Abstract
Background
Glucose regulating protein 78 (GRP78) is one member of the Heat Shock Protein family of chaperone proteins (HSPA5) found in eukaryotes. It acts as the master of the Unfolded Protein Response (UPR) process in the lumen of the Endoplasmic Reticulum (ER).
Scope
Under the stress of unfolded proteins, GRP78 binds to the unfolded proteins to prevent misfolding, while under the load of the unfolded protein, it drives the cell to autophagy or apoptosis. Several attempts reported the overexpression of GRP78 on the cell membrane of cancer cells and cells infected with viruses or fungi.
Major conclusions
Cell-surface GRP78 is used as a cancer cell target in previous studies. Additionally, GRP78 is used as a drug target to stop the progression of cancer cells by different compounds, including peptides, antibodies, and some natural compounds. Additionally, it can be used as a protein target to reduce the infectivity of different viruses, including the pandemic SARS-CoV-2. Besides, GRP78 targeting is used in diagnosis and imaging modalities using radionuclides.
General significance
This review summarizes the various attempts that used GRP78 both in therapy (fighting cancer, viral and fungal infections) and diagnosis (imaging).
Keywords: GRP78, BiP, SARS-CoV-2, Peptide inhibitors, Cancer-targeting, Natural compounds
Graphical abstract
1. Introduction
In eukaryotes, different mechanisms regulate the cell proteostasis, which can be understood as a cell's response to a signal [[1], [2], [3], [4], [5]]. Proteins represent the cell machines and tools used to perform specific functions or biochemical reactions harmonically [6]. With time, the biomacromolecules are affected by the cellular environment and may undergo partial unfolding or misfolding and need to be revisited by the cellular refolding or degradation mechanisms [1,5,7,8].
Glucose regulating protein 78 (GRP78) or Heat Shock Protein A5 (HSPA5) is the master protein responsible for directing the misfolded proteins in the ER for refolding or degradation mechanisms to keep the unfolded protein concentration at a minimum [2,4,9]. Cell degrades proteins either by the ubiquitin-proteasome system (UPS) or autophagy-lysosome pathway where, in both cases, chaperones play a crucial role [10].
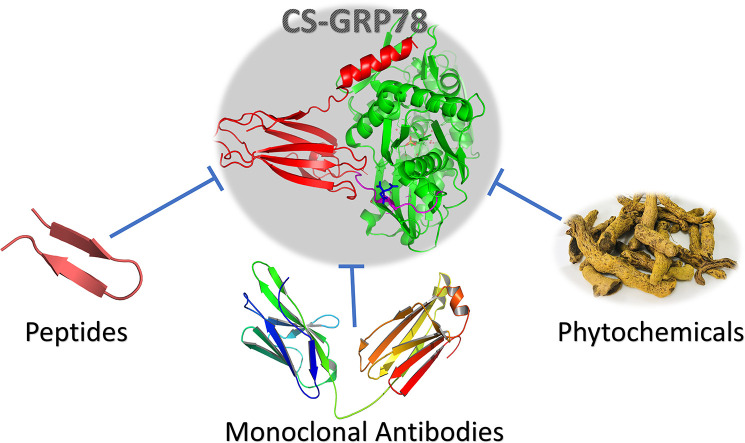
Chaperones in cancer cells play a significant role in adaptation in hypoxia cases and aid in improving resistance against anticancer drugs. Inhibitors of GRP78 as an anticancer agent are used as a cancer-fighting strategy [11]. The review discusses the three main approaches that are utilized to target GRP78; phytochemicals inhibitors, peptide inhibitors, and monoclonal antibodies (see the Graphical abstract). Inhibiting such target protein reduces the virulence of pathogens and reduces the therapeutic resistance in the case of cancer. We first come to the basic understanding of the role of GRP78 in healthy and diseased cells.
2. GRP78 in normal versus stressed cell
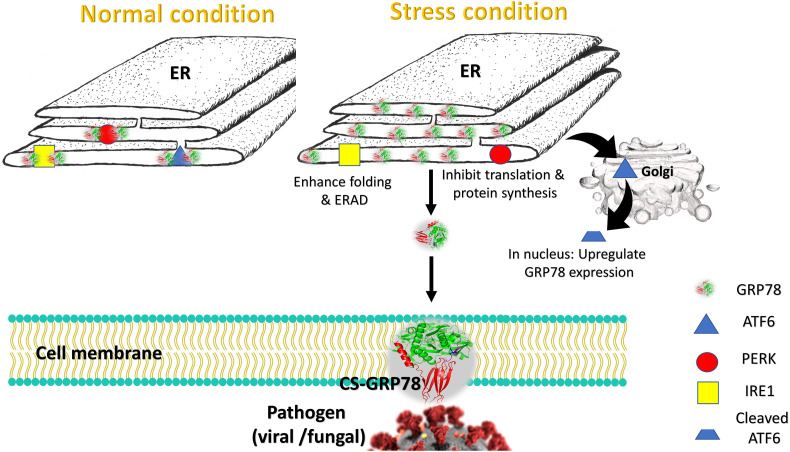
Under normal conditions, GRP78 is found bound to three essential enzymes that regulate cell growth, differentiation, apoptosis, and signaling [[12], [13], [14], [15], [16], [17]]. These enzymes are Activating transcription factor 6 (ATF6), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and Inositol-requiring enzyme 1 (IRE1) which are inactivated through binding to GRP78 in the ER lumen. Under the pressure of unfolded proteins in the ER, GRP78 releases ATF6, PERK, and IRE1, and the enzymes are activated. Once activated, the enzymes, ATF6, PERK, and IRE1 upregulate transcription of chaperones, inhibit the translation, and enhance protein folding, Endoplasmic Reticulum Assisted Degradation (ERAD), and other function that have been reviewed by others [4,[18], [19], [20]] (see also Fig. 1 ). If the pressure of the unfolded proteins is not relieved, the UPR will direct part of the ER to autophagy (ER-phagy). If it is not enough, the whole cell will undergo apoptosis [[21], [22], [23]].
Fig. 1.
Functional aspect of GRP78 in normal versus stress condition. In normal state (left), the GRP78 is located in the lumen of the endoplasmic reticulum (ER) bound to and inactivating ATF6 (blue triangle), PERK (red circle), and IRE1 (yellow square) enzymes. In the stress condition (right), the enzymes are free to do their jobs. ATF6 is translocated to Golgi apparatus to be cleaved then again translocated to the nucleus and helps in upregulating chaperones such as GRP78. PERK inhibits the translation and protein synthesis while IRE1 enhances the folding and ERAD. Under the pressure of the unfolded proteins, the GRP78 escapes the ER retention and translocate to the cytoplasm and the cell membrane. CS-GRP78 is subjected to the recognition of pathogenic proteins (Spike and envelope viral protein and coat proteins of fungi). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On the other hand, under the stress of unfolded proteins, GRP78 can escape the ER retention and translocates to the cytoplasm and on the cell membrane and become membrane-exposed, termed Cell-Surface CS-GRP78 (Fig. 1) [4,24]. This CS-GRP78 characterizes many aggressive types of cancers such as breast, ovarian, pancreatic, and colon cancers [4,16,17,[24], [25], [26], [27], [28], [29], [30], [31], [32]]. Additionally, CS-GRP78 was reported to facilitate pathogenic entry, both viral and fungal infections. Zika virus (ZIKV), Dengue Virus (DENV), Hepatitis C Virus (HCV), Human Papilloma Virus (HPV), Ebola Virus (EBOV), Middle-East Respiratory Syndrome Coronavirus (MERS CoV), Japanese Encephalitis Virus (JEV), Coxsackievirus A9, and Borna Disease Virus (BDV) are among viral infections that reported GRP78 association with viral proteins and GRP78 upregulation in infected cells [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. Additionally, recent studies hypothesized the association of CS-GRP78 with the spike protein of SARS-CoV-2 to help in virus attachment and host cell entry [44]. The primary binding viral protein to GRP78 is spike proteins in coronaviruses and envelope proteins for the other viruses [[44], [45], [46], [47]]. Besides, the spore coat protein homolog (CotH3) of Rhizopus oryzae, the causative fungus for Mucormycosis, is reported to bind to CS-GRP78 on endothelial cells and the binding is responsible for adherence and invasion of the fungus [48].
Since the association of the viral or pathogen infection and elevated levels of CS-GRP78 expression, researchers are focused on targeting GRP78 to prevent or even weaken the pathogenic infection. Reducing the concentration of GRP78 over the cell membrane would reduce the number of internalized pathogenic particles and hence reduce the infection. Additionally, when we target CS-GRP78, the pathogen virulence would be diminished. At the same time, cancer-associated resistance would also be dimensioned, which becomes of the highest priorities in dual diseases (such as viral or fungal infections in cancer patients).
3. GRP78 associated radio- and chemo-resistance
Chemoresistance is the resistance of a tumor to chemotherapy. It was an old observation, while the mechanism of GRP78-induced chemo-resistance in cancer cells was not fully understood [49]. Two mechanisms may be responsible for the chemo-resistance; the UPR pro-survival branch and the receptor-mediated activation of the Akt/PI3K (Phosphoinositide 3-kinase) pathway [50]. Alternatively, the proapoptotic action of the UPR could be compensated by the activation of the Akt/PI3K pathway, resulting in cell survival. The extracellular loop of Cleft Lip and Palate Transmembrane 1-Like (CLPTM1L) is essential for gemcitabine resistance and interaction with GRP78 [51]. Additionally, natural products such as isoliquiritigenin, a chalcone-type flavonoid, were able to reduce the chemoresistance and colony-forming ability of oral squamous cell carcinomas [52]. It is reported that the prior treatment of acidic stress protects the human dermal microvascular endothelial cells from apoptosis by reduced the cleavage of caspase 7, which was supposed to be due to the presence of GRP78 on the membrane of ER that suppress caspase 7 activation [53].
Non-small-cell lung cancer (NSCLC) and glioblastoma multiforme (GBM) have a low survival rate. The overexpressed GRP78 on the cell surface is the primary reason for the radio-resistance in NSCLC and GBM [54]. Targeting cell-surface GRP78 enhances the apoptosis and reduces cell proliferation, colony formation, and downregulates the crucial intracellular phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling essential in the cell cycle, growth and survival [25,30,31]. Besides, tumor growth is delayed with enhanced efficacy of the radiation treatment upon anti-GRP78 antibody administration in mice [54].
In breast cancer, GRP78 is overexpressed while the amount of cell-surface GRP78 is increased upon the treatment with the anti-angiogenic factor Combretastatin A4P [55]. Additionally, cancer cells treated with doxorubicin showed less resistance when treated with GRP78 neutralizing antibodies [50,[55], [56], [57]]. Generally, elevated levels of GRP78 are indicative of cancer aggressivity. Targeting cancer cell-surface GRP78 is a successful strategy to reduce the radio-resistance and chemo-resistance of tumors [15,28,29,58,59].
In this review, the focus is not only on some of the previous trials to use anti-GRP78 to treat cancer but also in the diagnosis (see the peptide inhibitors section below).
4. GRP78 targeting strategies
Different strategies are used to reduce the burden of overexpressed CS-GRP78 [60]. Different compounds show binding affinity to CS-GRP78. Once bound to a substrate, the CS-GRP78 will be internalized to the cell; hence the concentration of the membrane-bound GRP78 will be reduced. Once the level of GRP78 over the cell surface is dropped, the pathogens will not be able to enter the host cell through GRP78; hence the virulence will be reduced. The inhibitory molecules that can target CS-GRP78 include phytochemicals, peptides, and antibodies and will be discussed in detail in the next sections. The inhibitors compete with the pathogen recognizing proteins (such as spike, Envelope, or Coat proteins) for the CS-GRP78 substrate-binding domain β. This domain of the GRP78 is reported to be responsible for the binding of GRP78 to unfolded proteins inside the lumen of the ER through its hydrophobic batches [61,62].
4.1. Phytochemicals
Phytochemicals are compounds found in plants and have a variety of effects on protein function [63]. They are derived from fruits, vegetables, beans, grains, and some other plants [63]. Phytochemicals have a protective role because their antioxidant characteristics which play a vital role in the protection of cells against oxidative damage and decreasing the probability of cancer propagation via the reactive oxygen species (ROS), which can induce stress in ER. Apoptosis initiated by the ER If there is uncontrolled damage in cells [64]. We summarize some phytochemicals crucial in cell stress relief through inhibiting the master of UPR, GRP78.
4.1.1. Galangin and 6-Shogaol
Galangin is a flavonol produced from rhizomes of Alpinia officinarum, which belongs to the ginger family and grows in Southeast Asia. Galangin works as a suppressor for cell proliferation in hepatocellular carcinoma [65]. It raises ER stress through the upregulation of the UPR target genes C/EBP Homologous Protein (CHOP), GRP78, Glucose regulating protein 94 (GRP94), and cytosolic Ca2+ [3]. ER is the primary site for intercellular calcium ions, hence rising cytosolic Ca2+ disrupts the function of ER chaperones, which induce ER stress leading to the activation of UPR and subsequent upregulation of GRP78 [66]. Galangin upregulates ER stress, which inhibits tumor progression through inducing apoptosis [65].
6-Shogaol is produced by dehydration of 6-gingerol and generated from rhizomes of ginger. When treating hepatocellular carcinoma (HCC) cell-line with 6-Shogaol, cancer cells develop apoptotic phenotypes signs such as nuclear shrinkage and condensation in chromatin [11]. Activation of CHOP expression and PERK de-phosphorylation initiates reactions of caspase cascade, which induce apoptosis in HCC. Significant stimulation was observed in ER stress-related proteins, which induce apoptosis by 6-Shogaol through rising in the UPR expression (GRP94, GRP78, and HSP70) [67]. Studies proved that exposing cancer cells to 6-Shogaol and the activator of the PERK/eIF2α pathway, salubrinal, together for a specific time induce ER stress, which leads to cell apoptosis [11]. Salubrinal alone enhances the phosphorylation of eIF2α in the human hepatocarcinoma cell line SMMC-7721 with negligible toxicity [67]. This reveals the significant therapeutic effect of anti-GRP78 against malignancies.
4.1.2. Fungi
Sulphureuine B is produced from Laetiporus sulphureus and tested by glioma cells to detect anti-proliferative properties. Studies revealed that Sulphureuine B provides ER stress by raising the level of expression of CHOP, caspase-12, and GRP78, which prevents separation of GRP78 from PERK, ATF6, and IRE1 which initiates UPR [68,69]. Additionally, Mushrooms contain p-Coumaric acid and Caffeic acid that proved its binding affinity against GRP78 SBDβ in silico, hence suggested to be a possible inhibitor for overexpressed GRP78 in cancer cells or cell infected with viruses including SARS-CoV-2 [46].
4.1.3. Grape seeds and skin
Proanthocyanidins and resveratrol extracted from Grapeseeds. Exposing colorectal cancer cell (CRC) to Grape seed extract which has a high amount of proanthocyanidins and resveratrol leads to a modification in GRP78 and protein disulfide isomerase (PDI) which have a significant role in cell apoptosis which leads CRC to undergo apoptotic pathway leading to inhibition of the targeted cell to proliferation [11]. On the other hand, Caffeic acid and p-Coumaric acid polyphenols, found in the grape skin, have a protective role against photooxidative damage [70]. Additionally, it has a pre-exposure protective role for the human retinal pigment epithelial cells (ARPE-19) against blue light-associated apoptosis in a dose-dependent manner by promoting GRP78 expression. In contrast, GRP78 knockdown inhibited this protective role [70]. As mentioned before, Caffeic acid and p-Coumaric, are suitable binders to GRP78 SBDβ in silico [46].
4.1.4. Phytoestrogens
Estrogen receptor-positive breast cancer cells are responsive to hormonal therapy by blocking the estrogen synthesis leading to estrogen-starvation [71]. It was reported that GRP78 plays a vital role in resist estrogen-starvation induced apoptosis in breast cancer cells. Hence, it was suggested to dual-target the GRP78 during treating estrogen-positive breast cancer, if the expression level of the GRP78 is high, to improve the efficacy and reduce the resistance [71]. It was reported that GRP78 interacts with estrogen due to the critical role of GRP78 in folding the hormone-binding domain of estrogen receptors [71,72]. Additionally, GRP78 targeting was suggested as a therapeutic strategy to sensitize cancer cells to chemotherapy in endometrial cancer (estrogen induced GRP78 expression) [73]. Phytoestrogens are found in Cicer arietinum and include daidzein, genistein, formononetin, and biochanin A [74]. Both Estrogens (estriol and β-estradiol) and the four phytoestrogens are found to be recognized by GRP78 SBDβ and hence are suggested as possible GRP78 inhibitors in silico [46]. It was concluded that estrogens and phytoestrogens are the best binders to the GRP78, while the binding affinities range from −7.0 down to −8.5 kcal/mol. This indicates an excellent binding affinity to GRP78 SBDβ, even better than a selective cyclic peptide, Pep42 [46]. Despite its phytoestrogen activity, genistein activates the apoptosis process through UPR by upregulation of GRP78 and C/EBP homologous protein (CHOP), also termed growth arrest and DNA damage 153 (GADD153), and nuclear translation of GADD153 in HCC cells [75].
4.1.5. Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is a polyphenol found in green tea and has an anti-proliferative effect on breast cancer and melanoma [76]. Besides, it has an inhibition effect against GRP78 function through direct interaction with the ATP binding site of GRP78 competing against ATP binding [77]. EGCG increases the therapeutic efficacy of temozolomide when exposed to glioblastoma cells in vivo by inhibition of GRP78 [78].
4.1.6. Olive leaf extract and honeybee hive propolis
The olive leaf extracts active ingredient, hydroxytyrosol, show good binding affinity to the GRP78 SBDβ in silico [46]. Hydroxytyrosol proved its role as a prophylactic agent against myocardial infarction-mediated apoptosis [79]. Caffeic acid phenethyl ester (CAPE) is found in the hive propolis of the honeybee. CAPE shows in silico binding affinity against GRP78 SBDβ that is comparable to that of the cyclic Pep42, a selective GRP78 peptide [46]. Besides, CAPE induces ER stress in human SH-SY5Y neuroblastoma in an autophagy-dependent manner [80,81].
4.2. Peptides
Different peptides are used to target the cell-surface GRP78 specifically [60,82,83]. Peptides as anticancer drugs have two main types (i) short naked peptides to induce apoptosis (ii) conjugated peptides to deliver an anticancer drug into cells. For the first use, GRP78 serves as a receptor for the peptide and facilitates the internalization of the peptide, which can then modulate various pathways.
4.2.1. Peptides to induce apoptosis
-
a.
GMBP1 peptide
Multidrug Resistance (MDR) is drug resistance that happens when cancer cells treated with one anticancer drug develop resistance to different drugs that are different from the used drug in structure and function [84,85]. An example of binding peptides that use GRP78 as a receptor is GMBP1, which is used in reversing gastric cancer MDR. GRP78 facilitates GMBP1 internalization into cells through the transferrin-related pathway [86].
-
b.
Gonadotropin-releasing hormone analogs (GnRHa)
Gonadotropin-releasing hormone (GnRHa) is a hypothalamus secreted hormone that affects sex hormones, testosterone, and estrogen. Modified GnRHa is more efficient than the natural form; hence it is used as a drug depending on the analog [87]. GnRHa used as a drug against Endometriosis (a case in which cells like that lining the inside of the uterus grow outside it in other parts of the body) [88]. GnRHa inhibits proliferation and induces apoptosis of defected cells by inhibiting GRP78, thus leading to apoptosis [89].
4.2.2. Conjugated peptides
Cell targeting is the solution for the non-specific toxicity of anticancer drugs that affect cancer and healthy cells altogether and thus resulting in severe side effects [90]. Peptides can target cancer cells and deliver anticancer drugs into the cell. In cancer cells, the peptide can bind to the surface, a membrane-bound form of the overexpressed chaperone, GRP78 [27]. To choose the peptide for a particular cancer cell, in vitro trials are required, such as phage display. Phage display is a technique for studying molecular interactions such as protein-DNA, protein-protein, and protein-peptide utilizing the bacteriophages to encode peptides to genetic information [91]. A pool of cyclic peptides tested against the cancer cells, and then a peptide is chosen to be used for drug delivery [27].
-
a.
Pep42
Pep42 is a cyclic peptide (CTVALPGGYVRVC) identified by the phage display technique against human melanoma cell line Me6652/4 [27]. CS-GRP78 is the receptor for Pep42 and facilitates it's internalization to the cell [92]. Pep42-taxol and Pep42-doxorubicin conjugates bind to GRP78 in highly metastatic human melanoma cells leading to its death in vitro, leading to cancer cell death [4]. Pep42 selectively bind to GRP78 and enter the cell and thus make it a powerful tool to deliver anticancer drugs to various cancer cells [27,90,93]. Pep42 was used as a profiler for in silico predicting the CS-GRP78 and viral proteins of the Zika virus, Human papillomavirus, SARS-CoV-2, and Ebola virus [44,45,47,94].
-
b.
WIFPWIQL peptide
WIFPWIQL peptide binds to GRP78 expressed in breast cancer cells surface in the breast and metastatic cells. Subtilase cytotoxin is a toxin from the AB5 toxins family. Subtilase cytotoxin composed of two subunits; SubA, which is responsible for the toxicity, and SubB, which is responsible for Subtilase cytotoxin internalization to the cell [95]. SubA toxic effect is that it induces cell apoptosis by cleaving GRP78 between the amino acid residues Leu416 and Leu417 [96]. As indicated, WIFPWIQL peptide binds to GRP78 over cancer cells (CS-GRP78). WIFPWIQL-SubA fusion resulting in an efficient anticancer agent. WIFPWIQL-SubA works simultaneously, WIFPWIQL is responsible for GRP78 recognition and internalization to the cancer cells, while SubA is responsible for the toxic effect on the cell by cleaving GRP78 inside the cell and thus leading to apoptosis [97]. WIFPWIQL liposomes loaded with doxorubicin are used to target CS-GRP78 overexpressed over vascular endothelial growth factor (VEGF)-activated human umbilical vein endothelial cells [98]. WIFPWIQL bound N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer aminohexyl-geldanamycin conjugates were able to target CS-GRP78 and hence inhibit human prostate cancer cells [99]. Additionally, the genetic engineered mung bean trypsin inhibitor (GBP-TI) that includes the WIFPWIQL peptide was able to induce apoptosis in colorectal cancer cells [100].
-
c.
Bone Metastasis Targeting Peptide 78 (BMTP78)
BMTP78 composed of a peptide (WIFPWIQL) conjugated with proapoptotic moiety D(KLAKLAK)2 [101]. GRP78 facilitates the internalization of BMTP78 into the cytoplasm. In vitro trials showed that BMTP78 induces apoptosis in human and mouse mammary cell lines. D(KLAKLAK)2 after internalization disrupts mitochondrial membrane permeability and thus kills the cell [102]. BMTP78 induced dose-dependent cytotoxicity in human leukemia and lymphoma cell lines and acute myeloid leukemia patients [103,104]. Additionally, the GRP78 receptor/BMTP78 system was used to image breast tumors accurately. The adeno-associated virus-M13-derived phage (AAVP) can be used clinically to detect (imaging) and eradicate (targeted therapy) of Inflammatory breast cancer utilizing CS-GRP78 as a target [101].
-
d.
GIRLRG peptide
GIRLRG is a peptide identified using phage display and binds GRP78. GIRLRG conjugated to paclitaxel-encapsulated nanoparticles, specifically targeted breast cancer and glioblastoma [105]. It was predicted (in silico) that GIRLRG binds to the ATPase domain of GRP78. GIRLRG conjugated with Poly Ethylene Glycol (PEG) can efficiently target different tumor cell lines including, heterotopic cervical (HT3), esophageal (OE33), pancreatic (BXPC3), lung (A549), and glioma (D54) [106]. Additionally, the radio-labeled 111In-PEG-GIRLRG show specificity toward cervical, esophageal, pancreatic, lung, and brain tumors using SPECT imaging [107].
-
e.
VAP peptide
SNTRVAP (VAP) is a peptide identified using the phage display technique, and it binds to GRP78 specifically [108]. SNTRVAP coupling with a siRNA for GRP78, effectively downregulated its expression [109]. VAP modified micelles (RI-VAP (retro Inverso isomer of L-VAP) and D-VAP (retro isomer of L-VAP)) could effectively achieve glioma-targeted drug delivery, through GRP78. At the same time, it improved the therapeutic efficacy of paclitaxel for glioma [110].
4.2.3. Binding peptides in diagnostics
As we mentioned before, peptides could be used for drug delivery; it can be used as a carrier for radiolabels for imaging purposes, such as in the Positron Emission Tomography (PET), utilizing the same concept of targeting GRP78 over cancer cells.
Radiolabeled Polyethylene glycol (PEG)-GIRLRG is used in targeting many cancers as heterotopic cervical, esophageal, pancreatic, lung, and glioma tumors [106]. Triple-negative breast cancer (TNBC) resembles 15% of breast cancer cases, while the available diagnostic technology for its detection is by the invasive needle biopsy. For example, 68Ga, a radiolabel for PET imaging, can be conjugated with dodecane tetraacetic acid (DOTA)-VAP. GRP78-targeted PET imaging with [68Ga]-DOTA-VAP is a useful and accurate technique for imaging TNBC and differentiates it from other cancer types [111].
4.3. Monoclonal antibodies
Antibody (Ab), also called immunoglobulin (Ig), is a huge, Y-shaped protein produced mainly by plasma cells that are used by the immune system to neutralize pathogens such as viruses and bacteria. The pathogenic molecule that is recognized by the antibody is called an antigen [112]. The antibody binds with the antigen with a key-lock mechanism. Once the interaction established, the cell bearing the antigen triggers a response such as metabolic inhibition [113].
4.3.1. Monoclonal antibody 159 (MAb159)
MAb159 is a highly specific monoclonal antibody against the human GRP78 (Kd = 1.7 nM) [114]. When administered, MAb159 found localized on the membranes of cancer cells but not normal cell-lines. Upon glucose starvation stress, MAb159 is found more abundant on the cell membrane [115]. As the CS-GRP78 is PI3K/AKT signaling upstream regulator through its interaction with Crypto and alpha2-macroglobulin over the cell membrane, it is required for these factors to activate the PI3K/AKT signaling [116]. Once bound to CS-GRP78, MAb159 endocytosed and modulate the PI3K pathway leading to inhibition for cell proliferation, tumor growth, and metastasis. At the same time, it enhances tumor cell death both in vitro and in vivo [116]. The efficacy of MAb159 was examined in various tumor xenograft models, including HT29 (colon cancer), H249 (small cell lung carcinoma), and A549 (lung adenocarcinoma). These cells have relatively higher (4.6%–9.4%) surface GRP78 expression compared to healthy cells [117]. MAb159 treatment led to 50%, 58%, and 78% tumor growth inhibition in these models, respectively [116].
4.3.2. Monoclonal IgM antibody SAM-6
The fully human monoclonal IgM antibody, SAM-6, was isolated from a gastric cancer patient, and it binds to an O-glycosylated form of GRP78. SAM-6 is internalized via endocytosis and is finally responsible for a lethal accumulation of oxidized lipoproteins followed by apoptosis in cancer cells [118]. SAM-6 not only bind to GRP78 on the cancer cell membrane but also it reduces the drug resistance and kills the cancer cell [50].
4.3.3. Human IgM Antibody PAT-SM6
PAT-SM6 specifically binds to primary multiple myelomas cells. Staining the cells by immunohistochemistry reveals binding to GRP78 of the PAT-SM6. This binding induces apoptosis and complement-dependent cytotoxicity [119].
4.3.4. α 2-macroglobulin (α2M)
α2M is associated with the N-terminal region of cell-surface GRP78. The binding activates Akt to suppress apoptotic pathways and promotes cell proliferation [120,121].
4.3.5. Mouse MAb C38 and C107
The mouse monoclonal antibody C38 recognizes the C-terminal domain of the murine GRP78 exposed on the cell membrane. The binding induces inhibition of the Akt/PI3K proliferative pathway in melanoma cells [122]. A comparable experiment done on melanoma mouse model shows that the antibody C107 also binds to GRP78. In both experiments, the binging with the antibody decreases the tumor growth [122]. Anti GRP78-C-terminal domain (CTD) antibodies are tested against human prostate cancer cells. It significantly reduces tumor growth, inhibits cell proliferation, while promotes apoptosis. Besides, in the prostate cancer patients, the anti-CTD GRP78 antibody binds the cell-expressed, GRP78 in human prostate cancer cells [120,121].
5. Conclusion
GRP78, a master chaperone protein of the unfolded protein response, plays an essential role in cancer chemo-resistance and virulence of the pathogenic infections. Targeting GRP78 was utilized to defeat aggressive types of cancer like triple-negative breast cancer. Additionally, inhibiting GRP78 overexpressed in viral infections is suggested as a promising strategy to reduce the virulence of many viruses and fungal infections. The present review article summarizes the up to date targeting strategies used to inhibit cell-surface GRP78 illuminating the potential use of these strategies to defeat both cancer chemo-resistance and viral and fungal infections.
Declaration of competing interest
All the authors declare no conflict of interest for this work.
References
- 1.Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 2.Gething M.-J., Sambrook J. Protein folding in the cell. Nature. 1992;355:33. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 3.Hu H., Tian M., Ding C., Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019;9 doi: 10.3389/fimmu.2018.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim I.M., Abdelmalek D.H., Elfiky A.A. GRP78: a cell’s response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Little E., Ramakrishnan M., Roy B., Gazit G., Lee A.S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 6.Brocchieri L., De Macario E.C., Macario A.J. hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail A.M., Elfiky A.A., Elshemey W.M. Recognition of the gluconeogenic enzyme, Pck1, via the Gid4 E3 ligase: an in silico perspective. J. Mol. Recognit. 2020;33 doi: 10.1002/jmr.2821. [DOI] [PubMed] [Google Scholar]
- 8.Liao P.C., Tan S.K., Lieu C.H., Jung H.K. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J. Cell. Biochem. 2008;104:1509–1523. doi: 10.1002/jcb.21730. [DOI] [PubMed] [Google Scholar]
- 9.Haas I. BiP—a heat shock protein involved in immunoglobulin chain assembly. Curr. Top. Microbiol. Immunol. 1991;167:71–82. doi: 10.1007/978-3-642-75875-1_4. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Ni M., Lee B., Barron E., Hinton D., Lee A. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava S., Jain G., Dang S., Gupta S., Gabrani R. Springer; 2018. Phytochemicals Targeting Endoplasmic Reticulum Stress to Inhibit Cancer Cell Proliferation. Anticancer Plants: Natural Products and Biotechnological Implements; pp. 273–287. [Google Scholar]
- 12.Ge R., Kao C. Cell surface GRP78 as a death receptor and an anticancer drug target. Cancers. 2019;11:1787. doi: 10.3390/cancers11111787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopal U., Pizzo S.V. Cell Surface GRP78, a New Paradigm in Signal Transduction Biology. Elsevier; 2018. The endoplasmic reticulum chaperone GRP78 also functions as a cell surface signaling receptor; pp. 9–40. [Google Scholar]
- 14.Luo S., Mao C., Lee B., Lee A.S. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaffenbach K.T., Lee A.S. The critical role of GRP78 in physiologic and pathologic stress. Curr. Opin. Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan X., Dong M., Li X., Zhou J. GRP78 promotes the invasion of pancreatic cancer cells by FAK and JNK. Mol. Cell. Biochem. 2015;398:55–62. doi: 10.1007/s11010-014-2204-2. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hashimi A.A., Rak J., Austin R.C. Cell surface GRP78: a novel regulator of tissue factor procoagulant activity. Cell Surface GRP78, a New Paradigm in Signal Transduction Biology: Elsevier. 2018:63–85. [Google Scholar]
- 19.Lee A.S. GRP78 induction in Cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 20.Liu M., Spellberg B., Phan Q.T., Fu Y., Fu Y., Lee A.S., et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Invest. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha-Molstad H., Yu J.E., Feng Z., Lee S.H., Kim J.G., Yang P., et al. p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nat. Commun. 2017;8:102. doi: 10.1038/s41467-017-00085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji C.H., Kim H.Y., Heo A.J., Lee S.H., Lee M.J., Kim S.B., et al. The N-degron pathway mediates ER-phagy. Mol. Cell. 2019;75:1058–1072.e9. doi: 10.1016/j.molcel.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Mehrbod P., Ande S.R., Alizadeh J., Rahimizadeh S., Shariati A., Malek H., et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10:376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai Y.-L., Lee A.S. Elsevier; 2018. Cell Surface GRP78: Anchoring and Translocation Mechanisms and Therapeutic Potential in Cancer. Cell Surface GRP78, a New Paradigm in Signal Transduction Biology; pp. 41–62. [Google Scholar]
- 25.Chang Y.-J., Huang Y.-P., Li Z.-L., Chen C.-H. GRP78 knockdown enhances apoptosis via the down-regulation of oxidative stress and Akt pathway after epirubicin treatment in colon cancer DLD-1 cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T., Xu S. Chronic exposure of cisplatin induces GRP78 expression in ovarian cancer. Proceedings of the 2017 4th International Conference on Biomedical and Bioinformatics Engineering: ACM. 2017:35–38. [Google Scholar]
- 27.Kim Y., Lillo A.M., Steiniger S.C., Liu Y., Ballatore C., Anichini A., et al. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–9444. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Zhang L., Zhao Y., Li H., Xiao H., Fu R., et al. Cell-surface GRP78 facilitates colorectal cancer cell migration and invasion. Int. J. Biochem. Cell Biol. 2013;45:987–994. doi: 10.1016/j.biocel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Niu Z., Wang M., Zhou L., Yao L., Liao Q., Zhao Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015;5 doi: 10.1038/srep16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian S., Chang W., Du H., Bai J., Sun Z., Zhang Q., et al. The interplay between GRP78 expression and Akt activation in human colon cancer cells under celecoxib treatment. Anti-Cancer Drugs. 2015;26:964–973. doi: 10.1097/CAD.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 31.Xie J., Tao Z.-H., Zhao J., Li T., Wu Z.-H., Zhang J.-F., et al. Glucose regulated protein 78 (GRP78) inhibits apoptosis and attentinutes chemosensitivity of gemcitabine in breast cancer cell via AKT/mitochondrial apoptotic pathway. Biochem. Biophys. Res. Commun. 2016;474:612–619. doi: 10.1016/j.bbrc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J., Jiang Y., Jia Z., Li Q., Gong W., Wang L., et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin. Exp. Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen H.-H., Chen C.-C., Lin Y.-S., Chang P.-C., Lu Z.-Y., Lin C.-F., et al. AR-12 suppresses dengue virus replication by down-regulation of PI3K/AKT and GRP78. Antivir. Res. 2017;142:158–168. doi: 10.1016/j.antiviral.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Choukhi A., Ung S., Wychowski C., Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu H., Chan C.-M., Zhang X., Wang Y., Yuan S., Zhou J., et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells. J. Biol. Chem. 2018;293(30):11709–11726. doi: 10.1074/jbc.RA118.001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S., Laxminarayana S.V., Chandra N., Ravi V., Desai A. Heat shock protein 70 on Neuro2a cells is a putative receptor for Japanese encephalitis virus. Virology. 2009;385:47–57. doi: 10.1016/j.virol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Honda T., Horie M., Daito T., Ikuta K., Tomonaga K. Molecular chaperone BiP interacts with Borna disease virus glycoprotein at the cell surface. J. Virol. 2009;83:12622–12625. doi: 10.1128/JVI.01201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jindadamrongwech S., Thepparit C., Smith D. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch. Virol. 2004;149:915–927. doi: 10.1007/s00705-003-0263-x. [DOI] [PubMed] [Google Scholar]
- 39.Liberman E., Fong Y.-L., Selby M.J., Choo Q.-L., Cousens L., Houghton M., et al. Activation of the grp78 andgrp94 promoters by hepatitis C virus E2 envelope protein. J. Virol. 1999;73:3718–3722. doi: 10.1128/jvi.73.5.3718-3722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nain M., Mukherjee S., Karmakar S.P., Paton A.W., Paton J.C., Abdin M., et al. GRP78 is an important host-factor for Japanese encephalitis virus entry and replication in mammalian cells. J. Virol. 2017;91(6):1–21. doi: 10.1128/JVI.02274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pujhari S., Macias V.M., Nissly R.H., Nomura M., Kuchipudi S.V., Rasgon J.L. Heat shock protein 70 (Hsp70) is involved in the Zika virus cellular infection process. bioRxiv. 2017 doi: 10.1080/22221751.2018.1557988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes-del Valle J., Chávez-Salinas S., Medina F., Del Angel R.M. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J. Virol. 2005;79:4557–4567. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shurtleff A.C., Costantino J.A., Tritsch S.R., Retterer C., Spurgers K.B., Bavari S. HSPA5 is an essential host factor for Ebola virus infection. Antivir. Res. 2014;109:171–174. doi: 10.1016/j.antiviral.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elfiky A.A. Ebola virus glycoprotein GP1-host cell-surface HSPA5 binding site prediction. Cell Stress Chaperones. 2020;25:541–548. doi: 10.1007/s12192-020-01106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elfiky A.A., Ibrahim I.M. Zika virus envelope – heat shock protein A5 (GRP78) binding site prediction. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1784794. [DOI] [PubMed] [Google Scholar]
- 48.Gebremariam T., Liu M., Luo G., Bruno V., Phan Q.T., Waring A.J., et al. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Invest. 2014;124:237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang CY, Cusack JC, Jr., Liu R, Baldwin AS, Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat. Med.. 1999;5:412–7. [DOI] [PubMed]
- 50.Roller C., Maddalo D. The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front. Pharmacol. 2013;4:10. doi: 10.3389/fphar.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clarke W.R., Amundadottir L., James M.A. CLPTM1L/CRR9 ectodomain interaction with GRP78 at the cell surface signals for survival and chemoresistance upon ER stress in pancreatic adenocarcinoma cells. Int. J. Cancer. 2019;144:1367–1378. doi: 10.1002/ijc.32012. [DOI] [PubMed] [Google Scholar]
- 52.Hu F.-W., Yu C.-C., Hsieh P.-L., Liao Y.-W., Lu M.-Y., Chu P.-M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget. 2017;8:93912–93923. doi: 10.18632/oncotarget.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visioli F., Wang Y., Alam G.N., Ning Y., Rados P.V., Nör J.E., et al. Glucose-regulated protein 78 (Grp78) confers chemoresistance to tumor endothelial cells under acidic stress. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dadey D.Y.A., Kapoor V., Hoye K., Khudanyan A., Collins A., Thotala D., et al. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non–small cell lung cancer cell lines and tumor models. Clin. Cancer Res. 2017;23:2556. doi: 10.1158/1078-0432.CCR-16-1935. [DOI] [PubMed] [Google Scholar]
- 55.Gazit G., Lu J., Lee A.S. De-regulation of GRP stress protein expression in human breast cancer cell lines. Breast Cancer Res. Treat. 1999;54:135–146. doi: 10.1023/a:1006102411439. [DOI] [PubMed] [Google Scholar]
- 56.Hannun Y.A. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89:1845–1853. [PubMed] [Google Scholar]
- 57.Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., et al. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc. Natl. Acad. Sci. U. S. A. 1987;84:3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuroda K., Horiguchi A., Asano T., Ito K., Asakuma J., Sato A., et al. Glucose-regulated protein 78 positivity as a predictor of poor survival in patients with renal cell carcinoma. Urol. Int. 2011;87:450–456. doi: 10.1159/000330883. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L.-Y., Li P.-L., Xu A., Zhang X.-C. Involvement of GRP78 in the resistance of ovarian carcinoma cells to paclitaxel. Asian Pac. J. Cancer Prev. 2015;16:3517–3522. doi: 10.7314/apjcp.2015.16.8.3517. [DOI] [PubMed] [Google Scholar]
- 60.Bailly C., Waring M.J. Pharmacological effectors of GRP78 chaperone in cancers. Biochem. Pharmacol. 2019;163:269–278. doi: 10.1016/j.bcp.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez–Gronow M., Selim M.A., Papalas J., Pizzo S.V. GRP78: a multifunctional receptor on the cell surface. Antioxid. Redox Signal. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 62.Quinones Q.J., Ridder Ggd, Pizzo S.V. GRP78, a chaperone with diverse roles beyond the endoplasmic reticulum. Histol. Histopathol. 2008;23(11):1409–1416. doi: 10.14670/HH-23.1409. [DOI] [PubMed] [Google Scholar]
- 63.Dillard C.J., German J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. [Google Scholar]
- 64.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 65.Su L., Chen X., Wu J., Lin B., Zhang H., Lan L., et al. Galangin inhibits proliferation of hepatocellular carcinoma cells by inducing endoplasmic reticulum stress. Food Chem. Toxicol. 2013;62:810–816. doi: 10.1016/j.fct.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu R., Zhou P., Peng Y.-B., Xu X., Ma J., Liu Q., et al. 6-Shogaol induces apoptosis in human hepatocellular carcinoma cells and exhibits anti-tumor activity in vivo through endoplasmic reticulum stress. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biswas J., Roy M., Mukherjee A. Anticancer drug development based on phytochemicals. J Drug Disc Develop Delivery. 2015;2:1012. [Google Scholar]
- 69.Ren Z.-H., Zhang J.-W., Wen G.-L., Zhang L., Duan D.-M. Sulphureuine B, a drimane type sesquiterpenoid isolated from Laetiporus sulphureus induces apoptosis in glioma cells. Bangladesh Journal of Pharmacology. 2015;10:896–902. [Google Scholar]
- 70.Zhao Z., Sun T., Jiang Y., Wu L., Cai X., Sun X., et al. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem. Toxicol. 2014;74:216–224. doi: 10.1016/j.fct.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Fu Y., Li J., Lee A.S. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation–induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 72.Avila M.F., Cabezas R., Torrente D., Gonzalez J., Morales L., Alvarez L., et al. Novel interactions of GRP78: UPR and estrogen responses in the brain. Cell Biol. Int. 2013;37:521–532. doi: 10.1002/cbin.10058. [DOI] [PubMed] [Google Scholar]
- 73.Luvsandagva B., Nakamura K., Kitahara Y., Aoki H., Murata T., Ikeda S., et al. GRP78 induced by estrogen plays a role in the chemosensitivity of endometrial cancer. Gynecol. Oncol. 2012;126:132–139. doi: 10.1016/j.ygyno.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 74.Sayed A.A., Elfiky A.A. In silico estrogen-like activity and in vivo osteoclastogenesis inhibitory effect of Cicer arietinum extract. Cell. Mol. Biol. 2018;64:29–39. [PubMed] [Google Scholar]
- 75.Yeh T.-C., Chiang P.-C., Li T.-K., Hsu J.-L., Lin C.-J., Wang S.-W., et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem. Pharmacol. 2007;73:782–792. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 76.Nihal M., Ahmad N., Mukhtar H., Wood G.S. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int. J. Cancer. 2005;114:513–521. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 77.Ermakova S.P., Kang B.S., Choi B.Y., Choi H.S., Schuster T.F., Ma W.-Y., et al. (−)−Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 78.Chen D., Wan S.B., Yang H., Yuan J., Chan T.H., Dou Q.P. EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011;53:155–177. doi: 10.1016/b978-0-12-385855-9.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu L.-x., Xu Y.-y., Yang Z.-j., Feng Q. Hydroxytyrosol and olive leaf extract exert cardioprotective effects by inhibiting GRP78 and CHOP expression. J. Biomed. Res. 2018;32:371. doi: 10.7555/JBR.32.20170111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanida I., Ueno T., Kominami E. Springer; 2008. LC3 and Autophagy. Autophagosome and Phagosome; pp. 77–88. [DOI] [PubMed] [Google Scholar]
- 81.Tomiyama R., Takakura K., Takatou S., Le T.M., Nishiuchi T., Nakamura Y., et al. 3,4-Dihydroxybenzalacetone and caffeic acid phenethyl ester induce preconditioning ER stress and autophagy in SH-SY5Y cells. J. Cell. Physiol. 2018;233:1671–1684. doi: 10.1002/jcp.26080. [DOI] [PubMed] [Google Scholar]
- 82.Goldenberg-Cohen N., Raiter A., Gaydar V., Dratviman-Storobinsky O., Goldstein T., Weizman A., et al. Peptide-binding GRP78 protects neurons from hypoxia-induced apoptosis. Apoptosis. 2012;17:278–288. doi: 10.1007/s10495-011-0678-x. [DOI] [PubMed] [Google Scholar]
- 83.Palmeira A., Sousa E., Köseler A., Sabirli R., Gören T., Türkçüer İ., et al. Preliminary virtual screening studies to identify GRP78 inhibitors which may interfere with SARS-CoV-2 infection. Pharmaceuticals. 2020;13:132. doi: 10.3390/ph13060132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 85.Zhang D., Fan D. New insights into the mechanisms of gastric cancer multidrug resistance and future perspectives. Future Oncol. 2010;6:527–537. doi: 10.2217/fon.10.21. [DOI] [PubMed] [Google Scholar]
- 86.Wang X., Li Y., Xu G., Liu M., Xue L., Liu L., et al. Mechanism study of peptide GMBP1 and its receptor GRP78 in modulating gastric cancer MDR by iTRAQ-based proteomic analysis. BMC Cancer. 2015;15:358. doi: 10.1186/s12885-015-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bethesda . 2012. LiverTox: Clinical and Research Information on Drug-induced Liver Injury [Internet] [PubMed] [Google Scholar]
- 88.Bulun S.E. Endometriosis. N. Engl. J. Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 89.Weng H., Liu F., Hu S., Li L., Wang Y. GnRH agonists induce endometrial epithelial cell apoptosis via GRP78 down-regulation. J. Transl. Med. 2014;12:306. doi: 10.1186/s12967-014-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y., Steiniger S.C., Kim Y., Kaufmann G.F., Felding-Habermann B., Janda K.D. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol. Pharm. 2007;4:435–447. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith G.P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science (New York, N.Y.) 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 92.Tsai Y.-L., Lee A.S. In: Cell Surface GRP78, a New Paradigm in Signal Transduction Biology. Pizzo S.V., editor. Academic Press; 2018. Chapter 3 - cell surface GRP78: anchoring and translocation mechanisms and therapeutic potential in cancer; pp. 41–62. [Google Scholar]
- 93.Yoneda Y., Steiniger S.C., Capkova K., Mee J.M., Liu Y., Kaufmann G.F., et al. A cell-penetrating peptidic GRP78 ligand for tumor cell-specific prodrug therapy. Bioorg. Med. Chem. Lett. 2008;18:1632–1636. doi: 10.1016/j.bmcl.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elfiky A.A. Human papillomavirus E6: host cell receptor, GRP78, binding site prediction. J. Med. Virol. 2020 doi: 10.1002/jmv.25737. (n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beddoe T., Paton A.W., Le Nours J., Rossjohn J., Paton J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010;35:411–418. doi: 10.1016/j.tibs.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paton A.W., Beddoe T., Thorpe C.M., Whisstock J.C., Wilce M.C., Rossjohn J., et al. AB 5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L., Li Z., Shi T., La X., Li H., Li Z. Design, purification and assessment of GRP78 binding peptide-linked Subunit A of Subtilase cytotoxic for targeting cancer cells. BMC Biotechnol. 2016;16:65. doi: 10.1186/s12896-016-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katanasaka Y., Ishii T., Asai T., Naitou H., Maeda N., Koizumi F., et al. Cancer antineovascular therapy with liposome drug delivery systems targeted to BiP/GRP78. Int. J. Cancer. 2010;127:2685–2698. doi: 10.1002/ijc.25276. [DOI] [PubMed] [Google Scholar]
- 99.Larson N., Ray A., Malugin A., Pike D.B., Ghandehari H. HPMA copolymer-aminohexylgeldanamycin conjugates targeting cell surface expressed GRP78 in prostate cancer. Pharm. Res. 2010;27:2683–2693. doi: 10.1007/s11095-010-0267-7. [DOI] [PubMed] [Google Scholar]
- 100.Li Z., Zhao C., Li Z., Zhao Y., Shan S., Shi T., et al. Reconstructed mung bean trypsin inhibitor targeting cell surface GRP78 induces apoptosis and inhibits tumor growth in colorectal cancer. Int. J. Biochem. Cell Biol. 2014;47:68–75. doi: 10.1016/j.biocel.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 101.Dobroff A.S., Eckhardt B.L., Salmeron C.C., Cimino D.F., Arap W., Pasqualini R. Abstract 3543: ligand-directed and transcription-based molecular imaging and treatment of cancer. Cancer Res. 2015;75:3543. [Google Scholar]
- 102.Miao Y.R., Eckhardt B.L., Cao Y., Pasqualini R., Argani P., Arap W., et al. Inhibition of established micrometastases by targeted drug delivery via cell surface–associated GRP78. Clin. Cancer Res. 2013;19:2107–2116. doi: 10.1158/1078-0432.CCR-12-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Araujo N., Hebbar N., Rangnekar V.M. GRP78 is a targetable receptor on cancer and stromal cells. EBioMedicine. 2018;33:2–3. doi: 10.1016/j.ebiom.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Staquicini D.I., D’Angelo S., Ferrara F., Karjalainen K., Sharma G., Smith T.L., et al. Therapeutic targeting of membrane-associated GRP78 in leukemia and lymphoma: preclinical efficacy in vitro and formal toxicity study of BMTP-78 in rodents and primates. The Pharmacogenomics Journal. 2018;18:436–443. doi: 10.1038/tpj.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Passarella R.J., Spratt D.E., van der Ende A.E., Phillips J.G., Wu H., Sathiyakumar V., et al. Targeted nanoparticles that deliver a sustained, specific release of paclitaxel to irradiated tumors. Cancer Res. 2010;70:4550–4559. doi: 10.1158/0008-5472.CAN-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kapoor V., Dadey D.Y., Nguyen K., Wildman S.A., Hoye K., Khudanyan A., et al. Tumor-specific binding of radiolabeled PEGylated GIRLRG peptide: a novel agent for targeting cancers. J. Nucl. Med. 2016;57:1991–1997. doi: 10.2967/jnumed.115.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapoor V., Dadey D., Nguyen K., Li H., Rogers B., Thotala D., et al. Abstract 1791: targeting radiation-inducible cell surface GRP78 using GIRLRG peptide as a novel imaging and therapeutic strategy for tumors. Cancer Res. 2015;75:1791. [Google Scholar]
- 108.Mandelin J., Cardó-Vila M., Driessen W.H., Mathew P., Navone N.M., Lin S.-H., et al. Selection and identification of ligand peptides targeting a model of castrate-resistant osteogenic prostate cancer and their receptors. Proc. Natl. Acad. Sci. 2015;112:3776–3781. doi: 10.1073/pnas.1500128112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stone L. On target — theranostic imaging for aggressive disease. Nature Reviews Urology. 2017;14:7. doi: 10.1038/nrurol.2016.230. [DOI] [PubMed] [Google Scholar]
- 110.Ran D., Mao J., Shen Q., Xie C., Zhan C., Wang R., et al. GRP78 enabled micelle-based glioma targeted drug delivery. J. Control. Release. 2017;255:120–131. doi: 10.1016/j.jconrel.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 111.Zhao H., Meng H., Wen J., Wang C., Liu J., Huang G. Noninvasive classification of human triple negative breast cancer by PET imaging with GRP78-targeted molecular probe [68 Ga] DOTA-VAP. Mol. Imaging Biol. 2019:1–8. doi: 10.1007/s11307-019-01416-4. [DOI] [PubMed] [Google Scholar]
- 112.Janeway C.A., Capra J.D., Travers P., Walport M. 1999. Immunobiology: The Immune System in Health and Disease. [Google Scholar]
- 113.Tesniere A., Apetoh L., Ghiringhelli F., Joza N., Panaretakis T., Kepp O., et al. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Gill P, Liu R, Lee A. Antibodies that bind cell surface GRP78 and their use for detection of cancer. US Patent App. 16/438,289; 2020.
- 115.Wang M., Kaufman R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 116.Liu R., Li X., Gao W., Zhou Y., Wey S., Mitra S.K., et al. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin. Cancer Res. 2013;19:6802–6811. doi: 10.1158/1078-0432.CCR-13-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y., Liu R., Ni M., Gill P., Lee A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vercauteren D., Vandenbroucke R.E., Jones A.T., Rejman J., Demeester J., De Smedt S.C., et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Ther. 2010;18:561–569. doi: 10.1038/mt.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee A.S. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat. Rev. Cancer. 2014;14:263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Misra U.K., Pizzo S.V. Potentiation of signal transduction mitogenesis and cellular proliferation upon binding of receptor-recognized forms of α2-macroglobulin to 1-LN prostate cancer cells. Cell. Signal. 2004;16:487–496. doi: 10.1016/j.cellsig.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 121.Misra U.K., Mowery Y., Kaczowka S., Pizzo S.V. Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol. Cancer Ther. 2009;8:1350–1362. doi: 10.1158/1535-7163.MCT-08-0990. [DOI] [PubMed] [Google Scholar]
- 122.de Ridder G.G., Ray R., Pizzo S.V. A murine monoclonal antibody directed against the carboxyl-terminal domain of GRP78 suppresses melanoma growth in mice. Melanoma Res. 2012;22:225–235. doi: 10.1097/CMR.0b013e32835312fd. [DOI] [PubMed] [Google Scholar]