Abstract
Background & Aims:
Approximately one-third of patients with IBS-diarrhea (IBS-D) have increased bile acid (BA) synthesis or excretion. An open-label study showed benefits of colesevelam on bowel functions, consistent with luminal BA sequestration by colesevelam. We compared the effects of colesevelam vs placebo on symptoms and gene expression patterns in the sigmoid colon mucosa in patients with BA diarrhea associated with IBS-D.
Methods:
We performed a double-blind, parallel-group study of 30 adults with IBS-D and evidence of increased BA synthesis or fecal excretion, from December 2017 through December 2018 at a single center. Patients were randomly assigned (1:1) to groups given colesevelam (3 tablets, 625 mg each) or matching placebo, orally twice daily for 4 weeks. Stool diaries documented bowel functions for 8 days before and 28 days during colesevelam or placebo. Stool and fasting serum samples were collected for analyses of fecal BAs and serum levels of C4 and FGF19. We measured colonic transit by scintigraphy, mucosal permeability by in vivo excretion of saccharide probes, and mRNA levels in rectosigmoid biopsies. All measurements were made at baseline and on the last days of treatment. The primary endpoints were change in total fecal BA concentration and stool consistency.
Results:
Compared with placebo, colesevelam was associated with significant changes in sequestered fecal total BA excretion (P<.001) and serum levels of C4 and FGF19 (both P<.001), and with a mean increase in fecal level of deoxycholic acid (10%; P=.07) compared to placebo. Colesevelam decreased colon mucosal expression of NR1H4 and P2RY4 and increased expression of GPBAR1, compared with baseline. Stool frequency and consistency, colonic transit, and permeability did not differ significantly between groups. Colesevelam was well tolerated.
Conclusions:
In a randomized trial, we found that colesevelam increases delivery of total and secondary BAs to stool, hepatic BA synthesis, and colonic mucosal expression of genes that regulate BA, farnesoid X, and GPBAR1 receptors. Larger studies are needed to determine the effects on clinical responses. ClinicalTrials.gov no: NCT03270085
Keywords: sequestrant, hepatic synthesis, cholesterol, bile acid-binding
BACKGROUND
Irritable bowel syndrome (IBS) affects ~11% and IBS-diarrhea (IBS-D) affects ~5% of the U.S. population1 and often impairs quality of life.2 IBS is currently defined by symptoms (i.e., abdominal discomfort associated with bowel disturbances) in the absence of organic disease on routine testing. Current concepts on IBS focus on disturbances of peripheral mechanisms (colonic transit, mucosal immune function, visceral hypersensitivity), and central nervous system (CNS) hypervigilance.3,4,5 These peripheral perturbations may results from effects of intraluminal irritants [such as mal-digested carbohydrates (producing short chain fatty acids ((SCFA)) or fats], excess of bile acids (BAs), bioactive amines (e.g., serotonin),6 mucosal barrier function (TJ proteins),7 immune activation, increased small intestinal and colonic permeability, and alterations in microbiome. In fact, alterations in colonic BA levels have been shown to affect all of these mechanisms,8 and about one-third of patients with IBS have alterations in fecal BA excretion and hepatic BA synthesis rates compared to healthy controls.9
A meta-analysis showed BA diarrhea (BAD) in up to 50% of patients with chronic functional diarrhea or IBS-D based on 75SeHCAT retention test (not available in USA), and these studies also showed the test identifies patients most likely to respond to BA sequestrants.10 Efficacy of colestipol or cholestyramine or colesevelam has been documented predominantly in open-label trials, sometimes with flexible dosing schedules.11,12, 13
With new diagnostic tests for BAD, specifically fecal BA measurements and fasting serum C4,14 it is now possible to specifically diagnose idiopathic BAD in patients with symptoms of IBS-D.
In a previous study, we showed increased mucosal expression, in the 47 IBS-D patients (compared to healthy controls), of genes related to BA absorption (SLC10A2 for ASBT), BA synthesis (NR1H4 for FXR), ion transport (GUCA2B and PDZD3), immune function (C4BP4 and CCL20), and barrier function (CLDN1 and FN1). In contrast, no such differential expression was noted in mucosal biopsies from patients with IBS-C.15
Our study aim was to compare effects of the BA sequestrant, colesevelam, and placebo on bowel functions, BA synthesis and excretion, colonic transit, intestinal and colonic mucosal permeability, and mucosal expression of genes in sigmoid colon mucosa in patients with BAD in association with IBS-D.
METHODS
Study Design, Randomization, and Experimental Protocol
We conducted a single-center, randomized, double-blinded, single-dose level, parallel-group, controlled, 28-day trial of the effects of colesevelam, 1875mg (3 tablets, 625mg each), or matching placebo (ratio 1:1), taken orally, twice daily, in 30 patients with IBS-D and prior evidence of increased BA synthesis or fecal excretion. Placebo consisted of gelatin, titanium dioxide, and FDA/E172 red iron oxide.
The study was conducted at Mayo Clinic, Rochester Minnesota, and participants were recruited from December 2017 to December 2018. Identification and eligibility of participants are detailed in Supplemental Materials.
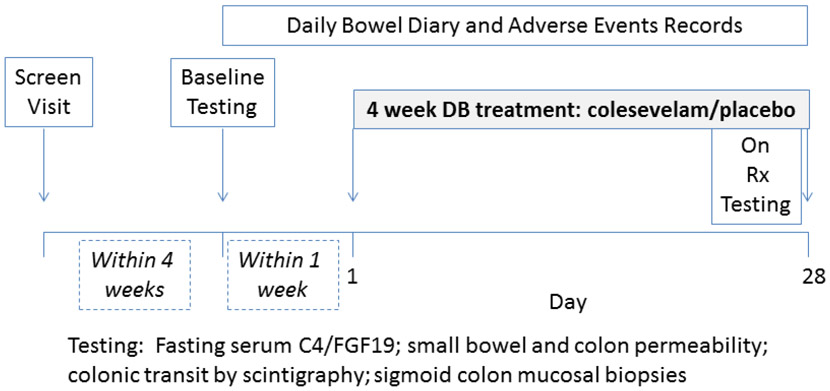
The experimental protocol is shown in Figure 1. All of the authors had access to the study data and approved the final manuscript. The study statistician generated by computer a stratified randomization sequence designed for equal distribution of patients on treatment or placebo and BMI (<35 and ≥35kg/m2). Further details are provided in Supplemental Materials under random allocation.
Figure 1.
Experimental design
Characterization of patient symptoms at screening, exclusions based on concomitant medications or illnesses and details on the colesevelam treatment are detailed in the Supplemental Materials. Patients who were on a bile acid sequestrant before the start of the study discontinued the sequestrant at least 30 days before initial study visit.
Small Intestinal and Colonic Permeability: In Vivo 13C Mannitol Excretion after Oral Load
After baseline urine collection, 100mg 13C mannitol and 1g lactulose dissolved in 250mL of water was administered. Urine collections were pooled for baseline (pre-test dose), 0–2, 2–8 and 8–24 hours following administration of test sugars, and excreted sugar concentrations were measured by HPLC-tandem MS as previously published.16
Biological Samples and Measurements of Bile Acid Parameters
Blood samples were collected in the morning after overnight fasting before starting the colesevelam and on the morning after the 28-day dosing with colesevelam.
Random stools samples were collected before starting the colesevelam and after day 28 of dosing with colesevelam. Participants provided a single random stool sample. The samples were sent to a central laboratory to be frozen immediately for subsequent testing for fecal BAs by HPLC, tandem mass spectrometry. Data are expressed as μmol/g stool.
Laboratory Measurements of Bile Acid Parameters
Additional information regarding serum 7α-hydroxy-4-cholesten-3-one (C4) testing and fecal bile acid excretion are available in the Supplemental Materials.
Rectosigmoid Mucosal Expression of Genes Using RNA-Sequencing
The methods used and selection of genes of interest (previously associated with BAD) are shown in the Supplemental Materials.
Statistical Power and Analysis
These are detailed in the Supplemental Materials.
Endpoints for analysis
The primary endpoint for analysis was the change in the total fecal BAs concentration (μmol/g stool) from baseline in response to treatment with colesevelam compared to placebo. Since the fecal BA measurement included an extraction step that freed the BAs into the stool water, the effect of colesevelam was measured as an increase in fecal BA excretion. The co-primary patient response endpoint was stool consistency. The secondary endpoints addressed other effects of colesevelam compared to placebo in patients with IBS-D and BAD; these endpoints were appraised primarily for the entire 4-week treatment period:
Percent fecal primary (CDCA and CA) and secondary (DCA and LCA) BA excretion
Fasting serum C4 and serum FGF19
Stool frequency
Colonic transit measured at 24h and 48h
Small intestinal and colonic permeability
Mucosal expression of genes of interest previously associated with BAD17
The comparisons of stool parameters (frequency and consistency) were performed as prespecified for the total duration (4 weeks) of treatment and in post-hoc analyses for the last 7 days of treatment, as well as for the 57% of patients with elevated C4 or decreased FGF19 compared to those with normal serum biomarkers.
RESULTS
Demographics
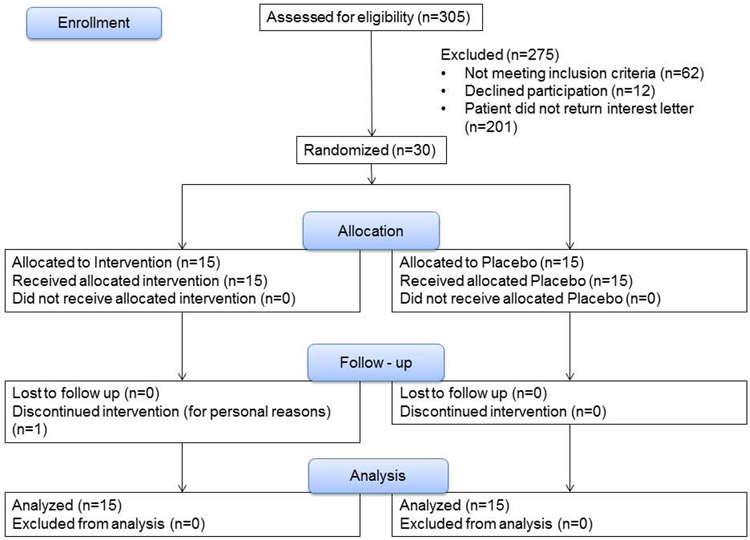
Table 1 shows the demographics and baseline data of participants in the study and documents the absence of any statistically significant differences between the 2 groups. The CONSORT flow chart and participant disposition are shown in Figure 2. One person withdrew from the study secondary to personal reasons, but was included within the analysis in accordance with intention to treat principles. Within this cohort, 30% (9/30) were diagnosed with BAD based on elevated total fecal BA, 57% (17/30) based on elevated primary fecal BA, and 13% (4/30) based on elevated C4.
Table 1. Demographics and baseline and post-treatment values in the two groups for bowel function endpoints, fecal bile acids, fecal weight and serum C4 and FGF19, permeability and GI transit.
There were no significant differences in the two groups at baseline. Data show median (IQR). At baseline, colonic transit at 24 h was the only transit parameter measured. P values compare data at end of treatment for colesevelam and placebo with baseline values as covariates.
| Group | Placebo, n=15 | Colesevelam, n=15 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | 44 (34-52) | 56 (40-67) | |||||||
| Sex, F/M | 11/4 | 13/2 | |||||||
| BMI kg/m2 | 33.2 (28.0-36.7) | 33.6 (28.2-36.4) | |||||||
| N | Baseline | N | Treatment | N | Baseline | N | Treatment | ||
| # stools per day | 15 | 2.4 (1.6-3.7) | 15 | 2.2 (1.6-2.9) | 14 | 3.8 (2.3-5.0) | 15 | 3.1 (1.6-3.8) | 0.91 |
| Stool form (BSFS) | 15 | 5.2 (4.8-6.0) | 15 | 4.6 (4.3-5.0) | 14 | 5.5 (5.0-6.4) | 15 | 4.6 (3.9-5.2) | 0.81 |
| Total fecal BA excretion (μmoles/g stool) | 15 | 5.2 (3.0-6.7) | 14 | 3.6 (2.6-7.2) | 13 | 3.2 (1.4-5.4) | 15 | 10.7 (8.2-17.4) | <0.001 |
| Fecal CDCA excretion % | 15 | 6.5 (0.6-17.4) | 14 | 6.04 (1.0-18.4) | 13 | 8.7 (1.4-20.3) | 15 | 2.7 (1.1-10.4) | 0.57 |
| Fecal CA excretion % | 15 | 13.8 (0.3-40.9) | 14 | 3.8 (0.4-26.4) | 13 | 19.8 (0.6-33.2) | 15 | 0.9 (0.4-7.7) | 0.34 |
| Fecal 1° BA (CDCA + CA) % | 15 | 15.7 (1 – 48.1) | 14 | 9.8 (1.2 – 37.1) | 13 | 25.8 (1.3 – 49.3) | 15 | 2.7 (1.3 – 15.6) | 0.41 |
| Fecal LCA excretion % | 15 | 23.5 (6.8-43.4) | 14 | 28.8 (10.3-33.7) | 13 | 22.8 (9.2-35.6) | 15 | 29.4 (22.7-31.8) | 0.89 |
| Fecal DCA excretion % | 15 | 46.8 (24-56) | 14 | 54.3 (41.7-64) | 13 | 52.6 (37.5-61.8) | 15 | 64.1 (57.3-67.5) | 0.07 |
| Fecal 2° BA (LCA + DCA) % | 15 | 83.4 (59 – 99) | 14 | 90.1 (61.2 – 98.8) | 13 | 71.8 (50.5-98.7) | 15 | 97.3 (83.7 – 98.7) | 0.43 |
| Serum C4 (ng/mL) | 15 | 31.4 (20-50) | 14 | 29 (17-52) | 13 | 45.5 (37-85) | 15 | 112.5 (89-205) | <0.001 |
| Serum FGF19 (pg/mL) | 15 | 84 (67-217) | 14 | 83.7 (67-161) | 13 | 48.3 (34-95) | 15 | 26 (18-33.5) | <0.001 |
| SB (0-2h) permeability, 13C-mannitol excretion (%)* | 14 | 12.6 (9.3-15.7) | 14 | 12.9 (8.9-14.4) | 15 | 10.3 (6.0-12.8) | 13 | 11.0 (7.4-18.2) | 0.73 |
| Colonic (8-24h) permeability, 13C-mannitol excretion (%)* | 15 | 3.4 (3.0-7.1) | 14 | 3.0 (2.2-3.8) | 14 | 3.84 (2.5-5.45) | 14 | 3.1 (1.0-4.1) | 0.98 |
| Gastric emptying T1/2, min | 14 | 116.4 (101.4-140.3) | 15 | 107.6 (92.3-125.4) | 0.23 | ||||
| Colonic filling at 6h, % | 14 | 55.5 (33.7-75.0) | 15 | 71.0 ( 27.0-86.0) | 0.38 | ||||
| Colonic transit GC24 | 14 | 2.7 (2.6 – 3.2) | 14 | 2.6 ( 2-4) | 15 | 3.3 (2.5 -4.4) | 15 | 2.7 (2.3-3.8) | 0.65 |
| Colonic transit GC48 | 14 | 4.6 (4-5) | 15 | 4.8 (3.9-5.0) | 0.57 | ||||
100mg dose of 13C-mannitol administered BMI=body mass index; BSFS=Bristol Stool Form Scale; BA=bile acid; CDCA=chenodeoxycholic acid; CA=cholic acid; DCA=deoxycholic acid; LCA=lithocholic acid; Primary = 1°; Secondary = 2°; SB=small bowel
Figure 2.
CONSORT flow chart and participant disposition
Thirty-five percent of patients (7/20) were receiving bile acid sequestrant before the first (screening) study visit. Four patients were on cholestyramine, 4 grams daily or BID; 2 were on colestipol, 1-2 grams BID; and 1 patient was on colesevelam, 1,875 mg BID. All patients stopped the sequestrant therapy at least 30 days before the initial study visit.
Effects of Colesevelam on Stool Form and Bowel Function
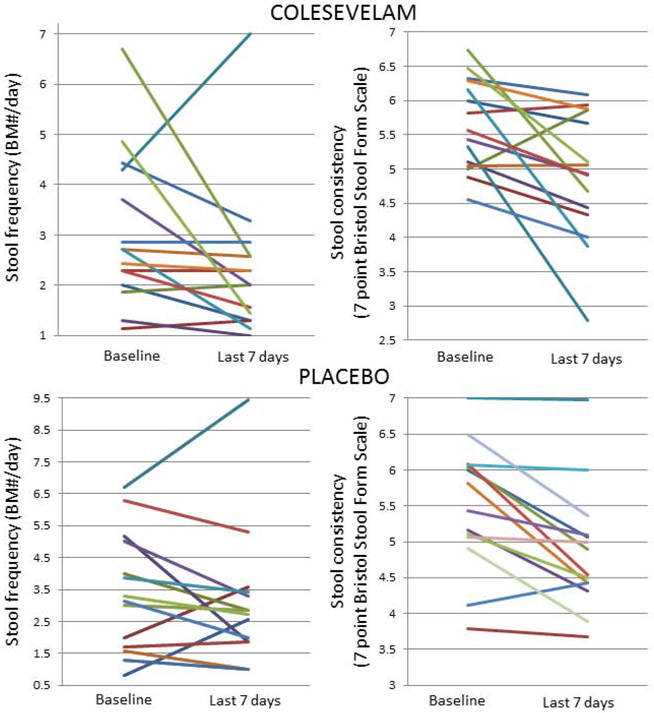
Table 1 and Figure 3 summarize the group data and individual participant data regarding the patient response outcomes. While there were numerical improvements in stool number and stool consistency in several participants, there were no significant group differences in the analysis of least square means, which account for the pre-study observations in each group.
Figure 3.
Effects of colesevelam on stool frequency and consistency in patients with diarrhea-predominant irritable bowel syndrome and bile acid diarrhea. Same color lines within each cohort represent the same patient.
During the last week of treatment compared to baseline (Figure 3), there was numerical, non-significant improvement in stool frequency with colesevelam [delta from baseline −0.65 (−1.6 to −0.5)] compared to placebo [delta from baseline −0.3 (−0.14 to 0.0)] (p=0.93). Average stool consistency during the last 7 days of treatment was not significant between the two treatment groups: colesevelam −0.6 (−1.7 to −0.1) and placebo −0.55 (−0.94 to −0.07) (p=0.41).
There were no significant differences in stool consistency or frequency in the 57% of participants with baseline elevated C4 or decreased FGF19 (p=0.46 and p=0.36, respectively).
Pharmacodynamic Effects of Colesevelam on Fecal BA Excretion and Fasting Serum C4 and FGF19
Binding and sequestration of BAs in stool with colesevelam are demonstrated by the increased fecal total BAs (Table 1), as well as an average 10% increase in sequestration of the secretory, secondary BA, deoxycholic acid; the increased sequestration and loss of BAs were associated with the expected increase in serum C4 and decease in serum FGF19. These data illustrate that colesevelam achieved its pharmacodynamics effects.
Correlation of Fecal BA Excretion and Bowel Functions
Based on non-linear regression analysis, increased fecal BA sequestration (expressed as concentration of total fecal bile acids per gram of stool) was associated with a higher reduction in stool frequency (Rs=0.28, p<0.01 for the entire 4 weeks and Rs=0.41, p <0.01 for the last 7 days of treatment compared to baseline) with colesevelam. In contrast, there was an increase in stool frequency observed with placebo during the last 7 days of treatment (Rs=0.47, p=0.14). There was no significant correlation seen with the amount of total fecal BA sequestered and improvement in stool consistency with colesevelam (Rs=0.11, p=0.75) or placebo (Rs=0.17, p=0.61).
Effects of Treatment on Mucosal Permeability and Gastrointestinal and Colonic Transit
Table 1 shows there were no significant differences in small intestinal or colonic permeability, or in stomach, small bowel and colonic transit. Data for 13C-mannitol excretion are included in Table 1.
Effects of Treatment on Mucosal Expression Based on RNA Sequencing
Using standard criteria of false discovery rate (FDR) <5% and log2 fold change ≥1 or ≤−1, there were 1051 genes that were different in the mucosal biopsies taken post-colesevelam compared to post-placebo, as illustrated in the heatmap of differential expression shown in Supplemental Figure 1 (left side: colesevelam [orange] and placebo [cyan] groups). Based on canonical pathway analysis (Supplemental Figure 1, right side), the vast majority of the differentially expressed mucosal genes are associated with immune functions, such as Th1 and Th2 activation pathways and signaling in T helper cells. Given that these mechanisms have not been previously definitively associated with BAD or functional diarrhea, we explored the genes previously reported to be associated with BAD.
Effects of Treatment on Mucosal mRNA Expression Related to Bile Acid Pathways
In order to account for potential intra-individual differences in mRNA expression, we analyzed the ratio of post- / pre-mRNA expression for each individual, and then compared the data for all participants available in the two treatment groups. These data were available for 8/15 patients in the colesevelam group and 9/15 patients in the placebo group. Biopsies were available at baseline for one additional patient in each group; however, this participant elected not to undergo repeat biopsy at the end of treatment.
RNA sequencing with ingenuity pathway analysis demonstrated significant or borderline differences in the colesevelam- and placebo-treated groups for the ratio between post- and pre-treatment mRNA expression in rectosigmoid mucosal biopsies (Figure 4). Colesevelam treatment was associated with reduced expression of NR1H4 (gene for FXR receptor) and P2RY4 (gene involved in glial cell function) and with increased expression of GPBAR1 (bile acid receptor). There were numerical increases in VIP (neurotransmitter, secretagogue) and CCL20 (cytokine) expression that did not reach statistical significance.
Figure 4.
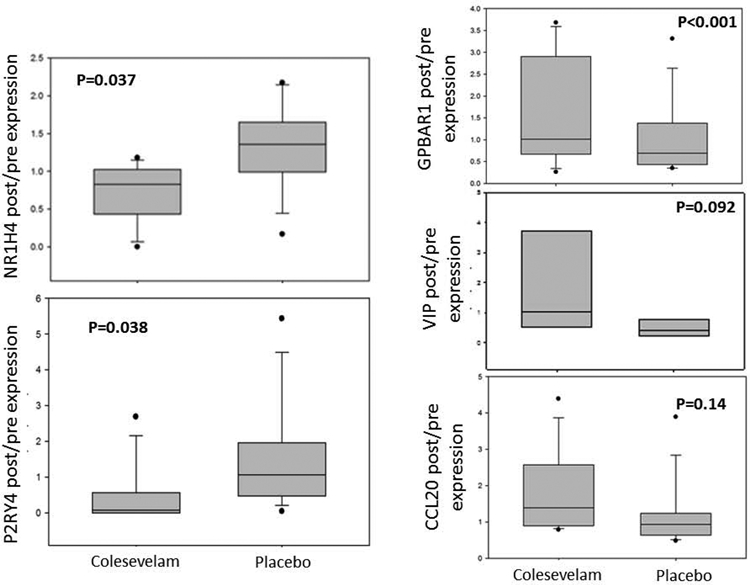
Expression of genes of interest in sigmoid colonic mucosa comparing two treatment groups for post- / pre-mRNA expression
Adverse Events
No serious adverse events were reported throughout the study.
DISCUSSION
Our study demonstrates expected pharmacodynamic effects of colesevelam such as increase in sequestered total fecal BAs, increase in fasting serum C4 reflecting compensatory BA synthesis, and the expected reciprocal reduction in serum FGF19. There was also a numerical increase in sequestration of deoxycholic acid in stool with colesevelam (p=0.07). However, there were no significant effects on stool frequency and consistency with colesevelam compared to placebo, although there was variable clinical responsiveness to colesevelam among patients with BAD. The pharmacodynamics observations reassure us that it is unlikely that noncompliance or inappropriate timing of medication contributed to the nonsignificant changes in patient response outcomes (stool frequency and consistency).
Small intestinal and colonic permeability and gastrointestinal and colonic transit were not different in the two treatment groups; in our prior study, colesevelam did not significantly alter colonic transit, although the ascending colon emptying T1/2 on colesevelam treatment correlated with baseline serum C4 level,18 and genetic variants in FGFR4 rs351855 and KLB rs4975017 identified a subset of IBS-D patients with beneficial response to colesevelam.19
In addition to the biochemical pharmacodynamic effects, our study demonstrates alterations in mucosal expression in genes of interest in the BA pathway and their biological effects. The upregulation of the CCL20 gene related to inflammation in response to colesevelam compared to placebo is surprising and unexplained since BAs can induce a histological picture similar to microscopic colitis, there is high prevalence of bile acid diarrhea in collagenous colitis (mean 41%and lymphocytic colitis (mean 29%; reviewed in ref.20), and 86% of the patients with BAD and microscopic colitis responded to BA sequestrant, cholestyramine.21
The positive transcriptomics findings were reduced expression of NR1H4 (nuclear receptor subfamily 1, group H, member 4 gene for FXR receptor) and P2RY4 (pyrimidergic receptor P2Y, G-protein coupled, 4 gene involved in glial cell function) and increased expression of GPBAR1 (G protein-coupled bile acid receptor 1; synonym TGR5 or Takeda G coupled BA receptor). These observations suggest that, in the presence of the BA sequestrant, a reduction in expression of the gene for FXR protein may reflect the reduction in BA absorption by colonocytes; normally, the absorbed BAs bind to the nuclear FXR receptor and result in the synthesis of FGF19. Consistent with this hypothesis is our observation of reduced FGF19 in fasting serum samples. Activation of P2RY4 is a downstream effect in BAD, based on our prior studies, and intraluminal sequestration of the BAs likely results in a reduced activation of glial signaling, which is involved in the control of mucosal secretion.22-24 Finally, the increased expression of GPBAR1 with colesevelam is consistent with the prior observation that this receptor (aka TGR5) is required for colesevelam to induce GLP-1 and alter hepatic glycogen metabolism, and that BAs bound to colesevelam activate a prolonged TGR5-mediated cAMP response.25 The latter observation may also explain the inter-individual variability in the response to colesevelam observed in our study, since the persistent activation of cAMP by the sequestered BAs in some patients may itself result in colonic secretion and variation in patient response outcomes.
Our data suggest that further large, placebo-controlled, randomized studies of longer duration are required, particularly given the numerical difference in stool frequency in the last week of the current trial, and the availability of only few placebo- or active-comparator controlled trials.26,27
In a previous double-blind, randomized, controlled trial, patients with IBS-D with and without BA diarrhea were given cholestyramine, 4 grams twice daily, compared to hydroxypropyl cellulose for 8 weeks. Although the study did not demonstrate efficacy on the primary endpoint of less than or equal to 3 bowel movements per day, there was a significant decrease in watery bowel movements compared to the hydroxypropyl cellulose. Based on previous evaluations, we know that hydryoxypropyl cellulose will also bind fecal BAs, and this may have complicated the assessment of the results.26 In a longer open-label study utilizing dose titration of cholestyramine, there was a significant improvement in stool consistency and frequency, and cholestyramine was utilized for a median of 35 months,11 far longer than the 4 week duration of our trial.
In addition, the optimal doses for the BA sequestrants or FXR agonists for BAD are still incompletely understood. For example, improvement in BA retention after cholestyramine occurred in 50% of patients with post-cholecystectomy BAD, and normalization was achieved in only 20% of patients,28 and only of 27 patients with BAD responded with colestipol treatment.12
A placebo-controlled trial of colesevelam in secondary bile acid diarrhea after ileal resection in Crohn's disease showed significant clinical efficacy; however, the level of BA excretion was likely different from the current study.27
The goal of BA sequestrants is not to bind all intestinal BAs, as that would conceivably cause diarrhea associated with fat malabsorption.29 In theory, an ileocolonic released form of BA sequestrant may have better efficacy, but this would need to be evaluated in prospective, clinical studies in patients with BA diarrhea. Cholestyramine enterocoated with cellulose acetate phthalate has been shown to be effective in patients after ileal resection.30
Limitations
Our assessment of clinical responses is clearly impacted by the sample size (15 per treatment group), missing mucosal gene expression data in a few participants, the fact that this is a single-center study and 3 patients had BSFS <5 and bowel frequency <3/day at baseline, but did meet Rome III IBS-D criteria; these factors reduce generalizability of the results and conclusions. Based on the variations in responses observed for bowel movements per day and consistency (standard deviation for each 0.7), with the sample size of 15 per treatment group, the study was powered to detect a change in bowel frequency of 0.75/day and stool consistency of 0.75 on the 7-point Bristol Stool Form Scale. Changes of 0.5 for both endpoints would require 32 patients per treatment arm, based on these data. Importantly, the colesevelam dose used in this study was selected from the approved dose for treatment of hyperlipidemia and type 2 diabetes, and further dose explorations may be required, especially in view of the documented variability of responsiveness to all BA sequestrants, and the potential role of mucosal genetic variation in pivotal genes19 in the bile acid synthesis pathway (specifically FGFR4 and KLB) may also require examination.
Conclusion
Our study is consistent with the literature documenting that, to date, the optimal dosing of BA sequestrants is unclear, despite the significant pharmacodynamics effects of serum C4, FGF19, and BA sequestration with colesevelam in this study, and the positive correlation between improved stool frequency and consistency and the binding and fecal excretion of BAs. However, stool frequency and consistency were not significantly different between colesevelam and placebo treatment. Larger studies with dose titration treatment for longer durations are required to further identify the correct dose of sequestrant to optimize or individualize therapy. Our observations suggest that differences in the expressions of mucosal genes associated with BAs and their proteins, especially FXR and GPBAR1, may contribute to the variable responses.
Supplementary Material
Acknowledgements:
The authors thank Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding support: Dr. Camilleri’s research on bile acid diarrhea and specifically this clinical trial are supported by grant R01-DK115950 from National Institutes of Health and by Mayo Foundation (Atherton and Winifred W. Bean Endowed Professorship). The study was conducted with the help of the Nursing Core of Mayo Clinic CCaTS grant #UL1-TR000135 from National Institutes of Health.
Abbreviations:
- BA
bile acid
- BAD
bile acid diarrhea
- BDQ
bowel disease questionnaire
- C4
7α-hydroxy-4-cholesten-3-one
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- FGF-19
Fibroblast growth factor 19
- GPBAR1
G protein-coupled bile acid receptor 1
- HAD
Hospital Anxiety and Depression Scale
- IBS
irritable bowel syndrome
- IBS-D
diarrhea-predominant irritable bowel syndrome
- KLB
klotho beta
- LCA
lithocholic acid
- NSAIDs
non-steroidal anti-inflammatory drugs
- SNRIs
serotonin and norepinephrine reuptake inhibitors
- UDCA
ursodeoxycholic acid
Footnotes
Conflicts of interest:
Michael Camilleri has received funding from Allergan, NGM Pharmaceuticals and Novartis to study treatment of patients with bile acid disorders and irritable bowel syndrome and from Takeda for studies in gastroparesis; he has no other personal conflicts of interest. The other authors have no conflicts of interest.
ClinicalTrials.Gov registration number: NCT03270085; Mayo Clinic IRB #17-004639
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–721. [DOI] [PubMed] [Google Scholar]
- 2.Mönnikes H Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol 2011;45(Suppl.):S98–S101. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychological and autonomic functions in 119 patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008;6:772–781, e.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367:1626–1635. [DOI] [PubMed] [Google Scholar]
- 6.Mawe GM, Hoffman JM. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 2011;106:2165–2173. [DOI] [PubMed] [Google Scholar]
- 8.Vijayvargiya P, Camilleri M. Update on bile acid malabsorption: finally ready for prime time? Curr Gastroenterol Rep 2018;20(3):10. [DOI] [PubMed] [Google Scholar]
- 9.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhoea in functional bowel disorder with diarrhoea: a systematic review and meta-analysis. Gut 2016;65:1951–1959. [DOI] [PubMed] [Google Scholar]
- 10.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–717. [DOI] [PubMed] [Google Scholar]
- 11.Stotzer P-O, Abrahamsson H, Bajor A, Sadik R. Effect of cholestyramine on gastrointestinal transit in patients with idiopathic bile acid diarrhea: a prospective, open-label study. Neuroenterology 2013;2:Article ID 235657. doi: 10.4303/ne/235657 [DOI] [Google Scholar]
- 12.Bajor A, Törnblom H, Rudling M, Ung KA, Simrén M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015;64:84–92. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Acosta A, Busciglio I, Boldingh A, Dyer RB, Zinsmeister AR, Lueke A, Gray A, Donato LJ. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2015;41:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayvargiya P, Camilleri M. Commentary: Practice change in the diagnosis of bile acid diarrhea. Gastroenterology 2019;156:1233–1238. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri M, Carlson P, Acosta A, Busciglio I. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 2015;309:G10–G20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grover M, Camilleri M, Hines J, Burton D, Ryks M, Wadhwa A, Sundt W, Dyer R, Singh RJ. 13C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 2016;28:1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilleri M, Carlson P, Acosta A, Busciglio I. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 2015;309:G10–G20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 2010;8:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong BS, Camilleri M, Carlson PJ, Odunsi-Shiyanbade S, McKinzie S, Busciglio I, Burton D, Zinsmeister AR. Pharmacogenetics of the effects of colesevelam on colonic transit in irritable bowel syndrome with diarrhea. Dig Dis Sci 2012;57:1222–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen MA1, Munck LK Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther 2012;36:79–90. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Bañares F, Esteve M, Salas A, Forné TM, Espinos JC, Martín-Comin J, Viver JM. Bile acid malabsorption in microscopic colitis and in previously unexplained functional chronic diarrhea. Dig Dis Sci 2001;46:2231–2238. [DOI] [PubMed] [Google Scholar]
- 22.Esposito G, Cirillo C, Sarnelli G, De Filippis D, D'Armiento FP, Rocco A, Nardone G, Petruzzelli R, Grosso M, Izzo P, Iuvone T, Cuomo R. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease.Gastroenterology 2007;133:918–925. [DOI] [PubMed] [Google Scholar]
- 23.MacEachern SJ, Patel BA, Keenan CM, Dicay M, Chapman K, McCafferty DM, Savidge TC, Beck PL, MacNaughton WK, Sharkey KA. Inhibiting inducible nitric oxide synthase in enteric glia restores electrogenic ion transport in mice with colitis. Gastroenterology 2015;149:445–455.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil 2011;23:e372–e382. [DOI] [PubMed] [Google Scholar]
- 25.Potthoff MJ, Potts A, He T, Duarte JA, Taussig R, Mangelsdorf DJ, Kliewer SA, Burgess SC. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol 2013;304:G371–G380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Bañares F, Rosinach M, Piqueras M, Ruiz-Cerulla A, Modolell I, Zabana Y, Guardiola J, Esteve M. Randomised clinical trial: colestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther 2015;41: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 27.Beigel F, Teich N, Howaldt S, Lammert F, Maul J, Breiteneicher S, Rust C, Göke B, Brand S, Ochsenkuhn T. Colesevelam for the treatment of bile acid malabsorption-associated diarrhea in patients with Crohn's disease: a randomized, double-blind, placebo-controlled study. J Crohns Colitis 2014;8:1471–1479. [DOI] [PubMed] [Google Scholar]
- 28.Sciarretta G, Furno A, Mazzoni M, Malaquti P. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol 1992;87:1852–1854. [PubMed] [Google Scholar]
- 29.Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology 1972;62:918–934. [PubMed] [Google Scholar]
- 30.Jacobsen O, Højgaard L, Hylander Møller E, Wielandt TO, Thale M, Jarnum S, Krag E. Effect of enterocoated cholestyramine on bowel habit after ileal resection: a double blind crossover study. Br Med J (Clin Res Ed) 1985;290:1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.