Abstract
Reactive oxygen species (ROS) including superoxide (O2•-) play an important role in a variety of diseases, including Alzheimer’s Disease, cancer, and atherosclerosis. Early reports showed that O2•- is a stimulant for collagen synthesis. However, the mechanism remains incompletely understood. Here we showed that LY83583 (6-anilinoquinoline-5,8-quinone), a substance known to induce O2•- production by smooth muscle cell (SMC), increases Type I collagen secretion. This effect could be blocked by treating the cells with Tiron, a scavenger for O2•−. LY83583-induced Type I collagen secretion required P4HA1 and P4HA2. Knockout of either P4ha1 or P4ha2 greatly reduced LY83583-stimulated Type I collagen maturation whereas silencing of both P4ha1 and P4ha2 completely blocked LY83583-induced Type I collagen maturation. Although significantly more hydroxyproline on purified Type I collagen was detected from LY83583 treated mouse embryonic fibroblast (MEF) cells by mass spectrometry, the level of prolyl 4-hydroxylases was not altered. Thus, LY83583 might increase the enzymatic activity of prolyl 4-hydroxylases to increase Type I collagen maturation. In addition, we found that LY83583 activated prolyl 4-hydrolases differed from ascorbate-activated prolyl 4-hydroxylase in two aspects: (1) LY83583 activated both P4HA1 and P4HA2 involved in collagen maturation whereas ascorbate mainly stimulated P4HA1 in collagen maturation; (2) LY83583 did not induce N259 glycosylation on P4HA1 as ascorbate did. The mechanisms remain to be investigated.
Keywords: Reactive oxygen species (ROS), Superoxide, Prolyl 4-Hydroxylases, Type I collagen
1.1. Introduction
Reactive oxygen species (ROS) including superoxide (O2•-), hydrogen peroxide (H2O2), hydroxyl anions (•OH) and nitric oxide (NO) have emerged as important molecules in a variety of diseases, including Alzheimer’s Disease, cancer, and atherosclerosis. ROS act as intracellular secondary messengers to regulate signal transduction pathways that ultimately control gene expression and post-translational modification of proteins [1].
Vascular endothelium is a major source of O2•- generation [2–4], but vascular smooth muscle cell (SMC) and fibroblasts also can generate ROS [5–7]. Several superoxide producing enzyme systems such as NAD(P)H oxidase [8, 9], xanthine oxidase [10], lipoxygenase, mitochondrial oxidases and NO synthases [11] are found in the vascular wall. NAD(P)H oxidase has been implicated as an important source for superoxide production in cell and animal models of hypercholesterolemia and hypertension [12, 13].
Intimal hyperplasia is a common cause of late vascular graft failure and is characterized by SMC accumulation and extracellular matrix deposition. Collagen is the major extracellular matrix protein in arterial wall and 70% of all collagen present in arteries is Type I collagen. Type I collagen protein is the product of two genes, COL1A1 and COL1A2, which are located on two different chromosomes. Two chains of Type I collagen α1 (col 1 α1) and one chain of Type I collagen α2 (col 1 α2) form a triple-stranded helix. There are some early reports to show that O2•- is a stimulant for collagen synthesis [14–16], but the mechanism of O2•--induced collagen production has not been fully explored.
In the present study, the effect O2•- on Type I collagen secretion was assessed. LY83583 (6-anilinoquinoline-5,8-quinone), a benzoquinone derivative a substance known to induce O2•- production by SMC [17] and inhibit soluble guanylate cyclase and modulate cGMP [18, 19], was used to generate O2•- in mouse embryonic fibroblast (MEF) cells and primary aortic SMC. LY83583 induced Type I collagen secretion that required prolyl 4-hydroxylasess(P4Hs). Silencing either P4ha1 or P4ha2 greatly reduced LY83583-induced Type I collagen secretion in the culture media whereas silencing both P4ha1 and P4ha2 blocked LY83583-induced Type I collagen secretion in the culture media. Most importantly, LY83583 addition resulted in significantly more proline hydroxylation on Type I collagen. However, unlike ascorbate-induced Type I collagen secretion, LY83583 stimulation did not induce N259 glycosylation on P4HA1 and amount of secreted Type I collagen was obviously less than that stimulated by ascorbate. The mechanism warrants further investigation.
1.2. Materials and Methods
The primers used in the paper were listed in Supplemental Table 1 and Table 2. The materials and methods were described in detail in supplemental information.
1.3. Results
1.3.1. Collagen secretion increased by LY83583 in aortic SMC and MEF cells is independent of Col1a1/1a2 transcription
LY83583 has been shown to stimulate O2•- in aortic SMC to produce Type I collagen [20]; however, the mechanism remains incompletely understood. Therefore, we investigated the mechanisms of LY83583-induced Type I collagen production in MEF cells as well as in mouse aortic SMC. When MEF cells were treated with LY83583, O2•- production was increased, which was blocked by Tiron (2.5 μM) (Fig. 1a). In addition, Type I collagen secretion by MEF cells and SMC was increased by 3 hrs after LY83583 treatment (Supplemental Fig. 1), similar to a previous report [21]. Immunoblotting with Type I collagen specific antibody confirmed that more Type I collagen could be detected in the culture medium 6 hrs post treatment (Fig. 1b). These results suggested that LY83583 modulated Type I collagen production, maturation, or secretion.
Figure 1. LY83583 stimulated O2•- dependent collagen secretion is independent of COL1a1/2 transcription.

(a) Incubation of MEF cells with LY83583 induced production of O2•- which could be blocked by O2•-scavenger Tiron. (b) Immunoblotting with Type I collagen specific antibody showed that more Type I collagen was secreted into the culture medium 6 hrs post LY83583 addition. (c) Addition of LY83583 for 6 hrs induced pepsin-resistant Type I collagen in the culture medium. (d) LY83583-induced pepsin-resistant Type I collagen secretion into the culture medium of MEF cells was blocked by O2•- scavenger Tiron. (e) Pepsin-resistant Type I collagen could be detected in the culture medium 3 hrs after addition of LY83583. Accordingly, less Type I collagen was detected from the cell lysates. (f) 0.5 μM of LY83583 induced detectable pepsin-resistant Type I collagen in the culture medium. Accordingly, less Type I collagen was detected in the cell lysates. (g) LY83583 stimulation did not result in significant differences in Col1a1 or Col1a2 mRNA level within 6 hrs of treatment based on RT-qPCR. (h) Immunoblotting with Type I collagen specific antibody showed that blocking mRNA transcription by Act D did not significantly reduce pepsin-resistant Type I collagen in the culture medium induced by LY83583. Laminin and actin were used as loading control for total proteins in the culture medium or cell lysates. Medium or cell lysate collagen to actin was quantified by IMAGE J software. SMC: smooth muscle cell; MEF: mouse embryonic fibroblast; Col 1 α1/2: Type I collagen α1/2; Actinomycin D: Act D. * indicated the position of col 1 α2 or pepsin-resistant col 1 α2. P values were indicated in each panel if available. Other than indicated, all experiments were independently repeated at least three times with similar results.
To differentiate the process responsible for the observed increase in collagen in the culture medium, pepsin was added to the culture medium to determine if the collagen present was mature collagen, which is relatively resistant to pepsin treatment. The collagen induced by LY83583 was resistant to pepsin treatment (Fig. 1c), suggesting that LY83583 stimulated collagen maturation.
To investigate if the enhanced secretion of mature collagen was due to increased production of O2•-, MEF cells were incubated with LY83583 and Tiron. Tiron totally blocked the production of pepsin-resistant collagen (Fig. 1d), suggesting that LY83583-induced O2•- production modulated collagen maturation and secretion.
If LY83583 addition simply increased Type I collagen secretion, more Type I collagen would be seen entering Golgi apparatus to be secreted and less would be retained intracellularly. Indeed, we detected more Type I collagen entry into Golgi apparatus after LY83583 addition (Supplemental Fig. 2). By immunoblotting, less Type I collagen in cell lysate was detected 3 and 6 hrs after treatment (Fig. 1e). Accordingly, more pepsin-resistant Type I collagen was detected in the culture medium (Fig.1e). These results suggested that LY83583 stimulated Type I collagen secretion as well as maturation. When MEF cells were treated with different concentrations of LY83583, 0.25 μM LY83583 increased pepsin-resistant Type I collagen in the culture medium (Fig. 1f).
Although the above results suggested that LY83583 stimulated Type I collagen secretion, ROS have also been shown to up-regulate collagen I and III gene expression [22–25]. Therefore, we investigated if the increased collagen in the culture media was due to enhanced transcription. RT-qPCR was performed to quantify the Type I collagen mRNA levels from MEF cells incubated with or without LY83583. No significant differences in Type I collagen transcripts were found (Fig 1g). In addition, treatment with actinomycin D did not reduce the amount of LY83583-induced pepsin-resistant type I collagen in the medium as measured by immunoblot analysis (Fig. 1h); and did not retain more intracellular Type I collagen (Fig. 1h). These results confirmed that LY83583-induced Type I collagen secretion was independent of Col1a1/1a2 transcription.
1.3.2. P4HA1 and P4HA2 are both contributing to LY83583-induced Type I collagen secretion
Two prolyl 4-hydroxylases P4HA1 and P4HA2 were detected in MEF cells [26]. Earlier reports showed that ROS increase prolyl hydroxylase activity and collagen production [27, 28]. Since LY83583-induced Type I collagen secretion is likely through prolyl hydroxylase, the effect of LY83583 on expression of P4HA1 and P4HA2 was assessed. Incubation of MEF cells with LY83583 did not enhance the mRNA levels of P4HA1 and P4HA2 (Fig. 2a), and the protein levels of P4HA1 and P4HA2 were not increased (Fig. 2b). On the contrary, the levels of P4HA1 and P4HA2 were significantly decreased 6 hrs after LY83583 treatment (Fig. 2b, right panels). Hence, increased Type I collagen secretion was not due to increased P4HA1 or P4HA2. To differentiate the role these two enzymes played in LY83583-stimulated collagen secretion, P4ha1 or/and P4ha2 were knocked-out in MEF cells with CRISPR/Cas 9. Silencing either P4ha1 or P4ha2 significantly diminished pepsin-resistant Type I collagen in the culture medium (Fig. 2c and 2d). Silencing P4ha1 slightly enhanced the protein level of P4HA2 whereas silencing P4ha2 slightly increased P4HA1 protein level (Fig. 2c and 2d). When both P4ha1 and P4ha2 were silenced, we could not detect any pepsin-resistant Type I collagen in the culture medium (Fig. 2e). These results suggested that both P4HA1 and P4HA2 are involved in LY83583-induced Type I collagen maturation, which is different from ascorbate-induced Type I collagen maturation [26].
Figure 2. LY83583 stimulated Type I collagen secretion depends on P4HA1 and P4HA2 activity but not their expression level.
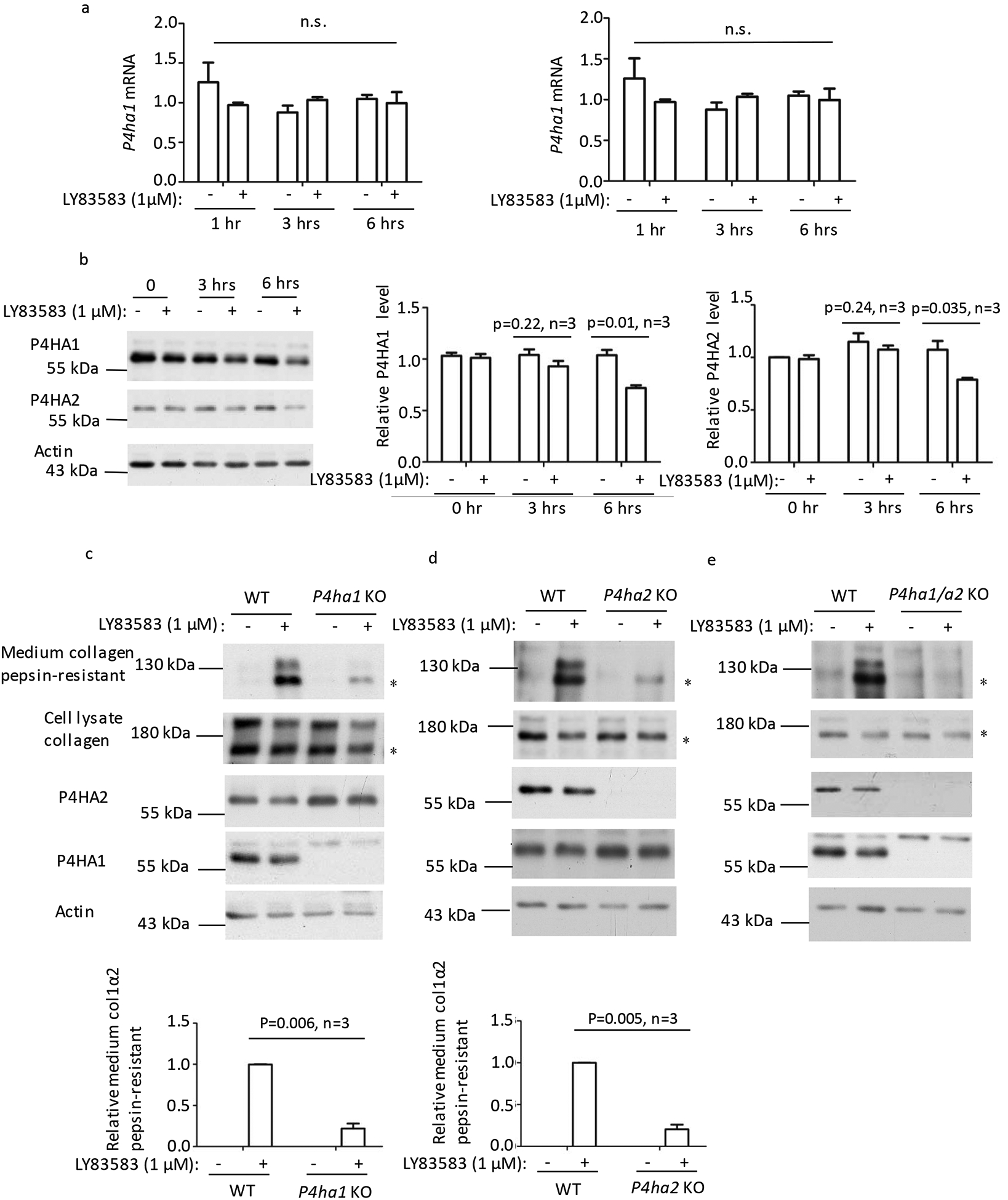
(a) Addition of LY83583 did not result in significant differences in P4ha1 or P4ha2 mRNA level within 6 hrs based on RT-qPCR. (b) Immunoblotting with P4HA1 or P4HA2 specific antibody showed that LY83583 did not enhance the levels of these two enzymes. In fact, significantly less P4HA1 or P4HA2 was detected by immunoblotting 6 hrs after LY83583 addition. P4HA1/actin or P4HA2/actin was quantified by IMAGE J software by arbitrary defining the signal at 0 hr after addition of LY83583 as 1. (c, d and e) Silencing P4ha1 (c) or P4ha2 (d) greatly decreased the amount of pepsin-resistant Type I collagen in the culture medium (panel 1) but slightly enhanced the level of P4HA2 (c-panel 3) or P4HA1 (d-panel 4). Silencing both p4ha1 and p4ha2 (e) totally blocked pepsin-resistant Type I collagen in the culture medium. Pepsin-resistant collagen in the culture medium to actin was quantified by IMAGE J software and is depicted graphically. * indicated pepsin-resistant col 1 α2. N.s.: not statistically significant. P values were indicated in each panel if available.
1.3.3. N259 glycosylation of P4HA1 is not required for LY83583-stimulated collagen secretion
Since P4HA1 participates in LY83583-induced Type I collagen maturation, and previously we showed that N259 glycosylation on P4HA1 is required to alter P4HA1 binding Type I collagen when MEF cells were treated with ascorbate [26], the effect of LY83583 on N259 glycosylation on P4HA1 was investigated. LY83583 did not appear to induce N259 glycosylation as evidenced by the lack of band shift seen with ascorbate (Fig. 3a, panel 3 indicated by an arrow).
Figure 3. LY83583 stimulated collagen secretion is independent of P4HA1 N259 glycosylation.
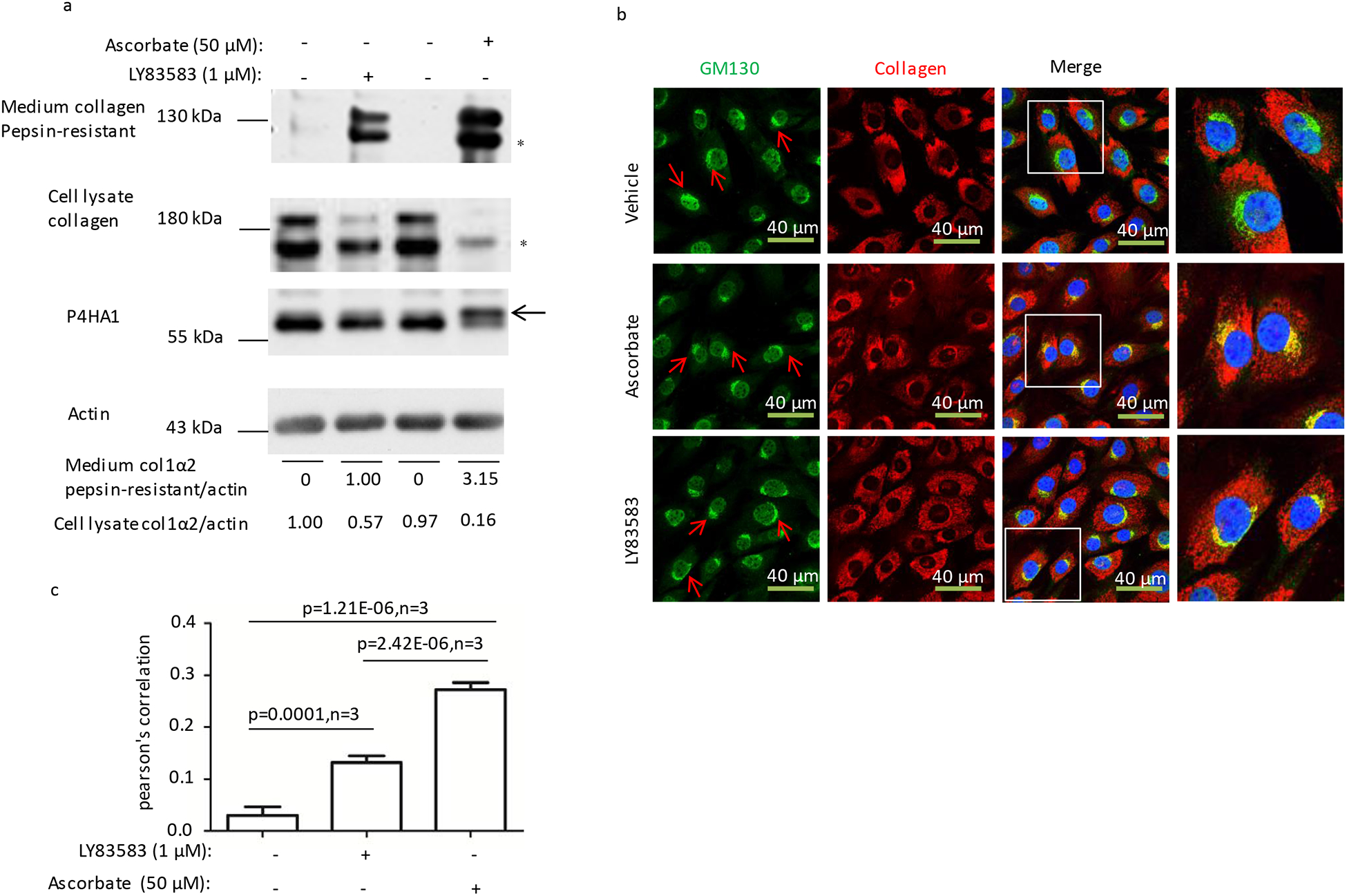
(a) Ascorbate but not LY83583 treatment for 6 hrs induced N259 glycosylation on P4HA1 (panel 3, indicated by an arrow) and more pepsin-resistant Type I collagen in the culture medium (panel 1). (b and c) Ascorbate treatment induced significantly more type I collagen (red) entering Golgi apparatus (green stained by GM130, indicated by red arrows). The higher resolution parts in the panels were indicated by the squares. Statistical analysis was performed from three different immunofluorescence staining experiments. Co-localization index (Pearson’s correlation) was calculated based on NIS-Elements AR software. Collagen in the culture medium or lysate to actin was quantified by IMAGE J software. * indicated pepsin-resistant col 1 α2. P values were indicated in each panel if available.
The level of pepsin-resistant Type I collagen in culture medium was lower than that induced by ascorbate (Fig. 3a, panel 1). Accordingly, more cell lysate collagen was detected (Fig. 3a, panel 2). To explore this further, the amount of collagen entering the Golgi apparatus was investigated. A lower percentage of collagen entered the Golgi apparatus when MEF were incubated with LY83583 compared with ascorbate (Fig. 3b), and hence less Type I collagen was secreted. Statistical analysis of 3 independent experiments confirmed that significantly more Type I collagen entered the Golgi apparatus when the cells were treated with LY83583 as compared to vehicle treated cells (Fig. 3c).
1.3.4. LY83583 treatment enhanced proline hydroxylation on Type I collagen.
The above observations suggested that LY83583 could increase the proline hydroxylation in Type I collagen. To test this, Type I collagen was concentrated and similar amounts of Type I collagen were loaded for electrophoresis and detected with a polyclonal antibody specific for hydroxyproline. LY83583 induced more proline hydroxylation on Type I collagen (Fig. 4a). In addition, 0.25 μM LY83583 was sufficient to increase the hydroxyproline in Type I collagen in the cell culture medium (Fig. 4b). Mass spectrometry demonstrated significantly more hydroxyproline on Type I collagen in the conditioned medium of cells incubated with LY83583 (Table 1 and Supplemental table 3). Therefore, LY83583 stimulates P4HA1/2 to hydroxylate proline on Type I collagen.
Figure 4. LY83583 treatment enhanced proline hydroxylation on Type I collagen.
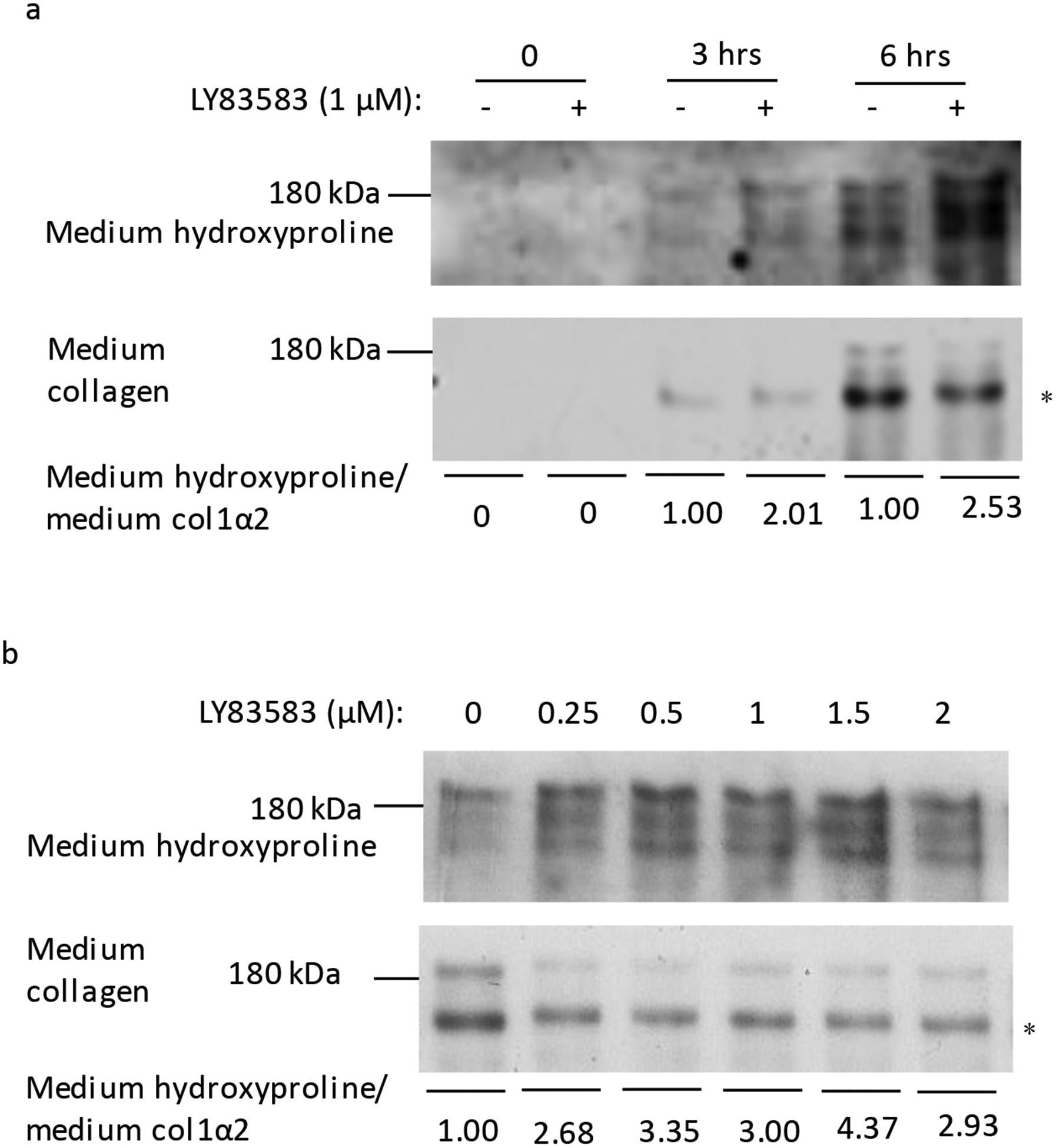
(a) Similar amount of affinity purified type I collagen from MEF cells culture medium was blotted with anti-hydroxyproline specific antibody (panel 1) or anti-Type I collagen specific antibody (panel 2). More hydroxyproline was detected from samples 6 hrs after addition of LY83583. (b) To assess the effect of LY83583 at 6 hrs on hydroxyproline content, protein from MEF cells culture medium was concentrated, separated by SDS-PAGE, probed with antibody to Type I collagen, and the blot stripped and re-probed with antibody specific for hydroxyproline. More hydroxyproline was detected after addition of LY83583. * indicated pepsin-resistant col 1 α2.
Table 1.
Type I collagen containing hydroxyproline and peptide hits by mass spectrometry.
| Type I collagen | Group | Number of identical peptide | Number of hydroxyproline | Ratio | Chi-square p-value |
|---|---|---|---|---|---|
| Col1α1 | Vehicle | 495 | 538 | 1.08 | 0.001 |
| LY83583 | 512 | 731 | 1.43 | ||
| Col1α2 | Vehicle | 267 | 246 | 0.92 | 0.033 |
| LY83583 | 247 | 296 | 1.20 |
1.4. Discussion
Many reports show that collagen or prolyl 4-hydroxylase is normally regulated at the level of transcription [27, 29–33]. Our data suggests that after an oxidative stress, collagen secretion is increased by enhanced activity of P4HA1 and P4HA2, Neither Type I collagen nor P4HA1 and P4HA2 mRNA level was elevated within 6 hrs following LY83583 addition. More pepsin-resistant Type I collagen could be detected in the culture medium after LY83583 addition. Silencing either P4ha1 or P4ha2 greatly reduced LY83583-induced pepsin-resistant Type I collagen in the culture medium. Silencing both P4ha1 and P4ha2 blocked LY83583-induced pepsin-resistant Type I collagen in the culture medium. Hydroxyproline specific antibody detected more signals from LY83583-induced Type I collagen from conditioned medium. Mass spectrometry identified significantly more hydroxyproline on Type I collagen purified from culture medium of cells incubated with LY83583. Blocking O2•- by Tiron prevented the increase in pepsin-resistant Type I collagen secretion, suggesting that the effect of LY83583 is mediated by its stimulation of O2•- production.
The ability of ROS to increase Type I collagen maturation has been observed [16], and it is hypothesized that prolyl 4-hydroxylase is involved [34]. We show that P4HA1 and P4HA2 participate in LY83583-induced Type I collagen maturation process. Although the exact mechanism by which O2•- enhances prolyl hydroxylase activity remains elusive, the enzymes involved in O2•--induced Type I collagen maturation and ascorbate-induced Type I collagen maturation are not identical. For O2•--induced Type I collagen, both P4HA1 and P4HA2 participate and N259 glycosylation of P4HA1 is not required. On the other hand, in ascorbate-induced Type I collagen maturation P4HA1 is mainly involved, and this effect is significantly enhanced by N259 glycosylation of P4HA1 [26]. The cause of the decreased amount of P4HA1 or P4HA2 protein but increased amount of secreted Type I collagen 6 hrs after LY83583 addition is unclear. The absolute amounts of P4HA1 and P4HA2 have not been measured, and immunoblot analysis might be misleading due to differences in the affinities of the P4HA1 or P4HA2 antibodies to their corresponding enzymes, but this should not affect the relative change in the amount of each prolyl hydroxylase.
Although we provide evidence that LY83583 increases O2•- production in SMC and MEF cells. This leads to enhanced Type I collagen maturation and secretion with increased hydroxylation of proline on Type I collagen via prolyl 4-hydroxylases. We do not identify specific proline residues that are hydroxylated. We can not exclude the possibility that other prolyl hydroxylases also contribute to LY83583-induced Type I collagen maturation. Furthermore, we cannot exclude other possible effects of O2•- in the production, maturation, and secretion of collagen.
Supplementary Material
Highlights.
Intimal hyperplasia is characterized by smooth muscle cell accumulation and Type I collagen deposition. Superoxide could induce Type I collagen maturation and secretion.
Type I collagen maturation induced by short period of superoxide was not due to transcription of Type I collagen and prolyl hyrdoxylases (P4HAs).
Superoxide activated Type I collagen maturation depends on P4HA1 and P4HA2, which is mechanistically different from ascorbate.
Superoxide could induce more hydroxyproline on Type I collagen.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH/NHLBI) (Grants HL41178 and HL64357 to LMG), by MOST (2018YFA0507201 to CL), by National Natural Science Foundation of China (30670170 to CL), and by Guangzhou Key Medical Discipline Construction Project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
Reference:
- [1].Kunsch C, Medford RM, Oxidative stress as a regulator of gene expression in the vasculature, Circulation research, 85 (1999) 753–766. [DOI] [PubMed] [Google Scholar]
- [2].Mohazzab KM, Kaminski PM, Wolin MS, NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium, The American journal of physiology, 266 (1994) H2568–2572. [DOI] [PubMed] [Google Scholar]
- [3].Sanders SP, Zweier JL, Kuppusamy P, Harrison SJ, Bassett DJ, Gabrielson EW, Sylvester JT, Hyperoxic sheep pulmonary microvascular endothelial cells generate free radicals via mitochondrial electron transport, The Journal of clinical investigation, 91 (1993) 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW, Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells, Circulation research, 74 (1994) 1141–1148. [DOI] [PubMed] [Google Scholar]
- [5].Miller FJ Jr., Gutterman DD, Rios CD, Heistad DD, Davidson BL, Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis, Circulation research, 82 (1998) 1298–1305. [DOI] [PubMed] [Google Scholar]
- [6].Meier B, Cross AR, Hancock JT, Kaup FJ, Jones OT, Identification of a superoxide-generating NADPH oxidase system in human fibroblasts, The Biochemical journal, 275 ( Pt 1) (1991) 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meier B, Radeke HH, Selle S, Younes M, Sies H, Resch K, Habermehl GG, Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha, The Biochemical journal, 263 (1989) 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG, Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone, The Journal of clinical investigation, 97 (1996) 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ushio-Fukai M, Zafari AM, Fukui T, Ishizaka N, Griendling KK, p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells, The Journal of biological chemistry, 271 (1996) 23317–23321. [DOI] [PubMed] [Google Scholar]
- [10].White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA, Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits, Proc. Natl. Acad. Sci. U. S. A, 93 (1996) 8745–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vásquez-Vivar J, Kalyanaraman B, Martásek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr., Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors, Proc. Natl. Acad. Sci. U. S. A, 95 (1998) 9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK, Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy, Hypertension (Dallas, Tex. : 1979), 32 (1998) 488–495. [DOI] [PubMed] [Google Scholar]
- [13].Channon KM, Qian H, Neplioueva V, Blazing MA, Olmez E, Shetty GA, Youngblood SA, Pawloski J, McMahon T, Stamler JS, George SE, In vivo gene transfer of nitric oxide synthase enhances vasomotor function in carotid arteries from normal and cholesterol-Fed rabbits, Circulation, 98 (1998) 1905–1911. [DOI] [PubMed] [Google Scholar]
- [14].Tanaka H, Okada T, Konishi H, Tsuji T, The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts, Archives of dermatological research, 285 (1993) 352–355. [DOI] [PubMed] [Google Scholar]
- [15].Chandrakasan G, Bhatnagar RS, Stimulation of collagen synthesis in fibroblast cultures by superoxide, Cellular and molecular biology, 37 (1991) 751–755. [PubMed] [Google Scholar]
- [16].Hussain MZ, Bhatnagar RS, Involvement of superoxide in the paraquat-induced enhancement of lung collagen synthesis in organ culture, Biochemical and biophysical research communications, 89 (1979) 71–76. [DOI] [PubMed] [Google Scholar]
- [17].Baas AS, Berk BC, Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells, Circulation research, 77 (1995) 29–36. [DOI] [PubMed] [Google Scholar]
- [18].Lee YS, Wurster RD, Mechanism of potentiation of LY83583-induced growth inhibition by sodium nitroprusside in human brain tumor cells, Cancer chemotherapy and pharmacology, 36 (1995) 341–344. [DOI] [PubMed] [Google Scholar]
- [19].Kontos HA, Wei EP, Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583, Stroke, 24 (1993) 427–434. [DOI] [PubMed] [Google Scholar]
- [20].Absood A, Furutani A, Kawamura T, Graham LM, A comparison of oxidized LDL-induced collagen secretion by graft and aortic SMCs: role of PDGF, American journal of physiology. Heart and circulatory physiology, 287 (2004) H1200–1206. [DOI] [PubMed] [Google Scholar]
- [21].Patel R, Cardneau JD, Colles SM, Graham LM, Synthetic smooth muscle cell phenotype is associated with increased nicotinamide adenine dinucleotide phosphate oxidase activity: effect on collagen secretion, Journal of vascular surgery, 43 (2006) 364–371. [DOI] [PubMed] [Google Scholar]
- [22].Lijnen P, Papparella I, Petrov V, Semplicini A, Fagard R, Angiotensin II-stimulated collagen production in cardiac fibroblasts is mediated by reactive oxygen species, Journal of hypertension, 24 (2006) 757–766. [DOI] [PubMed] [Google Scholar]
- [23].Chen K, Chen J, Li D, Zhang X, Mehta JL, Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone, Hypertension (Dallas, Tex. : 1979), 44 (2004) 655–661. [DOI] [PubMed] [Google Scholar]
- [24].Cao Q, Mak KM, Lieber CS, Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways, Journal of cellular biochemistry, 97 (2006) 188–197. [DOI] [PubMed] [Google Scholar]
- [25].Saxena NK, Saliba G, Floyd JJ, Anania FA, Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells, Journal of cellular biochemistry, 89 (2003) 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shi R, Hu W, Zhang Y, Gao S, Smith AH, Ye J, Cai L, Graham LM, Li C, Ascorbate inducible N259 glycans on prolyl 4-hydroxylase subunit α1 promote hydroxylation and secretion of type I collagen, Cellular and molecular life sciences : CMLS, 76 (2019) 3449–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hussain MZ, Watson JA, Bhatnagar RS, Increased prolyl hydroxylase activity and collagen synthesis in hepatocyte cultures exposed to superoxide, Hepatology (Baltimore, Md.), 7 (1987) 502–507. [DOI] [PubMed] [Google Scholar]
- [28].DeNichilo MO, Panagopoulos V, Rayner TE, Borowicz RA, Greenwood JE, Evdokiou A, Peroxidase enzymes regulate collagen extracellular matrix biosynthesis, The American journal of pathology, 185 (2015) 1372–1384. [DOI] [PubMed] [Google Scholar]
- [29].Nieto N, Friedman SL, Greenwel P, Cederbaum AI, CYP2E1-mediated oxidative stress induces collagen type I expression in rat hepatic stellate cells, Hepatology (Baltimore, Md.), 30 (1999) 987–996. [DOI] [PubMed] [Google Scholar]
- [30].Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C, Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide, Hepatology (Baltimore, Md.), 25 (1997) 361–367. [DOI] [PubMed] [Google Scholar]
- [31].Fähling M, Perlewitz A, Doller A, Thiele BJ, Regulation of collagen prolyl 4-hydroxylase and matrix metalloproteinases in fibrosarcoma cells by hypoxia, Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, 139 (2004) 119–126. [DOI] [PubMed] [Google Scholar]
- [32].Li J, Ghazwani M, Zhang Y, Lu J, Li J, Fan J, Gandhi CR, Li S, miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression, Journal of hepatology, 58 (2013) 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Feng G, Shi H, Li J, Yang Z, Fang R, Ye L, Zhang W, Zhang X, MiR-30e suppresses proliferation of hepatoma cells via targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) mRNA, Biochemical and biophysical research communications, 472 (2016) 516–522. [DOI] [PubMed] [Google Scholar]
- [34].Giri SN, Misra HP, Chandler DB, Chen ZL, Increases in lung prolyl hydroxylase and superoxide dismutase activities during bleomycin-induced lung fibrosis in hamsters, Experimental and molecular pathology, 39 (1983) 317–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


