Abstract
We report here that a fluorescent benzobisimidazolium salt (TBBI) can be used for the fluorescent and colorimetric detection of carbonyl sulfide (COS) over related heterocumulenes including CO2 and CS2 in wet MeCN. The reaction between TBBI and COS in the presence of fluoride yields a highly fluorescent (λem = 354 nm) and colored product (λmax = 321, 621 nm), that is readily observed by the naked eye. We view these results as a first step toward developing activity-based probes for COS detection.
Graphical Abstract

Carbonyl sulfide (COS) is an important organosulfur species in the global sulfur cycle1 that is generated during the burning of biomass,2, 3 combustion of biofuels,4 and in different production processes in the pulp and paper industries.5 In addition, geochemical production of COS arises from volcanic activity, emission from hot springs, and deep sea thermal vents.6 Although the chemical properties and reactivity of COS have long been established,7 the biological implications of COS have only recently begun to emerge.8 As an example, treatment of α-amino acids with COS under prebiotic reaction conditions results in the formation of dipeptides, demonstrating the potential role of COS in origin of life chemical ligation.9 Aligned with our interests in studying biological reactive sulfur species,10 our group has recently advanced the use of COS-releasing compounds for the controlled release of hydrogen sulfide (H2S) due to the rapid catalyzed hydrolysis of COS by carbonic anhydrase, a ubiquitous metalloenzyme.11 Although enzymatic pathways for endogenous COS production in mammals remain undiscovered, COS has been detected in porcine cardiovascular tissues12 and exhaled breath from cystic fibrosis patients13 by using gas chromatography mass spectrometry. More recently, in our investigation of esterase-sensitive COS-based H2S donors in human lung epithelial (BEAS2B) cells, we observed COS-dependent inhibition of mitochondrial bioenergetics. This finding suggests that COS may possess biological activities and properties akin to H2S.14 As the potential roles of COS in biological systems and investigations into COS releasing compounds expand, a currently unmet need to further such investigations is the development of minimally invasive and sensitive methods for COS detection. Based on the significant impacts of colorimetric and fluorescent activity-based probes for the detection of reactive sulfur species, such as cysteine, reduced glutathione, and H2S, we envisioned that analogous platforms could be developed for COS detection.15, 16 To the best of our knowledge, no activity-based probes for COS detection or differentiation from the related heterocumulenes CO2 or CS2 have been reported.
Based on the electrophilicity and structural similarities between carbon dioxide (CO2), carbon disulfide (CS2), and COS,7 we anticipated that a nucleophilic platform could be used to develop chemical tools for COS detection. For example, N-heterocyclic carbenes can insert into heterocumulenes, which has been demonstrated separately for CO2,17 CS2,18 and COS,19 respectively. Bridging the gap to heterocumulene detection, the vast majority of work has aimed at the spectroscopic20 or electrochemical detection21 of CO2. Of these reported methods, we were drawn to the prior use of a fluorescent, colorimetric benzobisimidazolium probe (TBBI) for CO2 detection.22 In this system, treatment of TBBI with tetra-n-butylammonium fluoride (TBAF) produces a “carbine-like” intermediate23 that readily reacts with CO2 through carbene insertion into CO2 and subsequent hydrolysis to form bicarbonate (Figure 1a). This reactivity was also investigated for CS2 by 13C NMR spectroscopy and shown to result in the generation of an imidazolium-dithiocarboxylate betaine through carbene insertion into CS2 (Figure 1b). Building from structural similarities between these compounds, we hypothesized the treatment of TBBI with COS in the presence of fluoride would provide a unique spectroscopic response for COS that would be distinguishable from that of CO2 and CS2. Based on this motivation, we report here the first demonstration of selective COS detection by UV-Vis and fluorescence spectroscopy.
Figure 1.

(a) Reaction of CO2 and TBBI in the presence of fluoride. (b) Reaction between CS2 and TBBI in the presence of fluoride. (c) Proposed reaction between COS and TBBI in the presence of fluoride.
One challenge when working with COS is that commercial sources often contain H2S as a common impurity. To overcome this challenge, we prepared COS through the acidic hydrolysis of KSCN using modifications of known methods (Figure 2a).24 Based on the growing interest in various aspects of COS chemistry, including delivery, organometallic chemistry, and chemical biology, we have included this method here and in the Supporting Information. A key requirement when preparing COS is purification of the product, which can be accomplished by sparging through aqueous KOH, aniline in anhydrous ethanol, ice water, and neat H2SO4. The purified COS can be collected and stored in a gas storage flask or added directly into a solution or NMR tube for use as a reactant or reagent (Figure 2b). COS has a characteristic 13C chemical shift at δ = 154.3 ppm (CD3CN) and IR stretch at 2927 and 2909 cm−1, which match prior reports.25
Figure 2.
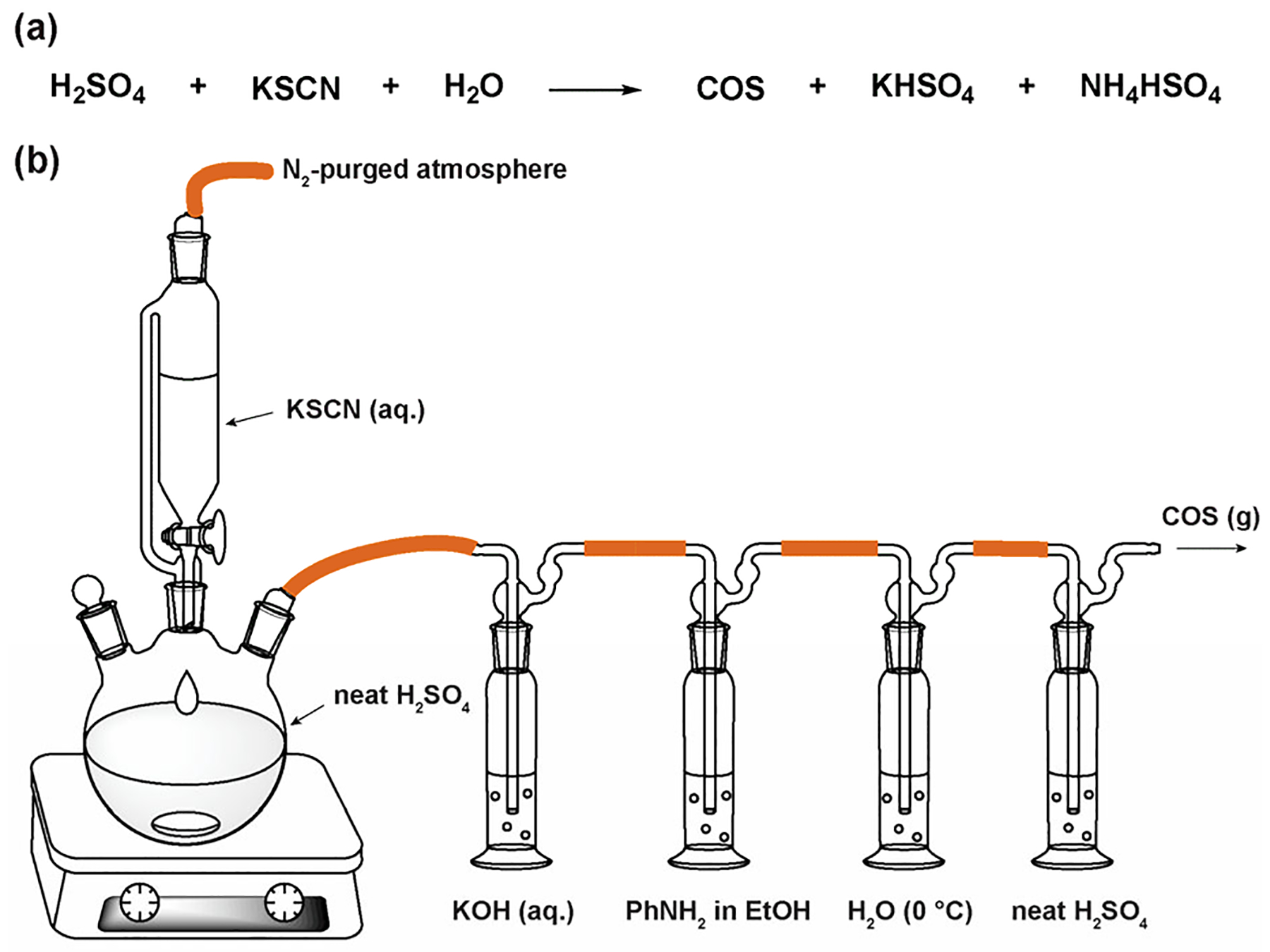
(a) Acid-mediated hydrolysis of KSCN to generate COS. (b) Schematic of laboratory-scale COS synthesis and purification.
To investigate the proposed strategy of carbene insertion for COS detection, we prepared TBBI22 and monitored its reactivity toward COS in the presence of TBAF by UV-Vis spectroscopy (Figure 3a). The parent TBBI compound (15 μM in MeCN containing 1% (v/v) H2O) has an absorption band centered at 290 nm, which shifts to 344 nm upon addition of TBAF (6.0 equiv.). This change in absorbance is consistent with the previous report of hydrogen bond formation between the C1-H in TBBI and F−.22 Subsequent addition of COS in 0.5 mL increments resulted in a decrease in the 344 nm absorbance and concomitant increase of a new absorbance at 321 nm. We note that this observed reaction with COS did not occur if anhydrous MeCN was used, which highlights that residual H2O is required. The new absorbance at 321 nm is unique to COS and is not observed upon reaction of TBBI with CO2 or CS2 in the presence of TBAF.
Figure 3.

(a) Reaction of TBBI with COS. (b) UV-Vis spectra and (c) fluorescence spectra for TBBI (15 μM), TBAF (90 μM), and COS (5.0 mL).
Building from this observation, we next investigated the reaction by fluorescence spectroscopy. The parent TBBI compound (15 μM, MeCN containing 1% (v/v) H2O) showed negligible fluorescence when excited at 321 nm, but addition of TBAF (6.0 equiv.) resulted in the appearance of a broad emission centered at 434 nm, which we attribute to formation of the hydrogen bonded TBBI intermediate. Subsequent addition of COS in 0.5 mL increments led to a clean ratiometric response, with a decrease at the 434 nm emission and concomitant increase at 354 nm (Figure 3c). The product formed upon COS addition is persistent, unlike in the case for CO2 in which rapid hydrolysis to bicarbonate is observed. A plot of COS concentrations against the ratio of fluorescence intensities at 354 and 434 nm is linear (R2 = 0.986), which suggests that this detection method could be used to quantify COS concentrations (see Supporting Information, Figure S3). Taken together, these results demonstrate that TBBI can be used to spectroscopically distinguish between CO2, CS2, and COS in the presence of the fluoride.
To confirm the formation of the proposed thiocarboxylate product, we accessed this compound by treatment of TBBI with KHMDS (2.2 equiv.) and subsequent addition of COS (g). The addition of COS (g) to a solution of doubly deprotonated TBBI in THF-d8 yielded a yellow-orange solution and the appearance of a new resonance in the 13C NMR spectrum at 190.34 ppm corresponding to thiocarboxylate formation (Figure 4a and b). This observed chemical shift is within the range of previously synthesized imidazolium-2-thiocarboxylates.19 We further characterized this compound by IR spectroscopy and observed C-S and C=O stretching frequencies at 990 and 1524 cm−1, respectively (see Supporting Information, Figure S6). We next measured the fluorescence spectrum of this product in MeCN (λex = 321 nm, λem = 330–600 nm) and observed a nearly identical match to the spectroscopic properties observed under experimental conditions (Figure 4c). When taken together, the combined 13C NMR data and fluorescence spectroscopy data both support the formation of the proposed thiocarboxylate upon treatment of TBBI with F− and COS (g).
Figure 4.

(a) The 13C{1H} NMR spectrum of TBBI (30 mM) treated with KHMDS (2.2 equiv.) in THF-d8. (b) The 13C{1H} NMR spectrum following the addition of COS (g) to a solution of doubly deprotonated TBBI. (c) Normalized fluorescence spectra comparing emission from TBBI + TBAF + COS and the synthesized thiocarboxylate adduct (TBBI∙(COS)2).
The stability and unique spectroscopic properties of product formed upon COS addition prompted us to investigate whether this TBBI system could be used for naked-eye optical detection of COS. The recognition of CS2 by 10 mM TBBI in the presence of fluoride was previously reported to generate a color change from clear to yellow red and form an imidazolium-dithiocarboxylate betaine.22 To further investigate this reactivity, we charged a series of cuvettes with 10 mM TBBI and 60 mM TBAF in MeCN containing 1% (v/v) H2O to generate the hydrogen bonded intermediate; this was followed by addition of CO2, CS2, or COS (Figure 5). Consistent with previous results, the cuvette containing CO2 yielded a white precipitate corresponding to the bicarbonate salt of TBBI, whereas the cuvette containing CS2 yielded a pale orange solution. The reaction between TBBI and COS yielded a teal green color and transitioned to a dark forest green upon standing for an extended period of time. This color is due to the combination of the major broad absorbance at 321 nm, which tails into the visible region of the spectrum, and a significantly weaker absorbance at 621 nm that appears upon COS addition (See Supporting Information, Figure S7). These results demonstrate the unique ability of TBBI to serve as a versatile platform for distinct recognition of CO2, COS, and CS2 by naked eye detection.
Figure 5.

Naked-eye detection and differentiation of CO2, CS2, and COS using TBBI (10 mM) in the presence of TBAF (60 mM).
In summary, we have demonstrated that the TBBI platform can be used for the colorimetric and fluorescent detection of COS. Importantly, these spectroscopic responses for COS are distinct from those generated by CO2 or CS2. We view that these advances provide a first step toward developing activity-based chemical tools for COS detection and imaging, which we anticipate can be used to investigate the growing evidence for a biological role of COS in complex environments.
Supplementary Material
Acknowledgements
This research was supported by the NIH (R01GM113030). NMR instrumentation in the UO CAMCOR facility is supported by the NSF (CHE-1427987 and CHE-1625529).
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: Experimental procedures and COS synthesis details. See DOI: 10.1039/x0xx00000x
References
- 1.Lee C-L and Brimblecombe P, Earth Sci Rev, 2016, 160, 1–18. [Google Scholar]
- 2.Andreae MO and Merlet P, Global Biogeochem Cycles, 2001, 15, 955–966. [Google Scholar]
- 3.Andreae MO, Atmos Chem Phys, 2019, 19, 8523–8546. [Google Scholar]
- 4.Campbell JE, Whelan ME, Seibt U, Smith SJ, Berry JA and Hilton TW, Geophys Res Lett, 2015, 42, 3004–3010. [Google Scholar]
- 5.Vainio E, Brink A, Demartini N, Hupa M, Vesala H, Tormonen K and Kajolinna T, J. Pulp Pap. Sci, 2010, 36, 135–142. [Google Scholar]
- 6.Watts SF, Atmos Environ, 2000, 34, 761–779. [Google Scholar]
- 7.Ferm RJ, Chem Rev, 1957, 57, 621–640. [Google Scholar]
- 8.Steiger AK, Zhao Y and Pluth MD, Antioxid Redox Signal, 2018, 28, 1516–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leman L, Orgel L and Ghadiri MR, Science, 2004, 306, 283–286. [DOI] [PubMed] [Google Scholar]
- 10.Lau N and Pluth MD, Curr Opin Chem Biol, 2019, 49, 1–8. [DOI] [PubMed] [Google Scholar]
- 11.Levinn CM, Cerda MM and Pluth MD, Acc Chem Res, 2019, 52, 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balazy M, Abu‐Yousef IA, Harpp DN and Park J, Biochem Biophys Res Commun, 2003, 311, 728–734. [DOI] [PubMed] [Google Scholar]
- 13.Kamboures MA, Blake DR, Cooper DM, Newcomb RL, Barker M, Larson JK, Meinardi S, Nussbaum E and Rowland FS, Proc Natl Acad Sci U S A, 2005, 102, 15762–15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steiger AK, Marcatti M, Szabo C, Szczesny B and Pluth MD, ACS Chem Biol, 2017, 12, 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J and Ma H, Chem Sci, 2016, 7, 6309–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao X, Li Y, Niu J, Xie X, Wang X and Tang B, Anal Chem, 2018, 90, 533–555. [DOI] [PubMed] [Google Scholar]
- 17.Tudose A, Demonceau A and Delaude L, Journal of Organometallic Chemistry, 2006, 691, 5356–5365. [Google Scholar]
- 18.Delaude L, Demonceau A and Wouters J, Eur J Inorg Chem, 2009, 2009, 1882–1891. [Google Scholar]
- 19.Hans M, Wouters J, Demonceau A and Delaude L, Eur J Org Chem, 2011, 2011, 7083–7091. [Google Scholar]
- 20.Zhou X, Lee S, Xu Z and Yoon J, Chem Rev, 2015, 115, 7944–8000. [DOI] [PubMed] [Google Scholar]
- 21.Neethirajan S, Jayas DS and Sadistap S, Food Bioprocess Tech, 2008, 2, 115–121. [Google Scholar]
- 22.Guo Z, Song NR, Moon JH, Kim M, Jun EJ, Choi J, Lee JY, Bielawski CW, Sessler JL and Yoon J, J Am Chem Soc, 2012, 134, 17846–17849. [DOI] [PubMed] [Google Scholar]
- 23.Hopkinson MN, Richter C, Schedler M and Glorius F, Nature, 2014, 510, 485–496. [DOI] [PubMed] [Google Scholar]
- 24.Svoronos PDN and Bruno TJ, Indust Eng Chem Res, 2002, 41, 5321–5336. [Google Scholar]
- 25.Zhang C-J and Zhang X-H, Macromolecules, 2019, 53, 233–239. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


