Abstract
Over the last 18 months, 3 immunotherapy combination regimens (ipilimumab + nivolumab, pembrolizumab + axitinib, and axitinib + avelumab) were approved by the US Food and Drug Administration for the first-line treatment of metastatic renal cell carcinoma (mRCC), making selection of the optimal first-line treatment regimen very challenging. As of April 2020, preferred first-line treatment options for mRCC are pembrolizumab + axitinib and ipilimumab + nivolumab, based on the improvement in overall survival and progression-free survival compared to sunitinib, as observed in pivotal phase III clinical trials. Because the combination of 2 drugs is typically more toxic than a monotherapy, careful attention must be given to overlapping toxicities. The pembrolizumab + axitinib combination led to grade ≥3 adverse events in 75.8% of patients (vs 70.6% in the sunitinib group), while grade ≥3 adverse events were less frequent in the nivolumab + ipilimumab group compared to the sunitinib group. Discontinuation rates due to toxicity were 10.7% for pembrolizumab + axitinib (both drugs), 22% for ipilimumab + nivolumab and were comparable with sunitinib in both studies (13.9% and 12%, respectively). The combination of pembrolizumab + axitinib may have immune-modulating functions that may provide clinical benefit without the additional toxicity observed with ipilimumab + nivolumab. In addition, this tyrosine kinase inhibitor + immune checkpoint combination should have faster treatment response in patients with larger disease burden or in more symptomatic patients, which makes this combination an excellent choice for the first-line treatment regimen for mRCC. These combinations have proven to be tolerable, though long-term results are still lacking. As treatment options for mRCC are rapidly expanding, immunotherapy combinations could potentially change the treatment paradigm, with the ultimate goal of prolonging life and eventually curing mRCC.
Keywords: pembrolizumab, axitinib, renal cell carcinoma
Introduction
Each year worldwide, renal cell carcinoma (RCC) affects more than 393,000 people and leads to 139,000 deaths. The United States has approximately 73,750 new cases and 14,830 deaths annually from RCC,1 with increasing incidence related to obesity2 and smoking.3
RCC can be idiopathic or hereditary; people with a family history of RCC tend to have a risk of developing RCC of less than 1:114 (0.07%).4 The major histologic subtypes per World Health Organization classification are clear cell RCC (ccRCC) and non-clear cell RCC (nccRCC), which includes papillary, chromophobe, collecting duct tumors, other rarer molecular subcategories, and an unclassified category.
At initial presentation, approximately 19% of RCC patients either have advanced locoregional disease (stage III; 8%) or distant metastases (stage IV; 11%).5 While surgery is the only curative option for early-stage disease, cytoreductive nephrectomy can be considered for mRCC patients who have a long-term sustained benefit from systemic therapy, though the CARMENA6,7 and SURTIME8 trials suggest that systemic therapy should be prioritized. Also, a subset of patients with slow-growing oligometastatic RCC with a prolonged time interval from removal of primary tumor to metastases development can safely be surveilled before starting systemic therapy, as reported by Rini et al.9 In those patients, metastasectomy could also be considered since it is associated with survival benefit in the era of targeted therapy.10
Vascular Endothelial Growth Factor Antibodies and Mammalian Target of Rapamycin Inhibitors as First-Line Therapy in mRCC
Over the last few decades, several drugs have been approved as first-line therapy to treat patients with mRCC, including vascular endothelial growth factor (VEGF) antibodies, tyrosine kinase inhibitors (TKIs), and agents targeting the mammalian target of rapamycin (mTOR)11 (as summarized in Table 1).
Table 1.
Front-Line Therapies Targeting VEGF and mTOR Pathways in mRCC
| Drug | Advantages | Disadvantages |
|---|---|---|
| Sorafenib | Improves PFS (5.5 vs 2.8 months) | Less tolerable than sunitinib or pazopanib |
| Sunitinib | Improves PFS (11 vs 5 months) Good ORR 47% |
Lack of OS benefit |
| Temsirolimus | Improves OS (10. 9 vs 7.3 months) Good option for intermediate- to poor-risk mRCC and non-clear cell histology |
Weekly IV dosing Lack of objective responses by RECIST criteria |
| Bevacizumab + IFN-α | Improves PFS (10.2 vs 5.4 months) | IFN-α is not well-tolerated No OS benefit |
| Pazopanib | Improves PFS (9.2 vs 4.2 months) Noninferior to sunitinib (COMPARZ) |
Higher incidence of hepatotoxicity |
| Cabozantinib | Improves PFS (8.6 vs 5.3 months) | Not an option for good-risk mRCC |
Abbreviations: VEGF, vascular endothelial growth factor; mTOR, mammalian target of rapamycin; mRCC, metastatic renal cell carcinoma; OS, overall survival; PFS, progression-free survival; ORR, overall response rate; IFN-α, interferon-alpha.
In 2005, sorafenib became the first TKI approved by the US Food and Drug Administration (FDA) for the treatment of mRCC, based on a phase III trial that randomized mRCC patients to sorafenib or placebo.12 The median progression-free survival (PFS) with sorafenib was 5.5 months vs 2.8 months with placebo (hazard ratio [HR]=0.44, 95% confidence interval [CI] 0.35–0.55; p<0.01). Although overall survival (OS) also favored sorafenib over placebo (HR=0.72, 95% CI 0.54–0.94; p=0.02), this benefit was not statistically significant per predefined statistical parameters. The most common side effects, among others, were palmar-plantar erythrodysesthesia, diarrhea, rash, and fatigue. Although sorafenib is FDA-approved, it is not commonly used since it is less tolerable than sunitinib and pazopanib.
Sunitinib, a multikinase inhibitor of the VEGF receptor (VEGFR), c-Kit and platelet-derived growth factor receptor (PDGFR), was FDA-approved for treatment of mRCC in 2006 and has been the standard of care in subsequent mRCC clinical trials.13 In a phase III study comparing sunitinib and interferon-alfa (IFN-α), patients treated with sunitinib had significantly longer PFS (11 months vs 5 months) (HR=0.42, 95% CI 0.32–0.54; p<0.001).14 When results were updated, there was a trend toward improved OS in the sunitinib group (26.4 vs 21.8 months, respectively; HR=0.821, 95% CI 0.673–1.001; p=0.051).15 Overall response rate (ORR) for sunitinib was 47% compared to 12% for IFN-α (p<0.001).16 Sunitinib is generally well tolerated; hypertension, nausea, fatigue, and diarrhea are the most common adverse events (AEs).
Temsirolimus, an inhibitor of mTOR, was FDA-approved in 2007 for first-line treatment of intermediate- to poor-risk mRCC, including nccRCC. In a phase III trial, 626 patients with untreated mRCC were randomized to one of the 3 arms: combination of temsirolimus 15 mg weekly and IFN-α 3x/week, temsirolimus 25 mg i.v. weekly, or IFN-α 3 MIU s.c. 3x/week.17 Median OS was 10.9 months for temsirolimus monotherapy, 7.3 months for IFN-α, and 8.4 months for the combination therapy. Temsirolimus yielded more hyperglycemia, rash, hyperlipidemia, and peripheral edema. Interestingly, improvement in OS was most pronounced in patients with non-clear-cell histology (n=73).18
In 2009, the FDA-approved bevacizumab in combination with IFN-α for first-line treatment of mRCC. In a phase III trial (AVOREN), 649 patients were randomized: 327 were treated with bevacizumab 10 mg/kg i.v. every 2 weeks plus IFN-α-2a 9 MIU s.c. 3x/week, while 322 received placebo plus IFN-α-2a.19 The primary endpoint, median PFS, was longer in the bevacizumab plus IFN-α-2a group (10.2 months vs 5.4 months; HR=0.63, 95% CI 0.52–0.75; p=0.0001), irrespective of risk category. The most commonly reported grade ≥3 AEs were fatigue (12%) and asthenia (10%). Unfortunately, in a follow-up report, there was no significant difference in median OS between the 2 arms.20
Pazopanib, a multikinase inhibitor of VEGFR-1, −2, and −3, PDGFR-α and -β, and c-KIT, was FDA-approved for treatment of mRCC in 2009. In a phase III trial, pazopanib increased PFS over placebo (9.2 months vs 4.2 months, respectively; HR=0.46, 95% CI 0.34–0.62; p<0.0001).21 Pazopanib had an ORR of 30% vs 3% for placebo (p<0.001). Common AEs included anorexia, nausea, vomiting, diarrhea, hypertension, and changes in hair color. A large phase III study (COMPARZ) comparing sunitinib to pazopanib found similar clinical outcomes for each, although quality of life and toxicity profile favored pazopanib.22
Cabozantinib, a multikinase inhibitor of VEGFRs, MET, and AXL, was FDA-approved in 2017 as first-line treatment for mRCC based on the results of the CABOSUN trial. In this phase II study, patients with intermediate- or poor-risk mRCC were randomized to cabozantinib or sunitinib.23–25 Median PFS was improved at 8.6 months for cabozantinib (95% CI 6.8–14.0) compared to 5.3 months for sunitinib (95% CI 3.0–8.2) (HR=0.48, 95% CI 0.31–0.74; p=0.0008). ORR for cabozantinib was 20% vs 9% for sunitinib. Common side effects with cabozantinib are hypertension (28%), diarrhea (10%), palmar-plantar erythrodysesthesia (8%), fatigue (6%), and hematologic toxicities (3%).18
Immunotherapy as First-Line Treatment for mRCC
Before the advent of the immune checkpoint inhibitors in the last 5 years, the first immunotherapy agent for ccRCC was a cytokine, high-dose interleukin (IL)-2, approved in 1992 due to its ability to cure a small subset of mRCC patients.26,27 McDermott et al reported superior survival with high-dose IL-2 compared to s.c. IL-2 plus IFN-α-2b for patients with primary tumor in situ (p=0.040) or with liver or bone metastases (p=0.001).28 Interestingly, a durable complete response (CR) was observed in 5–9% of patients.27,29 The most common side effects were capillary leak syndrome, urinary tract and catheter-site infections, hypotension, tachycardia, dyspnea, renal dysfunction, hyperbilirubinemia, transaminase elevations, and neurological changes,30 leading to death in 4% of patients in the initial studies.26 A recently published retrospective analysis of 170 patients treated with high-dose IL-2 from 2005 to 2012 using the PROCLAIM registry showed that duration of median OS was longer than historically reported (median OS for treatment-naïve patients was 48.9 months) and that high-dose IL-2 can be administered safely and still has a role in the treatment of eligible patients.31 Moreover, according to National Comprehensive Cancer Network (NCCN) Guidelines, high-dose IL-2 is still recommended for first-line treatment of favorable-risk ccRCC in patients with excellent Karnofsky performance status (KPS) and normal organ function.18
From their initial discovery, immune checkpoint inhibitors have been studied extensively, since metastatic kidney cancer is considered immunogenic due to the increased infiltration of different immune cells such as T cells and NK cells.32 However, regulatory T cells (Tregs), tumor-associated macrophages, and myeloid-derived suppressor cells (MDSCs) are responsible for immunosuppression frequently observed in mRCC.33 In addition to immunosuppressive cells, increased levels of pro-inflammatory cytokines such as TNF-α, IL-6, and interleukin-1 beta (IL-1β) are also associated with worse prognosis and advanced disease.34 Anti-PD-1 and anti-CTLA4 antibodies improve T-cell responses and allow upregulation of anticancer activity by negative Treg regulation and increased IFN-γ and IL-2 production. One of the key mechanisms of resistance to immune checkpoint inhibitors is MDSCs,35 which are known to accumulate in kidney tumors and inhibit activation of CD4+ and CD8+ T cells.36
Pembrolizumab, a selective, fully humanized immunoglobulin G4-κ monoclonal antibody against PD-1, is FDA-approved for the treatment of many cancers, including head and neck squamous cell carcinoma, melanoma, non-small cell and small cell lung cancer, gastric cancer, urothelial cancer, triple-negative breast cancer, microsatellite instability-high (MSI-high) colorectal cancer, and Hodgkin lymphoma.37,38 Pembrolizumab was also approved in 2017 for the treatment of mismatch repair-deficient or MSI-high solid tumors, which was the first tissue-agnostic approval in oncology.38
KEYNOTE-427 was an single arm, open-label, phase II trial to evaluate pembrolizumab as first-line treatment for ccRCC (cohort A).39,40 The primary endpoint was ORR, which was reported to be 33.6% (95% CI 24.8–43.4), with 1 CR and 35 partial responses (PRs).39 Updated results showed that ORR was 36.4%, with 37 PRs and 3 CRs.40 Median duration of response (DOR) and median OS were not reached. Median PFS was 7.1 months (95% CI 5.6–11.0). The toxicity profile was consistent with previous experiences. Grade 3–5 treatment-related adverse events (TRAEs) occurred in 73.6% of patients, including fatigue (23.6%), pruritis (21.8%), diarrhea (16.4%), rash (13.6%), and arthralgia (11.8%).
In cohort B of KEYNOTE-427, 165 patients with nccRCC were treated with pembrolizumab 200 mg i.v. every 3 weeks for up to 35 cycles.41 The primary endpoint was ORR. Within this cohort, 68% of patients had intermediate- to poor-risk disease per International Metastatic RCC Database Consortium (IMDC) criteria, and 62% had PD-L1-positive disease. ORR was 24.8% (95% CI 18.5–32.2); median DOR was not reached. By histology, ORR was 25.4% (95% CI 17.9–34.3) for papillary, 9.5% (95% CI 1.2–30.4) for chromophobe, and 34.6% (95% CI 17.2–55.7) for unclassified nccRCC. ORR was 28.3% (95% CI 16.8–42.3) for patients with favorable-risk disease vs 23.3% (95% CI 15.8–32.1) for patients with intermediate- to poor-risk disease. TRAEs occurred in 11% of patients and led to a discontinuation rate of 6%. As with cohort A, the full results of cohort B have not been published. Pembrolizumab monotherapy is not currently FDA-approved for the treatment of mRCC.18
Nivolumab, a programmed cell death protein 1 (PD-1) inhibitor, was FDA-approved in 2015 as second-line treatment of metastatic kidney cancer after treatment with a VEGF-targeting agent, based on the results of the CheckMate 025 study which showed improved OS in patients treated with nivolumab (25 months) compared to everolimus. Nivolumab monotherapy is not approved as first-line mRCC treatment.
The combination of nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) was studied as first-line therapy in a phase III trial (CheckMate 214).42 At the 18-month point, OS was 75% vs 60% for nivolumab/ipilimumab compared to sunitinib, respectively. The median OS was not reached in the nivolumab/ipilimumab group vs 26.0 months with sunitinib (HR=0.63; p<0.001). In an extended follow-up study, nivolumab/ipilimumab continued to be better than sunitinib in terms of OS (median OS not reached [95% CI 35.6–not estimable] vs 26.6 months [95% CI 22.1–33.4]; HR=0.66, 95% CI 0.54–0.80; p<0.0001) and the fraction of patients achieving an objective response (42% vs 29%; p=0.0001).43 Discontinuation due to TRAEs occurred in 22% of the nivolumab/ipilimumab group vs 12% of the sunitinib group. Of patients with TRAEs in the nivolumab/ipilimumab group, 35% received high-dose glucocorticoids (≥40 mg of prednisone per day or equivalent). This immunotherapy combination is not ideal for good-risk mRCC patients since the risk of serious toxicity outweighs potential treatment benefits. TRAEs in the nivolumab/ipilimumab group were observed in 93% of patients, including immune-related AEs such as pruritis, rash, and lipase elevation. In 2018 this pivotal trial led to FDA approval of the nivolumab/ipilimumab combination for intermediate- and poor-risk treatment naïve mRCC patients.18
Combination of VEGF-Targeted Therapy and Immune Checkpoint Inhibitors as First-Line Treatment for ccRCC
Most patients treated with VEGFR inhibitors will eventually develop resistance to therapy. In preclinical models, resistance to antiangiogenic therapy is mediated through a variety of mechanisms:44 1) production of redundant and alternative angiogenic factors by tumor cells; 2) induction of hypoxia, which in turn upregulates HIF-1 and leads to transcription of proangiogenic genes; 3) mobilization of bone marrow-derived proangiogenic cells, including endothelial and pericyte progenitors, tumor-associated macrophages, immature monocytic cells, and myeloid cells; and 4) the formation of collateral circulation. Additional preclinical evidence suggests that tumor vasculature is an important barrier to T cells.45 Angiogenesis inhibition can increase T-cell infiltration and enhance antitumor activity by decreasing the effect of macrophages and MDSCs. In a murine model of colon adenocarcinoma, treatment with anti-VEGFR-2 and anti-PD-1 monoclonal antibodies simultaneously inhibited tumor growth synergistically in vivo. Specifically, combining anti-PD-1 and anti-VEGFR-2 therapy increased the number of infiltrating CD8+ and CD4+ T cells.46 These findings suggest that the combination of anti-VEGF and anti-PD-1 therapies may have immune-modulating functions and clinical benefits greater than either modality alone.
The combination of anti-VEGF and anti-PD-1 therapy was tested in multiple trials with variable success. CheckMate 016 was an open-label phase I study that examined the efficacy and safety of nivolumab combined with sunitinib or pazopanib.47,48 The nivolumab plus sunitinib arm had high incidence of AEs (TRAEs in 100% of patients), making this combination too toxic for further development. Another phase I/II study tested the combination of pembrolizumab and pazopanib, but this combination was also not further developed due to dose-limiting hepatotoxicity.49 It was thought that observed toxicities were related to off-target effects of the TKIs and that a more selective VEGF inhibitor, such as axitinib, may be better tolerated. Axitinib was a clear choice since it is a second-generation multikinase receptor inhibitor of VEGFR-1, −2, and −350 and is already approved as second-line therapy for mRCC as monotherapy.51
Avelumab Plus Axitinib
The JAVELIN Renal 100 trial was an open-label, multicenter, phase Ib trial52 that tested the combination of axitinib 5 mg p.o. twice daily for a lead-in of 7 days, followed by combined avelumab 10 mg/kg i.v. every 2 weeks and axitinib 5 mg p.o. twice daily. At the data cutoff date, 100% of patients (6/6) in the dose-finding cohort and 53% of patients (26/49) in the dose-expansion cohort had confirmed objective responses (58%; 32/55 total patients). Promising results from this study led to a phase III study (JAVELIN Renal 101).53 Patients were randomized to avelumab 10 mg/kg i.v. every 2 weeks plus axitinib 5 mg p.o. twice daily or sunitinib 50 mg p.o. daily. Median PFS was 13.8 months vs 7.2 months favoring the avelumab/axitinib group (HR=0.61; 95% CI 0.47–0.79; p<0.001). It is important to note that although this study met its primary endpoint for PFS, OS was not statistically significant. The main secondary endpoint was PFS in the overall population, which was 13.8 months for the avelumab/axitinib group and 8.4 months for the sunitinib group (HR=0.69, 95% CI 0.56–0.84; p<0.001). Grade ≥3 AEs were observed in 71.2% of patients in the avelumab/axitinib group compared to 71.5% in the control group. The most common AEs were diarrhea (62.2% vs 47.6%, respectively) and hypertension (49.5% vs 36.0%, respectively). For patients in the avelumab/axitinib group, 38.2% experienced immune-related AEs and 9.0% had grade ≥3 AEs. High-dose glucocorticoids (≥40 mg of prednisone per day or equivalent) were given to 11.1% of patients who received avelumab/axitinib. Based on the results of this trial, the FDA approved this combination for first-line treatment of ccRCC in 2019.
Atezolizumab Plus Bevacizumab
In a multicenter, open-label, phase III trial (IMmotion 151), patients with ccRCC or sarcomatoid histology were randomized to atezolizumab 1200 mg and bevacizumab 15 mg/kg i.v. every 3 weeks or sunitinib 50 mg p.o. daily for 4 weeks on and 2 weeks off.54 The study met the co-primary endpoints of PFS in patients with PD-L1+ disease and OS in the intention-to-treat population. Median PFS was 11.2 months with atezolizumab/bevacizumab and 7.7 months with sunitinib (stratified HR=0.74, 95% CI 0.57–0.96; p=0.0217). In the intention-to-treat population, OS was not significantly different between the 2 groups. Atezolizumab/bevacizumab group had fewer grade 3–4 TRAEs than patients in the sunitinib group (40% vs 56%, respectively), and fewer patients discontinued therapy due to TRAEs (5% vs 8%, respectively).54 The most common grade 3 or 4 TRAE in the atezolizumab/bevacizumab group was hypertension (14%). In the sunitinib group, the most common side effects were hypertension (17%) and palmar-plantar erythrodysesthesia (9%). Interestingly, observed immune-related AEs, including hypothyroidism, rash, and liver function test abnormalities, were low-grade. Of patients receiving atezolizumab/bevacizumab, 9% received high-dose glucocorticoids.
Pembrolizumab Plus Axitinib
In an open-label phase 1b trial testing the combination of pembrolizumab 2 mg/kg i.v. every 3 weeks and axitinib 5 mg p.o. twice daily in patients with advanced RCC (predominantly clear cell type), 73% of patients achieved either a CR or PR with acceptable toxicity.55 This led to KEYNOTE-426, a pivotal open-label, phase III trial in which 861 patients with ccRCC were randomized to receive pembrolizumab/axitinib (pembrolizumab 200 mg i.v. every 3 weeks plus axitinib 5 mg p.o. twice daily) or sunitinib (50 mg p.o. daily, 4 weeks on and 2 off).56 Eligible patients had recurrent or newly diagnosed stage IV ccRCC, were 18 years and older, were treatment-naïve, had a KPS score of ≥70%, and had measurable disease and tumor tissue available for biomarker analysis. Exclusion criteria included history of autoimmune disease, CNS metastases, uncontrolled hypertension (≥150/90 mmHg), cardiovascular ischemic disease, New York Heart Association class III or IV congestive heart failure diagnosed within 1 year before screening, or current systemic immunosuppressive therapy. Patients were stratified by IMDC risk groups, including favorable, intermediate, or poor risk. The median duration of treatment was 10.4 months in the pembrolizumab/axitinib group and 7.8 months in the sunitinib group. Disease progression was the most common reason for discontinuation. Dual primary outcomes of OS and PFS favored the pembrolizumab/axitinib group. At 12 months, 89.9% of patients (95% CI 86.4–92.4) were alive in the pembrolizumab/axitinib group vs 78.3% of patients (95% CI 73.8–82.1) in the sunitinib group. The pembrolizumab/axitinib group had a 47% lower risk of death compared to the sunitinib group (HR=0.53; 95% CI 0.38–0.74; p<0.0001). At 18 months, 82.3% of patients (95% CI 77.2–86.3) were alive in the pembrolizumab/axitinib group vs 72.1% of patients (95% CI 66.3–77.0) in the sunitinib group. Median PFS was also significantly longer (15.1 months in the pembrolizumab/axitinib group vs 11.1 months in the sunitinib group; HR for disease progression or death=0.69; 95% CI 0.57–0.84; p<0.001). Interestingly, the improved OS and PFS were seen in all IMDC risk groups and in all PD-L1 expression categories. The secondary outcome of ORR also favored the pembrolizumab/axitinib group (59.3%) over the sunitinib group (35.7%; 95% CI 31.1–40.4; p<0.001). Median DOR was not reached in the pembrolizumab/axitinib group (1.4–18.2 months) and was 15.2 months (1.1–15.4) in the sunitinib group. These intriguing data demonstrated that the combination of pembrolizumab and axitinib could be used as first-line treatment for ccRCC.
In the KEYNOTE-426 trial, 98.4% of patients in the pembrolizumab/axitinib group experienced an AE vs 99.5% of patients in the sunitinib group.56 Grade ≥3 TRAEs were noted in 62.9% of patients in the pembrolizumab/axitinib group vs 58.1% of patients in the sunitinib group. In both groups, the most common AEs were diarrhea and hypertension. Grade ≥3 AEs that occurred in ≥10% of patients were hypertension in the sunitinib group and increased ALT and hypertension in the pembrolizumab/axitinib group. The discontinuation rate for pembrolizumab/axitinib was 10.7% vs 13.9% for sunitinib. AEs of interest were observed in 51.3% of patients given pembrolizumab/axitinib and in 36.2% of patients treated with sunitinib.
Taken together, results from these trials show that the combination of pembrolizumab and axitinib improves both PFS and OS compared to sunitinib. Also, this combination seems to work in patients with mRCC with sarcomatoid features who historically do not respond well to VEGF- and mTOR-targeted therapies. This study led to FDA approval in 2019 of pembrolizumab/axitinib as first-line treatment for ccRCC.
Future Directions and Conclusions
Immunotherapy has transformed the treatment paradigm for many cancers. The approval of first-line immunotherapy combinations in mRCC has dramatically changed the role of agents targeting the VEGF and mTOR pathways. Multiple research groups are actively seeking more effective treatments for mRCC, as evidenced by 321 clinical trials listed at ClinicalTrials.gov as of February 10, 2020,57 several of them testing combinations of anti-VEGF therapy and immune checkpoint inhibitors (Table 2). Three recently approved immunotherapeutic first-line combination treatments for mRCC have substantially improved treatment options for patients with RCC. Still, several issues remain to be addressed, including appropriate patient selection, side-effect monitoring, and biomarker development to determine who will benefit the most from immunotherapy treatment combinations.
Table 2.
Ongoing Phase III Clinical Trials Combining Checkpoint Inhibitors and Anti-VEGF Therapy
| Phase | Title | NCT Number | Status | Patients (n) | Primary Endpoint(s) |
|---|---|---|---|---|---|
| Phase III, multicenter, randomized, open-label | Study of Nivolumab Combined with Cabozantinib Compared to Sunitinib in Previously Untreated Advanced or Metastatic RCC (CheckMate 9ER) | NCT03141177 | Active, not recruiting | 701 | PFS per blinded independent review |
| Phase III, multicenter, randomized, double-blinded | Study of Cabozantinib with Nivolumab and Ipilimumab in Patients with Previously Untreated Advanced or Metastatic RCC (COSMIC-313) | NCT03937219 | Recruiting | 676 | PFS per blinded independent review |
| Phase III, multicenter, randomized, open-label | Immunotherapy with Nivolumab and Ipilimumab Followed by Nivolumab or Nivolumab with Cabozantinib for Patients with Advanced Kidney Cancer | NCT03793166 | Recruiting | 1046 | OS |
| Phase III, multicenter, randomized, open-label | Lenvatinib/Everolimus or Lenvatinib/Pembrolizumab versus Sunitinib Alone as Treatment of Advanced Renal Cell Carcinoma (CLEAR) | NCT02811861 | Active, not recruiting | 1069 | PFS per independent review |
| Phase III, randomized, open-label | Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination with Axitinib versus Sunitinib Monotherapy in Participants with RCC (MK-3475-426/KEYNOTE-426) | NCT02853331 | Active, not recruiting | 861 | PFS per blinded independent review and OS |
| Phase III, multicenter, randomized, open-label | A Study of Atezolizumab in Combination with Bevacizumab versus Sunitinib in Participants with Untreated Advanced RCC (IMmotion 151) | NCT02420821 | Active, not recruiting | 915 | Percentage of participants with disease progression or death from any cause; PFS in PD-L1 selected population; percentage of participants who died of any cause in ITT population; and OS in ITT population |
| Phase III, multicenter, randomized, open-label | Study of Avelumab with Axitinib versus Sunitinib in Advanced RCC (JAVELIN Renal 101) | NCT02684006 | Active, not recruiting | 886 | PFS and OS in PD-L1-positive patients |
Abbreviations: ITT, intention to treat; PD-L1, programmed death-ligand 1; PFS, progression-free survival; NCT, National Clinical Trial; OS, overall survival; RCC, renal cell carcinoma.
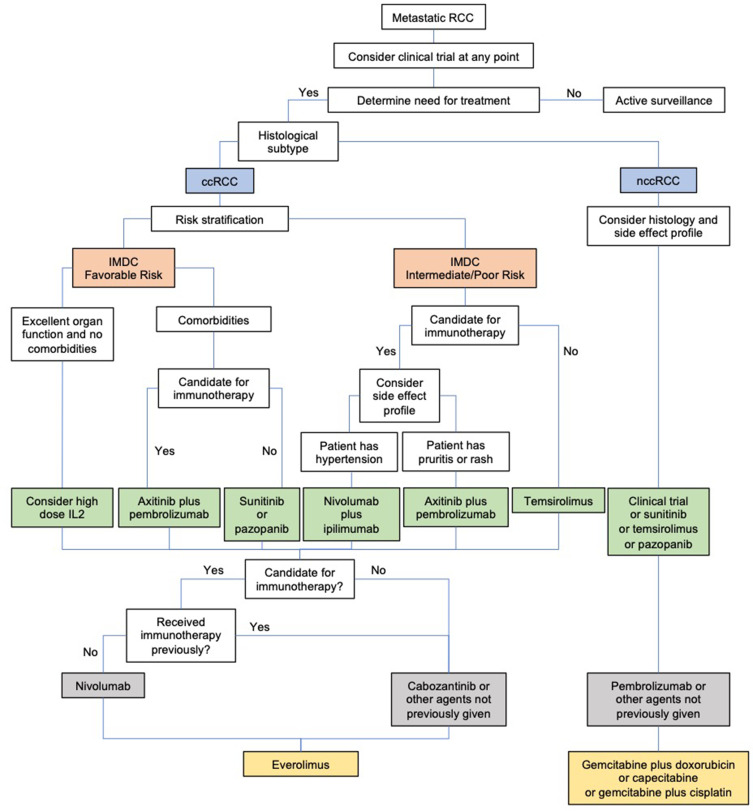
One of the most difficult challenges for a practicing clinician is choosing among approved therapies in the first-line setting (Figure 1). We believe that all mRCC patients with no contraindications to immunotherapy should receive an immunotherapy-based regimen as first-line treatment. Patients with excellent organ function and no comorbidities can be considered for high-dose IL-2, and responses may be assessed quickly. For mRCC patients with favorable-risk disease, axitinib plus pembrolizumab is the preferred treatment option because this combination improves OS and has an acceptable toxicity profile. For patients who are not candidates for immunotherapy, sunitinib or pazopanib are still considered treatments of choice.
Figure 1.
Proposed algorithm for determining treatment for mRCC.
For intermediate/poor-risk disease, the situation is more complicated since both nivolumab/ipilimumab and axitinib/pembrolizumab are good choices. Axitinib/pembrolizumab may be a superior option given its toxicity profile (lower discontinuation rate than for nivolumab/ipilimumab), but these two combination therapies have not been compared directly. Some axitinib and pembrolizumab toxicities may be overlapping, although caused by a different mechanism (eg, diarrhea or liver function test abnormalities). However, management is easier with axitinib as one can hold the drug for a few days to see if toxicity improves before starting steroids, while with the nivolumab/ipilimumab combination both drugs have to be discontinued.
For second-line therapy in patients with metastatic ccRCC, we again prefer immunotherapy if not previously received. Nivolumab improves OS compared to everolimus, but patients would need to be monitored for immune-related AEs. For those who have already received immunotherapy, cabozantinib or other TKIs can be considered, while recognizing that these agents may not necessarily invoke a significant response.
Additional trials are needed to address therapy sequencing and should include patients with nccRCC as it is essential that we help these patients find an appropriate clinical trial. Alternatively, first-line agents such as sunitinib, temsirolimus, or pazopanib can be considered. Second-line agents include pembrolizumab or other agents that have not been previously given. Other than pembrolizumab, immunotherapy remains relatively unexplored in nccRCC.
The rate at which treatments are being developed for RCC is impressive and accelerating.
Overall, combining specific anti-VEGF therapy with immunotherapy is an important strategy for improving survival in patients with mRCC.
Acknowledgments
The authors acknowledge Kathryn Cappell, MD, PhD for helpful suggestions during the preparation of this manuscript and Bonnie L. Casey for editorial assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105(24):1862–1870. doi: 10.1093/jnci/djt310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011;29(15):2027–2031. doi: 10.1200/jco.2010.30.9484 [DOI] [PubMed] [Google Scholar]
- 4.Zbar B, Glenn G, Merino M, et al. Familial renal carcinoma: clinical evaluation, clinical subtypes and risk of renal carcinoma development. J Urol. 2007;177(2):461–465. doi: 10.1016/j.juro.2006.09.037 [DOI] [PubMed] [Google Scholar]
- 5.Patel HD, Gupta M, Joice GA, et al. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur Urol Oncol. 2019;2(4):343–348. doi: 10.1016/j.euo.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 6.Mejean A, Thezenas S, Chevreau C, et al. Cytoreductive nephrectomy (CN) in metastatic renal cancer (mRCC): update on Carmena trial with focus on intermediate IMDC-risk population. J Clin Oncol. 2019;37(suppl 15):4508. doi: 10.1200/JCO.2019.37.15_suppl.4508 [DOI] [Google Scholar]
- 7.Mejean A, Ravaud A, Thezenas S, et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2018;379(5):417–427. doi: 10.1056/NEJMoa1803675 [DOI] [PubMed] [Google Scholar]
- 8.Bex A, Mulders P, Jewett M, et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: the SURTIME Randomized Clinical Trial. JAMA Oncol. 2019;5(2):164–170. doi: 10.1001/jamaoncol.2018.5543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, Phase 2 trial. Lancet Oncol. 2016;17(9):1317–1324. doi: 10.1016/s1470-2045(16)30196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito S, Kinoshita H, Kondo T, et al. Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: a multicenter study of 556 patients.. Urology. 2013;82(4):846–851. doi: 10.1016/j.urology.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 11.Merza H, Bilusic M. Current Management Strategy for Metastatic Renal Cell Carcinoma and Future Directions. Curr Oncol Rep. 2017;19(4):27. doi: 10.1007/s11912-017-0583-8 [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655 [DOI] [PubMed] [Google Scholar]
- 13.Mena AC, Pulido EG, Guill├⌐n-Ponce C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anticancer Drugs. 2010;21(Suppl 1):S3–S11. doi: 10.1097/01.cad.0000361534.44052.c5 [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/jco.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drugs@FDA: FDA-Approved Drugs. U.S. Food & Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=201275. 2006. Accessed 19February 2020..
- 17.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 18.NCCN Guidelines for Treatment of Cancer by Site. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. 2019. Accessed 20April 2020..
- 19.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/s0140-6736(07)61904-7 [DOI] [PubMed] [Google Scholar]
- 20.Escudier B, Bellmunt J, Grier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–2150. doi: 10.1200/jco.2009.26.7849 [DOI] [PubMed] [Google Scholar]
- 21.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/jco.2009.23.9764 [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Reeves J, et al. LBA8_PR - Randomized, Open-Label, Phase III Trial Of Pazopanib Versus Sunitinib In First-Line Treatment Of Patients With Metastatic Renal Cell Carcinoma (MRCC): results of the Comparz Trial. Ann Oncol. 2012;23(suppl9):ixe13. doi: 10.1016/S0923-7534(20)34325-8 [DOI] [Google Scholar]
- 23.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: the Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35(6):591–597. doi: 10.1200/jco.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer. 2018;94:115–125. doi: 10.1016/j.ejca.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choueiri TK, Hessel C, Halabi S, et al. Corrigendum to ‘Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update’ [Eur J Cancer 94 (May 2018) 115-125]. Eur J Cancer. 2018;103:287. doi: 10.1016/j.ejca.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi: 10.1200/jco.1995.13.3.688 [DOI] [PubMed] [Google Scholar]
- 27.Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Fyfe G. High-dose aldesleukin in renal cell carcinoma: long-term survival update. Cancer J Sci Am. 1997;3(Suppl 1):S70–S72. [PubMed] [Google Scholar]
- 28.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23(1):133–141. doi: 10.1200/jco.2005.03.206 [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228(3):307–319. doi: 10.1097/00000658-199809000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology. 2002;16(11Suppl 13):11–20. [PubMed] [Google Scholar]
- 31.Alva A, Daniels GA, Wong MK, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer Immunol Immunother. 2016;65(12):1533–1544. doi: 10.1007/s00262-016-1910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy KA, James BR, Guan Y, Torry DS, Wilber A, Griffith TS. Exploiting natural anti-tumor immunity for metastatic renal cell carcinoma. Hum Vaccin Immunother. 2015;11(7):1612–1620. doi: 10.1080/21645515.2015.1035849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finke JH, Rayman PA, Ko JS, Bradley JM, Gendler SJ, Cohen PA. Modification of the tumor microenvironment as a novel target of renal cell carcinoma therapeutics. Cancer J. 2013;19(4):353–364. doi: 10.1097/PPO.0b013e31829da0ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida N, Ikemoto S, Narita K, et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002;86(9):1396–1400. doi: 10.1038/sj.bjc.6600257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3K╬│ in myeloid cells. Nature. 2016;539(7629):443–447. doi: 10.1038/nature20554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodaczewska KK, Szczylik C, Kieda C. Immune consequences of anti-angiogenic therapy in renal cell carcinoma. Contemp Oncol. 2018;22(1a):14–22. doi: 10.5114/wo.2018.73878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoja L, Butler MO, Kang SP, Ebbinghaus S, Joshua AM. Pembrolizumab. J Immunother Cancer. 2015;3:36. doi: 10.1186/s40425-015-0078-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcus L, Lemery SJ, Keegan P, Pazdur R, Approval Summary: FDA. Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi: 10.1158/1078-0432.Ccr-18-4070 [DOI] [PubMed] [Google Scholar]
- 39.McDermott D, Lee J, Szczylik C, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort A of KEYNOTE-427. J Clin Oncol. 2018;36(suppl 15):4500. doi: 10.1200/JCO.2018.36.15_suppl.4500 [DOI] [Google Scholar]
- 40.Tykodi SS, Donskov F, Lee J, et al. First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): updated results for KEYNOTE-427 cohort A. J Clin Oncol. 2019;37(suppl15):4570. doi: 10.1200/JCO.2019.37.15_suppl.4570 [DOI] [Google Scholar]
- 41.McDermott D, Lee J, Ziobro M, et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): results from KEYNOTE-427 cohort B. J Clin Oncol. 2019;37(suppl7):546. doi: 10.1200/JCO.2019.37.7_suppl.546 [DOI] [Google Scholar]
- 42.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, Phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. doi: 10.1016/s1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grepin R, Pages G. Molecular mechanisms of resistance to tumour anti-angiogenic strategies. J Oncol. 2010;2010:835680. doi: 10.1155/2010/835680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. 2015;33:55–63. doi: 10.1016/j.coi.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500–506. doi: 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amin A, Plimack ER, Ernstoff MS, et al. Correction to: safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer. 2019;7(1):73. doi: 10.1186/s40425-019-0559-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer. 2018;6(1):109. doi: 10.1186/s40425-018-0420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury S, McDermott D, Voss MH, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2017;35(suppl 15):4506. doi: 10.1200/JCO.2017.35.15_suppl.4506 [DOI] [Google Scholar]
- 50.Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D. 2011;11(2):113–126. doi: 10.2165/11591240-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403 [DOI] [PubMed] [Google Scholar]
- 52.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19(4):451–460. doi: 10.1016/s1470-2045(18)30107-4 [DOI] [PubMed] [Google Scholar]
- 53.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/s0140-6736(19)30723-8 [DOI] [PubMed] [Google Scholar]
- 55.Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19(3):405–415. doi: 10.1016/s1470-2045(18)30081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 57.Study of TAK-228 In Patients With Previously Treated Metastatic Renal Cell Carcinoma. https://clinicaltrials.gov/ct2/show/NCT03097328?recrs=a&cond=renal+cell+carcinoma&draw=2&rank=1.2020. Accessed 10February 2020..