Abstract
Purpose
Fluorescence lifetime imaging ophthalmoscopy (FLIO) is a novel modality to investigate the human retina. This study aims to characterize the effects of age, pigmentation, and gender in FLIO.
Methods
A total of 97 eyes from 97 healthy subjects (mean age 37 ± 18 years, range 9–85 years) were investigated in this study. This study included 47 (49%) females and 50 males. The pigmentation analysis was a substudy including 64 subjects aged 18 to 40 years (mean age 29 ± 6 years). These were categorized in groups A (darkly pigmented, 8), B (medium pigmented, 20), and C (lightly pigmented, 36). Subjects received Heidelberg Engineering FLIO and optical coherence tomography imaging. Retinal autofluorescence lifetimes were detected in two spectral channels (short spectral channel [SSC]: 498–560 nm; long spectral channel [LSC]: 560–720 nm), and amplitude-weighted mean fluorescence lifetimes (τm) were calculated. Additionally, autofluorescence lifetimes of melanin were measured in a cuvette.
Results
Age significantly affected FLIO lifetimes, and age-related FLIO changes in the SSC start at approximately age 35 years, whereas the LSC shows a consistent prolongation with age from childhood. There were no gender- or pigmentation-specific significant differences of autofluorescence lifetimes.
Conclusions
This study confirms age-effects in FLIO but shows that the two channels are affected differently. The LSC appears to show the lifelong accumulation of lipofuscin. Furthermore, it is important to know that neither gender nor pigmentation significantly affect FLIO lifetimes.
Translational Relevance
This study helps to understand the FLIO technology better, which will aid in conducting future clinical studies.
Keywords: FLIO, fluorescence lifetime imaging, melanin
Introduction
Fluorescence lifetime imaging ophthalmoscopy (FLIO) emerges as a novel imaging modality that may be highly beneficial in diagnosing a variety of eye diseases. It was first developed in 2002 by Schweitzer et al.,1,2 and a few prototypes were built in 2012 by Heidelberg Engineering. Since then, clinical knowledge has expanded immensely.3,4 Various studies have shown that different retinal diseases, such as age-related macular degeneration and macular telangiectasia type 2 (MacTel), can be detected with FLIO even before other clinical signs of the disease appear, and well before damage is manifest.5–9 Furthermore, FLIO is able to detect disease progression, which was especially highlighted in various cases of Stargardt disease, in which FLIO detects progression approximately 1 to 2 years before these changes are visible in conventional autofluorescence imaging.10,11 In patients with early retinal toxicity due to drugs, such as hydroxychloroquine (Plaquenil), FLIO may also be helpful.12 Furthermore, patients with diabetes and Alzheimer disease were recently described to show altered fluorescence lifetimes in even early diseases stages, which leads to the assumption that FLIO may be able to assess metabolic states of the human retina in vivo.13–15 FLIO imaging holds the advantage of being a fast and noninvasive imaging modality, allowing for quick detection of retinal abnormalities.
The first clinical study using a prototype FLIO device by Heidelberg Engineering was published in 2014 by Dysli et al.16 The authors included 31 healthy, phakic subjects aged 22 to 61 years. They found a high test–retest reliability for FLIO measurements, described changes between miosis and mydriasis, and found a prolongation of lifetimes with increasing age. The authors also found that there is no significant difference between the two eyes of healthy individuals.16
Because of the noninvasive nature of FLIO, the discussion of the origin of different fluorescence signals is still ongoing and not well understood. The retinal carotenoids lutein and zeaxanthin have been investigated extensively with FLIO, and knowledge regarding a short lifetime fluorescence signal originating from these substances in the foveal center has been established.17,18 Furthermore, researchers believe that lipofuscin from within the retinal pigment epithelium (RPE) may account for the intermediate lifetimes that can be found all across the retina,19 and long lifetimes may originate from collagen or connective tissue, as well as fibrotic or atrophic areas.3,4 It is possible that other fluorophores, such as melanin, may also influence fluorescence signals obtained with FLIO. A recent study by Hammer et al.20 discussed this possibility, suggesting that melanin may account for a portion of the fluorescence from within the RPE.
Based on the knowledge available on FLIO imaging, the aim of this study was to further understand what is being measured with FLIO. To increase the knowledge regarding the influence of age, a larger number of patients with a broader age-range were studied. In addition, males are compared with females, and a substudy investigates the effect of different pigmentation types. Finally, an ex vivo measurement of melanin was included as well. These analyses are targeted to better understand the FLIO technology and to explore the important criteria to know when matching healthy controls to study patients.
Methods
This cross-sectional study was approved by the University of Utah Institutional Review Board and adhered to the Declaration of Helsinki. Informed written consent was obtained from all adult patients prior to any investigations. For minors (ages 9–17), informed written consent was obtained from a parent or legal guardian, and assent was obtained from the minor. All subjects were examined between March 2017 and January 2020 at the Moran Eye Center.
Study Protocol
Healthy subjects were recruited from clinic at the Moran Eye Center. Inclusion criteria were a healthy fundus examination, as well as healthy optical coherence tomography (OCT) imaging, which was used for documentation. Patients with history of previous retinal surgeries were excluded, but uncomplicated cataract surgery was not an exclusion criterion. Patients with existing eye diseases or even a family history of inherited retinal diseases, including but not limited to retinitis pigmentosa, Stargardt disease, MacTel, and others, were not included. Diabetes or any severe systemic disease was another exclusion criterion.
After inclusion and consent, the best corrected visual acuity was obtained, which was 20/25 or better for every included patient. Intraocular pressure was measured with a Tonopen, and no topical or intravenous fluorescein was applied before imaging. As FLIO investigates the fluorescence lifetimes from the fundus, fluorescein use should be avoided prior to imaging. Pupils were maximally dilated prior to imaging, as this is also important for consistent FLIO imaging.16,21 Each patient received FLIO (Heidelberg Engineering, Heidelberg, Germany) and OCT imaging (Spectralis, Heidelberg Engineering) after pupil dilation.
FLIO-Setup and Image Acquisition
Based on the Heidelberg Engineering Spectralis, FLIO records autofluorescence lifetimes in vivo in a 30° retinal field, relying on the principle of time-correlated single photon counting.22,23 The detailed setup and safety of FLIO have been described previously.16,18,22 Briefly, retinal autofluorescence is excited with a pulsed diode laser at 473 nm wavelength, and fluorescence photons are detected with two hybrid photomultipliers (HPM-100-40; Becker & Hickl GmbH, Berlin, Germany). This results in the detection of photons within two separate spectral channels: the short spectral channel (SSC; 498–560 nm) and the long spectral channel (LSC; 560–720 nm). A high-contrast confocal infrared reflectance image is included for eye tracking. To ensure reliable image quality, minimal signal thresholds of at least 1000 photons were recorded for each pixel. Typically, 2 minutes of acquisition time were required for each eye.
The fluorescence data were analyzed using the Software SPCImage 4.4.2 (Becker & Hickl GmbH), and the mean fluorescence decay was approximated. The amplitude-weighted mean fluorescence decay time (τm) was used for further analysis, representing the average of three time constants from the fit, weighted by their amplitude. Further details have been described elsewhere.18,23
The FLIMX software was used to obtain the mean FLIO lifetimes over regions of interest.24 This software is documented and freely available for download online under the open source BSD-license (http://www.flimx.de). Areas of interest were obtained from a standardized Early Treatment of Diabetic Retinopathy Study grid (ETDRS grid). In this study, the central area (C), as well as the inner ring (IR), the outer ring (OR), and the full grid (Full) were investigated.
Melanin Measurements Ex Vivo
For the ex vivo melanin measurement, synthetic melanin powder (MP Biomedicals LLC, Solon, OH) was dissolved in a 0.1 mol/L NaOH solution at a final concentration of 0.05 mg/mL. Both the empty cuvette, as well as NaOH solution without melanin, were measured as a reference. Neither of these measurements showed any fluorescence. For the ex vivo FLIO measurement of melanin, the 0.05 mg/mL solution was placed in a 1- to 2-mm path-length quartz cuvette, which was then placed in a special cuvette holder attachment at the FLIO device. For the investigations of ex vivo melanin fluorescence, a triexponential approach resulted in the most accurate fit and was therefore utilized here for data analyses. Acquisition times of 2 and 10 minutes were used, and average photon counts were recorded. Measurements were performed in complete darkness. Mean autofluorescence lifetimes from each measurement were obtained from a rectangular region of the cuvette. As FLIO lifetimes should not change with different concentrations, only one measurement with a melanin solution was performed.
Statistical Analysis
For all statistical analyses, SPSS 21 (IBM Corp., Armonk, NY) was employed. A Pearson correlation was used to correlate mean FLIO lifetimes from different areas of interest with age. Independent sample t-tests were used to compare FLIO lifetimes from male subjects to those from female subjects. Finally, an analysis of variance was conducted to check for significant FLIO lifetime differences between the three pigmentation groups of subjects. Data were checked to confirm normal distributions, and Bonferroni correction was applied in cases of multiple testing. All results are reported with their mean ± standard deviation (± SD).
Results
Subjects: 97 eyes from 97 healthy subjects were investigated in this study. Mean age over all subjects was 37 ± 18 years, age ranging from 9 to 85 years. This study included 47 (49%) females and 50 males. Six patients had an artificial intraocular lens (IOL); all other patients had natural lenses without significant cataracts. None of these subjects reported supplementation with lutein or zeaxanthin. The pigmentation analysis was a substudy that included 64 eyes from 64 subjects aged 18 to 40 years (mean age 29 ± 6 years). These were categorized as either African (8), Indian and Hispanic (20), or Caucasian (31). This grouping was based on the darkness of skin pigmentation of included subjects. Table 1 provides further information regarding each group of patients used for the different analyses in this study.
Table 1.
Subject Characteristics
| Subjects | Number of Patients | Number of Eyes | Age (years) | Lens | Eye | Gender | Analysis |
|---|---|---|---|---|---|---|---|
| All healthy subjects | 97 | 97 | 37 ± 18 (range 9–85) | 91 natural lens (94%) 6 IOL (6%) | 73 OD (75%) 24 OS (25%) | 47 females (49%) 50 males (51%) | Gender |
| Healthy subjects with natural lens | 91 | 91 | 34 ± 15 (range 9–71) | 91 natural lens (100%) | 69 OD (76%) 22 OS (24%) | 46 females (50%) 45 males (50%) | Age |
| Young healthy subjects (age ≤ 35 years) | 61 | 61 | 26 ± 7 (range 9–35) | 61 natural lens (100%) | 45 OD (74%) 16 OS (26%) | 31 females (51%) 30 males (49 %) | |
| Healthy subjects groups A, B, and C | 64 | 64 | 29 ± 6 (range 18–40) | 64 natural lens (100%) | 49 OD (77%) 15 OS (23%) | 31 females (48%) 33 males (52%) | Pigmentation |
| Group A Darkly pigmented | 8 | 8 | 30 ± 8 (range 18–40) | 8 natural lens (100%) | 6 OD (75%) 2 OS (25%) | 1 female (13%) 7 males (87%) | |
| Group B Medium pigmented | 20 | 20 | 30 ± 6 (range 18–40) | 20 natural lens (100%) | 16 OD (80%) 4 OS (20%) | females (%) males (%) | |
| Group C Lightly pigmented | 36 | 36 | 28 ± 8 (range 20-40) | 36 natural lens (100%) | 27 OD (75%) 9 OS (25%) | 21 females (58%) 15 males (42%) |
OD, oculus dextrus; OS, oculus sinister.
Fluorescence Lifetimes and Age
Fundus autofluorescence lifetimes are significantly influenced by age. In this study, we investigated patients with natural lenses ranging from 9 to 71 years. We also investigated six patients who had undergone cataract surgery; however, these were not included in any statistical analysis and only served as reference cases. In general, older subjects showed longer lifetimes. This is depicted in Figures 1 and 2. The corresponding Pearson correlations are reported in Table 2.
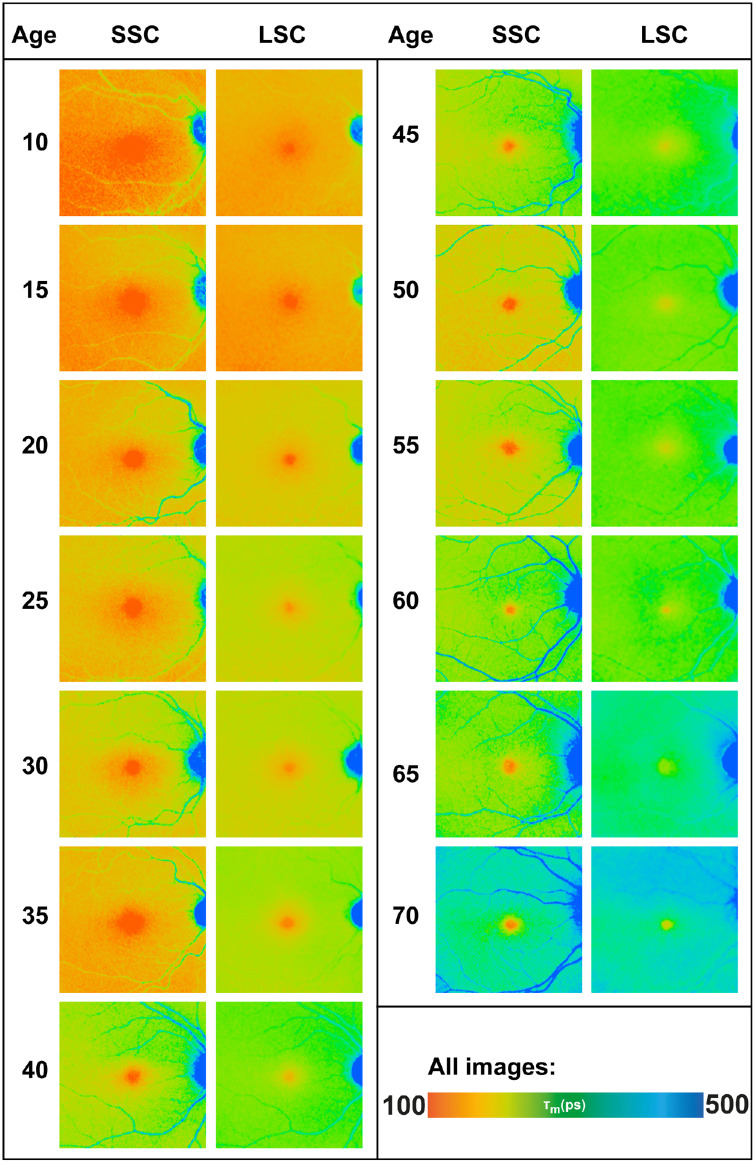
Figure 1.
FLIO lifetimes from both spectral channels for different ages. The SSC shows age-related changes (prolongation of FLIO lifetimes) only after age 35 years, whereas the LSC prolongs consistently with increasing age. All images are shown in the same color scale.
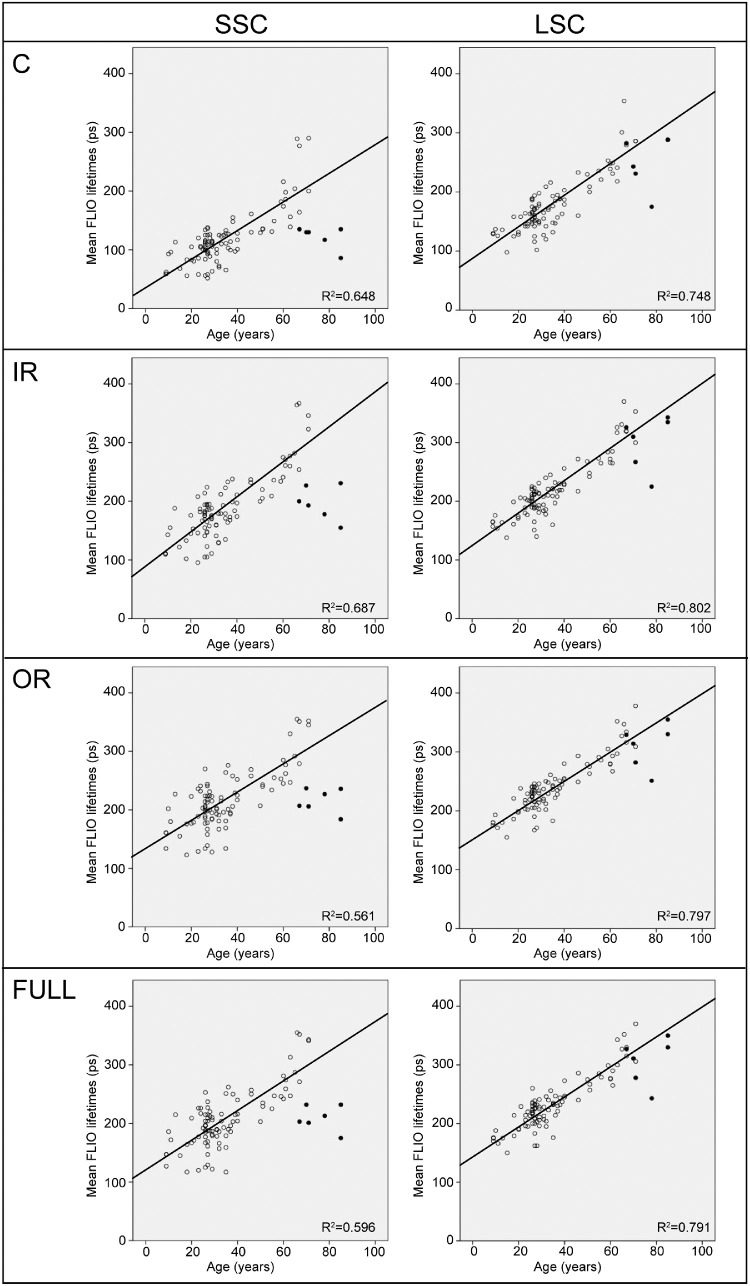
Figure 2.
Pearson correlations of age with FLIO lifetimes from different areas of interest (C, central; IR, inner ring; OR, outer ring; Full, full ETDRS grid) for the entire study population with natural lenses. For completion, patients with artificial IOLs are shown as well (black dots), these patients are not included in the correlation. Patients with IOLs show a separate cloud of lifetimes in the SSC, but not in the LSC, which indicates that the impact of the lens is strong in the SSC but that FLIO lifetimes from the LSC are relatively independent of the lens.
Table 2.
The Effect of Age on FLIO Lifetimes
| All Probands with | All Young Probands | ||||
|---|---|---|---|---|---|
| Natural Lenses (n = 91) | Age ≤35 years (n = 61) | ||||
| Correlation of Age with Area | Channel | Pearson R2 | P Value | Pearson R2 | P Value |
| C | SSC | 0.805 | <0.001 | 0.390 | <0.01 |
| LSC | 0.865 | <0.001 | 0.462 | <0.001 | |
| IR | SSC | 0.829 | <0.001 | 0.392 | <0.01 |
| LSC | 0.896 | <0.001 | 0.549 | <0.001 | |
| OR | SSC | 0.749 | <0.001 | 0.212 | 0.101 |
| LSC | 0.893 | <0.001 | 0.609 | <0.001 | |
| Full grid | SSC | 0.772 | <0.001 | 0.236 | 0.134 |
| LSC | 0.889 | <0.001 | 0.576 | <0.001 | |
Pearson correlation of age with different areas of interest for the entire study population with natural lenses, as well as subjects aged 35 years or younger. Bonferroni corrected P values are shown. P values <0.05 are considered significant and highlighted in bold.
C, central; OR, outer ring; IR, inner ring; Full grid, full circle (all areas from standardized ETDRS grid); SSC, 498–560 nm; LSC, 560–720 nm.
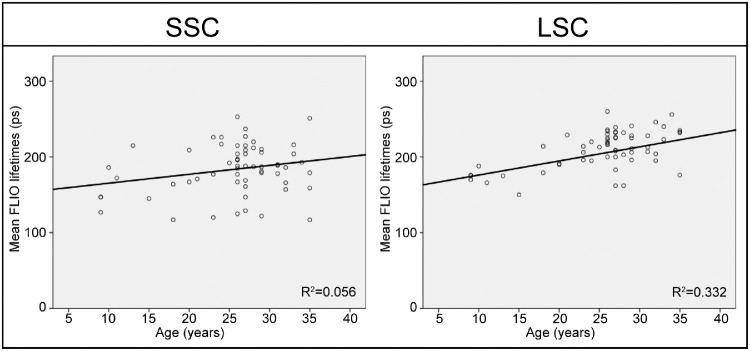
The two spectral channels are differently affected by age, which can also be seen in Figures 1 and 2. In this study, we found that the LSC shows an age dependency from childhood on. We found a strong correlation of age and autofluorescence lifetimes in the LSC for all areas of interest. This was relatively independent of the lens status, as can be seen in Figure 2: the black dots represent patients who had undergone cataract surgery, and with one exception, they are similar to their age-matched controls in the LSC. The SSC, however, showed age-specific changes starting at approximately the age of 35 years. Analyzing only patients age 35 years or younger resulted in no significant correlation for the SSC (P = 0.134), but a significant correlation of lifetimes with age in the LSC (P < 0.001). Figure 3 shows this subanalysis. In addition, patients who had undergone cataract surgery can be found as a separate cloud of dots for every area of interest in the SSC (black dots, Fig. 2). This highlights that lifetime changes with age are strongly influenced by the lens in the SSC, whereas actual age-related changes within the retina are detected in the LSC.
Figure 3.
Pearson correlations of age with FLIO lifetimes from the full ETDRS grid in subjects aged 35 years or younger. In the SSC, no significant correlation was found between age and FLIO lifetimes (P = 0.134). The LSC showed a significant correlation (P < 0.001), further details are shown in Table 2.
Fluorescence Lifetimes and Gender
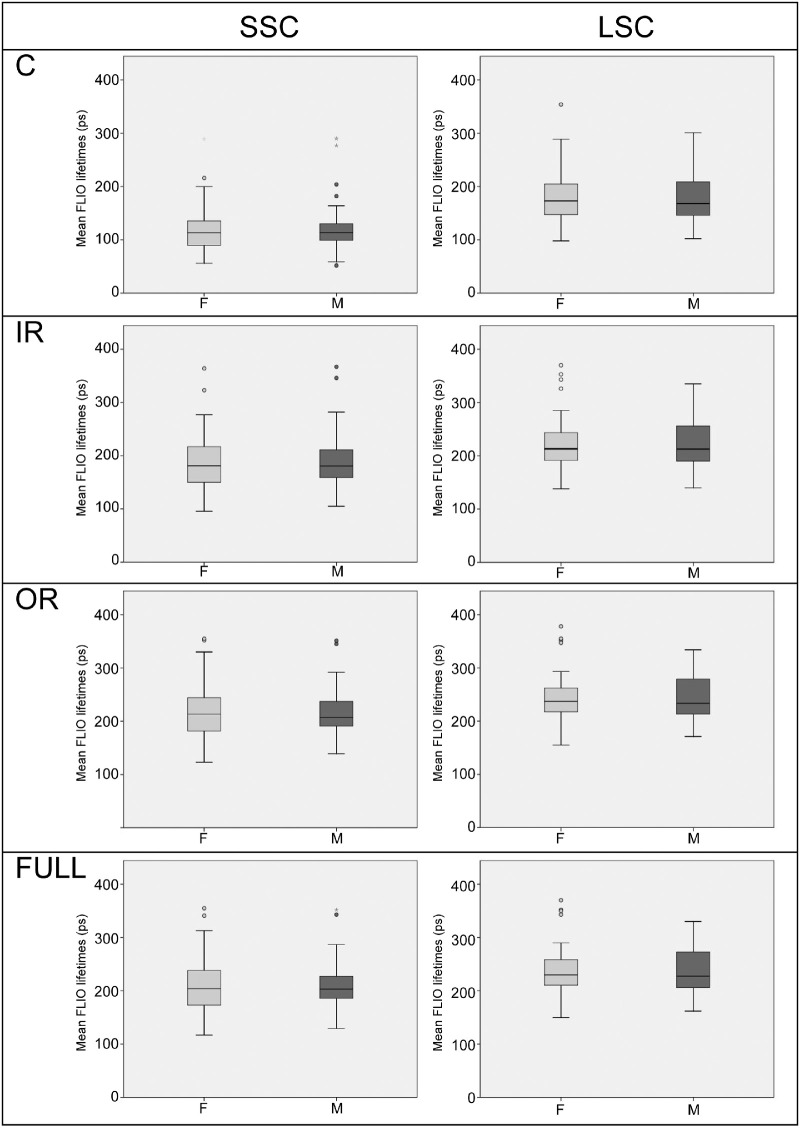
All subjects were included in the gender analysis, and no significant differences were found between males and females in any analysis. When comparing all areas of interest (C, IR, OR, and Full macula of a standardized ETDRS grid) between males and females, P values ranged from 0.711 to 0.989. Table 3 shows these findings, and Figure 4 depicts these findings graphically. Similar findings with no significant P values were observed when investigating only the young subcohort age 35 years or younger (P values from 0.161–0.709), or when analyzing all patients with natural lenses (P values from 0.634–0.951).
Table 3.
The Effect of Gender on FLIO Lifetimes
| Area | Channel | Female | Male | P Value |
|---|---|---|---|---|
| C | SSC | 118 ± 47 ps | 120 ± 45 ps | 0.804 |
| LSC | 181 ± 50 ps | 184 ± 51 ps | 0.839 | |
| IR | SSC | 188 ± 57 ps | 190 ± 51 ps | 0.874 |
| LSC | 223 ± 52 ps | 225 ± 51 ps | 0.829 | |
| OR | SSC | 214 ± 55 ps | 218 ± 42 ps | 0.733 |
| LSC | 243 ± 48 ps | 243 ± 45 ps | 0.979 | |
| Full grid | SSC | 205 ± 56 ps | 209 ± 43 ps | 0.711 |
| LSC | 237 ± 49 ps | 237 ± 46 ps | 0.989 |
Independent sample t-test of FLIO lifetimes from different areas of interest for the entire study population, males compared with females. P values <0.05 are considered significant.
C, central; OR, outer ring; IR, inner ring; Full grid, full circle (all areas from standardized ETDRS grid); SSC, 498–560 nm; LSC, 560–720 nm.
Figure 4.
Boxplots showing FLIO lifetimes from different areas of interest (C, central; IR, inner ring; OR, outer ring; Full, full ETDRS grid) for the entire study population, males compared with females. No significant differences were found in any region.
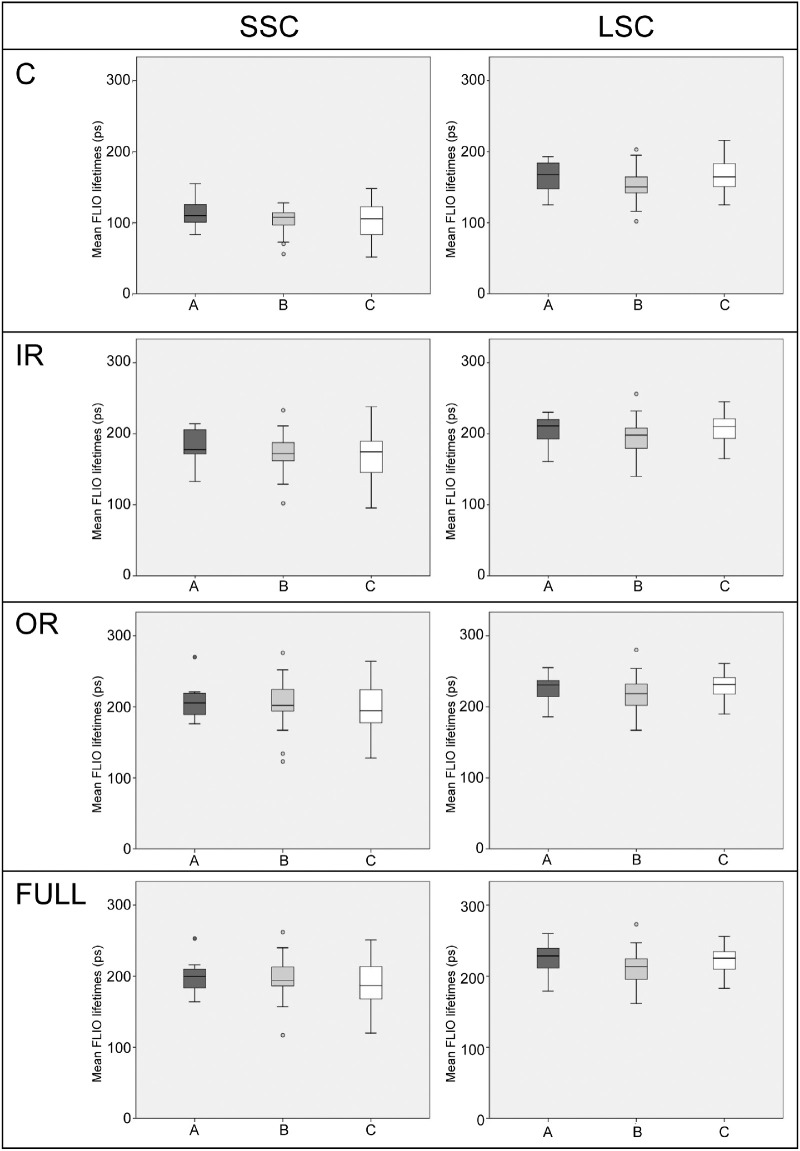
Fluorescence Lifetimes and Pigmentation
For the pigmentation subanalysis, we included only young subjects of ages 18 to 40 years. This cutoff in age was set as an inclusion criterion before this study was analyzed. We investigated three different groups: group A with darkly pigmented subjects, typically of African descent (8 subjects); group B with medium pigmented subjects, typically of Hispanic and Indian descent (20 subjects); and group C with lightly pigmented subjects, typically of Caucasian descent (31 subjects). No significant differences were found between subjects of different pigmentation. When comparing all areas of interest (C, IR, OR, and Full macula of a standardized ETDRS grid) between different groups, P values ranged from 0.106 to 0.552. Table 4 shows these findings, and Figure 5 depicts these findings graphically.
Table 4.
The Effect of Pigmentation on FLIO Lifetimes
| Area | A (n = 8) | B (n = 20) | C (n = 36) | P Value | |
|---|---|---|---|---|---|
| C | SSC | 114 ± 22 | 103 ± 19 | 102 ± 26 | 0.422 |
| LSC | 165 ± 23 | 153 ± 25 | 166 ± 23 | 0.137 | |
| IR | SSC | 182 ± 26 | 173 ± 30 | 166 ± 34 | 0.422 |
| LSC | 205 ± 22 | 195 ± 27 | 207 ± 19 | 0.148 | |
| OR | SSC | 209 ± 29 | 205 ± 36 | 197 ± 34 | 0.548 |
| LSC | 226 ± 21 | 218 ± 27 | 230 ± 17 | 0.132 | |
| Full grid | SSC | 200 ± 27 | 194 ± 36 | 187 ± 34 | 0.552 |
| LSC | 225 ± 26 | 211 ± 27 | 223 ± 17 | 0.106 | |
Analysis of variance of FLIO lifetimes from different areas of interest for the different subgroups: A (darkly pigmented), B (medium pigmented), and C (lightly pigmented). P values <0.05 are considered significant.
C, central; OR, outer ring; IR, inner ring; Full grid, full circle (all areas from standardized ETDRS grid); SSC, 498–560 nm; LSC, 560–720 nm.
Figure 5.
Boxplots showing FLIO lifetimes from different areas of interest (C, central; IR, inner ring; OR, outer ring; Full, full ETDRS grid) or the different subgroups: A (darkly pigmented), B (medium pigmented), and C (lightly pigmented). No significant differences were found in any region.
Fluorescence Lifetimes of Melanin Ex Vivo
The lifetimes of melanin measured in a cuvette were found to be approximately 1400 ps (SSC) and 850 picoseconds (LSC). Fluorescence signals are slightly stronger in the SSC. Table 5 shows the results.
Table 5.
Autofluorescence Lifetimes of Ex Vivo Melanin
| Time | Channel | Mean Lifetime | Average Photon Count | |
|---|---|---|---|---|
| Empty cuvette | 2 min | SSC | 0 ± 0 ps | 0 photons |
| 2 min | LSC | 0 ± 0 ps | 0 photons | |
| NaOH solution | 2 min | SSC | 0 ± 0 ps | 0 photons |
| 2 min | LSC | 0 ± 0 ps | 0 photons | |
| Melanin | 2 min | SSC | 1447 ± 109 ps | 700–850 photons |
| 2 min | LSC | 865 ± 46 ps | 600–750 photons | |
| Melanin | 10 min | SSC | 1369 ± 44 ps | 6000–7000 photons |
| 10 min | LSC | 821 ± 25 ps | 5000–6000 photons |
Analysis of ex vivo FLIO lifetimes from melanin.
SSC, 498–560 nm; LSC, 560–720 nm.
Discussion
Imaging is key in the diagnosis of many retinal diseases, and new imaging modalities constantly improve the diagnostic potential. Among the novel modalities on the horizon is FLIO. FLIO has shown promising results as a prototype; it holds the potential to diagnose retinal diseases at early stages and may be on its way to become an important imaging modality in the future.3–12,25 To use FLIO in clinical studies, background knowledge regarding specific influences on FLIO is important. A previous study by Dysli et al.16 nicely demonstrated that FLIO has good test–retest reliability, which holds true for miosis and mydriasis. This has later been found by other groups as well.21 Dysli et al.16 also investigated the difference between right and left eyes and found that both eyes of healthy individuals show similar FLIO lifetimes. In our study, we included only one eye for each subject. As there is no difference between right and left eyes, we always chose the right eye if both eyes were imaged. This results in 75% right eyes included in this study. We investigated four areas of interest from a standardized ETDRS grid—C, IR, OR, or Full—focusing on differences in age, gender, and pigmentation.
Dysli et al.16 found a prolongation of lifetimes with increasing age, which has been found by many other groups as well.18,21 In contrast to other studies, we were able to include pediatric subjects, with the youngest subject aged 9 years. This allows for a broader and more detailed analysis of FLIO lifetimes over the span of life. Interestingly, we found differences in the age effect on the two spectral channels. The SSC seems to be fairly unaffected by age until the age of approximately 35 years, and no significant correlation was found between age and FLIO lifetimes for subjects aged 35 years or younger, as can be seen in Figure 3. Interestingly, a previous study that also included only young healthy subjects did not find a significant correlation of age and FLIO lifetimes in the SSC.18 In that study, only the C of a standardized ETDRS grid was analyzed. Here 48 patients aged 20 to 37 years were included, and a significant correlation of FLIO lifetimes and age was only reported for the LSC. In contrast, we found a significant correlation of age and FLIO lifetimes within the C of the SSC, which is likely because we included a wider age span. This again shows that FLIO lifetimes from the SSC are only significantly affected by age when investigating patients above a certain age, and that it is likely that these changes start at approximately age 35 years. FLIO lifetimes from LSC show a much stronger correlation with age than those from the SSC. Furthermore, in the LSC, age and FLIO lifetimes significantly correlated in all areas of interest, whereas within the SSC, the correlation of age and FLIO lifetimes was significant for the C, as well as the IR, but not for the OR. These differences may seem very subtle, but we think that it can help us understand the different influences we have when using FLIO. First, the two spectral channels were initially chosen based on the fluorescence emission spectrum of lipofuscin.26 Therefore by investigating both spectral channels, it is likely that we may be able to distinguish between a channel with a strong influence of lipofuscin fluorescence (LSC), and a channel without or at least with significantly reduced lipofuscin influence (SSC).1 We therefore strongly believe that we observe the accumulation of lipofuscin within the RPE over time in the LSC. This would explain the very strong correlation of lifetimes with age in the LSC, starting at a very young age and increasing continuously with advancing age. The SSC, however, might be relatively independent of lipofuscin. However, it has been shown in many studies that the SSC is influenced by the autofluorescence of the lens.3,5,16,27,28 As young individuals typically have a clear lens, this influence is not as strong when investigating younger or middle-aged groups of patients.6,18,19 We therefore may consider that the influence of the lens is not linearly increasing with age, but rather affects older individuals more strongly. If we assume that the lens causes the changes over time in the SSC, this would explain why there is a weaker correlation in young individuals (ages 35 and younger). Furthermore, it is interesting that the OR does not show a significant correlation in these young patients, whereas the IR and C do. This could be due to a confounder, such as the centrally localized MP.18 However, it may be possible that the lens influence is not uniform across the fundus, but that the C may be more strongly affected by the lens due to a weaker retinal fluorescence within the C, which again is mostly caused by macular pigment.18,29 Although most of the MP should be located within the C of the ETDRS grid, there may be a small influence of this in the IR as well. The OR, however, should be independent of this. If we assume this to be true, there might be a small influence of the lens even before age 35 years. This should be investigated in larger studies focusing on the lens effect specifically. Furthermore, patients with artificial IOLs seem to show much shorter FLIO lifetimes. Figure 2 shows these individuals as black dots, and these patients seem to form a separate cloud of lifetimes that appears to be less affected by age in the SSC, whereas these dots fit well in the correlation in the LSC for the most part. This shows that the lens likely has a smaller influence on the LSC and may mostly be important in patients with cataracts.27,28 A further study including larger numbers of patients with artificial IOLs would be helpful to better understand this influence. All of these findings, however, support that the SSC is likely strongly affected by the influence of the lens, and that an impact of the lens can especially be expected in patients and subjects starting at an age of 35 years. The LSC, however, shows a constant prolongation of FLIO lifetimes with increasing age, starting in childhood, supported by the significant correlation of FLIO lifetimes from the LSC with age even in young subjects. Based on this, we can conclude that the LSC is less influenced by the lens and may show age-related changes more reliably.
This study also investigates the impact of gender and pigmentation on FLIO lifetimes. No significant differences were found when comparing males to females in any analysis. Therefore it is safe to assume that in the process of matching healthy controls, it is not necessary to match the gender. Pigmentation does not seem to cause significant differences either, although a nonsignificant trend of longer lifetimes in the SSC was observed for group A. To understand this in more detail, it is important to pay attention to the ex vivo analysis of melanin. We were able to show that melanin has very long FLIO lifetimes of approximately 1400 ps in the SSC and 850 ps in the LSC. These times are somewhat in accordance with autofluorescence lifetimes reported from single- and multiphoton excitation techniques.30 However, excitation wavelengths in these studies were different from FLIO imaging (473 nm), especially the two-photon excitation, which are somewhat similar to FLIO imaging and had excitation wavelengths of 750 nm and above. Therefore this study provides a unique approach of analyzing FLIO lifetimes in melanin with the Heidelberg Spectralis device. However, the previous studies on melanin fluorescence highlight that there is a fluorescence that may influence FLIO lifetimes in vivo as well, and we found a nonsignificant trend of longer lifetimes in the darker pigmented group A. This was found when investigating the SSC of ex vivo melanin, which shows long lifetimes and a stronger fluorescence intensity. Because group A consisted of eight subjects, which is a relatively small number, we believe it would make sense to repeat this study with a larger number of individuals. Furthermore, the impact of melanin may be stronger in older patients, as there may be a connection between the increase of lipofuscin and an increase of melanin. Studies investigating human donor RPE found that the fluorescence intensity of both melanin, as well as lipofuscin, increased with donor age, whereas this increase was even stronger for melanin.31 The characteristics of melanin itself may also change with advancing age.31 Therefore the influence of melanin needs to be further investigated in larger studies including older patients as well. Our study does not show any significant differences between different pigmentation in young subjects.
This study has some limitations. Despite a total number of 97 patients, the subgroup analysis shows small numbers for group A (African descent). As this study was conducted in Utah, it was challenging to obtain diversity in our study population. This study should be repeated in the setting of higher diversity. Furthermore, we did not use a quantitative measure to characterize the pigmentation status. For example, this could be done by using fundus photography, which we did not obtain. Additionally, we did not investigate iris color, which might be of interest in future studies as well. In addition, this study was focused on phakic patients, and only six patients with artificial IOLs were included for comparison. It would be interesting to evaluate the effect of age, gender, and pigmentation within a study population exclusively with IOLs. Finally, we investigated four areas of interest (C, IR, OR, and Full), but decided against analyzing all subfields of the ETDRS grid. We do not believe that more areas of interest would show different results from the ones presented in this study, especially because all of these subfields are included in the investigated areas of interest.
Conclusions
FLIO is a new imaging modality that may be very helpful in detecting and diagnosing retinal, as well as systemic, diseases. FLIO lifetimes are influenced by age, but both spectral wavelength channels are influenced differently. FLIO lifetimes from the SSC are independent of age until approximately 35 years, whereas the LSC shows a constant prolongation of FLIO lifetimes with increasing age. Finally, FLIO lifetimes are independent of gender and pigmentation. This study helps to better understand how to match healthy controls to patients, and further studies may show the full potential of this novel imaging modality.
Acknowledgments
The authors thank Heidelberg Engineering for providing the FLIO, as well as for their technical assistance, especially Yoshihiko Katayama, PhD. The authors thank the Lowy family and the Lowy Medical Research Institute (LMRI) for their support. Additional support was provided by National Institutes of Health grants EY11600 and EY14800, and Research to Prevent Blindness. The authors thank all coworkers from the John A. Moran Eye Center who helped recruit and image patients.
Disclosure: L. Sauer, None; A.S. Vitale, None; C.M. Milliken, None; N.K. Modersitzki, None; J.D. Blount, None; P.S. Bernstein, None
References
- 1. Schweitzer D, Jentsch S, Schenke S, Hammer M, Biskup C, Gaillard E. Spectral and time-resolved studies on ocular structures. Proceedings of the SPIE. 2007; 6628: 662807. [Google Scholar]
- 2. Schweitzer D, Kolb A, Hammer M, Anders R. [Time-correlated measurement of autofluorescence. A method to detect metabolic changes in the fundus]. Ophthalmologe. 2002; 99: 774–779. [DOI] [PubMed] [Google Scholar]
- 3. Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS. Fluorescence lifetime imaging ophthalmoscopy. Prog Retin Eye Res. 2017; 60: 120–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauer L, Andersen KM, Dysli C, Zinkernagel MS, Bernstein PS, Hammer M. Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy. J Biomed Opt. 2018; 23: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauer L, Gensure RH, Andersen KM, et al.. Patterns of fundus autofluorescence lifetimes in eyes of individuals with nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2018; 59: AMD65–AMD77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmol Retina. 2018; 2: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sauer L, Vitale AS, Andersen KM, Hart B, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy (FLIO) patterns in clinically unaffected children of macular telangiectasia type 2 (MacTel) patients. Retina. 2020; 40: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dysli C, Fink R, Wolf S, Zinkernagel MS. Fluorescence lifetimes of drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 4856–4862. [DOI] [PubMed] [Google Scholar]
- 9. Solberg Y, Dysli C, Wolf S, Zinkernagel MS. Fluorescence lifetime patterns in macular telangiectasia type 2. Retina. 2020; 40: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dysli C, Wolf S, Hatz K, Zinkernagel MS. Fluorescence lifetime imaging in Stargardt disease: potential marker for disease progression. Invest Ophthalmol Vis Sci. 2016; 57: 832–841. [DOI] [PubMed] [Google Scholar]
- 11. Solberg Y, Dysli C, Escher P, Berger L, Wolf S, Zinkernagel MS. Retinal flecks in Stargardt disease reveal characteristic fluorescence lifetime transition over time. Retina. 2019; 39: 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sauer L, Calvo CM, Vitale AS, Henrie N, Milliken CM, Bernstein PS. Imaging of hydroxychloroquine toxicity with fluorescence lifetime imaging ophthalmoscopy. Ophthalmol Retina. 2019; 3: 814–825. [DOI] [PubMed] [Google Scholar]
- 13. Sadda SR, Borrelli E, Fan W, Ebraheem A, Marion KM, Harrington M, Kwon S.. A pilot study of fluorescence lifetime imaging ophthalmoscopy in preclinical Alzheimer's disease. Eye (Lond). 2019; 33: 1271–1279. PMID: 30923356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jentsch S, Schweitzer D, Schmidtke KU, et al.. Retinal fluorescence lifetime imaging ophthalmoscopy measures depend on the severity of Alzheimer's disease. Acta Ophthalmol. 2015; 93: e241–e247. [DOI] [PubMed] [Google Scholar]
- 15. Schweitzer D, Deutsch L, Klemm M, et al.. Fluorescence lifetime imaging ophthalmoscopy in type 2 diabetic patients who have no signs of diabetic retinopathy. J Biomed Opt. 2015; 20: 61106. [DOI] [PubMed] [Google Scholar]
- 16. Dysli C, Quellec G, Abegg M, et al.. Quantitative analysis of fluorescence lifetime measurements of the macula using the fluorescence lifetime imaging ophthalmoscope in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 2106–2113. [DOI] [PubMed] [Google Scholar]
- 17. Sauer L, Andersen KM, Li B, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy (FLIO) of macular pigment. Invest Ophthalmol Vis Sci. 2018; 59: 3094–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauer L, Schweitzer D, Ramm L, Augsten R, Hammer M, Peters S. Impact of macular pigment on fundus autofluorescence lifetimes. Invest Ophthalmol Vis Sci. 2015; 56: 4668–4679. [DOI] [PubMed] [Google Scholar]
- 19. Sauer L, Peters S, Schmidt J, et al.. Monitoring macular pigment changes in macular holes using fluorescence lifetime imaging ophthalmoscopy. Acta Ophthalmol. 2017; 95: 481–492. [DOI] [PubMed] [Google Scholar]
- 20. Hammer M, Sauer L, Klemm M, Peters S, Schultz R, Haueisen J. Fundus autofluorescence beyond lipofuscin: lesson learned from ex vivo fluorescence lifetime imaging in porcine eyes. Biomed Opt Express. 2018; 9: 3078–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon S, Borrelli E, Fan W, Ebraheem A, Marion KM, Sadda SR. Repeatability of fluorescence lifetime imaging ophthalmoscopy in normal subjects with mydriasis. Transl Vis Sci Technol. 2019; 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schweitzer D, Hammer M, Schweitzer F, et al.. In vivo measurement of time-resolved autofluorescence at the human fundus. J Biomed Opt. 2004; 9: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 23. Becker W. The bh TCSPC Handbook. 6th ed. Berlin: Becker & Hickl GmbH; 2014. [Google Scholar]
- 24. Klemm M, Schweitzer D, Peters S, Sauer L, Hammer M, Haueisen J. FLIMX: a software package to determine and analyze the fluorescence lifetime in time-resolved fluorescence data from the human eye. PLoS One. 2015; 10: e0131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dysli C, Wolf S, Tran HV, Zinkernagel MS. Autofluorescence lifetimes in patients with choroideremia identify photoreceptors in areas with retinal pigment epithelium atrophy. Invest Ophthalmol Vis Sci. 2016; 57: 6714–6721. [DOI] [PubMed] [Google Scholar]
- 26. Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995; 36: 718–729. [PubMed] [Google Scholar]
- 27. Klemm M, Blum J, Link D, Hammer M, Haueisen J, Schweitzer D. Combination of confocal principle and aperture stop separation improves suppression of crystalline lens fluorescence in an eye model. Biomed Opt Express. 2016; 7: 3198–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schweitzer D, Hammer M, Schweitzer F. [Limits of the confocal laser-scanning technique in measurements of time-resolved autofluorescence of the ocular fundus]. Biomed Tech (Berl). 2005; 50: 263–267. [DOI] [PubMed] [Google Scholar]
- 29. Delori FC. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch Biochem Biophys. 2004; 430: 156–162. [DOI] [PubMed] [Google Scholar]
- 30. Dancik Y, Favre A, Loy CJ, Zvyagin AV, Roberts MS. Use of multiphoton tomography and fluorescence lifetime imaging to investigate skin pigmentation in vivo. J Biomed Opt. 2013; 18: 26022. [DOI] [PubMed] [Google Scholar]
- 31. Docchio F, Boulton M, Cubeddu R, Ramponi R, Barker PD. Age-related changes in the fluorescence of melanin and lipofuscin granules of the retinal pigment epithelium: a time-resolved fluorescence spectroscopy study. Photochem Photobiol. 1991; 54: 247–253. [DOI] [PubMed] [Google Scholar]