Abstract
Transmission of coronavirus disease 2019 (COVID-19) in healthcare settings has significant implications for patients and healthcare workers, may amplify local outbreaks, and may place additional burden on already stretched resources. Risk of missed or late diagnosis of COVID-19 was high during the UK's initial ‘containment phase’, because of strict criteria for testing. The risk remains due to asymptomatic/pre-symptomatic transmission, complicated by challenges faced with laboratory testing. We present a case study of potential nosocomial transmission associated with the first case of COVID-19 at a large acute NHS Trust in South-West London, and we describe the prevailing burden of nosocomial infections.
Keywords: COVID-19, SARS-CoV-2, Nosocomial transmission, Infection control, Outbreak investigation, Healthcare-associated infection
Introduction
During the initial ‘containment phase’ of the UK's coronavirus disease 2019 (COVID-19) response, only patients with compatible signs/symptoms and epidemiological risk factors (relevant travel history or contact with known case) were eligible for testing [1]. Prior to testing on clinical suspicion of COVID-19, there was a brief period during which all critical care patients with severe acute respiratory syndrome were recommended for testing [2]. Surveillance soon revealed evidence of COVID-19 in inpatients, which posed major infection control challenges.
Epsom and St Helier University Hospitals NHS Trust is a large acute provider, delivering secondary care to more than 490,000 people in South-West London and Surrey. This report describes the Trust's investigation, supported by Public Health England (PHE), into potential nosocomial transmission arising from exposure to the first inpatient diagnosed with COVID-19, identified through critical care surveillance.
As possible nosocomial transmission was established, a rapid retrospective analysis of readily available data was undertaken on all inpatients diagnosed with COVID-19 to determine the extent of nosocomial infections.
Methods
Patient journey and exposures
Details of the index case (P0) patient journey and exposure information were obtained from case notes, electronic patient records, and occupational health records (Figure 1 ). Exposed staff included all staff caring for the patient from admission to discharge and laboratory staff who handled samples. Exposed patients were classed as those on open bays with the index case.
Figure 1.
Index case patient journey. ITU, intensive therapy unit; A&E, accident and emergency; CCU, coronary care unit; CPAP, continuous airway positive pressure; HCW, healthcare worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Early retrospective trust-wide inpatient review
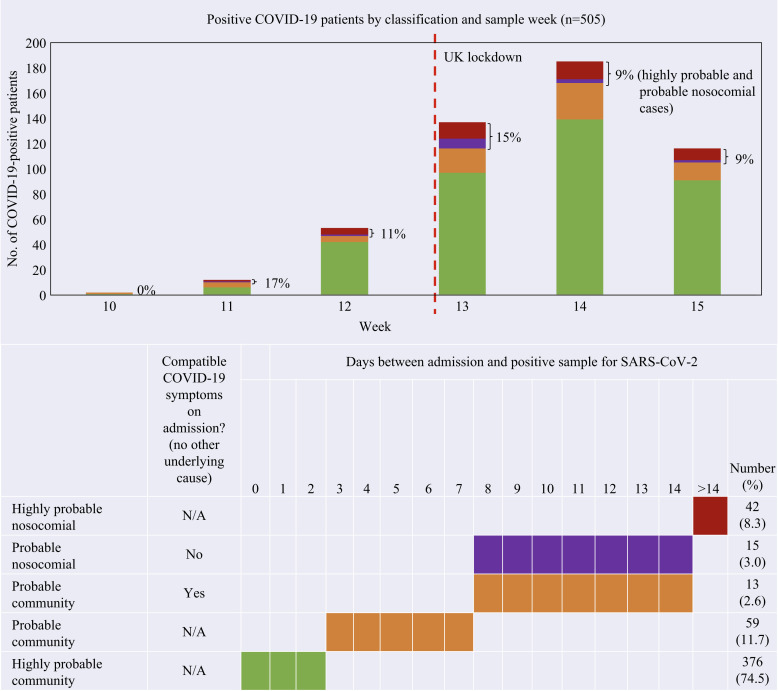
Admission and sample dates were retrospectively retrieved for all inpatients diagnosed with COVID-19 between March 6th and April 12th, 2020. Definitions were adapted from guidelines in development from the European Centre for Disease Prevention and Control and PHE (Figure 2 ) [3,4]. Cases were classified according to time between admission and first positive sample (nose/throat swab for SARS-CoV-2 followed by reverse transcription–polymerase chain reaction), while giving due importance to compatible symptom onset (fever/cough/shortness of breath) to differentiate between probable community and nosocomial cases.
-
–
Highly probable community: positive sample ≤2 days after admission.
-
–
Probable community: positive sample 3–7 days after admission, or 8–14 days after with compatible symptoms on admission.
-
–
Probable nosocomial: positive sample 8–14 days after admission with no compatible symptoms on admission.
-
–
Highly probable nosocomial: positive sample >14 days after admission.
Figure 2.
Number of coronavirus disease 2019 (COVID-19)-positive patients by sample week and algorithm for classification of nosocomial and community-acquired cases (N = 505).
Where patients had multiple admissions within the study period (N = 21); cases were reviewed jointly and a clinical judgement agreed as to which admission/sample dates were most meaningful for classification. Where a patient was discharged and readmitted within 48 h, the earlier admission date was used.
Ethics approval
This report covers a public health investigation and is exempt from NHS ethics review requirements, as defined by the NHS Health Research Authority, most recently in October 2017.
Results
The first confirmed COVID-19 case in London was imported on February 11th; no further cases were identified until 24th February. By February 28th there were 13 confirmed cases, and by March 8th there were 127 [5]. The index case (P0) was a 72-year-old male with multiple underlying health conditions, who presented on February 28th, 2020 with an apparent exacerbation of chronic obstructive pulmonary disease (COPD). He had no relevant travel history or epidemiological links and did not meet the national case definition, hence was not suspected/tested for COVID-19 on admission [1]. He had a week-long stay on medical and respiratory wards before admission to intensive therapy unit (ITU) where nose and throat swabs were taken as part of recently introduced critical care surveillance [2]. A positive result was reported 2 days later. The index case unfortunately died the following night; cause of death was certified as COVID-19 leading to type 1 respiratory failure, with COPD as the significant contributing condition.
Exposures
Following admission, the index case was placed in a side-room with clinical suspicion of influenza, then moved to open bays on coronary care unit (CCU) and ITU, where aerosol-generating procedures (AGPs) were performed.
Of 150 exposed staff, no laboratory (N = 53) or mortuary (N = 1) staff reported developing symptoms and were not tested. Of the 96 clinical and domestic staff, three developed compatible symptoms within 14 days of last exposure. None reported wearing respiratory PPE (surgical masks or respirators) during exposures.
Healthcare worker 1 (HCW1), an accident and emergency (A&E) nurse, had direct physical contact and developed compatible symptoms 9 days post exposure, testing positive 12 days post exposure. They had no other known exposures, implying probable nosocomial acquisition, possibly from the index case.
HCW2, a doctor exposed on ward 1, performed respiratory examinations on the index patient and developed compatible symptoms 13 days post exposure, testing positive 22 days post exposure. HCW2 had no relevant travel history but had exposure to another COVID-19 patient (confirmed subsequently) five days before symptom onset. Thus, HCW2 may have acquired COVID-19 nosocomially, but less likely from the index case.
HCW3, a doctor exposed on CCU, performed respiratory examinations on the index patient and developed highly compatible symptoms 6 days post exposure, but was unable to access testing (community testing withdrawn and HCW testing had not yet been established) [6]. HCW3 likely acquired COVID-19 nosocomially from the index case. HCW3 declined antibody testing.
The infection control team identified 16 exposed patients. None had had direct contact with the index case, and those exposed on CCU/ITU were bed-bound >2 m from the index case's bed. Patient 1 (P1) was exposed on A&E where he was nursed with the index case in the resuscitation area by the same team for >2 h. Upon diagnosis of COVID-19, the index case was isolated, and after enhanced cleaning, exposed patients were cohorted and enhanced infection control precautions and active surveillance were established. Active surveillance continued while patients remained in hospital. On discharge, this was switched to passive surveillance (patients asked to report any symptoms). Four patients completed 14 days of active surveillance, four completed >7 days, eight were discharged <7 days post exposure.
Two patient-contacts developed symptoms, and both tested positive; neither had any other known epidemiological risk factors. One (P10) had compatible symptoms on admission (shortness of breath, fever, bilateral patchy consolidation on chest X-ray) and tested positive 6 days post admission, suggesting probable community acquisition. The other (P1) tested positive 11 days post exposure. They likely acquired COVID-19 from healthcare exposure to the index case, or another unidentified source.
None of the remaining patients developed or reported compatible symptoms in the 14 days following exposure, but asymptomatic infection cannot be excluded. As of May 22nd, 2020, nine had been discharged home, two had been transferred to another hospital, and three had died (not COVID-19-related).
Inpatient review
As the Trust's incident risk assessment identified potential secondary transmission events, an early retrospective Trust-wide review of all confirmed COVID-19 inpatient cases was convened (late March), to quantify potential nosocomial COVID-19 burden.
This rapid review identified 505 COVID-19-positive inpatients between March 6th and April 12th, 2020 (Figure 2). Forty-two (8.3%) tested positive >14 days after admission to hospital. These ‘long stay’ patients (average 35.6 days, range 15,106) were classified as highly probable nosocomial cases. In addition, 15 (3.0%) patients tested positive 8–14 days post admission and had no compatible symptoms on admission. Combined, highly probable and probable nosocomial cases were on average older (mean: 77.4 years; range: 31.99) compared to highly and probable community cases (mean: 67.7 years; range: 0.99). They represented 11.3% of confirmed COVID-19 cases within the Trust, which is below the 20% modelled estimates for a typical UK hospital and within NHS England's estimate of 10–20% reported in the national press [7,8].
Thirteen (2.6%) patients testing positive 8–14 days post admission had compatible symptoms on admission and were classified as probable community cases, along with cases testing positive 3–7 days after admission.
Discussion
Accurate attribution of nosocomial COVID-19 is challenging, particularly given asymptomatic/subclinical infection, wide variation in incubation periods, and limited testing capacity. However, describing and quantifying nosocomial infections is essential to informing infection control, patient safety, and quality improvement measures.
Our case study identified limited nosocomial transmission from a confirmed COVID-19 case, cared for largely without respiratory PPE and in open bays on CCU and ITU, despite AGPs being performed. In line with another recent study, we did not identify patient–patient transmission from open bay exposures where AGPs had been performed [9]. Better ventilation with greater air changes in CCU and ITU may have contributed. The only probable secondary patient-case (P1) was nursed alongside the index case for >2 h. However, it is possible that P1 acquired infection from another, unidentified, nosocomial source.
Given increasing community transmission, we cannot exclude community acquisition among the subsequent HCW cases, as reported internationally [10]. Similarly, we cannot exclude other unidentified healthcare sources, and our secondary case attribution may therefore be an overestimate in this respect. Transmission resulting in asymptomatic carriage is unlikely to have been captured, as limited early testing capacity meant that exposed staff and patients were not regularly tested post exposure, unless symptomatic. Active surveillance of patient contacts was initially deemed imperative; however, as cases increased, cohorting with passive surveillance was more sustainable during this period.
Inpatients identified as highly probable nosocomial cases in the retrospective review were not routinely tested on admission. Of these cases, 40% were admitted in March, when local community transmission was increasing and they may have been asymptomatic carriers on admission. The accuracy and scope of our analysis is limited by its rapid nature and does not capture detailed dynamics such as patient–patient versus HCW–patient transmission. Increased accuracy would necessitate increased resource for patient-level investigation of every case, including of atypical presentations and all possible epidemiological risk factors.
The reliability of testing for SARS-CoV-2 (particularly in relation to the timing and quality of samples) is also a consideration. Of the 39 confirmed cases reviewed in detail, 13 (33.3%) had respiratory symptoms but tested negative on/early in admission.
Despite the inherent challenges, a practical Trust-level approach to quantifying and refining nosocomial acquisition data, which focuses stretched resources efficiently, is imperative. As accurate symptom onset dates are often difficult to ascertain, sample dates are a useful proxy when testing capacity is high. True estimation of nosocomial COVID-19 is challenging and our approach provides a real-world, pragmatic solution for routine use in healthcare settings.
Acknowledgements
The authors would like to thank all staff at the Trust and PHE for their ongoing work to respond to the COVID-19 pandemic, in particular, S. McLean, Infection Prevention & Control Matron and A.-M. Escoffery, Occupational Health Technician for their role in the initial incident management outlined in this paper. We would also like to thank the index case's family for their permission to publish the Trust's experience related to their loved one.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Public Health England . PHE; London: January 10th, 2020. COVID-19: investigation and initial clinical management of possible cases.https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases#history (updates ongoing). Current version and documented updates available at: [Google Scholar]
- 2.NHS England . NHSE; London: March 3rd, 2020. COVID-19 preparedness and response letter to the NHS.https://www.england.nhs.uk/wp-content/uploads/2020/03/covid-19-nhs-preparedness-and-response-letter-to-the-nhs.pdf Available at: [Google Scholar]
- 3.European Centre for Disease Prevention and Control . ECDC; 20 July 2020. Surveillance definitions for COVID-19 – source of infection: healthcare (nosocomial) vs community transmission. Available at: https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitionstitle="https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions">https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions. [Google Scholar]
- 4.NHS England . NHSE; London: 19 May 2020. Letter to Trust CEOs, medical directors and chief nurses – interim data collection – hospital-onset COVID-19. [Google Scholar]
- 5.Public Health England Coronavirus (COVID-19) dashboard, London Region. 2020. https://coronavirus-staging.data.gov.uk/cases?areaType=region&areaName=London Available at: [Google Scholar]
- 6.NHS England . NHSE; London: March 29th, 2020. COVID-19 testing and staff retention.https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/covid-19-testing-and-staff-retention-letter-29-march-2020.pdf Available at: [Google Scholar]
- 7.Evans S., Agnew E., Vynnycky E., Robotham J.V. medRxiv; 2020. The impact of testing and infection prevention and control strategies on within-hospital transmission dynamics of COVID-19 in English hospitals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding L., Campbell D. The Guardian; May 17th, 2020. Up to 20% of hospital patients with Covid-19 caught it at hospital.https://www.theguardian.com/world/2020/may/17/hospital-patients-england-coronavirus-covid-19 Available at: [Google Scholar]
- 9.Wong S.C., Kwong R.T., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105:119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluytmans M., Buiting A., Pas S., Bentvelsen R., van den Bijllaardt W., van Oudheusden A. medRxiv; 2020. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. [DOI] [Google Scholar]