Abstract
Background
Transfusion-related sepsis remains an important hospital infection control challenge. Investigation of septic transfusion events is often restricted by the limitations of bacterial culture in terms of time requirements and low yield in the setting of prior antibiotic administration.
Methods
In 3 gram-negative septic transfusion cases, we performed metagenomic next-generation sequencing (mNGS) of direct clinical blood specimens in addition to standard culture-based approaches utilized for infection control investigations. Pathogen detection leveraged IDSeq, a new open-access microbial bioinformatics portal. Phylogenetic analysis was performed to assess microbial genetic relatedness and understand transmission events.
Results
mNGS of direct clinical blood specimens afforded precision detection of pathogens responsible for each case of transfusion-related sepsis and enabled discovery of a novel Acinetobacter species in a platelet product that had become contaminated despite photochemical pathogen reduction. In each case, longitudinal assessment of pathogen burden elucidated the temporal sequence of events associated with each transfusion-transmitted infection. We found that informative data could be obtained from culture-independent mNGS of residual platelet products and leftover blood specimens that were either unsuitable or unavailable for culture or that failed to grow due to prior antibiotic administration. We additionally developed methods to enhance accuracy for detecting transfusion-associated pathogens that share taxonomic similarity to contaminants commonly found in mNGS library preparations.
Conclusions
Culture-independent mNGS of blood products afforded rapid and precise assessment of pathogen identity, abundance, and genetic relatedness. Together, these challenging cases demonstrated the potential for metagenomics to advance existing methods for investigating transfusion-transmitted infections.
Keywords: healthcare infections, platelet transfusion, septic transfusion, metagenomic sequencing, mNGS
Transfusion-transmitted infections cause significant morbidity and are challenging to prevent and diagnose. We found that culture-independent metagenomic sequencing of blood products afforded rapid and precise assessment of pathogen identity, abundance, and genetic relatedness, enhancing traditional hospital infection control strategies.
While transfusion-associated infections have globally decreased over the past 50 years, bacterial contamination of the platelet supply remains a significant public health challenge, with approximately 1 in 1500–5000 units containing detectable organisms [1–5]. Room temperature storage facilitates more bacterial growth in platelets compared with other blood products, with skin flora, asymptomatic donor bacteremia, and direct introduction from environmental sources accounting for the majority of contaminants [3, 6]. Even though current culture-based screening approaches may miss a significant fraction of contaminated units, clinically reported sepsis occurs in only 1 in 15 000–100 000 transfusions, presumably due to frequent concurrent antibiotic administration, low bacterial inoculum, or attribution of transfusion-related outcomes to a patient’s preexisting infection or illness [3, 7].
Pathogen reduction of platelet products using amotosalen, a nucleic acid cross-linking agent activated by ultraviolet A irradiation, has been routinely performed in Europe for more than a decade and recently received US Food and Drug Administration approval [8]. Photoinactivation is broadly effective against diverse bacterial pathogens, and while failure at high bacterial loads has been described, few reports of sepsis have been reported following treatment [9, 10].
Here, we describe 3 transfusion-related gram-negative sepsis investigations, including 1 that involved a pathogen-reduced platelet product. For each, we performed metagenomic next-generation sequencing (mNGS) in addition to standard microbiologic diagnostics and found that mNGS enabled rapid and precise taxonomic identification and phylogenetic analysis of bacterial isolates, as well as culture-independent assessment of direct clinical samples.
METHODS
Microbial Culture
Patient blood cultures were performed via inoculation into BD Bactec Plus Aerobic and Lytic Anaerobic media (Becton Dickinson). This same approach was used for residual platelet transfusion cultures in cases 1 and 3 and for residual red blood cell segment cultures in case 2. Species identification was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker). Details on culture time to positivity are provided in Supplementary Table 1. Sterility testing was carried out as described in the Supplementary Methods.
Sequencing
Sample processing and DNA extraction were carried out as described in the Supplementary Methods. Ten to 100 ng of DNA from each sample was sheared with fragmentase (New England Biolabs) and used to construct sequencing libraries with the NEBNext Ultra II Library Prep Kit (New England Biolabs). Adaptor ligated samples underwent amplification with dual unique indexing primers. Libraries were quantified and pooled and underwent paired end 150 base pair sequencing on an Illumina MiSeq or NextSeq 550. Supplementary Table 2 lists the number of reads obtained for each sample.
Bioinformatics and Phylogenetic Analyses
Detection, taxonomic identification, and abundance quantitation of microbes from raw sequencing reads was first performed using the IDseq pipeline according to described protocols [11]. To control for background environmental and reagent contaminants, no-template water control samples were incorporated alongside extracted nucleic acid and carried forward throughout library preparation and sequencing. Genome assembly was performed by first trimming the raw sequencing reads in fastq files using TrimGalore [12] and assembling using Unicycler [13] with default parameters. To identify the closest related species in cases 1 and 2, BLAST+ [14] against the National Center for Biotechnology Information (NCBI) nt database and the Mash/Mini Hash search via PATRIC [15, 16] were used to analyze assembled and annotated contiguous sequences (contigs). For case 3, sequence type was determined using SRST2 [17]; other Klebsiella pneumoniae sequences in NCBI databases belonging to the same sequence type were found using a Mash/Mini Hash search via PATRIC [15, 16].
Phylogenetic analysis for case 1 and case 3 was used to determine the relationship between the samples in this study and the most closely related NCBI genomes. First, trimmed reads were aligned against a reference genome (case 1: Acinetobacter baumannii CP017642.1, case 3: K. pneumoniae CP015392.1) using Snippy v4.3.6 [18]. Then, a single-nucleotide polymorphism (SNP) alignment was obtained by variant calling using bcftools v1.9 [19]. We filtered SNPs with QUAL <30, and filtered genotypes with major allele depth (FMT/AD) <10 or major allele frequency (FMT/AF) <9. Finally, the maximum likelihood phylogeny based on the SNP alignments was built in RAxML v8.2.12 [20] using ‘-m ASC_GTRCAT --asc-corr=lewis’ options.
Ethics Statement
Investigations were carried out according to a no-subject-contact study protocol approved by the University of California–San Francisco Institutional Review Board, which permitted analysis of deidentified leftover clinical microbiology samples from collaborating institutions and subsequent review of study participants’ electronic medical records. No decisions regarding antibiotics or other patient-specific treatment interventions were made using sequencing data.
RESULTS
Investigation 1: Acinetobacter Septic Platelet Transfusion
Patient A, a 59-year-old man with relapsed acute lymphoblastic leukemia undergoing chemotherapy, received 2 doses of pathogen-reduced apheresis platelets on the day of planned home discharge. No side effects were experienced after transfusion of the first platelet unit. However, after receipt of the second unit 2 hours later, he developed chills and rigors followed by fever and critical hypotension. He received fluids and vancomycin before being transferred to the intensive care unit where meropenem, vasopressors, respiratory support, and continuous renal replacement therapy were administered. He recovered fully and was discharged home 16 days later on parenteral antibiotics.
Blood cultures obtained 4.5 hours posttransfusion returned positive for Acinetobacter calcoaceticus/baumannii (ACB) complex. Residual material obtained from a saline rinse of the returned platelet bag also grew ACB complex as well as Staphylococcus saprophyticus. Culture of a nontransfused platelet cocomponent from the same donor, concurrently pathogen-inactivated with the transfused product 5 days earlier, was negative. At the time of the investigation, hypotheses considered included failure of the pathogen-reduction process, contamination of the pathogen-reduced platelet bag during transport, or preexisting subclinical bacteremia in the patient.
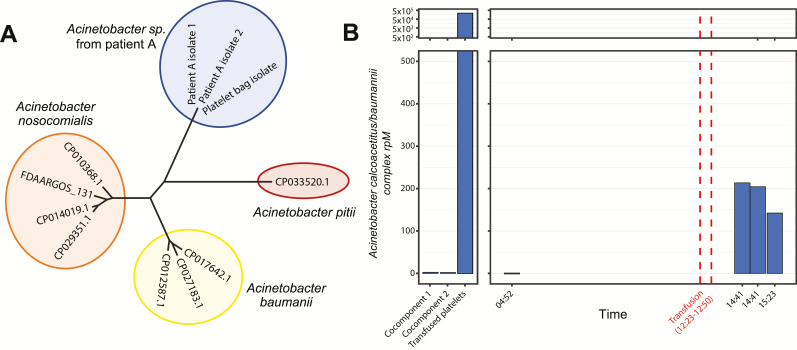
To investigate this case further, we first sequenced the cultured ACB complex isolate from the patient’s blood and the ACB complex isolate from a wash of the transfused platelet bag. In total, sequencing and analysis for this case was performed in 45 hours. De novo genome assembly revealed a novel Acinetobacter species most closely related (92% average nucleotide identity) to Acinetobacter nosocomialis and Acinetobacter pittii, members of the ACB complex (Figure 1A). Phylogenetic analysis revealed zero SNPs over the 3.9 Mb genome between the novel Acinetobacter species cultured from the blood and from the platelet bag, indicating that the 2 isolates were identical.
Figure 1.
Acinetobacter septic transfusion investigation. A, Maximum likelihood phylogenetic tree based on single-nucleotide polymorphism alignments demonstrates relatedness of the novel Acinetobacter species isolated from both patient A and the residual transfused platelet product relative to closely related species within the Acinetobacter calcoaceticus/baumannii (ACB) complex. B, Abundance of ACB complex in the transfused platelet product and cocomponents (left panel) and in patient A’s plasma (right panel), determined by culture-independent metagenomic sequencing and measured in rpM. Abbreviation: rpM, reads per million.
Culture-independent mNGS detected a high abundance of ACB complex in the transfused product (230 000 reads per million [rpM]) and in the patient’s plasma following transfusion (190 rpM; Figure 1B), as determined by summing the reads found by IDSeq aligning to species within the ACB complex (NCBI taxid 909768), which includes A. calcoaceticus, A. baumannii, A. nosocomialis, and A. pittii, among others. By comparison, the untransfused cocomponents and patient’s plasma before transfusion had very few alignments to ACB complex, in the range of expected background levels (0.25 and 2.2 rpM, respectively). Assessment of these low-abundance alignments at the species level revealed that they most likely represented misassigned reads from other Acinetobacter species, notably Acinetobacter johnsonii. More specifically, the cocomponents and pretransfusion samples had only a small percentage of Acinetobacter genus reads that best aligned to an ACB species, while in the transfused product and posttransfusion samples, the vast majority of genus Acinetobacter reads aligned to ACB species (Supplementary Figure 1A). The orders-of-magnitude difference in percent reads mapping to ACB complex, along with the change in composition of Acinetobacter assignments between samples, indicated that only the transfused product and patient’s posttransfusion plasma were contaminated by a species within the ACB complex. Although the sequence of the cultured isolate in this case allowed us to properly identify the strain phylogenetically, it was not required to complete the mNGS analysis that distinguished pathogen and environmental contaminant.
Investigation 2: Fatal Pseudomonas aeruginosa Septic Transfusion
Patient B, a 77-year-old man, was admitted for management of acute on chronic heart failure and implantation of a right ventricular assist device. During surgery, he received 3 units of platelets, 3 units of fresh-frozen plasma, and 2 units of cross-matched packed red blood cells (PRBCs). Intraoperatively, the patient became hemodynamically unstable. Cultures drawn 3 hours postsurgery ultimately returned positive for Pseudomonas aeruginosa, raising concern for a septic transfusion reaction (Figure 2).
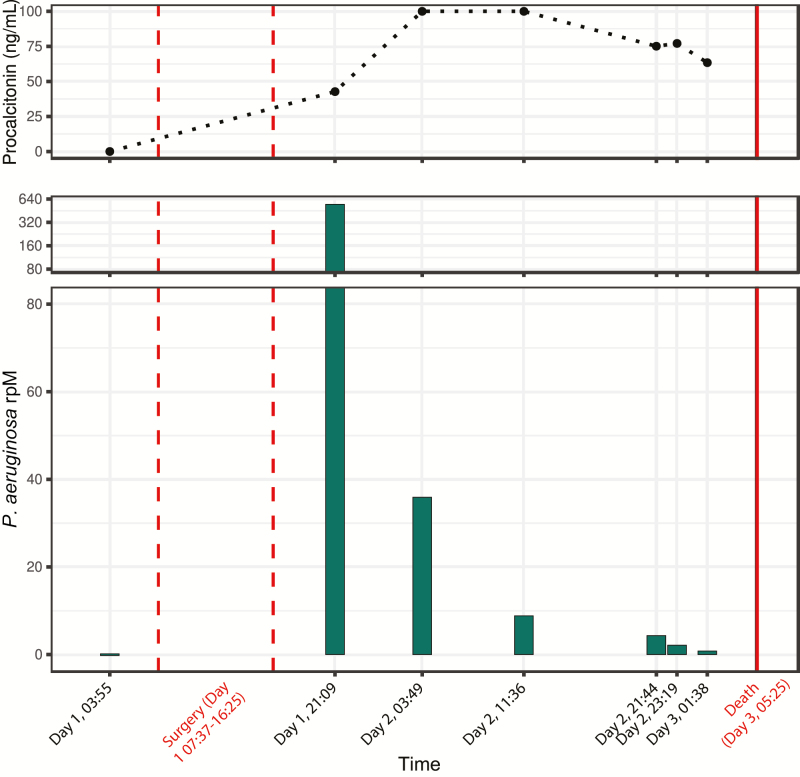
Figure 2.
Pseudomonas septic transfusion investigation. Abundance of Pseudomonas aeruginosa in patient B’s plasma throughout the course of the fatal septic transfusion event, determined by culture-independent metagenomic sequencing and measured in rpM (lower panel). Procalcitonin level (ng/mL) over the course of the septic transfusion event is plotted in the upper panel. Abbreviation: rpM, reads per million.
Despite administration of antipseudomonal antibiotics, the patient’s clinical stability continued to deteriorate, and he did not survive beyond postoperative day 3. mNGS was retrospectively performed on plasma samples collected pre and posttransfusion from the patient, on aliquots from the blood culture bottles that eventually turned positive, and on residual PRBCs that remained following transfusion. Following receipt of samples from the affected hospital, library preparation, sequencing, and preliminary analysis time totaled 72 hours. No remaining material from the platelets or transfused plasma was available for mNGS or other diagnostic testing. Cultured bacterial isolates were unavailable for sequencing.
mNGS revealed no P. aeruginosa in the pretransfusion plasma sample nor in the residual PRBCs but identified a high abundance of P. aeruginosa in posttransfusion plasma (Figure 2) that decreased over time in the setting of antibiotic treatment. Serial measurement of procalcitonin demonstrated a normal pretransfusion level (0.034 ng/mL; reference interval, <0.15 ng/mL) but significantly elevated concentrations following surgery (range, 42.73 to >100 ng/mL [above detection limit]). Phylogenetic analysis indicated strong relatedness to P. aeruginosa strain BWHPSA041.
Several environmentally ubiquitous Pseudomonas species are common contaminants of mNGS library preparation reagents. Thus, we used IDSeq [11] to determine the percent of Pseudomonas genus reads that mapped specifically to P. aeruginosa. In all 6 posttransfusion plasma samples, as well as the blood culture bottle samples, more than 99% of genus Pseudomonas reads mapped best to P. aeruginosa, while in water controls, an average of 7.2% (range, 0%–12.1%) mapped best to P. aeruginosa (Supplementary Figure 1B). This result indicated that the P. aeruginosa observed in the posttransfusion plasma samples was not the result of contamination from mNGS library preparation reagents.
Investigation 3: Fatal Klebsiella Septic Platelet Transfusion
Two immunocompromised pediatric patients, aged 2 and 3 years, developed septic shock following transfusion of platelets derived from a single donor, as recently reported [6]. Patient C, who had undergone successful autologous hematopoietic stem cell transplantation, developed hypotension, tachycardia, and vomiting 15 minutes into the transfusion. Despite initiation of vancomycin and cefepime, fluid resuscitation, vasopressor support, and intubation, the patient died within 5 hours. Blood cultures ultimately returned positive for K. pneumoniae, as did a culture from residual material in the platelet bag.
Five hours earlier, patient D, who was receiving empiric cefepime for neutropenic fever, had undergone transfusion with the second platelet unit derived from the same donor. He decompensated into septic shock 9 hours following transfusion but ultimately survived following fluid resuscitation and vasopressor support. Blood cultures remained negative in the setting of concurrent antibiotic treatment, initially precluding determination of whether sepsis was due to a Klebsiella-contaminated platelet transfusion or to another etiology.
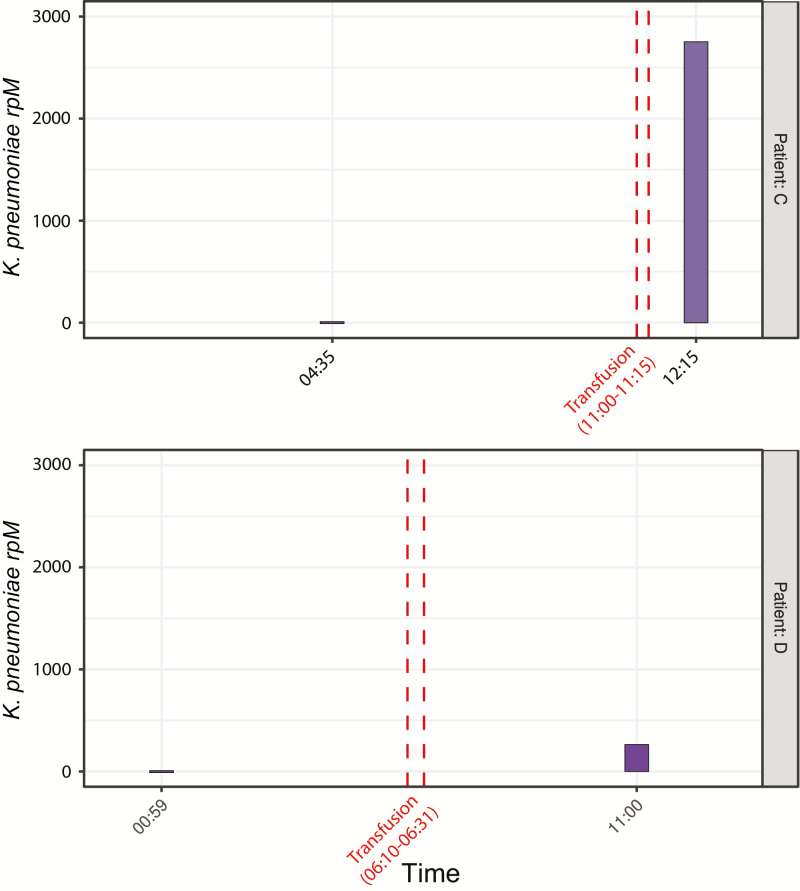
Culture-independent mNGS of pre and posttransfusion plasma or serum samples revealed a marked increase in K. pneumoniae following transfusion in both patients, although patient D, who had negative blood cultures, had 12.9-fold fewer Klebsiella rpM detected compared with patient C (Figure 3). Genome assembly and phylogenetic analysis revealed only 1 SNP across the 4.2 Mb core genome of K. pneumoniae among patient C’s plasma sample, patient C’s blood culture isolate, and the residual platelet bag culture isolate. The residual platelet product in the bag given to patient D was discarded by the hospital nursing staff and unavailable for either culture or mNGS. Across approximately 2700 bases of the K. pneumoniae genome detected in patient D’s plasma with a read depth greater than 10, zero SNPs relative to the other 3 samples were identified. The sequence type of K. pneumoniae in all 4 samples was determined to be ST491, and the closest ST491 sequence on GenBank differed from these sequences by 212 SNPs.
Figure 3.
Klebsiella septic transfusion investigation. Abundance of Klebsiella pneumoniae in plasma from patients C (upper panel) and D (lower panel) during the course of related septic transfusion events, as determined by culture-independent metagenomic sequencing and measured in rpM. Patient C, who did not survive the event, had posttransfusion blood cultures return positive for K. pneumoniae that was highly related (1 single-nucleotide polymorphism across the 4.2 Mb core genome) to the K. pneumoniae isolated from the residual transfused platelet product. Patient D, who was receiving antibiotics with activity against Klebsiella prior to transfusion, survived but had negative posttransfusion blood cultures, precluding definitive confirmation of a related second septic transfusion event in the absence of culture-independent metagenomic sequencing. Abbreviation: rpM, reads per million.
Together, these data confirmed that the platelet unit represented a single source of infection and that despite patient D having negative blood cultures, both patients became bacteremic with the same strain of K. pneumoniae transfused from the contaminated platelet components. As previously reported, routine culture-based screening of the donor’s platelets at 24 hours performed by the blood supplier remained negative at 5 days, although an additional platelet unit from the same donor shipped to a different hospital and was quarantined before transfusion grew K. pneumoniae that was highly related based on whole-genome sequencing analysis [6].
DISCUSSION
Bacterial contamination of platelet products remains an important and underrecognized hospital infection control challenge despite existing screening methods and psoralen-based pathogen-reduction strategies [1–5]. Rapid recognition of potential transfusion-associated sepsis can permit quarantine of untransfused cocomponents from a potentially contaminated supply chain and assist with root cause analysis. Traditionally, culture-based methods including pulsed-field gel electrophoresis and, more recently, whole-genome sequencing have been the central diagnostic tools for septic transfusion investigations. Here, we found that culture-independent mNGS extended the utility of these methods by directly detecting pathogens from clinical samples to assess genetic relatedness, obtain precise strain information, and interrogate levels of pathogen in a patient’s bloodstream throughout the course of a septic transfusion event.
In each case examined, mNGS provided detailed and precise information that clarified the sequence of events that resulted in transfusion-related sepsis. In case 1, for example, potential explanations considered included failure of the pathogen inactivation process, contamination of the platelet bag from an environmental source after pathogen reduction, and preexisting occult bacteremia resulting in retrograde introduction of bacteria into the platelet bag during transfusion. Temporal mNGS assessment of patient plasma demonstrated abundant Acinetobacter DNA in posttransfusion samples and in washes of the transfused platelet bag but not in any pretransfusion samples, consistent with a septic transfusion event and not preexisting bacteremia.
The findings of high-abundance Acinetobacter sp. in the transfused unit but not untransfused cocomponents suggested that contamination occurred following pathogen-reduction treatment, potentially from an environmental source during product handling, transport, or storage. Transfusion-related sepsis from pathogen-reduced platelet products has been reported but is extremely rare [9, 10]. This case suggests that pathogen-reduced platelet products should still be accompanied by rigorous infection control precautions and potentially undergo the same culture-based sterility testing as other platelet products. Discovery that the implicated pathogen in case 1 represented a novel species of Acinetobacter also highlighted the unique ability of sequencing-based diagnostics for unbiased microbe discovery.
In case 2, the possibility of an occult but developing bloodstream infection present prior to transfusion was also considered as a potential explanation for the patient’s postsurgical sepsis. As in case 1, assessment of plasma samples collected before and after transfusion clarified the sequence of events and demonstrated that P. aeruginosa was only detectable posttransfusion. Evidence for a septic transfusion event was further corroborated by temporal measurement of procalcitonin, a host inflammatory biomarker with specificity for bacterial infection [21, 22]. Posttransfusion procalcitonin levels above the upper limit of detection in the context of normal presurgical levels provided further evidence that a septic transfusion event had occurred. The absence of detectable P. aeruginosa in the transfused PRBC segments suggested that platelets or plasma may have been the source of contamination, although neither sample type was available for confirmation.
All 3 cases notably demonstrated that mNGS afforded high-resolution taxonomic identification without the need to isolate a pathogen in culture. This allowed for post hoc analyses of banked clinical specimens obtained both pre and posttransfusion that were either unsuitable or unavailable for culture. For instance, even though no cultured isolates were available in case 2, direct mNGS of leftover blood products allowed for precise identification of the most closely related P. aeruginosa strain, which incidentally was recovered in 2013 from a patient’s wound in Massachusetts.
Confirmation of transmission of a pathogen during septic transfusion events is essential for hospital infection control but, in some cases, is not possible because culture fails to identify a microbe. This problem was highlighted by case 3 in which blood cultures from patient D remained negative despite the development of posttransfusion septic shock. Patient D was receiving a prophylactic antibiotic with activity against Klebsiella, which likely inhibited bacterial growth in culture, precluding definite confirmation of a septic transfusion event related to that experienced by patient C. Culture-independent mNGS not only confirmed the presence of K. pneumoniae in the blood of both patients but also established that it was identical to the isolate derived from the transfused platelet product. This unfortunate fatal transfusion case demonstrated the capability of mNGS to provide definitive confirmation and characterization of septic transfusion events in cases where culture fails to yield an isolate.
Acinetobacter and Pseudomonas are environmentally ubiquitous and common contaminants of mNGS and 16S rRNA gene sequencing library preparation reagents [23, 24]. As such, they can add considerable complexity to investigations in which accurate assessment of the abundance of transfused pathogens belonging to these genera is critical. To address this, we used 2 complementary approaches that may be broadly useful for future investigations: assessment of compositional changes of species within the relevant genus as a proxy for environmental contamination and exclusive analysis on the exact species implicated in the transfusion event. In case 1, for example, these approaches clarified that the novel Acinetobacter species was present only in the residual transfused product and in the patient’s plasma following transfusion but not in the other cocomponents nor in the patient’s bloodstream prior to the event. In case 2, the lack of a Pseudomonas cultured isolate limited our ability to perform phylogenetic analyses. However, mNGS clearly identified the most closely related species to the causative agent and exhibited a stark compositional difference between the pre and posttransfusion samples, demonstrating that metagenomic bioinformatics tools like IDSeq can make species-level assignments of individual reads or contigs even in the absence of culture-based whole-genome sequencing.
Rapid assessment of septic transfusion events is critical to ensure that related contaminated products are swiftly quarantined and probable sources of contamination are identified. We found that mNGS and pathogen analysis could be reliably performed in less than 48 hours, faster than the turnaround time for blood culture at many institutions. Cost, time, and infrastructure requirements currently make sequencing impractical at many healthcare institutions; however, new platforms such as the Illumina iSeq and Oxford Nanopore Minion will undoubtedly increase the broad applicability of this technology for rapid hospital epidemiologic investigations.
As genomic approaches become more widely used for investigating transfusion-related infections, rapid exchange of pathogen genomic information via open access databases could accelerate identification of related cases, enhancing infection control efforts of emerging outbreaks. Indeed, the findings described here have contributed to a multicenter Centers for Disease Control and Prevention investigation that has identified the novel Acinetobacter sp. from patient A in related cases from Utah and Connecticut [25].
In summary, transfusion-related sepsis continues to cause excess mortality and morbidity despite the introduction of pathogen-reduction technologies. We found that culture-independent mNGS complemented current best available methods for investigation of transfusion-related sepsis by extending traditional whole-genome sequencing–based phylogenetics of cultured isolates and by permitting longitudinal assessment of pathogen abundance pre and posttransfusion from direct clinical specimens. While additional studies are needed to validate these methods, implementation of mNGS for both investigation and prevention of transfusion-related infections may enhance existing practices.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. L., E. C., J. K., and S. M. wrote the article. J. K. and L. L. performed phylogenetic analyses. C. L., E. C., J. K., L. L., and P. C. performed additional data analyses. A. Ni., A. Na., D. Y., M. M., M. Z., N. T., and S. B. directed clinical aspects of the investigations. C. T. and J. D. directed molecular aspects of the investigations. A. L., B. P., C. L., E. C., P. H., and J. Q. performed sample extractions and metagenomic library preparation. M. P. served as clinical research coordinator. M. T. and R. S. performed sequencing and quality control.
Acknowledgments. The authors thank Norma Neff for her guidance on metagenomic sequencing methodology and P. K. for his support and input on the study. The authors thank Amy Kistler and Renuka Kumar for their efforts with data analysis and sample processing. The authors thank Michael Busch for sharing his expertise in evaluating transfusion-transmitted infections.
Data availability. Raw sequencing data are available via National Center for Biotechnology Information BioProject Accession ID: PRJNA544865.
Financial support. This work was funded by the National Heart, Lung and Blood Institute (grant K23HL138461-01A1).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Eder AF, Dy BA, DeMerse B, et al. Apheresis technology correlates with bacterial contamination of platelets and reported septic transfusion reactions: : Apheresis Technology and Bacterial Contamination of PLTs. Transfusion 2017; 57:2969–76. [DOI] [PubMed] [Google Scholar]
- 2. Kaufman RM, Assmann SF, Triulzi DJ, et al. Transfusion-related adverse events in the platelet dose study. Transfusion 2015; 55:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood 2019; 133:1854–64. [DOI] [PubMed] [Google Scholar]
- 4. Tormey CA, Sweeney JD, Champion MH, Pisciotto PT, Snyder EL, Wu Y. Analysis of transfusion reactions associated with prestorage-pooled platelet components. Transfusion 2009; 49:1242–7. [DOI] [PubMed] [Google Scholar]
- 5. Kuehnert MJ, Roth VR, Haley NR, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 2001; 41:1493–9. [DOI] [PubMed] [Google Scholar]
- 6. Horth RZ, Jones JM, Kim JJ, et al. Fatal sepsis associated with bacterial contamination of platelets—Utah and California, August 2017. MMWR Morb Mortal Wkly Rep 2018; 67:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuller AK, Uglik KM, Savage WJ, Ness PM, King KE. Bacterial culture reduces but does not eliminate the risk of septic transfusion reactions to single-donor platelets. Transfusion 2009; 49:2588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin L, Dikeman R, Molini B, et al. Photochemical treatment of platelet concentrates with amotosalen and long-wavelength ultraviolet light inactivates a broad spectrum of pathogenic bacteria. Transfusion 2004; 44:1496–504. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt M, Hourfar MK, Sireis W, et al. Evaluation of the effectiveness of a pathogen inactivation technology against clinically relevant transfusion-transmitted bacterial strains: efficiency of pathogen inactivation. Transfusion 2015; 55:2104–12. [DOI] [PubMed] [Google Scholar]
- 10. Benjamin RJ, Braschler T, Weingand T, Corash LM. Hemovigilance monitoring of platelet septic reactions with effective bacterial protection systems: hemovigilance for transfusion sepsis. Transfusion 2017; 57:2946–57. [DOI] [PubMed] [Google Scholar]
- 11. Ramesh A, Nakielny S, Hsu J, et al. Etiology of fever in Ugandan children: identification of microbial pathogens using metagenomic next-generation sequencing and IDseq, a platform for unbiased metagenomic analysis. 2018; Available at: http://biorxiv.org/lookup/doi/10.1101/385005. Accessed 3 November 2018.
- 12. TrimGalore. Available at: https://github.com/FelixKrueger/TrimGalore. Accessed 1 February 2019.
- 13. Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 2017; 13:e1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Camacho C, Coulouris G, Avagyan V, et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ondov BD, Treangen TJ, Melsted P, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol 2016; 17 Available at: http://genomebiology.biomedcentral.com/articles/10.1186/s13059-016-0997-x. Accessed 21 March 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wattam AR, Abraham D, Dalay O, et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res 2014; 42:D581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inouye M, Dashnow H, Raven LA, et al. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 2014; 6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snippy. Available at: https://github.com/tseemann/snippy/releases. Accessed 1 February 2019.
- 19. bcftools v1.9. Available at: https://github.com/samtools/bcftools. Accessed 1 February 2019.
- 20. Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014; 30:1312–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vijayan AL, Vanimaya, Ravindran S, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 2017; 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adult patients with sepsis: a systematic review and meta-analysis. PLoS One 2015; 10:e0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson MR, O’Donovan BD, Gelfand JM, et al. Chronic meningitis investigated via metagenomic next-generation sequencing. JAMA Neurol 2018; 75:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014; 12 Available at: https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-014-0087-z. Accessed 10 May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones SA, Jones JM, Leung V, et al. Septic transfusion reactions attributed to bacterial contamination of platelets associated with a potential common source—multiple states, 2018. MMWR Morb Mortal Wkly Rep 2019; 68:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.