Abstract
We used a constant total N application base rate to conduct a two-year field experiment comparing the effects of three organic fertilizers (rapeseed meal (RSM), soybean meal (SBM), and cattle manure (CM)) on the crop yield, economic growth, and soil quality of a winter wheat-summer maize rotation system. Winter wheat and summer maize in rapeseed meal treatment (RSMT), soybean meal treatment (SBMT), and cattle manure treatment (CMT) showed yield increases of 161%, 299%, and 256%, respectively, when compared to no organic fertilizer treatment (CK) (P < 0.05). The annual net incomes of SBMT and CMT were 1.46 and 1.42 times higher, respectively, than RSMT. Compared to the results of the CK group, RSM, SBM, and CM stimulated the soil physically, chemically, and biologically. We found the highest soil macroaggregate proportions, soil organic matter (SOM) levels, total N (TN) levels, and phospholipid fatty acid (PLFA) levels in SBMT. The highest soil pH, microbial biomass carbon (MBC) levels, and microbial biomass nitrogen (MBN) levels were observed in CMT. We used a soil quality index (SQI) to evaluate soil quality. After the two-year fertilization treatments, we calculated the SQI using a minimum data set (MDS). We used SOM levels and actinomycete quantity for the MDS properties. The SQI values were significantly different across the four treatments, with the highest values occurring in SBMT, then CMT and RSMT. In conclusion, SBM and CM were more effective than RSM at maintaining crop yield, economic growth, and soil quality.
Keywords: Rapeseed meal, Soybean meal, Cattle manure, Crop yield, Soil chemical characteristics, Soil aggregate, Microbial biomass, PLFA, Soil quality
Introduction
The world population has reached 7.6 billion people and is expected to reach 8.6 billion by 2030, 9.8 billion by 2050, and 11.2 billion by 2100 (Gerliani, Hammami & Aïder, 2019; Tripathi et al., 2019). To meet the global anticipated food demand caused by this population growth, various agricultural practices have been proposed and tested (Godfray et al., 2010), including the excessive application of chemical fertilizers, pesticides, and herbicides (Ju et al., 2007); large-scale utilization of water resources (Tilman et al., 2002); and intensive plowing (Koch & Stockfisch, 2006). Although these practices can increase food production, they also pose serious problems, including the decrease in organic matter content (Koch & Stockfisch, 2006; García-Orenes et al., 2012; Fernández-Romero et al., 2016), soil acidification (Guo et al., 2010; Zhu et al., 2018), greenhouse gas emissions (McGill, 2015), and loss of biodiversity (Clark & Tilman, 2008; Yu et al., 2015). Many reports have emphasized the need for major changes to the agricultural system to meet the challenge of feeding the growing population while minimizing environmental impacts (Godfray et al., 2010; Foley et al., 2011). Organic farming, which largely relies on organic materials instead of chemical fertilizers, pesticides, herbicides, or other synthetics (Luttikholt, 2007), has been increasingly recognized by both researchers and consumers (Ma & Joachim, 2006; Chen et al., 2014). High-efficiency organic fertilizers can increase crop yield without depleting soil quality, making their application a means of supporting both long-term food security and environmental preservation.
Previous studies have shown that the periodic application of organic amendments in an organic farming system can increase soil macroaggregate proportions (Zhang et al., 2020), soil organic matter (SOM) and total N (TN) levels (Liu et al., 2016; Guo et al., 2016), earthworm density (Guo et al., 2016; Meng et al., 2016), microbial biomass carbon (MBC) levels, microbial biomass nitrogen (MBN) levels, and enzymatic activities (Liu et al., 2010), which help to maintain crop yield and food quality (Liu et al., 2016; Tejada & Benítez, 2020). Although organic farming has numerous advantages, many farmers are still hesitant to change their systems, primarily due to the concern that their yields may be reduced during the conversion from conventional farming (Reganold et al., 2001; Tu et al., 2006). Additionally, using organic materials such as crop residues, compost, and poultry manure requires a great amount of human labor. Therefore, it is essential for organic farming development to identify new and better organic fertilizers that both minimize crop yield losses and enhance soil quality during the conversion period. Traditional organic fertilizers mainly include human excreta (Akram et al., 2019), compost, and poultry manure (Guo et al., 2016; Liu et al., 2016). Ecologists have suggested more available, effective, and cheaper organic fertilizers to completely or partially replace chemical fertilizers, such as different types of straw (Yang et al., 2015; Zhao et al., 2018), biochar (Zhang et al., 2020), sewage sludge, and municipal solid waste (Tejada & Benítez, 2020). Soybean meal (SBM) and rapeseed meal (RSM) are the residues created after oil is extracted from soybean (Glycine max (Linn.) Merr.) and rapeseed (Brassica campestris Linn.) seeds. They are high in N, which helps to increase vegetable, fruit, tea, and tobacco crop yields (Peng et al., 2008; Shan et al., 2010; Liu et al., 2012; Barker et al., 2019). However, few studies have investigated the effects of using SBM and RSM to fertilize a winter wheat (Triticum aestivum L.)-summer maize (Zea mays L.) rotating plantation.
According to data collected from the National Bureau of Statistics of China, the annual average sown areas of wheat and maize over the past 5 years were 24.5 and 43.3 million ha, respectively. Additionally, the annual yields of wheat and maize were 5,386.2 and 5,976.7 kg ha−1, respectively. The North China Plain (NCP) is a major grain production region containing about 35 million ha of croplands, 40% of which are dominated by a winter wheat-summer maize rotation system. This accounts for 61% and 45% of China’s total wheat and maize production, respectively (Yang et al., 2015). It is crucial to study the ecological and economic effects of SBM and RSM application in winter wheat-summer maize rotation systems in the NCP.
Soil quality is an indicator of sustainable soil management (Herrick, 2000), defined as “the capacity of soil to function within ecosystem boundaries to sustain productivity, maintain environmental quality, and promote plant and animal health” (Doran & Parkin, 1994). Fertilizers and soil management practices can affect soil quality by altering the physical, chemical, and microbiological indicators (Boafo et al., 2020). The most common method used to evaluate soil quality is the development of a soil quality index (SQI) (Qi et al., 2009; Cheng et al., 2016), which typically integrates the composite scores of multiple physical, chemical, and biological indicators (Andrews et al., 2002; Qi et al., 2009). The SQI method is considered to be effective at evaluating the effects of management practices on soil quality in agricultural lands (Zhang et al., 2004; Rojas et al., 2016; Nabiollahi et al., 2017; Boafo et al., 2020).
Fertilizer efficacy is typically evaluated by analyzing changes in crop yield and soil quality. In this study, we hypothesized that organic fertilizers could improve soil quality, crop yield, and the farmers’ profit. We carried out a two-year field experiment using a constant total N application rate (66.7 kg N ha−1 yr−1) from October 2017 to October 2019. Our objectives were to evaluate the effects of RSM, SBM, and cattle manure (CM) on a winter wheat-summer maize rotation system’s: (1) crop yield and economic growth; (2) physical, chemical, and biological soil characteristics; and (3) soil quality, evaluated using the SQI method.
Materials and Methods
Study area
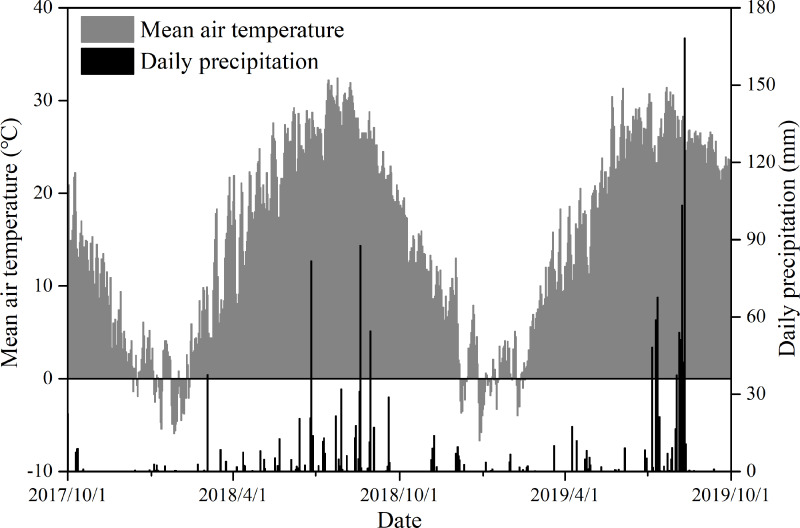
We conducted this experiment over two years in Jiang Jiazhuang Village, Pingyi County, Shandong Province, Eastern China (35°26′21″N, 117°50′11″E). Our study was approved by the Plant Eco-physiological Research Group (PERG) in the Institute of Botany, Chinese Academy of Sciences (project number: 201707.8). In this study, the name of the farmer is Jianjin Jiang who has a verbal agreement with the PERG in the Institute of Botany, Chinese Academy of Sciences. Although the farmland belongs to Jianjin Jiang, he has already rented it to the PERG. So, we should only get permission of the PERG. Any other permission from the individual and company is not needed. Shandong Province is an important source of food production in the NCP, and its main cropping system is a winter wheat-summer maize rotation. The study area has a typical temperate and monsoonal climate. In 2018 and 2019, the total annual rainfall was 685.1 mm and 842.2 mm, and the average mean air temperature was 15.2 °C and 15.1 °C, respectively. Figure 1 shows the daily precipitation and mean air temperature during our study from 1 October 2017 to 1 October 2019. The experimental soil was identified as an alfisol according to its soil taxonomy (IUSS Working Group WRB, 2014). The main soil (0–20 cm) characteristics are shown in Table 1.
Figure 1. Daily precipitation (mm) and mean air temperature (°C) during the experimental period in the study area.
Table 1. Properties of the experimental soil (0–20 cm) and the three organic fertilizers.
| □ | Water content (%) | OM (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | pH |
|---|---|---|---|---|---|---|
| Soil | 17.70 ± 0.47 | 15.41 ± 0.37 | 0.97 ± 0.01 | 0.81 ± 0.06 | 17.38 ± 0.54 | 6.95 ± 0.03 |
| RSM | 8.48 ± 0.38 | 791.49 ± 0.51 | 60.14 ± 0.06 | 20.64 ± 1.01 | 19.04 ± 0.67 | □– |
| SBM | 16.32 ± 0.54 | 798.60 ± 0.50 | 70.89 ± 0.12 | 11.21 ± 0.65 | 21.30 ± 0.78 | □– |
| CM | 65.02 ± 3.20 | 420.47 ± 20.11 | 18.67 ± 0.23 | 5.23 ± 0.32 | 17.78 ± 0.82 | □– |
Notes.
Data were means ± standard error (n = 3). RSM, SBM and CM represent rapeseed meal, soybean meal and cattle manure, respectively.
- OM
- organic matter
- TN
- total nitrogen
- TP
- total phosphorus
- TK
- total potassium
Experimental design
We set up our study using a randomized block design with four treatments and three replicates based on previous related research (Zhen et al., 2014; Guo et al., 2016). Each plot had an area of 80 m2 (10 m ×8 m). The four treatments were: (1) RSMT, rapeseed meal treatment, 1,212 kg ha−1 yr−1 (fresh weight) of RSM; (2) SBMT, soybean meal treatment, 1,125 kg ha−1 yr−1 (fresh weight) of SBM; (3) CMT, cattle manure treatment, 10,215 kg ha−1 yr−1(fresh weight) of CM; and (4) CK, no organic fertilizer treatment. We applied the fertilizers according to the local farmers’ average SBM application rate. We distributed equivalent amounts of N (66.7 kg ha−1 yr−1) across the three organic fertilizer treatments. The three organic fertilizers were solid, and their nutrient and water levels are shown in Table 1. All fertilizers were applied one time before winter wheat seeding. The winter wheat cultivar Shannong 32 was sown in mid-October and harvested in early June the following year. The summer maize cultivar Jinhai 5 was sown in mid-June and harvested in early October. 172.5 kg ha−1 and 37.5 kg ha−1 of wheat and maize seeds were planted, respectively. The distance between rows of wheat was 0.3 m, and the distances between rows and between individual maize were 0.6 m and 0.3 m, respectively. All other field management practices remained unchanged. A rotary tiller ridged the plots one time before winter wheat seeding at a plowing layer depth of 30 cm. Crops were irrigated twice during the winter wheat season. No irrigation was necessary during the summer maize season because of the abundance of rainfall. Crops were physically weeded twice during the winter wheat season and once during the summer maize season.
Yield assessment
Three 1 m2 crops were randomly harvested from each plot to determine winter wheat yield on 7 June 2018 and 10 June 2019. Three replicates of 10 consecutive summer maize plants in the same row were harvested on 9 October 2018 and 8 October 2019. The seeds of the harvested winter wheat and summer maize had a water content of < 14%.
Farm profitability
We recorded in detail each of the total inputs and outputs of the four treatments. The treated wheat and maize were sold online for $0.89/kg−1 and $0.59/kg−1, respectively. We used the annual average net income to assess economic benefits.
Soil sampling
We sampled the soils for chemical and biological characteristic analysis on 9 October 2018 and 8 October 2019. We used a 5 cm soil auger to select five random sampling points in the 0–20 cm layer, and then mixed the samples together into one. All soils were immediately transported to the laboratory, where all visible stones, roots, and undegraded fertilizer were removed. We divided the soil samples into two groups. One group was air-dried for chemical analysis, and the other group was passed through a 10-mesh sieve for biological analysis.
Soil chemical analysis
We passed a portion of the air-dried soil through a 10-mesh sieve to analyze its soil pH, available N (AN), available P (AP), and available K (AK). The other group of air-dried soils was passed through a 100-mesh sieve to analyze its SOM, TN, total phosphorus (TP), and total potassium (TK). We used a digital pH meter (PB-10, Sartorius, Germany) and a 1:2.5 soil:water ratio to measure soil pH. AN was determined using NaOH hydrolysis (Bao, 2000). We extracted AP by applying 0.5 M NaHCO3 (1:10, w/v) for 30 min, and determined colorimetrically using the molybdate method (Watanabe & Olsen, 1965). AK was measured using flame photometry (Schollenberger & Simon, 1945). We measured SOM using the dichromate oxidation method (Nelson & Sommers, 1996). TN was determined using the Kjeldahl method (Bremner & Mulvaney, 1982) and the FOSS Kjeltec 8200 (Foss, Hilleroed, Denmark). We used nitric-perchloric digestion (1:1) to extract TP and TK, and used EPA Method 3050 (USEPA, 1986) for analysis.
Soil aggregate distribution
We gathered our soil samples for aggregate distribution analysis from five subsamples (20 cm ×20 cm ×20 cm) of each plot on 8 October 2019. In the laboratory, we gently broke apart the moist soil samples along their natural break points and removed all visible stones, roots, and undegraded fertilizer. The soil samples were then air-dried for aggregate distribution analysis.
The soil aggregates were separated using the wet-sieve method (Elliott, 1986), and four aggregate-size classes were determined (large macroaggregate, >2 mm; small macroaggregate, 0.25–2 mm; microaggregate, 0.053–0.25 mm; and silt + clay, <0.053 mm). A subsample of 100 g air-dried soil was placed on top of a 2 mm sieve gently slaked with deionized water for 5 min. We then passed the slaked soil sample through three sieves with different mesh sizes (2 mm, 0.25 mm, and 0.053 mm) by automatically moving the sieves up and down for 15 min. All fractions left on each sieve and the bottom pan were thoroughly rinsed and transferred to aluminum boxes. We dried and weighed all aggregates to determine their size class.
Soil biological analysis
We analyzed MBC and MBN levels using a chloroform fumigation-direct extraction procedure. We estimated the levels using the difference between the fumigated and non-fumigated soil samples, and a conversion factor of 0.45 (Vance, Brookes & Jenkinson, 1987; Brookes, 1995). We extracted 25 g of fresh soil from each soil sample using 50 ml of 0.5 mol L−1 K2SO4 solution. The other 25 g of fresh soil was fumigated with chloroform at room temperature for 24 h, after being extracted with the K2SO4 solution. We immediately stored all extracts at −20 °C to be measured using a total organic carbon (TOC) analyzer (multi N/C 3100, Analytik Jena, Germany). We weighed out 10 g of fresh soil to measure its water content and to calculate its MBC and MBN levels.
Phospholipid fatty acid (PLFA) analysis was performed following the procedure described by Bossio et al. (1998). We extracted lipids from freeze dried soil samples at −80 °C using a single-phase chloroform-methanol-citrate buffer mixture (1:2:0.8, v/v/v). The PLFAs were then purified and identified using an Agilent 6850 gas chromatograph with the Sherlock Microbial Identification System (MIDI) V4.5 with the internal standard set at PLFA 19:0. In this study, the PLFA group was made up of three components (Bossio et al., 1998; Fierer, Schimel & Holden, 2003): (1) bacteria, most commonly 14:0, 15:0, 16:0, 17:0, and 18:0; gram-negative bacteria (G–) cy17:0, cy19:0, 15:1 ω6c, 16:1 ω7c, 16:1 ω9c, 17:1 ω8c, 18:1 ω7c, and 18:1 ω9c; and gram-positive bacteria (G+) i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, and a17:0; (2) fungi, most commonly 18:2 ω6c, 18:3 ω6c, 20:1 ω9c (Joergensen & Wichern, 2008), and arbuscular mycorrhizae fungi (AMF) 16:1 ω5c (Olsson, 1999); and (3) actinomycetes 10Me16:0, 10Me17:0, and 10Me18:0.
Soil quality evaluation
We assessed soil quality using the SQI method. The SQI was developed according to the following three steps: (1) selecting a minimum data set (MDS) for soil indicators, (2) scoring the MDS indicators, and (3) integrating the scored indicators into one SQI value (Qiu et al., 2019; Boafo et al., 2020).
We performed one-way analysis of variance on 18 soil indicators. All indicators showed significant differences (P < 0.05) across the four treatments and were included in the total data set (TDS). The TDS soil indicators were employed in principal component analysis (PCA) and selected the components with eigenvalues >1 as principal components (PCs). For each PC, we considered highly loaded indicators as those with absolute loading values within 10% of the highest factor loading. When a PC contained only one highly loaded indicator, this indicator was selected for the MDS. When a PC contained multiple indicators, we determined each indicator’s relevance using Pearson’s correlation analysis. If they were not correlated (r < 0.70), all highly loaded indicators were selected for the MDS. Otherwise, only the indicator with the highest sum of correlation coefficients (absolute value) was selected for the MDS (Qiu et al., 2019; Boafo et al., 2020).
After MDS selection, we used a linear scoring method to transform each soil indicator into a unitless score ranging from 0.00 to 1.00 (Boafo et al., 2020). Indicators were divided into two types according to their soil parameter concentration thresholds: “more is better” and “less is better”. In this study, considering the contributions of the MDS indicators, we only applied the “more is better” function:
| (1) |
where Y stands for the indicator linear score, X is the soil indicator value, and Xmax is the maximum value for each soil indicator.
We weighed the MDS indicators using the PCA results where the percentage of PC variation was divided by the total percentage of variation. We integrated the transformed indicator scores into the SQI using the following weighted equation:
| (2) |
where SQI is the soil quality index, Si is the indicator score, Wi is the weight of each indicator, and n is the number of soil indicators in the MDS. In this study, higher SQI values reflected better organic fertilizer treatment outcomes.
Statistical analyses
We performed statistical analysis using Microsoft Excel (2016) and SPSS 22.0 (IBM Corp, Armonk, New York, USA). Crop yield and soil property differences across the four treatments over the same year were determined using one-way analysis of variance and Duncan’s test. We used an independent sample t-test to assess the differences between different years, 2018 and 2019, over the same treatment. We set the significance level at P < 0.05. Soil microbial community composition (PLFAs) differences across the four treatments were evaluated using a PCA by correlation matrix (Past Version 3.25, Hammer; University of Oslo, Oslo, Norway). During the soil quality evaluation, SPSS 22.0 used both Pearson’s correlation and PCA to determine the MDS indicators and their weights. Figures were created using OriginPro 9.2 (OriginLab, Massachusetts, USA).
Results
Crop yield and economic benefits
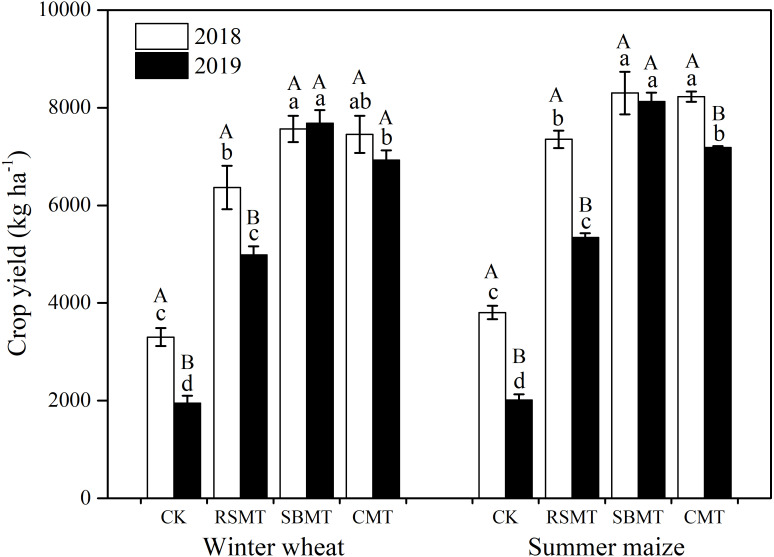
Compared to the CK group, RSMT, SBMT, and CMT showed significantly increased yields over the two-year study (P < 0.05, Fig. 2). In 2018, winter wheat and summer maize crop yields were 3,301.5 and 3,805.4 kg ha−1 in the CK group, 6,368.0 and 7,351.8 kg ha−1 in RSMT, 7,565.1 and 8,299.9 kg ha−1in SBMT, and 7,453.8 and 8,227.5 kg ha−1 in CMT, respectively. In 2019, winter wheat and summer maize in RSMT, SBMT, and CMT had 161%, 299%, and 256% greater yields, respectively, than the CK group (P < 0.05). However, the 2019 winter wheat and summer maize CK and RSMT crop yields were significantly lower than those in 2018 (P < 0.05).
Figure 2. Winter wheat and summer maize yields under different fertilizer treatments in 2018 and 2019.
CK, RSMT, SBMT and CMT represent no organic fertilizer treatment, rapeseed meal treatment, soybean meal treatment and cattle manure treatment, respectively. Data were means ± standard error (n = 3). Different lowercase letters indicate significant differences at P < 0.05 level among the four treatments in the same year. Different capital letters indicate significant difference at P < 0.05 level between 2018 and 2019 in the same treatment.
As shown in Table 2, RSMT, SBMT, and CMT had much greater outputs than the CK group. We noted the highest annual average net income from SBMT and the lowest annual production cost from CMT. The inputs (materials, equipment, and labor) totaled $1,695.1, $2,431.0, $2,312.2 and $1,900.0/ha−1 for the CK, RSMT, SBMT, and CMT groups, respectively. The annual average production costs for the CK, RSMT, SBMT, and CMT groups were $0.33, $0.21, $0.15 and $0.13/kg−1, respectively. Excluding fertilizer and fertilization costs, the other inputs were consistent across the four treatments. RSM and SBM were priced higher than CM. However, CM required more manual labor during the fertilization process. The annual average net incomes in RSMT, SBMT, and CMT were $6,367.2, $9,318.7, and $9,047.0/ha−1, and were 2.70, 3.95, and 3.84 times higher than the CK group ($2,357.9/ ha−1), respectively. The annual average net incomes in SBMT and CMT were 1.46 and 1.42 times higher, respectively, than RSMT.
Table 2. Comprehensive economic benefits under different fertilizer treatments.
| □ | □ | Details | CK ($ ha−1) | RSMT ($ ha−1) | SBMT ($ ha−1) | CMT ($ ha−1) |
|---|---|---|---|---|---|---|
| Input | Materials | Fertilizer | 0 | 715.1 | 596.3 | 90.9 |
| Seed | 194.3 | 194.3 | 194.3 | 194.3 | ||
| Water | 222.0 | 222.0 | 222.0 | 222.0 | ||
| Equipment | Ploughing | 177.6 | 177.6 | 177.6 | 177.6 | |
| Seeding | 133.2 | 133.2 | 133.2 | 133.2 | ||
| Harvesting | 377.4 | 377.4 | 377.4 | 377.4 | ||
| Labor | Fertilization | 0 | 20.8 | 20.8 | 114.0 | |
| Irrigation | 124.4 | 124.4 | 124.4 | 124.4 | ||
| Weed control | 466.2 | 466.2 | 466.2 | 466.2 | ||
| Annual average input | 1,695.1 | 2,431.0 | 2,312.2 | 1,900.0 | ||
| Output | 2018 | 5,182.8 | 10,005.2 | 11,629.9 | 11,488.6 | |
| 2019 | 2,923.2 | 7,591.1 | 11,630.7 | 10,405.4 | ||
| Annual average output | 4,053.0 | 8,798.1 | 11,630.3 | 10,947.0 | ||
| Annual average net income | 2,357.9 | 6,367.2 | 9,318.1 | 9,047.0 | ||
| Production cost ($ kg−1) | 2018 | 0.24 | 0.18 | 0.15 | 0.12 | |
| 2019 | 0.43 | 0.24 | 0.15 | 0.13 | ||
| Annual average production cost ($ kg−1) | 0.33 | 0.21 | 0.15 | 0.13 | ||
Notes.
Data were upscaled to 1 ha based on the data collected from this experiment. US$1.0=6.756 Chinese Yuan. The outputs of wheat and maize were calculated at organic prices, respectively, $0.89/kg−1 and $0.59/kg−1. CK, RSMT, SBMT and CMT represent no organic fertilizer treatment, rapeseed meal treatment, soybean meal treatment and cattle manure treatment, respectively.
Soil chemical characteristics
In this study, we measured 8 soil chemical characteristics (Table 3). CMT had the highest soil pH, followed by the SBMT, RSMT, and CK groups (P < 0.05). SBMT had the highest amounts of SOM, TN, AN, AP, and AK, followed by the CMT, RSMT, and CK groups (P < 0.05, Table 3). RSMT, SBMT, and CMT in 2019 had SOM and TN levels that were 54% and 71%, 102% and 90%, and 88% and 86% higher, respectively, than the CK group (P < 0.05). SBMT displayed the highest TP and TK levels, followed by RSMT and CMT. Soil chemical levels in 2019 were higher than those in 2018 across the three organic fertilizer treatments.
Table 3. Soil physicochemical and biological properties under different fertilizer treatments in 2018 and 2019.
| □ | □ | CK | RSMT | SBMT | CMT |
|---|---|---|---|---|---|
| pH | 2018 | 6.81 ± 0.03bA | 6.92 ± 0.06bA | 6.94 ± 0.03bA | 7.18 ± 0.05aA |
| 2019 | 6.80 ± 0.03cA | 7.01 ± 0.01bA | 7.01 ± 0.01bA | 7.10 ± 0.00aA | |
| SOM (g kg−1) | 2018 | 13.38 ± 0.36cA | 14.89 ± 0.17bB | 17.36 ± 0.66aB | 16.82 ± 0.09aB |
| 2019 | 11.12 ± 0.51dB | 17.16 ± 0.52cA | 22.49 ± 0.67aA | 20.85 ± 0.12bA | |
| TN (g kg−1) | 2018 | 0.78 ± 0.02cA | 0.93 ± 0.02bB | 1.07 ± 0.01aB | 0.96 ± 0.02bB |
| 2019 | 0.63 ± 0.01cB | 1.08 ± 0.01bA | 1.20 ± 0.03aA | 1.17 ± 0.01aA | |
| TP (g kg−1) | 2018 | 0.74 ± 0.03bA | 0.83 ± 0.05bB | 1.02 ± 0.09aA | 0.81 ± 0.03bA |
| 2019 | 0.68 ± 0.03cA | 0.99 ± 0.01bA | 1.13 ± 0.02aA | 0.98 ± 0.02bA | |
| TK (g kg−1) | 2018 | 17.25 ± 0.12bA | 17.49 ± 0.15bA | 18.62 ± 0.29aA | 17.43 ± 0.27bB |
| 2019 | 17.04 ± 0.34bA | 17.89 ± 0.15aA | 18.34 ± 0.25aB | 17.75 ± 0.13abA | |
| AN (mg kg−1) | 2018 | 48.15 ± 2.28cA | 63.75 ± 2.73bB | 79.54 ± 3.48aB | 71.92 ± 4.51abB |
| 2019 | 43.04 ± 2.10dA | 74.90 ± 2.56cA | 90.06 ± 1.14aA | 82.06 ± 2.01bA | |
| AP (mg kg−1) | 2018 | 49.22 ± 2.87dA | 68.22 ± 2.87cB | 109.29 ± 3.33aB | 80.73 ± 3.33bB |
| 2019 | 39.53 ± 2.66dA | 90.64 ± 1.18cA | 122.60 ± 2.00aA | 99.56 ± 1.35bA | |
| AK (mg kg−1) | 2018 | 128.76 ± 1.59dA | 142.72 ± 1.64cB | 162.31 ± 0.34aB | 158.23 ± 0.72bB |
| 2019 | 123.28 ± 1.93cA | 237.95 ± 5.00bA | 263.01 ± 5.66aA | 229.88 ± 0.91bA | |
| MBC (mg kg−1) | 2018 | 88.22 ± 2.11cA | 115.98 ± 5.13bB | 125.24 ± 0.82bB | 138.76 ± 4.95aB |
| 2019 | 79.62 ± 2.98cA | 137.56 ± 1.28bA | 238.05 ± 3.19aA | 245.54 ± 6.85aA | |
| MBN (mg kg−1) | 2018 | 12.93 ± 0.04cA | 18.87 ± 1.28bB | 21.79 ± 1.12abB | 22.93 ± 1.26aB |
| 2019 | 13.40 ± 0.62dA | 22.91 ± 0.54cA | 38.10 ± 0.22bA | 40.00 ± 0.66aA | |
| Bacteria (nmol g−1) | 2018 | 8.27 ± 1.39bA | 9.46 ± 0.20abA | 12.79 ± 1.25aA | 11.42 ± 0.79abA |
| 2019 | 6.94 ± 0.94bA | 9.38 ± 0.23abA | 11.95 ± 1.16aA | 10.70 ± 0.26aA | |
| Fungi (nmol g−1) | 2018 | 0.90 ± 0.16aA | 1.01 ± 0.05aA | 1.13 ± 0.11aA | 1.09 ± 0.08aA |
| 2019 | 0.66 ± 0.11cA | 0.94 ± 0.04bA | 1.20 ± 0.08aA | 1.14 ± 0.05abA | |
| Actinomycetes (nmol g−1) | 2018 | 0.55 ± 0.10bA | 0.69 ± 0.19bB | 1.60 ± 0.14aA | 0.99 ± 0.25bA |
| 2019 | 1.00 ± 0.13bA | 1.34 ± 0.05abA | 1.75 ± 0.21aA | 1.58 ± 0.06aA | |
| AMF (nmol g−1) | 2018 | 0.35 ± 0.07bA | 0.42 ± 0.00abA | 0.57 ± 0.06aA | 0.54 ± 0.04aA |
| 2019 | 0.28 ± 0.05cA | 0.42 ± 0.03bA | 0.58 ± 0.05aA | 0.52 ± 0.01abA | |
| Total PLFA (nmol g−1) | 2018 | 9.72 ± 1.52bA | 11.16 ± 0.09bA | 15.52 ± 1.50aA | 13.50 ± 0.91abA |
| 2019 | 8.60 ± 1.18bA | 11.66 ± 0.31abA | 14.89 ± 1.45aA | 13.42 ± 0.33aA | |
| Soil aggregate fractions | M | 41.13% ± 0.04b | 57.46% ± 0.00a | 60.93% ± 0.01a | 59.02% ± 0.0a |
| m | 39.99% ± 0.02a | 30.74% ± 0.01b | 29.01% ± 0.03b | 28.42% ± 0.01b | |
| S + C | 18.88% ± 0.02a | 11.80% ± 0.01b | 10.06% ± 0.02b | 12.56% ± 0.02b |
Notes.
Data were means ± standard error (n = 3). Different lowercase letters indicate significant differences at P < 0.05 level among the four treatments in the same year. Different capital letters indicate significant difference at P < 0.05 level between 2018 and 2019 in the same treatment. CK, RSMT, SBMT and CMT represent no organic fertilizer treatment, rapeseed meal treatment, soybean meal treatment and cattle manure treatment, respectively.
- SOM
- soil organic matter
- TN
- total nitrogen
- TP
- total phosphorus
- TK
- total potassium
- AN
- available nitrogen
- AP
- available phosphorus
- AK
- available potassium
- MBC
- microbial biomass carbon
- MBN
- microbial biomass nitrogen
- AMF
- arbuscular mycorrhizae fungi
- M
- large macroaggregate + small macroaggregate
- m
- microaggregate
- S + C
- silt + clay
Soil aggregate fractions
Large and small macroaggregates were the most represented aggregate-size classes. We noted that SBMT had the highest amount of macroaggregates, while the CK group had the highest amounts of microaggregates and silt + clay (P < 0.05). The proportion of macroaggregates in RSMT, SBMT, and CMT were 40%, 48%, and 43% greater, respectively, than in the CK group. The proportion of microaggregates in RSMT, SBMT, and CMT were 23%, 27%, and 29% lower, respectively, and the proportion of silt + clay was 38%, 47%, and 33% lower, respectively, than in the CK group (P < 0.05, Table 3).
Soil biological characteristics
Organic fertilizers significantly increased soil MBC and MBN levels (P < 0.05, Table 3). In 2018 and 2019, CMT had the highest MBC and MBN levels, followed by SBMT and RSMT. We found the lowest MBC and MBN levels in the CK group. At the end of the experiment, MBC levels in RSMT, SBMT, and CMT were 73%, 199%, and 208% (P < 0.05) greater, respectively, than in the CK group. MBN levels in RSMT, SBMT, and CMT were 71%, 184%, and 199% greater, respectively, than in the CK group (P < 0.05). RSMT, SBMT, and CMT had MBC levels in 2019 that were 19%, 90%, and 77% greater, respectively, than MBC levels in 2018, and MBN levels in 2019 that were 21%, 75%, and 74% greater, respectively, than MBN levels in 2018 (P < 0.05, Table 3).
The total amount of PLFAs, bacteria, fungi, actinomycetes, and AMF in 2018 and 2019 were highest in SBMT, followed by the CMT, RSMT, and CK groups (Table 3). At the end of the experiment, RSMT, SBMT, and CMT had 36%, 73%, and 56% more PLFAs; 35%, 72%, and 54% more bacteria; 43%, 83%, and 74% more fungi; 34%, 74%, and 58% more actinomycetes; and 52%, 106%, and 86% more AMF, respectively, than the CK group (P < 0.05, Table 3).
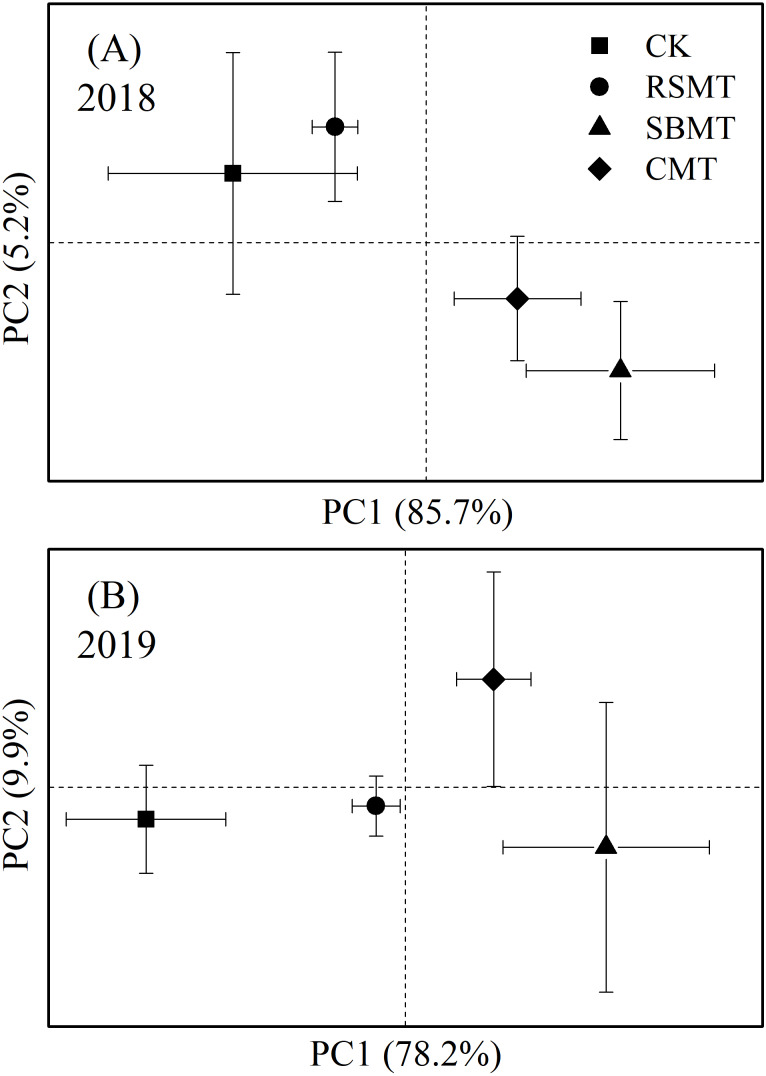
We performed 27 PLFAs to PCA in 2018 and in 2019 (Fig. 3). In 2018, principle component 1 (PC1) and principle component 2 (PC2) accounted for 85.7% and 5.2% of the total variance, respectively. SBMT and CMT demonstrated a clear separation along PC1 and PC2 from the RSMT and CK groups (Fig. 3A). In 2019, the first two PCs accounted for 78.2% and 9.9% of the total variance, respectively. Microbial communities from the CK group and the three organic fertilizer treatments showed significant separation in PC1 (P < 0.05). In PC2, CMT was isolated from the other three treatments (P > 0.05, Fig. 3B).
Figure 3. PCA for PLFAs in topsoil samples under different fertilizer treatments in 2018 (A) and 2019 (B).
In 2018, PC1 and PC2 accounted for 85.7% and 5.2% of the total variance, respectively; In 2019, PC1 and PC2 accounted for 78.2% and 9.9% of the total variance, respectively. CK, RSMT, SBMT and CMT represent no organic fertilizer treatment, rapeseed meal treatment, soybean meal treatment and cattle manure treatment, respectively. Data were means ± standard error (n = 3).
Soil quality evaluation
The PCA results (Table 4) showed that only the first two PCs had eigenvalues >1.0 and 90.917% of the total variability: 82.253% for PC1 and 8.664% for PC2. In PC1, the highly loaded indicators were SOM, TN, TP, AN, AP, AK, MBC, MBN, bacteria, fungi, AMF, total PLFA, and macroaggregates (Table 4). These 13 indicators showed significant correlations among each other (r > 0.70). Since the SOM had the highest absolute loading sum, it was selected for the MDS (Table 5). The highly loaded indicators in PC2 were TK, bacteria, actinomycetes, and total PLFA. We also selected actinomycetes for the SQI MDS because they had the highest absolute correlation coefficient (Table 5).
Table 4. Loading, eigenvalue and variance explained of principal component analysis (PCA) using eighteen soil properties.
| Soil quality indicators | PC1 | PC2 |
|---|---|---|
| pH | 0.859 | −0.119 |
| SOM | 0.977 | −0.018 |
| TN | 0.968 | −0.132 |
| TP | 0.953 | −0.106 |
| TK | 0.778 | −0.415 |
| AN | 0.979 | −0.159 |
| AP | 0.977 | −0.066 |
| AK | 0.950 | −0.175 |
| MBC | 0.923 | 0.059 |
| MBN | 0.923 | 0.061 |
| Bacteria | 0.883 | 0.445 |
| Fungi | 0.906 | 0.398 |
| Actinomycetes | 0.862 | 0.457 |
| AMF | 0.920 | 0.352 |
| Total PLFA | 0.886 | 0.444 |
| M | 0.907 | −0.386 |
| Microaggregate | −0.848 | 0.392 |
| Silt + clay | −0.793 | 0.296 |
| Eigenvalue | 14.806 | 1.559 |
| Explained variance (%) | 82.253 | 8.664 |
| Cumulative variance (%) | 82.253 | 90.917 |
Notes.
Boldface factor loadings are considered highly weighted. Boldface and underlined loadings correspond to the indicators retained in the MDS.
- SOM
- soil organic matter
- TN
- total nitrogen
- TP
- total phosphorus
- TK
- total potassium
- AN
- available nitrogen
- AP
- available phosphorus
- AK
- available potassium
- MBC
- microbial biomass carbon
- MBN
- microbial biomass nitrogen
- AMF
- arbuscular mycorrhizae fungi
- M
- large macroaggregate + small macroaggregate
Table 5. Person correlation coefficients for highly weighed variables under PC1 and PC2 (n = 12).
| □ | SOM | TN | TP | TK | AN | AP | AK | MBC | MBN | B | F | A | AMF | TPLFA | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOM | 1 | 0.952** | 0.926** | 0.724** | 0.958** | 0.970** | 0.904** | 0.945** | 0.950** | 0.841** | 0.867** | 0.808** | 0.877** | 0.843** | 0.886** |
| TN | 1 | 0.936** | 0.723** | 0.979** | 0.965** | 0.967** | 0.884** | 0.889** | 0.781** | 0.835** | 0.740** | 0.828** | 0.784** | 0.913** | |
| TP | 1 | 0.822** | 0.955** | 0.981** | 0.965** | 0.810** | 0.808** | 0.817** | 0.811** | 0.782** | 0.829** | 0.815** | 0.884** | ||
| TK | 1 | 0.818** | 0.787** | 0.809** | 0.639** | 0.625** | 0.540** | 0.536** | 0.566** | 0.597** | 0.545** | 0.878** | |||
| AN | 1 | 0.981** | 0.966** | 0.898** | 0.896** | 0.792** | 0.821** | 0.759** | 0.841** | 0.793** | 0.937** | ||||
| AP | 1 | 0.963** | 0.881** | 0.879** | 0.838** | 0.852** | 0.797** | 0.865** | 0.837** | 0.888** | |||||
| AK | 1 | 0.799** | 0.796** | 0.771** | 0.804** | 0.737** | 0.816** | 0.772** | 0.914** | ||||||
| MBC | 1 | 0.998** | 0.796** | 0.847** | 0.782** | 0.861** | 0.803** | 0.804** | |||||||
| MBN | 1 | 0.797** | 0.850** | 0.783** | 0.854** | 0.804** | 0.806** | ||||||||
| B | 1 | 0.970** | 0.987** | 0.972** | 0.999** | 0.639** | |||||||||
| F | 1 | 0.963** | 0.979** | 0.975** | 0.668** | ||||||||||
| A | 1 | 0.960** | 0.990** | 0.625** | |||||||||||
| AMF | 1 | 0.975** | 0.710** | ||||||||||||
| TPLFA | 1 | 0.642** | |||||||||||||
| M | 1 |
Notes.
indicates P < 0.01.
- SOM
- soil organic matter
- TN
- total nitrogen
- TP
- total phosphorus
- TK
- total potassium
- AN
- available nitrogen
- AP
- available phosphorus
- AK
- available potassium
- MBC
- microbial biomass carbon
- MBN
- microbial biomass nitrogen
- B
- bacteria
- F
- fungi
- A
- actinomycetes
- AMF
- arbuscular mycorrhizae fungi
- TPLFA
- total PLFA
- M
- large macroaggregate + small macroaggregate
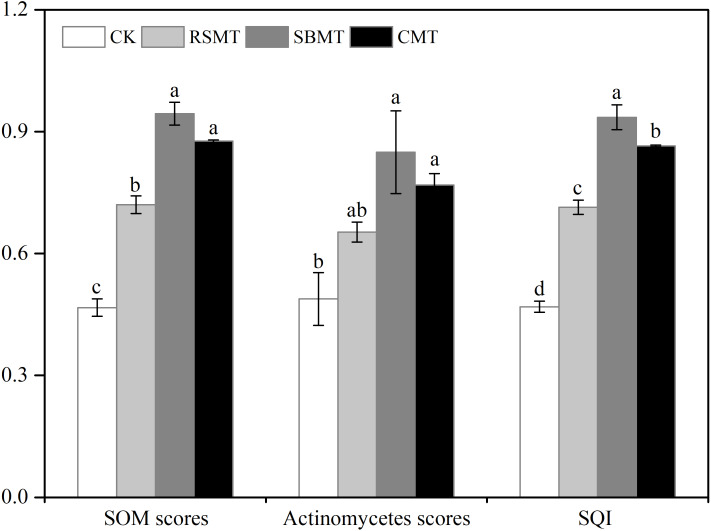
After selecting SOM and actinomycetes for the MDS, we applied a “more is better” linear scoring function. The highest SOM and actinomycete score values were found in SBMT, followed by CMT, RSMT, and CK (Fig. 4). We determined the weight of each MDS indicator by calculating its contribution percentage in each PC. The weights of SOM and actinomycetes were 0.905 and 0.095, respectively. After scoring the selected MDS indicators, we calculated the SQI using the following weighted equation:
| (3) |
Figure 4. Scored values of MDS indicators (SOM, actinomycetes) and soil quality index.
CK, RSMT, SBMT and CMT represent no organic fertilizer treatment, rapeseed meal treatment, soybean meal treatment and cattle manure treatment, respectively. Data were means ± standard error (n = 3). Different lowercase letters indicate significant differences at P < 0.05 level among the four treatments. SOM, soil organic matter; SQI, soil quality index.
The average SQI values for the CK, RSMT, SBMT, and CMT groups were 0.47, 0.71, 0.94, and 0.87, respectively. The differences across the four treatments were significant (P < 0.05, Fig. 4).
Discussion
Crop yield and economic benefits
Previous studies have claimed that using organic fertilizers results in an average of 19.8–25% lower crop yield than chemical fertilizers (Ponti, Rijk & Ittersum, 2012; Seufert, Ramankutty & Foley, 2012; Ponisio et al., 2015). However, yield losses have not been the case across all climate zones, organic fertilizers, or crops (Seufert, Ramankutty & Foley, 2012; Gattinger et al., 2013). When using best management practices and suitable organic fertilizers, an organic system’s yield can meet or even surpass a chemical system (Liu et al., 2016). Our results clearly indicate that wheat and maize in SBMT and CMT produced much higher yields than the average in Shandong Province (6,035.0 kg ha−1 for wheat and 6,469.0 kg ha−1 for maize). Therefore, we suggest that the proper application of SBM and CM in a winter wheat-summer maize rotation system would reduce yield losses.
For many farmers, however, crop yield benefits are second to economic benefits. In our previous studies, we found that organic systems presented much higher economic benefits than conventional systems (Liu et al., 2016; Meng et al., 2016). Using economic benefit analysis, we observed the highest input in RSMT, and the lowest annual production costs in CMT, followed by SBMT. Net incomes were higher for SBMT ($9,318.1/ha−1) and CMT ($9,047.0/ha−1), and they were 1.46 and 1.42 times greater, respectively, than RSMT, as well as 4.82 and 4.68 times greater, respectively, than conventionally farmed local crops (Liu et al., 2016). Our results indicate that SBM and CM fertilizers generate more economic benefits than RSM and chemical fertilizers.
Soil chemical characteristics
We found that soil pH in RSMT, SBMT, and CMT was significantly higher than in the CK group (P < 0.05), indicating that organic fertilizers, specifically CM, can alleviate the soil acidification caused by long-term chemical fertilizer application (Guo et al., 2010; Zhu et al., 2018). At the end of our study, we found much higher concentrations of SOM and the minerals essential for plant growth (N, P, and K) in organic fertilizer treatments than in the CK group, which corresponded with the results of Liu et al. (2016). The soil chemical levels for the three organic fertilizer treatments in 2019 were significantly higher than those in 2018, indicating organic fertilizers’ ability to nurse cultivated land. This effect was more obvious over time.
Soil aggregate fractions
Soil aggregates are important indicators of soil quality and the effects of soil management (Barthès & Roose, 2002). Organic fertilizer treatments showed significantly higher proportions of macroaggregates and lower levels of microaggregates and silt + clay than the CK group, which was consistent with the findings of Liao, Boutton & Jastrow (2006) and Yu et al. (2012). These results may be due to the higher amounts of AMF and other fungi (Li et al., 2020) that predominantly proliferate in larger soil pores and contribute to macroaggregate formation (De Gryze et al., 2005; Wilson et al., 2009; Ding & Han, 2014).
Soil biological characteristics
MBC representing about 1–5% of the soil’s TOC could provide an effective warning of the soil quality (Powlson & Brooks, 1987). Our results clearly demonstrated that organic fertilizers significantly increased MBC and MBN levels, which was consistent with the findings of Kaur, Kapoor & Gupta (2005), who found that MBC levels tended to be lower in unfertilized soil compared to soil treated with organic fertilizers. Increased MBC and MBN levels may be caused by organic fertilizers’ abundant metabolizable carbon and N that promote root growth and increase soil microbial biomass (Ekblad & Nordgren, 2002). More importantly, organic fertilizers directly supply energy to microbes, further increasing soil microbial biomass and activity (Vance & Chapin, 2001; Ekblad & Nordgren, 2002).
Our PCA results indicated that organic fertilizers significantly affected the soil microbial community. SBMT and CMT showed higher amounts of total PLFAs, bacteria, fungi, and actinomycetes than RSMT and the CK group. Bacteria represented about 80–85% of the total PLFAs in this study. The increase of bacteria in SBMT and CMT had a positive effect on soil properties, owing to the fact that many types of bacteria (G+ and G–) can easily adapt to environmental changes (McCann et al., 1994; Zhang et al., 2018). G+ and G–produce spores, have thicker cell walls, and can adjust their cell wall structure, making them more adept at surviving environmental changes (McCann et al., 1994; Zhang et al., 2018). Therefore, SBM and CM significantly affected soil properties by changing the soil’s microbial communities.
AMF are an important group of soil microorganisms. In this study, SBMT and CMT had more AMF than RSMT or the CK group, which corresponded with previous research (Oehl et al., 2004; Manoharan et al., 2017). AMF help protect plants against soil pathogens (Sikes, Cottenie & Klironomos, 2009), increase plant drought tolerance (Auge et al., 2001), and provide plants with nutrients (Van der Heijden et al., 1998; Hill et al., 2010; Wagg et al., 2011), meaning that the lower amounts of AMF found in RSMT and CK groups may lead to reduced agricultural land quality and nutrient utilization in the follow-up crops.
Soil quality
In this study, we selected 18 indicators as potential soil quality indicators. Previous studies have also adopted an MDS when using an SQI (Qiu et al., 2019; Boafo et al., 2020), with the most commonly selected indicators being SOM, TN, and MBC (Abid & Lal, 2008; Liu et al., 2014; Bera et al., 2016). Selecting MDS indicators is contingent on various factors. In this study, we selected SOM and actinomycetes as MDS indicators. The SQI values varied across the four treatments and ranged from 0.47 to 0.94. The highest SQI value occurred in SBMT, followed by CMT and RSMT, indicating that SBM application is the most capable at maintaining soil quality.
Conclusions
Using a constant total N application rate, we compared RSMT, SBMT, and CMT to determine which fertilizer achieved the highest crop yields, economic growth, and levels of SOM and essential minerals (N, P, and K). We found the highest MBC and MBN levels in CMT, and the highest amount of PLFAs in SBMT. We selected SOM and actinomycetes as the MDS properties, and observed the highest SQI values in SBMT, followed by CMT and RSMT. Our results demonstrated that SBM and CM organic fertilizers may be better than RSM at improving crop yields and soil quality. This study provides a scientific basis for selecting effective organic fertilizers that can replace chemical fertilizers.
Supplemental Information
Acknowledgments
We thank Jianjin Jiang and his wife for providing facilities and accommodations. We are also grateful to the researchers who helped us during the study period.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2016YFC050708 and 2017YFC1700701) and the National Natural Science Foundation of China (No. 31670413). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Liyue Guo, Email: guoliyue@ibcas.ac.cn.
Gaoming Jiang, Email: jgm@ibcas.ac.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yu Cen and Liyue Guo conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Meizhen Liu and Xian Gu performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Caihong Li analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Gaoming Jiang conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiment was approved by the Plant Eco-physiological Research Group (PERG) in the Institute of Botany, Chinese Academy of Sciences (project number: 201707.8).
In this study, the name of the farmer is Jianjin Jiang who has a verbal agreement with the PERG in the Institute of Botany, Chinese Academy of Sciences. Although the farmland belongs to Jianjin Jiang, he has already rented it to the PERG. So, we should only get permission of the PERG. Any other permission from the individual and company is not needed.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File.
References
- Abid & Lal (2008).Abid M, Lal R. Tillage and drainage impact on soil quality: I. Aggregate stability, carbon and nitrogen pools. Soil and Tillage Research. 2008;100:89–98. doi: 10.1016/j.still.2008.04.012. [DOI] [Google Scholar]
- Akram et al. (2019).Akram U, Quttineh NH, Wennergren U, Tonderski K, Metson GS. Enhancing nutrient recycling from excreta to meet crop nutrient needs in Sweden-a spatial analysis. Scientific Reports. 2019;9:10264. doi: 10.1038/s41598-019-46706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews et al. (2002).Andrews SS, Mitchell JP, Mancinelli R, Karlen DL, Hartz TK, Horwath WR, Pettygrove GS, Scow KM, Munk DS. On-farm assessment of soil quality in California’s central valley. Agronomy Journal. 2002;94:12–23. doi: 10.2134/agronj2002.0012. [DOI] [Google Scholar]
- Auge et al. (2001).Auge RM, Stodola AJW, Tims JE, Saxton AM. Moisture retention properties of a mycorrhizal soil. Plant and Soil. 2001;230(1):87–97. doi: 10.1023/a:1004891210871. [DOI] [Google Scholar]
- Bao (2000).Bao SD. Soil and agricultural chemistry analysis. third ed. China Agriculture Press; Beijing: 2000. [in Chinese] [Google Scholar]
- Barker et al. (2019).Barker AV, Meagym MJ, Eaton TE, Jahanzad E, Bryson G. Improvement of mineral nutrient content of tomato through selection of cultivars and soil fertility. Agriculture, Ecosystems and Environment. 2019;42(8):928–941. doi: 10.1080/01904167.2019.1579840. [DOI] [Google Scholar]
- Barthès & Roose (2002).Barthès B, Roose E. Aggregate stability as an indicator of soil susceptibility to runoff and erosion, validation at several levels. Catena. 2002;47(2):133–149. doi: 10.1016/S0341-8162(01)00180-1. [DOI] [Google Scholar]
- Bera et al. (2016).Bera T, Collins HP, Alva AK, Purakayastha TJ, Patra AK. Biochar and manure effluent effects on soil biochemical properties under corn production. Applied Soil Ecology. 2016;107:360–367. doi: 10.1016/j.apsoil.2016.07.011. [DOI] [Google Scholar]
- Boafo et al. (2020).Boafo DK, Kraisornpornson B, Panphon S, Owusu BE, Amaniampong PN. Effect of organic soil amendments on soil quality in oil palm production. Applied Soil Ecology. 2020;147:103358. doi: 10.1016/j.apsoil.2019.09.008. [DOI] [Google Scholar]
- Bossio et al. (1998).Bossio DA, Scow KM, Gunapala N, Graham KJ. Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecology. 1998;36(1):1–12. doi: 10.1007/s002489900087. [DOI] [PubMed] [Google Scholar]
- Bremner & Mulvaney (1982).Bremner JM, Mulvaney CS. Nitrogen-total. In: A L, editor. Methods of soil analysis, Part 2. 2nd edition. ASA and SSSA; Madison: 1982. [Google Scholar]
- Brookes (1995).Brookes PC. The use of microbial parameters in monitoring soil pollution by heavy metals. Biology and Fertility of Soils. 1995;19(4):269–279. doi: 10.1007/bf00336094. [DOI] [Google Scholar]
- Chen et al. (2014).Chen XP, Cui ZL, Fan MS, Vitousek P, Zhao M, Ma WQ, Wang ZL, Zhang WJ, Yan XY, Yang JC, Deng XP, Gao Q, Zhang Q, Guo SW, Ren J, Li SQ, Ye YL, Wang ZH, Huang JL, Tang QY, Sun YX, Peng XL, Zhang JW, He MR, Zhu YJ, Xue JQ, Wang GL, Wu L, An N, Wu LQ, Ma L, Zhang WF, Zhang FS. Producing more grain with lower environmental costs. Nature. 2014;514(7523):486–489. doi: 10.1038/nature13609. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2016).Cheng JJ, Ding CF, Li XG, Zhang TL, Wang XX. Soil quality evaluation for navel orange production systems in central subtropical China. Soil and Tillage Research. 2016;155:225–232. doi: 10.1016/j.still.2015.08.015. [DOI] [Google Scholar]
- Clark & Tilman (2008).Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451(7179):712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- De Gryze et al. (2005).De Gryze S, Six J, Brits C, Merckx R. A quantification of short-term macroaggregate dynamics: influences of wheat residue input and texture. Soil Biology and Biochemistry. 2005;37(1):55–66. doi: 10.1016/j.soilbio.2004.07.024. [DOI] [Google Scholar]
- Ding & Han (2014).Ding XL, Han XZ. Effects of long-term fertilization on contents and distribution of microbial residues within aggregate structures of a clay soil. Biology and Fertility of Soils. 2014;50(3):549–554. doi: 10.1007/s00374-013-0867-6. [DOI] [Google Scholar]
- Doran & Parkin (1994).Doran JW, Parkin TB. Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA, editors. Defining soil quality for a sustainable environment. Soil Science Society of America Journal. Madison: 1994. pp. 3–21. [DOI] [Google Scholar]
- Ekblad & Nordgren (2002).Ekblad A, Nordgren A. Is growth of soil microorganisms in boreal forests limited by carbon or nitrogen availability? Plant and Soil. 2002;242(1):115–122. doi: 10.1023/a:1019698108838. [DOI] [Google Scholar]
- Elliott (1986).Elliott ET. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Science Society of America Journal. 1986;50(3):627–633. doi: 10.2136/sssaj1986.03615995005000030017x. [DOI] [Google Scholar]
- Fernández-Romero et al. (2016).Fernández-Romero ML, Lozano-García B, Parras-Alcántara L, Collins CD, Clark JM. Effects of land management on different forms of soil carbon in olive groves in Mediterranean areas. Land Degradation and Development. 2016;27(4):1186–1195. doi: 10.1002/ldr.2327. [DOI] [Google Scholar]
- Fierer, Schimel & Holden (2003).Fierer N, Schimel JP, Holden PA. Variations in microbial community composition through two soil depth profiles. Soil Biology and Biochemistry. 2003;35(1):167–176. doi: 10.1016/s0038-0717(02)00251-1. [DOI] [Google Scholar]
- Foley et al. (2011).Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC, Balzer C, Bennett EM, Carpenter SR, Hill J, Monfreda C, Polasky S, Rockstrom J, Sheehan J, Siebert S, Tilman D, Zaks DPM. Solutions for a cultivated planet. Nature. 2011;478(7369):337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- García-Orenes et al. (2012).García-Orenes F, Roldán A, Mataix-Solera J, Cerdà A, Campoy M, Arcenegui V, Caravaca F. Soil structural stability and erosion rates influenced by agricultural management practices in a semi-arid Mediterranean agro-ecosystem. Soil Use and Management. 2012;28(4):571–579. doi: 10.1111/j.1475-2743.2012.00451.x. [DOI] [Google Scholar]
- Gattinger et al. (2013).Gattinger A, Muller A, Haeni M, Skinner C, Fließbacha A, Buchmann N, Mäder P, Stolze M, Smith P, Scialabba NEH, Niggli U. Reply to leifeld et al.: enhanced top soil carbon stocks under organic farming is not equated with climate change mitigation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(11):E985–E985. doi: 10.1073/pnas.1221886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerliani, Hammami & Aïder (2019).Gerliani N, Hammami R, Aïder M. Assessment of the extractability of protein-carbohydrate concentrate from soybean meal under acidic and alkaline conditions. Food Bioscience. 2019;28:116–124. doi: 10.1016/j.fbio.2019.01.004. [DOI] [Google Scholar]
- Godfray et al. (2010).Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. Food security: the challenge of feeding 9 billion people. Science. 2010;327(5967):812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2010).Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS. Significant acidification in major Chinese croplands. Science. 2010;327(5968):1008–1010. doi: 10.1126/science.1182570. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2016).Guo LY, Wu GL, Li Y, Li CH, Liu WJ, Meng J, Liu HT, Yu XF, Jiang GM. Effects of cattle manure compost combined with chemical fertilizer on topsoil organic matter, bulk density and earthworm activity in a wheat-maize rotation system in Eastern China. Soil and Tillage Research. 2016;156:140–147. doi: 10.1016/j.still.2015.10.010. [DOI] [Google Scholar]
- Herrick (2000).Herrick JE. Soil quality: an indicator of sustainable land management? Applied Soil Ecology. 2000;15(1):75–83. doi: 10.1016/s0929-1393(00)00073-1. [DOI] [Google Scholar]
- Hill et al. (2010).Hill JO, Simpson RJ, Ryan MH, Chapman DF. Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop and Pasture Science. 2010;61(2):122–131. doi: 10.1071/CP09217. [DOI] [Google Scholar]
- IUSS Working Group WRB (2014).IUSS Working Group WRB . World Soil Resources Reports No. 106. FAO, Rome; 2014. World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. [Google Scholar]
- Joergensen & Wichern (2008).Joergensen RG, Wichern F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biology and Biochemistry. 2008;40(12):2977–2991. doi: 10.1016/j.soilbio.2008.08.017. [DOI] [Google Scholar]
- Ju et al. (2007).Ju XT, Kou CL, Christile P, Dou ZX, Zhang FS. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environmental Pollution. 2007;145(2):497–506. doi: 10.1016/j.envpol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Kaur, Kapoor & Gupta (2005).Kaur K, Kapoor KK, Gupta AP. Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. Journal of Plant Nutrition and Soil Science. 2005;168(1):117–122. doi: 10.1002/jpln.200421442. [DOI] [Google Scholar]
- Koch & Stockfisch (2006).Koch HJ, Stockfisch N. Loss of soil organic matter upon ploughing under a loses soil after several years of conservation tillage. Soil and Tillage Research. 2006;86(1):73–83. doi: 10.1016/j.still.2005.02.029. [DOI] [Google Scholar]
- Li et al. (2020).Li X, Zhang HH, Sun ML, Xu N, Sun GY, Zhao MC. Land use change from upland to paddy field in Mollisols drives soil aggregation and associated microbial communities. Applied Soil Ecology. 2020;146:103351. doi: 10.1016/j.apsoil.2019.09.001. [DOI] [Google Scholar]
- Liao, Boutton & Jastrow (2006).Liao JD, Boutton TW, Jastrow JD. Organic matter turnover in soil physical fractions following woody plant invasion of grassland: evidence from natural 13C and 15N. Soil Biology and Biochemistry. 2006;38(11):3197–3210. doi: 10.1016/j.soilbio.2006.04.004. [DOI] [Google Scholar]
- Liu et al. (2010).Liu EK, Yan CR, Mei XR, He WQ, Bing SH, Ding LP, Liu Q, Liu S, Fan TL. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma. 2010;158(3–4):0–180. doi: 10.1016/j.geoderma.2010.04.029. [DOI] [Google Scholar]
- Liu et al. (2016).Liu HT, Meng J, Bo WJ, Cheng D, Li Y, Guo LY, Li CH, Zheng YH, Liu MZ, Ning TY, Wu GL, Yu XF, Feng SF, Tana WY, Li J, Li LJ, Zeng Y, Liu SV, Jiang GM. Biodiversity management of organic farming enhances agricultural sustainability. Scientific Reports. 2016;6:23816. doi: 10.1038/srep23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu QW, Wang WL, Xu FL, Liu X. Effects of the application of biogas manure and soybean cake compost on yields and fruit quality of pear-jujube in mountainous region of loess plateau. Journal of northwest forestry university. 2012;27(4):39–42. [in Chinese] [Google Scholar]
- Liu et al. (2014).Liu ZJ, Wei Z, Shen JB, Li ST, Liang GQ, Wang XB, Sun JW, Chao A. Soil quality assessment of acid sulfate paddy soils with different productivities in Guangdong Province, China. Journal of Integrative Agriculture. 2014;13(1):177–186. doi: 10.1016/S2095-3119(13)60594-8. [DOI] [Google Scholar]
- Luttikholt (2007).Luttikholt LWM. Principles of organic agriculture as formulated by the International Federation of Organic Agriculture Movements. NJAS Wageningen Journal of Life Sciences. 2007;54(4):347–360. doi: 10.1016/S1573-5214(07)80008-X. [DOI] [Google Scholar]
- Ma & Joachim (2006).Ma SM, Joachim S. Review of history and recent development of organic farming worldwide. Agricultural Sciences in China. 2006;5(3):169–178. doi: 10.1016/S1671-2927(06)60035-7. [DOI] [Google Scholar]
- Manoharan et al. (2017).Manoharan L, Rosenstock NP, Williams A, Hedlund K. Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Applied Soil Ecology. 2017;115:53–59. doi: 10.1016/j.apsoil.2017.03.012. [DOI] [Google Scholar]
- McCann et al. (1994).McCann MC, Shi J, Roberts K, Carpita NC. Changes in pectin structure and localization during the growth of unadapted and nacl-adapted tobacco cells. The Plant Journal. 1994;5(6):773–785. doi: 10.1046/j.1365-313X.1994.5060773.x. [DOI] [Google Scholar]
- McGill (2015).McGill B. Land use matters. Nature. 2015;520(7549):38–39. doi: 10.1038/520038a. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2016).Meng J, Li LJ, Liu HT, Li Y, Li CH, Wu GL, Yu XF, Guo LY, Cheng D, Muminov MA, Liang XT, Jiang GM. Biodiversity management of organic orchard enhances both ecological and economic profitability. PeerJ. 2016;4:e2137. doi: 10.7717/peerj.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabiollahi et al. (2017).Nabiollahi K, Taghizadeh-Mehrjardi R, Kerry R, Moradian S. Assessment of soil quality indices for salt-affected agricultural land in Kurdistan Province, Iran. Ecological Indicators. 2017;83:482–494. doi: 10.1016/j.ecolind.2017.08.001. [DOI] [Google Scholar]
- Nelson & Sommers (1996).Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnson CT, Sumner ME, editors. Methods of soil analysis Part 3-chemical methods. Soil Science Society of America books series. vol. 5. Soil Science Society of America, Inc.; Madison: 1996. pp. 961–1010. [Google Scholar]
- Oehl et al. (2004).Oehl F, Sieverding E, Mäder P, Dubois D, Ineichen K, Boller T, Wiemken A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia. 2004;138(4):574–583. doi: 10.1007/s00442-003-1458-2. [DOI] [PubMed] [Google Scholar]
- Olsson (1999).Olsson PA. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiology Ecology. 1999;29(4):303–310. doi: 10.1016/s0168-6496(99)00021-5. [DOI] [Google Scholar]
- Peng et al. (2008).Peng Y, Zhou JH, Yang HQ, Hu ZM, Xiao S, Li Liu. Effects of specialty fertilizer combined with different organic manure fertilizers on growth and quality of flue-cured tobacco. Journal of Hunan Agricultural University. 2008;34(2):159–163. [in Chinese] [Google Scholar]
- Ponisio et al. (2015).Ponisio LC, M’Gonigle LK, Mace KC, Palomino J, Valpine Pde, Kremen C. Diversification practices reduce organic to conventional yield gap. Proceedings of the Royal Society B-Biological Sciences. 2015;282(1799):UNSP 20141396. doi: 10.1098/rspb.2014.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti, Rijk & Ittersum (2012).Ponti TD, Rijk B, Ittersum MKV. The crop yield gap between organic and conventional agriculture. Agricultural Systems. 2012;108:1–9. doi: 10.1016/j.agsy.2011.12.004. [DOI] [Google Scholar]
- Powlson & Brooks (1987).Powlson DS, Brooks PC. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biology and Biochemistry. 1987;19(2):159–164. doi: 10.1016/0038-0717(87)90076-9. [DOI] [Google Scholar]
- Qi et al. (2009).Qi YB, Darilek JL, Huang B, Zhao YC, Sun WX, Gu ZQ. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma. 2009;149:325–334. doi: 10.1016/j.geoderma.2008.12.015. [DOI] [Google Scholar]
- Qiu et al. (2019).Qiu XC, Peng DL, Wang HB, Wang ZY, Cheng S. Minimum data set for evaluation of stand density effects on soil quality in larix principis-rupprechtii plantations in north china. Ecological Indicators. 2019;103:236–247. doi: 10.1016/j.ecolind.2019.04.010. [DOI] [Google Scholar]
- Reganold et al. (2001).Reganold JP, Glover JD, Andrews PK, Hinman HR. Sustainability of three apple production systems. Nature. 2001;410(6831):926–930. doi: 10.1038/35073574. [DOI] [PubMed] [Google Scholar]
- Rojas et al. (2016).Rojas JM, Prause J, Sanzano GA, Arce OEA, Sánchez MC. Soil quality indicators selection by mixed models and multivariate techniques in deforested areas for agricultural use in NW of Chaco, Argentina. Soil and Tillage Research. 2016;155:250–262. doi: 10.1016/j.still.2015.08.010. [DOI] [Google Scholar]
- Schollenberger & Simon (1945).Schollenberger CJ, Simon RH. Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Science. 1945;59(1):13–24. doi: 10.1097/00010694-194501000-00004. [DOI] [Google Scholar]
- Seufert, Ramankutty & Foley (2012).Seufert V, Ramankutty N, Foley JA. Comparing the yields of organic and conventional agriculture. Nature. 2012;485(7397):229–232. doi: 10.1038/nature11069. [DOI] [PubMed] [Google Scholar]
- Shan et al. (2010).Shan WX, Luo W, Xiao RL, He QH, Chen P, Xu HQ. Effect of 5-year rapeseed cake fertilization and straw mulching on tea plantation soil ecosystem. Chinese Journal of Eco-Agriculture. 2010;18(3):472–476. doi: 10.3724/SP.J.1011.2010.00472. [in Chinese] [DOI] [Google Scholar]
- Sikes, Cottenie & Klironomos (2009).Sikes BA, Cottenie K, Klironomos JN. Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. Journal of Ecology. 2009;97(6):1274–1280. doi: 10.2307/27754321. [DOI] [Google Scholar]
- Tejada & Benítez (2020).Tejada M, Benítez C. Effects of different organic wastes on soil biochemical properties and yield in an olive grove. Applied Soil Ecology. 2020;146:103371. doi: 10.1016/j.apsoil.2019.103371. [DOI] [Google Scholar]
- Tilman et al. (2002).Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Tripathi et al. (2019).Tripathi AD, Mishra R, Maurya KK, Singh RB, Wilson DW. Estimates for world population and global food availability for global health. In: Singh RB, Watson RR, Takahashi T, editors. The role of functional food security in global health. London: Academic Press; 2019. [Google Scholar]
- Tu et al. (2006).Tu C, Louws FJ, Creamer NG, Mueller JP, Brownie C, Fager K, Bell M, Hu SJ. Responses of soil microbial biomass and N availability to transition strategies from conventional to organic farming systems. Agriculture Ecosystem and Environment. 2006;113(1–4):206–215. doi: 10.1016/j.agee.2005.09.013. [DOI] [Google Scholar]
- USEPA (1986).United States Environmental Protection Agency (USEPA) National Technical Information Service; EPA/SW-846, Springfield: 1986. Test methods for evaluating solid waste (Volume IA, 3rd ed.) [Google Scholar]
- Van der Heijden et al. (1998).Van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemkenand A, Sanders IR. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature. 1998;396(6706):69–72. doi: 10.1038/23932. [DOI] [Google Scholar]
- Vance, Brookes & Jenkinson (1987).Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry. 1987;19(6):703–707. doi: 10.1016/0038-0717(87)90052-6. [DOI] [Google Scholar]
- Vance & Chapin (2001).Vance ED, Chapin FS. Substrate limitations to microbial activity in taiga forest floors. Soil Biology and Biochemistry. 2001;33(2):173–188. doi: 10.1016/s0038-0717(00)00127-9. [DOI] [Google Scholar]
- Wagg et al. (2011).Wagg C, Jansa J, Stadler M, Schmid B, van der Heijden MGA. Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology. 2011;92(6):1303–1313. doi: 10.1890/10-1915.1. [DOI] [PubMed] [Google Scholar]
- Watanabe & Olsen (1965).Watanabe FS, Olsen SR. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society of America Journal. 1965;29(6):677–678. doi: 10.2136/sssaj1965.03615995002900060025x. [DOI] [Google Scholar]
- Wilson et al. (2009).Wilson GWT, Rice CW, Rillig MC, Springer A, Hartnett DC. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: results from long-term field experiments. Ecology Letters. 2009;12(5):452–461. doi: 10.1111/j.1461-0248.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang ZC, Zhao N, Huang F, Lv YZ. Long-term effects of different organic and inorganic fertilizer treatments on soil organic carbon sequestration and crop yields on the North China Plain. Soil and Tillage Research. 2015;146:47–52. doi: 10.1016/j.still.2014.06.011. [DOI] [Google Scholar]
- Yu et al. (2015).Yu C, Hu XM, Deng W, Li Y, Xiong C, Ye CH, Han GM, Li X. Changes in soil microbial community structure and functional diversity in the rhizosphere surrounding mulberry subjected to long-term fertilization. Applied Soil Ecology. 2015;86:30–40. doi: 10.1016/j.apsoil.2014.09.013. [DOI] [Google Scholar]
- Yu et al. (2012).Yu HY, Ding WX, Luo JF, Geng RL, Cai ZC. Long-term application of organic manure and mineral fertilizers on aggregation and aggregate-associated carbon in a sandy loam soil. Soil and Tillage Research. 2012;124:170–177. doi: 10.1016/j.still.2012.06.011. [DOI] [Google Scholar]
- Zhang et al. (2020).Zhang QQ, Song YF, Wu Z, Yan XY, Gunina A, Kuzyakov Y, Xiong ZQ. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. Journal of Cleaner Production. 2020;242:UNSP 118435. doi: 10.1016/j.jclepro.2019.118435. [DOI] [Google Scholar]
- Zhang et al. (2018).Zhang XF, Xin XL, Zhu AN, Yang WL, Zhang JB, Ding SJ, Mu L, Shao LL. Linking macroaggregation to soil microbial community and organic carbon accumulation under different tillage and residue managements. Soil and Tillage Research. 2018;178:99–107. doi: 10.1016/j.still.2017.12.020. [DOI] [Google Scholar]
- Zhang et al. (2004).Zhang B, Zhang Y, Chen D, White RE, Li Y. A quantitative evaluation system of soil productivity for intensive agriculture in China. Geoderma. 2004;123:319–331. doi: 10.1016/j.geoderma.2004.02.015. [DOI] [Google Scholar]
- Zhao et al. (2018).Zhao HL, Shar AG, Li S, Chen YL, Shi JL, Zhang XY, Tian XH. Effect of straw return mode on soil aggregation and aggregate carbon content in an annual maize-wheat double cropping system. Soil and Tillage Research. 2018;175:178–186. doi: 10.1016/j.still.2017.09.012. [DOI] [Google Scholar]
- Zhen et al. (2014).Zhen Z, Liu HT, Wang N, Guo LY, Meng J, Ding N, Wu GL, Jiang GM. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLOS ONE. 2014;9(10):e108555. doi: 10.1371/journal.pone.0108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. (2018).Zhu QC, Liu XJ, Hao TX, Zeng MF, Shen JB, Zhang FS, Vries WD. Modeling soil acidification in typical Chinese cropping systems. Science of the Total Environment. 2018;613–614:1339–1348. doi: 10.1016/j.scitotenv.2017.06.257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available as a Supplemental File.