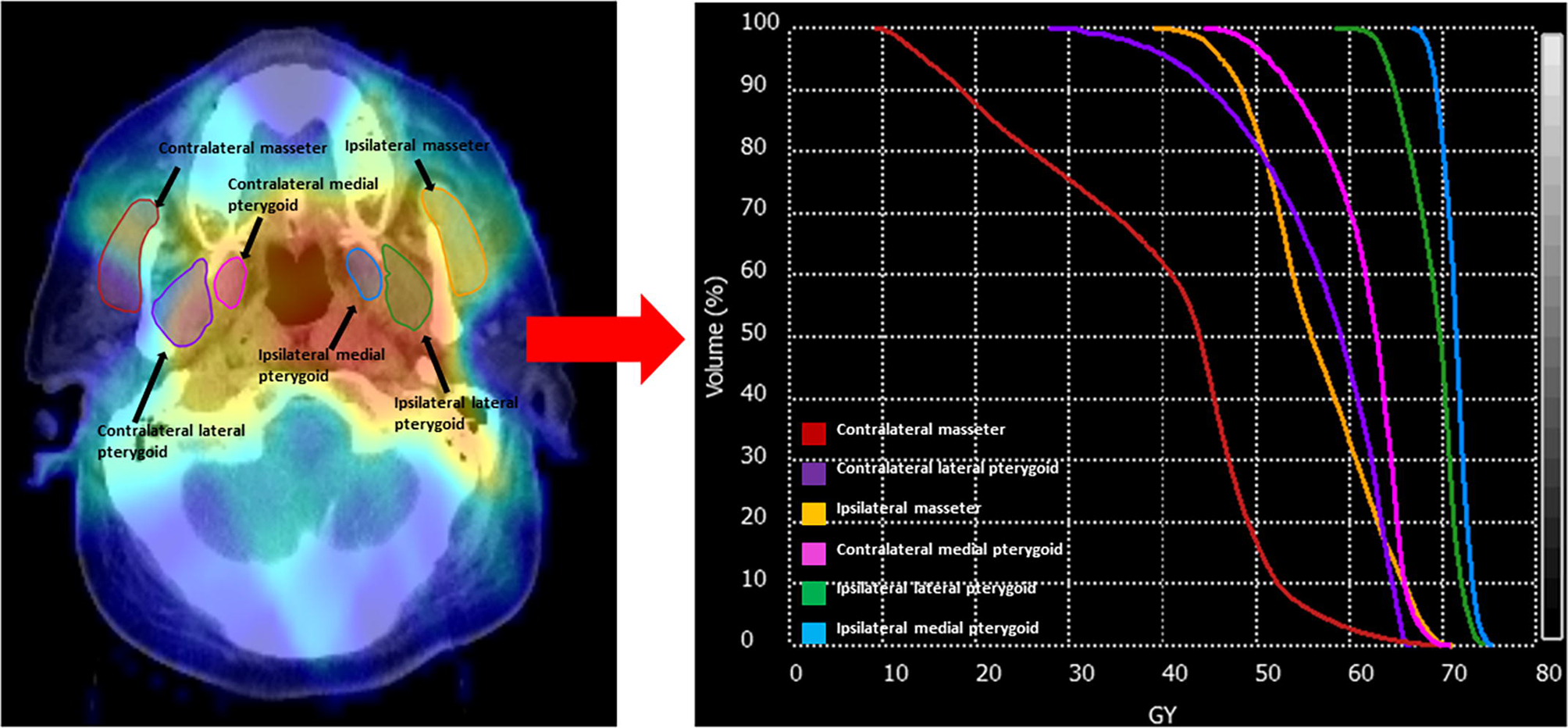
Patient CT with superimposed treatment plan dose intensity map and segmented structures of interest (left) from which dose volume histograms (DVH) can be produced for analysis (right).
Statistical Analysis
Descriptive statistics were used. Furthermore, patient and treatment characteristics were characterized via trismus severity stratification using the Kruskal-Wallis test and Pearson chi-square test for group means and categorical variables, respectively. Univariate Cox proportional hazard analysis was performed to identify the impact of clinical characteristics on the prevalence of trismus. Regression analysis was used to assess the association between the clinical variables and prevalence and severity of trismus. The Dmean delivered to each ROIs and its stander deviation (SD) were calculated and compared collectively across trismus severities using the Kruskal-Wallis test. The Dmean was further analyzed for each ROI by comparing possible combinations of trismus severity (none vs. any, none/mild vs. moderate/severe, and none vs. mild vs. moderate/severe) using a Pairwise Wilcoxon rank-sum test.
The patient cohort was then dichotomized according to the presence or absence of trismus, and cumulative group dose DVHs were produced and analyzed using the Wilcoxon rank sum test. Decision tree partitioning analysis or recursive partitioning analysis (RPA) was used to define dosimetric thresholds for the prevalence of trismus as a function of doses delivered to the ROIs. RPA gives the ability to assess the relative contribution of multiple ROIs and to drive exploratory non-model-dependent dose-volume constraints. RPA allows identifying dose-threshold from continuous dose distributions, and omits the effect of multi-collinearity and/or potential hyper-dimensional interactions within/between clinical candidates and dosimetric covariates. With trismus as the discriminant variable, the ROI specific Dmean and volume receiving 1–75 Gy (V1-V75) were used as candidate dosimetric parameters. Receiver operating curve analysis and K-fold cross-validation, with ROC/ AUCs optimization, were used to define dose-volume thresholds associated with late trismus. All statistical analyses were performed using the JMP Pro software program (version 12.1; SAS Institute Inc, Cary, NC).
Results
A total of 587 patients were included (Supplementary Figure 1). Their median age was 58 years. A total of 84% were male and 58% had HPV-associated OPC. The most common primary tumor site was the base of the tongue (49 %) and the great majority (92%) of the patients had a nodal disease at the time of diagnosis. Seventeen percent, 38%, 22% and 24 % of the patients underwent induction chemotherapy (IC) alone, concurrent chemotherapy (CCT), IC+CCRT and IMRT alone, respectively. The mean (± SD) radiation dose was 68 ± 2.7Gy. At a median follow-up time of 72 months (range, 12–192), a total of 168 patients (29%) reported late trismus (mouth opening smaller than 3 fingers). The patients’ trismus profile according to their clinicopathological and treatment characteristics is detailed in Table 1. Higher T-category (p<0.001), receipt of chemotherapy (p<0.001), and higher total radiation dose (p<0.0001) were significantly associated with higher trismus severity in univariate analysis, Table 1. Table 2 shows the associations between the clinical variables and the prevalence of late trismus. In multivariate analysis, higher radiation dose, advanced T stage and presentation with tonsillar carcinoma showed a statistically significant association with the presence of late trismus.
Table 1.
Patient, tumor, and treatment characteristics
| All patients | No trismus (≥3 fingers) | Mild trismus (≥2 fingers) | Moderate/Severe trismus (<2 fingers) | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n=587 | n=419 (71%) | n=154 (26%) | n=14 (3%) | ||||||
| Covariate | n | % | n | % | n | % | n | % | |
| Sex | |||||||||
| Male | 492 | 83.8 | 348 | 71 | 132 | 27 | 12 | 2 | 0.73 |
| Female | 95 | 16.2 | 71 | 75 | 22 | 23 | 2 | 2 | |
| Mean age (years) ± SD | 57.69 | 8.59 | 57.52 | 8.62 | 58.07 | 8.53 | 58.57 | 8.58 | 0.67 |
| Initial disease site | |||||||||
| Base of tongue | 288 | 49.1 | 220 | 76 | 64 | 22 | 4 | 2 | 0.59 |
| Tonsil | 267 | 45.5 | 178 | 67 | 81 | 30 | 8 | 3 | |
| NOS | 17 | 2.9 | 10 | 60 | 6 | 35 | 1 | 5 | |
| Soft palate | 7 | 1.2 | 7 | 100 | - | - | - | ||
| GPS | 6 | 1.0 | 3 | 50 | 2 | 33 | 1 | 17 | |
| Pharyngeal wall | 2 | 0.3 | 1 | 50 | 1 | 50 | - | - | |
| HPV/p16 status | |||||||||
| Positive | 338 | 57.6 | 248 | 73 | 84 | 25 | 6 | 2 | 0.33 |
| Negative | 42 | 7.1 | 29 | 69 | 13 | 31 | - | - | |
| Unknown | 207 | 35.3 | 142 | 68 | 57 | 28 | 8 | 4 | |
| T status | |||||||||
| T1 | 203 | 34.6 | 166 | 82 | 34 | 17 | 3 | 1 | <0.0001* |
| T2 | 229 | 39.0 | 161 | 70 | 66 | 29 | 2 | 1 | |
| T3 | 91 | 15.5 | 61 | 67 | 27 | 30 | 3 | 3 | |
| T4 | 61 | 10.4 | 29 | 47 | 26 | 42 | 6 | 1 | |
| Tx | 3 | 0.5 | 2 | 67 | 1 | 33 | |||
| N status | |||||||||
| N0 | 46 | 7.8 | 31 | 67 | 14 | 30 | 1 | 2 | 0.35 |
| N1 | 81 | 13.8 | 61 | 75 | 17 | 2 | 3 | 3 | |
| N2a | 62 | 10.6 | 47 | 76 | 14 | 22 | 1 | 2 | |
| N2b | 283 | 48.2 | 204 | 72 | 76 | 27 | 3 | 1 | |
| N2c | 103 | 17.5 | 67 | 65 | 30 | 29 | 6 | 6 | |
| N3 | 12 | 2.0 | 9 | 75 | 3 | 25 | - | - | |
| Mean radiation dose ± SD, Gy | 68.12 | 2.67 | 67.85 | 2.46 | 68.69 | 3.15 | 69.84 | 1.23 | <0.0001* |
| Mean no. fractions ± SD | 32.17 | 2.59 | 31.9 | 2.34 | 32.77 | 3.10 | 33.43 | 2.13 | <0.0001* |
| Chemotherapy | |||||||||
| Induction | 98 | 16.7 | 76 | 78 | 22 | 22 | - | - | 0.0001* |
| Concurrent | 220 | 37.5 | 155 | 71 | 63 | 29 | 2 | <1 | |
| Induction + Concurrent | 129 | 21.8 | 75 | 58 | 45 | 36 | 8 | 6 | |
| None | 141 | 24.0 | 113 | 80 | 24 | 17 | 4 | 3 | |
| Surgery | |||||||||
| Yes | 12 | 2.0 | 11 | 92 | 1 | 8 | - | - | 0.29 |
| No | 575 | 98.0 | 408 | 71 | 153 | 27 | 14 | 2 | |
| Mean survival time at survey (years) ± SD | 5.94 | 2.86 | 5.75 | 2.87 | 6.41 | 2.79 | 6.14 | 2.71 | 0.0321* |
Abbreviations: SD, Standard deviation; NOS, Not otherwise specified; GPS, Glossopharyngeal sulcus; Gy, Gray Group mean and categorical variables were compared with Kruskal-Wallis test and Pearson’s chi-square test, respectively.
Table 2.
Association between the clinical variables and the presence of trismus
| Covariate | OR, 95% CI | Univariate analysis p-value | Multivariate analysis FDR-p-value | |
|---|---|---|---|---|
| Sex | 0.42 | |||
| Male | 1.12(0.74–2) | |||
| Female | 1 | |||
| Mean age (years) ± SD | 0.11 | |||
| Initial disease site* | 0.01 | 0.04 | ||
| Base of tongue | 1 | |||
| Tonsil | 1.6(1.1–2.3) | |||
| Smoking status | 0.12 | |||
| Never | 1 | |||
| Current/Former Smoker | 1.3(0.9–1.9) | |||
| HPV/p16 status* | 0.56 | |||
| Positive | 1 | |||
| Negative | 1.2(0.1–2.5) | |||
| T status* | ||||
| T1–2 | 1 | <0.0001* | 0.01 | |
| T3–4 | 2.14(1.45–3.7) | |||
| N status | ||||
| N0–1 | 1 | 0.76 | ||
| N2–3 | 1.1(0.6–1.6) | |||
| Mean radiation dose ± SD, Gy | <0.0001* | 0.01 | ||
| Chemotherapy | ||||
| None | 1 | 0.0001* | 0.06 | |
| Concurrent | 1.69(1.0–2.8) | |||
| Induction + Concurrent | 2.85(1.6–4.9) | |||
| Induction | 1.68(0.6–2.2) | |||
patients with unknown/NOS status were excluded.
The Dmean delivered to all masticatory ROI was statistically associated with the presence and severity of trismus (p<0.05) in univariate analysis, Table 3. Multivariate analysis demonstrated an association between the severity of trismus (none/mild vs. moderate/severe) and Dmean delivered to the ILP and CMP (p <0.01 and 0.02, respectively).
Table 3:
Dmean of ROIs according to presence and severity of trismus.
| Trismus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Mean ± SD | P Value* | Mean ± SD | P Value* | Mean ± SD | P Value* | ||||
| Yes | Any | None | Mild | Moderate/Severe | None/Mild | Moderate/Severe | ||||
| IM | 39.29 ± 9.41 | 34.62 ± 8.89 | <0.0001 | 34.61 ± 8.89 | 38.76 ± 9.30 | 45.15 ± 8.94 | <.0001 | 45.15 ± 8.94 | 35.73 ± 9.18 | 0.0005 |
| IMP | 64.72 ± 8.65 | 60.71 ± 9.48 | <0.0001 | 60.71 ± 9.48 | 64.32 ± 8.68 | 69.08 ± 7.25 | <.0001 | 69.08 ± 7.25 | 61.68 ± 9.40 | <.0001 |
| ILP | 49.50 ± 14.87 | 40.72 ± 14.95 | <0.0001 | 40.72 ± 14.95 | 48.75 ± 14.72 | 57.69 ± 14.51 | <.0001 | 57.69 ± 14.51 | 42.87 ± 15.29 | 0.0003 |
| CM | 25.29 ± 8.10 | 22.61 ± 8.59 | 0.0008 | 22.61 ± 8.59 | 24.91 ± 7.99 | 29.44 ± 8.47 | 0.0014 | 29.44 ± 8.47 | 23.23 ± 8.47 | 0.0317 |
| CMP | 47.36 ± 13.10 | 43.11 ± 14.64 | 0.0002 | 43.11 ± 14.64 | 46.54 ± 13.15 | 56.30 ± 8.70 | <.0001 | 56.30 ± 8.70 | 44.03 ± 14.32 | 0.0006 |
| CLP | 29.93 ± 12.78 | 25.72 ± 12.29 | <0.0001 | 25.79 ± 12.31 | 29.50 ± 12.17 | 36.61 ± 13.84 | <0.0001 | 36.61 ± 13.84 | 26.79 ± 12.37 | 0.0157 |
Abbreviations: ROI, Region of n=interest; IM, Ipsilateral masseter; IMP, Ipsilateral medial pterygoid; ILP, Ipsilateral lateral pterygoid; CM, Contralateral masseter; CMP, Contralateral medial pterygoid; CLP, Contralateral lateral pterygoid; SD, Standard deviation.
Mean values of trismus compared with Wilcoxon rank sum test.
Figure 2 shows that the Dmean delivered to each ROI was higher in patients who had moderate/severe trismus compared to those with none/mild trismus. Specifically, the Dmeans delivered to IM (45 vs. 36 Gy), IMP (69 vs. 62 Gy), ILP (58 vs. 43 Gy), CM (29 vs. 23 Gy), CMP (56 vs. 44 Gy), and CLP (37 vs. 27 Gy).
Fig. 2.
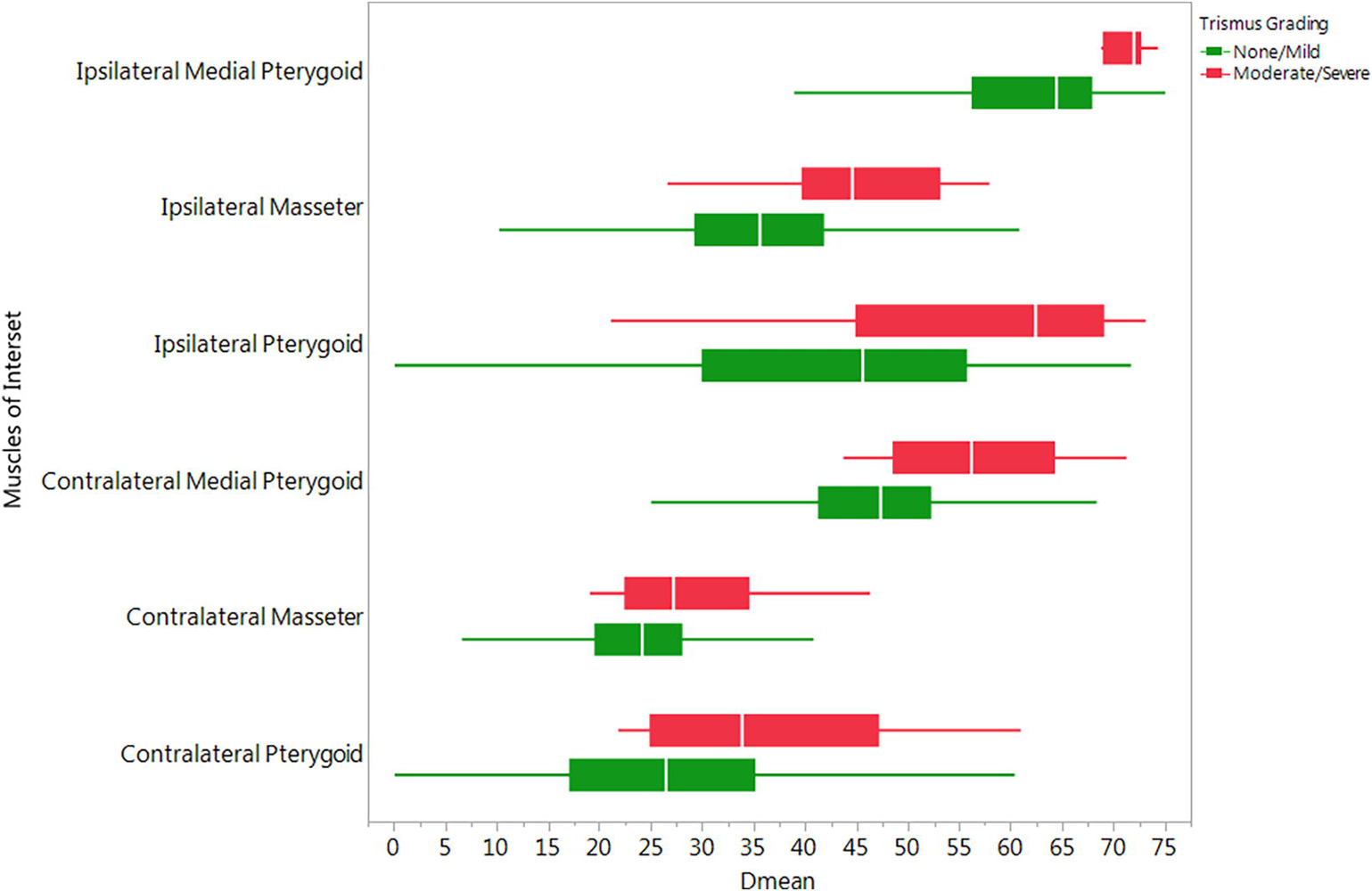
Whole ROI Dmean dose differentials by Trismus Severity Grouping.
Composite DVHs shown in Figure 3 illustrate that patients with late trismus had higher radiation dose delivered to all ROIs compared to those with no self-reported late trismus, with the greatest separation seen in the ILP (Figure 3). We identified ROI-specific dose-volume thresholds associated with the persistent trismus using RPA and decision tree analysis. RPA demonstrated that DVH-derived ILP V27 and Dmean were associated with persistent RT-attributable trismus, specifically a V27 of at least 98.6% and Dmean of 61 Gy (LogWorth 10 and 3.6 respectively). A significant association between the dosimetric parameters of the ILP and the presence of late trismus was maintained after adjustment for clinical variables.
Fig. 3.
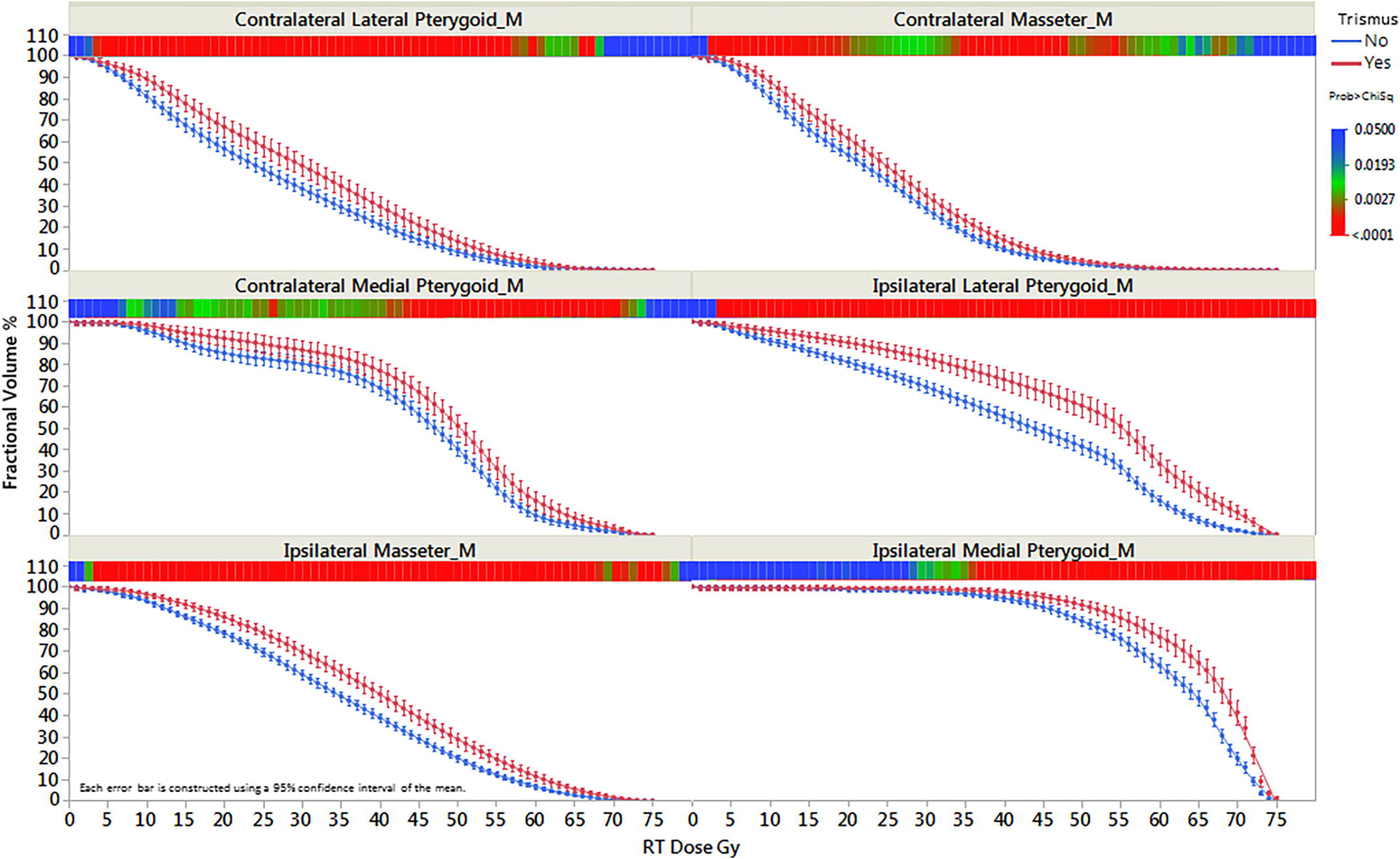
Trismus-related structures DVH stratified by presence of trismus.
Discussion
In this study, we utilized a prospectively administered patient-reported survey and we found that the 1) prevalence of trismus in long-term OPC survivors treated with IMRT is associated with delivery of higher Dmean to all of the evaluated masticatory muscles, including the ipsilateral and contralateral masseters, medial pterygoids, and lateral pterygoids; 2) Marked separation in cumulative DVHs between patients with and without late trismus was observed in the ILP, even in the low-dose range; and 3) the dose-volume thresholds most associated with persistent trismus include ILP V27 of at least of 98.6 % and Dmean of 61 Gy to ILP.
In the clinical setting, we could only apply the proposed constraints without compromising the tumor control and whenever it is technically feasible. Out of this research effort, it would be of great value if we could bring the clinicians’ attention to segment ILP and apply the proposed Dmen constraint. Yet, we cannot ignore the impact of the low integral dose of IMRT beams on the normal tissues.
Multiple factors likely contributed to the development of trismus in patients undergoing RT for HNC. First, malignant tumors can directly invade or cause inflammation of the muscles of mastication or the temporomandibular joint (TMJ) [17]. Second, tumors can impinge upon nerve tracts in the pharynx and oral cavity and induce trismus of the muscles of mastication [8]. Finally, trismus can be a direct sequela of RT which when delivered to the TMJ or muscles of mastication causing fibrosis leading to restricted mobility and decreased mouth opening [18–21], even with treatment advances.
IMRT is an irradiation technique that uses dynamic beam arrangements to create a highly conformal three-dimensional dose field. In the field of HNC, IMRT has most notably been used for parotid sparing treatment and associated with decreased rates of RT-induced xerostomia [22]. Beyond the parotid glands, IMRT may deliver lower radiation doses to other surrounding normal structures, including the TMJ and muscles of mastication, as evidenced by a 2010 systematic review in which the weighted prevalence trismus rates of in HNC patients were 25.4% for conventional RT but just 5% for IMRT [18]. In our study, we limited study inclusion to OPC patients given IMRT as a component of definitive treatment. While 29% of patients reported any degree of late trismus, only 2.4% of them had late trismus categorized as moderate or severe, which compares favorably with previous reports. Conversely, other studies have demonstrated the trismus incidence rates to be as high as 45% [5]. Such higher reported rate could be explained by differences in the RT modalities used; we included a homogenous group of patients who underwent IMRT rather than a mixed group of patients receiving conformal RT/IMRT, the study outcome (incidence vs. prevalence) and the study design; our study is cross sectional study to determine the prevalence of patient-reported late trismus in OPC survivors.
Currently, standard or universally accepted treatment of trismus is lacking. Proposed interventions (e.g., use of jaw-mobilizing devices, stretching techniques, electrotherapy, hyperbaric oxygen therapy, manual therapies, and pentoxifylline) have had variable success rates [23] [24] [25].
Undoubtedly, the most effective strategy to improve the QOL in HNC survivors, as it relates to jaw and mouth opening, would be to prevent the development of trismus. Thus, implementation of the dose constraints to the non-target normal structures whose irradiation contributes to trismus may help guide decision making during RT planning. Some studies have explored the dose-effect relationship for trismus in HNC patients [7, 10, 26]. Van der Molen et al. showed that the Dmean, Dmax, V20, V40, and V60 for each masticatory structure (masseter, temporalis, and pterygoid muscles and mandibular condyle) were significant predictors of the presence of trismus at 10 weeks after RT in patients with advanced HNC. However, to date, no investigations have established a radiation dose threshold for the muscles of mastication in a cohort limited to patients with OPC receiving IMRT or in long-term OPC survivors. By limiting our analysis to patients with OPC receiving IMRT, we focused on a relatively homogenous group of patients at increased risk for trismus (as compared with patients with laryngeal, HPC, or NPC cancers) who underwent modern RT techniques (IMRT as compared with 3D conformal RT). We also sought to include statistical analyses with a large range of dose-volume parameters to retain some spatial information rather than focusing on the Dmean or pre-determined volumetric parameters. With this focused methodology, we successfully established dose-volume thresholds for the IM, IMP, ILP, CM, CMP, CLP, and C-PGs, above which the prevalence of long-term trismus increased substantially. Moreover, we found trismus to be significantly associated with higher Dmean delivered to the ILP and CMP in the multivariate analysis. In OPC patients with and without trismus, due to the spatial location of the medial masticator muscles (MPs), the IMP muscles are usually within the high/ intermediate dose region. Thus, there was substantially an observed significant differential radiation doses delivered to the ILP and CMP muscles (between patients with and without late trismus), which are relatively far away from the high-dose beam path but still within the low/intermediate dose region.
This analysis has some limitations, including those inherent to a single-center observational study at a tertiary cancer care center. Another potential limitation was the risk of misclassification of trismus as the prevalence and severity of trismus in our study were solely subjective, reported by the patients, as required in this large survivorship survey. More accurate stratification of trismus would require objective measurement of the maximum inter-incisal opening as well as physician and/or dental assessment. However, mandating in-person assessment would likely limit the sample size considerably. Such measurements would ensure capture of even very mild cases of trismus and allow for analyses of continuous rather than binary variables. Also, given the cross-sectional study design, we did not have baseline trismus status uniformly collected but have controlled on regression analysis for T-stage and tumor site, which are the most likely surrogates for baseline trismus. Nonetheless, another analysis of these survey data found this trismus measure to be highly related to quality of life, health utility, symptom interference, and functional status, lending support to the validity of the measure. These data provide insight into the psychosocial and clinical relevance of the presence and severity of trismus.
In conclusion, to the best of our knowledge, this study is the first to identify masticatory muscles dose-volume thresholds associated with persistent trismus in patients with OPC treated with IMRT. The most striking dosimetric differences among patients with and without late trismus were obsevered to be related to the ILP muscles (specifically, V27 of at least of 98.6 % and Dmean of 61 Gy), both the low dose bath to this muscle and mean dose. These findings may help guide future IMRT treatment planning and decrease the risk of late trismus in this population.
Supplementary Material
Highlights:
Trismus remains a highly prevalent survivorship concern in the IMRT era.
Incorporation of patient-reported outcomes into the predictive toxicity models is a must.
Utilization of the proposed dosimetric constraints may reduce the risk of late trismus after IMRT in oropharyngeal cancer patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
This work was directly supported by the Charles and Daneen Stiefel Oropharynx Fund. This work is also directly supported by the Andrew Sabin Family Foundation; Dr. Fuller is a Sabin Family Foundation Fellow. Drs. Mohamed, Lai, Hutcheson, and Fuller receive (d) funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248–01/R56DE025248–01) and NIH/NCI Early Phase Clinical Trials in Imaging and Image Guided Interventions Program (1R01CA218148–01). Dr. Fuller receives federal grant and/or salary support from: the NIH/National Cancer Institute (NCI) Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50CA097007–10) and a National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825–01); and the Cancer center Support Grant Radiation Oncology/Cancer Imaging Program Seed Grant (5P30CA016672). Dr. Fuller receives (d) industry grant support and speaker travel funding from Elekta AB.
References
- [1].van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Hilgers FJ. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009;266:889–900. [DOI] [PubMed] [Google Scholar]
- [2].Kocak-Uzel E, Gunn GB, Colen RR, Kantor ME, Mohamed ASR, Schoultz SH, et al. Beam path toxicity in candidate organs–at-risk: Assessment of radiation emetogenesis for patients receiving head and neck intensity modulated radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2014;111:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: Dose–volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiotherapy and Oncology. 2016;118:304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosenthal DI, Chambers MS, Fuller CD, Rebueno NCS, Garcia J, Kies MS, et al. Beam Path Toxicities to Non-Target Structures During Intensity-Modulated Radiation Therapy for Head and Neck Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Louise Kent M, Brennan MT, Noll JL, Fox PC, Burri SH, Hunter JC, et al. Radiation-induced trismus in head and neck cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2008;16:305–9. [DOI] [PubMed] [Google Scholar]
- [6].Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral oncology. 2004;40:879–89. [DOI] [PubMed] [Google Scholar]
- [7].Satheeshkumar PS, Mohan MP, Jacob J. Restricted mouth opening and trismus in oral oncology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:709–15. [DOI] [PubMed] [Google Scholar]
- [8].Tveteras K, Kristensen S. The aetiology and pathogenesis of trismus. Clinical otolaryngology and allied sciences. 1986;11:383–7. [DOI] [PubMed] [Google Scholar]
- [9].Greco E, Simic T, Ringash J, Tomlinson G, Inamoto Y, Martino R. Dysphagia Treatment for Patients With Head and Neck Cancer Undergoing Radiation Therapy: A Meta-analysis Review. International Journal of Radiation Oncology*Biology*Physics. 2018;101:421–44. [DOI] [PubMed] [Google Scholar]
- [10].Rao SD, Saleh ZH, Setton J, Tam M, McBride SM, Riaz N, et al. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncologica. 2016;55:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CR, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106:364–9. [DOI] [PubMed] [Google Scholar]
- [12].Gebre-Medhin M, Haghanegi M, Robért L, Kjellén E, Nilsson P. Dose-volume analysis of radiation-induced trismus in head and neck cancer patients. Acta Oncologica. 2016;55:1313–7. [DOI] [PubMed] [Google Scholar]
- [13].Garden AS, Kies MS, Morrison WH, Weber RS, Frank SJ, Glisson BS, et al. Outcomes and patterns of care of patients with locally advanced oropharyngeal carcinoma treated in the early 21st century. Radiat Oncol. 2013;8:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys. 2005;63:1000–5. [DOI] [PubMed] [Google Scholar]
- [15].Dillman DA, Smyth JD, Christian LM. Internet, phone, mail, and mixed-mode surveys : the tailored design method. 4th edition ed. Hoboken: Wiley; 2014. [Google Scholar]
- [16].Mohamed ASR, Ruangskul M-N, Awan MJ, Baron CA, Kalpathy-Cramer J, Castillo R, et al. Quality Assurance Assessment of Diagnostic and Radiation Therapy–Simulation CT Image Registration for Head and Neck Radiation Therapy: Anatomic Region of Interest–based Comparison of Rigid and Deformable Algorithms. Radiology. 2015;274:752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ichimura K, Tanaka T. Trismus in patients with malignant tumours in the head and neck. The Journal of laryngology and otology. 1993;107:1017–20. [DOI] [PubMed] [Google Scholar]
- [18].Bensadoun RJ, Riesenbeck D, Lockhart PB, Elting LS, Spijkervet FK, Brennan MT, et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1033–8. [DOI] [PubMed] [Google Scholar]
- [19].Goldstein M, Maxymiw WG, Cummings BJ, Wood RE. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1999;88:365–73. [DOI] [PubMed] [Google Scholar]
- [20].Lindblom U, Garskog O, Kjellen E, Laurell G, Levring Jaghagen E, Wahlberg P, et al. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta oncologica. 2014;53:620–7. [DOI] [PubMed] [Google Scholar]
- [21].Teguh DN, Levendag PC, Voet P, van der Est H, Noever I, de Kruijf W, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head & neck. 2008;30:622–30. [DOI] [PubMed] [Google Scholar]
- [22].Eisbruch A IMRT reduces xerostomia and potentially improves QoL. Nature Reviews Clinical Oncology. 2009;6:567. [DOI] [PubMed] [Google Scholar]
- [23].Scherpenhuizen A, van Waes AM, Janssen LM, Van Cann EM, Stegeman I. The effect of exercise therapy in head and neck cancer patients in the treatment of radiotherapy-induced trismus: A systematic review. Oral oncology. 2015. [DOI] [PubMed] [Google Scholar]
- [24].King GE, Scheetz J, Jacob RF, Martin JW. Electrotherapy and hyperbaric oxygen: promising treatments for postradiation complications. The Journal of prosthetic dentistry. 1989;62:331–4. [DOI] [PubMed] [Google Scholar]
- [25].Chua DT, Lo C, Yuen J, Foo YC. A pilot study of pentoxifylline in the treatment of radiation-induced trismus. American journal of clinical oncology. 2001;24:366–9. [DOI] [PubMed] [Google Scholar]
- [26].van der Molen L, Heemsbergen WD, de Jong R, van Rossum MA, Smeele LE, Rasch CRN, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose–effect relationships for swallowing and mastication structures. Radiotherapy and Oncology. 2013;106:364–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


