SUMMARY
Recent work shows that thalamocortical (TC) inputs are plastic after the developmental critical period has closed, but the mechanism of such plasticity is unknown. Here we show in post-critical period rodent barrel cortex, long term potentiation (LTP) at thalamocortical (TC) inputs is restored in spared cortex following either unilateral infra-orbital nerve (ION) lesion, unilateral whisker trimming or unilateral ablation of barrel cortex. Furthermore, unilateral ION lesion also reactivates anatomical map plasticity induced by whisker follicle ablation. The reactivation of TC LTP is accompanied by the transient reappearance of silent synapses. Both LTP and silent synapse formation is preceded by a transient re-expression of synaptic GluN2B-containing NMDA receptors, the activation of which in vivo is required for the reappearance of TC plasticity. Thus peripheral sensory deprivation reactivates synaptic plasticity in layer 4 barrel cortex with the same features as developmental critical period plasticity and is triggered by re-expression of GluN2B.
INTRODUCTION
Thalamocortical (TC) inputs to primary sensory cortex exhibit robust synaptic plasticity during early postnatal development that correlates with the critical period for experience-dependent plasticity. After the end of the critical period, plasticity is greatly reduced such that sensory maps in the thalamorecipient layer 4 primary sensory cortex become resistant to experience-driven plasticity (Barth and Malenka, 2001; Crair and Malenka, 1995; Hubel and Wiesel, 1970). However, after the end of the critical period, plasticity of sensory maps still occurs in superficial cortex driven largely by intracortical synaptic plasticity (Diamond et al., 1993; Diamond et al., 1994; Fox, 1992, 2002; Hickmott and Merzenich, 2002; Kaas and Catania, 2002; Nudo et al., 1990; Qi et al., 2014; Snow et al., 1988; Wallace and Fox, 1999).
Recent work has challenged the view that plasticity does not occur at TC inputs to layer 4 in the adult whisker barrel cortex. For example, studies on rodent somatosensory barrel cortex show that the number of TC synapses are reduced by whisker trimming during adulthood (Oberlaender et al., 2012; Wimmer et al., 2010). Unilateral lesion of the infra-orbital nerve (ION), which contains sensory afferents from the whisker pad, induces robust plasticity of TC inputs in spared layer 4 barrel cortex a month after the end of the critical period (Yu et al., 2012). These results and others from visual cortex and auditory cortex (Alvarez et al., 2007; Dringenberg et al., 2007; Gagolewicz and Dringenberg, 2011; Heynen and Bear, 2001; Kuo and Dringenberg, 2008; Mainardi et al., 2010; Montey and Quinlan, 2011; Petrus et al., 2014) indicate that TC inputs in layer 4 can express plasticity after the end of the critical period. In none of these cases has the detailed synaptic mechanisms underlying this plasticity been described.
A useful paradigm for studying the mechanisms for post-critical period reactivation of TC synaptic plasticity has recently been described (Yu et al., 2012). Unilateral lesion of the ION in 4–6 week-old rats produced an increase in activation of spared barrel cortex evoked by electrical stimulation in vivo assayed using either BOLD fMRI or in vivo electrophysiology. This increased activation was associated with increased synaptic strength of TC synaptic input to glutamatergic stellate cells in layer 4 of barrel cortex as measured in vitro in slice. During development, LTP at TC inputs produces synaptic strengthening in a mechanism requiring NMDA receptor activation and the activation of silent synapses (Crair and Malenka, 1995; Isaac et al., 1997; Kirkwood and Bear, 1995) of the critical period, TC inputs lose their ability to express LTP; this loss of plasticity correlates with the disappearance of silent synapses and a switch from GluN2B- to GluN2A-containing NMDA receptors at TC synapses (Barth and Malenka, 2001; Crair and Malenka, 1995; Daw et al., 2007a; Isaac et al., 1997; Kirkwood and Bear, 1995; Lu et al., 2001).
Here the mechanisms for the strengthening of the TC input in spared layer 4 barrel cortex following unilateral ION lesion in post-critical period rats was studied. 11 days following ION lesion GluN2B is re-expressed at TC synapses. 13 days after ION lesion LTP reappears and this is associated with formation of silent synapses, both of which coincide with strengthening of the TC input. TC input strength remains increased for up to at least 18 days after ION lesion, but the ability to induce LTP and the existence of silent synapses is lost, indicating a transient window of reactivation of plasticity mechanisms. In addition, we found that unilateral ION lesion restores structural map plasticity in spared barrel cortex induced by lesioning of the whisker follicles. Furthermore, the transient restoration of TC synaptic plasticity is also produced by unilateral peripheral sensory deprivation or ablation of S1 barrel cortex (in the absence of ION lesion). The earliest functional change detected was re-expression of GluN2B suggesting that GluN2B is required for reactivation of the plasticity. Consistent with this idea, chronic in vivo blockade of GluN2B, by infusing ifenprodil into layer 4 barrel cortex, prevented the ION lesion-induced TC plasticity. Therefore, peripheral nerve injury leads to a TC synaptic plasticity program being reactivated in the post-critical period somatosensory cortex that is similar to that observed during the critical period.
RESULTS
Transient reappearance of LTP at TC inputs in layer 4 of spared barrel cortex following unilateral ION lesion
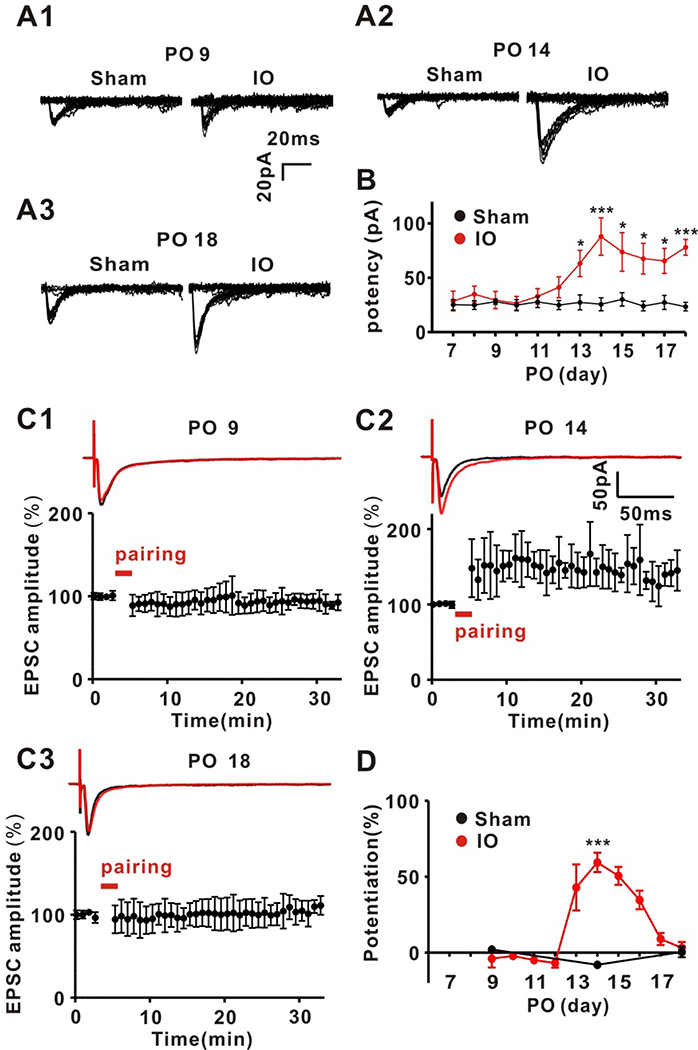
Previous work showed that unilateral ION lesion in 4 week-old rats leads to a potentiation of TC inputs in layer 4 in the spared barrel cortex two weeks after the lesion (Yu et al., 2012). During normal development, TC inputs in layer 4 barrel cortex exhibit a well-defined critical period in which they are plastic only during the first postnatal week (Barth and Malenka, 2001; Crair and Malenka, 1995). Therefore, the hypothesis that following unilateral ION lesion developmental TC plasticity may be reactivated in spared cortex was tested. To investigate this idea, the timing of the increase in TC synaptic strength relative to onset of ION lesion was defined. Using TC brain slices prepared from rats at various days following ION lesion the potency (the amplitude of EPSCs excluding failures) of TC EPSCs in stellate cells (SCs) using whole-cell patch-clamp recordings and single TC axon stimulation was measured as previously described (Yu et al., 2012). The potency of the TC input begins to increase 12 days post-ION lesion (PO) and this increase in synaptic strength was maintained until at least PO18 (Figure 1A, B). The potency in slices from rats that underwent sham surgery was unchanged throughout this time period.
Figure 1.
Unilateral infra-orbital nerve (ION) lesion restores LTP capability to adult thalamocortical inputs transiently
(A1–3) Representative TC EPSC traces evoked by minimal stimulation at PO 9, 14 and 18 in Sham and ION-lesion (IO) groups. (B) Time courses for potencies of single fiber-activated TC EPSCs in both groups (IO group: n = 6, 6, 5, 6, 7, 6, 10, 8, 7, 7, 9, 8 at each PO from 7 to 18, respectively; Sham group: n = 9, 9, 7, 7, 7, 8, 7, 7, 7, 7, 7, 7 at each PO from 7 to 18, respectively) (Two-way ANOVA with post hoc Bonferroni test: Sham vs IO; *P <0.05, **P <0.01, ***P <0.001). (C1–3) LTP induction at TC inputs induced by pairing in slices from PO 9, 14 and 18 following ION lesion. Upper trace, representative traces showing of EPSCs before (average of 20 traces, black line) and after (average of 180 sweeps, red line). Lower trace, averaged time-courses for EPSC amplitude during LTP induction at PO 9, 14 and 18 following ION in IO group. (D) Summarized time-course of TC LTP in ION lesion or sham (IO group: n = 6, 6, 6, 6, 8, 6, 6, 6, 6, 6 at each POs from 9 to 18 respectively; sham group: n = 6, 6, 7 at PO 9, 14, 18 respectively). The amount of LTP induced in ION at PO14 was compared to the equivalent PO14 time point for sham (two-way ANOVA with post hoc Bonferroni test: Sham vs IO; ***P <0.001).
LTP is well established as an important mechanism for experience-dependent plasticity in layer 4 primary sensory cortex during the critical period, including in whisker barrel cortex (Crair and Malenka, 1995; Daw et al., 2007a; Dudek and Friedlander, 1996; Feldman et al., 1998; Kirkwood and Bear, 1995). In developing layer 4 barrel cortex, LTP at TC inputs exhibits a critical period, disappearing by postnatal day 7 (Crair and Malenka, 1995). Thus, it was determined whether LTP could be induced at TC inputs following unilateral ION lesion as a candidate mechanism for the strengthening of TC inputs. LTP could be induced in stellate cells (SCs) using a pairing protocol in slices prepared from rats following ION lesion at 13 to 17 days post lesion (PO), but not in slices from sham-operated rats (Figure 1C, D). Notably, this reappearance of LTP was transient, lasting for about 5 days and disappearing by PO 18.
Re-expression of GluN2B at TC synapses following unilateral ION lesion
Similar to other cortical areas, GluN2B-containing NMDA receptors are prominent at TC synapses early postnatally in barrel cortex, are required for LTP, but are lost during development (Crair and Malenka, 1995; Harlow et al., 2010; Lu et al., 2001; Philpot et al., 2001; Quinlan et al., 1999a). To determine whether TC inputs exhibit changes in NMDA receptor subtype following ION lesion, the relative contribution of NMDA receptors to TC EPSCs was determined by measuring the amplitude of the AMPA receptor-mediated EPSC (AMPA EPSC) measured at a holding potential of −70 mV and that of the pharmacologically-isolated NMDA receptor-mediated EPSC (NMDA EPSC) at +40 mV in the same cells. This NMDA:AMPA ratio increased transiently at PO13, returning to baseline levels by PO18, but was unaffected in slices from sham-operated rats (Figure 2A, B). To determine the contribution of GluN2B-containing NMDA receptors, the sensitivity of the NMDA EPSC to 5 μM ifenprodil was measured. The NMDA EPSC showed a transient increase in ifenprodil sensitivity from PO10 – PO15 that was not detected in controls. (Figure 2C, D). Associated with the electrophysiological evidence for increases in GluN2B, increases in GluN2B protein were also detected transiently in layer 4 barrel cortex synaptosomes during this same time period (Figure S1). Thus, unilateral ION lesion elicits an increase in GluN2B expression and an increase in GluN2B-containing NMDA receptor function at TC synapses in spared barrel cortex.
Figure 2.
Re-expression of GluN2B at TC synapses following unilateral infra-orbital nerve lesion
(A1–3) Representative traces showing NMDA and AMPA TC EPSCs at PO 9, 15, 21 respectively following ION lesion. (B) Time-course for NMDA:AMPA ratios in both groups (IO group: n = 8, 10,12, 6, 15, 10, 12, 6, 12, 6, 12 at each POs from 9 to 18 and 21; sham group: n = 6, 6, 6 at PO 9, 14, 21) (Two-way ANOVA with post hoc Bonferroni test: Sham vs IO; ***P <0.001). (C1–3) Representative traces of NMDA EPSCs recorded in slices from animals at PO 9, 12 and 18 before (black lines) and after application (red lines) of 5 μM ifenprodil. (D) Time-course for ifenprodil sensitivity of TC NMDA EPSCs following ION lesion or for sham (IO group: n = 6, 6, 6, 6, 6, 8, 6, 6, 6, 6, 6, 6 at each PO from 7 to 18 respectively; Sham group: n = 6, 6 and 6 at PO 7, 12, 18 respectively) (Two-way ANOVA with post hoc Bonferroni test: Sham vs IO; ***P <0.001).
Reappearance of silent synapses following unilateral ION lesion
The enhancement in the NMDA:AMPA ratio of the TC EPSC following unilateral ION lesion may reflect the re-emergence of silent synapses that contain NMDA receptors but no AMPA receptors (Isaac et al., 1995; Kullmann, 1994; Liao et al., 1995). Notably, silent synapses are evident at TC inputs onto SCs in layer 4 barrel cortex early in postnatal development, are converted to AMPA receptor-containing synapses during developmental LTP, and are developmentally down-regulated and lost by the end of the first postnatal week (Ashby and Isaac, 2011; Isaac et al., 1997). To test whether silent synapses re-emerge following unilateral ION lesion, the proportion of silent synapses was measured using minimal stimulation comparing failure rates at holding potentials of −70 mV and +40 mV (Liao et al., 1995). There was no evidence for silent synapses at TC inputs in slices from sham operated controls, but silent synapses could be readily detected by PO13 following ION lesion and persisted until PO17 (Figure 3).
Figure 3.
Reappearance of silent synapses following unilateral infra-orbital nerve lesion
(A1, B1, C1) Representative traces for EPSCs evoked by minimal stimulation for 50 trials at holding potentials of −70 mV or +40- mV in slices from animals at PO 9, 14 and 21 for IO and sham groups. (A2, B2, C3) Time course of EPSC amplitudes for examples cells shown in A1, B1 and C1 collected at −70 mV (blue symbols) and +40 mV (red symbols). (A3, B3, C3) Failure rates for EPSCs at −70 mV or +40- mV in slices from animals PO 9, 14 and 21 for IO and sham groups. (D) Time course for percentage silent synapse proportions for IO and sham groups (IO group: n = 8, 10, 12, 6, 15, 10, 12, 6, 12, 6, 12 at each PO 9, 11, 13, 14, 15, 17, 21; Sham group: n = 6, 6, 6 at PO 9, 14, 21) (Two-way ANOVA with post hoc Bonferroni test: Sham vs IO; ***P <0.001).
One explanation for the re-appearance of GluN2B-containing NMDA receptors and silent synapses is that GluN2B-containing NMDA receptor re-expression occurs preferentially at silent synapses following ION lesion. This was tested by comparing the failure rates at −70 mV and at +40 mV in slices from ION lesioned animals (at PO14) in the presence or absence of ifenprodil (5 μM). Ifenprodil did not affect the number of silent synapses detected, indicating that GluN2B-containing NMDA receptors are not selectively expressed at silent synapses (Figure 4). We also confirmed this finding by measuring silent synapses directly using a minimal stimulation intensity at which no AMPA EPSCs were detected (at −70 mV) and then depolarising the neuron to +40 mV to detect NMDA-only EPSCs (Isaac et al., 1997; Isaac et al., 1995) (Figure S2A, B). NMDA-only EPSCs were detected in all TC inputs recorded at PO 14 following ION lesion (n = 12); however, NMDA-only TC EPSCs were never detected in slices sham-operated controls (n = 7). To directly test whether there is any contribution from GluN2B-containing NMDA receptors to the NMDA EPSC at silent synapses, ifenprodil was applied to the isolated silent synapses. Ifenprodil caused a small but significant reduction in the amplitude of the NMDA EPSC at isolated silent synapses but no change in failure rate, indicating that the majority of the NMDA EPSC is mediated by GluN2A-containing NMDARs (Figure S2C, D). Taken together these findings show there is a transient re-expression of silent synapses at TC inputs following unilateral ION lesion, however, these synapses do not preferentially contain GluN2B-containing NMDA receptors. This suggests that the majority of the NMDA current at silent synapses is mediated by GluN2A-containing NMDA receptors.
Figure 4.
NMDA EPSCs at silent synapses are not primarily mediated by GluN2B-containing NMDA receptors.
(A1) Representative traces for EPSCs evoked by minimal stimulation at holding potentials of −70 mV or +40- mV in slices from animals at PO 14 following ION lesion recorded in the absence (left traces) or presence of 5 μM ifenprodil (right traces; these example experiments are from different cells). (A2) Time course of EPSC amplitudes for examples cells shown in A1. (A3) Failure rates for the responses at −70 mV and +40 mV at PO 14 in the absence or presence of 5 μM ifenprodil. (B) Summary of ifenprodil effect on percent silent synapses (n = 10).
GluN2B-containing NMDA receptor activation is not required for LTP induction but is required in vivo for reactivation of TC plasticity
In neocortex, including layer 4 barrel cortex, a loss of synaptic GluN2B-containing NMDA receptors correlates with the end of the critical period and a loss of the ability of TC synapses to express LTP. However, the precise role of GluN2B in this developmental down regulation of plasticity is unclear (Carmignoto and Vicini, 1992; Crair and Malenka, 1995; Harlow et al., 2010; Lu et al., 2001; Philpot et al., 2001; Quinlan et al., 1999a; Quinlan et al., 1999b). To determine the role of the re-expressed GluN2B in the plasticity following unilateral ION lesion the effect of ifenprodil on LTP induction was measured. In slices prepared from animals at PO14, ifenprodil (5 μM) had no effect on LTP induction; however, LTP was prevented by the broad-spectrum NMDA receptor antagonist, D-AP5 (10 μM; Figure 5A, B). Thus, NMDA receptor activation is necessary for LTP induction but GluN2B is not required. Considering that the earliest changes detected were an increase in GluN2B, the role of GluN2B-containing NMDA receptors during the two weeks after ION was investigated. Ifenprodil or saline as control was infused continuously into layer 4 of the barrel cortex for two weeks after ION using osmotic minipumps implanted the day of the ION lesion surgery. In vivo infusion of ifenprodil prevented the re-emergence of both TC LTP and silent synapses as assessed in subsequent brain slice experiments (Figure 6). In the saline infused animals, the re-expression of LTP and silent synapses was the same as animals that had had no infusion. Thus, even though the re-activated TC LTP following unilateral ION lesion is NMDA receptor-dependent, it does not require acute activation of GluN2B-containing NMDA receptors. Rather GluN2B re-expression is required to reactivate plasticity at TC synapses, possibly by triggering the re-expression of silent synapses.
Figure 5.
TC LTP following ION lesion is NMDA receptor-dependent, but GluN2B-independent.
(A1) Upper traces, representative traces for the effect of 10 μM D-AP5 on LTP induction at PO 14 following ION lesion. Lower traces, time course of EPSC amplitude for same experiment. (A2) Averaged time-course of EPSC amplitude for LTP experiments in the presence of AP-5 at PO 14 following ION lesion. (A3) Summary of AP5 effects on LTP induction at PO 14 following ION lesion (ION group: n = 6; ION + AP5 group: n = 5; unpaired T-test; ***P <0.001). (B1) Upper traces, representative traces for the effect of 5 μM ifenprodil on LTP induction at PO 14 following ION lesion. Lower traces time-course for EPSC amplitude for same experiment. (B2) Averaged time-course for EPSC amplitude for LTP experiments in the presence of ifenprodil at PO 14 following ION lesion (B3) Summary of ifenprodil effects on LTP induction at PO 14 in following ION lesion (ION group: n = 6; ION + ifenprodil group: n = 7).
Figure 6.
Activation of GluN2B-containing NMDA receptors is required in vivo for reappearance of silent synapses and TC LTP
(A) Left, bright field image of a TC slice showing the injection site (red box) in a methylene blue-injected rat. Right, bright field image of a TC slice showing the recording electrode and injection site (red arrow head). (B1) Upper traces, representative traces for the effect of in vivo saline injection on LTP induction at PO 14 following ION lesion. Lower panel, time-course of EPSC amplitude for the same experiment (B2) Averaged time-course of EPSC amplitude for LTP experiments in slices from PO 14 ION lesion rats in the saline-injected group (n = 6). (B3) Upper traces, Representative traces for the effect of in vivo ifenprodil injection on LTP induction at PO 14 following ION lesion. Lower panel, time-course of EPSC amplitude for the same experiment (B4) Averaged time-course of EPSC amplitude for LTP experiments in slices from PO 14 ION lesion rats in the ifenprodil-injected group (n = 6). (C) Summary of the effect of saline or ifenprodil injection on LTP induction at PO 14 in sham and ION lesion groups (sham+saline: n = 7; IO+saline: n = 6; IO+ifenprodil: n = 6; one-way ANOVA with post hoc Tukey test; *P <0.05; ***P <0.001). (D1) Representative traces for EPSCs at −70 mV and +40 mV evoked by minimal stimulation at PO 14 in ION lesion rats in the saline-injected group. (D2) Time course of EPSC amplitude for experiment shown in D1. (D3) Failure rates for the responses at −70 mV and +40 mV at PO 14 in ION lesion rats in the saline-injected group. (D4) Representative traces for EPSCs at −70 mV and +40 mV evoked by minimal stimulation at PO 14 in ION lesion rats in the ifenprodil-injected group. (D5) Time course of EPSC amplitude for experiment shown in 4. (D6) Failure rates for the responses at −70 mV and +40 mV at PO 14 in ION lesion rats in the ifenprodil-injected group. (E) Summary data for the effect of saline or ifenprodil injection on % silent synapses at PO 14 following ION lesion (saline: n = 5; ifenprodil: n = 5; unpaired T test; ***P <0.001).
Role of whisker-evoked activity in the reactivation of plasticity at TC inputs
To determine whether other manipulations besides unilateral ION lesion can reactivate plasticity in spared barrel cortex, the effect of unilateral whisker trimming (UWT) every other day for 13 days, starting at 4 weeks of age, on TC synaptic plasticity in spared barrel cortex was investigated. Similar to unilateral ION lesion, the potency of minimal stimulation-evoked AMPA EPSCs at TC inputs in layer 4 SCs was increased in spared barrel cortex when measured at PO14 (the day after the last UWT; Figure 7A, C). Furthermore, the ability to induce LTP was also restored (Figure 7C1, D)
Figure 7.
Unilateral whisker trimming or ablation of barrel field cortex restores long-term plasticity to TC input in spared barrel cortex similar to unilateral ION lesion.
(A) (From top to bottom) Representative TC EPSC traces evoked by minimal stimulation at PO 14 in Sham, ION lesion (IO), unilateral whisker-trimmed (UWT), ION lesion with spared whisker-trimmed (SWT+IO) and unilateral BFC-lesioned (UBL) groups. The traces for Sham and IO groups are the same as Figure 1A2. Unilateral whisker trimming and BFC lesion were performed at the age of 4 weeks, similar to IO and Sham operation. Spared whiskers were trimmed daily from PO 12 to PO 14 in spared whisker-trimmed ION-lesioned rats. (B) Averaged summary for potencies of single fiber-activated TC EPSCs in each groups (Sham group: 25.6 + 6.0 pA, n = 7; IO group: 88.6 + 17.2 pA, n = 7; UWT group: 72.4 + 10.4 pA, n = 7; SWT+IO group: 24.0 + 5.1 pA, n = 7; UBL group: 76.6 + 12.0 pA, n = 8) (One-way ANOVA with post hoc Tukey test, *P <0.05, **P <0.01). (C1–2) LTP induction at TC inputs induced by pairing in slices from PO 14 following unilateral whisker trimming (C1) or BFC lesion (C2). Upper traces, representative traces showing of EPSCs before (average of 20 traces, black line) and after (average of 180 sweeps, red line). Lower trace, averaged time-courses for EPSC amplitude during LTP induction at PO 14 following unilateral whisker trimming (C1) or BFC lesion (C2). (D) Averaged summary for TC LTP at PO 14 in Sham, IO, UWT and UBL groups. (Sham group: −8.0 + 2.2 %, n = 6; IO group: 59.4 + 6.4 %, n = 6; UWT group: 54.2 + 7.7 %, n = 7; UBL group: 43.9 + 4.6 pA, n = 6) (One-way ANOVA with post hoc Tukey test, *P <0.05, **P <0.01).
To further understand the contribution of whisker-evoked sensory activity in gating the reactivation of TC synaptic plasticity, we investigated whether whisker-evoked sensory input is required for the reactivation of plasticity following unilateral ION. We found that the increase in AMPA EPSC potency observed at PO14 was prevented by daily trimming the whiskers on the contralateral (spared) whisker pad starting at PO 12 (Figure 7A, B), indicating that sensory experience is necessary for the ION lesion-induced reactivation of TC plasticity.
We also tested whether a unilateral reduction of barrel cortex activity alone induces TC plasticity in the spared barrel cortex by unilaterally ablating the BFC (Barrel field cortex) in 4 week old rats (Figure S3) and measuring TC synaptic strength and LTP in layer 4 of spared barrel cortex 2 weeks later. Unilateral ablation lesion of BFC, led to synaptic potentiation of TC inputs in spared barrel cortex and a restoration of LTP, to a similar extent as observed for unilateral ION lesion (Figure 7). Thus, three different manipulations that produce a unilateral decrease in activity of barrel cortex (unilateral ION, UWT, unilateral BFC ablation) produce reactivation of plasticity of TC inputs in spared barrel cortex.
Unilateral ION reactivates anatomical map plasticity in L4 barrel cortex
Peripheral sensory manipulations can produce an anatomical re-organization of barrel cortex as well as functional plasticity during the critical period (Belford and Killackey, 1980; Crair and Malenka, 1995; Durham and Woolsey, 1984; Schlaggar et al., 1993; Van der Loos and Woolsey, 1973). To determine whether unilateral ION lesion in 4 week-old rats also reactivates anatomical barrel map plasticity, we first measured whether unilateral ION lesion changed the total size of the barrels in layer IV in the posterior medial barrel subfield (PMBSF) measured using cytochrome oxidase (CO)-staining. At PO 18 following unilateral ION lesion the total area occupied by the barrels (‘barrel field area’ [BFA] measured for rows B - D, arcs 1 – 4) was increased compared to sham and the individual barrels, C1 and C2 were significantly increased (Figure S4A–C). To determine whether the anatomical map plasticity in spared barrel cortex is reactivated by unilateral ION lesion, follicles of the C row whiskers of spared barrel cortex were electrocauterized at PO 11 following unilateral ION lesion or the sham operation. Using a map plasticity index (MPI; see Experimental Procedures) to quantify any plasticity, we found that this electrocauterization of the C-row whiskers caused a significantly reduction in the C row BFA compared to the adjacent B and D rows in the ION lesioned but not the sham control animals (Figure S4D, E). Thus unilateral ION lesion both induces a change in the size of the anatomical map and also restores anatomical map plasticity in 4–6 week-old rats.
DICUSSION
Here we have shown that unilateral ION lesion in four week-old rats causes a re-emergence of silent synapses and reactivates synaptic plasticity of the TC input to layer 4 in the spared barrel cortex. This occurs at an age that is three weeks after the end of the developmental critical period for TC synaptic plasticity. TC plasticity required transient re-expression of GluN2B-containing NMDA receptors starting at ten days post-lesion. This was followed by the transient re-appearance of silent synapses and LTP two days later, resulting in a stable increase in strength of the TC input in layer 4. Notably, activation of GluN2B-containing NMDA receptors was required in vivo for the plasticity mechanism to occur; however, the GluN2B-containing NMDA receptor subtype is not necessary for acute LTP induction. These findings are summarized in Figure S5 which illustrates a working model for the sequence of synaptic changes that occur at TC inputs following unilateral ION.
Although previous work has suggested that LTP can be induced at adult TC synapses in primary sensory cortex under certain conditions in vivo (Cooke and Bear, 2010; Hogsden and Dringenberg, 2009; Lee and Ebner, 1992), a cellular mechanism for such adult TC plasticity had not been described. The present work demonstrates that unilateral ION lesion re-activates a plasticity mechanism that appears identical to that observed early in development during the critical period. Moreover, the re-appearance of the various features of plasticity - GluN2B expression, silent synapses and LTP - is transient with a similar duration (five to six days) as observed during development in layer 4 barrel cortex (Daw et al., 2007b).
Previous studies of plasticity during development in barrel cortex have noted co-incident loss of GluN2B-containing NMDA receptor expression at TC synapses and the end of the critical period for LTP. Moreover, induction of developmental TC LTP is prevented by the GluN2B-selective antagonist, ifenprodil (Barth and Malenka, 2001; Lu et al., 2001). Nevertheless, it has also been shown using GluN2A knockout mice, in which GluN2B expression persists at TC synapses beyond the end of the critical period, that the loss of GluN2B-containing NMDA receptors is not required for the loss of LTP (Lu et al., 2001). Thus, the mechanistic role of GluN2B in regulating the timing of the critical period for TC LTP is unclear. In the present study GluN2B re-expression at TC synapses in barrel cortex was a requirement for the re-activation of TC plasticity, arguing for a role of GluN2B in re-opening TC plasticity. The data indicate that the role of GluN2B is not for the acute induction of LTP, but suggests rather that GluN2B is required to induce new silent synapses at TC inputs onto L4 SCs. We hypothesize that these silent synapses provide the requisite substrate for LTP. A role for GluN2B in inducing silent synapses is supported by genetic gain and loss of function studies showing that GluN2B expression can drive synaptogenesis and the formation of silent synapses in hippocampal neurons (Gambrill and Barria, 2011; Gray et al., 2011; Hall et al., 2007). Furthermore, GluN2B has been shown to preferentially recruit an intracellular signalling complex via its C-terminus that drives new synapse formation and synaptic plasticity (Foster et al., 2010; Kim et al., 2005; Ryan et al., 2013; Wang et al., 2011). The fact that LTP opens and then closes again in this post-critical period model of plasticity should enable future studies on the detailed cellular and molecular mechanisms causing these transitions.
It remains unclear what the neural mechanisms are by which unilateral ION lesion produces the activation of plasticity in spared barrel cortex. Unilateral ION lesion produces a loss of sensory-evoked activity in deprived barrel cortex that may lead to a reduction of cross hemispheric inhibition onto spared barrel cortex, allowing increased activation of spared barrel cortex by ascending sensory input (Adam and Gunturkun, 2009; Levy and Trevarthen, 1976; Levy et al., 1972; Urgesi et al., 2005). This is consistent with hypotheses from previous work on unilateral denervation of sensory input (Pelled et al., 2007) and also consistent with visual cortex studies showing that loss of sensory input can lead to reactivation of experience-dependent plasticity in adult animals (Eaton et al., 2016; Montey et al., 2013; Montey and Quinlan, 2011). In the present study, we show that either unilateral ablation of the barrel field cortex or unilateral whisker trimming produce reactivation of plasticity similar to unilateral ION lesion. Moreover, trimming the whiskers on the spared side also prevented the unilateral ION lesion-induced plasticity, suggesting a possibility that the plasticity may be reactivated in response to increased behavioral use of the whiskers on the spare side, and not directly by the damage or deprivation of the other side. Therefore, these findings all point to an important role for the abrupt and sustained change in sensory-evoked activity in deprived cortex as the initiator of the plasticity reactivation.
Peripheral sensory manipulations lead to anatomical and/or functional re-organization of layer 4 barrel cortex during the developmental critical period, with lesions typically producing more profound anatomical map plasticity whereas whisker trimming/plucking produces functional map plasticity with little anatomical change. (Belford and Killackey, 1980; Crair and Malenka, 1995; Durham and Woolsey, 1984; Fox, 1992, 2002; Li and Crair, 2011; Schlaggar et al., 1993; Van der Loos and Woolsey, 1973). The dissociation between the effects of certain peripheral sensory manipulations of anatomical plasticity and functional plasticity indicates that these two forms of plasticity likely share some, but not all, mechanisms of induction. Notably, in the present study we now show that the anatomical plasticity in layer 4 can be reactivated and that the same manipulation also produces a reactivation of TC synaptic plasticity. Further work will be needed to determine whether the two processes are mechanistically distinct or if the TC plasticity is a necessary requirement for the anatomical map plasticity.
It is not clear what the effects of this plasticity are on whisker related behaviours or whether the plasticity is adaptive or maladaptive. It could be there is increased acuity in whisker sensation, similar to enhanced hearing in mice that have been made blind which is a manipulation that also induced TC plasticity in the auditory pathway (Petrus et al., 2014). While not specifically studied here, there is also increased potentiation from the spared whisker cortex to the deprived whisker cortex via the corpus callosum following ION lesion (Yu et al., 2012). Recently this has been shown to block takeover of deprived whisker barrel cortex by neighbouring somatosensory areas, suggesting that the plasticity in the spared cortex may be important for this protection of cortex for whisker processing (Yu and Koretsky, 2014). Independent of the behavioural relevance for the plasticity, the mechanisms clearly demonstrate that LTP and silent synapses can re-appear at this central synapse in response to peripheral nerve damage.
In summary, a synaptic plasticity program is re-activated at TC synapses in spared barrel cortex following unilateral lesion of sensory afferent input, unilateral whisker trimming or unilateral barrel field cortex ablation. This reactivated plasticity appears to be identical to that which occurs during the critical period of development of this neural pathway. The sequence of events is a transient increase in GluN2B, followed by formation of silent synapses that coincide with the ability to induce LTP in slices and increased synaptic strength and in vivo potentiation. Increased synaptic strength is maintained even though the appearance of silent synapses and LTP are transient. In addition, unilateral peripheral sensory lesion also restores whisker lesion-induced anatomical map plasticity in the spared barrel cortex. The re-opening and subsequent closing of synaptic plasticity should enable a detailed analysis of the factors controlling plasticity in the adult brain. Studying such mechanisms holds promise for manipulating plasticity in adults to aid recovery from injury or identifying ways to recover synaptic function in neurodegenerative diseases.
EXPERIMENTAL PROCEDURES
All animal procedures were approved by the Institutional Animal Care and Use Committee of the National Institutes of Neurological Disorders and Stroke, National Institutes of Health (Bethesda, MD, USA) and Yonsei University Health System (Seoul, South Korea).
ION denervation
Four week-old male Sprague-Dawley rats were anesthetized with isoflurane. The unilateral ION lesion procedure was similar to that employed previously (Dietrich et al., 1985; Yu et al., 2012). Briefly, the infraorbital branch of the trigeminal nerve was first exposed as it emerges from the infraorbital foramen in a broad band of fibers fanning out in all directions to the skin of the snout and to the vibrissal roots. The infraorbital fiber bundles were ligated just distal to the infraorbital foramen to prevent regeneration. 2–3 mm distal to the ligature was cauterized towards the vibrissal roots. For animals undergoing a sham procedure, incisions were made and the nerve exposed, but nerve bundle ligation and cauterization was not performed.
Electrophysiology
TC slices (450 μm thick) were prepared from adult Sprague-Dawley Rats (6 −7 weeks-old) with some modifications of the method described previously (Agmon and Connors, 1991; Isaac et al., 1997; Yu et al., 2012). Briefly, after rats were anesthetized with isoflurane, the brain was rapidly cooled via transcardial perfusion with ice-cold sucrose- artificial cerebrospinal fluid (ACSF). The brain was removed and placed in ice-cold sucrose ACSF. Paracoronal slices were prepared at an angle of 50° relative to the midline on a ramp at an angle of 10°. Then, slices were incubated in ACSF at 35°C for 30 min to recover. Slices were then incubated in ACSF at room temperature (23°C −25°C) for 1–4 hours before being placed in the recording chamber for experiments. The standard ACSF contained (mM) 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.0 NaH2PO4, 26.2 NaH2CO3, 11 glucose, 1 Na pyruvate, 0.4 Na ascorbate saturated with 95% O2/5% CO2. Sucrose ACSF contained (mM) 198 sucrose, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose, 1 Na pyruvate, 0.4 Na ascorbate saturated with 95% O2/5% CO2. All experiments were conducted at 27°C – 29°C.
For electrophysiological experiments, electrodes with 3–6 MΩ resistance were used and stimuli were applied to the ventral posteromedial nucleus (VPM) using a concentric bipolar electrode (FHC, Bowdoin, ME). The somatosensory cortex was identified by the presence of barrels under 4x magnification and differential interference contrast (DIC) optics, and by the ability to evoke short and constant latency field excitatory postsynaptic potentials (fEPSPs) by VPM stimulation (Agmon and Connors, 1991). Whole-cell voltage-clamp recordings were made from stellate cells (SC) in layer IV of the somatosensory barrel cortex using infrared illumination and differential interference contrast (DIC) optics. The whole cell recording solution was as follows (mM): 135 Cs methanesulfonate, 8 NaCl, 10 Hepes, 0.5 EGTA, 4 Mg-ATP, 0.3 Na-GTP and 5 QX-315 Cl (pH 7.25 with CsOH, 285 mOsm). Cells were held at −70 mV during recordings unless otherwise indicated. Recordings were made using a multiclamp 700B (Molecular devices, Sunnyvale, CA) digitized at 10 KHz and filtered at 2 KHz. Input resistance and series resistance were monitored continuously during recordings, as previously described (Isaac et al., 1995). TC EPSCs were evoked at 0.1 Hz by VB stimulation and were accepted as monosynaptic if they exhibited a short and constant latency that did not change with increasing stimulus intensity as previously described (Feldman et al., 1998; Isaac et al., 1997).
For the minimal-stimulation protocol, VB thalamic stimulation intensity was adjusted to find the lowest intensity that elicited a mixture of synaptic responses and failures. Failure rate was calculated as number of failures/total number of trials. Potency was calculated as the mean EPSC peak amplitude excluding failures (Chittajallu and Isaac, 2010; Isaac et al., 1997; Stevens and Wang, 1995). Percent silent synapses were calculated using the following equation: 1-Ln(F-70)/Ln(F+40), in which F-70 was the failure rate at −70 mV and F+40 was the failure rate at +40 mV (Huang et al., 2009; Liao et al., 1995). The criteria for single-axon stimulation were (1) all or none synaptic events, (2) no change in the mean amplitude of the EPSC evoked by small increases in stimulus intensity, as previously reported (Chittajallu and Isaac, 2010; Gil et al., 1999). For the experiments measuring NMDA:AMPA ratio and ifenprodil sensitivity of the NMDA EPSC, pharmacologically-isolated NMDA EPSCs were recorded in the presence of 100 μM pictotoxin and 5 μM NBQX. For the LTP experiments, LTP was induced by pairing stimuli at 2Hz for 2 min with postsynaptic depolarization to 0mV.
Whisker trimming
For unilateral whisker trimming, all whiskers in the left whisker pad were cut every other day and the electrophysiology experiments were performed at PO 14 following unilateral whisker trimming. For some experiments, all whiskers on the spared whisker pad were trimmed at PO 12 following unilateral ION lesion to deprive whisker-evoked sensory input to spared cortex.
Osmotic pump implantation
Rats were anesthetized with isoflurane during surgery. The rat was then placed in a stereotaxic frame (Harvard apparatus, USA). The parietal and the frontal bones were exposed by a sagittal incision of the scalp at the midline from the level of the eyes to the occipital protuberance. A small bur hole was drilled after exposing the skull. A glass injection cannula was placed at the following coordinates: (Bregma −5.45, lateral −2.38, and ventral 2mm). Sterile saline solution (+/− 5μM ifenprodil) was perfused at a constant flow rate of 0.5 uL/hr through the injection cannula using a mini-osmotic pump (DURECT Corporation ALZET Osmotic Pumps, CA). Implantation was done in layer 4 of the barrel cortex ipsilateral to the ION lesion. To estimate extent of ifenprodil diffusion in layer 4 barrel cortex during pump injection, we first injected methylene blue to stain the ifenprodil-diffusion area. On average methylene blue diffused to 1040.0 ± 50.5 μm (n =6) away from the injection site. Based on this analysis, we only recorded from SCs located within 1000 μm from injection site.
Data analysis
All data were presented as mean ± SEM. ‘n’ represents the number of cells used in each experiments. Typically one slice was used from each animal; one recording was made from each slice. We used unpaired Student’s t-test to compare means values from the two groups and one-way ANOVA from the three or more groups. Two-way ANOVA was used to compare mean values during the specific time course between two groups. P-values < 0.05 were considered statistically significant. These statistical analyses were all performed using Prism 5.0 (GraphPad Software, Inc).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, NINDS and the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2012-0009525) and the “Kiturami” faculty Research Assistance Program of Yonsei University College of Medicine (6-2011-0168). We are grateful to Dr. Chris McBain, Dr. Ramesh Chittajallu and Dr. Emily Petrus for their scientific comments and insightful discussions. We also thank Ms. Kathryn Sharer and Ms. Nadia Boraoud for their technical support.
REFERENCES
- Adam R, and Gunturkun O (2009). When one hemisphere takes control: metacontrol in pigeons (Columba livia). PloS one 4, e5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmon A, and Connors BW (1991). Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Ridenour DA, and Sabatini BL (2007). Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 7365–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, and Isaac JT (2011). Maturation of a recurrent excitatory neocortical circuit by experience-dependent unsilencing of newly formed dendritic spines. Neuron 70, 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, and Malenka RC (2001). NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4, 235–236. [DOI] [PubMed] [Google Scholar]
- Belford GR, and Killackey HP (1980). The sensitive period in the development of the trigeminal system of the neonatal rat. The Journal of comparative neurology 193, 335–350. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, and Vicini S (1992). Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science (New York, NY) 258, 1007–1011. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, and Isaac JT (2010). Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci 13, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, and Bear MF (2010). Visual experience induces long-term potentiation in the primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 16304–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, and Malenka RC (1995). A critical period for long-term potentiation at thalamocortical synapses. Nature 375, 325–328. [DOI] [PubMed] [Google Scholar]
- Daw MI, Ashby MC, and Isaac JT (2007a). Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci 10, 453–461. [DOI] [PubMed] [Google Scholar]
- Daw MI, Scott HL, and Isaac JT (2007b). Developmental synaptic plasticity at the thalamocortical input to barrel cortex: mechanisms and roles. Molecular and cellular neurosciences 34, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, and Ebner FF (1993). Experience-dependent plasticity in adult rat barrel cortex. Proceedings of the National Academy of Sciences of the United States of America 90, 2082–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, and Ebner FF (1994). Laminar comparison of somatosensory cortical plasticity. Science (New York, NY) 265, 1885–1888. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Ginsberg MD, Busto R, and Smith DW (1985). Metabolic alterations in rat somatosensory cortex following unilateral vibrissal removal. The Journal of neuroscience : the official journal of the Society for Neuroscience 5, 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Hamze B, Wilson A, Speechley W, and Kuo MC (2007). Heterosynaptic facilitation of in vivo thalamocortical long-term potentiation in the adult rat visual cortex by acetylcholine. Cerebral cortex (New York, NY : 1991) 17, 839–848. [DOI] [PubMed] [Google Scholar]
- Dudek SM, and Friedlander MJ (1996). Developmental Down-Regulation of LTD in Cortical Layer IV and Its Independence of Modulation by Inhibition. Neuron 16, 1097–1106. [DOI] [PubMed] [Google Scholar]
- Durham D, and Woolsey TA (1984). Effects of neonatal whisker lesions on mouse central trigeminal pathways. The Journal of comparative neurology 223, 424–447. [DOI] [PubMed] [Google Scholar]
- Eaton NC, Sheehan HM, and Quinlan EM (2016). Optimization of visual training for full recovery from severe amblyopia in adults. Learning & memory (Cold Spring Harbor, NY) 23, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, and Isaac JT (1998). Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron 21, 347–357. [DOI] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, and Sheng M (2010). Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K (1992). A critical period for experience-dependent synaptic plasticity in rat barrel cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 12, 1826–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K (2002). Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience 111, 799–814. [DOI] [PubMed] [Google Scholar]
- Gagolewicz PJ, and Dringenberg HC (2011). NR2B-subunit dependent facilitation of long-term potentiation in primary visual cortex following visual discrimination training of adult rats. The European journal of neuroscience 34, 1222–1229. [DOI] [PubMed] [Google Scholar]
- Gambrill AC, and Barria A (2011). NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proceedings of the National Academy of Sciences of the United States of America 108, 5855–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George Paxinos CW (2013). The Rat Brain in Stereotaxic Coordinates In The Rat Brain in Stereotaxic Coordinates (USA: George Paxinos,), p. 472. [Google Scholar]
- Gil Z, Connors BW, and Amitai Y (1999). Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron 23, 385–397. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, and Nicoll RA (2011). Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron 71, 1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, and Ghosh A (2007). NR2B signaling regulates the development of synaptic AMPA receptor current. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 13446–13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, and Contractor A (2010). Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, and Bear MF (2001). Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 9801–9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickmott PW, and Merzenich MM (2002). Local circuit properties underlying cortical reorganization. J Neurophysiol 88, 1288–1301. [DOI] [PubMed] [Google Scholar]
- Hogsden JL, and Dringenberg HC (2009). Decline of long-term potentiation (LTP) in the rat auditory cortex in vivo during postnatal life: involvement of NR2B subunits. Brain research 1283, 25–33. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. (2009). In Vivo Cocaine Experience Generates Silent Synapses. Neuron 63, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, and Wiesel TN (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of physiology 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, and Malenka RC (1997). Silent synapses during development of thalamocortical inputs. Neuron 18, 269–280. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, and Malenka RC (1995). Evidence for silent synapses: implications for the expression of LTP. Neuron 15, 427–434. [DOI] [PubMed] [Google Scholar]
- Kaas JH, and Catania KC (2002). How do features of sensory representations develop? BioEssays : news and reviews in molecular, cellular and developmental biology 24, 334–343. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, and Sheng M (2005). Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron 46, 745–760. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, and Bear MF (1995). Elementary forms of synaptic plasticity in the visual cortex. Biological research 28, 73–80. [PubMed] [Google Scholar]
- Kullmann DM (1994). Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron 12, 1111–1120. [DOI] [PubMed] [Google Scholar]
- Kuo MC, and Dringenberg HC (2008). Histamine facilitates in vivo thalamocortical long-term potentiation in the mature visual cortex of anesthetized rats. The European journal of neuroscience 27, 1731–1738. [DOI] [PubMed] [Google Scholar]
- Lee SM, and Ebner FF (1992). Induction of high frequency activity in the somatosensory thalamus of rats in vivo results in long-term potentiation of responses in SI cortex. Experimental brain research 90, 253–261. [DOI] [PubMed] [Google Scholar]
- Levy J, and Trevarthen C (1976). Metacontrol of hemispheric function in human split-brain patients. Journal of experimental psychology Human perception and performance 2, 299–312. [DOI] [PubMed] [Google Scholar]
- Levy J, Trevarthen C, and Sperry RW (1972). Reception of bilateral chimeric figures following hemispheric deconnexion. Brain : a journal of neurology 95, 61–78. [DOI] [PubMed] [Google Scholar]
- Li H, and Crair MC (2011). How do barrels form in somatosensory cortex? Annals of the New York Academy of Sciences 1225, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, and Malinow R (1995). Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404. [DOI] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, and Crair MC (2001). Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron 32, 619–634. [DOI] [PubMed] [Google Scholar]
- Mainardi M, Landi S, Gianfranceschi L, Baldini S, De Pasquale R, Berardi N, Maffei L, and Caleo M (2010). Environmental enrichment potentiates thalamocortical transmission and plasticity in the adult rat visual cortex. Journal of neuroscience research 88, 3048–3059. [DOI] [PubMed] [Google Scholar]
- Montey KL, Eaton NC, and Quinlan EM (2013). Repetitive visual stimulation enhances recovery from severe amblyopia. Learning & memory (Cold Spring Harbor, NY) 20, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montey KL, and Quinlan EM (2011). Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nature communications 2, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, and Merzenich MM (1990). Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosensory & motor research 7, 463–483. [DOI] [PubMed] [Google Scholar]
- Oberlaender M, Ramirez A, and Bruno RM (2012). Sensory experience restructures thalamocortical axons during adulthood. Neuron 74, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled G, Chuang KH, Dodd SJ, and Koretsky AP (2007). Functional MRI detection of bilateral cortical reorganization in the rodent brain following peripheral nerve deafferentation. NeuroImage 37, 262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus E, Isaiah A, Jones AP, Li D, Wang H, Lee HK, and Kanold PO (2014). Crossmodal induction of thalamocortical potentiation leads to enhanced information processing in the auditory cortex. Neuron 81, 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Sekhar AK, Shouval HZ, and Bear MF (2001). Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29, 157–169. [DOI] [PubMed] [Google Scholar]
- Qi Y, Klyubin I, Harney SC, Hu N, Cullen WK, Grant MK, Steffen J, Wilson EN, Do Carmo S, Remy S, et al. (2014). Longitudinal testing of hippocampal plasticity reveals the onset and maintenance of endogenous human Ass-induced synaptic dysfunction in individual freely behaving pre-plaque transgenic rats: rapid reversal by anti-Ass agents. Acta neuropathologica communications 2, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, and Bear MF (1999a). Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proceedings of the National Academy of Sciences of the United States of America 96, 12876–12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, and Bear MF (1999b). Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci 2, 352–357. [DOI] [PubMed] [Google Scholar]
- Rema V, and Ebner FF (2003). Lesions of mature barrel field cortex interfere with sensory processing and plasticity in connected areas of the contralateral hemisphere. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 10378–10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Kopanitsa MV, Indersmitten T, Nithianantharajah J, Afinowi NO, Pettit C, Stanford LE, Sprengel R, Saksida LM, Bussey TJ, et al. (2013). Evolution of GluN2A/B cytoplasmic domains diversified vertebrate synaptic plasticity and behavior. Nat Neurosci 16, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Fox K, and O’Leary DD (1993). Postsynaptic control of plasticity in developing somatosensory cortex. Nature 364, 623–626. [DOI] [PubMed] [Google Scholar]
- Snow PJ, Nudo RJ, Rivers W, Jenkins WM, and Merzenich MM (1988). Somatotopically inappropriate projections from thalamocortical neurons to the SI cortex of the cat demonstrated by the use of intracortical microstimulation. Somatosensory research 5, 349–372. [DOI] [PubMed] [Google Scholar]
- Stevens CF, and Wang Y (1995). Facilitation and depression at single central synapses. Neuron 14, 795–802. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Bricolo E, and Aglioti SM (2005). Hemispheric metacontrol and cerebral dominance in healthy individuals investigated by means of chimeric faces. Brain research Cognitive brain research 24, 513–525. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, and Woolsey TA (1973). Somatosensory cortex: structural alterations following early injury to sense organs. Science (New York, NY) 179, 395–398. [DOI] [PubMed] [Google Scholar]
- Wallace H, and Fox K (1999). Local cortical interactions determine the form of cortical plasticity. Journal of neurobiology 41, 58–63. [PubMed] [Google Scholar]
- Wang CC, Held RG, Chang SC, Yang L, Delpire E, Ghosh A, and Hall BJ (2011). A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron 72, 789–805. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Broser PJ, Kuner T, and Bruno RM (2010). Experience-induced plasticity of thalamocortical axons in both juveniles and adults. The Journal of comparative neurology 518, 4629–4648. [DOI] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen D-Y, Wang S, Dodd Stephen J., Walters Judith R., Isaac John T.R., and Koretsky Alan P. (2012). Thalamocortical Inputs Show Post-Critical-Period Plasticity. Neuron 74, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, and Koretsky AP (2014). Interhemispheric plasticity protects the deafferented somatosensory cortex from functional takeover after nerve injury. Brain connectivity 4, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.