Abstract
Purpose:
Preclinical studies suggest PARP inhibition (PARPi) induces immunostimulatory micromilieu in ovarian cancer thus complementing activity of immune checkpoint blockade. We conducted a phase 2 trial of PARPi olaparib and anti-PD-L1 durvalumab and collected paired fresh core biopsies and blood samples to test this hypothesis.
Experimental Design:
In a single-center, proof-of-concept phase 2 study, we enrolled women aged ≥18 with recurrent ovarian cancer. All patients were immune-checkpoint inhibitor naïve and had measurable disease per RECISTv1.1, ECOG performance status 0–2, and adequate organ and marrow function. Patients received olaparib 300mg twice daily and durvalumab 1500mg intravenously every 4 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. Primary endpoint was overall response rate (ORR). Secondary objectives were safety and progression-free survival (PFS). Translational objectives included biomarker evaluation for relationships with clinical response and immunomodulatory effects by treatment.
Results:
35 ovarian cancer patients (median 4 prior therapies [IQR 2-5.5], predominantly platinum-resistant [86%], BRCA wild-type [77%]) received at least one full cycle of treatment. ORR was 14% (5/35;95%CI,4.8%-30.3%). Disease control rate (PR+SD) was 71% (25/35;95%CI,53.7%-85.4%). Treatment enhanced IFNγ and CXCL9/CXCL10 expression, systemic IFNγ/TNFα production, and tumor-infiltrating lymphocytes, indicating an immunostimulatory environment. Increased IFNγ production was associated with improved PFS (HR:0.37[95%CI,0.16-0.87], p=0.023) while elevated VEGFR3 levels were associated with worse PFS (HR=3.22[95%CI,1.23-8.40], p=0.017).
Conclusions:
The PARPi and anti-PD-L1 combination showed modest clinical activity in recurrent ovarian cancer. Our correlative study results suggest immunomodulatory effects by olaparib/durvalumab in patients and indicate that VEGF/VEGFR pathway blockade would be necessary for improved efficacy of the combination.
INTRODUCTION
Ovarian cancer is the most fatal gynecologic malignancy worldwide1,2. The majority of women with epithelial ovarian cancer present at an advanced stage and frequently recur, leading to incurable disease with limited treatment options1. A critical need remains for new effective therapeutic strategies. Immune checkpoint inhibition, such as programmed death (PD)-1 and PD-ligand 1 (PD-L1) pathway blockade, has led to important clinical advances in various malignancies and has also been tested in recurrent ovarian cancer3. To date, the monotherapy activity of immune checkpoint inhibitors has been limited in ovarian cancer, leaving opportunity to test combination strategies3.
An active therapeutic target for combination treatment is the DNA damage response pathway, such as poly (ADP-ribose) polymerase (PARP)4. Successful introduction of PARP inhibitors (PARPi) has led to a new treatment paradigm in ovarian cancer, in particular for patients with BRCA mutation (BRCAm)4. PARP inhibition has been shown to cause DNA damage via catalytic inhibition of the PARP enzyme and trapping of DNA-PARP complexes, resulting in synthetic lethality in cells deficient in homologous recombination (HR) repair4.
Emerging data also suggest the efficacy of PARPi may be associated with immunomodulation5–8. DNA damage by PARPi may enhance tumor mutational load and neoantigen expression, leading to antitumor immune responses9. Also, high levels of interferon-γ (IFNγ) increases the cytotoxic effect of PARPi in a BRCA1-deficient ovarian cancer model10 and increased DNA damage by PARPi activates the stimulator of interferon genes (STING) pathway, resulting in systemic antitumor immunity5–8. Specifically, the PARPi olaparib promotes accumulation of cytosolic DNA fragments, which enter the cytoplasm and bind to cyclic GMP-AMP synthase (cGAS), leading to upregulation of the cGAS-STING pathway in both BRCA1-deficient and BRCA-proficient ovarian cancer cell lines and mouse models5,6. PARPi also upregulates PD-L1 expression through a variety of mechanisms, including IFNγ stimulation, STING pathway activation, or inactivation of glycogen synthase kinase-3β (GSK3β) in ovarian, breast and lung cancer preclinical models6,11,12. Hence, addition of PARPi may complement the clinical activity of immune checkpoint blockade by creating a more immunogenic tumor microenvironment.
There are now multiple clinical trials combining PARPi with immune checkpoint blockade therapy in ovarian cancer1. However, it is unknown whether PARPi actually induce the immunostimulatory milieu in recurrent ovarian cancer patients and prime the immune microenvironment for the PD-1/PD-L1 pathway inhibitors. To test this hypothesis, we prospectively designed a proof-of-concept, investigator-initiated phase 2 study of the PARPi olaparib and the PD-L1 inhibitor durvalumab with collection of paired pre-and on-treatment fresh tissue and blood samples in women with recurrent ovarian cancer. Here we show immunostimulatory changes induced by treatment, including increased expression of IFNγ, CXCL9 and CXCL10, systemic production of IFNγ and TNFα, and tumoral infiltration by lymphocytes. Enhanced plasma IFNγ levels were associated with response and improved progression free survival (PFS). Our findings also suggest the VEGF/VEGFR pathway may act to counterbalance immunostimulatory changes by PARPi and serve as a target to further improve the efficacy of the PARPi and anti-PD-L1 combination. Overall, our results showed modest clinical activity of this therapeutic combination but indicate that PARPi creates an immunostimulatory environment that may augment immune responses to anti-PD-L1 among subsets of patients, warranting further investigation.
MATERIALS AND METHODS
Study design and participants
This trial was designed as a proof-of-concept, signal-seeking phase 2 study with 4 independent cohorts: ovarian, triple negative breast, prostate and lung cancers. This report describes the ovarian cancer cohort. Eligible patients were aged ≥18 years and had histologically confirmed recurrent or metastatic ovarian, fallopian tube, or primary peritoneal cancer. Patients must have had measurable disease based on response evaluation criteria in solid tumors (RECIST) v1.1 criterion and at least one lesion safely accessible for a mandatory percutaneous baseline biopsy. Documentation of germline BRCAm status was requested at enrollment. Patients may have received any number of other systemic therapies including prior PARPi. Other key inclusion criteria included Eastern Cooperative Oncology Group performance status 0–2, adequate organ and marrow function, demonstrated by absolute neutrophil count ≥1,500/mcL; platelets ≥100,000/mcL; hemoglobin ≥ 9 gm/dL; total bilirubin ≤1.5 times the institutional upper limit of normal (ULN); aspartate aminotransferase and alanine aminotransferase ≤2.5 times ULN; creatinine ≤ULN or a creatinine clearance ≥50 mL/min/1.73 m² (Supplementary material). Key study exclusion criteria included concurrent anticancer therapy, prior immune checkpoint inhibitors, any investigational anticancer therapy ≤ 3 weeks before first doses of study drugs; central nervous system metastases ≤ 1 year prior to enrollment; severe prior immune-related AEs requiring steroid maintenance, or active or prior documented inflammatory bowel disease; and/or, baseline features suggestive of myelodysplastic syndrome or acute myelogenous leukemia (Supplementary material).
All patients provided written informed consent before enrollment. The trial was approved by the Institutional Review Board of the Center for Cancer Research (CCR), National Cancer Institute (NCI). The study has been conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with the International Council on Harmonization guidelines on Good Clinical Practice, all applicable laws and regulatory requirements, and all conditions required by a regulatory authority and/or institutional review board. ClinicalTrials.gov identifier: NCT02484404.
Procedures
Treatment consisted of olaparib 300mg twice daily and durvalumab 1500mg by intravenous infusion every 4 weeks (−4 to +8 days; one cycle is defined as 28 days) until radiologic progression or unacceptable toxicity (Supplementary Figure 1, Supplementary material). Laboratory assessments (including hematology, fasting serum chemistry, endocrine function and urinalysis) were done before each cycle. Clinical response was assessed every two cycles by imaging using RECISTv1.1 guidelines. Patients were evaluated for toxicity per Common Terminology Criteria for Adverse Events version (CTCAE) v4.0. Study treatment was discontinued for progression of disease, intercurrent illness, AEs not recovering to ≤ grade 1 within 14 days, or patient withdrawal of consent.
For correlative studies, we collected pretreatment fresh frozen core biopsies and paired blood samples (at baseline and on cycle 1 day 15; Supplementary material). A pre-treatment fresh core biopsy was mandatory for all patients and second biopsy on cycle 1 day 15 was optional because of patient’s refusal or safety concerns. Mutations in DNA repair genes were identified by targeted sequencing of tumor DNA with a BROCA-HR sequencing assay13 on pre-treatment tissue samples for 29 patients without gBRCAm. Homologous recombination deficiency (HRD) was defined based on the published literature14,15 as a deleterious germline or somatic mutation identified by BROCA-HR sequencing present in >10% of the neoplastic fraction in one of the following genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK12, NBN, PALB2, RAD51C, RAD51D13. RNA-sequencing (RNA-seq) was performed using a HiSeq3000 sequencing system (Illumina, San Diego, CA, USA) at the CCR sequencing facility, NCI (Supplementary material). A multiplex enzyme-linked immunosorbent assay (ELISA) angiome assay for 33 cytokines was performed on plasma samples (Supplementary material). Immunohistochemistry was used for PD-L1 expression (clone SP142) and TIL analysis using standard procedure16 (Supplementary material), and for STING pathway expression (ab92605; Supplementary material). Whole exome sequencing on DNA samples were performed on the Novaseq6000-S2 system at the CCR sequencing facility, NCI (Supplementary material).
Outcomes
The primary objective was overall response rate (ORR) by RECISTv1.1 in evaluable patients who had undergone computed tomography (CT) imaging at baseline and at least one protocol-specified follow-up timepoint. Secondary objectives included safety evaluation and PFS. Prespecified exploratory objectives were to investigate potential predictive biomarkers described in the correlative studies.
Statistical analyses
The study was conducted using Simon’s optimal two-stage phase 2 design to rule out a 10% ORR in favor of a 30% ORR, with α=0.10 and β=0.10. These parameters were chosen for this single arm, signal-seeking study to minimize the number of women exposed to a potentially inactive combination and to target a sufficiently high ORR to support moving into a definitive trial should this trial be positive. The null hypothesis of 10% was selected to accommodate the inclusion of heavily-pretreated platinum-resistant BRCAwt patients. A response in two of the first 12 patients sufficed to move to the second stage of accrual, adding another 23 patients. The regimen would be considered sufficiently interesting if ≥ 6/35 patients had a complete response or PR. The probability of early termination was 65.9% under the null hypothesis. PFS was estimated using the Kaplan-Meier method beginning at the on-study date and continuing until progression or death without progression. Patients who have not progressed had their follow-up censored at February 12, 2019 for this evaluation. Differences between Kaplan-Meier curves tests were determined using a log-rank test. Evaluations for the effect of cytokine parameters on PFS were determined using a Cox proportional hazards model after adjusting for standard clinical factors including BRCAm positivity, platinum sensitivity, previous lines of therapy, and previous bevacizumab. Safety evaluation included all enrolled patients. Patients considered non-evaluable had either no post-baseline CT scan or discontinued after less than 8 weeks without documented progression.
RESULTS
Study Design, Enrollment, and Patient Demographics
Between February 2016 and April 2018, 35 patients were enrolled and received at least one full cycle of treatment (Supplementary Figure 1a). One patient with BRCA2m platinum-sensitive disease was receiving treatment at the time of data cutoff (February 12, 2019), at >21 months continuous treatment. Clinical characteristics of patients are summarized in Table 1. Patients were heavily-pretreated with a median of 4 prior therapies [IQR 2-5.5] and all were immune-checkpoint inhibitor naïve. The majority of patients (86% [30/35]) had platinum-resistant disease and six (17%) had known germline BRCAm (gBRCAm) confirmed by commercial BRCA testing prior to enrollment. Six of 29 germline BRCA wild type (BRCAwt) patients were found to have somatic DNA repair pathway mutations suggestive of HRD including BRCA1 (1), BRCA2 (1), BRIP1 (2), PALB2 (1), CDK12 (1) (Supplementary Table 1). Higher coverage BROCA-HR sequencing did not identify BRCA reversion mutations in 6 gBRCAm carriers who had prior PARPi (rucaparib, n=1), and platinum-based therapy (all).
Table 1.
Baseline patients’ characteristics
| Baseline characteristics | |
|---|---|
| Characteristics | Olaparib and durvalumab (n=35)* |
| Age, years, median (range) | 63 (40-85) |
| ECOG performance status, 0/1/2 | 9 (26%) / 25 (71%) / 1 (3%) |
| Tumor type | |
| Ovarian carcinoma/Primary peritoneal carcinoma | 34 (98%)/ 1 (2%) |
| Platinum-sensitive / Platinum-resistant** | 5 (14 %)/ 30 (86%) |
| High grade serous / Endometrioid / Mucinous | 31 (88%) / 3 (9%) / 1 (3%) |
| BRCA mutation status | |
| Germline / somatic / wild-type | 6 (17%)/ 2 (6%) / 27 (77%) |
| Lines of prior therapy | |
| 1 | 4 (11%) |
| 2-3 | 13 (37%) |
| ≥ 4 | 18 (52%) |
| Prior PARP inhibitor | 2 (6%) |
| Prior bevacizumab | 16 (46%) |
36 patients were enrolled. One patient was found to have brain metastases three days after her first durvalumab infusion and olaparib. At baseline, she had no symptoms or signs suggestive of brain metastasis but brain MRI due to new onset of dizziness confirmed metastases. She was thus taken off treatment for being ineligible and also for intercurrent illness, thus was not evaluable for outcome.
Patients were categorized as platinum-sensitive (progression ≥ 6 months after last platinum-based therapy) or platinum-resistant (progressed <6 months after last platinum-based therapy).
Abbreviations: ECOG = Eastern Cooperative Oncology Group; PARP = poly (ADP-ribose) polymerase.
Efficacy
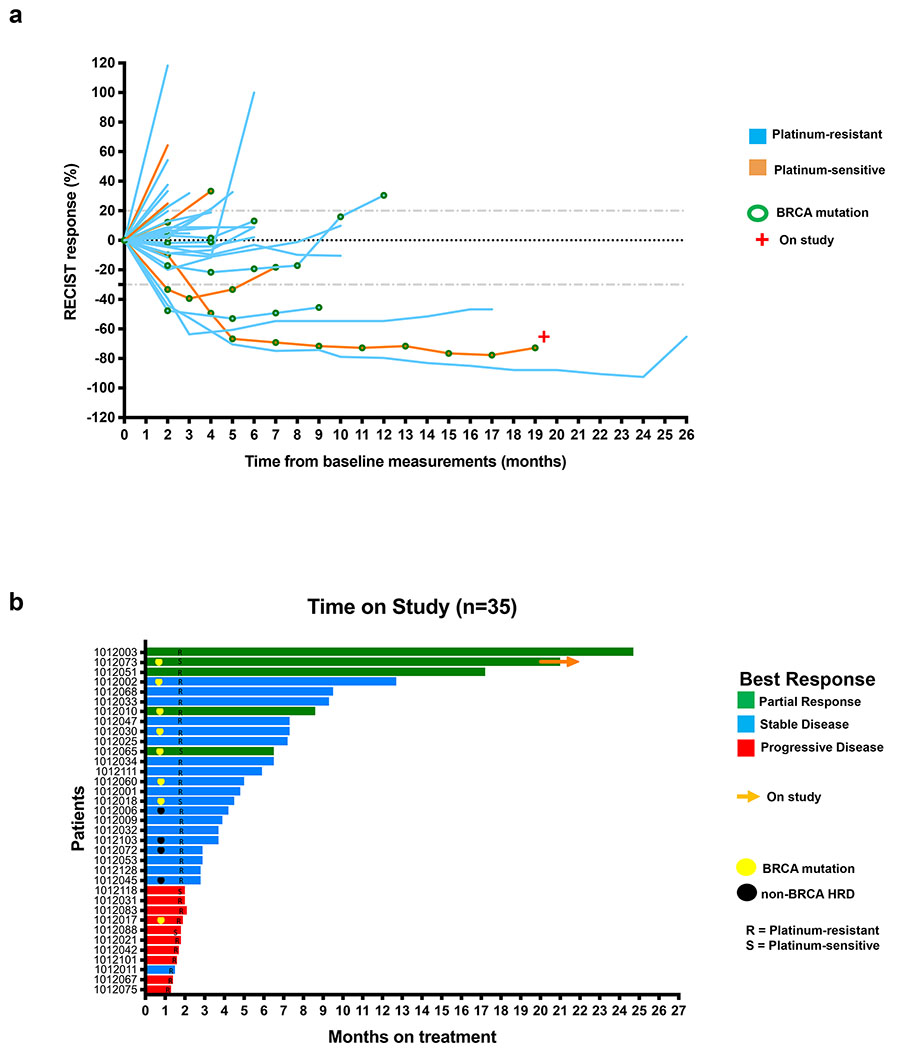
Changes in tumor size from baseline and duration on study are shown in Figure 1. Among 35 evaluable patients, five attained a partial response (PR; ORR 14%, 95%CI 4.8%-30.3%) with a median duration on study of 17.2 months (IQR 8.6-21); 2 gBRCAm/ 1 somatic BRCAm [sBRCAm]/ 2 BRCAwt and non-HRD. The overall median PFS was 3.9 months (IQR 2-7.25, Supplementary Fig 1b). This study thus did not meet the pre-specified primary endpoint of ≥17.1% ORR (6 or more complete or partial responses among 35 patients). However, the disease control rate (DCR=PR+stable disease [SD]) was 71% (25/35; 95%CI 53.7%-85.4%). 12 of 35 (34%; 95%CI 19.1%-52.2%) had clinical benefit (defined as PR+SD≥6 months), including ten of 30 (33.3%) heavily-pretreated platinum-resistant patients. Three of 30 (10%) platinum-resistant patients attained a PR with a median duration on study of 17.2 months (IQR 12.5-20.9) and seven (23.3%) achieved disease stabilization lasting at least 6 months (median 7.3 months on study, IQR 7.25-9.4). This indicates combination treatment may provide durable clinical benefit (≥6 months) in subsets of heavily-pretreated patients for whom either PARPi or immune checkpoint blockade monotherapy have shown limited activity1,17.
Figure 1. Changes in tumor size and duration on the treatment.
a) Changes in tumor size on the study treatment.
b) Duration in the study.
Abbreviations: S = platinum-sensitive recurrent ovarian cancer, R = platinum-resistant recurrent ovarian cancer, HRD = homologous recombination deficiency.
Safety and Tolerability
Treatment with durvalumab and olaparib was overall well-tolerated. The most common treatment-related grade 3 or 4 adverse event (AE) was hematologic toxicity, predominantly anemia. We found approximately 31% of patients (11/35; 95% CI 17-49%) had grade ≥3 anemia, which was a higher frequency than reported (10-20%) in other PARPi and immune checkpoint blockade combination studies18,19. Clinical workup determined that none of the anemia cases were immune-related. We speculate that the higher frequency of grade ≥3 anemia is likely due to small sample size and the heavily pretreated population in our cohort with a median of 3 prior cytotoxic chemotherapy regimens [IQR 2-4.5]. All treated patients had at least one any grade treatment-associated AE, summarized in Table 2.
Table 2.
Treatment-related adverse events by maximum grade per patient
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematological | ||||
| Anemia* | 9 (26%) | 11 (31%) | 11 (31%) | 0 |
| Decreased platelets | 9 (26%) | 0 | 0 | 0 |
| Decreased leukocytes | 7 (20%) | 4 (11%) | 1 (3%) | 0 |
| Decreased lymphocytes | 4 (11%) | 14 (40%) | 7 (20%) | 0 |
| Decreased neutrophils | 0 | 3 (9%) | 1 (3%) | 0 |
| Gastrointestinal | ||||
| Nausea | 16 (46%) | 1 (3%) | 0 | 0 |
| Vomit | 12 (34%) | 0 | 0 | 0 |
| Diarrhea | 8 (23%) | 1 (3%) | 0 | 0 |
| Constipation | 4 (11%) | 0 | 0 | 0 |
| GERD | 5 (14%) | 0 | 0 | 0 |
| Anorexia | 8 (23%) | 1 (3%) | 0 | 0 |
| Proctitis | 0 | 1 (3%) | 0 | 0 |
| Endocrinology and Chemistry | ||||
| Hypothyroidism | 1 (3%) | 0 | 0 | 0 |
| Hyperthyroidism | 2 (6%) | 0 | 0 | 0 |
| Increased creatinine | 7 (20%) | 4 (11%) | 0 | 0 |
| Increased ALT/AST | 3 (9%) | 0 | 0 | 0 |
| Dermatological | ||||
| Rash | 2 (6%) | 1 (3%) | 1 (3%) | 0 |
| Hyperpigmentation | 2 (6%) | 0 | 0 | 0 |
| Erythema multiforme | 1 (3%) | 0 | 0 | 0 |
| Other | ||||
| Fatigue | 14 (40%) | 5 (14%) | 0 | 0 |
| Headache | 2 (6%) | 0 | 0 | 0 |
| Insomnia | 1 (3%) | 0 | 0 | 0 |
| Arthralgia | 2 (6%) | 0 | 0 | 0 |
| Myalgia | 1 (3%) | 0 | 0 | 0 |
| Weight loss | 1 (3%) | 0 | 0 | 0 |
| Weight gain | 1 (3%) | 0 | 0 | 0 |
| Dry eye | 1 (3%) | 0 | 0 | 0 |
| Dry mouth | 1 (3%) | 0 | 0 | 0 |
| Flushing** | 1 (3%) | 0 | 0 | 0 |
Three patients required olaparib dose reduction because of recurrent anemia. No one had durvalumab dose reduction or discontinuation due to adverse events. No treatment related deaths were recorded.
During durvalumab infusion
Abbreviations: GERD = gastroesophageal reflux disease, ALT = alanine aminotransferase, AST = aspartate aminotransferase
Correlative studies
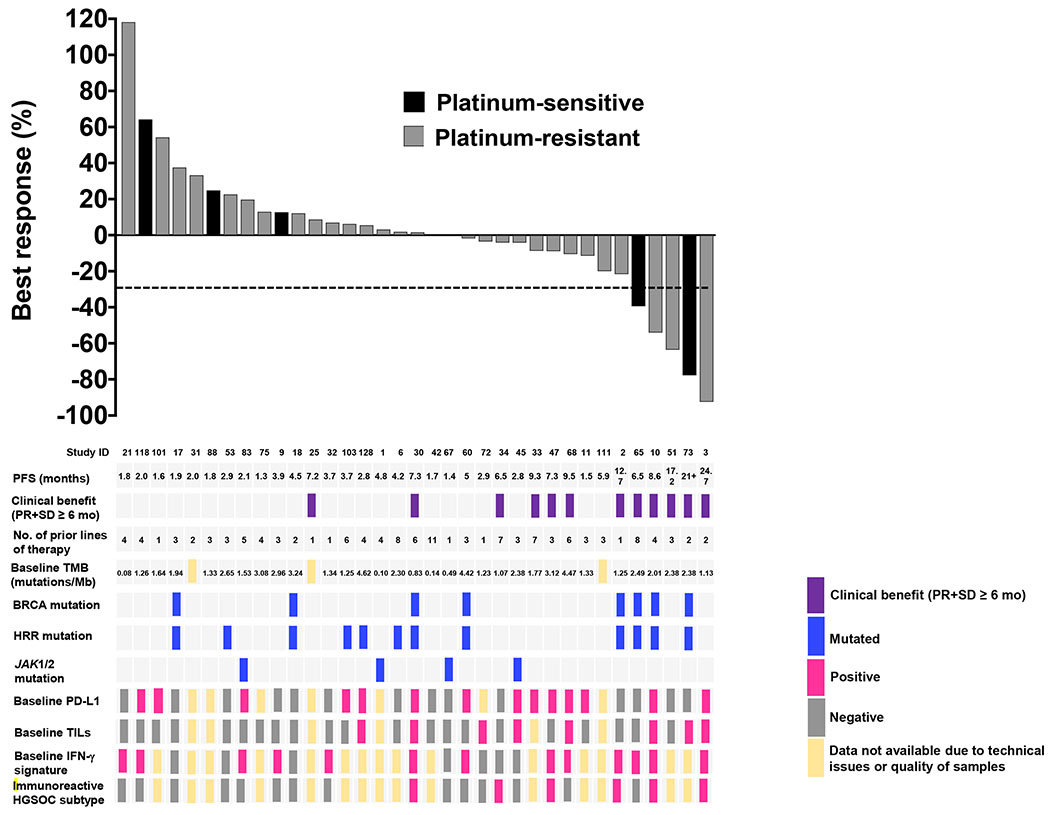
Baseline tissue samples were collected in 32 of 35 patients because three patients had the pretreatment biopsy procedure aborted for safety concerns (Supplementary material). 22 patients (7 BRCAm, 1 BRCAwt and HRD positive [PALB2 mutation], 14 BRCAwt and non-HRD) underwent the second biopsy on cycle 1 day 15. Paired blood samples were collected in all patients. A summary of the baseline biomarker endpoints with clinical response can be found in Figure 2. Changes in biomarker expression after treatment are summarized in Supplementary Figure 2.
Figure 2. Baseline biomarker endpoints in relation to RECIST best response.
Best responses are shown according to study ID, PFS, clinical response, number of prior lines of therapy, baseline TMB, BRCA mutation status, HRR mutation status, JAK1/2 mutation status, baseline tumor expression of PD-L1 and TILs, baseline expression of an IFNγ gene signature, and baseline immunoreactive HGSOC molecular subtype. TIL positive defined as >10%, PD-L1 positive defined as ≥1%.
Abbreviations: RECIST: response evaluation criteria in solid tumors, ID = identification, PFS = progression-free survival, PR = partial response, SD = stable disease, mo = months, TMB = tumor mutational burden, Mb = Megabase, IFNγ = interferon gamma, PD-L1 = programmed death-ligand 1, TIL = tumor infiltrating lymphocyte, No. = number, HRR = homologous recombination repair, HGSOC = high grade serous ovarian cancer.
Treatment upregulates IFNγ signaling resulting in an immune-inflamed tumor microenvironment
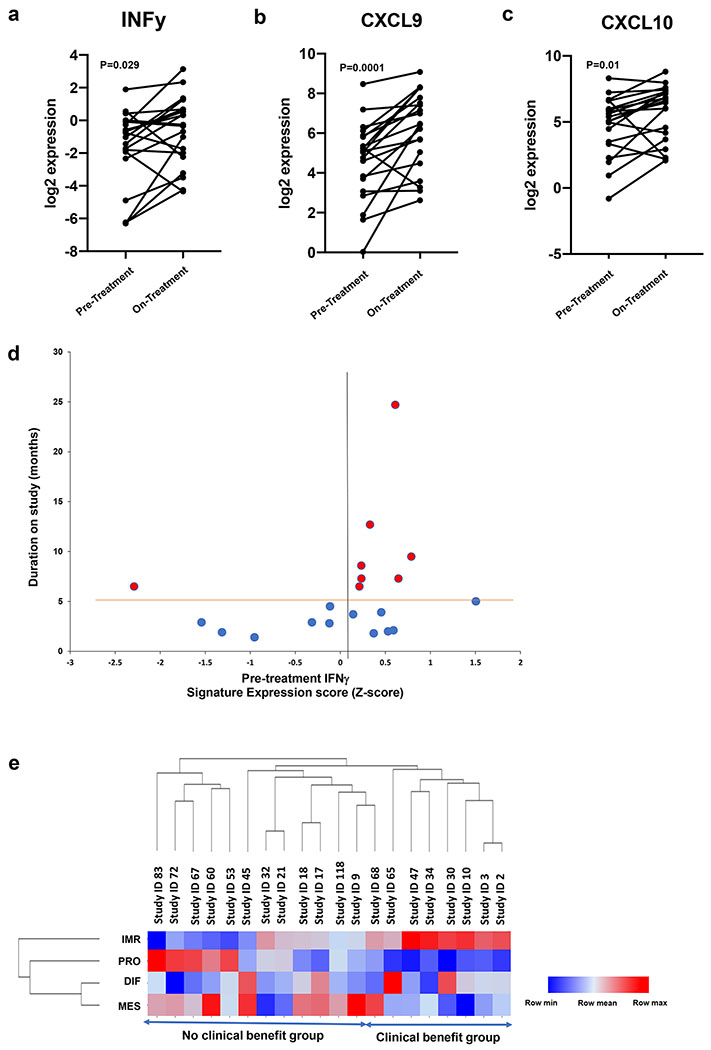
Exploratory analysis of RNA-seq data from 20 paired fresh frozen biopsy samples showed increased expression of IFNγ (median fold change 2.31, IQR 0.86-4.72, p=0.029, Figure 3a) and IFNγ-induced chemokines CXCL9 (median fold change 2.14, IQR 1.54-5.64, p=0.0001, Figure 3b) and CXCL10 (median fold change 2.10, IQR 1.13-3.92, p=0.01, Figure 3c) after treatment, suggesting the PARPi and anti-PD-L1 combination therapy induces an immune-inflamed tumor microenvironment. There was no association between high levels of local IFNγ with clinical benefit. Among eight evaluable patients achieving clinical benefit, seven had a positive baseline IFNγ signature previously determined by Ayers et al.20 (Figure 3d) and five had a positive baseline expanded 18-gene immune signature20, although there was no statistical association with clinical response. Lastly, we evaluated whether a predefined high grade serous ovarian cancer (HGSOC) immunoreactive (C2) subtype by transcriptomic analysis21 was associated with clinical benefit. Of the eight evaluable patients deriving clinical benefit, all expressed moderate to high levels of the pre-treatment immunoreactive signature. In contrast, patients with no clinical benefit had either low levels (n=4) or did not express immunoreactive signature (n=8) at baseline (p=0.0047, Figure 3e).
Figure 3. Gene expression analysis by RNA-seq.
a) Change in log2 IFNγ expression among 20 paired pre- vs on-treatment tumor biopsies (median pre −1.02 vs on-therapy −0.34, p=0.029, Wilcoxon signed-rank test).
b) Change in log2 CXCL9 expression among 20 paired pre- vs on-treatment tumor biopsies (median pre 5.10 vs on-therapy 6.47, p=0.0001, Wilcoxon signed-rank test).
c) Change in log2 CXCL10 expression among 20 paired pre- vs on-treatment tumor biopsies (median pre 5.39 vs on-therapy 6.46, p=0.01, Wilcoxon signed-rank test).
d) Baseline expression of a 6-gene IFNγ-related signature plotted against duration in months. 7 of 8 patients deriving clinical benefit (PR+SD≥6 months) have a positive expression score. Horizontal orange bar denotes 6 month cut-off and vertical black bar separates positive from negative expression Z-score.
e) Heatmap depicting HGSOC molecular subtype expression signatures, calculated by single sample gene set enrichment analysis on pre-treatment biopsies of the 20 patients with paired pre and on-treatment samples with evaluable RNA-seq.
Abbreviations: PR = partial response; SD = stable disease; PD = progressive disease; HGSOC = high grade serous ovarian cancer, IMR = immunoreactive, PRO = proliferative, DIF = differentiated, MES = mesenchymal, PREP = pre-treatment patient, NR = non-responder (no clinical benefit), R = responder (clinical benefit).
Treatment increases production of immunostimulatory cytokines and compensatory angiogenic factors
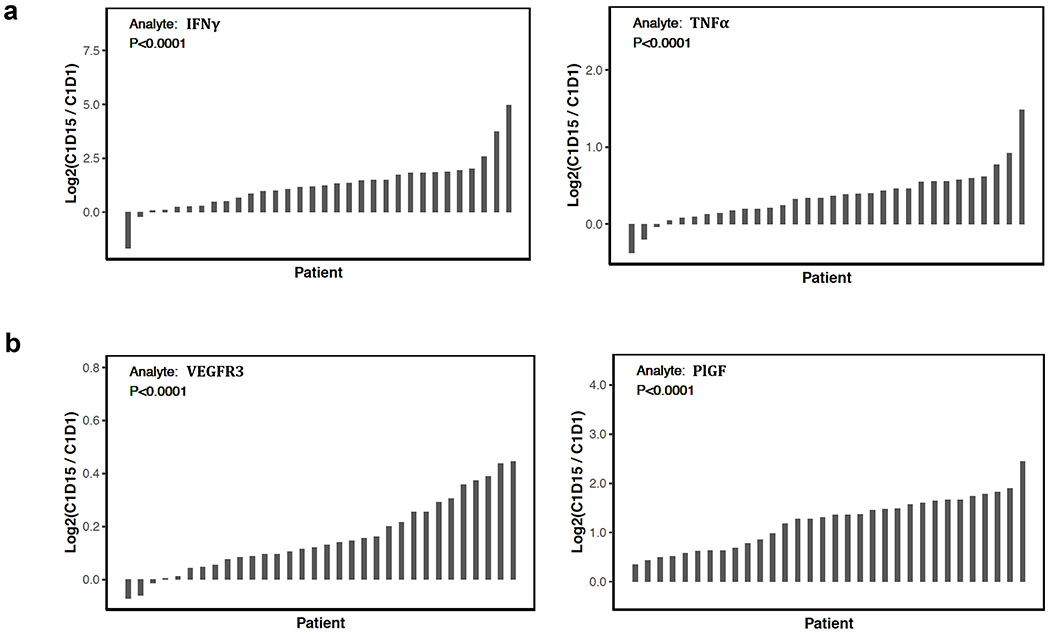
Using paired plasma samples from 32 patients, we identified systemic upregulation of several immunostimulatory cytokines after treatment such as IFNγ (median fold-change 2.31, IQR 1.41-3.52) and TNFα (median fold-change 1.27, IQR 1.11-1.46) (p<0.0001 for both after adjusting for multiple comparisons, Figure 4a). A greater fold-change increase in IFNγ was observed in patients deriving clinical benefit (n=12) compared to those who did not (n=20) (median fold-change 3.17 vs 1.97, p=0.029). Additionally, there was an association between higher levels of IFNγ after treatment and improved PFS (Hazard Ratio (HR):0.37 [95% CI: 0.16-0.87], p=0.023) using a univariate Cox proportional hazards model. This effect was maintained when clinical factors (BRCAm positivity, platinum sensitivity, previous lines of therapy, and previous bevacizumab) were included in a multivariable Cox proportional hazards model (HR:0.27 [95% CI: 0.10 – 0.72], p=0.0086). An increase in the circulating angiogenic factors e.g., vascular endothelial growth factor receptor 3 (VEGFR3; median fold-change 1.09, IQR 1.05-1.19) and placental growth factor (PlGF; median fold-change 2.52, IQR 1.59-3.06) (p<0.0001 for both, Figure 4b), was also observed with treatment. A multivariable Cox proportional hazards model demonstrated increased levels of VEGFR3 over baseline were associated with worse PFS (HR=3.22 [95%CI 1.23–8.40], p=0.017). No tumoral increase in expression of VEGFR3 or PlGF was observed by RNA-seq analysis. Together, our findings suggest olaparib and durvalumab combination therapy induces systemic immune activation as well as a possible compensatory angiogenic response.
Figure 4. PARP and PD-L1 inhibition increase systemic immunostimulatory cytokines and angiogenic factors.
a) Waterfall plots showing significant changes from baseline (C1D1) to C1D15 in immune-stimulating cytokines: IFNγ and TNFα. Of the two patients with decreased IFNγ levels, one was a non-responder (SD 2.9 mo) and another had SD for 7.2 months. Of the three patients with decreased TNFα levels, two were non-responders (PD 1.6 mo, SD 4.2 mo) and one had SD for 7.2 months.
b) Waterfall plots showing significant changes from baseline to C1D15 in angiogenic factors: VEGFR3 and PlGF. All comparisons performed by Wilcoxon signed-rank test.
Abbreviations: C1D1 = cycle 1 day 1, C1D15 = cycle 1 day 15, IFNγ = interferon gamma, TNFα = tumor necrosis factor alpha, SD = stable disease, mo = months PD = progressive disease, VEGFR3 = vascular endothelial growth factor receptor 3, PlGF = placental growth factor.
Treatment induces immune cell infiltration and upregulates tumor PD-L1 expression
We next tested the hypothesis that PARPi could induce immune cell infiltration and PD-L1 upregulation as preclinically demonstrated in BRCAm ovarian tumors6. Tumor-infiltrating lymphocytes (TILs) and PD-L1 expression were analyzed in 22 paired paraffin-embedded biopsy samples (Supplementary material). There was an overall significant increase in TILs (median 5% pre vs 10% on-therapy, p=0.035), with higher %TILs observed in 11 of 15 (73%) evaluable on-treatment biopsy samples (Supplementary Figure 3a). Change in TILs was not associated with clinical benefit, BRCAm status, or immunoreactive subtype. However, higher TILs at baseline was associated with clinical benefit (Supplementary Figure 3b–e; Supplementary Table 2), as seen in other solid tumor reports22. The majority of patients (9/14 evaluable samples, 64%) contained PD-L1 positive carcinoma cells after treatment, defined as >1% PD-L1 staining (Supplementary Table 2). Seven (58%) carcinomas of 12 evaluable pairs gained PD-L1 expression (median 5% increase, IQR 5.0-16.25%; representative patient in Supplementary Figure 3f–i). No tumors completely lost PD-L1 expression (positive to negative). Additionally, neither baseline nor changes in PD-L1 expression were predictive of treatment response (Supplementary Table 2) consistent with other reports for immune checkpoint blockade combination treatments23.
PARPi unlikely modulates STING expression in recurrent ovarian cancer patients
To test if PARPi leads to STING pathway activation clinically as has been shown in a BRCA1-deficient syngeneic ovarian cancer mouse model6, STING expression was evaluated in 14 of 22 paired paraffin-embedded biopsy samples (4 BRCAm, 1 BRCAwt and HRD positive [PALB2], and 9 BRCAwt and non-HRD). Most patients had either decreased or unchanged STING expression after treatment (Supplementary Figure 4). Only four (29%) patients had increased STING expression after treatment (median 0.26% [pre] vs 2.6% [on-therapy], p=0.125), all who either had SD or progressive disease (PD). Overall, there was no association between the change in STING expression and BRCAm or clinical response. Baseline STING expression was not associated with clinical benefit. We also evaluated RNA-seq data for STING pathway-related gene levels e.g., STING, interferon regulatory factor 3 (IRF3) and TANK-binding kinase 1 (TBK1), and found no significant changes in pre- versus on-treatment samples. Furthermore, among the four patients with increased STING expression by immunohistochemistry, none showed an increase in STING RNA expression. Although levels of IFNβ were undetectable, increased expression of inflammatory chemokines activated downstream of type I interferons was observed24. Specifically, increased expression of CCL5 (median fold-change 3.30, IQR 1.48-4.43, p=0.008) and CCL4 (median fold-change 2.12, IQR 1.50-2.84, p=0.01) was seen among those attaining clinical benefit (Supplementary Figure 5a,b). Additionally, increased expression of CXCL10, induced by both IFNγ and type I IFNs25, was observed as described above (Figure 3c). Together, the data suggests that STING pathway activation is unlikely to be a predominant mechanism driving enhanced response to treatment although the increased expression of chemokines downstream of type I IFNs warrants further validation.
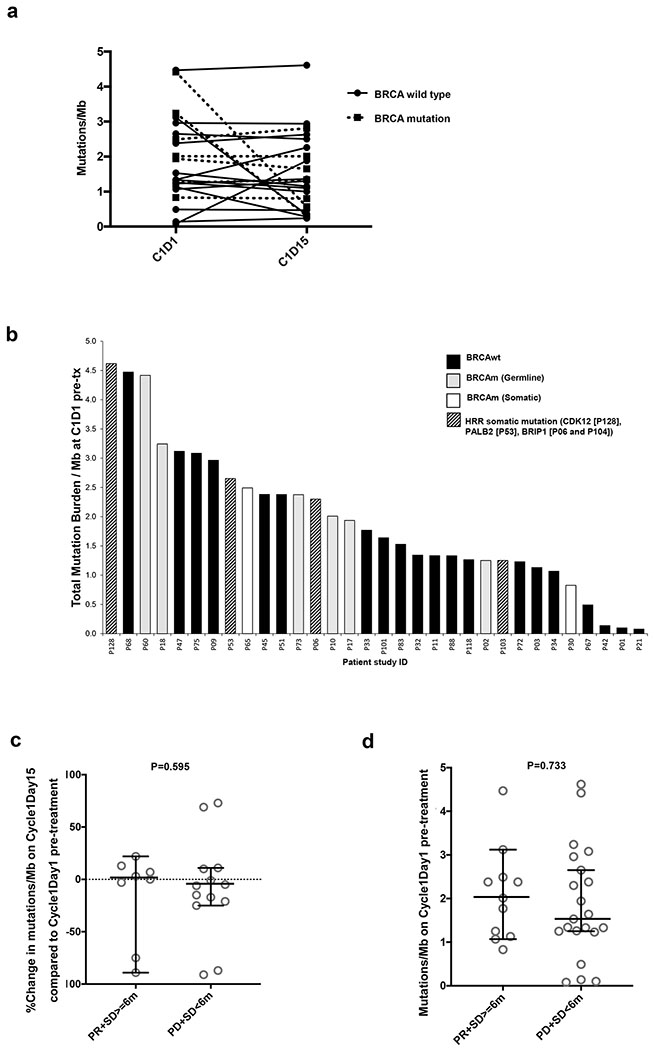
There was an overall low baseline TMB with no significant increase observed after PARPi
We observed no significant changes in tumor mutational burden (TMB; Figure 5a) and all remained below 5 somatic mutations/Mb after treatment. Additionally, we evaluated whether baseline TMB was associated with response to treatment as has been shown with immune checkpoint inhibition in lung and urothelial cancer26. We first observed that all 32 baseline tumors had less than 5 somatic mutations/Mb, consistent with the modest mutational load described in ovarian cancer26 (Figure 5b). We noted no difference in baseline mutational load between BRCAm and BRCAwt patients and no correlation of TMB either at baseline or after treatment was seen with clinical response (Figures 5c and 5d).
Figure 5.
Tumor mutation burden (TMB) as measured by somatic mutations/Megabase (Mb) exome coverage of patients at baseline (C1D1 pre-treatment) and on-treatment at C1D15. Total exome coverage was determined at 30x read coverage. p-values of <0.05 are considered significant.
a) Mutations/Mb of exome at C1D1 and C1D15 among 22 available matched pairs of patients.
b) Total TMB for all patients (n=32) measured as mutations/Mb of exome at baseline.
c) Percentage change in mutations/Mb within 22 matched samples, for the clinical benefit group (PR+SD≥6 mo; n=8) vs no clinical benefit group (PD+SD<6 mo; n=13). Patient ID 21 who had BRCAwt and PD showed 24-fold increase in TMB (mutations/Mb) at C1D15 (C1D1 0.08 vs C1D15 1.88) and was not included in the plot to prevent skew. Comparison made with unpaired Mann-Whitney U test.
d) Mutations/Mb at baseline for clinical benefit group (PR+SD≥6 mo) vs no clinical benefit group (PD+SD<6 mo) is shown. Horizontal bars and error bars indicate median ± 95% CI. Comparison made with unpaired Mann-Whitney U test.
Abbreviations: BRCAwt =BRCA wild-type, BRCAm = BRCA mutant, C1D1 = cycle 1 day 1, C1D15 = cycle 1 day 15, P = Patient, PR = partial response, SD = stable disease, mo = months PD = progressive disease, HRR = homologous recombination repair, tx = treatment, CI = confidence interval.
Resistance to immune checkpoint blockade
Finally, we examined baseline tumor biopsies for somatic JAK1/2 mutations as they have been shown to confer resistance to checkpoint blockade in melanoma models via impaired IFNγ signaling and downregulation of PD-L1 expression27. Four of 23 patients who had no clinical benefit were found to have at least one somatic JAK1/2 missense mutation (c.1976G>A; c.2371G>A; c.1789G>T; c.86C>T and c.397C>T), while no patients with clinical benefit had a JAK mutation. However, we did not see an association between JAK mutations and tumoral IFNγ gene expression, PD-L1 staining, or levels of IL6, which drives the JAK/signal transducer and activator of transcription (STAT) signaling pathway28. TGFβ signaling in the tumor microenvironment has also been associated with poor response to PD-1/PD-L1 blockade29. Consistent with this, RNA-seq analysis showed pre-treatment TGFB1 expression levels were significantly higher in non-responders (n=12) than responders (n=8) (median z-score 0.48 vs −0.43, p=0.017), while other pathway genes, including TGFB2 and TGFBR2, showed no significant difference.
DISCUSSION
Immunotherapy represents a paradigm shift in the treatment of various cancers although it has demonstrated modest activity in ovarian carcinoma1. Preclinical studies have suggested that PARP inhibition may increase mutational burden9 or activate the STING pathway5–8. The STING pathway is a potent activator of type I interferons (IFN) and elicits antitumor immune responses5–8,11, including increased activation of intratumoral CD4+ and CD8+ T cells, production of immune-stimulatory cytokines, and recruitment of antigen-presenting dendritic cells, thus making PARPi an attractive combination treatment strategy for the immune checkpoint inhibitors. However, in our recurrent ovarian cancer patients, we observed no significant changes in TMB or STING expression, independent of BRCAm status. We report a 14% ORR and 71% DCR, and found that the PARPi and anti-PD-L1 combination creates an immunostimulatory environment that may enhance durable anti-tumor immune responses to immune checkpoint blockade in subsets of patients.
The PARPi and anti-PD-L1 combination therapy did not show significant improvement in clinical efficacy per RECIST criteria. However, we noted one third of our patients received clinical benefit lasting longer than 6 months although they were mostly platinum-resistant and heavily pretreated, with over half having received four or more previous treatment regimens. Therefore, these findings are encouraging and suggest that improved patient selection may further enhance PARPi and anti-PD-L1 efficacy in this difficult-to-treat population, as nearly all patients with recurrent ovarian cancer ultimately develop platinum resistance and are left with limited treatment options30.
There are several ongoing clinical trials combining the PD1/PD-L1 inhibitor and PARPi or other DNA damaging agents in recurrent ovarian cancer. The results of those studies reported to date are consistent with our findings in predominantly platinum-resistant patients. In the phase 1/2 TOPACIO study, the PARPi niraparib and anti-PD-1 pembrolizumab yielded an 18% ORR and 65% DCR independent of BRCAm and HRD status18 and the phase 3 JAEVELIN Ovarian 200 study of the combination of avelumab and pegylated liposomal doxorubicin showed a 13% RR31. The 72% RR observed in the phase 2 MEDIOLA study of olaparib and durvalumab likely reflects their cohort of exclusively platinum-sensitive, PARPi-naïve BRCAm ovarian cancer patients32. Unlike the MEDIOLA study, our trial did not include an olaparib lead-in period given our predominantly BRCAwt, platinum-resistant cohort for whom PARPi monotherapy RRs are <10% and may rapidly progress while on PARPi treatment alone33. Importantly, no comprehensive biomarker studies using paired fresh tissue and blood samples have been reported to date.
Our data indicates that PARPi does not significantly increase TMB in ovarian cancer patients, regardless of BRCAm status, consistent with preclinical findings that long-term treatment with the PARPi niraparib did not increase the mutational load in BRCA1m breast carcinoma cells34. This may be due to a short exposure to PARPi and also less mutagenic effects by PARPi. Moreover, neither baseline nor change in TMB after treatment were associated with clinical response, which likely reflects the overall low mutational load in ovarian cancer26. Most trials that report a survival benefit use a threshold of approximately 10 or more somatic mutations/Mb to define high TMB26 while all patients remained <5 mutations/Mb in our study.
We found that the PARPi and anti-PD-L1 combination induces systemic immune activation, possibly STING-independent, including increased expression of IFNγ and related immunostimulatory chemokines, systemic production of TNFα and IFNγ, and tumor infiltration by lymphocytes in ovarian cancer patients. These data were not unanticipated as Fenerty et al. also reported olaparib significantly increases tumor cell sensitivity to NK cell killing and antibody-dependent cellular cytotoxicity in both BRCAwt and BRCAm prostate carcinoma cells, independent of STING expression or modulation35. Furthermore, our findings are consistent with studies of DNA-damaging chemotherapy showing apoptotic ovarian cancer cells induced by carboplatin and paclitaxel are immunogenic and enhance T cell IFNγ secretion36. IFNγ causes tumor inflammation and is associated with antitumor activity, including inhibiting tumor cell proliferation and promoting apoptosis37,38, potentially allowing for more effective immunotherapy combinations. Although IFNγ RNA expression levels were not associated with clinical benefit possibly due to small sample size, an increase in IFNγ plasma levels after treatment was associated with improved response and PFS, supporting the hypothesis that immune activation following treatment may contribute to the observed clinical benefit. Moreover, the immunoreactive molecular subtype, characterized by enhanced cytokine expression, T-cell activation, and TILs21, was associated with clinical benefit, suggesting the immune-inflamed microenvironment induced by PARPi and anti-PD-L1 combination therapy may particularly benefit patients with immunoreactive tumors at baseline.
We also speculate that immunostimulatory effects may have been negated by various resistance mechanisms, e.g., JAK1/2 mutations, TGFβ signaling, or an increase in counter-regulatory angiogenic factors like VEGFR3, resulting in modest clinical activity. JAK1/2 mutations result in loss of the anti-tumor effects of IFNγ via lack of expression of downstream signaling receptors and have been associated with primary resistance to immunotherapy due to the absence of reactive PD-L1 expression27 while TGF-β signaling counteracts anti-tumor immunity by restricting T cell infiltration29. Consistent with our multivariable Cox proportional hazards model demonstrating increased post-treatment levels of VEGFR3 were associated with worse PFS, Chen et al. reported melanoma patients who had no response to immune checkpoint inhibitors had higher on-treatment RNA expression of VEGFA thus implicating angiogenesis as a potential mechanism of resistance to immunotherapy39. Also, overexpression of angiogenic factors in the tumor microenvironment has been shown preclinically to promote immunosuppression and facilitate cancer growth and metastases40. As such, tumorigenic factors e.g., the VEGF/VEGFR pathway may act to counterbalance the immunostimulatory changes and serve as a target to further modulate the immunosuppressive microenvironment in ovarian cancer for improved efficacy of the PARPi and PD-L1 blockade combination. We are testing this hypothesis in an ongoing phase 2 trial of durvalumab in combination with olaparib and the VEGFR1-3 inhibitor cediranib in ovarian cancer (NCT02484404).
Here, we conducted the first clinical investigation into the immunomodulatory effects of olaparib and durvalumab in heavily pretreated recurrent ovarian cancer patients. Our results show the combination induces antitumor immune responses in subsets of patients via an IFNγ-induced inflamed immune microenvironment. Limitations of our study include its small size and the heterogeneous patient population, including platinum-sensitive and resistant patients, BRCAm and BRCAwt patients, although a majority of patients had BRCAwt and platinum-resistant disease. We also acknowledge that the data on STING are suggestive but not definitive as activation of STING is not dependent on increased STING levels but rather on STING function. Furthermore, only a mandatory baseline and optional cycle 1 day 15 biopsy were collected, which may not capture changes in tumoral biology that occurred at other timepoints. Finally, based on our patient population we did not include an olaparib lead-in period and are therefore unable to definitively differentiate the immunomodulatory effects of PARPi alone versus combination therapy.
In summary, we found subsets of heavily pretreated platinum-resistant patients, for whom either PARPi or immune checkpoint blockade monotherapy have shown limited activity, had a long duration of response although this study did not meet the pre-specified ORR primary endpoint. Moreover, our data indicate combination may be best suited for the treatment of recurrent ovarian cancer patients with immunoreactive subtype and addition of VEGF/VEFGR pathway blockade may be necessary to improve the efficacy of the PARPi and PD-L1 blockade combination, warranting further investigation.
Supplementary Material
Translational relevance.
PARP inhibitors (PARPis) are now integral in the treatment of ovarian cancer while clinical activity of immune checkpoint inhibitor monotherapy has been modest thus far. Preclinical data suggest addition of PARPi may create a more immunogenic tumor milieu, thus complementing the clinical activity of immune checkpoint blockade. As such, PARPis and PD-1/PD-L1 inhibitors are currently being tested in combination in a number of clinical trials. In this study, using fresh core biopsy and blood samples, we found that tumoral and peripheral IFNγ increases were associated with durable clinical benefit from combination therapy. We also noted further exploration of immunoreactive gene signatures may improve patient selection. Furthermore, our results suggest VEGF/VEGFR pathway blockade would be necessary to further modulate the immunosuppressive milieu in ovarian cancer for improved efficacy of the PARPi and anti-PD-L1 combination.
Acknowledgements
We thank Drs. Elise C. Kohn, Bernard Parker, Lori Minasian and Farah Zia, Irene Ekwede RN, Nicole Houston RN, Kathryn Trewhitt NP and Ms. Mireya Gomez for their contributions in clinic. We thank Bao Tran, Maggie Cam, and Xiongfong Chen for their expertise in generating the RNA and WES data used in this study. We also thank Drs. Kohn and Steeg for their invaluable comments concerning the manuscript. This research was supported by the Intramural Research Program of the NCI, CCR (Grant Number: #ZIA BC011525) [J-M. Lee]. Durvalumab and olaparib were supplied to the CCR, NCI under a Cooperative Research and Development Agreement between the CCR/NCI and AstraZeneca. Research funding was also provided by AstraZeneca and Stand Up To Cancer-Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant (Grant Number: SU2C-AACR-DT16-15) [E. Swisher]. Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of Stand Up To Cancer. This research was made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (DDCF Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors [E. Lampert]. This project has also been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E [E. Nichols]. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflict of interest statement:
Ashley Cimino-Mathews: Research Funding: Bristol-Myers Squibb; Consultant for: Bristol-Myers Squibb.
Elizabeth M. Swisher: Research Funding: Grants from Stand up to Cancer/American Association for Cancer Research during the conduct of the study.
Andrew B. Nixon: Research Funding: Acceleron Pharma, Amgen, AstraZeneca/MedImmune, Eureka Therapeutics, Genentech, HTG Molecular Diagnostics, Leadiant Biosciences, MedPacto Inc, Novartis, Seattle Genetics, and Tracon Pharma; Consultant for: Eli Lilly, Kanghong Pharma, GlaxoSmithKline, and Promega.
Christina M. Annunziata: Research Funding: Precision Biologics (Institution).
Janis M. Taube: Research Funding: Bristol-Myers Squibb; Consultant/Advisory Board for: Bristol-Myers Squibb, Merck and AstraZeneca.
Jung-Min Lee: Research Funding: AstraZeneca and Eli Lilly (Institution).
All others: nothing to declare.
REFERENCES
- 1.González-Martín A, Sánchez-Lorenzo L. Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: Still promising? Cancer 2019; 125(S24): 4616–22. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 3.Lee JM, Ivy SP, Kohn EC. Challenges and Opportunities for Immunotherapies in Gynecologic Cancers. Oncology (Williston Park) 2016; 30(1): 67–9. [PubMed] [Google Scholar]
- 4.O’Sullivan CC, Moon DH, Kohn EC, Lee JM. Beyond Breast and Ovarian Cancers: PARP Inhibitors for BRCA Mutation-Associated and BRCA-Like Solid Tumors. Front Oncol 2014; 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen J, Zhao W, Ju Z, et al. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res 2019; 79(2): 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding L, Kim HJ, Wang Q, et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 2018; 25(11): 2972–80 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen T, Rodriguez BL, Chen L, et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantelidou C, Sonzogni O, de Oliveira Taveira M, et al. PARP inhibitor efficacy depends on CD8+ T cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer 2018; 118(3): 312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higuchi T, Flies DB, Marjon NA, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res 2015; 3(11): 1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabanon RM, Muirhead G, Krastev DB, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest 2019; 129(3): 1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao S, Xia W, Yamaguchi H, et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin Cancer Res 2017; 23(14): 3711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017; 18(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 14.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A 2011; 108(44): 18032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390(10106): 1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JM, Cimino-Mathews A, Peer CJ, et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women’s Cancers: A Dose-Escalation, Phase I Study. J Clin Oncol 2017; 35(19): 2193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzese E, Centonze S, Diana A, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev 2019; 73: 1–9. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drew Y, Jonge M, Hong SH, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA -mutated ( gBRCA m) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecologic Oncology 2018; 149: 246–7. [Google Scholar]
- 20.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017; 127(8): 2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konecny GE, Wang C, Hamidi H, et al. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst 2014; 106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. Journal for Immunotherapy of Cancer 2016; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17(12): e542–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer 2019; 18(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev 2018; 63: 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019; 30(1): 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov 2017; 7(2): 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billing U, Jetka T, Nortmann L, et al. Robustness and Information Transfer within IL-6-induced JAK/STAT Signalling. Commun Biol 2019; 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganesh K, Massague J. TGF-beta Inhibition and Immunotherapy: Checkmate. Immunity 2018; 48(4): 626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs 2011; 71(11): 1397–412. [DOI] [PubMed] [Google Scholar]
- 31.Pujade-Lauraine E, et al. Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: Primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial Society of Gynecologic Oncology 2019. [Google Scholar]
- 32.Drew Y, Kaufman B, Banerjee S, et al. 1190PDPhase II study of olaparib + durvalumab (MEDIOLA): Updated results in germline BRCA-mutated platinum-sensitive relapsed (PSR) ovarian cancer (OC). Annals of Oncology 2019; 30(Supplement_5). [Google Scholar]
- 33.Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011; 12(9): 852–61. [DOI] [PubMed] [Google Scholar]
- 34.Poti A, Berta K, Xiao Y, et al. Long-term treatment with the PARP inhibitor niraparib does not increase the mutation load in cell line models and tumour xenografts. Br J Cancer 2018; 119(11): 1392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenerty KE, Padget M, Wolfson B, et al. Immunotherapy utilizing the combination of natural killer- and antibody dependent cellular cytotoxicity (ADCC)-mediating agents with poly (ADP-ribose) polymerase (PARP) inhibition. J Immunother Cancer 2018; 6(1): 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Feng QM, Wang Y, Shi J, Ge HL, Di W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother 2010; 59(2): 279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Kohli K, Black RG, et al. Systemic Interferon-gamma Increases MHC Class I Expression and T-cell Infiltration in Cold Tumors: Results of a Phase 0 Clinical Trial. Cancer Immunol Res 2019; 7(8): 1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol 2018; 9: 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen PL, Roh W, Reuben A, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 2016; 6(8): 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15(5): 325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.