Abstract
Uterine infection is associated with infertility in women and dairy cows, even after the resolution of infection. However, the mechanisms causing this persistent infertility are unclear. Here, we hypothesized that induced endometritis in non-lactating dairy cows would reduce the developmental competence of oocytes. Non-lactating Holstein cows received an intrauterine infusion of endometrial pathogenic bacteria (Escherichia coli and Trueperella pyogenes; n = 12) or vehicle control (n = 11) on day 2 of the estrous cycle. Bacterial infusion increased expression of endometrial inflammatory mediators, and a mucopurulent discharge in the vagina confirmed the establishment of endometritis. Oocytes were collected by transvaginal ultrasound-guided ovum pickup on days 2, 24, 45, and 66 following infusion and subjected to in vitro fertilization and embryo culture. Bacterial infusion resulted in fewer cleaved oocytes developing to morulae compared to vehicle-infused controls (30.7 versus 45.0%), with the greatest effect observed in oocytes collected on day 24. Development to morula was inversely correlated with endometrial expression of IL6 on day 6. The expression of genes associated with embryo quality did not differ significantly between morulae from bacteria-infused and control cows. Artificial insemination 130 days after intrauterine infusion resulted in normal, filamentous embryos that produced interferon tau 16 days after conception in both infusion groups. This model of experimentally induced uterine infection successfully resulted in endometritis and a reduction in the proportion of oocytes that developed to morulae following in vitro fertilization. In conclusion, endometritis reduced the capacity of oocytes to develop to morulae.
Keywords: oocyte, inflammation, female infertility, embryo, endometritis
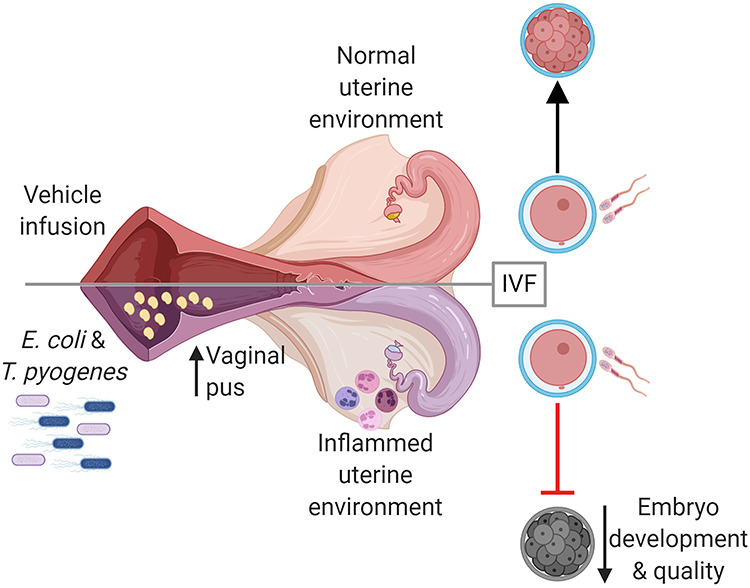
Induction of uterine infection has a long-term impact on the oocyte, reducing the capacity of the oocyte to develop to an embryo in dairy cows.
Graphical Abstract
Graphical Abstract.
Introduction
Uterine infections of women and dairy cows are associated with reduced fertility [1, 2], yet the mechanisms responsible for persistent infertility are unclear. An estimated 3.6 million cases of gonorrhea or chlamydia infections occur in women annually in the United States [3], of which 10% cause pelvic inflammatory disease [4]. Bacterial infections of the reproductive tract and pelvic inflammatory disease both result in uterine inflammation [2, 5]. Each case of pelvic inflammatory disease costs approximately $3000 and results in high rates of hospitalization [6]. Similarly, bacterial infection of the postpartum uterus is ubiquitous in dairy cows, with up to 40% of cows developing a clinical uterine disease [1, 7]. Cows diagnosed with postpartum uterine disease are less likely to become pregnant and are more likely to abort [8]. Uterine disease in cows costs approximately $900 million annually in the United States due to treatment cost, loss of milk production, infertility, increased culling, and cost of replacement cows [1, 9].
Although infections occur in the uterus, ovarian signaling and function are altered in cattle with active uterine infection. Cows with uterine disease have slower follicle growth, impaired ovulation, and delayed and irregular ovarian cyclicity [10–12]. Interestingly, infertility in cows is evident even after resolution of disease, as many cows remain unable to conceive [13–15].
The Gram-negative bacteria, Escherichia coli, and Gram-positive bacteria, Trueperella pyogenes, are common pathogens that cause clinical endometritis in dairy cows [16]. Outer membrane components of Gram-negative bacteria, including lipopolysaccharide (LPS), are detectable in follicular fluid, and follicular fluid LPS concentrations are correlated with the severity of uterine inflammation [17, 18]. Granulosa cells produce an inflammatory response to LPS and other bacterial components in vitro, and uterine infection changes the transcriptome of granulosa cells long after the clearance of infection [17, 19]. Oocytes cultured in the presence of bacterial components have an increased frequency of meiotic failure [13] and a decreased capacity to develop to the blastocyst [14]. Oocytes are a finite resource in the ovary and require in excess of 100 days to develop from the primordial follicle until ovulation [20, 21]; this allows for the intriguing possibility that oocytes could be affected during uterine infection and bear prolonged perturbations that could compromise their quality and affect fertility long after the resolution of infection.
Lactation, negative energy balance (when the metabolic energy required for maintenance and lactation exceeds the energy available from the diet), uterine environment, and common postpartum diseases can influence fertility of the postpartum dairy cow. To disentangle the effects of uterine infection on oocyte quality from other postpartum factors, we experimentally induced endometritis in non-lactating dairy cows and collected oocytes to assess their capacity to develop to morulae following in vitro fertilization and embryo culture. We hypothesized that induced endometritis in non-lactating dairy cows would reduce the competence of oocytes to develop into embryos following in vitro fertilization and embryo culture. We aimed to establish infection in non-lactating cows using pathogenic bacteria, determine the impact of uterine infection on oocyte quality and subsequent embryo development, and assess the ability of cows to conceive following artificial insemination.
Materials and methods
The University of Florida Institutional Animal Care and Use Committee approved all animal procedures (protocol number 201508884). The experiment was conducted from February to August 2018 at the University of Florida Dairy Research Unit.
Experimental protocol and establishment of uterine infection
Establishment of endometritis followed the protocol of Piersanti et al. [22], with minor modifications (Figure 1). Twenty-three 2-year-old first lactation Holstein cows were enrolled in the study. Cows were free of clinical disease following parturition (including uterine disease) prior to enrollment. At least 38 days prior to the beginning of the study, cows were vaccinated against bovine viral diarrhea, infectious bovine rhinotracheitis, parainfluenza, bovine respiratory syncytial virus, and multiple serovars of Leptospira (Bovi-Shield Gold FP 5 VL5 HB; Zoetis, Parsippany, NJ) and dewormed using moxidectin (Cydectin; Bayer HealthCare, LLC, Animal Health Division, Shawnee Mission, KS). Lactation was ended within 67 days of calving and at least 45 days prior to the initiation of the study by a final milking and intramammary treatment with ceftiofur hydrochloride (Spectramast, Zoetis), followed by a teat sealant (Orbeseal, Zoetis). No cows developed mastitis during the study period. Cows were maintained on pasture and fed a total mixed ration daily with free access to water. Cows were blocked by days postpartum and divided into two cohorts consisting of two infusion groups (vehicle or bacteria intrauterine infusion, see below). Random assignment to either treatment group was performed for each block by random number generation in Microsoft Excel.
Figure 1.
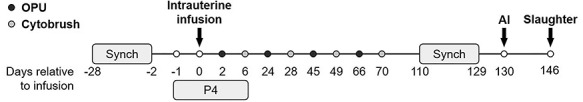
Timeline of major experimental events. Estrous cycles were synchronized with gonadotrophin-releasing hormone and prostaglandin F2α prior to intrauterine infusion of either LB broth vehicle medium (vehicle; n = 11) or pathogenic E. coli and T. pyogenes in LB broth (bacteria; n = 12) on experimental day 0. Major events include oocyte pickup (OPU,  ), endometrial cytobrush (
), endometrial cytobrush ( ) sampling, artificial insemination (AI), progesterone (P4) administration, and slaughter. Timeline is not drawn to scale.
) sampling, artificial insemination (AI), progesterone (P4) administration, and slaughter. Timeline is not drawn to scale.
Estrous cycles of cows were synchronized (Figure 1) using 100 μg GnRH (gonadorelin diacetate tetrahydrate; OvaCyst, Bayer) i.m., followed by 25 mg prostaglandin (PG) F2α (dinoprost tromethamine; Prostamate, Bayer) i.m. 7 days later, followed by GnRH after 3 days and 10 days, PGF2α 7 days later, and another, final GnRH injection 56 h following PGF2α to stimulate ovulation [23]. Progesterone (200 mg) in corn oil (Sigma-Aldrich, St. Louis, MO) was administered i.m. to cows daily starting on the final day of GnRH administration and continued for 7 days. On the day of intrauterine infusion (experimental day 0), cows received an epidural anesthetic of 60 mg lidocaine hydrochloride 2% (Aspen Veterinary Resources, Greeley, CO) injected into the intercoccygeal intervertebral space. External genitalia were cleaned with 1% Virkon solution (DuPont, Wilmington, DE), followed by 1% chlorohexidine solution (Aspen Veterinary Resources) and 70% ethanol. A sheathed Neilson catheter (450 mm; Supplies for Farmers, Lincolnshire, UK) was introduced transvaginally into the reproductive tract and guided into the uterine body via rectal palpation. Once in the uterine body, the sheath was retracted to expose the catheter port, which was rotated three times against the endometrial lining to debride the endometrium prior to intrauterine infusion. Bacteria-infused cows (n = 12) received 10 mL of Luria–Bertani (LB) broth containing 5.05 × 107 CFU/mL E. coli MS499 and 10 mL of LB containing 3.65 × 107 CFU/mL of T. pyogenes MS249, followed by 10 mL of LB to flush the catheter. Vehicle-infused cows (n = 11) received an intrauterine infusion of 30 mL of LB broth.
Propagation of pathogenic E. coli and T. pyogenes for intrauterine infusion
Bacterial cultures were prepared as previously described by Piersanti et al. [22]. Briefly, E. coli MS499 was cultured from frozen glycerol stocks on LB agar [24]. The day before intrauterine infusion, a single bacterial colony was picked from the plate and inoculated into LB broth containing 1% tryptone, 0.5% yeast extract, and 1% sodium chloride. The culture was incubated overnight at 37 °C with shaking at 200 rpm. In parallel, T. pyogenes MS249 was grown from frozen glycerol stocks on trypticase soy blood agar at 37 °C for 48 h [25]. The day prior to intrauterine infusion, a single bacterial colony was selected and inoculated into Bacto Brain Heart Infusion broth (BHI; Fisher Scientific, Pittsburgh, PA) supplemented with 5% fetal bovine serum (Fisher Scientific) and cultured overnight at 37 °C with shaking at 200 rpm. Bacterial growth was monitored by measuring optical density at 600 nm. A final preparation of 5.05 × 107 CFU/mL E. coli or 3.65 × 107 CFU/mL of T. pyogenes MS249 was diluted in sterile LB broth and loaded into 10-mL syringes for infusion. To measure the final concentrations of bacterial cells infused, tenfold serial diluted bacterial cultures were plated on agar and grown at 37 °C to count CFU. Sterile LB broth for flushing catheters and vehicle infusions was loaded into 10-mL syringes. Infusions were transported to the farm on ice.
Evaluation of uterine infection
Rectal temperature was measured (AG-102 thermometer, AG-Medix, Mukwonago, WI) between 7 AM and 9 AM on day −2, 1, 3, 5, and 10. Vaginal mucus samples were collected and examined using a clean, gloved hand on days −2, 3, 5, 10, 14, 20, 28, 49, and 70. Briefly, the vulva was cleaned with 70% alcohol and dried with paper towels. Mucus was collected by introducing a clean, gloved hand into the vagina and retrieving any contents from the lateral, dorsal, and ventral vaginal walls. Mucus was graded on a scale of 0–4 according to Sheldon et al. [1], where grade 0 was no mucus or clear/translucent mucus; grade 1 was mucus containing flecks of white or off-white pus; grade 2 was mucus containing ≤50% white or off-white mucopurulent material; grade 3 was mucus containing >50% purulent material; and grade 4 was mucus containing >50% purulent material and dark brown blood.
Endometrial cytobrush samples were collected on day 6, 28, 49 or 50, and 69 or 70 (Figure 1). Briefly, external genitalia were cleaned with 1% chlorohexidine solution followed by 70% ethanol. Guided by rectal palpation, the cytobrush tool (Medscand Medical, Cooper Surgical, Trumbull, CT) contained within a metal sheath and covered in a plastic chemise was introduced into the vagina and passed through the cervix. The plastic chemise was retracted over the tool exposing the brush to the endometrium. The brush was rotated three times to collect endometrial cells before being retracted into the metal sheath and removed from the cow. The cytobrush was smeared on a clean, glass slide for cytology, and then the brush was snap-frozen in liquid nitrogen and stored at −80 °C until used for real-time RT-PCR. For cytology, slides were air dried and stained with Rapid-Chrome Kwik-Diff (Thermo Fisher Scientific, Waltham, MA) to assess the proportion of polymorphonuclear cells present. A total of 200 cells were counted at both 10× and 40× magnification on a Nikon Optiphot microscope (Nikon Instruments, Melville, NY), and the proportion of polymorphonuclear cells was determined.
Blood was collected via coccygeal venipuncture on days −2, 5, and 145 into evacuated tubes containing lithium heparin (Becton Dickinson, Franklin Lakes, NJ) for plasma collection or Z serum clot activator (Greiner Bio-One, Monroe, NC) for serum collection and centrifuged for 10 min at 2400 × g at room temperature, aliquoted and stored at −20 °C. Plasma haptoglobin (Life Diagnostics, Inc., West Chester, PA) and serum progesterone (DRG International, Springfield Township, NJ) were quantified using commercially available ELISAs according to the manufacturer’s instructions. The haptoglobin and progesterone intra-assay coefficient of variation was 4.2 and 2.9%, and the limit of detection was 3.91 and 0.3 ng/mL, respectively. Serum anti-Müllerian hormone (AMH) was quantified by a commercial laboratory (Ansh Labs, Webster, TX). The intra-assay coefficient of variation was 1.5% for high-concentration (1721 pg/mL), 2.8% for medium-concentration (687 pg/mL), and 0.4% for low-concentration (336 pg/mL) bovine serum AMH controls. The inter-assay coefficient of variation was 9.3, 7.4, and 5.7% for high-, medium-, and low-concentration controls, respectively. The limit of detection for AMH was 22 pg/mL.
Follicle aspiration for oocyte pickup and follicle ablation
To assess the impact of endometritis on oocytes over time, transvaginal ultrasound-guided oocyte pickup was performed on experimental days 2, 24, 45, and 66 (Figure 1). Between each oocyte pickup procedure, dominant follicle ablation was performed 4 days prior to oocyte pickup to maximize the number of oocytes collected and facilitate estrous synchronization. In brief, cows were restrained and received epidural anesthetic in the intercoccygeal intervertebral space using 3 mg xylazine (AnaSed; Akorn, Lake Forest, IL) and 60 mg of lidocaine hydrochloride 2%. External genitalia were cleaned with 1% chlorhexidine solution, followed by 70% ethanol. The vagina was rinsed three times by lavage, first using 100 mL of 0.5% chlorhexidine solution and then twice with 100 mL sterile 0.9% saline. The oocyte pickup instrument, including a 7.5-MHz convex ultrasound probe (Choice Medical, South Pasadena, FL) covered in a disposable chemise, was introduced into the vagina using sterile lubricant. The ovary was visualized by ultrasound (Aloka SSD-500, Hitachi Healthcare Americas, Twinsburg, OH), and an 18-gauge needle and vacuum were employed for dominant follicle (>8 mm) ablation or follicle (<8 mm) aspiration for oocyte pickup. Follicular aspirates from dominant follicle (>8 mm) ablation were discarded. Follicle aspirates (<8 mm) were collected into ovum pickup medium (IVF Bioscience, Falmouth, UK) and subsequently filtered and rinsed using an embryo flush filter (Watanabe Tecnologia Aplicada, Brazil). Oocytes were isolated and washed in three drops of 39 °C HEPES-buffered oocyte maturation medium (IVF Bioscience) and matured in glass vials containing HEPES-buffered maturation medium at 38.5 °C for 24 ± 3 h. All procedures for oocyte maturation, fertilization, and embryo culture were performed keeping oocytes and subsequent embryos from each cow at each time point as an individual group.
In vitro fertilization and embryo culture
Following oocyte maturation, groups of 1 to 12 oocytes were transferred to 100 μL drops of BO-IVF medium overlaid with light mineral oil (IVF Bioscience). Oocytes were fertilized with sperm from the sire Monument 014HO04784 (Select Sires, Plain City, OH) to yield a final concentration of 2 × 106 sperm/mL and placed in a humidified incubator at 38.8 °C with 6% O2, 6% CO2, and balanced N2. After 22 ± 2 h of fertilization, oocytes were rinsed in oocyte wash medium (IVF Bioscience), and cumulus cells were removed by mechanical pipetting (CooperSurgical, Trumbull, CT). Subsequently, oocytes were moved to 100 μL drops of BO-IVC embryo culture medium (IVF Bioscience) overlaid with light mineral oil. Embryos were cultured in groups of 1 to 12 at 38.8 °C in a humidified environment of 6% O2 and 6% CO2 and balanced N2. Embryos were assessed for cleavage 3.5 days after fertilization and development to morula 6 days after fertilization. Six days after fertilization, morulae from each individual cow were washed three times in DPBS containing 0.2% polyvinylpyrrolidone, the zona pellucida removed in Tyrode acid solution (Sigma-Aldrich) and washed three times in DPBS before snap freezing in liquid nitrogen and storage at −80 °C. Embryo development was halted at the morula stage, prior to differentiation to inner cell mass and trophectoderm, in order to analyze a homogenous cell population.
Fixed-time artificial insemination
Beginning at experimental day 110, estrous cycles of cows were synchronized for fixed-time artificial insemination (Figure 1). The synchronization protocol was initiated using PGF2α, followed by injection of GnRH 48 h later and another GnRH injection 6 days later, two subsequent PGF2α injections 24 h apart, 1 week after the previous GnRH injection, and a final GnRH injection 12 h before insemination. On experiment day 130, all cows were inseminated with 500 μL of semen from the sire Passat 7HO12659 (Select Sires). Sixteen days following insemination, cows were euthanized on experimental day 146 (details below) to recover embryos and collect reproductive tissues.
Postmortem tissue collection
Cows were euthanized by captive bolt and exsanguination 146 days after intrauterine infusion (16 days following insemination; Figure 1). Reproductive tracts were collected, placed on ice, and processed within 1 h of slaughter. Excess tissue was trimmed and the reproductive tract (cervix, uterus, oviduct, and ovaries) was weighed. The uterine horn ipsilateral to the side of ovulation was identified by the corpus luteum in the ovary. The ovaries and oviducts were removed for further processing. The uterus was clamped with hemostats near the uterotubal junction and at the uterine bifurcation. The ipsilateral horn was flushed with 20 mL of 0.9% saline to collect uterine fluid and potential embryos. An additional three uterine flushes were performed to maximize the potential of recovering an embryo. The first uterine flush was examined for the presence of an embryo prior to centrifugation for 10 min at 1000 × g to remove debris, snap-frozen in liquid nitrogen, and stored at −80 °C. Embryo morphology was recorded prior to sampling the distal portion of trophectoderm using clean 20-gauge needles. Trophectoderm samples were washed twice in PBS, snap-frozen in liquid nitrogen, and stored at −80 °C.
Interferon tau quantification in uterine fluid
Interferon tau (IFNT) content of uterine fluid was quantified by ELISA (Bishop JV and Hansen TR, unpublished in collaboration with a Biopharma Company). Briefly, glycosylated recombinant bovine IFNT was purified from cultures of human HEK cells that were transformed with bovine IFNT cDNA (bTP509) and used to generate polyclonal antibodies in goats (#51; 3.5 μg/mL) and in rabbits (#5670; 9.6 μg/mL). These antibodies were used as capture and biotinylated detection antibodies, respectively, in a sandwich ELISA. The ELISA had a detection range of 7.8–500 pg/mL and limit of detection of 61 pg/mL. The intra-assay coefficient of variation was 0–1.4% for high-concentration (500 pg/mL), 0–3.9% for medium-concentration (100 pg/mL), and 0.9–2.2% for low-concentration (20 pg/mL) recombinant bovine IFNT controls. The inter-assay coefficient of variation was 1.1, 1.6, and 1.8% for high-, medium-, and low-concentration controls, respectively. The ELISA specifically detects IFNT and does not cross-react with IFNω, IFNα/β, or IFNγ. Samples were assayed undiluted or at dilutions in steer serum of 1:10, 1:100, 1:1000, 1:5000, or 1:10 000 to detect IFNT in the linear range of the assay. Operators were blind to the treatment of samples being assayed. Samples below the limit of detection were assigned a concentration of 61 pg/mL for statistical analysis.
RNA extraction and real-time RT-PCR
Total RNA was extracted from cytobrush and trophectoderm samples using the Trizol method (Life Technologies, Carlsbad, CA). Total RNA was extracted from morula that were pooled from a single cow at each time point using the RNeasy Micro Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Reverse transcription was performed using the Verso cDNA synthesis kit (Thermo Fisher Scientific). Morula cDNA underwent additional selective pre-amplification using SsoAdvanced PreAmp Supermix (Bio-Rad, Hercules, CA) prior to real-time RT-PCR.
Primers were designed using the NCBI database (Table 1). Amplification efficiency for each primer pair was evaluated and met MIQE guidelines of r2 > 0.98 and efficiency of 90–110% [26]. Real-time RT-PCR was performed in duplicate using a two-step PCR protocol for cytobrush samples and a three-step protocol for morula and trophectoderm. Each 20 μL reaction consisted of 500 nM of each forward and reverse primer and iTaq Universal SYBR Green Master Mix (Bio-Rad) and cDNA. A Bio-Rad CFX Connect light cycler (Bio-Rad) was employed with an initial denaturation step at 95 °C for 30 s followed by 40 cycles at 95 °C for 5 s, specific annealing temperature (Table 1) for 10 s, and final extension at 60 °C for 30 s. A no template negative control was used in place of cDNA to determine nonspecific amplification for each primer pair. Relative expression for genes of interest was calculated using the 2−ΔCt method relative to selected housekeeping genes (GAPDH for endometrial cytobrush data; geometric mean of GAPDH, RPL19, and SDHA for morula data; and geometric mean of ACTB and GAPDH for trophectoderm data). Reference gene expression was stable across experimental treatments (P > 0.05).
Table 1.
Primer sequence and annealing conditions used for real-time RT-PCR.
| Gene Symbol | Primer sequence | Annealing temperature (°C) | Accession number |
|---|---|---|---|
| ACTB | 5′—TTGGCCTTAGGGTTCAGGG | 60 | NM_173979.3 |
| 3′—CAGAAGCACTCGTACGTGGG | |||
| AKR1C4 | 5′—TGCAACCAGGTGGAATGTCA | 60 | NM_181027.2 |
| 3′—ACCCATTCTTTTAGTCGTTGGGA | |||
| BAX | 5′ – CAGGGTGGTTGGGACGG | 60 | NM_173894.1 |
| 3′ – CTTCCAGATGGTGAGCGAGG | |||
| BCL2 | 5′ – GAGTTCGGAGGGGTCATGTG | 60 | NM_001166486.1 |
| 3′ – ACAAAGGCGTCCCAGCC | |||
| CDKN1C | 5′ – GCCTCTCATCTCCGACTTCT | 60 | NM_001077903.2 |
| 3′ – CCCAGGAACCTCGTTCGAC | |||
| CXCL8 | 5′ – GCAGGTATTTGTGAAGAGAGCTG | 60 | NM_173925.2 |
| 3′ – CACAGAACATGAGGCACTGAA | |||
| DNMT3A | 5′ – CCATGTACCGCAAGGCTATCTA | 60 | XM_024998.68.1 |
| 3′ – CCTGTCATGGCACATTGGAA | |||
| GAPDH | 5′ – AGGTCGGAGTGAACGGATTC | 60 | NM_001034034.2 |
| 3′ – ATGGCGACGATGTCCACTTT | |||
| HSPA1A | 5′ – GACAAGTGCCAGGAGGTGATTT | 60 | NM_203322.3 |
| 3′ – CAGTCTGCTGATGATGGGGTTA | |||
| IFNT2 | 5′ – TCCATGAGATGCTCCAGCAGT | 60 | NM_001015511.4 |
| 3′ – TGTTGGAGCCCAGTGCAGA | |||
| IGF2R | 5′ – CAGGTCTTGCAACTGGTGTATGA | 60 | NM_174352.2 |
| 3′ – TTGTCCAGGGAGATCAGCATG | |||
| IL1B | 5′ – CTTCATTGCCCAGGTTTCTG | 60 | NM_174093.1 |
| 3′ – CAGGTGTTGGATGCAGCTCT | |||
| IL6 | 5′ – ATGACTTCTGCTTTCCCTACCC | 60 | NM_173923.2 |
| 3′ – GCTGCTTTCACACTCATCATTC | |||
| PPARG | 5′ – ATTATTCTCAGTGGAGACCGCC | 60 | NM_181024.2 |
| 3′ – CAAGGCTTGCAGCAGATTGT | |||
| PTGES | 5′ – GCTGCGGAAGAAGGCTTTTG | 60 | NM_174443.2 |
| 3′ – AAAGCCCAGGAACAGGAAGG | |||
| PTGS2 | 5′ – CGTGAAAGGCTGTCCCTTTA | 62 | NM_001105323.1 |
| 3′—ATCTAGTCCAGAGTGGGAAGAG | |||
| PTPRC | 5′ – CTCGATGTTAAGCGAGAGGAAT | 56 | NM_001206523.1 |
| 3′—TCTTCATCTTCCACGCAGTCTA | |||
| RPL19 | 5′ – ATGCCAACTCCCGCCAGCAGAT | 60 | NM_001040516.2 |
| 3′ – TGTTTTTCCGGCATCGAGCCCG | |||
| SDHA | 5′ – GGAACACTGACCTGGTGGAG | 60 | NM_174178.2 |
| 3′ – GGAACACTGACCTGGTGGAG | |||
| SLC2A1 | 5′ – AGCGTCATCTTCATCCCAGC | 60 | NM_174602.2 |
| 3′ – AGCTTCTTCAGCACGCTCTT | |||
| TNF | 5′ – CACATACCCTGCCACAAGGC | 62 | NM_173966.3 |
| 3′ – CTGGGGACTGCTCTTCCCTCT |
Statistical analysis
Rectal temperature, haptoglobin, polymorphonuclear cell number, progesterone, AMH, gene expression, and IFNT were analyzed using SPSS v25 (IBM Corporation, Armonk, NY). Data were analyzed with a generalized linear mixed model with repeated measures (if applicable) and autoregressive covariance structure. The fixed effects of treatment, day, and the interaction were analyzed using pairwise comparisons. Analysis of treatment combined all days and only studied an effect of bacterial infusion, while analysis of day combined treatments and only assessed effect of each day regardless of treatment. Rectal temperature and haptoglobin each had a pre-infusion data point (day −2) which was used as a covariate. Gene expression and haptoglobin data were log-transformed for normality. Vaginal mucus grade, oocyte cleavage, and morula development data were analyzed using logistic regression with Poisson and binomial distribution, respectively, with the GLIMMIX procedure in SAS (SAS Institute, Cary, NC). Cow within treatment was considered a random effect, and fixed effects of treatment, day, and the interaction were analyzed. Carcass weight, uterine weight, corpus luteum diameter, and number of embryos collected at slaughter were analyzed using the two-tailed t-test function in GraphPad Prism 7.04 (GraphPad Software, La Jolla, CA). Statistical significance was set at P ≤ 0.05.
Results
Establishment of uterine infection
Intrauterine infusion of bacteria induced clinical endometritis, as determined by an increased vaginal mucus grade compared to vehicle infusion (P ≤ 0.05; Figure 2A). Although the proportion of cows with polymorphonuclear cells present in uterine cytology samples on day 6 was not different between treatments, polymorphonuclear cells were detected in 5 of 12 bacteria-infused cows and only 1 of 11 vehicle-infused cows (P > 0.05; Figure 2B). Bacteria-infused cows did not have elevated rectal temperatures compared to vehicle-infused cows, and no cows exhibited fever (>39.5 °C, P > 0.05; Figure 2C). Circulating haptoglobin concentrations on day 5 were not significantly increased by bacterial infusion compared to vehicle infusion (P > 0.05; Figure 2D), but the four highest concentrations were all in the bacterial infusion group. Together these data show that clinical endometritis was induced by bacterial infusion without causing systemic disease.
Figure 2.
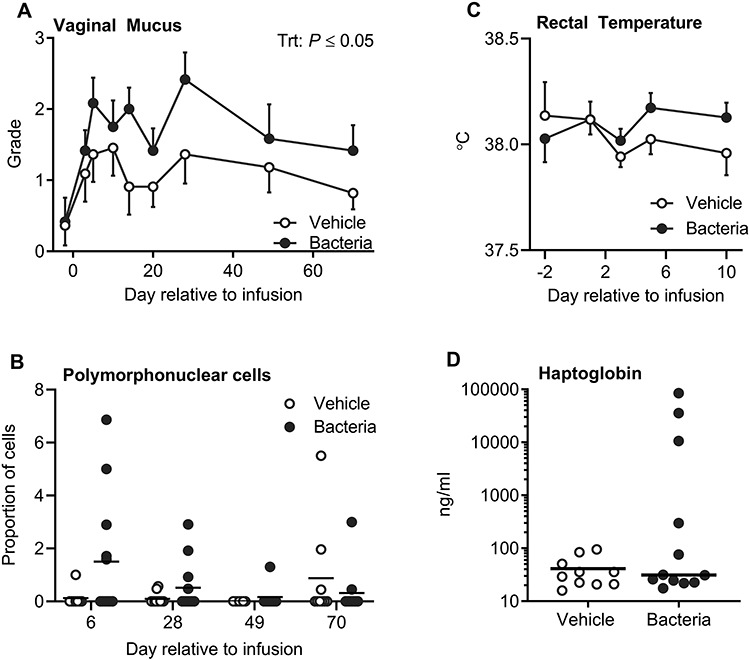
Establishment and quantification of uterine disease. Vaginal mucus (A) was collected and graded on a scale of 0 to 4 based on the presence of mucopurulent discharge. Data are the mean grade ± SEM. The proportion of polymorphonuclear cells in cytological samples (B) was assessed in a total of 200 cells per cow. Each dot represents a cow and the solid line represents the mean. Rectal temperatures (C) are displayed as mean ± SEM. Plasma haptoglobin (D) was evaluated on day 5 relative to infusion, and each dot represents an individual cow and the solid line represents the mean.
Endometrial inflammation was evaluated using samples collected on days 6, 28, 49, and 70 (Figure 3). Expression of endometrial CXCL8, IL1B, IL6, PTGS2, and TNF was increased (P ≤ 0.05; Figure 3B–D, F, and G) on day 6 in bacteria-infused cows compared to vehicle-infused controls. Expression of endometrial IL1B and PTGS2 remained increased on day 28 in bacteria-infused cows compared to vehicle-infused controls (P ≤ 0.05; Figure 3C and F). The expression of endometrial TNF was increased in bacteria-infused cows compared to vehicle controls overall (P ≤ 0.05; Figure 3H), while AKR1C4, CXCL8, IL1B, IL6, PTGES, PTGS2, PTPRC, and TNF expression was affected by day relative to infusion (P ≤ 0.05; Figure 3A–H), and CXCL8, IL6, and PTGS2 expression was affected by the interaction between treatment and day relative to infusion (P ≤ 0.05; Figure 3B, D, and F). The expression of CXCL8, IL1B, IL6, PTGES, and TNF was different on day 6 compared with days 28, 49, and 70, while PTGES, PTGS2, PTPRC, and TNF expression was different on day 28 compared to days 49 and 70. The expression of PTGS2 was different on day 6 compared to day 28, while expression of AKR1C4 was different on days 6 and 28 compared to day 70. Finally, the expression of PTPRC was different on day 6 compared to days 49 and 70, while expression of IL1B on day 70 was different compared to days 28 and 49. These findings provide further evidence of endometritis in the bacteria-infused cows.
Figure 3.
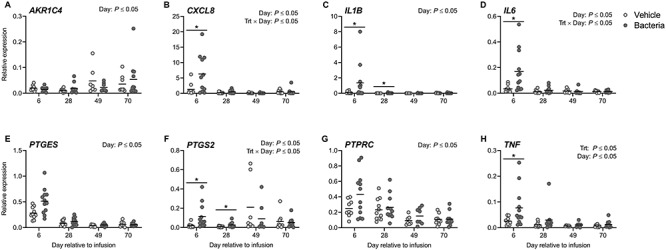
Endometrial expression of inflammatory mediators following intrauterine infusion. Expression of AKR1C4 (A), CXCL8 (B), IL1B (C), IL6 (D), PTGES (E), PTGS2 (F), PTPRC (G), and TNF (H) in cytobrush samples was evaluated by real-time RT-PCR. Data are presented as expression relative to GAPDH. Each dot represents a single cow and the solid line indicates the mean. Comparisons between treatments at a given day are indicated by * when P ≤ 0.05.
Developmental competence of oocytes following in vitro fertilization
Oocytes were collected from cows by aspiration of follicles (<8 mm) on days 2, 24, 45, and 66. There was no impact of intrauterine infusion on the total number of oocytes collected (vehicle: n = 438, vs. bacteria: n = 493; P > 0.05). There was variability in the number of oocytes (range 1 to 26) collected from each cow at any given time point, with an average of 10.7 ± 1.0 oocytes collected from vehicle-infused cows and 10.7 ± 0.9 oocytes collected from bacteria-infused cows at each time point (Supplementary Figure 1). Following IVF, the overall cleavage rate of oocytes on day 3.5 postfertilization was 62.9 ± 2.2% (Figure 4A), and there was no difference in oocyte cleavage rate between treatment groups (bacteria: 63.5 ± 3.3%, vehicle: 62.3 ± 3.0%, respectively; P > 0.05). However, the day of oocyte collection affected (P ≤ 0.05) the rate of oocyte cleavage, resulting in an increased rate of oocyte cleavage in the bacteria-infused group compared to vehicle infusion on day 2 (68.7 ± 8.4 and 53.1 ± 4.6%, respectively; P ≤ 0.05). The overall proportion of oocytes to develop to morulae was 24.3 ± 1.7% (Figure 4B), whereas the overall proportion of cleaved oocytes to develop to morulae was 37.5 ± 2.4% (Figure 4C). The proportion of oocytes that developed to morulae was not affected by bacterial infusion, day of oocyte collection, or the interaction between the two (Figure 4B). However, oocytes collected from bacteria-infused cows on day 24 had a reduced capacity to develop to morulae compared to those collected from vehicle-infused cows (15.5 ± 3.8 and 35.4 ± 4.0%, respectively; P ≤ 0.05). Bacterial infusion reduced the overall proportion of cleaved oocytes to develop to morulae compared to vehicle infusion (30.7 ± 3.0 and 45.0 ± 1.1%, respectively; P ≤ 0.05; Figure 4C). Specifically, bacterial infusion reduced the proportion of cleaved oocytes to develop to morulae day 24 compared to vehicle infusion (21.4 ± 5.0 and 45.6 ± 4.4%, respectively; P ≤ 0.05).
Figure 4.
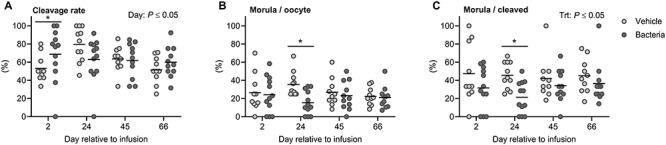
Effect of intrauterine infusion on developmental capacity of oocytes following in vitro fertilization and embryo culture. Oocytes were collected via ultrasound-guided transvaginal oocyte pickup on days 2, 24, 45, and 66 relative to infusion of either LB broth vehicle medium (vehicle; n = 11) or pathogenic E. coli and T. pyogenes in LB broth (bacteria; n = 12) and subjected to in vitro fertilization and embryo culture. Pooled oocytes from each cow were maintained as an individual replicate throughout insemination and culture. Each dot represents an individual cow, and the solid line represents the mean of the treatment. The proportion of oocytes that cleaved 3.5 days post-insemination (A), the proportion of oocytes to develop to morulae 6 days post-insemination (B), and the proportion of cleaved oocytes to develop to morulae 6 days post-insemination (C) are shown. Comparisons between treatments on a specific day are indicated by * when P ≤ 0.05.
Markers of embryo quality were assessed by real-time RT-PCR using pooled morulae derived from a single cow at a single time point (Figure 5). Genes related to stress response (HSPA1A), growth factor signaling and metabolism (IGF2R, SLC2A1), apoptosis (BAX, BCL2), and DNA methylation (DNMT3A) were analyzed. Bacterial infusion did not significantly alter the expression of any genes related to embryo quality (P > 0.05; Figure 5A–F). The expression of HSPA1A increased 112% in morulae developed from bacteria-infused cows compared to vehicle-infused cows (P = 0.08; Figure 5D). Day of oocyte collection affected expression of BAX and DNMT3A (P ≤ 0.05; Figure 5A and C). The expression of BAX on days 2 and 24 was different from days 45 and 66. DNMT3A expression was different on day 45 compared to days 24 and 66.
Figure 5.
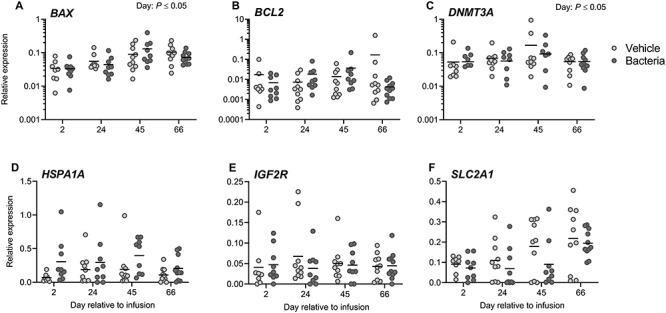
Effect of intrauterine infusion on gene expression of IVF derived morula stage embryos. Morula stage embryos derived by oocyte pickup, in vitro fertilization, and embryo culture from cows receiving intrauterine infusion of either LB broth vehicle medium (vehicle; n = 11) or pathogenic E. coli and T. pyogenes in LB broth (bacteria; n = 12) were probed for gene expression of BAX (A), BCL2 (B), DNMT3A (C), HSPA1A (D), IGF2R (E) and SLC2A1 (F) by real-time RT-PCR. Data are presented as expression relative to the geometric mean of the housekeeping genes GAPDH, SDHA, and RLP19. Each dot represents the average expression for an individual cow, and the solid line represents the mean of the treatment.
The association between morula development of cleaved embryos and endometrial expression of inflammatory mediators on day 6 was evaluated for all cows (Figure 6). There was a negative association between endometrial expression of IL6 and the capacity of cleaved oocytes to develop to morulae (P ≤ 0.05, r2 = 0.05; Figure 6C). There was no association between endometrial expression of CXCL8, IL1B, PTGES, PTGS2, or TNF and the capacity of cleaved oocytes to develop to morulae (P > 0.05; Figure 6A, B, and D–F).
Figure 6.
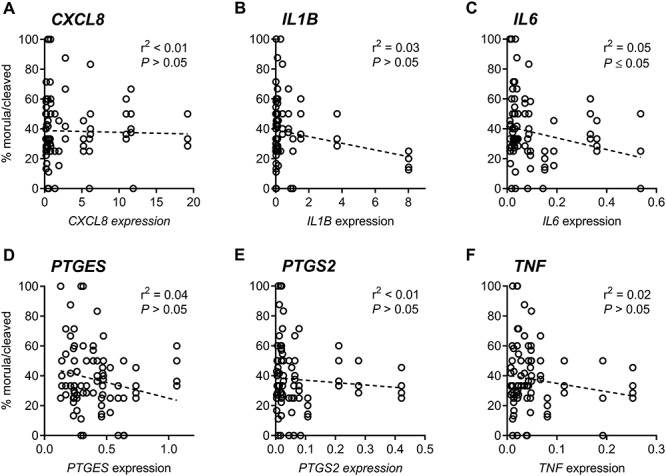
Association between morula development and endometrial inflammation. Endometrial expression of CXCL8 (A), IL1B (B), IL6 (C), PTGES (D), PTGS2 (E), and TNF (F) was determined by real-time RT-PCR on day 6 relative to infusion. Linear correlation was performed using the total proportion of cleaved oocytes to develop to morulae from all cows.
Effect of intrauterine infusion on in vivo embryo development
Cows were inseminated on day 130, following estrous synchronization (Figure 1). Sixteen days post-insemination, cows were euthanized, and uterine contents were collected by uterine flushing. Hot hanging carcass weights for vehicle- and bacteria-infused cows were not different (363.9 ± 6.6 versus 368.9 ± 8.0 kg, respectively; P > 0.05), and total reproductive tract weights were not different between treatments (vehicle: 466 ± 16 g, vs. bacteria: 449 ± 17 g; P > 0.05). A corpus luteum was present in all cows at euthanasia indicating a positive response to synchronization (corpus luteum diameter was 22.6 ± 0.8 and 22.5 ± 0.5 mm in vehicle- and bacteria-infused cows, respectively, P > 0.05). A total of 12 filamentous embryos (vehicle = 6 of 11, bacteria = 6 of 12) were recovered 16 days following insemination (Figure 7A). It was not possible to measure the length of each conceptus due to fragmentation. Interferon tau was measured in all uterine fluid samples and was detected in 17 of 23 samples (vehicle = 8 of 11, bacteria = 9 of 12). Uterine fluid IFNT concentrations ranged from 0.086 to 6858 ng/mL and were not different between bacteria-infused cows and vehicle-infused cows (P > 0.05, Figure 7B). There was no effect of infusion on serum progesterone on day 15 post-insemination (P > 0.05; Figure 7C), 1 day prior to euthanasia. There was no significant effect of infusion on the serum AMH concentration from −2 to 145 days (P > 0.05; Figure 7D). There was no effect of infusion on trophectoderm expression of CDKN1C, IFNT2, or PPARG in day 16 embryos (P > 0.05; Figure 8).
Figure 7.
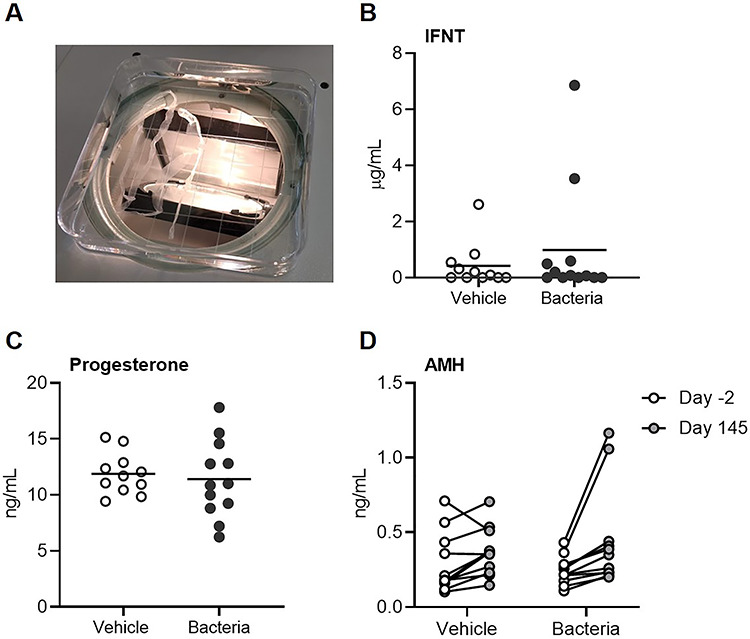
Effect of intrauterine infusion on embryo recovery, interferon tau concentration, serum progesterone, and anti-Müllerian hormone. Cows were synchronized and inseminated on day 130 post-infusion of either LB broth vehicle medium (vehicle; n = 11) or pathogenic E. coli and T. pyogenes in LB broth (bacteria; n = 12). Uterine content was collected ex vivo, 16 days post-insemination. All recovered embryos were filamentous in morphology (A). Interferon tau (IFNT) concentration (B) was quantified in uterine fluid by ELISA. Serum progesterone (C) was quantified 15 days after insemination. Each dot represents a cow and the solid line is the mean. Anti-Müllerian hormone (D) was quantified on day −2 prior to intrauterine infusion and day 145 after infusion. Each dot represents a cow.
Figure 8.
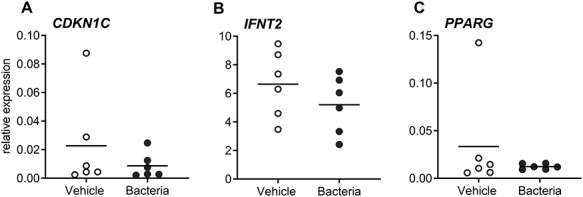
Effect of intrauterine infusion on gene expression of trophectoderm from in vivo-derived embryos. Cows were synchronized and inseminated on day 130 post infusion of either LB broth vehicle medium (vehicle; n = 11) or pathogenic E. coli and T. pyogenes in LB broth (bacteria; n = 12). Total RNA was isolated from trophectoderm of day 16 in vivo-derived embryos, and expression of CDKN1C (A), IFNT2 (B), and PPARG (C) was evaluated by real-time RT-PCR. Data displayed are expression relative to the geometric mean of the housekeeping genes ACTB and GAPDH. Each dot represents an embryo and the solid line depicts the mean.
Discussion
Infertility persists after resolution of uterine infections [1, 2, 15]. Women and cows previously diagnosed with uterine infection have lower conception rates compared to healthy counterparts [2, 13]. However, the culprit behind uterine infection-associated infertility remains elusive. Uterine infection alters the uterine environment and ovarian function [11, 27]. In order to determine the impact of uterine infection on the oocyte, the present study tested the hypothesis that endometritis reduces the capacity of the oocyte to develop to an embryo independent of a perturbed uterine environment. Our experiment successfully induced endometritis in non-lactating cows, and we found a reduction in the capacity of oocytes to develop to the morula stage during in vitro fertilization and culture. These findings demonstrate that uterine infection leaves a long-term impact on oocytes, even after clearance of infection.
The oocyte has been shown to have a greater influence on future blastocyst development when compared to culture conditions or the contribution of sperm [28]. We successfully collected oocytes from all cows, and there was no impact of uterine infection on the number of oocytes recovered or the fertilization rate; however, there was variability in the number of oocytes collected from each cow, but this was corrected for when assessing developmental competence using our statistical approach. Overall, fewer cleaved putative zygotes developed to morulae when oocytes were collected from bacteria-infused cows. Thus, even though cleavage rates were elevated in oocytes collected from cows 2 days after bacterial infusion, the relative ability to continue development to morulae was reduced compared to vehicle-infused cows. The largest reduction in developmental competence was observed when oocytes were collected 24 days following bacterial infusion, after endometrial inflammation was resolved. Bovine follicular development takes between 120 and 200 days, with approximately 42 days between antrum formation and ovulation [29]. All oocytes collected for in vitro fertilization and embryo culture were collected at the small antral follicle stage (<8 mm), 4 days after dominant follicle ablation, and approximately 20 days prior to potential ovulation. Thus, the timeline of follicular development indicates that small antral follicles aspirated from day 24 onwards would have likely been at the secondary stage of follicle development at the time of intrauterine infusion. Oocytes collected on day 24 were likely in the process of antrum formation at the time of uterine infusion. Oocytes collected on day 45 or 66 would have been earlier secondary stage follicles at the time of uterine infusion. While embryo development was numerically reduced on days 45 and 66, the enhanced reduction of developmental competence on day 24 suggests that specific stages of follicles are more susceptible to damage by uterine infection. Recovery from infertility following endometritis may simply require an extended period to clear negatively affected oocytes/follicles. However, spontaneous metritis causes altered transcription in granulosa cells of dominant follicles 63 days postpartum [17] and thus may require a longer period to recover oocyte health. The induction of bacterial uterine infection in isolation from other postpartum complications used here potentially underestimates the detrimental impact of spontaneous uterine infection on the oocyte.
In parallel, these studies established endometritis in healthy non-lactating cows. It is unclear if infertility in cows is mediated solely by uterine infection or if the confounding demands of lactation and other postpartum diseases also impact oocyte quality. We have previously established an experimental disease model comparable to clinical endometritis in virgin Holstein heifers by intrauterine infusion of pathogenic E. coli and T. pyogenes isolated from cows with active metritis [22, 24, 25]. In this model, cattle do not exhibit a systemic response to uterine infection, lacking pyrexia, but do display purulent vaginal discharge [22]. In this experiment, increased endometrial inflammation, characterized by increased expression of pro-inflammatory genes, occurred within 1 week of bacterial infusion and was resolved within 4 weeks. In addition, bacteria-infused cows had elevated vaginal mucus grade compared to vehicle-infused cows. Previous studies have demonstrated that other, nonreproductive diseases are detrimental to dairy cow fertility. Mastitis alters endocrine function and ovarian cyclicity, in addition to increasing the time to conception [30, 31]. Oocytes collected from dairy cows with mastitis are less likely to develop to a blastocyst compared to oocytes isolated from healthy cows [32], whereas lame cows have increased occurrence of ovarian cysts and a decreased ability to conceive compared to non-lame cows [33]. In general, cows diagnosed with one disease in the postpartum period, regardless if it is classified as uterine or non-uterine, have a reduced likelihood to conceive and an increased incidence of abortion [8, 15].
The mechanisms by which uterine infection reduces developmental competence of oocytes are unclear. Transfer of a healthy embryo into a cow with previous uterine infection does not resolve the negative impact of disease on fertility [8], suggesting that the uterus is in part responsible for infection-associated infertility. Herein, the inverse association between the degree of endometrial inflammation on day 6 of infection with reduced embryo development may be associated with inflammatory signals from the uterus that alter oocyte developmental competence either directly or by altering endocrine signaling to the ovary [34]. In vitro, bacterial LPS stimulates expression of inflammatory mediators by granulosa cells via the toll-like receptor 4 pathway [19], whereas bacterial LPS accumulates in the follicular fluid of cows with uterine infection and alters the follicular environment up to 63 days postpartum [17]. Similarly, exposure of oocytes to LPS during in vitro maturation reduces meiotic competence, increases reactive oxygen and apoptosis, and alters DNA methylation patterns of oocytes [19, 35]. It is unclear if cell wall components of Gram-positive bacteria are present in follicular fluid of cows with uterine disease or if these components effect oocyte competence. In parallel, uterine infection in these experiments may also disrupt hypothalamic–pituitary axis signaling, reducing GnRH and LH secretion which could negatively impact the growth and development of small follicles [36]; however hormonal signaling was not evaluated in the present study.
Embryo quality in those oocytes that could develop to morulae, as well as the ability of the cows to conceive following insemination, was examined. We selected a specific subset of genes to analyze known to be indicators of embryo quality [37]. Embryo development was halted at the morula stage in order to sample a homogeneous cell population instead of blastocyst embryos where treatment could affect the allocation of trophectoderm and inner cell mass cells, possibly confounding gene expression. Genes were chosen based on their function, including metabolism, measured by glucose transporter (GLUT1); stress, measured by heat shock protein 70 (HSPA1A); apoptosis, measured by BAX and BCL2; DNA methylation, measured by DNMT3A; and growth factor signaling, measured by insulin-like growth factor 2 receptor (IGF2R). In morulae derived from bacteria-infused cows, HSPA1A expression was elevated. Environmental stressors, such as heat stress, can increase HSPA1A gene expression in bovine embryos, even prior to embryonic genome activation implying altered expression is driven by maternal mRNA present in the oocyte [38]. Additionally, loss of function in the maternally inherited IGF2R gene results in developmental abnormalities, including large offspring syndrome [39, 40]. Conversely, increased IGF-2 signaling increases embryo development in vitro [41, 42]. It is important to note that embryo quality was assessed using a targeted approach with in vitro-produced embryos; thus, we cannot exclude the possibility that other factors important in embryo progression are altered as a result of uterine infection or that the uterine environment may aid in the recovery of perturbed embryos.
Finally, the effect of prior uterine infection on the health of embryos conceived by insemination was assessed. All recovered embryos on day 16 of pregnancy were filamentous. Our sample size here is small in terms of number of inseminations, and therefore, there is insufficient statistical power to make conclusions regarding the impact of experimental uterine infection on conception rate. Of embryos recovered, we did not observe an effect of infusion on trophectoderm expression of cell cycle regulation (CDKN1C), peroxisome proliferator-activated receptor gamma (PPARG), or interferon tau (IFNT2). The lack of difference in gene expression in recovered embryos is not surprising as these genes are critical to embryogenesis [43–45]; however, our results may be biased as we could only test embryos recovered at flushing which may exclude embryos that failed to develop earlier. Maternal recognition of pregnancy in the cow is driven by trophectoderm secretion of IFNT starting as early as the blastocyst [45, 46]. Here, the number of uterine flush samples with detectable IFNT was greater than the number of cows where an embryo was collected, suggesting that several embryos were not recovered due to technical error or that embryo development failed prior to collection on day 16. Regardless of the discrepancy, the rate at which embryo recovery and IFNT detection differed was similar between treatments based on the number of cows used here. Previous studies have demonstrated that uterine disease reduces IFNT concentration in uterine fluid [8], whereas we did not find any observable effect of bacterial infusion on IFNT concentration in uterine fluid on day 16 of pregnancy. It is important to note however that the method of IFNT quantification and variation of values reported are not consistent between experiments in the literature. Previous studies have used antiviral assays [8], RNA sequencing [47], or the same ELISA used here [48]. It is also unclear as to the minimum concentration of IFNT required for maternal recognition of pregnancy in the cow [49] or the best day or days to test for pregnancy status based on IFNT. However, it is believed that reduced IFNT secretion reflects poorer embryo quality and reduces pregnancy success.
In conclusion, intrauterine infusion of pathogenic bacteria to induce endometritis in dairy cows reduces the capacity of oocytes to develop to morulae. These novel findings demonstrate that uterine infection has a detrimental impact on the oocyte weeks after the occurrence of infection. These data aid in our understanding of the mechanisms of uterine infection-associated infertility in dairy cows and potentially women with reproductive tract infection or pelvic inflammatory disease. Future studies are required to determine the specific mechanism by which uterine disease diminishes oocyte quality.
Supplementary Material
Acknowledgments
The authors would like to thank Tod Pritchard, Miguel Torrado Vazquez, and the staff of the University of Florida Dairy Research Unit.
Conference presentation: Presented in part at the American Dairy Science Association 35th Discover Conference, 29 October–1 November 2018, Itasca, Illinois, and 52nd Annual Meeting of the Society for the Study of Reproduction, 18–21 July 2019, San Diego, California.
Footnotes
† Grant Support: This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD084316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth H-J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod 2009; 81:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsevat DG, Wiesenfeld HC, Parks C, Peipert JF. Sexually transmitted diseases and infertility. Am J Obstet Gynecol 2017; 216:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention Sexually Transmitted Disease Surveillance 2017. Atlanta: U.S. Department of Health and Human Services; 2018. doi: 10.15620/cdc.59237. [DOI] [Google Scholar]
- 4. Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age — United States, 2013-2014. Morb Mortal Wkly Rep 2017; 66:80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deb K, Chaturvedi MM, Jaiswal YK. Comprehending the role of LPS in gram-negative bacterial vaginosis: Ogling into the causes of unfulfilled child-wish. Arch Gynecol Obstet 2004; 270:133–146. [DOI] [PubMed] [Google Scholar]
- 6. Owusu-Edusei K, Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MCB, Kent CK. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40:197–201. [DOI] [PubMed] [Google Scholar]
- 7. Dohmen MJW, Joop K, Sturk A, Bols PEJ, Lohuis JACM. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology 2000; 54:1019–1032. [DOI] [PubMed] [Google Scholar]
- 8. Ribeiro ES, Gomes G, Greco LF, Cerri RLA, Vieira-Neto A, Monteiro PLJ, Lima FS, Bisinotto RS, Thatcher WW, Santos JEP. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J Dairy Sci 2016; 99:2201–2220. [DOI] [PubMed] [Google Scholar]
- 9. Lima FS, Snodgrass JA, De Vries A, Santos JEP. Economic comparison of systemic antimicrobial therapies for metritis in dairy cows. J Dairy Sci 2019; 102:7345–7358. [DOI] [PubMed] [Google Scholar]
- 10. Opsomer G, Gröhn YT, Hertl J, Coryn M, Deluyker H, De Kruif A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: A field study. Theriogenology 2000; 53:841–857. [DOI] [PubMed] [Google Scholar]
- 11. Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002; 123:837–845. [PubMed] [Google Scholar]
- 12. Ribeiro ES, Lima FS, Greco LF, Bisinotto RS, Monteiro APA, Favoreto M, Ayres H, Marsola RS. Prevalence of periparturient diseases and effects on fertility of seasonally calving grazing dairy cows supplemented with concentrates. J Dairy Sci 2013; 96:5682–5697. [DOI] [PubMed] [Google Scholar]
- 13. Borsberry S, Dobson H. Peripartureint diseases and their effect on reproductive performance in five dairy herds. Vet Rec 1989; 124:217–219. [DOI] [PubMed] [Google Scholar]
- 14. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 2002; 85:2223–2236. [DOI] [PubMed] [Google Scholar]
- 15. Santos JEP, Bisinotto RS, Ribeiro ES, Lima FS, Greco LF, Staples CR, Thatcher WW. Applying nutrition and physiology to improve reproduction in dairy cattle. Soc Reprod Fertil Suppl 2010; 67:387–403. [DOI] [PubMed] [Google Scholar]
- 16. Sheldon IM, Cronin JG, Bromfield JJ. Tolerance and innate immunity shape the development of postpartum uterine disease and the impact of endometritis in dairy cattle. Annu Rev Anim Biosci 2019; 7:361–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piersanti RL, Horlock AD, Block J, Santos JEP, Sheldon IM, Bromfield JJ. Persistent effects on bovine granulosa cell transcriptome after resolution of uterine disease. Reproduction 2019; 158:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 2007; 134:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bromfield JJ, Sheldon IM. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology 2011; 152:5029–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Britt JH, Cushman RA, Dechow CD, Dobson H, Humblot P, Hutjens MF, Jones GA, Ruegg PS, Sheldon IM, Stevenson JS. Invited review: Learning from the future—A vision for dairy farms and cows in 2067. J Dairy Sci 2018; 101:3722–3741. [DOI] [PubMed] [Google Scholar]
- 21. Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res 1951; 6:63–109. [Google Scholar]
- 22. Piersanti RL, Zimpel R, Molinari PCC, Dickson MJ, Ma Z, Jeong KC, Santos JEP, Sheldon IM, Bromfield JJ. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J Dairy Sci 2019; 102:2686–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Souza AH, Ayres H, Ferreira RM, Wiltbank MC. A new presynchronization system (double-Ovsynch) increases fertility at first postpartum timed AI in lactating dairy cows. Theriogenology 2008; 70:208–215. [DOI] [PubMed] [Google Scholar]
- 24. Goldstone RJ, Amos M, Talbot R, Schuberth H-J, Sandra O, Sheldon IM, Smith DGE. Draft genome sequence of Escherichia coli MS499, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc 2014; 2:e00217–e00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldstone RJ, Amos M, Talbot R, Schuberth H-J, Sandra O, Sheldon IM, Smith DGE. Draft genome sequence of Trueperella pyogenes, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc 2014; 2:e00194–e00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55:611–622. [DOI] [PubMed] [Google Scholar]
- 27. Herath S, Lilly ST, Fischer DP, Williams EJ, Dobson H, Bryant CE, Sheldon IM. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2α to prostaglandin E2 in bovine endometrium. Endocrinology 2009; 150:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61:234–248. [DOI] [PubMed] [Google Scholar]
- 29. Lussier JG, Matton P, Dufour JJ. Growth rates of follicles in the ovary of the cow. J Reprod Fertil 1987; 81:301–307. [DOI] [PubMed] [Google Scholar]
- 30. Moore DA, Cullor JS, Bondurant RH, Sischo WM. Preliminary field evidence for the association of clinical mastitis with altered interestrus intervals in dairy cattle. Theriogenology 1991; 36:257–265. [DOI] [PubMed] [Google Scholar]
- 31. Santos JEP, Cerri RL, Ballou MA, Higginbotham GE, Kirk JH. Effect of timing of first clinical mastitis occurrence on lactational and reproductive performance of Holstein dairy cows. Anim Reprod Sci 2004; 80:31–45. [DOI] [PubMed] [Google Scholar]
- 32. Roth Z, Dvir A, Kalo D, Lavon Y, Krifucks O, Wolfenson D, Leitner G. Naturally occurring mastitis disrupts developmental competence of bovine oocytes. J Dairy Sci 2013; 96:6499–6505. [DOI] [PubMed] [Google Scholar]
- 33. Melendez P, Bartolome J, Archbald LF, Donovan A. The association between lameness, ovarian cysts and fertility in lactating dairy cows. Theriogenology 2003; 59:927–937. [DOI] [PubMed] [Google Scholar]
- 34. Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, Bryant CE, Sheldon IM. Expression and function of toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2006; 147:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao SJ, Pang YW, Zhao XM, Du WH, Hao HS, Zhu HB. Effects of lipopolysaccharide on maturation of bovine oocyte in vitro and its possible mechanisms. Oncotarget 2017; 8:4656–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haziak K, Herman AP, Tomaszewska-Zaremba D. Effects of central injection of anti-LPS antibody and blockade of TLR4 on GnRH/LH secretion during immunological stress in anestrous ewes. Mediators Inflamm 2014; 2014:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sagirkaya H, Misirlioglu M, Kaya A, First NL, Parrish JJ, Memili E. Developmental and molecular correlates of bovine preimplantation embryos. Reproduction 2006; 131:895–904. [DOI] [PubMed] [Google Scholar]
- 38. Edwards JL, Ealy AD, Monterroso VH, Hansen PJ. Ontogeny of temperature-regulated heat shock protein 70 synthesis in preimplantation bovine embryos. Mol Reprod Dev 1997; 48:25–33. [DOI] [PubMed] [Google Scholar]
- 39. Barlow DP, Stoger R, Herrmann BG, Saitot K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 1991; 349:84–87. [DOI] [PubMed] [Google Scholar]
- 40. Young LE, Fernandes K, McEvoy TG, Butterwith SC, Gutierrez CG, Carolan C, Broadbent PJ, Robinson JJ, Wilmut I, Sinclair KD. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet 2001; 27:153–154. [DOI] [PubMed] [Google Scholar]
- 41. Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Horm Res 2006; 65:28–33. [DOI] [PubMed] [Google Scholar]
- 42. DeChiara TM, Efstratiadis A, Robertsen EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 1990; 345:78–80. [DOI] [PubMed] [Google Scholar]
- 43. Zhang P, Liégeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57(KIP2) indicates a role in Beckwith-Wiedemann syndrome. Nature 1997; 387:151–158. [DOI] [PubMed] [Google Scholar]
- 44. Ribeiro ES, Greco LF, Bisinotto RS, Lima FS, Thatcher WW, Santos JE. Biology of preimplantation conceptus at the onset of elongation in dairy cows. Biol Reprod 2016; 94:1–18. [DOI] [PubMed] [Google Scholar]
- 45. Thatcher WW, Hansen PJ, Gross TS, Helmer SD, Plante C, Bazer FW. Antiluteolytic effects of bovine trophoblast protein-1. J Reprod Fertil Suppl 1989; 37:91–99. [PubMed] [Google Scholar]
- 46. Hernandez-Ledezma JJ, Sikes JD, Murphy CN, Watson AJ, Schultz GA, Roberts RM. Expression of bovine trophoblast interferon in conceptuses derived by in vitro techniques. Biol Reprod 1992; 47:374–380. [DOI] [PubMed] [Google Scholar]
- 47. Mamo S, Mehta JP, Forde N, McGettigan P, Lonergan P. Conceptus-endometrium crosstalk during maternal recognition of pregnancy in cattle. Biol Reprod 2012; 87:1–9. [DOI] [PubMed] [Google Scholar]
- 48. Tríbulo P, Rabaglino MB, Bo MB, Carvalheira L d R, Bishop JV, Hansen TR, Hansen PJ. Dickkopf-related protein 1 is a progestomedin acting on the bovine embryo during the morula-to-blastocyst transition to program trophoblast elongation. Sci Rep 2019; 9:11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hansen TR, Sinedino LDP, Spencer TE. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017; 154:F45–F59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


