Abstract
Improved understanding of the genomic and molecular landscape of acute myeloid leukemia (AML) has resulted in significant evolution of our understanding of AML biology and allows refined prognostication for those receiving standard combination chemotherapy induction. This dramatic increase in knowledge preceded, and was somewhat responsible for, at least some of eight new FDA drug approvals for AML. This review discusses the impact of genomics on clinical care of AML patients and highlights newly approved FDA drugs. Despite these recent clinical advances, however, the outcome for most patients diagnosed with AML remains dire. Thus, we describe here some of the challenges identified with treating AML including off-target toxicity, drug transporters, clonal heterogeneity, and adaptive resistance, and some of the most promising opportunities for improved therapy.
Keywords: Acute myeloid leukemia, AML, genomic landscape, therapeutics, clinical implications
1. INTRODUCTION
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by an excess of immature clonal myeloblasts that are unable to differentiate into mature white blood cells, red blood cells, and platelets, thereby disrupting normal hematopoiesis. It is estimated that there will be approximately 19,940 new cases of AML in 2020 in the United States [1]. AML has the highest mortality rate of all leukemias, with an estimated 11,180 deaths annually [1]. While considerable progress has been made in understanding the genomic landscape of this disease, cytarabine in combination with anthracycline has remained the backbone treatment for young, fit patients for nearly five decades [2]. Recent developments have allowed for better prognostication and have stimulated the development of new therapies with improved patient outcomes. In this review, we describe the impact of genomics on clinical care of AML patients and 8 recently approved Food and Drug Administration (FDA) drugs. Despite these advances, it is difficult to cure AML. Thus, we highlight here some of the challenges associated with treating AML and describe potential new avenues for improving AML treatment.
2. DIAGNOSIS AND CLASSIFICATION
Assessments of morphology, cytogenetics, molecular abnormalities, and immunophenotyping are needed for the accurate diagnosis and classification of AML. According to current morphological guidelines, the disease is classified as AML when bone marrow or blood consists of at least 20% myeloblasts, with the exception of individuals with chromosomal abnormalities in t(15;17), t(8;21), inv(16), or t(16;16) [3,4]. Immunophenotyping is a critical step in diagnosing AML, as depicted in Table 1. It is also important to differentiate AML from acute lymphoblastic leukemia (ALL), identify acute promyelocytic leukemia (APL), and identify potential biomarkers to guide therapy (i.e. CD33). Improvements in our understanding of the genomic and molecular landscape of AML have naturally led to improvement in the classification of AML.
Table 1.
Expression of cell-surface antigens and cytoplasmic markers for the diagnosis of AML.
| Immunophenotype of Acute Myeloid Leukemia | |
|---|---|
| Precursor stage | CD34, CD117, CD33, CD13, HLA-DR |
| Granulocyte markers | CD65, cMPO |
| Monocytic markers | CD14, CD36, CD64, NSE |
| Megakaryocytic markers | CD41a, CD61 |
| Erythroid markers | CD36, CD235a |
From the 1970s to 2001, AML was diagnosed when patients had bone marrow with >30% myeloblasts and the morphologic appearance of blood cells was as described by a French-American-British working group (M0 through M7) [5]. This changed in 2001 when the World Health Organization (WHO) classification of myeloid neoplasms was developed, lowering the diagnosis cut-off to ≥20% myeloblasts and incorporating flow cytometry and cytogenetics into AML diagnosis and classification [6]. According to the new classification, three favorable-risk (t(8;21), inv(16), t(15;17)) and one unfavorable genetic abnormality (11q23) were recognized, as were AML with multilineage dysplasia and therapy-related AML (secondary AMLs). As our understanding of the molecular landscape advanced, in 2008 the revised WHO classification of myeloid neoplasms and acute leukemia added more adverse cytogenetic abnormalities to the classification (t(9;11), t(6;9), inv(3), t(1;22)) and recommended real-time polymerase chain reaction (RT-PCR) assays to detect mutations in nucleophosmin 1 (NPM1), fms-like tyrosine kinase 3 (FLT3) and CCAAT enhancer-binding protein alpha (CEBPA) in cytogenetically normal AML [7]. The most recent WHO revision occurred in 2016 (depicted in Table 2), incorporating findings from gene-expression studies and next-generation sequencing to specify biallelic CEBPA mutations as well as RUNX1 and BCR-ABL mutations in addition to classification of myeloid neoplasms with germ line predisposition [8]. The European Leukemia Network (ELN) incorporated information from the WHO classification system along with FLT3-internal tandem duplication (ITD) allelic ratio (<0.5 vs. ≥0.5) and the adverse prognostic mutations ASXL1 and TP53 into its 2017 risk stratification schema, as depicted in Table 3 [3].
Table 2.
2016 revision of the WHO classification of AML.
| AML with recurrent genetic abnormalities |
|---|
| AML with recurrent genetic abnormalities |
| AML with t(8;21)(q22;q22.1);RUNX1-RUNX1T1 |
| AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22);CBFB-MYH11 |
| APL with PML-RARA |
| AML with t(9;11)(p21.3;q23.3);MLLT3-KMT2A |
| AML with t(6;9)(p23;q34.1);DEK-NUP214 |
| AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2, MECOM |
| AML (megakaryoblastic) with t(1;22)(p13.3;q13.3);RBM15-MKL1 |
| Provisional entity: AML with BCR-ABL1 |
| AML with mutated NPM1 |
| AML with biallelic mutations of CEBPA |
| Provisional entity: AML with mutated RUNX1 |
| AML with myelodysplasia-related changes |
| Therapy-related myeloid neoplasms |
| AML, NOS |
| AML with minimal differentiation |
| AML without maturation |
| AML with maturation |
| Acute myelomonocytic leukemia |
| Acute monoblastic/monocytic leukemia |
| Pure erythroid leukemia |
| Acute megakaryoblastic leukemia |
| Acute basophilic leukemia |
| Acute panmyelosis with myelofibrosis |
| Myeloid sarcoma |
| Myeloid proliferations related to Down syndrome |
| Transient abnormal myelopoiesis (TAM) |
| Myeloid leukemia associated with Down syndrome |
Table 3.
2017 ELN genetic risk stratification of AML.
| Risk category | Genetic abnormality |
|---|---|
| Favorable | t(8;21)(q22;q22.1); RUNX1-RUNX1T1 |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 | |
| Mutated NPM1 without FLT3-ITD or with FLT3-ITDlow | |
| Biallelic mutated CEBPA | |
| Intermediate | Mutated NPM1 and FLT3-ITDhigh |
| Wild-type NPM1 without FLT3-ITD or with FLT3-ITDlow (without adverse-risk genetic lesions) | |
| t(9;11)(p21.3;q23.3); MLLT3-KMT2A | |
| Cytogenetic abnormalities not classified as favorable or adverse | |
| Adverse | t(6;9)(p23;q34.1); DEK-NUP214 |
| t(v;11q23.3); KMT2A rearranged | |
| t(9;22)(q34.1;q11.2); BCR-ABL1 | |
| inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2); GATA2,MECOM(EVI1) | |
| −5 or del(5q); −7; −17/abn(17p) | |
| Complex karyotype, monosomal karyotype | |
| Wild-type NPM1 and FLT3-ITDhigh | |
| Mutated RUNX1 | |
| Mutated ASXL1 | |
| Mutated TP53 | |
3. IMPACT OF GENOMICS ON CLINICAL CARE OF AML
Over the past three decades, risk stratification of AML has relied heavily on cytogenetics. However, it is becoming evident that molecular abnormalities that are not revealed by cytogenetic analysis play a pivotal role in the disease pathogenesis and prognosis of AML. The use of molecular techniques, particularly next-generation sequencing, has generated remarkable data depicting clonal evolution as well as the entire genomic, transcriptomic, epigenetic, and mutational landscape of the disease, and has largely replaced RT-PCR in clinical practice.
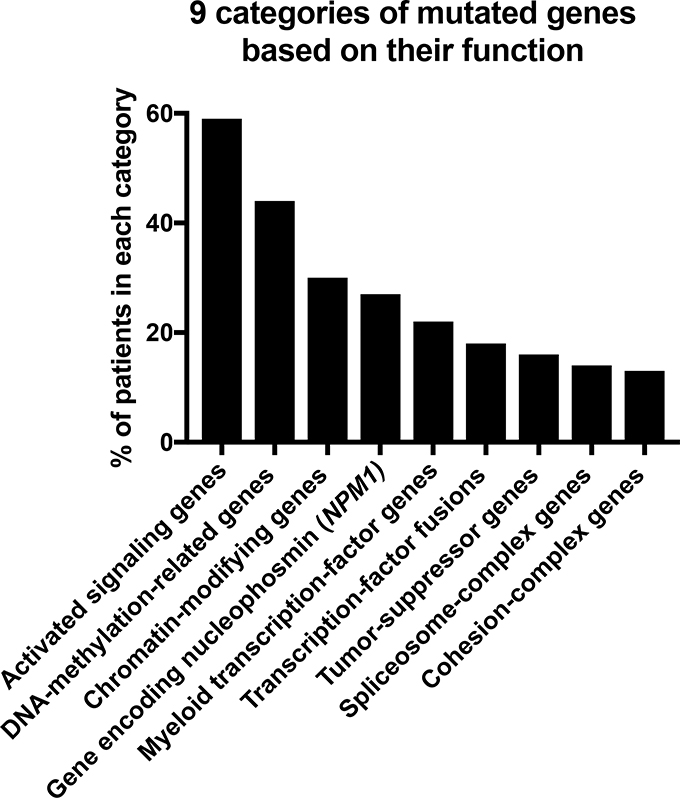
In 2013, The Cancer Genome Atlas (TCGA) Research Network analyzed 200 adult cases of de novo AML using whole-genome (n=50) or whole-exome (n=150) sequencing complemented by RNA and microRNA-sequencing and DNA-methylation analysis [9]. Mutated genes relevant to disease pathogenesis were divided into 9 categories based on their function, consisting of signaling genes (present in 59% of AML patients), DNA-methylation-related genes (44%), chromatin-modifying genes (30%), the gene encoding nucleophosmin, NPM1, (27%), myeloid transcription factor genes (22%), transcription factor fusions (18%), tumor-suppressor genes (16%), spliceosome-complex genes (14%), and cohesion-complex genes (13%) (Figure 1). In almost all cases of AML, patients had at least 1 nonsynonymous mutation (change in a nucleotide resulting in alteration of the amino acid sequence) in one of the 9 categories. While these genome-wide studies have clearly improved our understanding of the overall pathogenesis of AML, due to the heterogenous nature of AML, this sample size was not sufficient to decipher common patterns of evolution. However, these datasets are very valuable for use as a validation set for subsequent studies.
Fig. (1). Nine categories of mutated genes in AML patients based on their biological functions.
This figure was generated based on the mutational status of the 200 AML patients included in TCGA.
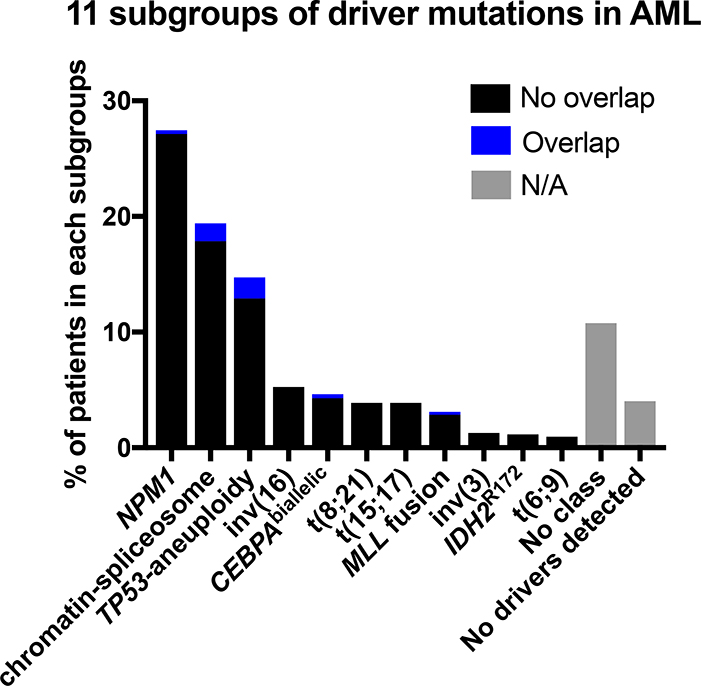
In 2016, Papaemmanuil, et al. reported post-hoc analysis of sequencing samples from 1540 AML patients who had been enrolled in trials utilizing intensive induction therapy [10]. Nearly half (48%) of their study cohort could not be classified according to the 2008 WHO classifications which utilized cytogenetics and morphology only, calling for a re-evaluation of the classification of AML. Papaemmanuil and colleagues genomically re-defined classes of AML into 11 subgroups consisting of NPM1 mutations (present in 27% of the AML patient cohort), chromatin-spliceosome (18%), TP53-aneuploidy (13%), inv(16) (5%), CEBPAbiallelic (4%), t(8;21) (4%), t(15;17) (4%), MLL fusion (3%), inv(3) (1%), IDH2R172 (1%), and t(6;9) (1%) (Figure 2). They further found overall survival differences among the subgroups. Among those with gene fusions, survival probability from poorest to best was as follows: inv(3), t(6;9), MLL fusion, t(8;21), t(15;17), and inv(16). Among those without gene fusions for whom molecular abnormalities were identified, survival probability from poorest to best was as follows: TP53-aneuploidy, chromatin-spliceosome, NPM1, IDH2R172, and CEBPAbiallelic.
Fig. (2). Eleven subgroups of driver mutations in AML.
This figure was generated based on the mutational status of the 1540 AML patients from the Papaemmanuil et al. report. Black bars indicate patients in the indicated subgroup, while the blue bars indicate patients in two or more subgroups. Gray bars indicate N/A (not applicable), for patients who either do not fall into any subgroups (No class) or had no identifiable driver mutations (No driver mutations detected).
Similarly, targeted sequencing has been used to define AML ontogeny, with mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR and STAG2 found to occur early and be highly specific for secondary AML with significantly worse clinical outcomes [11]. Also, underlying mutations in TP53 [12] and PPM1D [13] have been associated with therapy related myeloid neoplasms.
Clearly, cytogenetic and molecular abnormalities identified in this large genomic dataset have prognostic significance for patients with newly diagnosed AML being considered for intensive induction therapy. The next logical question is how to utilize this information to identify treatments that might improve outcomes over the standard cytarabine in combination with anthracycline treatment.
Beat AML is a multi-institutional collaboration that performed whole-exome sequencing (n=622), RNA-sequencing (n=451), and ex vivo drug sensitivity (n=409) assays on 672 tumor samples from 562 patients in 2018 [14]. Relationships between the drug sensitivities of leukemia cells and their molecular-genomic landscape were identified. More importantly, these generated datasets have been made accessible to the scientific community and will be beneficial for further study. The Beat AML dataset was recently utilized to improve understanding of the biology of primary refractory AML, a disease that carries a particularly dismal outcome and for which new therapies are desperately needed. Among all patients with primary refractory disease, three unique sub-groups with distinct transcriptional profiles were identified. Based on ex-vivo drug sensitivity data on 122 different drugs, flavopiridol was identified as a promising candidate for treatment of refractory AML [15,16]. In fact, several studies have been performed to test the drug alvocidib (formerly called flavopiridol) to treat AML patients [17–24]
More recently, the Beat AML group has launched a Master Trial (NCT03013998), a much-needed multi-institutional collaboration that utilizes rapid genomic screening to identify biomarker-based treatments for elderly patients (> 60 years of age) with newly diagnosed AML. Importantly, therapies targeting adverse mutations that carry a poor prognosis such as mixed lineage leukemia (MLL), TP53 and fms-like tyrosine kinase 3 (FLT3), are included. Testing of novel drugs tailored for each patient with AML based on their genetic and mutational signatures will undoubtedly advance our goal for precision medicine to greatly improve overall patient survival.
4. TREATMENT AND RECENTLY APPROVED FDA DRUGS
Standard induction chemotherapy for physically fit patients has essentially remained the same since the 1970s, with only 1 FDA approved drug (gemtuzumab ozogamicin) prior to 2017. A less intensive therapy, hypomethylating agents (HMA) (decitabine and azacytidine), were also available for those unfit or elderly patients who are unable to undergo an intensive induction chemotherapy. Associated in timing, and in some cases directly with our improved understanding of the pathophysiology of AML, has been the approval of 8 new AML drugs by the FDA within the past 3 years (Table 4). We describe these newly approved drugs below, and direct the interested reader to additional recent literature on this topic [25,26].
Table 4.
FDA approved drugs from 2017–2019.
| Drug | Year approved |
|---|---|
| Gemtuzumab Ozogamicin (GO) | 2017 |
| CPX-351 (Vyxeos) | 2017 |
| Midostaurin | 2017 |
| Enasidenib | 2017 |
| Venetoclax | 2018 |
| Glasdegib | 2018 |
| Ivosidenib | 2018 |
| Gilteritinib | 2019 |
4.1. Standard frontline therapy for AML
Generally, fit patients with newly diagnosed AML are treated with intensive induction chemotherapy. Prior to 2017, the standard of care was combining cytarabine (100–200 mg/m2 per day) for 7 days with an anthracycline (e.g., idarubicin 12 mg/m2, daunorubicin 60–90 mg/m2) for 3 days [27]. Among the 65–80% of patients who achieve a remission, post-remission therapy follows, consisting of consolidative chemotherapy or allogeneic stem cell transplant for high risk patients, although the risk of relapse is high. Among those who have primary refractory disease, outcomes are dismal as these patients are very poorly salvaged, and hence an unmet need for new therapy for these patients persists [27].
4.2. New FDA-approved drugs
4.2.1. Gemtuzumab ozogamicin (GO)
GO is a humanized CD33 monoclonal antibody toxin conjugate linked to the DNA-binding cytotoxin calicheamicin and is highly toxic to CD33-expressing leukemic cells. CD33 is a cell surface antigen present in most AML leukemia cells [28]. GO was FDA approved in 2000 as a novel AML monotherapy, but was withdrawn from the market in 2010, as it was associated with increased mortality and failed to benefit patients when combined with daunorubicin and cytarabine for induction therapy [29]. The effect of GO was further studied in a phase 3 trial comparing previously untreated de novo AML patients treated with standard treatment with or without five doses of intravenous GO [30]. The benefit of concomitant GO was seen in two-year estimates of event-free survival (40.8% as compared to 17.1% in the control group), relapse-free survival (50.3% as compared to 22.7% in the control group), and overall survival (53.2% as compared to 41.9% in the control group). The results of this study led to re-approval of GO in adults with newly diagnosed CD33-positive AML and for patients with CD33-positive AML that relapsed or whose disease did not respond to initial treatment. Given the discrepancy between this study and previous data, an individual patient data meta-analysis was performed incorporating 5 randomized trials that utilized GO in combination with standard induction therapy in adult patients with newly diagnosed AML [31]. While remission rates were not increased, 5-year overall survival was significantly improved owing to reductions in relapses and was most prominent among those with favorable cytogenetics. Those with intermediate cytogenetics also benefitted, albeit to a lower degree, and those with adverse cytogenetics did not benefit from GO. As a result of these data, it is recommended that physicians consider adding GO to the cytarabine in combination with anthracycline treatment for adult patients with CD33+ AML who are fit for intensive therapy and have favorable or intermediate cytogenetics. Close monitoring for myelosuppression, gastrointestinal toxicity, hepatotoxicity, and veno-occlusive disease must be undertaken, in particular for those who are allogeneic stem cell transplant candidates.
4.2.2. CPX-351 (Vyxeos)
CPX-351 is a liposomal co-formulation of cytarabine and daunorubicin approved in 2017 by the FDA for secondary AML and for AML with myelodysplasia-related changes. CPX-351 showed superior activity against leukemic cells as compared to free drugs when administered at the same ratio in animal models [32,33]. The trial leading to the approval of CPX-351 was a phase 3 study comparing 309 patients age 60 to 75 with newly-diagnosed high-risk/secondary AML receiving induction cycles of CPX-351 or standard cytarabine in combination with anthracycline induction chemotherapy followed by consolidation therapy with a similar regimen [34]. CPX-351 was found to be associated with a significant improvement in median overall survival (9.56 vs 5.95 months; hazard ratio = 0.69; p=0.003) and a significantly higher remission rate (47.7% vs 33.3%; two-sided p=0.016) as compared to cytarabine in combination with anthracycline treatment.
4.2.3. Midostaurin
Approximately 30% of patients with AML have an activating mutation in the transmembrane tyrosine kinase FLT3 [35]. Midostaurin (PKC412, CGP41251) is a multikinase inhibitor. Along with its active metabolites, it targets mutant forms of FLT3 and other protein kinases associated with leukemogenesis [36]. Initial studies investigating its potential as a PKC inhibitor showed that midostaurin acts to inhibit cellular proliferation by interfering with the cell cycle in non-small-cell lung cancer [37]. Further studies demonstrated the antiproliferative properties of midostaurin in various solid tumor lines (e.g., lung, colon, breast, melanoma, glioblastoma) [38]. FLT3 was later identified as a target of midostaurin [36]. The study leading to the FDA approval of midostaurin in AML was a phase 3 trial of 717 patients, 18.0 to 60.9 years of age, with newly diagnosed FLT3-mutated AML assigned to receive standard chemotherapy (induction therapy with daunorubicin and cytarabine and consolidation therapy with high-dose cytarabine) plus either midostaurin or placebo [39]. The addition of midostaurin led to a significant improvement in both overall survival and event-free survival of the patients. Midostaurin was approved by the FDA in 2017 for patients with newly-diagnosed AML with confirmed mutations in the FLT3 gene.
4.2.4. Enasidenib
Enasidenib is a specific inhibitor of mutated IDH2. A first-in-human phase 1/2 trial of 239 patients 18 years or older with mutant-IDH2 advanced myeloid malignancies was performed to assess the safety and maximum tolerated dose of enasidenib [40]. The overall response rate in patients with relapsed or refractory AML was 40.3% with a median response duration of 5.8 months. The median overall survival for relapsed/refractory patients was 9.3 months, and 19.7 months in those patients who attained complete remission. Enasidenib is generally well tolerated. This study prompted FDA approval in 2017 for treatment of recurrent or refractory IDH2-mutated AML.
4.2.5. Venetoclax
The B-cell leukemia/lymphoma-2 (BCL-2) proto-oncogene encodes for bcl-2, which regulates apoptosis [41]. Bcl-2 is associated with chemoresistance and survival of leukemic cells [41–43]. Venetoclax is a selective bcl-2 inhibitor that has shown promising results in treatment of AML and success in older, previously untreated patients with AML [42]. Results of a landmark multicenter phase 1b dose-escalation and expansion trial have largely shaped clinical practice regarding treatment of elderly or comorbid patients with AML [44]. Patients at least 65 years old who were ineligible for intensive chemotherapy received venetoclax partnered with either standard dosed azacitidine or decitabine. Half of the patients had poor-risk cytogenetics, highlighting a population that historically has been very challenging to treat. Responses were excellent, with 73% achieving a complete response (CR) or CR with incomplete count recovery (CRi), including CR and CRi rates of 60% and 65% respectively among elderly patients over 75 years old and those with poor risk cytogenetics. Responses were also durable, with a median duration of response of 11.3 months. In another phase 1b/2 study, patients 60 years and older with previously untreated AML ineligible for intensive chemotherapy [45] were treated with venetoclax in combination with low-dose cytarabine (LDAC). Responses were also high, with 54% of patients achieving either a CR or CRi. In both studies, side effect profiles of venetoclax-based treatments were manageable. Notably, with adequate monitoring, intravenous hydration and appropriate prophylaxis, there were no cases of clinical tumor lysis syndrome in either study. Based on the results of these studies, venetoclax was given accelerated approval by the FDA in 2018 for use in treating patients 75 years and older not eligible for intensive chemotherapy.
4.2.6. Glasdegib
Glasdegib (PF-04449913) is a potent and selective inhibitor of the Hedgehog (Hh) pathway that targets the essential pathway effector Smoothened (SMO) [46]. In vitro experiments showed the effectiveness of glasdegib at inhibiting Hh signaling [47]. Similarly, in a murine xenotransplant model of human AML, glasdegib inhibited tumor growth when used in combination with low dose cytarabine [47]. In a phase 2 trial of patients 55 years and older with AML or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy, patients were randomized to receive low-dose cytarabine (LDAC) with or without glasdegib [48]. Median overall survival for the glasdegib/LDAC group was 8.8 months as compared to 4.9 months in the LDAC group. Treatment with glasdegib/LDAC was also associated with a complete response in 17% of patients, along with a favorable benefit-risk profile. Glasdegib was approved by the FDA in 2018 for treatment of patients 75 years and older with newly-diagnosed AML and comorbidities that preclude use of intensive induction chemotherapy.
4.2.7. Ivosidenib
IDH1 and IDH2 are isocitrate dehydrogenase enzymes located in the cytoplasm and mitochondria that catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate. Recurring mutations of IDH1 and IDH2 are present in about 20% of individuals with AML [49]. From 7 to 14% are IDH1 mutations and from 8 to 19% are IDH2 mutations [49]. Mutations of IDH1 and IDH2 result in α-ketoglutarate being converted to the oncometabolite R2-hydroxyglutarate, which alters histone methylation of DNA in hematopoietic stem cells. Inhibition of mutant IDH enzymes reduces R2-hydroxyglutarate levels, thereby reducing aberrant histone hypermethylation [50]. Ivosidenib is a specific inhibitor of mutated IDH1. In a phase 1 trial including patients with relapsed or refractory IDH1-mutated AML, ivosidenib monotherapy was associated with a complete remission or complete remission with partial hematologic recovery in 30.4% of patients, and an overall response rate of 41.6% [51]. Ivosidenib was approved by the FDA in 2018 for treatment of relapsed or refractory IDH1-mutated AML.
4.2.8. Gilteritinib
Gilteritinib is a selective kinase that inhibits FLT3 and has shown activity against AML. The phase 1/2 trial enrolled patients 18 and older with relapsed AML and patients refractory to induction therapy [52]. 40% of patients that received gilteritinib achieved either a partial or complete response and the drug showed a favorable safety profile. The FDA approved gilteritinib in 2019 once preliminary results from the phase 3 trial were presented. In the ADMIRAL trial, patients with relapsed or refractory FLT3-mutated AML received either gilteritinib (n=247) or salvage chemotherapy (n=124). The results show that patients who received gilteritinib had significantly longer overall survival (9.3 months) compared to patients who had salvage chemotherapy (5.6 months) [53]. Currently, gilteritinib is approved for FLT3-mutated AML that has relapsed or is refractory to induction chemotherapy.
5. CHALLENGES ASSOCIATED WITH AML TREATMENT AND FUTURE AVENUES FOR IMPROVEMENT
Although the drugs recently approved by the FDA drugs have resulted in benefit to some patients, optimal therapy offers only incremental advantage over prior treatment and is often not curative. On average a 75-year-old individual living in the United States has more than a decade remaining to live [54]. In contrast, the life expectancy of a 75-year-old with AML is only a few months [55,56]. A few months of extended life expectancy from “optimal” therapy represents just a small fraction of the decade or more of lost life expectancy. What could be the underlying causes in the lack of significant progress towards curing AML? What could be done to improve the overall drug efficacy to treat AML? In this review, we highlight several potential causes and future avenues for the improvement of AML treatment.
5.1. Off-target toxicity – Incorrect identification of target and lack of proper understanding of a drug’s mechanism of action
Great efforts have been made to develop drugs to treat cancers. However, 97% of drug-indication pairs tested in clinical trials do not advance to receive FDA approval [57]. Such high failure rates are perplexing. Surprisingly, the incorrect identification of target genes and mischaracterization of drug mechanisms of action have contributed to this poor success rate. Thus, a more stringent analysis and validation of target genes could prevent such a high failure rate. The FDA approves drugs that show clinical benefit with tolerable side effects, even in the absence of a detailed mode of action. In addition, many high-throughput drug screens measure cell survival capability in the presence of drugs, but the drug’s mechanism of action is often overlooked. The potential off-target toxicity of these drugs may cause dangerous side effects. Thus, fully understanding the drug’s mechanism of action and adoption of more stringent genetic target and activity validation could reduce these off-target effects and increase the success rate of new cancer drugs.
5.2. Failure to account for the role of ABC transporters in AML resistance mechanisms.
One of the suggested mechanisms of chemotherapy resistance in AML is the efflux of chemotherapy drugs by the ATP-binding cassette (ABC) transporter P-glycoprotein (P-gp, MDR1, or ABCB1) [58]. Overexpression of P-gp has been associated with poor clinical outcome in AML [59,60]. One classic example is a study by the Southwest Oncology Group (SWOG) where inhibition of P-gp by cyclosporin A showed improvement in overall survival of high-risk AML patients [61], but many other studies have failed to show any improvement in survival with P-gp inhibitors [62–67]. We have recently reported that other ABC transporters, such as ABCG2, ABCA2, ABCA9, and ABCA6 are overexpressed in a small sub-population of refractory de novo AML patients [16]. This may explain why the SWOG trial [61] using cyclosporin A gave a promising result because cyclosporin A is able to inhibit ABCB1 and ABCG2 and potentially other ABC transporters while the E3999 trial [67] failed because zosuquidar is a P-gp specific inhibitor. As mentioned previously, noticing incorrect target identification and understanding a drug’s mode of action could have prevented this potentially important role of ABC transporters from being ignored.
5.3. Diverse patterns of clonal selection and alternation in clonal evolution in response to treatment
5.3.1. Clonal evolution of AML with t(8;21) treated with intensive cytarabine/anthracycline induction therapy and subsequent cytarabine-based consolidation therapy.
AML patients with the cytogenetic subtype t(8;21) are considered a favorable risk group [3]. t(8;21)(q22;q22) is a fusion of the RUNX1 gene on chromosome 21 with the RUNX1T1 gene on chromosome 8 [68]. This RUNX1-RUNX1T1 fusion inhibits the function of wild-type RUNX1 by competing with its DNA binding site. A recent study comprising 331 patients diagnosed with t(8;21) AML, the largest cohort of this type ever investigated, revealed a total of 729 mutations [69]. At least one mutation was identified in each patient. Most of the mutations occurred in RAS/RTK signaling pathways (accounting for 63.4% of the patients), followed by mutations in epigenetic regulation (chromatin remodeling and DNA methylation) (45%), cohesion complex (13.6%), MYC signaling (10.3%), and splicing machinery (7.9%). To further identify clonal evolution, each mutation was defined as stable if it was present both at the time of diagnosis and at relapse after treatment. It was revealed that some clones were successfully eradiated by induction therapy, but some clones successfully escaped, and were further selected for expansion. Interestingly, clonal evolution seems to have gene-related patterns. For example, mutations in epigenetic regulators and genes related to the cell cycle were either stable throughout evolution or were lost after treatment, but mutations in transcription factors, RAS/RTK signaling, cohesion complex, and splicing machinery either remained stable, gained, or were lost.
It is possible that founding clones were eradicated, and secondary clones outcompeted the other clones after induction therapy (acquired or adaptive resistance). It is also possible that there was actually no emergence of secondary clones, but simply selection of a very minor subclone that already existed (intrinsic resistance) but was below the limit of detection. Nonetheless, by the second remission, most patients had enrichment of clones that were different from the enriched founding clones. This explains why despite successful initial response to induction therapy, the majority of the AML patients relapse. Although this survival mechanism of AML may seem daunting, it is now possible to track the clonal evolution of AML to monitor its progression, and this could be taken advantage of to tailor treatment strategies based on the clonal mutational landscape for each individual patient. In addition, if the patients are alive when the clones eventually reach a more homogeneous population with a druggable target, it may be possible to fully eradicate their AML.
5.3.2. Clonal evolution of FLT3-mutated AML treated with the FLT3 inhibitor, gilteritinib.
Approximately 30% of AML patients have mutations in FLT3. Internal tandem duplications of FLT3 (FLT3-ITD) and the tyrosine kinase domain (TKD) result in constitutive activation of FLT3 and its downstream pathways [10]. Downstream pathways include RAS/MAPK and STAT5 pathways that can drive tumor growth [70]. Thus, mutations in FLT3, particularly, FLT3-ITD is associated with poor prognosis. Several inhibitors of FLT3 have been developed to prevent AML tumor progression [71–73]. Gilteritinib, as previously described, is an FLT3 inhibitor that has shown a modest improvement in survival as compared to salvage chemotherapy. Despite an initial successful response, patients usually relapse due to the development of secondary resistance.
A recent study utilizing targeted next-generation sequencing (NGS) on bulk paired samples pre- and post-gilteritinib treatment showed that AML patients treated with gilteritinib developed treatment-emergent RAS/MAPK pathway mutations in 36.6% of the patients [74]. Further single-cell DNA sequencing demonstrated early selection for RAS-mutant clonal populations as well as diverse patterns of clonal selection and evolution after selective pressure of gilteritinib treatment. This suggests that monitoring RAS/MAPK pathways in gilteritinib patients would be beneficial as an early intervention prior to development of resistance. In addition, patients who develop treatment-emergent RAS/MAPK pathway mutations may benefit from combined treatment of gilteritinib and MAPK inhibitors to prevent secondary mutations. Another striking observation in this study was that different concentrations of gilteritinib resulted in certain biases regarding selection for different clones. With low-dose gilteritinib treatment, FLT3-F691L cells became the predominant population, while with high-dose gilteritinib treatment, more NRAS-mutant cells thrived. Thus, it would be desirable to monitor the clonal evolution of the cells, which may drive the clonal population to become more druggable.
5.4. Adaptive resistance via activation of a compensatory innate immune stress pathway
Aside from mutations in the tyrosine kinase domain, which cause resistance to FLT3 inhibitors, there is also an adaptive resistance in which cancer cells activate parallel signaling pathways that can bypass FLT3 signaling to evade cell death. A recent study has shown that FLT3-ITD AML diminished sensitivity to FLT3 inhibitors (quizartinib or gilteritinib) even in the absence of treatment-mediated mutations in the tyrosine kinase domain of FLT3 (F691 and D835) or activating mutations (G12 and G13) in NRAS in FLT3-ITD AML cells [75]. This suggests cell dependency on adaptive signaling resistance mechanisms rather than acquired resistance. This study identified known compensatory signaling pathways such as MAPK signaling, but also identified Toll-like receptor (TLR) signaling (innate immune signaling) pathways that have not been implicated in adaptive resistance against FLT3 inhibition that provide protection of FLT3-mutant AML. In particular, the upstream effector of innate immune signaling, IL-1 receptor-associated kinase 1 (IRAK1) and IRAK4, are phosphorylated after FLT3 inhibition. Quizartinib increases phosphorylation of IRAK1/4 and gilteritinib increases phosphorylation of IRAK4. This compensatory activation of innate immune stress pathways is dependent on FLT3 signaling. Thus, when the FLT3-mutant AML is treated with FLT3 inhibitor and IRAK1/4 signaling together, it creates a synthetic lethality. Furthermore, NCGC1481, a multikinase FLT3-IRAK1/4 inhibitor, prevented the adaptive resistance of FLT3-mutant AML compared to current targeted FLT3 therapies. These results demonstrate that combinatorial therapies inhibiting FLT3 signaling and compensatory IRAK1/4 signaling have the potential to improve the clinical outcome of AML patients with FLT3 mutations as compared to targeted therapy alone.
6. NOVEL APPROACHES TO TARGETING AML
Standard chemotherapy is initially effective in eradicating the majority of AML. However, there are minor populations of cells with the resistance phenotype that can thrive and eventually dominate the population after chemotherapy treatment. Genomic techniques can be used to identify and track the evolutionary landscape of AML to better target these refractory cells that would likely result in better treatment outcomes for AML patients. Here, we provide some examples of potential targets.
6.1. Targeting glutamine metabolism and pre-existing leukemic stem cells in AML.
One of the hallmarks of cancer is rewiring of metabolism to sustain cell proliferation [76]. Like many other cancer cells [77], AML is also dependent on glutamine metabolism for energy production, synthesis of macromolecules, redox homeostasis, and cell survival. One study showed that inhibition of FLT3 in FLT3-mutant AML impairs glutamine metabolism, resulting in depletion of glutathione (GSH) synthesis and cell death due to increased oxidative stress in mitochondria [78]. A similar effect was also seen when FLT3-mutant AML was inhibited with glutaminase [79]. These studies, however, focused on FLT3-mutant AML. A subsequent study also demonstrated the efficacy of targeting glutamine metabolism in FLT3-wild-type AML [80]. It was shown that inhibiting glutaminase (CB-839) in combination with an oxidative stressor such as arsenic trioxide (ATO) and the protein translation inhibitor homo-harringtonine (HHT) resulted in effective apoptosis of AML cells irrespective of their FLT3 mutational status, representing a broad therapeutic utility.
Previously, it was shown that leukemia stem cells (LSCs) rely on amino acids to drive oxidative phosphorylation for survival [81]. More recently, Jones et al. found that inhibition of amino acid metabolism can selectively target LSCs [81,82]. Specifically, in this study, relative levels of reactive oxygen species (ROS) were used as a means to define LSCs (ROS-low) and AML blasts (ROS-high). They previously reported that ROS-low AML cells are enriched for LSCs [43]. They found that ROS-low LSCs have more amino acids and are dependent on amino acids to fuel oxidative phosphorylation for survival.
BCL2 is overexpressed in LSCs and inhibition of BCL2 can prevent oxidative phosphorylation, leading to cell death of LSCs without affecting hematopoietic stem cells (CD3+, CD7+, or CD19+) as normal stem cells are able to compensate for reduced oxidative phosphorylation through increasing glycolysis [83]. Combined treatment of LSCs with venetoclax (BCL2 inhibitor) and azacytidine (hypomethylating agent) reduces oxidative phosphorylation by decreasing amino acid uptake in ROS-low LSCs, thereby successfully killing LSCs [82]. Chemotherapy alone is unable to reduce amino acid levels in ROS-low LSCs, and this combined treatment would be more beneficial in treating LSCs. However, this combined treatment using venetoclax and azacytidine is ineffective in treating refractory LSCs. Unlike in de novo LSCs, oxidative phosphorylation in refractory LSCs is unaffected even in amino acid deprivation. Refractory LSCs are able to activate alternative metabolic pathways, particularly fatty acid metabolism, to compensate for the amino acid loss, retaining oxidative phosphorylation capability. To solve this problem, inhibiting fatty acid uptake using sorbitan sesquioleate (SSO), a CD36 inhibitor, re-sensitizes refractory LSCs to venetoclax and azacytidine. Together, these approaches allow successful killing of LSCs via LSC-specific metabolic activities, preventing residual AML cells from re-emerging after treatment.
6.2. Tumor heterogeneity in de novo refractory AML with targetable signatures.
While targeting metabolic pathways and leukemic stem cells would be beneficial, treatment should be tailored according to an individual’s AML signature. A recent study revealed tumor heterogeneity within de novo refractory AML with three refractory sub-populations (Ref1, Ref2, and Ref3 subpopulations) with distinct intrinsic resistance mechanisms [15,16]. While all refractory groups exhibited upregulation in cell cycle regulation, there was gene enrichment unique to each subpopulation. For example, the Ref1 subpopulations have increased metabolic pathways and decreased translation, Ref2 subpopulations are enriched with DNA replication and translation and decreased metabolic pathways, and Ref3 subpopulations have the most stem-cell signatures and overexpression of ABC transporters, with decreased metabolism and translation. This clearly shows tumor heterogeneity in de novo refractory AML, and yet, the same standard induction therapy is often used to treat these patients. Thus, for Ref1 patients, combination therapy of induction therapy with metabolic pathway inhibitors may provide promising results. However, for Ref2 and Ref3 subpopulations, it may not be as effective. In Ref3 populations, combination therapy with ABC transporter inhibitors may be more effective.
6.3. Targeting mutations in RNA splicing factors
Recent publications have shed light on the importance of mutations in RNA splicing factors that contribute to leukemogenesis [84,85]. One study focused on AML with mutations in both IDH2 and SRSF2, a combination that phenotypically resulted in a lethal myelodysplasia with proliferative features in vivo. The relationship of IDH2 and SRSF2 mutations acting in a coordinated fashion to cause a phenotypic myelodysplastic syndrome (MDS) was shown via the following: a murine bone marrow transplantation of double-mutant cells where mice transplanted with IDH2 and Srsf2 double-mutant cells developed a lethal MDS; and crossing of mice each expressing one mutant allele of the respective gene (e.g., Idh2, Srsf2) to create a model in which both mutants were expressed from endogenous loci, resulting in a MDS in double knock-in mice and not in the controls [84]. At the genotypic level, IDH2 and SRSF2 double-mutant cells were shown to aberrantly splice INTS3, a member of the integrator complex. While phenotypically in a double-mutant xenograft model, forced expression of INTS3 induced myeloid differentiation and slowed leukemia progression in vivo [84]. Another study determined the importance of aberrant RNA splicing via SF3B1 mutations that result in exonization of BRD9, an intronic sequence that, as a consequence of aberrant RNA splicing, is included as a poison exon [85]. The resultant degradation of BRD9 mRNA and depletion of BRD9 was shown to result in loss of non-canonical BAF, proteins important in nuclear assembly and chromatin organization. Correction of BRD9 aberrant splicing via antisense oligonucleotides (ASOs) blocking inclusion of the BRD9 poison exon resulted in suppressed tumor growth and induced tumor necrosis in an SF3B1 mutated xenograft of uveal melanoma [85]. These studies highlight the potential therapeutic implications of correcting RNA splicing aberrations in leukemia and potentially cancer in general.
Given the complex genetic disposition of AML, with each subtype composed of unique molecular drivers of leukemogenesis, future AML therapy must begin with a patient-specific, genetics-specific approach. The recent FDA-approved therapies are certainly a promising start. Future mechanisms of therapy could potentially include targeting mutations that contribute to RNA splicing aberrations [84], using antisense oligonucleotides to block inclusion of poison exons [85], and targeting amino acid metabolism [80,81]. As the scientific community continues to uncover the complex genomic landscape of this ailment, so too will the door open for continued therapeutic innovation.
7. CONCLUSION
There has been a clear shift in the study of AML, moving from the generation of large genomic datasets toward understanding the entire genomic landscape. Efforts now are more devoted to applying genomic data to clinical cases, and to tailoring drug treatments to individual patients based on genomic signatures. Previously, it was a challenge to dissect large genomic datasets and determine how they could be translated into clinical practice due to the complex nature of mutational patterns both within a particular patient and patterns occurring in many individuals afflicted with the disease. Patient response to various therapies has also been quite complex. However, with modern genomic studies and better understanding of the biology of AML, there is now hope that in the near future it may be possible to develop personalized treatment regimens for each patient, providing better treatment strategies for those afflicted with this disease.
ACKNOWLEDGEMENT
We thank C. Hourigan for his expertise and editorial comments and G. Leiman for editorial assistance.
FUNDING
This work was supported by the Intramural Research Program (NCI) of the National Institutes of Health, the National Cancer Institute.
LIST OF ABBREVIATIONS
- AML
Acute myeloid leukemia
- FDA
Food and Drug Administration
- ALL
Acute lymphoblastic leukemia
- APL
Acute promyelocytic leukemia
- WHO
World Health Organization
- RT-PCR
Real-Time Polymerase Chain Reaction
- NPM1
Nucleophosmin 1
- FLT3
Fms-Like Tyrosine Kinase 3
- CEBPA
CCAAT Enhancer-Binding Protein Alpha
- ELN
European Leukemia Network
- ITD
Internal Tandem Duplication
- TCGA
The Cancer Genome Atlas
- HMA
Hypomethylating Agent
- GO
Gemtuzumab Ozogamicin
- BCL-2
B-Cell Leukemia/Lymphoma-2
- LDAC
Low-Dose Cytarabine
- Hh
Hedgehog
- SMO
Smoothened
- MDS
Myelodysplastic Syndrome
- SWOG
Southwest Oncology Group
- ABC
ATP-Binding Cassette
- TDK
Tyrosine Kinase Domain
- NGS
Next Generation Sequencing
- IRAK1
IL-1 Receptor-Associated Kinase 1
- GSH
Glutathione
- ATO
Arsenic Trioxide
- HHT
Homo-Harringtonine
- LSC
Leukemia Stem Cell
- ROS
Reactive Oxygen Species
- SSO
Sorbitan Sesquioleate
- ASO
Antisense Oligonucleotide
Footnotes
CONFLICT OF INTEREST
All authors report no relevant financial conflicts of interest.
DISCLAIMER
This article is intended for the purpose of research and education and should not replace recommendations made by health care providers.
REFERENCES
- [1].Siegel RL; Miller KD; Jemal A Cancer statistics, 2020. CA Cancer J Clin, 2020, 70, 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Yates JW; Wallace HJ Jr.; Ellison RR; Holland JF Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep, 1973, 57, 485–488. [PubMed] [Google Scholar]
- [3].Dohner H; Estey E; Grimwade D; Amadori S; Appelbaum FR; Buchner T; Dombret H; Ebert BL; Fenaux P; Larson RA; Levine RL; Lo-Coco F; Naoe T; Niederwieser D; Ossenkoppele GJ; Sanz M; Sierra J; Tallman MS; Tien HF; Wei AH; Lowenberg B; Bloomfield CD Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood, 2017, 129, 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dohner H; Estey EH; Amadori S; Appelbaum FR; Buchner T; Burnett AK; Dombret H; Fenaux P; Grimwade D; Larson RA; Lo-Coco F; Naoe T; Niederwieser D; Ossenkoppele GJ; Sanz MA; Sierra J; Tallman MS; Lowenberg B; Bloomfield CD; European L Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood, 2010, 115, 453–474. [DOI] [PubMed] [Google Scholar]
- [5].Bennett JM; Catovsky D; Daniel MT; Flandrin G; Galton DA; Gralnick HR; Sultan C Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol, 1976, 33, 451–458. [DOI] [PubMed] [Google Scholar]
- [6].Vardiman JW; Harris NL; Brunning RD The World Health Organization (WHO) classification of the myeloid neoplasms. Blood, 2002, 100, 2292–2302. [DOI] [PubMed] [Google Scholar]
- [7].Vardiman JW; Thiele J; Arber DA; Brunning RD; Borowitz MJ; Porwit A; Harris NL; Le Beau MM; Hellstrom-Lindberg E; Tefferi A; Bloomfield CD The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 2009, 114, 937–951. [DOI] [PubMed] [Google Scholar]
- [8].Arber DA; Orazi A; Hasserjian R; Thiele J; Borowitz MJ; Le Beau MM; Bloomfield CD; Cazzola M; Vardiman JW The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 2016, 127, 2391–2405. [DOI] [PubMed] [Google Scholar]
- [9].Cancer Genome Atlas Research, N.; Ley TJ; Miller C; Ding L; Raphael BJ; Mungall AJ; Robertson A; Hoadley K; Triche TJ Jr.; Laird PW; Baty JD; Fulton LL; Fulton R; Heath SE; Kalicki-Veizer J; Kandoth C; Klco JM; Koboldt DC; Kanchi KL; Kulkarni S; Lamprecht TL; Larson DE; Lin L; Lu C; McLellan MD; McMichael JF; Payton J; Schmidt H; Spencer DH; Tomasson MH; Wallis JW; Wartman LD; Watson MA; Welch J; Wendl MC; Ally A; Balasundaram M; Birol I; Butterfield Y; Chiu R; Chu A; Chuah E; Chun HJ; Corbett R; Dhalla N; Guin R; He A; Hirst C; Hirst M; Holt RA; Jones S; Karsan A; Lee D; Li HI; Marra MA; Mayo M; Moore RA; Mungall K; Parker J; Pleasance E; Plettner P; Schein J; Stoll D; Swanson L; Tam A; Thiessen N; Varhol R; Wye N; Zhao Y; Gabriel S; Getz G; Sougnez C; Zou L; Leiserson MD; Vandin F; Wu HT; Applebaum F; Baylin SB; Akbani R; Broom BM; Chen K; Motter TC; Nguyen K; Weinstein JN; Zhang N; Ferguson ML; Adams C; Black A; Bowen J; Gastier-Foster J; Grossman T; Lichtenberg T; Wise L; Davidsen T; Demchok JA; Shaw KR; Sheth M; Sofia HJ; Yang L; Downing JR; Eley G Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med, 2013, 368, 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Papaemmanuil E; Gerstung M; Bullinger L; Gaidzik VI; Paschka P; Roberts ND; Potter NE; Heuser M; Thol F; Bolli N; Gundem G; Van Loo P; Martincorena I; Ganly P; Mudie L; McLaren S; O’Meara S; Raine K; Jones DR; Teague JW; Butler AP; Greaves MF; Ganser A; Dohner K; Schlenk RF; Dohner H; Campbell PJ Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med, 2016, 374, 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lindsley RC; Mar BG; Mazzola E; Grauman PV; Shareef S; Allen SL; Pigneux A; Wetzler M; Stuart RK; Erba HP; Damon LE; Powell BL; Lindeman N; Steensma DP; Wadleigh M; DeAngelo DJ; Neuberg D; Stone RM; Ebert BL Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood, 2015, 125, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong TN; Ramsingh G; Young AL; Miller CA; Touma W; Welch JS; Lamprecht TL; Shen D; Hundal J; Fulton RS; Heath S; Baty JD; Klco JM; Ding L; Mardis ER; Westervelt P; DiPersio JF; Walter MJ; Graubert TA; Ley TJ; Druley T; Link DC; Wilson RK Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature, 2015, 518, 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hsu JI; Dayaram T; Tovy A; De Braekeleer E; Jeong M; Wang F; Zhang J; Heffernan TP; Gera S; Kovacs JJ; Marszalek JR; Bristow C; Yan Y; Garcia-Manero G; Kantarjian H; Vassiliou G; Futreal PA; Donehower LA; Takahashi K; Goodell MA PPM1D Mutations Drive Clonal Hematopoiesis in Response to Cytotoxic Chemotherapy. Cell Stem Cell, 2018, 23, 700–713 e706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tyner JW; Tognon CE; Bottomly D; Wilmot B; Kurtz SE; Savage SL; Long N; Schultz AR; Traer E; Abel M; Agarwal A; Blucher A; Borate U; Bryant J; Burke R; Carlos A; Carpenter R; Carroll J; Chang BH; Coblentz C; d’Almeida A; Cook R; Danilov A; Dao KT; Degnin M; Devine D; Dibb J; Edwards D.K.t.; Eide CA; English I; Glover J; Henson R; Ho H; Jemal A; Johnson K; Johnson R; Junio B; Kaempf A; Leonard J; Lin C; Liu SQ; Lo P; Loriaux MM; Luty S; Macey T; MacManiman J; Martinez J; Mori M; Nelson D; Nichols C; Peters J; Ramsdill J; Rofelty A; Schuff R; Searles R; Segerdell E; Smith RL; Spurgeon SE; Sweeney T; Thapa A; Visser C; Wagner J; Watanabe-Smith K; Werth K; Wolf J; White L; Yates A; Zhang H; Cogle CR; Collins RH; Connolly DC; Deininger MW; Drusbosky L; Hourigan CS; Jordan CT; Kropf P; Lin TL; Martinez ME; Medeiros BC; Pallapati RR; Pollyea DA; Swords RT; Watts JM; Weir SJ; Wiest DL; Winters RM; McWeeney SK; Druker BJ Functional genomic landscape of acute myeloid leukaemia. Nature, 2018, 562, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Horibata S Transcriptomic profile of intrinsically chemoresistant acute myeloid leukemia patients. Mol Cell Oncol, 2019, 6, e1650631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Horibata S; Gui G; Lack J; DeStefano CB; Gottesman MM; Hourigan CS Heterogeneity in refractory acute myeloid leukemia. Proc Natl Acad Sci U S A, 2019, 116, 10494–10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karp JE; Ross DD; Yang W; Tidwell ML; Wei Y; Greer J; Mann DL; Nakanishi T; Wright JJ; Colevas AD Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res, 2003, 9, 307–315. [PubMed] [Google Scholar]
- [18].Karp JE; Passaniti A; Gojo I; Kaufmann S; Bible K; Garimella TS; Greer J; Briel J; Smith BD; Gore SD; Tidwell ML; Ross DD; Wright JJ; Colevas AD; Bauer KS Phase I and pharmacokinetic study of flavopiridol followed by 1-beta-D-arabinofuranosylcytosine and mitoxantrone in relapsed and refractory adult acute leukemias. Clin Cancer Res, 2005, 11, 8403–8412. [DOI] [PubMed] [Google Scholar]
- [19].Karp JE; Smith BD; Levis MJ; Gore SD; Greer J; Hattenburg C; Briel J; Jones RJ; Wright JJ; Colevas AD Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res, 2007, 13, 4467–4473. [DOI] [PubMed] [Google Scholar]
- [20].Karp JE; Blackford A; Smith BD; Alino K; Seung AH; Bolanos-Meade J; Greer JM; Carraway HE; Gore SD; Jones RJ; Levis MJ; McDevitt MA; Doyle LA; Wright JJ Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res, 2010, 34, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Karp JE; Smith BD; Resar LS; Greer JM; Blackford A; Zhao M; Moton-Nelson D; Alino K; Levis MJ; Gore SD; Joseph B; Carraway H; McDevitt MA; Bagain L; Mackey K; Briel J; Doyle LA; Wright JJ; Rudek MA Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood, 2011, 117, 3302–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Karp JE; Garrett-Mayer E; Estey EH; Rudek MA; Smith BD; Greer JM; Drye DM; Mackey K; Dorcy KS; Gore SD; Levis MJ; McDevitt MA; Carraway HE; Pratz KW; Gladstone DE; Showel MM; Othus M; Doyle LA; Wright JJ; Pagel JM Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica, 2012, 97, 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zeidner JF; Foster MC; Blackford AL; Litzow MR; Morris LE; Strickland SA; Lancet JE; Bose P; Levy MY; Tibes R; Gojo I; Gocke CD; Rosner GL; Little RF; Wright JJ; Doyle LA; Smith BD; Karp JE Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica, 2015, 100, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zeidner JF; Karp JE Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk Res, 2015, 39, 1312–1318. [DOI] [PubMed] [Google Scholar]
- [25].DeStefano CB; Hourigan CS Personalizing initial therapy in acute myeloid leukemia: incorporating novel agents into clinical practice. Ther Adv Hematol, 2018, 9, 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lai C; Doucette K; Norsworthy K Recent drug approvals for acute myeloid leukemia. J Hematol Oncol, 2019, 12, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dohner H; Weisdorf DJ; Bloomfield CD Acute Myeloid Leukemia. N Engl J Med, 2015, 373, 1136–1152. [DOI] [PubMed] [Google Scholar]
- [28].Khan N; Hills RK; Virgo P; Couzens S; Clark N; Gilkes A; Richardson P; Knapper S; Grimwade D; Russell NH; Burnett AK; Freeman SD Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia, 2017, 31, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Petersdorf SH; Kopecky KJ; Slovak M; Willman C; Nevill T; Brandwein J; Larson RA; Erba HP; Stiff PJ; Stuart RK; Walter RB; Tallman MS; Stenke L; Appelbaum FR A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood, 2013, 121, 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castaigne S; Pautas C; Terre C; Raffoux E; Bordessoule D; Bastie JN; Legrand O; Thomas X; Turlure P; Reman O; de Revel T; Gastaud L; de Gunzburg N; Contentin N; Henry E; Marolleau JP; Aljijakli A; Rousselot P; Fenaux P; Preudhomme C; Chevret S; Dombret H; Acute Leukemia French A Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet, 2012, 379, 1508–1516. [DOI] [PubMed] [Google Scholar]
- [31].Hills RK; Castaigne S; Appelbaum FR; Delaunay J; Petersdorf S; Othus M; Estey EH; Dombret H; Chevret S; Ifrah N; Cahn JY; Recher C; Chilton L; Moorman AV; Burnett AK Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol, 2014, 15, 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lim WS; Tardi PG; Dos Santos N; Xie X; Fan M; Liboiron BD; Huang X; Harasym TO; Bermudes D; Mayer LD Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res, 2010, 34, 1214–1223. [DOI] [PubMed] [Google Scholar]
- [33].Tardi P; Johnstone S; Harasym N; Xie S; Harasym T; Zisman N; Harvie P; Bermudes D; Mayer L In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res, 2009, 33, 129–139. [DOI] [PubMed] [Google Scholar]
- [34].Lancet JE; Uy GL; Cortes JE; Newell LF; Lin TL; Ritchie EK; Stuart RK; Strickland SA; Hogge D; Solomon SR; Stone RM; Bixby DL; Kolitz JE; Schiller GJ; Wieduwilt MJ; Ryan DH; Hoering A; Banerjee K; Chiarella M; Louie AC; Medeiros BC CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol, 2018, 36, 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakao M; Yokota S; Iwai T; Kaneko H; Horiike S; Kashima K; Sonoda Y; Fujimoto T; Misawa S Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia, 1996, 10, 1911–1918. [PubMed] [Google Scholar]
- [36].Weisberg E; Boulton C; Kelly LM; Manley P; Fabbro D; Meyer T; Gilliland DG; Griffin JD Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell, 2002, 1, 433–443. [DOI] [PubMed] [Google Scholar]
- [37].Ikegami Y; Yano S; Nakao K Antitumor effect of CGP41251, a new selective protein kinase C inhibitor, on human non-small cell lung cancer cells. Jpn J Pharmacol, 1996, 70, 65–72. [DOI] [PubMed] [Google Scholar]
- [38].Fabbro D; Buchdunger E; Wood J; Mestan J; Hofmann F; Ferrari S; Mett H; O’Reilly T; Meyer T Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharmacol Ther, 1999, 82, 293–301. [DOI] [PubMed] [Google Scholar]
- [39].Stone RM; Mandrekar SJ; Sanford BL; Laumann K; Geyer S; Bloomfield CD; Thiede C; Prior TW; Dohner K; Marcucci G; Lo-Coco F; Klisovic RB; Wei A; Sierra J; Sanz MA; Brandwein JM; de Witte T; Niederwieser D; Appelbaum FR; Medeiros BC; Tallman MS; Krauter J; Schlenk RF; Ganser A; Serve H; Ehninger G; Amadori S; Larson RA; Dohner H Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med, 2017, 377, 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stein EM; DiNardo CD; Pollyea DA; Fathi AT; Roboz GJ; Altman JK; Stone RM; DeAngelo DJ; Levine RL; Flinn IW; Kantarjian HM; Collins R; Patel MR; Frankel AE; Stein A; Sekeres MA; Swords RT; Medeiros BC; Willekens C; Vyas P; Tosolini A; Xu Q; Knight RD; Yen KE; Agresta S; de Botton S; Tallman MS Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood, 2017, 130, 722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Campos L; Rouault JP; Sabido O; Oriol P; Roubi N; Vasselon C; Archimbaud E; Magaud JP; Guyotat D High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood, 1993, 81, 3091–3096. [PubMed] [Google Scholar]
- [42].Del Poeta G; Venditti A; Del Principe MI; Maurillo L; Buccisano F; Tamburini A; Cox MC; Franchi A; Bruno A; Mazzone C; Panetta P; Suppo G; Masi M; Amadori S Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood, 2003, 101, 2125–2131. [DOI] [PubMed] [Google Scholar]
- [43].Lagadinou ED; Sach A; Callahan K; Rossi RM; Neering SJ; Minhajuddin M; Ashton JM; Pei S; Grose V; O’Dwyer KM; Liesveld JL; Brookes PS; Becker MW; Jordan CT BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell, 2013, 12, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DiNardo CD; Pratz K; Pullarkat V; Jonas BA; Arellano M; Becker PS; Frankfurt O; Konopleva M; Wei AH; Kantarjian HM; Xu T; Hong WJ; Chyla B; Potluri J; Pollyea DA; Letai A Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood, 2019, 133, 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wei AH; Strickland SA Jr.; Hou JZ; Fiedler W; Lin TL; Walter RB; Enjeti A; Tiong IS; Savona M; Lee S; Chyla B; Popovic R; Salem AH; Agarwal S; Xu T; Fakouhi KM; Humerickhouse R; Hong WJ; Hayslip J; Roboz GJ Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol, 2019, 37, 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Briscoe J; Therond PP The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol, 2013, 14, 416–429. [DOI] [PubMed] [Google Scholar]
- [47].Fukushima N; Minami Y; Kakiuchi S; Kuwatsuka Y; Hayakawa F; Jamieson C; Kiyoi H; Naoe T Small-molecule Hedgehog inhibitor attenuates the leukemia-initiation potential of acute myeloid leukemia cells. Cancer Sci, 2016, 107, 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cortes JE; Heidel FH; Hellmann A; Fiedler W; Smith BD; Robak T; Montesinos P; Pollyea DA; DesJardins P; Ottmann O; Ma WW; Shaik MN; Laird AD; Zeremski M; O’Connell A; Chan G; Heuser M Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia, 2019, 33, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Medeiros BC; Fathi AT; DiNardo CD; Pollyea DA; Chan SM; Swords R Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia, 2017, 31, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nassereddine S; Lap CJ; Haroun F; Tabbara I The role of mutant IDH1 and IDH2 inhibitors in the treatment of acute myeloid leukemia. Ann Hematol, 2017, 96, 1983–1991. [DOI] [PubMed] [Google Scholar]
- [51].DiNardo CD; Stein EM; de Botton S; Roboz GJ; Altman JK; Mims AS; Swords R; Collins RH; Mannis GN; Pollyea DA; Donnellan W; Fathi AT; Pigneux A; Erba HP; Prince GT; Stein AS; Uy GL; Foran JM; Traer E; Stuart RK; Arellano ML; Slack JL; Sekeres MA; Willekens C; Choe S; Wang H; Zhang V; Yen KE; Kapsalis SM; Yang H; Dai D; Fan B; Goldwasser M; Liu H; Agresta S; Wu B; Attar EC; Tallman MS; Stone RM; Kantarjian HM Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med, 2018, 378, 2386–2398. [DOI] [PubMed] [Google Scholar]
- [52].Perl AE; Altman JK; Cortes J; Smith C; Litzow M; Baer MR; Claxton D; Erba HP; Gill S; Goldberg S; Jurcic JG; Larson RA; Liu C; Ritchie E; Schiller G; Spira AI; Strickland SA; Tibes R; Ustun C; Wang ES; Stuart R; Rollig C; Neubauer A; Martinelli G; Bahceci E; Levis M Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol, 2017, 18, 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Perl AE, et al. Abstract CT184: Gilteritinib significantly prolongs overall survival in patients with FLT3-mutated (FLT3mut+) relapsed/refractory (R/R) acute myeloid leukemia (AML): Results from the Phase III ADMIRAL trial. Cancer Research, 2019, [Google Scholar]
- [54].Office of the Chief Actuary. Period Life Table. https://www.ssa.gov/oact/STATS/table4c6.html. (Accessed Jan 16, 2016)
- [55].Estey E ‘Looking beyond survival to define therapeutic value in acute myeloid leukemia’. Leuk Lymphoma, 2019, 60, 1107–1109. [DOI] [PubMed] [Google Scholar]
- [56].Surveillance Research Program NCI. SEER*Explorer: An interactive website for SEER cancer statistics. https://seer.cancer.gov/explorer/. (Accessed Jan 16, 2016)
- [57].Lin A; Giuliano CJ; Palladino A; John KM; Abramowicz C; Yuan ML; Sausville EL; Lukow DA; Liu L; Chait AR; Galluzzo ZC; Tucker C; Sheltzer JM Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med, 2019, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Patel C; Stenke L; Varma S; Lindberg ML; Bjorkholm M; Sjoberg J; Viktorsson K; Lewensohn R; Landgren O; Gottesman MM; Gillet JP Multidrug resistance in relapsed acute myeloid leukemia: evidence of biological heterogeneity. Cancer, 2013, 119, 3076–3083.23674237 [Google Scholar]
- [59].Campos L; Guyotat D; Archimbaud E; Calmard-Oriol P; Tsuruo T; Troncy J; Treille D; Fiere D Clinical significance of multidrug resistance P-glycoprotein expression on acute nonlymphoblastic leukemia cells at diagnosis. Blood, 1992, 79, 473–476. [PubMed] [Google Scholar]
- [60].Wilson CS; Davidson GS; Martin SB; Andries E; Potter J; Harvey R; Ar K; Xu Y; Kopecky KJ; Ankerst DP; Gundacker H; Slovak ML; Mosquera-Caro M; Chen IM; Stirewalt DL; Murphy M; Schultz FA; Kang H; Wang X; Radich JP; Appelbaum FR; Atlas SR; Godwin J; Willman CL Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood, 2006, 108, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].List AF; Kopecky KJ; Willman CL; Head DR; Persons DL; Slovak ML; Dorr R; Karanes C; Hynes HE; Doroshow JH; Shurafa M; Appelbaum FR Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood, 2001, 98, 3212–3220. [DOI] [PubMed] [Google Scholar]
- [62].Baer MR; George SL; Dodge RK; O’Loughlin KL; Minderman H; Caligiuri MA; Anastasi J; Powell BL; Kolitz JE; Schiffer CA; Bloomfield CD; Larson RA Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood, 2002, 100, 1224–1232. [PubMed] [Google Scholar]
- [63].Burnett AK; Milligan D; Goldstone A; Prentice A; McMullin MF; Dennis M; Sellwood E; Pallis M; Russell N; Hills RK; Wheatley K; United Kingdom National Cancer Research Institute Haematological Oncology Study, G. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. Br J Haematol, 2009, 145, 318–332. [DOI] [PubMed] [Google Scholar]
- [64].Liu Yin JA; Wheatley K; Rees JK; Burnett AK; Party UMALW Comparison of ‘sequential’ versus ‘standard’ chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-R trial. Br J Haematol, 2001, 113, 713–726. [DOI] [PubMed] [Google Scholar]
- [65].van der Holt B; Lowenberg B; Burnett AK; Knauf WU; Shepherd J; Piccaluga PP; Ossenkoppele GJ; Verhoef GE; Ferrant A; Crump M; Selleslag D; Theobald M; Fey MF; Vellenga E; Dugan M; Sonneveld P The value of the MDR1 reversal agent PSC-833 in addition to daunorubicin and cytarabine in the treatment of elderly patients with previously untreated acute myeloid leukemia (AML), in relation to MDR1 status at diagnosis. Blood, 2005, 106, 2646–2654. [DOI] [PubMed] [Google Scholar]
- [66].Greenberg PL; Lee SJ; Advani R; Tallman MS; Sikic BI; Letendre L; Dugan K; Lum B; Chin DL; Dewald G; Paietta E; Bennett JM; Rowe JM Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995). J Clin Oncol, 2004, 22, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cripe LD; Uno H; Paietta EM; Litzow MR; Ketterling RP; Bennett JM; Rowe JM; Lazarus HM; Luger S; Tallman MS Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood, 2010, 116, 4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Erickson P; Gao J; Chang KS; Look T; Whisenant E; Raimondi S; Lasher R; Trujillo J; Rowley J; Drabkin H Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood, 1992, 80, 1825–1831. [PubMed] [Google Scholar]
- [69].Christen F; Hoyer K; Yoshida K; Hou HA; Waldhueter N; Heuser M; Hills RK; Chan W; Hablesreiter R; Blau O; Ochi Y; Klement P; Chou WC; Blau IW; Tang JL; Zemojtel T; Shiraishi Y; Shiozawa Y; Thol F; Ganser A; Lowenberg B; Linch DC; Bullinger L; Valk PJM; Tien HF; Gale RE; Ogawa S; Damm F Genomic landscape and clonal evolution of acute myeloid leukemia with t(8;21): an international study on 331 patients. Blood, 2019, 133, 1140–1151. [DOI] [PubMed] [Google Scholar]
- [70].Hayakawa F; Towatari M; Kiyoi H; Tanimoto M; Kitamura T; Saito H; Naoe T Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene, 2000, 19, 624–631. [DOI] [PubMed] [Google Scholar]
- [71].Zarrinkar PP; Gunawardane RN; Cramer MD; Gardner MF; Brigham D; Belli B; Karaman MW; Pratz KW; Pallares G; Chao Q; Sprankle KG; Patel HK; Levis M; Armstrong RC; James J; Bhagwat SS AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood, 2009, 114, 2984–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Smith CC; Lasater EA; Lin KC; Wang Q; McCreery MQ; Stewart WK; Damon LE; Perl AE; Jeschke GR; Sugita M; Carroll M; Kogan SC; Kuriyan J; Shah NP Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci U S A, 2014, 111, 5319–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Galanis A; Ma H; Rajkhowa T; Ramachandran A; Small D; Cortes J; Levis M Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood, 2014, 123, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].McMahon CM; Ferng T; Canaani J; Wang ES; Morrissette JJD; Eastburn DJ; Pellegrino M; Durruthy-Durruthy R; Watt CD; Asthana S; Lasater EA; DeFilippis R; Peretz CAC; McGary LHF; Deihimi S; Logan AC; Luger SM; Shah NP; Carroll M; Smith CC; Perl AE Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov, 2019, 9, 1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Melgar K; Walker MM; Jones LM; Bolanos LC; Hueneman K; Wunderlich M; Jiang JK; Wilson KM; Zhang X; Sutter P; Wang A; Xu X; Choi K; Tawa G; Lorimer D; Abendroth J; O’Brien E; Hoyt SB; Berman E; Famulare CA; Mulloy JC; Levine RL; Perentesis JP; Thomas CJ; Starczynowski DT Overcoming adaptive therapy resistance in AML by targeting immune response pathways. Sci Transl Med, 2019, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hensley CT; Wasti AT; DeBerardinis RJ Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest, 2013, 123, 3678–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Altman BJ; Stine ZE; Dang CV From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer, 2016, 16, 619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gregory MA; D’Alessandro A; Alvarez-Calderon F; Kim J; Nemkov T; Adane B; Rozhok AI; Kumar A; Kumar V; Pollyea DA; Wempe MF; Jordan CT; Serkova NJ; Tan AC; Hansen KC; DeGregori J ATM/G6PD-driven redox metabolism promotes FLT3 inhibitor resistance in acute myeloid leukemia. Proc Natl Acad Sci U S A, 2016, 113, E6669–E6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gregory MA; Nemkov T; Reisz JA; Zaberezhnyy V; Hansen KC; D’Alessandro A; DeGregori J Glutaminase inhibition improves FLT3 inhibitor therapy for acute myeloid leukemia. Exp Hematol, 2018, 58, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gregory MA; Nemkov T; Park HJ; Zaberezhnyy V; Gehrke S; Adane B; Jordan CT; Hansen KC; D’Alessandro A; DeGregori J Targeting Glutamine Metabolism and Redox State for Leukemia Therapy. Clin Cancer Res, 2019, 25, 4079–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jones CL; Stevens BM; D’Alessandro A; Reisz JA; Culp-Hill R; Nemkov T; Pei S; Khan N; Adane B; Ye H; Krug A; Reinhold D; Smith C; DeGregori J; Pollyea DA; Jordan CT Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell, 2018, 34, 724–740 e724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jones CL; Stevens BM; D’Alessandro A; Culp-Hill R; Reisz JA; Pei S; Gustafson A; Khan N; DeGregori J; Pollyea DA; Jordan CT Cysteine depletion targets leukemia stem cells through inhibition of electron transport complex II. Blood, 2019, 134, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pollyea DA; Stevens BM; Jones CL; Winters A; Pei S; Minhajuddin M; D’Alessandro A; Culp-Hill R; Riemondy KA; Gillen AE; Hesselberth JR; Abbott D; Schatz D; Gutman JA; Purev E; Smith C; Jordan CT Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med, 2018, 24, 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yoshimi A; Lin KT; Wiseman DH; Rahman MA; Pastore A; Wang B; Lee SC; Micol JB; Zhang XJ; de Botton S; Penard-Lacronique V; Stein EM; Cho H; Miles RE; Inoue D; Albrecht TR; Somervaille TCP; Batta K; Amaral F; Simeoni F; Wilks DP; Cargo C; Intlekofer AM; Levine RL; Dvinge H; Bradley RK; Wagner EJ; Krainer AR; Abdel-Wahab O Coordinated alterations in RNA splicing and epigenetic regulation drive leukaemogenesis. Nature, 2019, 574, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Inoue D; Chew GL; Liu B; Michel BC; Pangallo J; D’Avino AR; Hitchman T; North K; Lee SC; Bitner L; Block A; Moore AR; Yoshimi A; Escobar-Hoyos L; Cho H; Penson A; Lu SX; Taylor J; Chen Y; Kadoch C; Abdel-Wahab O; Bradley RK Spliceosomal disruption of the non-canonical BAF complex in cancer. Nature, 2019, 574, 432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]