Abstract
Background
In pandemics such as COVID-19, shortages of personal protective equipment are common. One solution may be to decontaminate equipment such as facemasks for reuse.
Aim
To collect and synthesize existing information on decontamination of N95 filtering facepiece respirators (FFRs) using microwave and heat-based treatments, with special attention to impacts on mask function (aerosol penetration, airflow resistance), fit, and physical traits.
Methods
A systematic review (PROSPERO CRD42020177036) of literature available from Medline, Embase, Global Health, and other sources was conducted. Records were screened independently by two reviewers, and data was extracted from studies that reported on effects of microwave- or heat-based decontamination on N95 FFR performance, fit, physical traits, and/or reductions in microbial load.
Findings
Thirteen studies were included that used dry/moist microwave irradiation, heat, or autoclaving. All treatment types reduced pathogen load by a log10 reduction factor of at least three when applied for sufficient duration (>30 s microwave, >60 min dry heat), with most studies assessing viral pathogens. Mask function (aerosol penetration <5% and airflow resistance <25 mmH2O) was preserved after all treatments except autoclaving. Fit was maintained for most N95 models, though all treatment types caused observable physical damage to at least one model.
Conclusions
Microwave irradiation and heat may be safe and effective viral decontamination options for N95 FFR reuse during critical shortages. The evidence does not support autoclaving or high-heat (>90°C) approaches. Physical degradation may be an issue for certain mask models, and more real-world evidence on fit is needed.
Keywords: Personal protective equipment, N95, Filtering facepiece respirator, Decontamination, Pandemic, COVID-19
Introduction
During the SARS-CoV-2 pandemic, protecting frontline healthcare workers is of the utmost importance. As SARS-CoV-2 can be transmitted through airborne particles, the US Centers for Disease Control and Prevention (CDC) and the Public Health Agency of Canada (PHAC) have recommended the use of N95 filtering facepiece respirators (FFRs) when performing aerosol-generating procedures on suspected COVID-19 patients [[1], [2], [3]]. N95 FFRs filter out a minimum of 95% of airborne particles and are the personal protective equipment (PPE) preferred by healthcare workers during serious outbreaks of aerosol-borne viruses [4,5].
It is widely understood that single-use of FFRs is not sustainable in a pandemic such as COVID-19 [[6], [7], [8]]. FFR decontamination has been proposed as a safer method than standard ‘limited reuse’ [9], which involves no disinfection between wears [10,11]. However, any decontamination method must preserve the structural and functional characteristics of the mask (namely fit, aerosol penetration, and airflow resistance) or it may increase risk to healthcare workers [12]. The lack of clear consensus on how to achieve safe decontamination of single-use FFRs has discouraged manufacturers and public health experts from endorsing decontamination protocols [13], although several analyses of FFR-decontamination methods have been published and the CDC has provided suggestions for decontamination in critical situations [14]. Previous work has evaluated methods including radiation (ultraviolet-C, microwaves), moist heat (autoclaves), and chemical disinfectants (bleach, ethanol, hydrogen peroxide) [[15], [16], [17], [18]], which vary in their relative efficacies and feasibilities. For example, not all institutions have access to large UV lamps, and chemical disinfection may cause significant damage to FFRs or leave hazardous residues [19,20]. Microwaves and heat are known to inactivate viruses and bacteria [[21], [22], [23], [24], [25]], including coronaviruses [26,27], and can be accessible and affordable [28]; however, heat and humidity may impact the electrostatic charges that confer the high filtration efficiency of the polypropylene filter in N95 FFRs [[29], [30], [31]].
To help inform FFR-reuse policies and procedures, our team conducted three systematic reviews to synthesize existing published data regarding the effectiveness of ultraviolet germicidal irradiation (UVGI), heat, microwave irradiation, and chemical disinfectants for N95 FFR decontamination [17,32]. This review will focus on microwave- and heat-based decontamination with the following objectives: (1) to assess the effects of microwave irradiation and heat on FFR performance, with specific foci on aerosol penetration and airflow resistance; (2) to determine how effectively microwave irradiation and heat reduce viral or bacterial load on FFRs; and (3) to describe changes in FFR fit or physical traits caused by microwave irradiation or heat.
Methods
The study methods were established a priori. The protocol was submitted to PROSPERO on 29th March 2020 (CRD42020177036) and uploaded to Open Science Framework (OSF) on 30th March 2020 (https://osf.io/4se6b/) [33]. This systematic review is reported according to PRISMA guidelines (Supplementary Material) [34].
Eligibility criteria
Eligible studies met the following criteria: (1) study was an original article or systematic review; (2) study investigated decontamination of N95 (including surgical N95) filtering facepiece respirators or their components; (3) study included a decontamination arm involving microwave irradiation or heat treatment; (4) at least one of the following post-treatment outcomes was reported: (i) FFR performance (aerosol penetration, airflow resistance); (ii) reduction in viral/bacterial load; (iii) mask fit; (iv) changes in physical traits. Articles also had to be available in English or French and published after 1972, the first year that an FFR was approved by the National Institute for Occupational Safety and Health (NIOSH) [35]. We excluded abstract-only publications, study protocols, guidelines, commissioned reports, editorials, narrative reviews, book chapters, and patents.
Database search and study selection
Two health sciences librarians (L.S. and M.S.) searched the following databases for relevant literature: Medline and Medline in Process via OVID, Embase Classic + Embase via OVID, and Global Health via CAB Direct. A search strategy was developed in Medline, and then translated into the other databases as appropriate (Supplementary Material). All databases were searched from 1st January 1972 to 29th March 2020 for English and French publications.
Two journals were also hand-searched, as they were particularly relevant to the review but are not indexed in any of the aforementioned databases: Journal of the International Society for Respiratory Protection and Journal of Engineered Fibers and Fabrics. Two authors (M. S. Bergman and D. J. Viscusi) had been previously identified as publishing frequently on mask decontamination; Scopus was searched 29th March 2020 for those authors and N95-related terms. A search of Google Scholar (29th March 2020) yielded 1630 hits. The first 1000 were downloaded to Publish or Perish and screened until 50 consecutive irrelevant records were found. Records up to that point were saved as an RIS file and edited to remove patents, reports and books. The WHO database on COVID-19 (29th March 2020 edition) was searched. Disaster Lit: Database for Disaster Medicine and Public Health, MedRxiv and OSF Registries were searched 29th March 2020 for the term “N95” and records pertaining to decontamination were selected and downloaded. All references were entered into an Endnote file where duplicate records were removed. Following screening, one librarian (M.S.) reviewed the reference lists of included studies to identify any potentially relevant studies not included in the screening set.
Citation screening and data extraction
Titles and abstracts were uploaded to InsightScope (www.insightscope.ca) for title/abstract and full-text screening. At both levels of screening, citations were assessed independently in duplicate by a team of six reviewers from CHEO (a pediatric academic hospital in Ottawa, Canada), the University of Ottawa, and McMaster University. To ensure that all reviewers understood the eligibility criteria, the study leads (S.G., A.A.) constructed a test set of 30 citations in which five met all study criteria (true positives) and 25 did not (true negatives). Before gaining access to title/abstract screening, each reviewer was required to complete the test set and achieve a sensitivity of at least 80%. At both title/abstract and full-text screening, records were removed only if both reviewers agreed to exclude; any conflicts were reviewed and resolved by one of the study leads. Subsequently, the study leads reviewed the eligible citations to eliminate duplicates and confirm eligibility. The study leads developed an extraction tool for demographic and methodology data using REDCap tools hosted at CHEO and piloted the tool on five eligible studies [36,37]. Based on the data collected on REDCap, the study leads created and piloted spreadsheets (Microsoft Excel) to collect data on post-decontamination aerosol penetration, airflow resistance, germicidal effects, fit, and physical traits. In both phases of data extraction, eligible studies were divided equally among the reviewers for duplicate, independent extraction, followed by conflict resolution by the study leads. Data from figures were extracted by one reviewer using SourceForge Plot Digitizer (http://plotdigitizer.sourceforge.net/) and cross-verified by a second reviewer. Extracted data and meta-data of all records screened are available on OSF [38].
Study analysis and statistics
All statistical analyses were performed using the R statistical programming language [39]. Where two or more studies measured the same outcome using the same intervention type, cross-study data was meta-analysed using a random effects model with the R package ‘meta’ [40]. Variability between point estimates of studies was calculated by taking the standard deviation (aerosol penetration and airflow resistance) or standard error (germicidal effects) across the means. Heterogeneity was assessed using an I 2 statistic; if I 2 ≥75%, the pooled estimate was not reported.
Where standard deviation or standard error were not reported and could not be calculated across the means, generic imputation was used. If no arms within a study had a value for uncertainty, the average value between studies was imputed for missing data. For studies that performed the same decontamination intervention on different study arms (e.g., variable mask types, durations of exposure, heat temperatures, transmission modes), within-study data were averaged.
Germicidal data was reported as log10 pathogen reduction factor calculated from absolute pathogen loads, or relative survival if the log10 reduction factor was not reported directly in the article. For values below minimum detectable limits, we adopted the strategy described by Heimbuch et al. for imputation of log10 reduction factor: “Based on a US Environmental Protection Agency guideline [41], half of the limit of detection was used to calculate log reductions for treated samples that had no detectable virus” [15]. For Fisher et al.’s 2011 study [42], the difference in viral load was used without a detection limit correction [41] as there was inadequate information to perform an adjustment. For studies that reported a final pathogen load of zero and no limit of detection, log10 reduction factor was calculated as the log10 of the control pathogen load (i.e. it was assumed that all virus was inactivated).
Outcomes
Mask performance was evaluated based on percentage aerosol penetration through the mask, equivalent to 100% minus the mask's filtration efficiency, and initial airflow resistance, which is the pressure drop across the mask. Evidence of success for mask performance outcomes was defined as less than 5% aerosol penetration (i.e. at least 95% filtration efficiency) and airflow resistance under 25 mmH2O in accordance with NIOSH certification standards [4,43]. Pathogen log10 reduction factor of at least three, which is sufficient to fully decontaminate the highest levels of viral contamination that are predicted to occur in hospital settings [44], was considered a successful germicidal effect. Success thresholds for fit and physical traits were a fit factor (FF) of at least 100 as per Occupational Safety and Health Administration (OSHA) testing guidelines [45], and no observable changes to the mask, respectively.
Risk of bias
Risk of bias was assessed for each outcome in all included studies using criteria that were predetermined by the authors relating to study design, methodological consistency, population heterogeneity, sampling bias, outcome evaluation, and selective reporting (Supplementary Material). Given the absence of an accepted standard risk of bias assessment tool for laboratory studies, we created a tool with domains applicable to FFR decontamination studies, adapted from the Cochrane risk-of-bias tool for randomized trials [46].
Results
Study selection
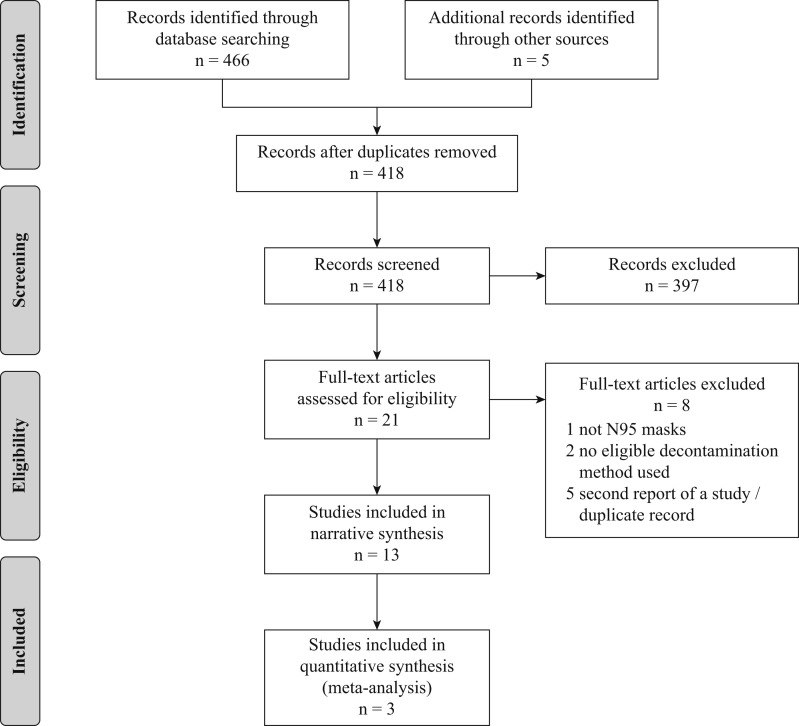
The initial database and journal searches identified 466 and three records, respectively, and two additional studies were identified via consultation with leaders in the field (Figure 1 ). After duplicate removal, 418 unique records remained for screening. All six reviewers achieved a sensitivity of 100% on the test set before beginning screening. The review team excluded 397 records at the title/abstract level (κ = 0.79). Three records were excluded after full-text review, resulting in 18 reports representing 13 unique studies eligible for inclusion (κ = 0.77). No additional studies were found from checking reference lists of included manuscripts.
Figure 1.
PRISMA flow diagram of search and screening process.
Study characteristics
Thirteen studies were included in this review (Table I ). The studies were published between 2007 and 2020, and all were performed in the USA except one from Canada and two from Taiwan. The two studies that investigated the novel coronavirus were published as pre-prints and not yet peer-reviewed at the time of inclusion [47,48]. Microwave and heat-based interventions were investigated in nine and 11 studies, respectively. Sixteen different mask models were used across the studies, with the 3M 1860 (N = 8), 3M 1870 (N = 7), 3M 8210 (N = 7), and 3M 8000 (N = 5) being the most commonly tested.
Table I.
Characteristics of studies included in this systematic review of microwave- and heat-based decontamination of N95 filtering facepiece respirators
| First author | Year | Region of origin | Number of decontamination conditionsa |
Number of inoculation conditionsa (varied parameter) | Number of unique N95 models | Outcomes evaluated |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | Heat | Aerosol penetration | Airflow resistance | Germicidal effect (pathogen) | Fit | Physical traits | |||||
| Bergman | 2010 | USA | 1 | 1 | – | 6 | Yes | Yes | No | No | Yes |
| Bergman | 2011 | USA | 1 | 1 | – | 3 | No | No | No | Yes | Yes |
| Fischer | 2020 | USA | – | 4 | 1 | 2 | No | No | Yes (SARS-CoV-2) | Yes | No |
| Fisher | 2009 | USA | 5 | – | 2 (inoculation medium) | 1 | No | No | Yes (MS2) | No | No |
| Fisher | 2011 | USA | 2 | – | 1 | 6 | Yes | No | Yes (MS2) | No | Yes |
| Heimbuch | 2011 | USA | 1 | 1 | 2 (transmission mode) | 6 | No | No | Yes (H1N1) | No | Yes |
| Kumar | 2020 | Canada | – | 1 | 1 | 4 | No | No | Yes (SARS-CoV-2) | Yes | Yes |
| Lin | 2017 | Taiwan | – | 2 | – | 1 | Yes | Yes | No | No | Yes |
| Lin | 2018 | Taiwan | – | 2 | 1 | 1 | No | No | Yes (Bacillus subtilis) | No | No |
| Lore | 2012 | USA | 1 | 1 | 1 | 2 | Yes | No | Yes (H5N1) | No | No |
| Viscusi | 2007 | USA | 2 | 4 | – | 1 | Yes | No | No | No | Yes |
| Viscusi | 2009 | USA | 1 | 5 | – | 6 | Yes | Yes (MW only) | No | No | Yes |
| Viscusi | 2011 | USA | 1 | 1 | – | 6 | No | No | No | Yes | Yes |
MW, microwave.
Numbers of decontamination and inoculation conditions were determined by the authors of this systematic review. Distinct decontamination conditions were defined as any treatments with differing parameters (e.g., variable temperature or duration) excluding different numbers of cycles. Distinct inoculation conditions included the use of different inoculation media or variable modes of transmission (i.e. droplet versus aerosol).
Microwave and heat treatments were performed in dry conditions or with the addition of moisture. Two studies used dry microwave treatment [18,49], and seven included a reservoir of water or steam bag within the microwave chamber, creating microwave-generated steam (MGS) [15,42,[50], [51], [52], [53], [54]]. Five studies used dry heat (oven or rice cooker) [16,18,19,47,49], five employed moist heat incubation (MHI) by adding water reservoirs inside ovens or using laboratory incubators [15,[50], [51], [52], [53]], and four used an autoclave [16,19,48,49].
N95 mask function (aerosol penetration and airflow resistance)
Aerosol penetration
Almost all studies that measured aerosol penetration [18,42,49,50,53] utilized a neutralized solid polydisperse sodium chloride aerosol (count median diameter (CMD) = 75 ± 20 nm, geometric standard deviation (GSD) ≤1.86) and a flow rate of 85 L/min over full masks as per NIOSH certification testing procedures [4]. The exception was Lin et al., who used a lower flow rate (5.95 L/min) to generate equivalent surface velocity on smaller mask segments, and measured penetration of a range of particle sizes using a neutralized potassium sodium tartrate tetrahydrate aerosol (CMD = 101 ± 10 nm, GSD = 2.01 ± 0.08) [19].
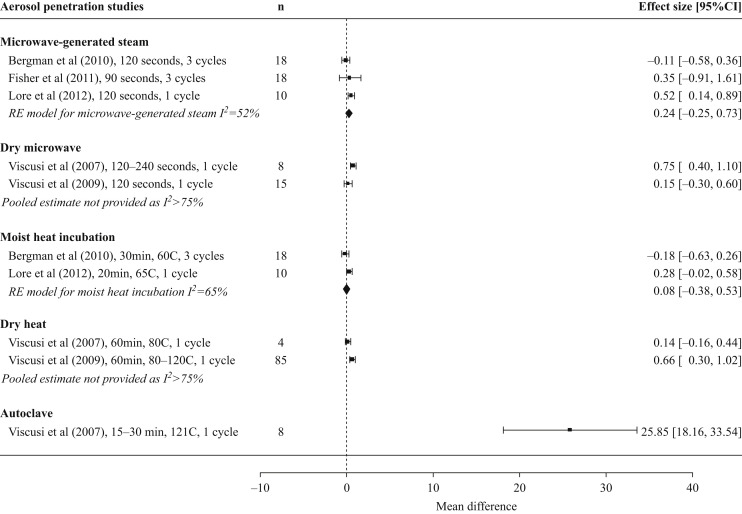
There were five studies that assessed aerosol penetration post-microwave intervention (Figure 2 , Table II ) [18,42,49,50,53], three using moist conditions (MGS) and two using dry conditions. All microwave interventions, which ranged from 90 to 240 s in duration, led to small increases in aerosol penetration, but post-treatment values maintained NIOSH certification standards (<5% penetration) [4].
Figure 2.
Impact of microwave and heat decontamination interventions on aerosol penetration through N95 filtering facepiece respirators. Treatment replicates are denoted by n. Horizontal axis and effect sizes represent the differences in percentage aerosol penetration between untreated and treated masks. Within-study data for different masks and treatment parameters are averaged to yield a single effect size. Results are only depicted for studies that used National Institute for Occupational Safety and Health certification testing procedures. RE, random effects.
Table II.
Microwave and heat intervention parameters and N95 filtering facepiece respirators for which post-decontamination performance outcomes (aerosol penetration and airflow resistance) were evaluated
| First author, year | Powera/Temperature | Time | Cycles | Moisture | Pressure | N95 models | Performance outcomes | Summary of resultsb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Microwave |
Bergman, 2010 |
750 W/ft3 |
120 s |
3 |
MGS |
Room |
3M 8000c 3M 8210c Moldex 2201c |
KC PFR95-174c 3M 1860c 3M 1870c |
Aerosol penetration Airflow resistance |
%P<3 for all models AR <15 mmH2O for all models |
| Fisher, 2011 | 750 W/ft3 | 90 s | 1 or 3 | MGS, steam bag Xd | Room | Cardinal Health 3M 8210 Moldex 2200 |
KC PFR95 3M 1860 3M 1870 |
Aerosol penetration | %P<5 for all models/conditions | |
| 3 |
MGS, steam bag Yd |
|||||||||
| Lore, 2012 |
1250 We |
120 s |
1 |
MGS |
Room |
3M 1860 3M 1870 |
Aerosol penetration |
%P<2 for all models |
||
| Viscusi, 2007 | 750 W/ft3 | 120 s | 1 | Dry | Room | 3M 8000c | Aerosol penetration | %P<2 for all conditions | ||
| 240 s | ||||||||||
| Viscusi, 2009 |
750 W/ft3 |
120 s |
1 |
Dry |
Room |
3M 8000c 3M 8210c Moldex 2200c |
KC PFR95-270c 3M 1860c 3M 1870c |
Aerosol penetration Airflow resistance |
%P<2 for all models AR ≤9 mmH2O for all models (3M 1870 not measured due to melting) |
|
| Heat | Bergman, 2010 |
60°C |
30 min |
3 |
MHI (80% RH) |
Room |
3M 8000c 3M 8210c Moldex 2201c |
KC PFR95-174c 3M 1860c 3M 1870c |
Aerosol penetration Airflow resistance |
%P<3 for all models AR ≤15 mmH2O for all models |
| Lin, 2017 | 149–164°C |
3 min |
1 |
Dry |
Room |
3M 8210 | Aerosol penetration Airflow resistance |
%P<3 for all conditions AR <11 mmH2O for all conditions |
||
| 121°C |
15 min |
1 |
Steam (autoclave) |
1.06 kg/cm2 |
||||||
| Lore, 2012 |
65°C |
20 min |
1 |
MHI (RH unspecified) |
Room |
3M 1860 3M 1870 |
Aerosol penetration |
%P<2 for all models |
||
| Viscusi, 2007 | 80°C | 60 min | 1 | Dry | Room | 3M 8000c | Aerosol penetration | 80°C: %P<1 160°C: mask not measured due to melting |
||
| 160°C | ||||||||||
| 121°C | 15 min | 1 | Steam (autoclave) | 1.05 kg/cm2 | Autoclave: %P>18 for both conditions | |||||
| 30 min | ||||||||||
| Viscusi, 2009 | 80°C | 60 min | 1 | Dry | Room | 3M 8000c 3M 8210c Moldex 2200c |
KC PFR95-270c 3M 1860c 3M 1870c |
Aerosol penetration | 80–100°C: %P ≤ 2 for all models 110–120°C: %P<5 for all models except KC PFR95-270 at 110°C (%P=5.4)f |
|
| 90°C | ||||||||||
| 100°C | ||||||||||
| 110°C | ||||||||||
| 120°C | ||||||||||
%P, percentage aerosol penetration; AR, airflow resistance; KC, Kimberly–Clark; MGS, microwave-generated steam; MHI, moist heat incubation; RH, relative humidity.
If both manufacturer-rated and experimentally-determined microwave power were provided, the experimental value is reported here.
Greater-/less-than values are reported to the nearest whole number. Outcome successes are defined as aerosol penetration <5% and airflow resistance <25 mmH2O.
Mask models were anonymized in article. Model names were obtained by e-mail from R. Shaffer in April 2020.
Steam Bag X = Medela Quick Clean™ MICRO-STEAM™ BAGS; Steam Bag Y = Munchkin® Steam Guard™ Bags.
Power units per volume not specified.
Data for the KC PFR95-270 at 110oC was obtained from only one replicate instead of three due to melting of the other two.
Five studies assessed aerosol penetration after heat treatment (Figure 2, Table II) [18,19,49,50,53], four of which had at least one moist condition (MHI or autoclave). MHI was applied for 20–30 min [50,53] and, for all mask models, the increase in aerosol penetration was small (<1%) and remained within NIOSH certification standards (<5% penetration) [4]. Results in autoclave conditions varied: in one study no increase was noted after 15-min treatment [19], while another noted increases of over 18% and 34% for 15- and 30-min treatments, respectively [49]. Three studies examined aerosol penetration post-dry heat treatment and reported small increases with all final values remaining within NIOSH certification standards except the Kimberly–Clark PFR95-270 after 60 min at 110°C (5.4% penetration) [18,19,49].
Airflow resistance (pressure drop)
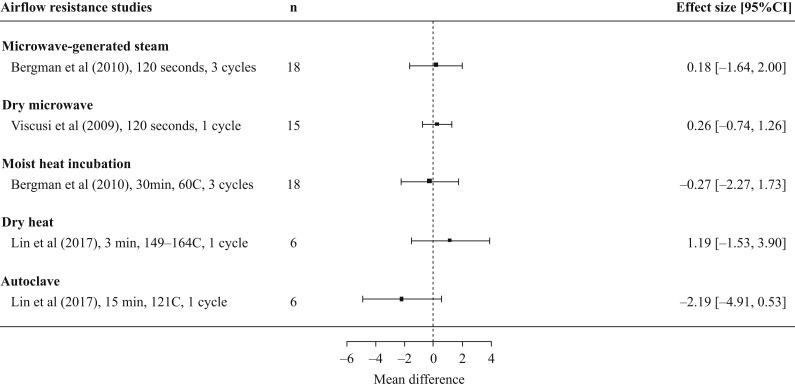
Three studies examined airflow resistance simultaneously with aerosol penetration (Figure 3 , Table II) [18,19,50]; of these, there were two microwave decontamination arms (one moist and one dry) and three heat arms (one MHI, one dry, and one autoclave). Initial resistance to airflow was reported in millimetres of water column height pressure. Where testing was performed, minimal to no increase in airflow resistance was noted, and all final values were within NIOSH guidelines (<25 mmH2O) [43].
Figure 3.
Impact of microwave and heat decontamination interventions on airflow resistance (pressure drop) of N95 filtering facepiece respirators. Treatment replicates are denoted by n. Horizonal axis and effect sizes represent the differences in airflow resistance between untreated and treated masks, expressed in mmH2O. Within-study data for different masks and treatment parameters are averaged to yield a single effect size.
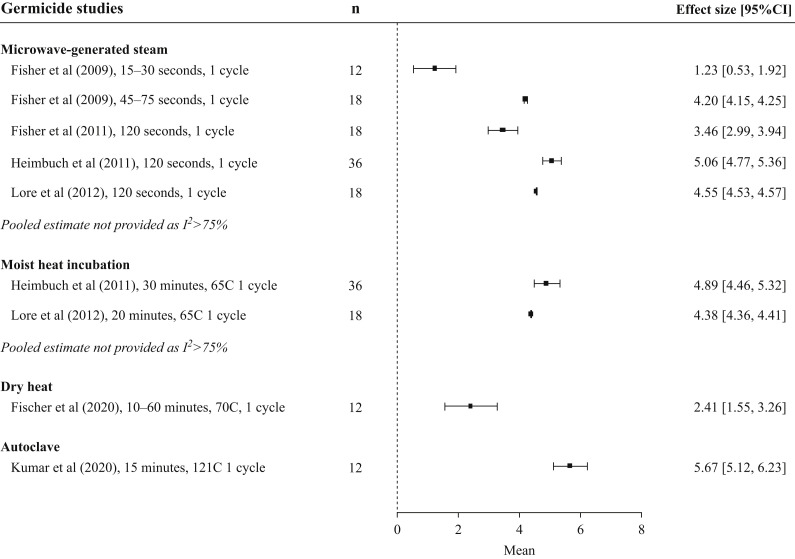
Germicidal effects
Seven studies evaluated reductions in pathogen load after microwave or heat interventions (Figure 4 , Table III ) [15,16,42,47,48,53,54]. One study used a bacterial pathogen (Bacillus subtilis) [16] and all others used viruses: SARS-CoV-2 [47,48], Influenza A subtype H1N1 [15] or H5N1 [53], and Escherichia virus MS2 [42,54].
Figure 4.
Germicidal effect of microwave and heat decontamination interventions on viral pathogens. Treatment replicates are denoted by n. Horizontal axis and effect sizes represent log10 viral reduction factors between untreated and treated masks. Within-study data for different masks and treatment parameters are averaged to yield a single effect size for all studies except Fisher et al. (2009), which was divided into two time ranges due to a significant increase in germicidal effect after the 30-s timepoint. Bactericidal measurements are not shown.
Table III.
Microwave and heat intervention parameters, inoculation conditions, and N95 filtering facepiece respirators for which germicidal effect was evaluated
| First author, year | Powera or temperature | Time | Cycles | Moisture | Pressure | Inoculation parameters |
N95 models | Summary of germicidal results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Inoculation medium | Transmission mode | ||||||||||
| Microwave |
Fisher, 2009 |
750 W/ft3 |
15 s 30 s 45 s 60 s 75 s |
1 |
MGS |
Room |
MS2 |
1% ATCC 271 |
Aerosol |
Cardinal Healthb |
Log10 reduction <2 after 15- and 30-s treatments Log10 reduction >4 after 45-, 60-, 75-s treatments |
|
| 100% ATCC 271 | ||||||||||||
| Fisher, 2011 |
750 W/ft3 |
90 s |
1 |
MGS, steam bag Xc |
Room |
MS2 |
100% ATCC 271 |
Droplet |
KC PFR95 3M 1870 Moldex 2200 |
Log10 reduction >3 for all models/conditions |
||
| MGS, steam bag Yc | ||||||||||||
| Heimbuch, 2011 |
1250 Wd |
120 s |
1 |
MGS |
Room |
H1N1 |
Mucin |
Aerosol |
3M 8000e 3M 8210e Moldex 1500e |
KC PFRe 3M 1860e 3M 1870e |
Log10 reduction >4 for all models/conditions |
|
| Droplet | ||||||||||||
| Lore, 2012 |
1250 Wd |
120 s |
1 |
MGS |
Room |
H5N1 |
NR |
Aerosol |
3M 1860 3M 1870 |
Log10 reduction >4 for both models |
||
| Heat | Fischer, 2020 |
70°C |
10 min 20 min 30 min 60 min |
1 |
Dry |
Room |
SARS-CoV-2 |
NR |
Droplet |
AOSafety N9504C |
Log10 reduction <3 after 10-, 20-, 30-min treatmentsf Log10 reduction >3 after 60-min treatmentf |
|
| Heimbuch, 2011 |
65°C |
30 min |
1 |
MHI (85% RH) |
Room |
H1N1 |
Mucin |
Aerosol | 3M 8000e 3M 8210e Moldex 1500e |
KC PFRe 3M 1860e 3M 1870e |
Log10 reduction >3 for all models/conditions |
|
| Droplet | ||||||||||||
| Kumar, 2020 |
121°C |
15 min |
1 |
Steam (autoclave) |
High (unspecified) |
SARS-CoV-2 |
NR |
Droplet |
3M 1860 3M Aura 1870 3M Vflex 1804S AOSafety 1054S |
Log10 reduction >5 for all modelsg |
||
| Lin, 2018 |
149–164°C |
3 min |
1 |
Dry |
Room |
Bacillus subtilis |
Water |
Aerosol |
3M 8210 |
Log10 reduction <3 after dry heath 0 cfu after AC (0% relative survival)i |
||
| 121°C |
15 min |
1 |
Steam (autoclave) |
1.05 kg/cm2 |
||||||||
| Lore, 2012 | 65°C | 20 min | 1 | MHI (RH unspecified) | Room | H5N1 | NR | Aerosol | 3M 1860 3M 1870 |
Log10 reduction >4 for both models | ||
AC, autoclave; cfu, colony-forming units; KC, Kimberly–Clark; MGS, microwave-generated steam; MHI, moist heat incubation; NR, not reported; RH, relative humidity.
If both manufacturer-rated and experimentally-determined microwave power were provided, the experimental value is reported here.
Mask models were anonymized in study. Model name was obtained by e-mail from R. Shaffer in April 2020.
Steam bag X = Medela Quick Clean™ MICRO-STEAM™ BAGS; steam bag Y = Munchkin® Steam Guard™ Bags.
Power units per volume not specified.
Mask models were anonymized in article. Model names were obtained by e-mail from B. Heimbuch in April 2020.
Log10 reduction calculated as Log10(TCID50/mL)control – Log10(TCID50/mL)treatment.
Study reported the mean viral recovery post-decontamination as 0 for each model, with no specified limit of detection. Therefore, the log10 reduction factor is assumed to be equivalent to the Log10(TCID50)control.
Log10 reduction calculated from relative survival rate.
Control bacterial load not reported, therefore log reduction cannot be calculated.
In the four studies that examined the germicidal effect of MGS [15,42,53,54], all arms demonstrated a log10 viral reduction factor greater than three except the two rapid-treatment arms (30 s or less) in Fisher et al.’s 2009 study [54]. All studies using heat treatment against viral pathogens (dry, MHI, and autoclave) also reported log10 reduction factors in excess of three [15,47,48,53], although this only occurred after 60 min at 70°C dry heat in Fischer et al.’s SARS-CoV-2 study and not at 10-, 20-, or 30-min timepoints [47]. Bacterial decontamination using rapid (3-min) high-temperature dry heat in Lin et al.’s study resulted in a log10 reduction factor of only 2.5, although this was increased to three after a 24-h incubation at ‘worst case’ temperature/humidity (37°C, 95% relative humidity) [16]. In the same study, no colonies grew post-autoclave treatment.
Fit
Four studies assessed FFR fit after microwave and/or heat treatment (Table IV ) [46,47,51,52]. Viscusi et al. abbreviated the standard OSHA fit test [45] from eight exercises to six [52]. An FF, scored from 1 (poor fit) to 200 (best fit), was calculated by measuring the ratio of ambient particle concentration outside the respirator to the particle concentration inside. Each subject donned each mask five times, with two replicates per model-treatment combination, and a multi-donning fit factor (MDFF10) was calculated as the harmonic mean of the 10 FFs. MDFF10 exceeded the passing threshold of 100 for all models after MGS and MHI treatments. Bergman et al. used an abbreviated OSHA fit test similar to Viscusi et al., but performed three cycles of decontamination with a single-donning fit test before the first treatment and after each of the three cycles [51]. The fit test pass rate after three MGS or MHI cycles was 95% for all models. Fischer et al. incorporated 2-h wear periods between each of three dry heat rounds and performed fit-testing using the official four-exercise modified OSHA protocol initially and after each decontamination-wear cycle; deterioration of fit was only seen in two (of six) replicates after the third treatment [47]. Kumar et al. fit-tested four N95 models after one, three, five, and ten autoclave cycles using normal and deep breathing exercises only [48]. Across all four studies, most replicates of all tested models maintained adequate fit for all interventions tested, with the exception of the 3M 1860 after multiple (>1) cycles of Kumar et al.’s autoclave treatment.
Table IV.
Impacts of microwave- and heat-based decontamination strategies on physical traits and fit of N95 filtering facepiece respirators
| First author, year | Intervention parametersa | N95 models | Method of physical assessment | Physical traitsb | Odour | Fitc | ||
|---|---|---|---|---|---|---|---|---|
| Microwave | Bergman, 2010 | Power: 750 W/ft3 Time: 120 s Cycles: 3 Moisture: MGS |
3M 8000d 3M 8210d Moldex 2201d |
KC PFR95-174d 3M 1860d 3M 1870d |
Physical traits: visual inspection Odour: smelling the mask |
3M 1870: slight separation of inner foam nose cushion (N = 3/3) KC PFR95-174: strap melted after first cycle (N = 2/3) |
No odour reported | – |
| Bergman, 2011 | Power: 750 W/ft3 Time: 120 s Cycles: 3 Moisture: MGS |
3M 1860 3M 1870 KC PFR95-270 |
Physical traits: visual inspection Fit: author-modified OSHA protocol FF ≥100 = pass; faceseal leakage = 1/FF |
3M 1870: slight separation of inner foam nose cushion (same degree after all cycles) KC PFR95-270: strap melted after third cycle (N = 1/21) |
– | Fit test pass rate remained ≥90% for all models (N = 20) Mean faceseal leakage remained <1% for all models (N = 20), no significant difference |
||
| Fisher, 2011 | Power: 750 W/ft3 Time: 90 s Cycles: 1, 3 Moisture: MGS (steam bag) |
Cardinal Health 3M 8210 3M 1860 3M 1870 KC PFR95 Moldex 2200 |
Physical traits: water retention evaluated by comparing initial mask weight to weight after MGS treatment and drying in room conditions | Cardinal Health, 3M 8210, 3M 1860: significant water (≥8 g) retained after 60 min drying (N = 1) Moldex 2200, KC PFR95, 3M 1870: low water absorbency, dry (≤0.1 ± 0.1 g) within 30 min (N = 3) |
– | – | ||
| Heimbuch, 2011 | Power: 1250 We Time: 120 s Cycles: 1 Moisture: MGS |
3M 8000f 3M 8210f Moldex 1500f |
KC PFRf 3M 1860f 3M 1870f |
Physical traits: visual inspection | 3M 1870: slight separation of inner foam nose cushion | – | – | |
| Viscusi, 2007 | Power: 750 W/ft3 Time: 120 s, 240 s Cycles: 1 Moisture: Dry |
3M 8000d | Physical traits: visual and tactile inspection | 120s: no visible changes (N = 4/4) 240s: filter media melted at ends of metallic nosebands and formed holes |
– | – | ||
| Viscusi, 2009 | Power: 750 W/ft3 Time: 120 s Cycles: 1 Moisture: Dry |
3M 8000d 3M 8210d Moldex 2200d |
KC PFR95-270d 3M 1860d 3M 1870d |
Physical traits: visual and tactile inspection Odour: smelling the mask |
3M 1870: filtration media melted in areas adjacent to metallic nosebands (N = 3/3) | No odour reported | – | |
| Viscusi, 2011 |
Power: 750 W/ft3 Time: 120 s Cycles: 1 Moisture: MGS |
3M 8000 3M 8210 3M 1860 3M 1870 KC PFR 95-270 Moldex 2200 |
Physical changes: visual and tactile inspection (researchers) Odour: smelling the mask (researchers); blinded visual analogue scale and verbal reports (subjects) Fit: multiple-donning fit test using author-modified OSHA protocol. MDFF10 ≥100 = pass Comfort/donning ease: blinded visual analogue scale rating and verbal reports (subjects) |
Moldex 2200: strap breaks in both treatment (N = 1/21) and control (N = 2/22) 3M 1870: slight separation of inner foam nose cushion (N = NR); strap break in treatment (N = 1/21) but not control |
Quantitative – no significant difference Qualitative – researchers detected no odour changes. No treatment-based patterns in participants' verbal reports |
Fit: mean MDFF10 passed for all models (N = 20), no significant difference Comfort/donning ease: Quantitative – no significant differences Qualitative – no treatment-based patterns in participants' verbal reports |
||
| Heat | Bergman, 2010 | Temperature: 60°C Time: 30 min Cycles: 3 Moisture: MHI (80% RH) |
3M 8000d 3M 8210d Moldex 2201d |
KC PFR95-174d 3M 1860d 3M 1870d |
Physical traits: visual inspection Odour: smelling the mask |
3M 1870: slight separation of inner foam nose cushion (N = 3/3) | No odour reported | – |
| Bergman, 2011 | Temperature: 60°C Time: 15 min Cycles: 3 Moisture: MHI (80% RH) |
3M 1860 3M 1870 KC PFR95-270 |
Physical traits: visual inspection Fit: author-modified OSHA protocol. Fit factor ≥100 = pass; faceseal leakage = 1/FF |
3M 1870: slight separation of inner foam nose cushion (same amount after all cycles) | – | Fit test pass rate remained ≥90% for all models (N = 20) Mean faceseal leakage remained <1% for all models (N = 20), no significant difference |
||
| Fischer, 2020 | Temperature: 70°C Time: NR Cycles: 3 (2 h wear between each) Moisture: dry |
3M Aura 9211+/37193 | Fit: fit testing using official modified OSHA-standard protocol. Fit factor ≥100 = pass | – | – | 100% pass rate after 1st and 2nd cycles, 66% pass rate after 3rd cycle (N = 6) | ||
| Heimbuch 2011 | Temperature: 65°C Time: 30 min Cycles: 1 Moisture: MHI (85% RH) |
3M 8000f 3M 8210f Moldex 1500f |
KC PFRf 3M 1860f 3M 1870f |
Physical traits: visual inspection | No significant changes | – | – | |
| Kumar, 2020 | Temperature: 121°C Time: 15 min at peak temp Cycles: 1, 3, 5, 10 Moisture: Steam (AC) Pressure: NR | 3M 1860 3M Aura 1870 3M Vflex 1804S AOSafety 1054S |
Physical traits: visual and tactile inspection Fit: normal and deep breathing tests only; Fit factor ≥100 = pass |
3M Vflex 1804S: mild bleeding of ink label after 1 cycle (N = 1/1) | – | 3M 1860: passed after 1 cycle (N = 1), failed after subsequent cycles All other models: passed after all cycles (N = 1) |
||
| Lin, 2017 | Temperature: 149–164°CTime: 3 minCycles: 1Moisture: Dry | 3M 8210 | Physical traits: visual and tactile inspection | No changes reported | – | – | ||
| Temperature: 121°C Time: 15 min Cycles: 1 Moisture: Steam (AC) Pressure: 1.06 kg/cm2 |
3M 8210 | Physical traits: visual and tactile inspection | Folds of inner/outer filter supports. Outer layers of masks were deformed, shrunken, and stiff with no remarkable mottle | – | – | |||
| Viscusi, 2007 | Temperature: 80°C and 160°C Time: 60 min Cycles: 1 Moisture: Dry |
3M 8000d |
Physical traits: visual and tactile inspection |
80°C: No significant changes (N = 4/4) 160°C: Masks melted and unusable after 22 min |
– |
– |
||
| Temperature: 121°C Time: 15 min and 30 min Cycles: 1 Moisture: Steam (AC) Pressure: 1.05 kg/cm2 |
3M 8000d | Physical changes: visual and tactile inspection | Masks deformed, shrunken, stiff, and mottled at both durations | – | – | |||
| Viscusi, 2009 | Temperature: 80°C, 90°C, 100°C, 110°C, 120°C Time: 60 min Cycles: 1 Moisture: dry |
3M 8000d 3M 8210d Moldex 2200d |
KC PFR95-270d 3M 1860d 3M 1870d |
Physical traits: visual and tactile inspection Odour: smelling the mask |
KC PFR95-270 samples at 100°C, 110°C, and 120°C: inner moisture barrier melted into the filtration media (N = 1/3, 2/3, 2/3) | No odour reported | – | |
| Viscusi, 2011 | Temperature: 60°C Time: 30 min Cycles: 1 Moisture: MHI (80% RH) |
3M8000 3M8210 3M1860 3M1870 KC PFR 95-270 Moldex 2200 |
Physical changes: visual and tactile inspection (researchers) Odour: smelling the mask (researchers); blinded visual analogue scale and verbal reports (subjects) Fit: multiple-donning fit test using author-modified OSHA-protocol. MDFF10 ≥100 = pass. Comfort/donning ease: blinded visual analogue scale rating and verbal reports (subjects) |
3M 1870: slight separation of inner foam nose cushion Moldex 2200: strap breaks in both treatment (N = 3/23) and control (N = 2/22) |
Quantitative – 3M 1860 had significantly increased odour (+5.94 out of 100); no other significant differences Qualitative – researchers detected no odour changes. No treatment-based patterns in participants' verbal reports |
Fit: Mean MDFF10 passed for all models (N = 20), though 3M 8210 and Moldex 2200 had significant reductions in MDFF10 (-29 and -59, respectively) Comfort/donning ease: Quantitative – no significant differences Qualitative – no treatment-based patterns in participants' verbal reports |
||
AC, autoclave; FF, fit factor; KC, Kimberly–Clark; MDFF10, multi-donning fit factor; MGS, microwave-generated steam; MHI, moist heat incubation; OSHA, Occupational Safety and Health Administration; RH, relative humidity;–, outcome not assessed.
Room pressure unless otherwise specified. If both manufacturer-rated and experimentally-determined microwave power were provided, the experimental value is reported here.
Where replicate numbers are provided as N = x/y, x is the number of replicates per model in which the physical change was observed and y is the total number of replicates per model; absence of N values in the table indicates that replicate numbers were not reported for the observation.
N values represent numbers of replicates that underwent fit testing for each model.
Mask models were anonymized in article. Model names were obtained by e-mail from R. Shaffer in April 2020.
Power units per volume not specified.
Mask models were anonymized in article. Model names were obtained by e-mail from B. Heimbuch in April 2020.
Physical traits
Nine studies reported on changes in physical traits after treatment, including mask appearance, feel, odour, and water retention (Table IV) [15,18,19,42,[48], [49], [50], [51], [52]]. Seven used microwave interventions (dry or MGS), and eight used at least one heat intervention (dry, MHI, and/or autoclave).
Physical changes were both treatment- and model-dependent. The 3M 1870 displayed consistent separation of the inner foam nose cushion after MGS and MHI, with this change not observed in any other mask model [15,[50], [51], [52]]. Melting of some models occurred after MGS, dry microwaving, or dry heat at temperatures of 100°C or greater [18,[49], [50], [51]]. Autoclaving led to significant mask deformation in two of three studies [19,49].
Changes in odour were assessed in three studies [18,50,52]; the only significant increase in odour was noted in the 3M 1860 after MHI in one study [52]. Unacceptable water retention, defined as over 1 g of water retained after drying for 1 h, was observed in three (3M 1860, 3M 8210, Cardinal Health) of six models tested [42].
Risk of bias and strength of evidence
A full risk of bias assessment for all study outcomes is presented in the Supplementary Material. Overall risks of bias across all studies for aerosol penetration and airflow resistance outcomes were low. Risk of bias for germicidal outcomes was moderate for most studies, primarily due to risk of population heterogeneity (i.e. masks not from same lot) and the use of unblinded visual assays. Risk of bias for fit was moderate in all studies, due either to high risk of sampling bias or moderate risk for both population heterogeneity and methodology. Risks of bias for physical traits varied between studies, but unblinded/subjective outcome evaluation and potential population heterogeneity were common reasons for increased risk.
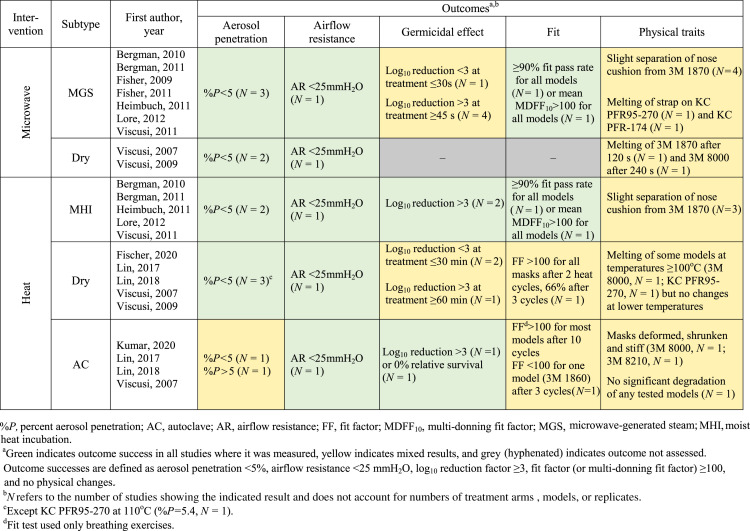
A summary of results for all treatments and outcomes can be found in Table V .
Table V.
Summary of reported outcomes.
Discussion
In response to PPE shortages during the COVID-19 pandemic, we systematically reviewed the existing literature on N95 FFR decontamination using microwave irradiation and heat. Our results indicate that moist/dry microwave irradiation and moist/dry heat between 60 and 90°C can effectively deactivate viral pathogens on certain N95 FFR models while maintaining mask fit and function within acceptable ranges. General use of high heat (greater than 90°C) and autoclaving are not supported by the evidence in review as these interventions compromised the integrity of multiple mask models.
Decontamination of N95 masks for reuse is worthwhile only if the masks retain their ability to remove at least 95% of viral particles from the air (i.e. aerosol penetration <5%) [4]. In the six studies that evaluated aerosol penetration after microwave and/or heat treatment, only two studies showed an increase in penetration above the standard 5% threshold [18,49]. The decontamination conditions in these studies (temperature above 100°C and autoclaving) were also associated with significant physical degradation of the mask. Interestingly, despite observing physical degradation, Lin et al. reported no significant change to aerosol penetration after autoclaving [19]. This discrepancy may be explained by the non-standard protocol used by Lin et al., which involved mask fragments, modified flow rate, and a different aerosol solution, precluding direct comparison with NIOSH aerosol penetration guidelines.
Mask usability does not only depend on filtration efficiency. N95 FFRs cause breathing resistance and reduce air exchange volume at baseline [55]; thus, if microwave- or heat-treatment were to increase airflow resistance significantly, this could render the masks intolerable, especially when worn for extended periods during PPE rationing [10]. Three studies in this review evaluated airflow resistance in a total of five different decontamination conditions (MGS, dry microwave, MHI, dry heat, and autoclave) [18,19,50]. The final average airflow resistance never reached even 50% of the maximum allowable resistance indicated in NIOSH-established guidelines for any mask model [43], and most models demonstrated slight reductions in resistance after decontamination, making airflow resistance an unlikely obstacle to N95 decontamination using microwave irradiation or heat.
Microwave irradiation and heat both effectively reduced viral load on FFRs, with all interventions displaying a log10 viral reduction factor greater than three when applied for sufficient duration. Although studies used masks that were artificially contaminated in the lab rather than those that had been contaminated during clinical use, viral loading titres that are sufficient for observation of a three log10 reduction factor meet or exceed the highest levels of viral contamination modelled to occur in hospital settings [44]. Germicidal impact can be further bolstered by leaving the masks for several days after decontamination before reuse: there is evidence that SARS-CoV-2 naturally decays over time on surfaces [56], and Lin et al. demonstrated that bacterial load was further reduced 24 h after incubation, even in warm, humid conditions [16]. For SARS-CoV-2, a wait time of at least three days is advisable as viable virus is detectable up to 72 h after application on some surfaces [56].
N95 FFRs must fit with a tight seal to ensure that air passes directly through the filter. Data regarding post-decontamination mask fit was promising, but most study protocols did not account for the impacts of repeated donning–wearing–doffing cycles. Previous research indicates that fit failure is associated with extended use and limited reuse of masks even without any decontamination treatment [57,58]. Thus, applying microwave/heat treatment to unused masks, as three of the four studies did, has limited generalizability. The exception was the protocol used by Fischer et al., which included 2-h wear cycles between each treatment and demonstrated that fit deteriorated after the third decontamination-donning cycle [47]. Kumar et al.’s positive post-autoclave fit results, which did not include wear-periods between cycles, must be interpreted with additional caution as they only tested fit using breathing exercises, which are not representative of functional movements of healthcare workers [48]. Overall, the results of these studies indicate that a limited number of microwave or heat decontamination cycles may not compromise fit; however, further testing is required using masks that have undergone prolonged wear time and multiple donning–doffing cycles. Regardless, a careful user seal check should be performed by any healthcare worker who dons a decontaminated FFR, just as would be done when donning a new one [59].
Physical degradation of an N95 FFR will almost invariably cause changes in fit, function, and tolerability. Melting of mask components was observed in some microwave and heat arms and depended on the mask model, temperature, and treatment duration. Frequent adverse physical changes were observed at temperatures over 90°C, which corresponds to the maximum operating temperature of polypropylene, the polymer that comprises the N95 filter [60]. High temperature was also the likely cause of melting during microwave treatments: a previous study demonstrated that wet kitchen sponges can exceed 90°C after 1 min of microwave irradiation [25]. Separation of the inner foam nose cushion was a consistent issue for the 3M 1870 after microwave and heat treatments, but did not lead to a significant reduction in fit and so may not preclude reuse if the mask feels tolerable to the user [52].
Autoclaving does not appear to be a suitable decontamination option for rigid FFRs as it caused significant physical deformations to the 3M 8000 and 3M 1820 [19,49]. Although Kumar et al. did not notice any significant physical changes after their autoclave intervention, functional degradation did occur in the one rigid mask model (3M 1860) while the three flexible ‘pleated’ mask models maintained their structural and functional integrity [48]. Thus, it is possible that autoclaving may be effective for pleated N95 varieties, although this needs further study.
Fisher et al.'s 2011 study contained the sole examination of water retention post-MGS treatment [42]. Notably, their results corresponded with hydrophobicity evaluations performed by Viscusi et al. [18] for the five mask models that were shared between the two studies: only the masks with all hydrophobic filtering layers (i.e. water droplets applied by Viscusi et al. beaded on each layer's surface and were not absorbed) showed acceptably low water retention levels in Fisher et al.’s study. As residual moisture may occlude mask pores and increase breathing resistance, hydrophobicity should be a consideration when choosing which mask models to sterilize using moist microwave or heating methods if drying time is limited [61,62].
Future directions
While the results of this review provide a starting point for the development of institutional microwave- or heat-based FFR decontamination protocols, there are several key gaps in the existing evidence. For example, few studies investigated fit; without a tight seal, air will flow through the gaps between the mask and the wearer's face, bypassing the filter altogether and making outcomes of aerosol penetration and airflow resistance irrelevant.
The characteristics of the micro-organisms used in several of the germicidal studies must also be taken into account when extrapolating these results to the SARS-CoV-2 coronavirus. Influenza A viruses, such as H1N1 and H5N1, are enveloped, approximately 120 nm in diameter, and covered in glycoproteins [63]; coronaviruses share all of these characteristics and may plausibly respond in a similar manner to heat and radiation [64]. Notably, effective decontamination was reached by Heimbuch et al. for H1N1 at a lower temperature and shorter timepoint than in Fischer et al.’s experiment using SARS-CoV-2 [15,47]. It is unclear whether the presence of moisture in Heimbuch et al.’s treatment may have increased germicidal efficacy, or if SARS-CoV-2 is more resistant to heat than influenza. MS2, as investigated by Fisher et al. in two studies [42,54], is less comparable as it consists of a non-enveloped 26-nm virion [65], and the bacterial species (Bacillus subtilis) in Lin et al.’s evaluation is further distinct [16]. The use of different viruses also necessitates the use of different assays (e.g., plaque or TCID50) and cell types according to the infectious properties of each virus, and these different tests may not have comparable sensitivities. However, consistent strong germicidal effects across interventions and pathogens support the notion that these methods should reduce the load of SARS-CoV-2 to undetectable levels if applied for sufficient time. Additionally, it should be noted that no studies using moderate-temperature MHI interventions quantified growth of non-target bacteria, which could increase under moist heat conditions and pose a separate infectious risk.
Risk of bias
The moderate risk of bias seen in most studies for germicidal outcomes arises from the fact that all studies quantified pathogens using plaque, colony, or TCID50 assays; while these are widely accepted means of quantifying viral and bacterial load, they involve visual procedures that are not fully objective, and no studies stated that the lab technicians were blinded to treatment and control designations. Two studies that evaluated fit replaced and re-tested masks when their straps broke or melted [51,52]; it is possible that this discounted data from the samples that were most vulnerable to physical damage, which could positively skew the fit scores. Similarly, there were two models (3M 1870, 3M 8000) for which aerosol penetration and/or airflow resistance could not be measured after certain treatments due to melting [18,49]; these were appropriately accounted for within the articles' conclusions and so did not significantly increase within-study risk of bias, but the absence of measurements from these more-vulnerable masks could positively bias the results of the systematic review. For physical trait outcomes, several studies reported observations in the results without indicating physical evaluation in their objectives or methods, and/or only commented on changes in some mask models without indicating that unmentioned models were unaffected, making it difficult to rule out methodological inconsistencies and selective reporting.
Strengths and limitations
This is a rigorous systematic review, involving a peer-reviewed search strategy, an a priori registered protocol, training and testing of the screening/extraction team, and adherence to PRISMA reporting guidelines [34]. However, the heterogeneity of the microwave and heat parameters across the 13 studies limits the ability to draw overarching conclusions about any one set of conditions. Temperature, pressure, and moisture all influenced outcomes, especially in heat-decontamination arms where an autoclave provides a vastly different environment than a dry heat rice cooker. There was evidence that different mask models have different physical vulnerabilities, indicating that the response of a given mask model to a particular treatment does not predict how any other model will react. Germicidal outcomes showed consistent viral reduction, but the artificial contamination of samples limits extrapolation to the clinical setting. The results of this review should therefore be used as a resource for determining which microwave and heat conditions may be most auspicious but cannot guarantee the success of any specific protocol.
In conclusion, in situations where sufficient new PPE is available, reuse of N95 FFRs should not be considered. However, in a situation where procurement of new masks is not possible, this systematic review indicates that microwaves and heat may both be suitable options for FFR decontamination. Microwave irradiation and moderate-temperature heat (up to 90°C), in both moist and dry conditions, demonstrated effective decontamination of viral pathogens without compromising mask performance or function. The most significant limitations to the application of available evidence are the differential effects on specific mask models, particularly regarding physical deterioration, and the lack of real-world data regarding changes in fit. Autoclaving is an effective germicide, but caused significant degradation and reduction of filter efficiency in some mask types, and so its use is not supported by the results of this review. Overall, any hospital implementing these decontamination methods would benefit from monitoring the physical responses of their mask models to determine which, if any, are durable in these treatment conditions, and for how many treatment cycles.
NB: The Association of Home Appliance Manufacturers has emphasized the importance of not using home appliances to microwave or heat facemasks due to risk of damage or injury [66].
Acknowledgements
Dr. Jemila Hamid and Kristin Konnyu provided advice regarding data cleaning and meta-analysis design. Dr. Marc-André Langlois offered insight on interpretation of germicidal results. Dr. Ron Shaffer provided identities of anonymized mask models and offered information and resources regarding mask performance and decontamination testing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.08.016.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
This research was supported by funding provided by the Ontario Ministry of Health, received from the successful application to the CHAMO COVID-19 grant opportunity.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Centers for Disease Control and Prevention . 2020. Strategies for optimizing the supply of N95 respirators: conventional capacity strategies.https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/conventional-capacity-strategies.html Available at: [last accessed March 2020] [Google Scholar]
- 2.Government of Canada . 2020. Infection prevention and control for novel coronavirus (2019-nCoV): interim guidance for acute healthcare settings.https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/interim-guidance-acute-healthcare-settings.html Available at: [last accessed March 2020] [Google Scholar]
- 3.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Government Publishing Office . 1999. Non-powered air-purifying particulate filter efficiency level determination.https://www.ecfr.gov/cgi-bin/text-idx?SID=5b2666d4940f00b38d815af01a2e7044&mc=true&node=pt42.1.84&rgn=div5#se42.1.84_1181 Available at: [last accessed April 2020] [Google Scholar]
- 5.Tan N.C., Goh L.G., Lee S.S. Family physicians' experiences, behaviour, and use of personal protection equipment during the SARS outbreak in Singapore: Do they fit the Becker Health Belief Model? Asia Pac J Public Health. 2006;18:49–56. doi: 10.1177/10105395060180030901. [DOI] [PubMed] [Google Scholar]
- 6.Carias C., Rainisch G., Shankar M., Adhikari B.B., Swerdlow D.L., Bower W.A., et al. Potential demand for respirators and surgical masks during a hypothetical influenza pandemic in the United States. Clin Infect Dis. 2015;60:S42–S51. doi: 10.1093/cid/civ141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A., D'Alessandro M., Ireland K., Burel W.G., Wencil E.B., Rasmussen S.A. Personal protective equipment supply chain: Lessons learned from recent public health emergency responses. Health Secur. 2017;15:244–252. doi: 10.1089/hs.2016.0129. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan A., Jernign D.B., Liedtke L., Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: Views from a national survey of infectious diseases consultants. Clin Infect Dis. 2004;39:272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- 9.Bauchner H., Fontanarosa P.B., Livingston E.H. Conserving supply of personal protective equipment—a call for ideas. JAMA. 2020;323:1911. doi: 10.1001/jama.2020.4770. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . 2020. Strategies for optimizing the supply of N95 respirators: crisis/alternate strategies.https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/crisis-alternate-strategies.html Available at: [last accessed March 2020] [Google Scholar]
- 11.Fisher E.M., Shaffer R.E. Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings. J Occup Environ Hyg. 2014;11:D115–D128. doi: 10.1080/15459624.2014.902954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Occupational Safety and Health . 1996. NIOSH guide to the selection and use of particulate respirators.https://www.cdc.gov/niosh/docs/96-101/default.html Available at: [last accessed March 2020] [Google Scholar]
- 13.3M . 2020. Disinfection of filtering facepiece respirators: considerations for healthcare organizations and occupational health professionals; pp. 1–3.https://multimedia.3m.com/mws/media/1816576O/disinfection-of-disposable-respirators-technical-bulletin.pdf Available at: [last accessed March 2020] [Google Scholar]
- 14.Centers for Disease Control and Prevention . 2020. Decontamination and reuse of filtering facepiece respirators.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed March 2020] [Google Scholar]
- 15.Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C.Y., Woo M.H., et al. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Lin T.H., Tang F.C., Hung P.C., Hua Z.C., Lai C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;28:754–762. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Hearn K., Gertsman S., Sampson M., Webster R.J., Tsampalieros A., Ng R., et al. Decontaminating N95 masks with ultraviolet germicidal irradiation (UVGI) does not impair mask efficacy and safety: A systematic review. J Hosp Infect. 2020;106:163–175. doi: 10.1016/j.jhin.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viscusi D., Bergman M.S., Eimer B., Shaffer R. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin T.H., Chen C.C., Huang S.H., Kuo C.W., Lai C.Y., Lin W.Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: Effects of five decontamination methods. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salter W.B., Kinney K., Wallace W.H., Lumley A.E., Heimbuch B.K., Wander J.D. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–445. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J.X., Wang F., Li X., Sun Y.Y., Wang Y., Ou C.R., et al. The influence of microwave sterilization on the ultrastructure, permeability of cell membrane and expression of proteins of Bacillus cereus. Front Microbiol. 2018;9:1870. doi: 10.3389/fmicb.2018.01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elhafi G., Naylor C.J., Savage C.E., Jones R.C. Microwave or autoclave treatments destroy the infectivity of infectious bronchitis virus and avian pneumovirus but allow detection by reverse transcriptase-polymerase chain reaction. Avian Pathol. 2004;33:303–306. doi: 10.1080/0307945042000205874. [DOI] [PubMed] [Google Scholar]
- 23.Siddharta A., Pfaender S., Malassa A., Doerrbecker J., Anggakusuma, Engelmann M., et al. Inactivation of HCV and HIV by microwave: a novel approach for prevention of virus transmission among people who inject drugs. Sci Rep. 2016;6:36619. doi: 10.1038/srep36619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y., Yao M. In situ airborne virus inactivation by microwave irradiation. Chin Sci Bull. 2014;59:1438–1445. doi: 10.1007/s11434-014-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park D.K., Bitton G., Melker R. Microbial inactivation by microwave radiation in the home environment. J Environ Health. 2006;69:17–24. quiz 39-40. [PubMed] [Google Scholar]
- 26.Leclercq I., Batéjat C., Burguière A.M., Manuguerra J.C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8:585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swenson V.A., Stacy A.D., Gaylor M.O., Ushijima B., Philmus B., Cozy L.M., et al. Assessment and verification of commercially available pressure cookers for laboratory sterilization. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J., Hinestroza J.P., Jasper W., Barker R.L. Effect of solvent exposure on the filtration performance of electrostatically charged polypropylene filter media. Textile Research Journal. 2009;74:343–350. [Google Scholar]
- 30.Alam M., Yuanxiang X., Zhu G. The effect of temperature and humidity on electric charge amount of polypropylene melt-blown nonwoven fabric. IOSR J Polymer Textile Eng. 2019;6:1–6. [Google Scholar]
- 31.Motyl E., Lowkis B. Effect of air humidity on charge decay and lifetime of PP electret nonwovens. Fibres Text East Eur. 2006;14:39–42. [Google Scholar]
- 32.O’Hearn K., Gertsman S., Webster R., Tsampalieros A., Ng R., Gibson J., et al. Efficacy and safety of disinfectants for decontamination of N95 and SN95 filtering facepiece respirators: A systematic review. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNally J.D., O’Hearn K., Gertsman S., Agarwal A., Sikora L., Sampson M., et al. Microwave- and heat-based decontamination for facemask personal protective equipment (PPE): Protocol for a systematic review. OSF Preprints. 2020 doi: 10.31219/osf.io/4se6b. [Pre-Print] [DOI] [Google Scholar]
- 34.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 35.3M . 2012. 3M disposable filtering facepiece respirators.https://media3.webcollage.net/1e96a9b0c631453265362cd7c1e9c4f7628d4740?response-content-type=application%2Fpdf&AWSAccessKeyId=AKIAIIE5CHZ4PRWSLYKQ&Expires=1893527854&Signature=x%2BVkOaESU5N547lbOv5cMkFHIJc%3D Available at: [Google Scholar]
- 36.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hearn K., Gertsman S., Webster R., Sampson M., Sikora L., Agarwal A., et al. OSF Preprints; 2020. Decontamination of N95 and SN95 filtering facepiece respirators.https://osf.io/bv9gp/ [Pre-Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Development Core Team . R Foundation for Statistical Computing; Vienna: 2010. A language and environment for statistical computing: reference index. [Google Scholar]
- 40.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh A., Nocerino J. Robust estimation of mean and variance using environmental data sets with below detection limit observations. Chemometr Intell Lab Syst. 2002;60:69–86. [Google Scholar]
- 42.Fisher E.M., Williams J.L., Shaffer R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Government Publishing Office . 1999. Airflow resistance tests.https://www.ecfr.gov/cgi-bin/text-idx?SID=5b2666d4940f00b38d815af01a2e7044&mc=true&node=pt42.1.84&rgn=div5#se42.1.84_1180 Available at: [last accessed April 2020] [Google Scholar]
- 44.Fisher E.M., Noti J.D., Lindsley W.G., Blachere F.M., Shaffer R.E. Validation and application of models to predict facemask influenza contamination in healthcare settings. Risk Anal. 2014;34:1423–1434. doi: 10.1111/risa.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Occupational Safety and Health Administration . United States Department of Labor; Washington: 1998. Fit testing procedures (mandatory), code of federal regulations title 29, Part 1910.124 app A.https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 Available at: [Google Scholar]
- 46.Higgins J.P.T., Savović J., Page M.J., Elbers R.G., Sterne J.A.C. In: Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019): Cochrane. Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al., editors. 2019. Chapter 8: Assessing risk of bias in a randomized trial.www.training.cochrane.org/handbook [Google Scholar]
- 47.Fischer R., Morris D.H., van Doremalen N., Sarchette S., Matson J., Bushmaker T., et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv. 2020 doi: 10.1101/2020.04.11.20062018. [Pre-Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., et al. N95 mask decontamination using standard hospital sterilization technologies. medRxiv. 2020 doi: 10.1101/2020.04.05.20049346. [Pre-Print] [DOI] [Google Scholar]
- 49.Viscusi D.J., King W.P., Shaffer R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007;24:93–107. [Google Scholar]
- 50.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R., Shaffer R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Eng Fibers and Fabrics. 2010;5:33–41. [Google Scholar]
- 51.Bergman M.S., Viscusi D.J., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J Int Soc Respir Prot. 2011;28:48–59. [Google Scholar]
- 52.Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A., Powell J., et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 53.Lore M., Heimbuch B., Brown T., Wander J., Hinrichs S. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 54.Fisher E., Rengasamy S., Viscusi D., Vo E., Shaffer R. Development of a test system to apply virus-containing particles to filtering facepiece respirators for the evaluation of decontamination procedures. Appl Environ Microbiol. 2009;75:1500–1507. doi: 10.1128/AEM.01653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H.P., Wang D.Y. Objective assessment of increase in breathing resistance of N95 respirators on human subjects. Ann Occup Hyg. 2011;55:917–921. doi: 10.1093/annhyg/mer065. [DOI] [PubMed] [Google Scholar]
- 56.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergman M.S., Viscusi D.J., Zhuang Z., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J Infect Control. 2012;40:375–380. doi: 10.1016/j.ajic.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Degesys N.F., Wang R.C., Kwan E., Fahimi J., Noble J.A., Raven M.C. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94–96. doi: 10.1001/jama.2020.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krah J., Shamblin M., Shaffer R. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Pittsburgh: 2018. Filtering out confusion: frequently asked questions about respiratory protection, User seal check. [Google Scholar]
- 60.Hutten I.M. Handbook of nonwoven filter media. Butterworth-Heinemann; Oxford: 2016. Chapter 3 – Properties of nonwoven filter media; pp. 108–157. [Google Scholar]
- 61.Weiss M.M., Weiss P.D., Weiss D.E., Weiss J.B. Disrupting the transmission of influenza A: Face masks and ultraviolet light as control measures. Am J Public Health. 2007;97:S32–S37. doi: 10.2105/AJPH.2006.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mardimae A., Slessarev M., Han J., Sasano H., Sasano N., Azami T., et al. Modified N95 mask delivers high inspired oxygen concentrations while effectively filtering aerosolized microparticles. Ann Emerg Med. 2006;48:391–399. doi: 10.1016/j.annemergmed.2006.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ViralZone. Alphainfluenzavirus. Available at: https://viralzone.expasy.org/6?outline=all_by_species [last accessed June 2020].
- 64.ViralZone. Betacoronavirus. Available at: https://viralzone.expasy.org/764?outline=all_by_species; [last accessed June 2020].
- 65.ViralZone. Levivirus. Available at: https://viralzone.expasy.org/291?outline=all_by_species; [last accessed June 2020].
- 66.Association of Home Appliance Manufacturers . 2020. Statement on microwave ovens for sanitizing face masks.https://www.aham.org/AHAM/News/Latest_News/Microwave_Ovens_Face%20Mask_Cleaning.aspx?WebsiteKey=c0a5e5a1-ea1c-42f1-9b84-d62256c16ea2 Available at: [last accessed May 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.