Abstract
Background
Human pegivirus (HPgV) is a single-strand RNA virus belonging to the Flaviviridae. Although no definitive association between HPgV infection and disease has been identified, previous studies have suggested an association of HPgV viremia with risk of lymphomas.
Methods
We conducted a systematic review and meta-analysis, including 1 cohort study and 14 case-control studies, assessing the association of HPgV viremia with adult lymphomas. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using a random-effects model, overall and by geographic region and lymphoma subtype.
Results
The overall OR for lymphoma was 2.85 (95% CI, 1.98–4.11), with statistically significantly elevated ORs observed in 8 of 15 studies. There was a small amount of heterogeneity among studies (I2 = 28.9%; Q = 18.27, P = .16), and the funnel plot provided no evidence for publication bias. The strongest association with lymphoma risk was observed for studies from Southern Europe (OR, 5.68 [95% CI, 1.98–16.3]), whereas weaker ORs (with 95% CIs) were observed for studies from North America (2.24 [1.76–2.85]), Northern Europe (2.90 [.45–18.7), and the Middle East (2.51 [.87–7.27]), but all of similar magnitude. Participants with HPgV viremia had statistically significantly increased risks (OR [95% CI]) for developing diffuse large B-cell (3.29 [1.63–6.62]), follicular (3.01 [1.95–4.63]), marginal zone (1.90 [1.13–3.18]), and T-cell (2.11 [1.17–3.89]) lymphomas, while the risk for Hodgkin lymphoma (3.53 [.48–25.9]) and chronic lymphocytic leukemia (1.45 [.45–4.66]) were increased but did not achieve statistical significance.
Conclusions
This meta-analysis supports a positive association of HPgV viremia with lymphoma risk, overall and for the major lymphoma subtypes.
Keywords: human pegivirus, lymphoma, risk, meta-analysis
In a meta-analysis of 15 studies, human pegivirus (HPgV) viremia was associated with an increased risk of lymphoma. As about 2% of US blood donors are viremic with HPgV at donation, these results raise issues regarding the safety of the blood supply.
(See the Editorial Commentary by Kandathil and Balagopal on pages 1229–31.)
Lymphoid neoplasms, including Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), and plasma cell neoplasms, are a heterogeneous group of malignancies. Overall, this group of neoplasms represents the sixth most common cancer in the United States (US), with an estimated 136 690 new cases in 2016, the majority represented by NHL [1]. Several factors have been clearly shown to increase the risk of developing a lymphoid neoplasm, including genetic susceptibility, immunodeficiency, autoimmune disorders, and exposure to physical, chemical, and infectious agents [2]. Among the latter, several large recent studies suggest an association between a common, otherwise nonpathogenic virus called human pegivirus (HPgV) and NHL [3–5]. HPgV was initially called by 2 names when it was discovered in 1995, GB virus type C (GBV-C) [6] and hepatitis G virus (HGV) [7]. As it was shown not to cause hepatitis or any other known illness, nor was infection identified in the presumed hepatitis patient with the initials “G. B.,” neither name accurately described the virus, and it was reclassified by the International Committee on the Taxonomy of Viruses as a member of the Pegivirus genus within the Flaviviridae family [8–10]. A second HPgV found in hepatitis C virus (HCV)–infected humans has more recently been identified, and it is referred to as either HHPgV or HPgV-2 [11, 12].
HPgV is a single-stranded RNA virus transmitted through parenteral, sexual, and perinatal routes [10, 13]. Phylogenetically, HPgV and HPgV-2 are related to HCV, which is also associated with risk of NHL [9, 14–17]. HPgV replicates at high titer, with an average of 3.28 × 106 and 4.76 × 106 copies/mL in people with and without human immunodeficiency virus (HIV) infection, respectively [18]. Active HPgV infection may persist for decades, although most infections clear within 2 years in immunocompetent hosts [19–21]. The lack of associated hepatic disease presumably relates to its tissue tropism, as HPgV does not appear to replicate in the liver. Based on both ex vivo and in vitro studies, HPgV is known to replicate in B and T lymphocytes, including CD4+ and CD8+ lymphocytes, spleen, and bone marrow [22–31]. Recently, a rhesus monkey animal model of pegivirus was developed and bone marrow tropism was confirmed [32].
Using a large North American case-control study, we recently reported a positive association of HPgV active infection with risk of lymphoma overall and for all major lymphoma subtypes except HL and CLL [3]. The aim of the present study was to systematically review epidemiologic data on the association between lymphoid neoplasms with HPgV viremia, assess major sources of heterogeneity in the available data, and conduct a meta-analysis.
METHODS
Literature Search
Two authors (A. F. and J. T. S.) independently performed a web-based literature search with PubMed/Medline and Google Scholar. The search strategy was based on the following words (all fields): [“hepatitis G virus” (Medical Subject Heading [MeSH]) or “HGV” or “GB virus type C” or “GBV-C” or “human pegivirus” or “HPgV”] and [“lymphoma” (MeSH) or “lymphoproliferative neoplasms” or “Hodgkin lymphoma” (MeSH) or “non-Hodgkin lymphoma” or “chronic lymphatic leukemia”].
Inclusion Criteria
We included only articles and reports published in English in the period 1997–2018. Eligible publications had to include the prevalence of HGV/GBV-C/HPgV viremia (active infection) in adult lymphoma patients and in a control group. The definition of control groups varied greatly between studies, including patients undergoing a given hospital procedure, blood donors, people living with HCV or HIV, or population-based samples.
Data Extraction
Data extraction was performed independently by 2 reviewers (A. F. and J. R. C.) and included author, year of publication, country of origin, sample size, method of HPgV RNA detection, and lymphoma classification used. For the case-control studies, we extracted the estimates of odds ratios (ORs) and 95% confidence intervals (CIs) and the variables used for matching and adjustment. For cohort studies, we extracted the source of the cohort, years of follow-up, the outcome measured with 95% CIs, and the variables used for adjustment. Findings for specific lymphoma subtypes were abstracted for studies where the data were reported. Very few discrepancies were identified between the 2 independent reviews, and these were addressed by a joint reevaluation of the original article.
The method of HPgV RNA detection differed among the studies, which leads to some heterogeneity. Specifically, nested reverse-transcription polymerase chain reaction (RT-PCR) increases sensitivity but reduces specificity, while real-time RT-PCR reduces sensitivity [33]. HPgV RNA titers are biphasic in humans [18], and therefore studies that use real-time RT-PCR will identify only a portion of those with lower titer virus. However, these results will be specific to HPgV (HPgV-1) as the PCR protocols do not detect the more recently described HPgV-2 [11, 12] or HCV [33]. Because HPgV E2 antibodies are detected only after clearance of viremia in >90% of subjects, studies examining E2 antibodies as inclusion criteria, or combined detection of HPgV RNA and E2 antibody, were excluded. Commercial E2 antibody testing methods are no longer available; thus, recent studies do not identify prior infection using a validated methodology [34].
Quality Assessment
The quality of each study was assessed independently by 2 reviewers (A. F. and J. R. C.) with the Newcastle-Ottawa Scale, consisting of 3 parameters: selection, comparability, and exposure/outcome assessment [35].
Data Synthesis and Analysis
To provide a quantitative estimate of the association of HPgV active infection with lymphoma risk, the ORs (adjusted, if available) and the corresponding 95% CIs were abstracted from published articles. When the ORs were not given, they were computed from tabular data. We then calculated the summary OR and corresponding 95% CI using a random-effects model of DerSimonian-Laird. The random-effects model accounts for heterogeneity between studies, which is expected in an analysis of this nature. Subset analyses were performed by lymphoma subtype and geographic area. Publication bias was also assessed by direct examination of a funnel plot. Heterogeneity among studies was assessed using the I2 statistic and Cochran Q statistic. According to Higgins, I2 values of 25%, 50%, and 75% are arbitrarily considered to have to low, moderate, and high heterogeneity, respectively [36]. All statistics and graphs were obtained using the R packages meta [37] and rmeta [38] in R version 3.4.2.
RESULTS
Search Results
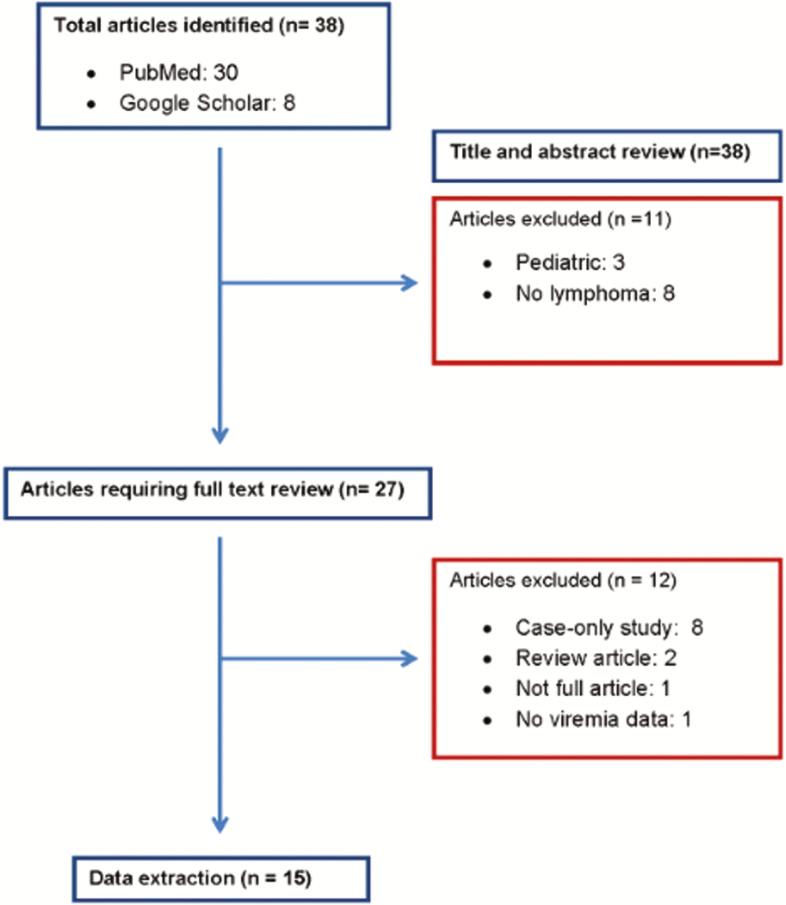
The results of our search strategy are summarized in Figure 1. A total of 38 articles were identified. We excluded 11 studies after review of the title and abstract: 8 had no reference to lymphoma and 3 were conducted in pediatric patients. After a detailed full text review of the 27 remaining articles, we excluded 12 articles: 8 were case-only studies, 2 were reviews, 1 was not available in full form (abstract only), and 1 did not report any viremia data. A total of 15 articles (14 case-control studies [3, 5, 39–50] and 1 cohort study [4]) met the inclusion criteria.
Figure 1.
Search results.
Characteristics of the Studies Included
The main characteristics of the published studies that evaluated the association of active HPgV infection with lymphoma risk are shown in Table 1. Geographically, 5 studies originated from Southern Europe, 2 from Northern Europe, 4 from North America, 3 from the Middle East, and 1 from Japan. Nine of the studies included HCV- or HIV-coinfected subjects and controls. The prevalence of HPgV viremia varied widely across studies, ranging from 0% reported in the small Japanese study [43] (33 cases and 45 controls) to 30% reported in the study conducted in 33 people with HIV infection [48]. Fourteen of 15 studies showed an increased lymphoma risk with HPgV active infection (all OR > 1.40), 8 of which were statistically significant. Four studies included analyses by NHL subtype.
Table 1.
Characteristics of the Studies Evaluating the Association Between Human Pegivirus Viremia and Lymphoma Risk
| First Author | Year | Country | Type of Study | Laboratory Method | Classification | Cases | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Period | No. | HCV | HIV | HPgV | Time Period | No. | HCV | HIV | HPgV | Source | Matching | ||||||
| Zignego | 1997 | Italy | Case-control | PCR | IWF | … | 150 | 24.6% | … | 6% | … | 165 | … | … | 1.2% | Healthy subjects | Age, sex |
| Minton | 1998 | UK | Case-control | RT-PCR | Not reported | Jul–Aug 1996 | 76 | … | … | 9.2% | Jul 96 | 100 | … | … | 1% | Healthy blood donors | None |
| Persico | 1998 | Italy | Case-control | RT-PCR | Not reported | … | 71 | 4.2% | … | 16.9% | … | 71 | 1.4% | … | 1.4% | Healthy subjects | None |
| Civardi | 1998 | Italy | Case-control | RT-PCR | Not reported | … | 33 | 100% | … | 3% | … | 249 | … | … | 2.8% | Not specified | None |
| Ogino | 1999 | Japan | Case-control | RT-PCR | IWF | 1991–1997 | 33 | 12.1% | … | 0% | 1991–1997 | 45 | 4.4% | … | 0% | Hospital patients | Age, sex |
| Collier | 1999 | Canada | Case-control | Ligase C | IWF | Feb–May 1997 | 100 | 1% | … | 5% | Feb–May 1997 | 100 | 1% | … | 3% | Cancer patients | Age, sex |
| De Renzo | 2002 | Italy | Case-control | PCR | Not reported | … | 227 | … | 0% | 6% | … | 110 | … | 0% | 0.9% | Healthy blood donors | Age, sex |
| Kaya | 2002 | Turkey | Case-control | PCR | IWF | … | 70 | 1.4% | … | 7.1% | … | 70 | 1.4% | … | 1.4% | Healthy subjects | Age, sex |
| Giannoulis | 2004 | Greece | Case-control | RT-PCR | WHO | 1999–2000 | 108 | 1.9% | … | 9.2% | 1999–2000 | 285 | … | … | 0.7% | Blood donors | None |
| Ernst | 2011 | Germany | Case-control | … | Not reported | … | 33 | … | 100% | 30.3% | … | 584 | … | 100% | 23.6% | HIV patients | None |
| Krajden | 2010 | Canada | Case-control | RT-PCR | WHO | 2000–2004 | 553 | 2.2% | … | 4.5% | … | 438 | 0.9% | … | 1.8% | Healthy subjects | Age, sex, region |
| Khodavandi | 2011 | Iran | Case-control | RT-PCR | Not reported | … | 70 | … | … | 2.8% | … | 100 | … | … | 1% | Other patients | None |
| Rezaeian | 2012 | Iran | Case-control | PCR | WHO | 2007–2011 | 140 | 2.9% | … | 4.3% | 2007–2011 | 120 | 0% | … | 2.5% | Hospital patients | None |
| Chang | 2014 | US | Cohort | RT-PCR | WHO | 1993–2001 | 658 | … | … | 1.8% | 1993–2001 | 1316 | … | … | 0.5% | Healthy subjects | Age, sex, race/ ethnicity |
| Fama | 2018 | US | Case-control | RT-PCR | WHO | 2002–2015 | 2094 | … | … | 10.1% | 2002–2015 | 1572 | … | … | 5% | Hospital patients | Age, sex, region |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPgV, human pegivirus; IWF, International Working Formulation; PCR, polymerase chain reaction; RT-PCR, reverse-transcription polymerase chain reaction; UK, United Kingdom; US, United States; WHO, World Health Organization.
Quality Assessment Results
The quality assessment of the studies included in this meta-analysis is shown in Supplementary Table 1; only 3 studies [3–5] were rated as good based on the Newcastle-Ottawa Scale.
Main Effect
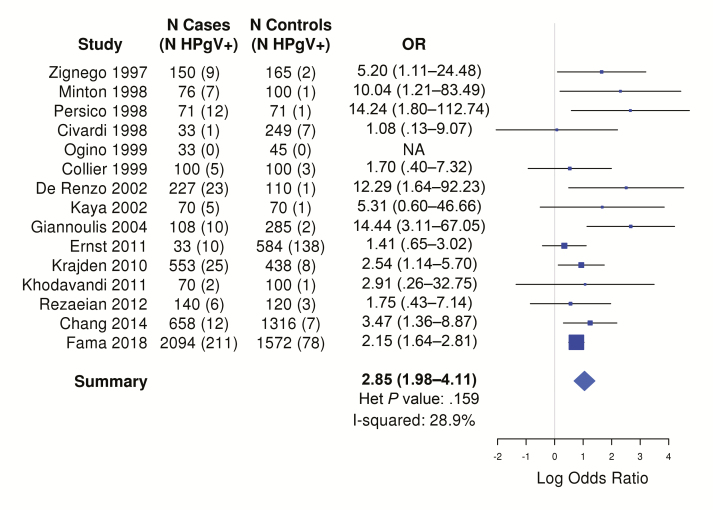
The summary OR from the 15 studies, based on 4416 cases (338 [7.6%] HPgV viremia positive) and 5502 controls (258 [4.6%] HPgV viremia positive), was 2.85 (95% CI, 1.98–4.11; I2 = 28.9%), with statistically significantly elevated ORs observed in half of the studies (Figure 2). The funnel plot showed a good symmetrical distribution of the studies, consistent with the absence of publication bias (Supplementary Figure 1). If this was a causal association, then using an OR or 2.85 as an estimate of the relative risk and prevalence ranges based on the study from British Columbia (1.8%) [5] and the study from the United States (US) (5.0%) [3] , the population attributable risk would be 3.2% or 8.5%, respectively.
Figure 2.
Odds ratios and corresponding 95% confidence intervals for risk of lymphoma by human peg0ivirus viremia positivity in case-control and cohort studies. Abbreviations: Het, heterogeneity; HPgV, human pegivirus; NA, not applicable; OR, odds ratio.
Subset Analyses
Coinfections
After excluding known HIV-positive cases and controls (leaving 8 studies with available data), the meta-analysis of HPgV and lymphoma revealed an OR of 2.83 (95% CI, 1.64–4.74; I2 = 32.6%; Supplementary Figure 2A), largely unchanged from the overall meta-analysis. After excluding known HCV-positive cases and controls (leaving 10 studies with available data), the meta-analysis of HPgV and lymphoma revealed an OR of 4.43 (95% CI, 2.75–7.15; I2 = 3.6%; Supplementary Figure 2B), much stronger than the overall meta-analysis. Only 1 study had data on hepatitis B virus (HBV) infection [49], and after excluding HBV+ cases and controls it found a weak positive association of HPgV and lymphoma (OR, 1.46 [95% CI, .09–23.7]), but was based on small numbers (1 exposed case and 1 exposed control).
Good-Quality Studies
When the analysis was restricted to the 3 studies rated as “good” on the Newcastle-Ottawa Scale (Supplementary Figure 3), there was an approximately 20% attenuation of the association to an OR of 2.25, with 95% CIs (1.76–2.88) that were similar to the overall meta-analysis.
Geographic Area
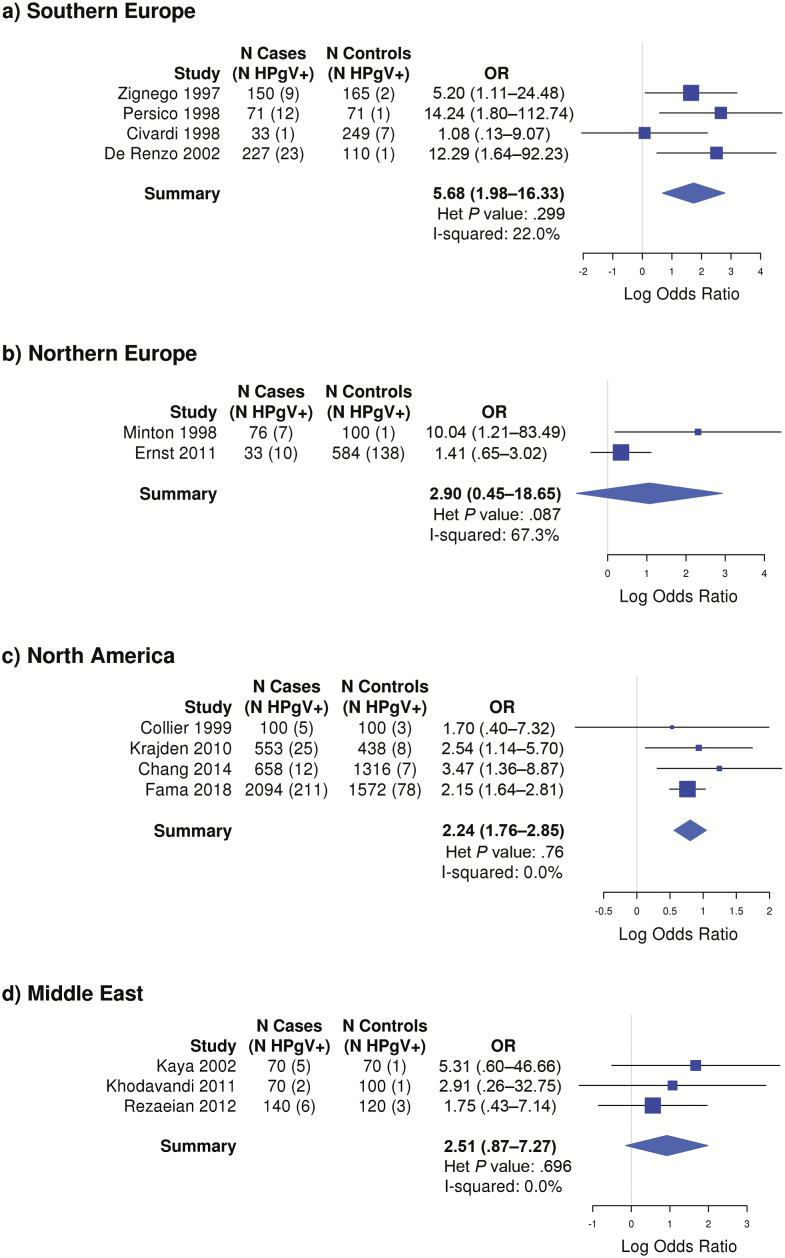
The strongest association was observed for studies from Southern Europe (OR, 5.68 [95% CI, 1.98–16.33]), while similar ORs were observed for studies from North America (OR, 2.24 [95% CI, 1.76–2.85]), Northern Europe (OR, 2.90 [95% CI, .45–18.65]), and the Middle East (OR, 2.51 [95% CI, .87–7.27]), although the latter 2 estimates were not statistically significant (Figure 3).
Figure 3.
Odds ratios and corresponding 95% confidence intervals for risk of lymphoma by human pegivirus viremia positivity, by geographic region. Abbreviations: Het, heterogeneity; HPgV, human pegivirus; OR, odds ratio.
Lymphoma Subtypes
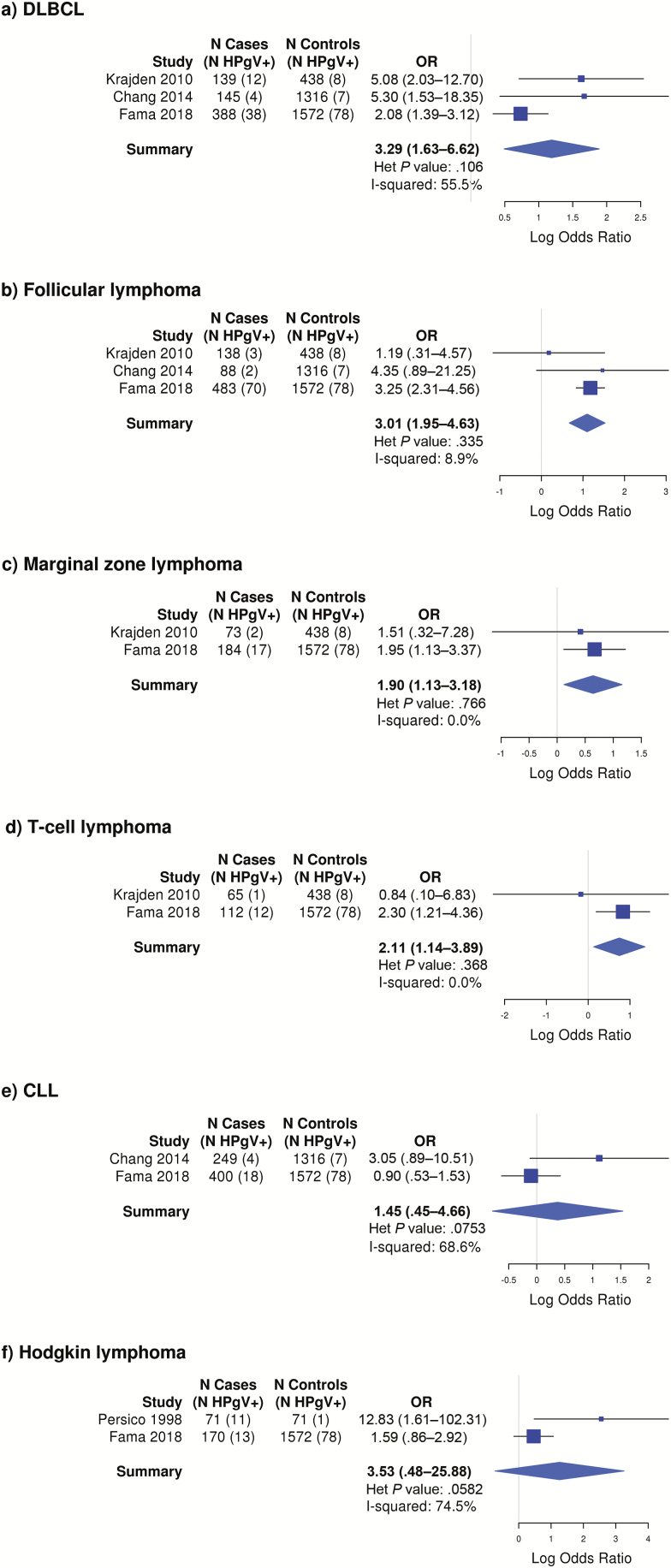
Elevated risk was observed for follicular lymphoma based on 3 studies (OR, 3.01 [95% CI, 1.95–4.63]), diffuse large B-cell lymphoma based on 3 studies (OR, 3.29 [95% CI, 1.63–6.62]), marginal zone lymphoma based on 2 studies (OR, 1.90 [95% CI, 1.13–3.18]), T-cell lymphoma based on 2 studies (OR, 2.11 [95% CI, 1.14–3.89]), CLL based on 2 studies (OR, 1.45 [95% CI, .45–4.66]), and HL based on 2 studies (OR, 3.53 [95% CI, .48–25.88]), with the latter 2 estimates not achieving statistical significance at P < .05 (Figure 4). None of the studies identified a characteristic clinical presentation or prognostic significance to lymphomas found in HPgV-infected patients.
Figure 4.
Odds ratios and corresponding 95% confidence intervals for risk of lymphoma by human pegivirus viremia positivity, by lymphoma subtype. Abbreviations: CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; Het, heterogeneity; HPgV, human pegivirus; OR, odds ratio.
DISCUSSION
In this first meta-analysis of HPgV viremia with lymphoma risk, we found a strong and statistically significant association with lymphoma risk overall (OR, 2.85) and for all of the major subtypes, except for CLL/SLL and HL. The association was much stronger in Southern Europe, and while risk estimates were weaker for North America, Northern Europe, and the Middle East, they were all of similar magnitude. While the results were robust, only 3 of 15 studies were rated as being of good methodologic quality, and the association attenuated approximately 20% to an OR of 2.25, suggesting the poorer quality studies slightly overestimated the association of HPgV viremia and lymphoma risk. We also did not find evidence for a strong role of coinfection by either HIV or HCV in explaining the HPgV and lymphoma association, noting we were not able to simultaneously assess HIV and HCV nor assess coinfection by HBV based on the published data. All but 1 study [4] was a case-control study and collected specimens at diagnosis, raising concerns about reverse causality. However, the results from case-control studies were consistent with the findings from a prospective cohort study [4] that used specimens banked before lymphoma diagnosis.
These results could have public health significance related to the safety of the blood supply. Approximately 2% of healthy US blood donors are viremic with HPgV at the time of blood donation, while individuals with sexually transmitted infections, people living with HIV, and intravenous drug users have a much higher rate of exposure and viremia, with a cross-sectional viremia prevalence of approximately 20%, as reviewed by Mohr and Stapleton [51]. To date, no definitive association between HPgV and clinical disease has been identified, and thus the US Food and Drug Administration has not recommended nor required screening of blood donors for HPgV viremia [52, 53]. This means that up to 200 000 units of GBV-C–contaminated blood products are transfused into recipients each year in the US [54]. If HPgV infection is truly a risk for lymphoma, the virus might represent a biomarker that potentially can be treated or prevented, raising a legitimate concern about the need to screen for and exclude HPgV-viremic donors from blood donation.
The overall association estimated by the random-effects model should be interpreted as the average of the ORs across the studies, and the main sources of heterogeneity of OR estimates between available studies must be considered before drawing conclusions on the real strength of the association between HPgV active infection and lymphoma. The most relevant source of heterogeneity was the prevalence of HPgV across the study subjects. Its wide variation might depend on the source of controls between the studies, including healthy blood donors, patients undergoing a given hospital procedure, people with HCV or HIV infection, or population-based samples. Another possible source of heterogeneity is the sensitivity of different methods for HPgV RNA detection, as noted above. We also only included results for viremic participants, and not those who were seropositive alone given concerns about the reproducibility of nonvalidated antibody test methods [34]. Furthermore, the relationship between prior viral clearance and NHL has not been clearly demonstrated and the prospective cohort study suggested that the association was more highly associated with active viremia [4]. If prior viremia is associated with NHL, our meta-analysis may underrepresent the contribution of HPgV to lymphoma. We were not able to fully assess the potential role of coinfections, particularly by HBV or simultaneous HCV and HBV. While most studies did not have data on transfusion history, a positive association was observed in studies that either excluded prior transfusion [45, 46] or adjusted for it the analysis [3, 5, 44]. An additional limit of our meta-analysis is the lack of adjustment for other potential confounding factors in most of the studies included in the analysis; however, the methodologically strongest studies in the analysis [3–5] observed positive associations after adjusting for a variety of potential confounders.
The biological mechanisms that may contribute to a role for HPgV viremia in lymphomagenesis include its being a lymphotropic virus that may cause persistent infection in both T and B lymphocytes [24, 30, 31]. In cell lines derived from HIV-infected patients, HPgV reduces Fas-mediated apoptosis [55] and virus impairs T-cell receptor and interleukin 2 receptor signaling in primary and transformed T-cell lines [56, 57] and in patients with HCV infection [58], all of which may contribute to subclinical impairment of immune surveillance. HPgV also influences cytokine and chemokine gene expression in lymphocytes in vitro and in cells obtained from infected individuals, potentially influencing immune response [59–62]. Finally, persistent lymphocyte infection could lead to DNA mutation and potentially malignant transformation. Alternatively, it could simply be a epiphenomenon attributable to both uncleared HPgV and lymphoma being more likely to be found in patients with suboptimal immune function.
In conclusion, this meta-analysis confirms that HPgV active infection is associated with an increased risk of developing lymphoma, including the major NHL subtypes except HL and CLL. Further epidemiological studies using banked samples from prospective cohort studies are needed to rule out reverse causality and understand latency, along with a comprehensive assessment of the potential role of coinfections (particularly for HBV) and blood transfusion. In addition, studies are needed to clarify biologic mechanisms underlying this association.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. F., J. T. S., and J. R. C. designed the study, identified the relevant publications, and abstracted key data elements. M. L., M. J. M., and S. L. S. conducted the statistical analysis. All authors interpreted the results, reviewed an initial draft of the manuscript, and approved the final version.
Financial support. This work was supported by the Department of Veterans Affairs (Merit Review grant numbers CX00821 and BX000207 to J. T. S.) and the National Institutes of Health (grant numbers P50 CA97274 and U01 CA195568 to J. R. C. and B. K. L.).
Potential conflicts of interest. M. J. M. has received grants from Celgene and Nanostring and personal fees from Morphosys. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016; 66:443–59. [DOI] [PubMed] [Google Scholar]
- 2. Cerhan JR, Vajdic CM, Spinelli JJ. The non-Hodgkin lymphomas. In: Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Schottenfeld and Fraumeni cancer epidemiology and prevention. 4th ed. New York: Oxford University Press, 2018:767–96. [Google Scholar]
- 3. Fama A, Xiang J, Link BK, et al. . Human pegivirus infection and lymphoma risk and prognosis: a North American study. Br J Haematol 2018; 182:644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang CM, Stapleton JT, Klinzman D, et al. . GBV-C infection and risk of NHL among U.S. adults. Cancer Res 2014; 74:5553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krajden M, Yu A, Braybrook H, et al. . GBV-C/hepatitis G virus infection and non-Hodgkin lymphoma: a case control study. Int J Cancer 2010; 126:2885–92. [DOI] [PubMed] [Google Scholar]
- 6. Simons JN, Leary TP, Dawson GJ, et al. . Isolation of novel virus-like sequences associated with human hepatitis. Nat Med 1995; 1:564–9. [DOI] [PubMed] [Google Scholar]
- 7. Linnen J, Wages J Jr, Zhang-Keck ZY, et al. . Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science 1996; 271:505–8. [DOI] [PubMed] [Google Scholar]
- 8. Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: a review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol 2011; 92:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simmonds P, Becher P, Bukh J, et al. . ICTV virus taxonomy profile: Flaviviridae. J Gen Virol 2017; 98:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adams MJ, King AM, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013). Arch Virol 2013; 158:2023–30. [DOI] [PubMed] [Google Scholar]
- 11. Berg MG, Lee D, Coller K, et al. . Discovery of a novel human pegivirus in blood associated with hepatitis C virus co-infection. PLoS Pathog 2015; 11:e1005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Wan Z, Xu R, et al. . A novel human pegivirus, HPgV-2 (HHpgV-1), Is tightly associated with hepatitis C virus (HCV) infection and HCV/human immunodeficiency virus type 1 coinfection. Clin Infect Dis 2018; 66:29–35. [DOI] [PubMed] [Google Scholar]
- 13. Heuft HG, Berg T, Schreier E, et al. . Epidemiological and clinical aspects of hepatitis G virus infection in blood donors and immunocompromised recipients of HGV-contaminated blood. Vox Sang 1998; 74:161–7. [PubMed] [Google Scholar]
- 14. Giordano TP, Henderson L, Landgren O, et al. . Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007; 297:2010–7. [DOI] [PubMed] [Google Scholar]
- 15. Engels EA, Chatterjee N, Cerhan JR, et al. . Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer 2004; 111:76–80. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci 2004; 95:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Sanjose S, Benavente Y, Vajdic CM, et al. . Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008; 6:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horemheb-Rubio G, Ramos-Cervantes P, Arroyo-Figueroa H, et al. . High HPgV replication is associated with improved surrogate markers of HIV progression. PLoS One 2017; 12:e0184494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tacke M, Schmolke S, Schlueter V, et al. . Humoral immune response to the E2 protein of hepatitis G virus is associated with long-term recovery from infection and reveals a high frequency of hepatitis G virus exposure among healthy blood donors. Hepatology 1997; 26:1626–33. [DOI] [PubMed] [Google Scholar]
- 20. Thomas DL, Nakatsuji Y, Shih JW, et al. . Persistence and clinical significance of hepatitis G virus infections in injecting drug users. J Infect Dis 1997; 176:586–92. [DOI] [PubMed] [Google Scholar]
- 21. Tillmann HL, Heringlake S, Trautwein C, et al. . Antibodies against the GB virus C envelope 2 protein before liver transplantation protect against GB virus C de novo infection. Hepatology 1998; 28:379–84. [DOI] [PubMed] [Google Scholar]
- 22. Berg T, Müller AR, Platz KP, et al. . Dynamics of GB virus C viremia early after orthotopic liver transplantation indicates extrahepatic tissues as the predominant site of GB virus C replication. Hepatology 1999; 29:245–9. [DOI] [PubMed] [Google Scholar]
- 23. Fan X, Xu Y, Solomon H, Ramrakhiani S, Neuschwander-Tetri BA, Di Bisceglie AM. Is hepatitis G/GB virus-C virus hepatotropic? Detection of hepatitis G/GB virus-C viral RNA in liver and serum. J Med Virol 1999; 58:160–4. [PubMed] [Google Scholar]
- 24. George SL, Varmaz D, Stapleton JT. GB virus C replicates in primary T and B lymphocytes. J Infect Dis 2006; 193:451–4. [DOI] [PubMed] [Google Scholar]
- 25. Xiang J, Wünschmann S, Schmidt W, Shao J, Stapleton JT. Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4-positive T cells. J Virol 2000; 74:9125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. George SL, Xiang J, Stapleton JT. Clinical isolates of GB virus type C vary in their ability to persist and replicate in peripheral blood mononuclear cell cultures. Virology 2003; 316:191–201. [DOI] [PubMed] [Google Scholar]
- 27. Kisiel E, Cortez KC, Pawełczyk A, et al. . Hepatitis G virus/GBV-C in serum, peripheral blood mononuclear cells and bone marrow in patients with hematological malignancies. Infect Genet Evol 2013; 19:195–9. [DOI] [PubMed] [Google Scholar]
- 28. Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Detection of hepatitis G virus replication sites by using highly strand-specific Tth-based reverse transcriptase PCR. J Virol 1998; 72:3072–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jabłońska J, Ząbek J, Pawełczyk A, et al. . Hepatitis C virus (HCV) infection of peripheral blood mononuclear cells in patients with type II cryoglobulinemia. Hum Immunol 2013; 74:1559–62. [DOI] [PubMed] [Google Scholar]
- 30. Tucker TJ, Smuts HE, Eedes C, et al. . Evidence that the GBV-C/hepatitis G virus is primarily a lymphotropic virus. J Med Virol 2000; 61:52–8. [PubMed] [Google Scholar]
- 31. Chivero ET, Bhattarai N, Rydze RT, Winters MA, Holodniy M, Stapleton JT. Human pegivirus RNA is found in multiple blood mononuclear cells in vivo and serum-derived viral RNA-containing particles are infectious in vitro. J Gen Virol 2014; 95:1307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bailey AL, Lauck M, Mohns M, et al. . Durable sequence stability and bone marrow tropism in a macaque model of human pegivirus infection. Sci Transl Med 2015; 7:305ra144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Souza IE, Allen JB, Xiang J, et al. . Effect of primer selection on estimates of GB virus C (GBV-C) prevalence and response to antiretroviral therapy for optimal testing for GBV-C viremia. J Clin Microbiol 2006; 44:3105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blankson JN, Klinzman D, Astemborski J, Thomas DL, Kirk GD, Stapleton JT. Low frequency of GB virus C viremia in a cohort of HIV-1-infected elite suppressors. AIDS 2008; 22:2398–400. [DOI] [PubMed] [Google Scholar]
- 35. Wells GA, Shea B, O’Connell DL, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Canada: Ottawa Hospital Research Institute; Available at: www.ohri.ca/programs/ clinical_epidemiology/oxford.htm Accessed 1 March 2019. [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwarzer G. meta: An R package for meta-analysis. R News 2007; 7:40–5. Available at: https://cran.r-project.org/doc/Rnews/Rnews_2007-3. Accessed 2 January 2019. [Google Scholar]
- 38. Lumley T. rmeta: Meta-analysis. R package version 2.16 2012. Available at: https://CRAN.R-project.org/package=rmeta. Accessed 2 January 2019.
- 39. Zignego AL, Ferri C, Giannini C, et al. . Hepatitis C virus infection in mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma: evidence for a pathogenetic role. Arch Virol 1997; 142:545–55. [DOI] [PubMed] [Google Scholar]
- 40. Minton J, Iqbal A, Eskiturk A, Irving W, Davies J. Hepatitis G virus infection in lymphoma and in blood donors. J Clin Pathol 1998; 51:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Persico M, De Renzo A, Persico E, Notaro R, Torella R, Rotoli B. Hepatitis G virus in patients with Hodgkin’s lymphoma. Br J Haematol 1998; 103:1206–7. [DOI] [PubMed] [Google Scholar]
- 42. Civardi G, Tanzi E, Ferrari B, Vallisa D, Zanetti A, Cavanna L. High prevalence of anti-HGV/E2 antibodies in HCV-positive patients with non-Hodgkin’s lymphoma. Haematologica 1998; 83:957–8. [PubMed] [Google Scholar]
- 43. Ogino H, Satomura Y, Unoura M, et al. . Hepatitis B, C and G virus infection in patients with lymphoproliferative disorders. Hepatol Res 1999; 14:187–94. [Google Scholar]
- 44. Collier JD, Zanke B, Moore M, et al. . No association between hepatitis C and B-cell lymphoma. Hepatology 1999; 29:1259–61. [DOI] [PubMed] [Google Scholar]
- 45. De Renzo A, Persico E, de Marino F, et al. . High prevalence of hepatitis G virus infection in Hodgkin’s disease and B-cell lymphoproliferative disorders: absence of correlation with hepatitis C virus infection. Haematologica 2002; 87:714–8; discussion 8. [PubMed] [Google Scholar]
- 46. Kaya H, Polat MF, Erdem F, Gündogdu M. Prevalence of hepatitis C virus and hepatitis G virus in patients with non-Hodgkin’s lymphoma. Clin Lab Haematol 2002; 24:107–10. [DOI] [PubMed] [Google Scholar]
- 47. Giannoulis E, Economopoulos T, Mandraveli K, et al. . Hellenic Cooperative Oncology Group Study The prevalence of hepatitis C and hepatitis G virus infection in patients with B cell non-Hodgkin lymphomas in Greece: a Hellenic Cooperative Oncology Group Study. Acta Haematol 2004; 112:189–93. [DOI] [PubMed] [Google Scholar]
- 48. Ernst D, Pischke S, Greer M, Wedemeyer H, Stoll M. No increased incidence for GB-virus C infection in a cohort of HIV-positive lymphoma patients. Int J Cancer 2011; 128:3013; author reply 3012. [DOI] [PubMed] [Google Scholar]
- 49. Khodavandi A, Alizadeh F, Harmal NS, et al. . Expression analysis of SIR2 and SAPs1-4 gene expression in Candida albicans treated with allicin compared to fluconazole. Trop Biomed 2011; 28:589–98. [PubMed] [Google Scholar]
- 50. Rezaeian AA, Yaghobi R, Nia MR, et al. . Etiology of hepatitis G virus (HGV) and hepatitis type C virus (HCV) infections in non-Hodgkin’s lymphoma patients in Southern Iran. Afr J Biotechnol 2012; 11:11659–64. [Google Scholar]
- 51. Mohr EL, Stapleton JT. GB virus type C interactions with HIV: the role of envelope glycoproteins. J Viral Hepat 2009; 16:757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alter HJ. G-pers creepers, where’d you get those papers? A reassessment of the literature on the hepatitis G virus. Transfusion (Paris) 1997; 37:569–72. [DOI] [PubMed] [Google Scholar]
- 53. Theodore D, Lemon SM. GB virus C, hepatitis G virus, or human orphan flavivirus? Hepatology 1997; 25:1285–6. [DOI] [PubMed] [Google Scholar]
- 54. Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377:1261–72. [DOI] [PubMed] [Google Scholar]
- 55. Moenkemeyer M, Schmidt RE, Wedemeyer H, Tillmann HL, Heiken H. GBV-C coinfection is negatively correlated to Fas expression and Fas-mediated apoptosis in HIV-1 infected patients. J Med Virol 2008; 80:1933–40. [DOI] [PubMed] [Google Scholar]
- 56. Bhattarai N, McLinden JH, Xiang J, Kaufman TM, Stapleton JT. GB virus C envelope protein E2 inhibits TCR-induced IL-2 production and alters IL-2-signaling pathways. J Immunol 2012; 189:2211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhattarai N, McLinden JH, Xiang J, Landay AL, Chivero ET, Stapleton JT. GB virus C particles inhibit T cell activation via envelope E2 protein-mediated inhibition of TCR signaling. J Immunol 2013; 190:6351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bhattarai N, McLinden JH, Xiang J, et al. . Hepatitis C virus infection inhibits a Src-kinase regulatory phosphatase and reduces T cell activation in vivo. PLoS Pathog 2017; 13:e1006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stapleton JT, Chaloner K, Martenson JA, et al. . GB virus C infection is associated with altered lymphocyte subset distribution and reduced T cell activation and proliferation in HIV-infected individuals. PLoS One 2012; 7:e50563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lanteri MC, Vahidnia F, Tan S, et al. . NHLBI REDS III Study Downregulation of cytokines and chemokines by GB virus C after transmission via blood transfusion in HIV-positive blood recipients. J Infect Dis 2015; 211:1585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT. Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1alpha, MIP-1beta, and SDF-1. Lancet 2004; 363:2040–6. [DOI] [PubMed] [Google Scholar]
- 62. Stapleton JT, Martinson JA, Klinzman D, Xiang J, Desai SN, Landay A. GB virus C infection and B-cell, natural killer cell, and monocyte activation markers in HIV-infected individuals. AIDS 2013; 27:1829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.