In 2011 the American Academy of Pediatrics (AAP) and NHLBI recommended universal cholesterol testing at age 9 – 11 years, discussing 2 rationales.1 The first rationale was identification of familial hypercholesterolemia, a severe disease with a prevalence of approximately 1:300.2 The long-term safety and benefits of cholesterol-lowering medications for youth with severe hypercholesterolemia have been established.3 These known benefits increase the value of early disease identification. The second rationale was identification of less severe dyslipidemias associated with pediatric obesity that may represent a modifiable risk for cardiovascular disease.1 Before 2011, pediatric cholesterol testing was most common among children with known cardiovascular risks, particularly obesity.4 Questions remain about the role of universal testing in pediatrics and the USPSTF has not endorsed universal testing.2,5 This uncertainty may influence guideline uptake.
Prior reports examining cholesterol testing in pediatric cohorts predate the 2011 guideline, evaluate specific efforts to adopt the guideline, or were unable to evaluate other cardiovascular risk factors that may be associated with testing.6–9 This study asks whether, and to what extent, universal testing has been adopted since the 2011 guideline.
Methods and Results
This retrospective cohort study included children from 2 primary care networks (51 practices in 3 US states) that participate in the Comparative Effectiveness Research through Collaborative Electronic Reporting (CER2), an AAP-led electronic health record (EHR) consortium.10 We included children who turned 12 years old from 2011 – 2016 who had one or more visits in the primary care setting at age 6 – 8 years and at age 9 – 11 years.
The primary outcome was any total cholesterol or LDL result in the EHR that was completed in primary care for children age 9 – 11 years.
Exposures of interest were cardiovascular risks, including Body Mass Index (BMI) and blood pressure (BP). For each, we used the first measurement after age 9 years, converting values into percentiles based on age, sex, and height specific norms. We stratified BMI as <85th% (not overweight), 85th – 94th% (overweight), and ≥95% (obese). We classified BP readings >90th% as elevated. Demographic covariates included birth cohort (the year the child turned 12 years old), race/ethnicity, and sex.
The 2011 guideline specifically recommended testing at age 9 – 11 years.1 Therefore, we would not expect testing at younger ages to change in response to this recommendation. In order to assess guideline-relevant changes, we assessed testing at age 9 – 11 years versus age 7 – 8 years. To focus on screening tests, as opposed to follow-up on existing conditions or comorbidities, children who completed cholesterol testing at ages 7 – 8 were excluded from further analyses. Next, examining children turning 12 years from 2011 – 2016, we assessed the proportion of children in each BMI category who completed testing from age 9 – 11 years.
For birth cohorts turning 12 years from 2012 – 2016, we used logistic regression adjusting for demographic covariates with variances accounting for clustering at the practice level to identify whether the primary exposures of BMI or elevated BP were associated with the dependent variable of cholesterol testing at age 9 – 11 years. An interaction term between birth cohort and BMI assessed whether the association between BMI and cholesterol testing changed over time. Analyses were conducted using R (Version 3.6.0).
This study was approved by the Institutional Review Boards at the Children’s Hospital of Philadelphia and the AAP. No informed consent was required. Use of study data may be requested from the AAP by qualified researchers trained in human subjects confidentiality (contact gregorye@email.chop.edu for details about requesting data).
The final cohort for regression included 55,610 children who were primarily non-Hispanic white (54%) and non-Hispanic black (30%). Testing increased by birth cohort. In 2011, 9% of children turning 12 years had been tested, while in 2016 this proportion was 20% (p-value < 0.001). This increase was driven by increased testing at age 9 – 11 years (Figure 1a). For all birth cohorts, prevalence of testing was greatest among children with obesity (Figure 1b). However, considering the BMI distribution within the total number of tests completed, children with normal weight represented a greater proportion of those tested in later cohorts (35% in 2011 versus 52% in 2016, p-value <0.001) (Figure 1c). Rates of testing varied across practices. For children turning 12 in 2016, rates of testing ranged from 0 – 67% across the 51 included practices.
Figure 1:
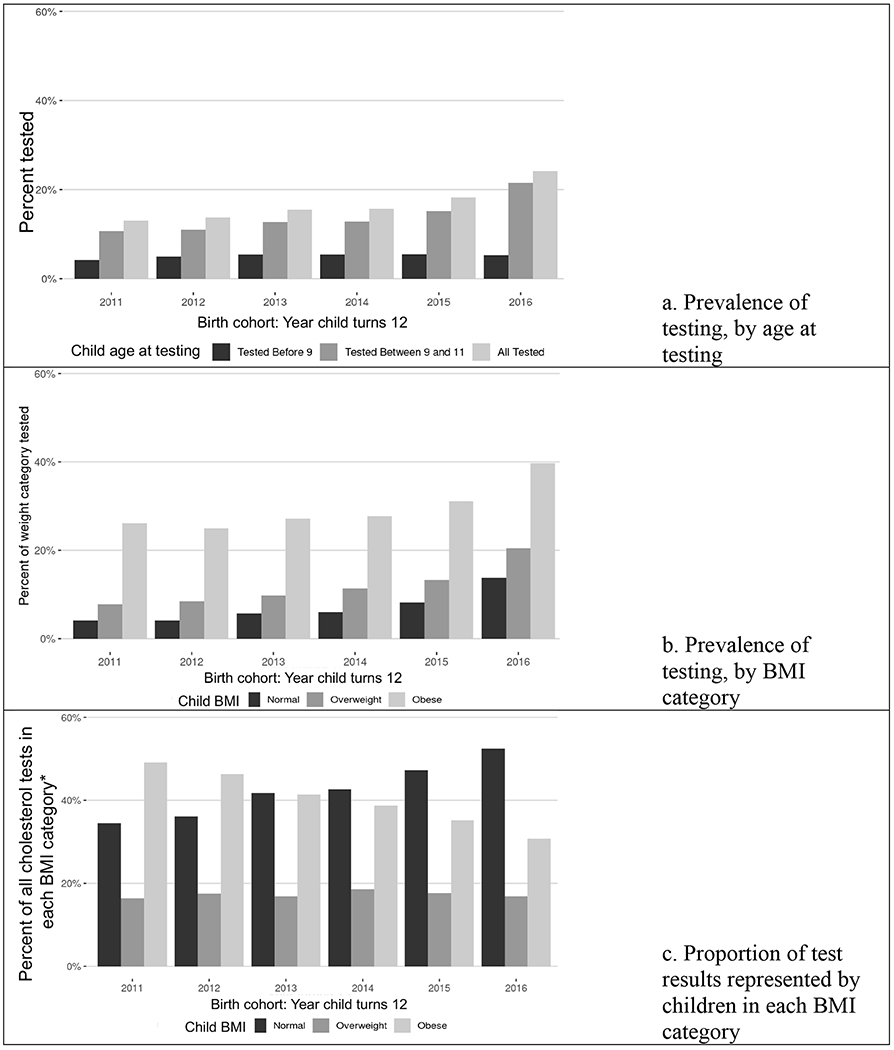
Cholesterol testing by age 12 by birth cohort (year child turns 12)
Note: Figures 1a and 1b show percent of all included children, whereas Figure C focuses on the BMI distribution within total 9 – 11 year cholesterol tests for that birth cohort. Figures 1b and 1c exclude children tested at age 7 – 8 years.
* For each year, total percent of tests across birth cohorts adds to 100%.
In logistic regression, birth cohort and BMI were both associated with increased odds of cholesterol testing (birth cohort OR 1.38, 95% CI 1.20 – 1.60; BMI 85th – 95th% OR 2.50, 95% CI 1.25 – 5.00; BMI ≥95th% OR 11.71, 95% CI 5.03 – 27.24). The interaction of birth cohort and BMI ≥95th% was associated with decreased testing, suggesting elevated BMI was less strongly associated with testing over time (OR 0.85, 95% CI 0.72 – 0.99). Elevated BP was not associated with testing (OR 0.99, 95% CI 0.86 – 1.13).
Comment
In this cohort, cholesterol testing by age 12 years increased between 2011 and 2016. Children with elevated BMI were more likely to complete testing for all birth cohorts. However, testing among children with normal BMI has become increasingly prevalent, suggesting a shift towards universal cholesterol testing in pediatrics since the 2011 recommendation.
Our study captured a diverse population across 3 states. However, our cohort was not representative of the US as a whole. In particular, Hispanic and Asian children were under-represented, and our study is limited to children seeking care at offices affiliated with academic centers. Nonetheless, our results provide insights into meaningful changes in cholesterol testing for children.
Though testing increased over time in our study population, most children in this cohort were never tested. Early medical treatment has now been shown to reduce complications associated with familial hypercholesterolemia.3 Given our findings of inconsistent testing at ages 9 – 11 years, there are likely still children who could benefit from early treatment who are not being identified in this timeframe.
Multiple factors influence the speed and consistency of guideline uptake.11 With regards to cholesterol testing, one recent report found increased testing following EHR modifications and targeted physician education.8 The USPSTF determination that there was insufficient evidence to support universal testing was issued in the last year of our study period, and may be an additional barrier to rapid implementation of universal testing. However, consistent implementation of universal testing could benefit children in need of early management, while providing further data about outcomes related to a universal testing approach.
Acknowledgements:
We thank the CER2 Consortium Research Team.
Sources of funding
Our project was supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services under grant number R40MC24943 “Primary Care Drug Therapeutics CER in a Pediatric EHR Network,” and grant number UB5MC20286 “Pediatric Primary Care for EHR Network for CER.” Support was also provided by the Agency for Health Research and Quality (AHRQ) under grant number P30HS021645: “National Center for Pediatric Practice Based Research and Learning” and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under the Best Pharmaceuticals for Children Act. Additional infrastructure funding was provided by the American Academy of Pediatrics. The content and conclusions are those of the authors and should not be construed as the official policy or position of, nor should any endorsements be inferred by AAP, HRSA, AHRQ, HHS, NICHD, or the US Government.
Footnotes
Disclosures: None
References
- 1.Daniels SR, Benuck I, Christakis DA. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and ad. Pediatrics. 2011;128:S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, Gillman MW, Kemper AR, Krist AH, Kurth AE, Lendefeld CS, LeFevre M, Mangione CM, Owens DK, Philips WR, Phipps MG, Pignone MP, Siu AL. Screening for Lipid Disorders in Children and Adolescents. JAMA. 2016;316:625–33. [DOI] [PubMed] [Google Scholar]
- 3.Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med. 2019;381:1547–1556. [DOI] [PubMed] [Google Scholar]
- 4.Vinci SR, Rifas-Shiman SL, Cheng JK, Mannix RC, Gillman MW, de Ferranti SD. Cholesterol testing among children and adolescents during health visits. Jama. 2014;311:1804–1807. [DOI] [PubMed] [Google Scholar]
- 5.Gregory EF, Miller JM, Wasserman RC, Seshadri R, Rubin DM, Fiks AG. Routine Cholesterol Tests and Subsequent Change in BMI Among Overweight and Obese Children. Acad Pediatr. 2019;19:773–779. [DOI] [PubMed] [Google Scholar]
- 6.Zachariah JP, McNeal CJ, Copeland LA, Fang-Hollingsworth Y, Stock EM, Sun FF, Song JJ, Gregory ST, Tom JO, Wright EA, VanWormer JJ, Cassidy-Bushrow AE. Temporal trends in lipid screening and therapy among youth from 2002 to 2012. J Clin Lipidol. 2015;9:S77–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DP, Davis S, Matches S, Shah D, Leung-Pineda V, Mou M, Hamilton L, McNeal CJ, Bowman WP. Universal cholesterol screening of children in community-based ambulatory pediatric clinics. J Clin Lipidol. 2015;9:S88–S92. [DOI] [PubMed] [Google Scholar]
- 8.DeSantes K, Dodge A, Eickhoff J, Peterson AL. Improving Universal Pediatric Lipid Screening. J Pediatr. 2017;188:87–90. [DOI] [PubMed] [Google Scholar]
- 9.Sriram S St. Sauver JL, Jacobson DJ, Fan C, Lynch BA, Cristiani V, Kullo IJ, Lteif AN, Kumar S. Temporal trends in lipid testing among children and adolescents: A population based study. Prev Med Reports. 2017;8:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiks AG, Grundmeier RW, Steffes J, Adams WG, Kaelber DC, Pace WD, Wasserman RC, Comparative Effectiveness Research Through a Collaborative Electronic Reporting Consortium (CER2). Pediatrics. 2015;136:e215–24. [DOI] [PubMed] [Google Scholar]
- 11.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why Don’t Physicians Follow Clinical Practice Guidelines? A Framework for Improvement. JAMA. 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]


