Abstract
Background
Children and adolescents with perinatal human immunodeficiency virus (HIV) infection and with low bone mineral density (BMD) may be at higher risk of osteoporosis and fractures in later life than their uninfected peers. Bisphosphonate therapy has been shown to reduce fractures in adults with osteoporosis, but has not been formally studied in youths living with HIV.
Methods
Fifty-two children and adolescents (aged 11–24 years) perinatally infected with HIV with low lumbar spine (LS) BMD (Z score < −1.5) were randomized to receive once-weekly alendronate or placebo in a double-blind cross-over study designed to assess the safety and efficacy of 48 and 96 weeks of alendronate in the United States and Brazil. All participants received daily calcium carbonate and vitamin D supplementation and were asked to engage in regular weight-bearing exercise. Safety and efficacy are summarized for the initial 48 weeks of the trial.
Results
Grade 3 or higher abnormal laboratory values, signs, or symptoms developed in 5 of 32 (16%) participants on alendronate and 2 of 18 (11%) on placebo (P > .99). No cases of jaw osteonecrosis, atrial fibrillation, or nonhealing fractures were reported. Mean increases (95% confidence interval) in LS BMD over 48 weeks were significantly larger on alendronate (20% [14%–25%]) than placebo (7% [5%–9%]) (P < .001). Similar improvements were seen for whole body BMD.
Conclusions
In this small study in children and adolescents perinatally infected with HIV with low LS BMD, 48 weeks of alendronate was well-tolerated, showed no safety concerns, and significantly improved LS and whole body BMD compared to participants on vitamin D/calcium supplementation and exercise alone.
Clinical Trials Registration
Keywords: HIV infection, children, low bone mineral density, alendronate
Forty-eight weeks of alendronate with calcium/vitamin D supplementation and exercise in children/adolescents with perinatal HIV infection with low lumbar spine (LS) bone mineral density (BMD) significantly increased LS and whole body BMD compared to supplementation and exercise alone; no safety concerns were identified.
(See the Editorial Commentary by Kalayjian and McComsey on pages 1289–91.)
Children and adolescents perinatally infected with human immunodeficiency virus (HIV) may have lower bone mineral density (BMD) compared to their uninfected peers [1, 2], which may in part be explained by poorer linear growth [3]. However, low BMD is also linked to antiretroviral therapy (ART) itself, particularly if the regimen contains tenofovir disoproxil fumarate (TDF) and/or protease inhibitors [4, 5]. Other factors that adversely impact bone health are common in children perinatally infected with HIV, including vitamin D insufficiency [6], delayed puberty [7], body composition abnormalities [8], and concomitant medications (corticosteroids, medroxyprogesterone) [9, 10]. Improving bone mineral accrual in children and adolescents living with HIV may decrease the risk of fracture later in life.
In adults with osteopenia or osteoporosis, bisphosphonate therapy can improve BMD, slow BMD loss, and reduce the risk of fractures. Use of bisphosphonates in children has mostly been limited to children with osteogenesis imperfecta, cerebral palsy, and other conditions that cause bone fragility [11]. Bisphosphonates have been effective in preventing or reversing BMD loss in ART-treated adults with HIV infection [12, 13], but they have not been well-studied in youths living with HIV. Bisphosphonates such as alendronate are incorporated into bone and result in reduced bone resorption. The effect of bisphosphonates may differ (exaggerated or attenuated) in juvenile bone, which has much greater bone turnover compared to adult bone. The effects may also differ in youths living with perinatal HIV because of chronic inflammation and long-term exposure to ART. The purpose of this trial was to evaluate the safety of alendronate, an oral bisphosphonate, and its effect on BMD in children and adolescents with HIV infection and low BMD.
METHODS
Study Design
The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1076 study (ClinicalTrials.gov identifier NCT00921557) was a randomized, placebo-controlled, double-blind, cross-over study designed to assess the safety and efficacy of 48 and 96 weeks of alendronate (Merck and Co, Rahway, New Jersey) in children and adolescents living with HIV. Inclusion criteria were 11–24 years of age, HIV acquisition before puberty, on the same ART regimen for ≥12 weeks (or not on ART for ≥12 weeks), and either a lumbar spine (LS) BMD Z score < −1.5 or history of fragility fracture within 12 months of study entry (baseline). Participants who were receiving TDF or medroxyprogesterone (both of which have been linked to decreases in BMD, particularly in the first months after initiation) must have been taking the drug for at least 6 months. Participants were not eligible if they had a 25-hydroxyvitamin D (25-OHD) concentration <10 ng/mL in combination with elevated intact parathyroid hormone.
The study was designed to achieve 80% power to detect a difference between alendronate and placebo of 3.6% in percentage change from baseline to week 48 in LS BMD, based on a standard deviation (SD) of 4%. Target enrollment was 51, with equal proportions randomized to 3 groups: (1) group A/A, 96 weeks of once-weekly oral alendronate (70 mg if >30 kg or 35 mg if ≤30 kg); (2) group A/P, alendronate for 48 weeks followed by placebo for 48 weeks; and (3) group P/A, placebo for 48 weeks followed by alendronate for 48 weeks. All participants received co-formulated calcium carbonate (600 mg) and vitamin D (400 IU) either once (if 25-OHD concentrations ≥20 ng/mL) or twice daily for those with 25-OHD concentration <20 ng/mL. All participants were asked to perform 60 minutes of weight-bearing exercise each day. After 96 weeks on study, participants were taken off study treatment (but continued on vitamin D/calcium), unblinded, and followed for an additional 48 weeks. Study-mandated reasons for discontinuing study treatment included an LS BMD Z score improving to >1.0, an unacceptable study drug-related toxicity, nonadherence with study drug, pregnancy, or having 25-OHD concentrations <10 ng/mL for >8 weeks.
Study Evaluations
Study visits were scheduled every 12 weeks, with telephone contact visits at 1, 4, 28, 49, 52, 76, and 100 weeks. Signs, symptoms, and diagnoses were collected at each study visit. Complete blood count, serum chemistries, and BMD of the LS and whole body (WB) with and less head were assessed at entry, every 24 weeks to week 96 and at week 144. LS and WB BMD were measured using dual-energy X-ray absorptiometry (DXA) on Hologic machines (models QDR4500A, QDR4500W, or Delphi A, Hologic, Bedford, Massachusetts). Analyses were based on results read in a standardized manner at the Tufts Body Composition Analysis Center at Tufts University School of Medicine. Age-, sex-, and race-adjusted Z scores were calculated from a variety of sources that covered the age range in P1076 [14–17]; Z scores were not available for WB BMD less head. Bone age was determined locally from a radiograph of the wrist unless the participant had reached the limit of maturity standards for bone age [18].
Statistical Methods
This manuscript summarizes the initial 48 weeks of follow-up on study and addresses the study’s primary objectives. Results are presented for participants on alendronate (groups A/A and A/P combined) and placebo (group P/A). Statistical significance was reported as nominal 2-sided P values unadjusted for interim analyses and multiple comparisons. In general, statistical tests with P < .05 were highlighted in the text. Analyses were done in SAS version 9.4.
Safety and efficacy analyses were modified intent-to-treat, including all participants who started study treatment. The primary safety outcome was any new or worsening grade 3 or higher laboratory values, signs, symptoms, or new cases of jaw osteonecrosis (JON), atrial fibrillation, or nonhealing fractures. Signs, symptoms, diagnoses, and laboratory values were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, version 1.0 [19]. Proportions of participants experiencing (1) a primary safety outcome, (2) new events grade 3 or higher, (3) new events grade 2 or higher, and (4) any events possibly/probably/definitely related to study treatment (as assessed at the site or by the protocol team) were presented by treatment with exact 95% confidence intervals (CIs). Differences in proportions between the 2 treatment groups were reported and treatments compared using Fisher exact test.
The primary efficacy outcome was percentage change in LS BMD from baseline to week 48. Secondary outcomes were percentage change in WB BMD and absolute changes in LS and WB BMD Z scores to week 48. Changes in all efficacy outcomes from baseline (percentage change in BMD and absolute change in Z scores) were summarized and compared within and between groups using paired and unpaired t tests, respectively, after reviewing distributional assumptions. Within-group changes need to be interpreted with caution as with no intervention, BMD should increase over time as participants were still growing, whereas age-adjusted Z scores should remain stable.
Other factors including ethnicity, sex, entry age, Tanner stage, bone age, TDF use, serum 25-OHD concentrations, nadir CD4+ T-lymphocyte (CD4) count (cells/μL), and height Z score were identified by the protocol team as potential effect modifiers of the association of treatment with the BMD outcomes. We did not include effect modification by baseline BMD because it is correlated with percentage change in BMD. Acknowledging the small sample size and lower power to detect other than large effects, to assess effect modification, a slope (representing average change in outcome over 48 weeks) was fit to each participant’s (1) percentage change (for BMD) or (2) change (for Z scores) from entry including weeks 0, 24, and 48. Linear regression models were fit for each outcome and each covariate, including main effects for treatment and the covariate, and an interaction term for treatment by the covariate. Statistically significant interactions would indicate different treatment effects across levels of the covariate. In models with no significant interaction (P > .10), the treatment difference was assessed adjusted for the covariate.
RESULTS
Baseline Characteristics
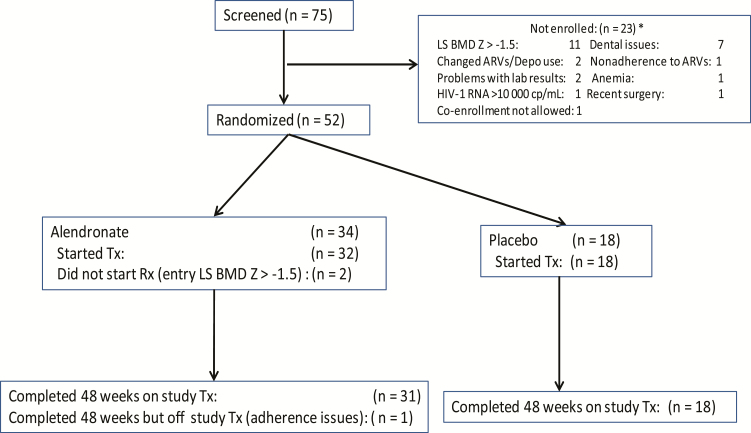
Fifty-two participants enrolled at 10 sites (2 in Brazil and 8 in the United States) between November 2009 and March 2014 (Figure 1). Two participants randomized to alendronate never started treatment because although their screening LS BMD Z score was < −1.5, their entry value was > −1.5. Analyses only include the 50 participants who started treatment.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. *Multiple reasons possible. Abbreviations: ARVs, antiretrovirals; BMD, bone mineral density; cp, copies; HIV-1, human immunodeficiency virus type 1; LS, lumbar spine; Tx, treatment.
At baseline, most enrollees were male (68%), with a median age of 16 years (range, 11–23 years) (Table 1). The route of HIV infections was breastfeeding for 1 participant and perinatal infection for the rest. Nine participants had a history of traumatic bone fractures, but none had history of nontraumatic (fragility) or nonhealing fractures. A majority were Centers for Disease Control and Prevention disease category C/3 (76%), with a higher proportion with more advanced HIV disease in the alendronate group (91% vs 50% on placebo) and most had HIV type 1 (HIV-1) RNA levels <400 copies/mL (82%). Six participants had serum 25-OHD concentrations <20 ng/mL. Baseline BMD levels by age are summarized in Supplementary Table 1.
Table 1.
Baseline Characteristics
| Characteristic | Treatment | |
|---|---|---|
| Alendronate (n = 32) | Placebo (n = 18) | |
| Country | ||
| United States | 14 (44) | 7 (39) |
| Brazil | 18 (56) | 11 (61) |
| Sex | ||
| Male | 21 (66) | 13 (72) |
| Female | 11 (34) | 5 (28) |
| Race/ethnicity | ||
| White non-Latino/Latina | 6 (19) | 1 (6) |
| Black non-Latino/Latina | 6 (19) | 1 (6) |
| Latino/Latina (regardless of race) | 20 (63) | 16 (89) |
| Age, y | ||
| Median (Min, Max) | 16.1 (11.1, 23.4) | 16.3 (11.2, 22.4) |
| 11–14 | 11 (34) | 6 (33) |
| 15–18 | 15 (47) | 8 (44) |
| ≥19 | 6 (19) | 4 (22) |
| Tanner stage | ||
| 1 | 1 (3) | 2 (11) |
| 2 | 5 (16) | 2 (11) |
| 3 | 6 (19) | 2 (11) |
| 4 | 9 (28) | 6 (33) |
| 5 | 11 (34) | 6 (33) |
| Smoker at entry | ||
| Yes | 2 (6) | 1 (6) |
| No | 30 (94) | 17 (94) |
| CDC disease category | ||
| A/1 | 1 (3) | 3 (17) |
| B/2 | 2 (6) | 6 (33) |
| C/3 | 29 (91) | 9 (50) |
| CD4 count, cells/μL | ||
| Median (Q1, Q3) | 692.5 (542.5, 776.5) | 875.5 (599.0, 1152.0) |
| 200–499 | 6 (19) | 2 (11) |
| 500–999 | 20 (63) | 10 (56) |
| ≥1000 | 6 (19) | 6 (33) |
| HIV-1 RNA, copies/mL | ||
| <400 | 26 (81) | 15 (83) |
| ≥400 | 6 (19) | 3 (17) |
| On tenofovir | ||
| No | 18 (56) | 9 (50) |
| Yes | 14 (44) | 9 (50) |
| Lumbar spine BMD, g/m2 | ||
| No. | 32 | 18 |
| Median (Q1, Q3) | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.8) |
| Lumbar spine BMD Z score | ||
| No. | 32 | 18 |
| Median (Q1, Q3) | −2.4 (−3.1, −1.9) | −2.5 (−3.6, −2.0) |
| Whole body BMD with head, g/m2 | ||
| No. | 30 | 16 |
| Median (Q1, Q3) | 0.9 (0.8, 1.0) | 0.9 (0.8, 0.9) |
| Whole body BMD less head, g/m2 | ||
| No. | 30 | 16 |
| Median (Q1, Q3) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.8) |
| Whole body BMD with head Z score | ||
| No. | 30 | 16 |
| Median (Q1, Q3) | −2.6 (−3.3, −1.9) | −2.5 (−3.9, −1.7) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMD, bone mineral density; CDC, Centers for Disease Control and Prevention; HIV-1, human immunodeficiency virus type 1 Q1, first quartile; Q3, third quartile.
Twenty-three (46%) participants were receiving TDF and 40 (80%) were receiving protease inhibitors. This was a heavily HIV-treated population: 1 participant was not on ART, 1 was on nucleoside reverse transcriptase inhibitors only, and the rest were on between 2 and 4 classes of ARTs (Supplementary Table 2). No females were receiving medroxyprogesterone, and 1 participant was receiving long-term oral corticosteroids at entry.
Treatment and Study Status
The 1 participant on alendronate who did not complete the 48 weeks on study treatment stopped alendronate temporarily at week 24 because of grade 1 tooth pain and was taken off study treatment permanently because of poor compliance with study visits (Figure 1). One participant on alendronate missed 5 doses and 1 participant was found not to have taken study drug during the last 24 weeks, both a result of nonadherence. One participant on placebo missed 1 dose and 1 other missed 3 doses, both due to tooth pain. All 50 participants completed the 48 weeks of follow-up. Over 48 weeks, at least half of each treatment group reported exercising at least 2–3 times/week and took at least 80% of the supplements (pill count).
Safety
Five of 32 (16%) participants experienced 1 primary safety event in the alendronate group (1 grade 3 abdominal pain, 2 grade 3 bilirubin elevation, 1 grade 3 low phosphorus, and 1 grade 4 low platelet count) compared to 2 of 18 (11%) in the placebo group (1 grade 3 bilirubin elevation and 1 participant with multiple primary safety outcomes [grade 3 weight loss, chest pain, and pain and difficulty swallowing]) (Table 2; P > .99). There were no cases of JON, atrial fibrillation, or nonhealing fractures. All events were judged by the Core Protocol Team and site personnel to be not related to study treatment with the exception of the grade 3 low phosphorus, which was judged to be probably not related. There were no statistically significant differences between participants on alendronate compared to those on placebo during the first 48 weeks on study in rates of any safety outcomes (Table 2).
Table 2.
Number of Participants Experiencing at Least 1 Safety Event
| Toxicities | P Valuea | Treatment | ParticipantsWith Events, No. | Total | % With Event | Alendronate – Placebo | ||
|---|---|---|---|---|---|---|---|---|
| % | (95% CI) | % | (95% CI) | |||||
| Primary safety events | > .99 | Alendronate | 5 | 32 | 15.6 | (5.3–32.8) | 4.5 | (−24.2 to 33.0) |
| Placebo | 2 | 18 | 11.1 | (1.4–34.7) | … | … | ||
| New events grade 3 or higher | > .99 | Alendronate | 5 | 32 | 15.6 | (5.3–32.8) | 4.5 | (−24.2 to 33.0) |
| Placebo | 2 | 18 | 11.1 | (1.4–34.7) | … | … | ||
| New events grade 2 or higher | .77 | Alendronate | 15 | 32 | 46.9 | (29.1–65.3) | 8.0 | (−21.2 to 35.9) |
| Placebo | 7 | 18 | 38.9 | (17.3–64.3) | … | … | ||
| Any possibly/probably/definitely related | .54 | Alendronate | 3 | 32 | 9.4 | (2.0–25.0) | 9.4 | (−19.5 to 37.4) |
| Placebo | 0 | 18 | 0.0 | (.0–18.5) | … | … |
Abbreviation: CI, confidence interval.
aFisher exact P value.
There was no evidence of HIV disease progression in either treatment group during the first 48 weeks of follow-up and no statistically significant changes from baseline in CD4 cell count or CD4 percentage within treatment groups (or differences between groups), and the percentage of participants with HIV-1 RNA ≤400 copies/mL did not change from entry.
Efficacy
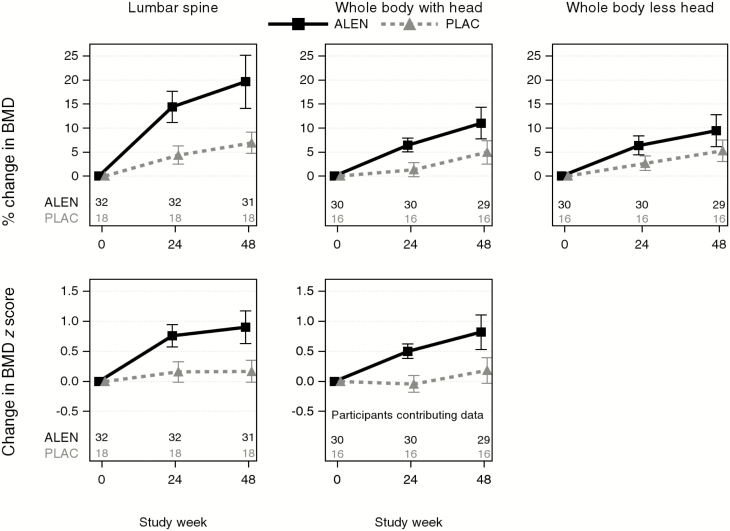
Percentage changes from baseline to weeks 24 and 48 are summarized for all LS and WB outcomes in Table 3 and illustrated in Figure 2. For the primary outcome of percentage change from baseline in LS BMD, there were statistically significant increases from baseline to weeks 24 and 48 in both treatment groups, but the alendronate group increased significantly more than the placebo group at both time points (P < .001). Mean increases in LS BMD in the alendronate group were 14% (95% CI, 11%–18%) to week 24 and 20% (95% CI, 14%–25%) to week 48. In the placebo group, these increases were 4% (95% CI, 2%–6%) and 7% (95% CI, 5%–9%) to weeks 24 and 48, respectively. Average treatment difference (alendronate minus placebo) in percentage change in LS BMD to week 24 was 10% (95% CI, 6%–14%) and to week 48 was 13% (95% CI, 7%–19%). A similar pattern was seen for LS BMD Z scores, with larger increases in the alendronate group than in the placebo group to both time points (P < .001). Mean increases in the alendronate group to week 48 were 0.90 SD (95% CI, .63–1.17; P < .001) compared to 0.17 SD (95% CI, −.02 to .35; P = .07) in the placebo group, with average treatment differences of 0.73 SD (95% CI, .41–1.05) to week 48. By week 48, 48% in the alendronate group and 6% in the placebo group had achieved LS Z scores > −1.5.
Table 3.
Lumbar Spine and Whole Body Percentage Change in Bone Mineral Density and Change in Z Score From Baseline to Weeks 24 and 48
| Location and Outcome | Time Point | Alendronate – Placebo | Treatment | No. | Mean | (95% CI) | Within-treatment P Valueb | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Between-treatment P Valuea | |||||||
| Lumbar spine | |||||||||
| % change BMD | 24 | 10.00 | (6.32–13.68) | < .001 | Alendronate | 32 | 14.38 | (11.14–17.62) | < .001 |
| Placebo | 18 | 4.38 | (2.47–6.29) | < .001 | |||||
| 48 | 12.72 | (6.83–18.60) | < .001 | Alendronate | 31 | 19.64 | (14.10–25.18) | < .001 | |
| Placebo | 18 | 6.92 | (4.71–9.14) | < .001 | |||||
| Change in Z score | 24 | 0.60 | (.36–.85) | < .001 | Alendronate | 32 | 0.76 | (.57–.95) | < .001 |
| Placebo | 18 | 0.16 | (−.01 to .32) | .07 | |||||
| 48 | 0.73 | (.41–1.05) | < .001 | Alendronate | 31 | 0.90 | (.63–1.17) | < .001 | |
| Placebo | 18 | 0.17 | (−.02 to .35) | .07 | |||||
| Whole body less head | |||||||||
| % change BMD | 24 | 3.71 | (1.30–6.12) | .003 | Alendronate | 30 | 6.40 | (4.41–8.39) | < .001 |
| Placebo | 16 | 2.69 | (1.22–4.17) | .001 | |||||
| 48 | 4.20 | (.27–8.13) | .037 | Alendronate | 29 | 9.46 | (6.10–12.82) | < .001 | |
| Placebo | 16 | 5.26 | (3.02–7.51) | < .001 | |||||
| Whole body with head | |||||||||
| % change BMD | 24 | 5.11 | (3.11–7.11) | < .001 | Alendronate | 30 | 6.48 | (5.04–7.93) | < .001 |
| Placebo | 16 | 1.38 | (−.10 to 2.85) | .07 | |||||
| 48 | 6.05 | (2.07–10.02) | .004 | Alendronate | 29 | 11.01 | (7.73–14.29) | < .001 | |
| Placebo | 16 | 4.96 | (2.52–7.41) | < .001 | |||||
| Change in Z score | 24 | 0.54 | (.36–.72) | < .001 | Alendronate | 30 | 0.50 | (.38–.62) | < .001 |
| Placebo | 16 | −0.04 | (−.18 to .10) | .52 | |||||
| 48 | 0.64 | (.29–.98) | < .001 | Alendronate | 29 | 0.82 | (.53 −1.10) | < .001 | |
| Placebo | 16 | 0.18 | (−.03 to .39) | .09 |
Abbreviations: BMD, bone mineral density; CI, confidence interval.
a t test with unequal variances for treatment differences.
b t test for within-group change.
Figure 2.
Mean (95% confidence interval) change from baseline in lumbar spine and whole body bone mineral density (percentage change) and Z scores (change). Abbreviations: ALEN, alendronate; BMD, bone mineral density; PLAC, placebo.
WB BMD with head and less head and WB BMD Z scores also increased significantly more on alendronate than the placebo arm. Average treatment differences in percentage change from baseline to week 48 were 4% (95% CI, 0%–8%) for WB less head (P = .037) and 6% (95% CI, 2%–10%) for WB with head (P = .004). Average treatment differences in changes in Z scores for WB with head were 0.64 SD (95% CI, .29–.98; P < .001).
Efficacy–Effect Modification
In this exploratory analysis with limited power to detect other than large differences, there was no evidence of effect modification of treatment on average change in BMD outcomes over 48 weeks by sex, ethnicity (Latina/Latino), baseline TDF use, vitamin D concentration (<30 or ≥30 ng/mL), or nadir CD4 count (<200, 200–499, ≥500 cells/μL). Age (<15, 15–18, ≥19 years), Tanner stage (1–3 or 4–5), and bone age (continuous) were correlated and showed similar patterns. Results are summarized for age in Supplementary Table 3. Among younger participants, alendronate had larger predicted mean improvements compared to placebo in LS BMD and LS Z scores than among older participants. Predicted mean increases in LS BMD over 1 year for alendronate compared to placebo in participants 11–14 years of age at entry were 27% (95% CI, 16%–37%), compared to 6% (95% CI, –7% to 20%) in those aged ≥19 years at entry. LS Z scores showed a similar pattern, with younger participants having larger predicted improvements on alendronate (1.47 [95% CI, .84–2.11]) relative to placebo than older participants (0.31 [95% CI, −.50 to 1.11]). For WB outcomes, there was no evidence of effect modification by age.
DISCUSSION
In this small, randomized placebo-controlled trial, 48 weeks of alendronate treatment in children and adolescents living with HIV with low BMD resulted in no safety issues and in significantly greater improvements in LS and WB BMD and in LS and WB BMD Z scores compared to placebo. Alendronate has been safe and well-tolerated in clinical trials of alendronate in children and adolescents with other chronic diseases such as osteogenesis imperfecta [20], Duchenne muscular dystrophy [21], glucocorticoid-induced low bone mass [22, 23], cystic fibrosis [24], and fibrous dysplasia [25]. Alendronate was well-tolerated in all of these studies, with no reported serious adverse events. In 2 studies that reported side effects, mild gastrointestinal symptoms were the most common [21, 24]. In 1 study, nausea and vomiting resulted in discontinuation of alendronate [25], but no laboratory abnormalities were observed.
Prior studies of oral alendronate in children and adolescents living with HIV have been limited to reports of compassionate use in a small number of children; the drug was well-tolerated, with no adverse events observed [26, 27]. The results of this first published placebo-controlled clinical trial of the safety and efficacy of alendronate in children and adolescents living with HIV are reassuring. Over the initial 48 weeks, we found no statistically significant difference in the proportion of participants experiencing primary safety events between those randomized to alendronate compared to placebo; none of the events reported were deemed related to alendronate treatment. In 1 case, an abnormal phosphorus concentration in a participant receiving alendronate was judged to be probably not related to treatment. None of the participants developed new cases of JON, atrial fibrillation, or nonhealing fractures. In adults, bisphosphonates reduce the risk of hip, nonvertebral, and vertebral fractures, particularly in postmenopausal women, and are commonly considered to be a first-line treatment [28]. The risk of JON is very low and has been mostly observed in adults who received high-dose intravenous bisphosphonate as supportive treatment during cancer therapy [29]. The risk of atrial fibrillation as a consequence of bisphosphonate treatment is low [30], as is the risk for atypical femoral fractures [31]. Similarly, no serious adverse events were reported in a trial of alendronate in adults with HIV [12].
While participants randomized to alendronate had significantly greater improvements in BMD compared to the placebo group, those randomized to placebo also experienced gains in BMD Z scores over the trial period. Vitamin D/calcium and regular strength-building exercises may have contributed to gains in BMD [32, 33], as age-adjusted Z scores should remain stable for children with no interventions as they grow.
In the current trial, alendronate produced greater BMD increases in less mature (lower age, Tanner stage, or bone age) participants, suggesting that a targeted approach to intervention during critical periods of bone accrual may have the greatest impact. This is consistent with a placebo-controlled trial of vitamin D supplementation in youths living with HIV, where vitamin D was associated with greater BMD increases compared to placebo among the younger children, but not among participants of all ages [34]. The study size was not large enough for a thorough exploration of other potential effect modifiers.
Although this study was well-powered for the primary efficacy outcome, it was a small study with limited ability to detect less common safety outcomes. DXA measurements were used to determine areal BMD, which may underestimate bone size in children with smaller bones for age and sex. However, in this randomized study, the distribution of participants by bone size should be balanced across treatment group assignment and treatment differences should be unbiased. We conducted a sensitivity analysis adjusting for height Z score to account for bone size, which did not change the results.
Earlier and better ART options may mean that low BMD is becoming less common in children and adolescents perinatally infected with HIV, decreasing the need for this intervention. However for those who do develop bone fragility or low BMD, this study has demonstrated the efficacy of alendronate over 1 year, with no apparent safety concerns. Further analysis of the secondary objectives over 96 and 144 weeks of follow-up will inform durability of the beneficial alendronate effects. Although impact on fractures and peak bone mass is unknown, alendronate treatment decreases fracture risk in children with other chronic diseases affecting bone [35], and similar long-term outcomes are anticipated in children and adolescents living with HIV.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the participants, their families, and caregivers who agreed to participate in the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1076 study, and the site clinical study teams who supported this work. Clinical sites: University of California, Los Angeles (Jaime G. Deville, MD; Francisco Ramos-Gomez, DDS, MS, MPH; Michele F. Carter, RN), Lurie Children’s Hospital of Chicago (Ram Yogev, MD; Ruth Williams, RN; Lisa Keys, APN), University of Miami (Grace A. Alvarez, FMD, MPH; Charles Mitchell, MD; Gwendolyn Scott, MD), University of South Florida–Tampa (Carina Rodriguez, MD; Alicia Marion, ARNP; Patricia Emmanuel, MD), San Juan City Hospital, Puerto Rico (Nicolas Rosario-Matos, MD; Lizbeth Fabregas-Troche, BS, MS; Ramón González-Garcia, DDS), Federal University of Minas Gerais Brazil (Jorge Pinto, MD; Juliana Romeiro, PhD; Maria Tavares, RN, MSc), University of Sao Paulo, Brazil (Marisa M. Mussi-Pinhata, MD; Marcia De Lima Isaac, MD; Fernanda Tome Sturzbecher, MD), Johns Hopkins University, Baltimore, Maryland (Allison Agwu, MD, ScM; Thuy Anderson, RN, BSN; Todd Noletto, MPH), St Jude Children’s Research Hospital, Memphis, Tennessee (Aditya Gaur, MD; Nehali Patel, MD; Sandra Jones, RN, PNP), Western New England Maternal Pediatric Adolescent Clinical Research Site Worcester (Katherine Luzuriaga, MD; Margaret McManus, MPH). Radiology: D. C. Colosi (Division of Radiology, Stony Brook University School of Dental Medicine). We also acknowledge Merck for the generous provision of the alendronate and placebo used in the study.
Disclaimer. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), the Department of Health and Human Services, or the US Agency for International Development.
Financial support. Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), all components of the NIH, under award numbers UM1AI068632 (IMPAACT Leadership and Operations Center), UM1AI068616 (IMPAACT Statistical and Data Management Center), and UM1AI106716 (IMPAACT Laboratory Centers), and by NICHD contract number HHSN275201800001I.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Negredo E, Domingo P, Ferrer E, et al. Peak bone mass in young HIV-infected patients compared with healthy controls. J Acquir Immune Defic Syndr 2014; 65:207–12. [DOI] [PubMed] [Google Scholar]
- 2. Yin MT, Lund E, Shah J, et al. Lower peak bone mass and abnormal trabecular and cortical microarchitecture in young men infected with HIV early in life. AIDS 2014; 28:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiMeglio LA, Wang J, Siberry GK, et al. Pediatric HIVAIDS Cohort Study (PHACS) Bone mineral density in children and adolescents with perinatal HIV infection. AIDS 2013; 27:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cassetti I, Madruga JV, Suleiman JM, et al. Study 903E Team The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naïve HIV-1-infected patients. HIV Clin Trials 2007; 8:164–72. [DOI] [PubMed] [Google Scholar]
- 5. Grund B, Peng G, Gibert CL, et al. INSIGHT SMART Body Composition Substudy Group Continuous antiretroviral therapy decreases bone mineral density. AIDS 2009; 23:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson DL, Stephensen CB, Miller TL, et al. Pediatric HIV/AIDS Cohort Study Associations of low vitamin D and elevated parathyroid hormone concentrations with bone mineral density in perinatally HIV-infected children. J Acquir Immune Defic Syndr 2017; 76:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams PL, Abzug MJ, Jacobson DL, et al. International Maternal Pediatric and Adolescent AIDS Clinical Trials P219219C Study and the Pediatric HIVAIDS Cohort Study Pubertal onset in children with perinatal HIV infection in the era of combination antiretroviral treatment. AIDS 2013; 27:1959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobson DL, Patel K, Siberry GK, et al. Pediatric HIV/AIDS Cohort Study Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr 2011; 94:1485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American College of Obstetricians and Gynecologists. Committee opinion no. 602: depot medroxyprogesterone acetate and bone effects. Obstet Gynecol 2014; 123:1398–402. [DOI] [PubMed] [Google Scholar]
- 10. Sarinho ESC, Melo VMPP. Glucocorticoid-induced bone disease: mechanisms and importance in pediatric practice. Rev Paul Pediatr 2017; 35:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim MJ, Kim SN, Lee IS, et al. Effects of bisphosphonates to treat osteoporosis in children with cerebral palsy: a meta-analysis. J Pediatr Endocrinol Metab 2015; 28:1343–50. [DOI] [PubMed] [Google Scholar]
- 12. Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr 2005; 38:426–31. [DOI] [PubMed] [Google Scholar]
- 13. McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007; 21:2473–82. [DOI] [PubMed] [Google Scholar]
- 14. Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 2007; 92:2087–99. [DOI] [PubMed] [Google Scholar]
- 15. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 2009; 4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly TJ. Bone mineral density reference databases for American men and women. J Bone Miner Res 1990; 5(Suppl 1):S249. [Google Scholar]
- 17. Kelly TL. Pediatric BMD reference database for US white children. Bone 2005; 36(Suppl 1):S30. [Google Scholar]
- 18. Greulich WW, Pyle SI.. Radiographic atlas of skeletal development of hand wrist. 2. Stanford, California: Stanford University Press, 1971. [Google Scholar]
- 19. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, version 1.0 (2004, clarification 2009) Available at: https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables. Accessed 30 September 2018.
- 20. DiMeglio LA, Peacock M. Two-year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. J Bone Miner Res 2006; 21:132–40. [DOI] [PubMed] [Google Scholar]
- 21. Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 2005; 86:284–8. [DOI] [PubMed] [Google Scholar]
- 22. Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford) 2005; 44:813–8. [DOI] [PubMed] [Google Scholar]
- 23. Wiernikowski JT, Barr RD, Webber C, Guo CY, Wright M, Atkinson SA. Alendronate for steroid-induced osteopenia in children with acute lymphoblastic leukaemia or non-Hodgkin’s lymphoma: results of a pilot study. J Oncol Pharm Pract 2005; 11:51–6. [DOI] [PubMed] [Google Scholar]
- 24. Bianchi ML, Colombo C, Assael BM, et al. Treatment of low bone density in young people with cystic fibrosis: a multicentre, prospective, open-label observational study of calcium and calcifediol followed by a randomised placebo-controlled trial of alendronate. Lancet Respir Med 2013; 1:377–85. [DOI] [PubMed] [Google Scholar]
- 25. Boyce AM, Kelly MH, Brillante BA, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab 2014; 99:4133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fortuny C, Noguera A, Alsina L, Villaronga M, Vidal-Sicart S, Sánchez E. Long-term use of bisphosphonates in the treatment of HIV-related bone pain in perinatally infected pediatric patients. AIDS 2008; 22:1888–90. [DOI] [PubMed] [Google Scholar]
- 27. Soler Palacin P, Torrent A, Rossich R, et al. Osteoporosis and multiple fractures in an antiretroviral-naive, HIV-positive child. J Pediatr Endocrinol Metab 2007; 20:933–8. [DOI] [PubMed] [Google Scholar]
- 28. Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med 2016; 374:2096–7. [DOI] [PubMed] [Google Scholar]
- 29. Soutome S, Hayashida S, Funahara M, et al. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: is tooth extraction a risk factor? PLoS One 2018; 13:e0201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma A, Chatterjee S, Arbab-Zadeh A, et al. Risk of serious atrial fibrillation and stroke with use of bisphosphonates: evidence from a meta-analysis. Chest 2013; 144:1311–22. [DOI] [PubMed] [Google Scholar]
- 31. Schilcher J, Koeppen V, Aspenberg P, Michaëlsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Havens PL, Stephensen CB, Van Loan MD, et al. Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) 109 Study Team Vitamin D3 supplementation increases spine bone mineral density in adolescents and young adults with human immunodeficiency virus infection being treated with tenofovir disoproxil fumarate: a randomized, placebo-controlled trial. Clin Infect Dis 2018; 66:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eckard AR, OʼRiordan MA, Rosebush JC, et al. Effects of vitamin D supplementation on bone mineral density and bone markers in HIV-infected youth. J Acquir Immune Defic Syndr 2017; 76:539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics 2009; 123:e121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lv F, Liu Y, Xu X, et al. Effects of long-term alendronate treatment on a large sample of pediatric patients with osteogenesis imperfecta. Endocr Pract 2016; 22:1369–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.