Abstract
The rapid breakout of the coronavirus disease of 2019 (COVID-19) has been declared pandemic with serious global concern due to high morbidity and mortality. As we enter the phase beyond limitations there is an urgent need for explicit treatment against COVID-19. To face this immediate global challenge, drug development from scratch is a lengthy process and unrealistic to conquer this battle. Drug repurposing is an emerging and practical approach where existing drugs, safe for humans, are redeployed to fight this harder to treat disease. A number of multi clinical studies have repurposed combined cocktail (remdesivir + chloroquine and favipiravir + chloroquine) to be effective against COVID-19. However, the exact mechanistic aspect has not yet been revealed. In the present study, we have tried to decipher the mechanistic aspects of existing medicines at the viral entry and replication stage via the structural viroinformatics approach. Here we implied the molecular docking and dynamic simulations with emphasis on the unique structural properties of host receptor angiotensin-converting enzyme 2 (ACE2), SARS-CoV2 spike protein and RNA dependent RNA polymerase enzyme (RdRp) of the SARS-CoV2. Deep structural analysis of target molecules exposed key binding residues and structural twists involved in binding with important pharmacophore features of existing drugs [(7-chloro-N-[5-(diethylamino)pentan-2-yl]quinolin-4-amine (chloroquine),N-[[4-(4-methylpiperazin-1-yl)phenyl]methyl]-1,2-oxazole-5-carboxamide N-[[4-(4-methylpiperazin-1-yl)phenyl]methyl]-1,2-oxazole-5-carboxamide) (SSAA09E2), 2-ethylbutyl (2S)-2-{[(S)-{[(2R,3S,4R,5R)-5-{4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl}-5-cyano-3 (remdesivir) and 6-Fluor-3-oxo-3,4-dihydro-2-pyrazincarboxamid (favipiravir)]. It is evident from this structural informatics study that combo of chloroquine + SSAA09E2 with remdesivir or favipiravir could significantly restrain the virus at the entry and replication stage. Thus, drug repurposition is an attractive approach with reduced time and cost to treat COVID-19, we don't have enough time as the whole world is lockdown and we are in urgent need of an obvious therapeutics' measures.
Keywords: COVID-19, Drug repurposition, Molecular docking, Molecular dynamics simulations, Spike protein, RdRp, ACE2
1. Introduction
In late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) was identified in Wuhan, China (Li et al., 2020) and named COVID-19 by WHO. COVID-19 (coronavirus disease of 2019) has surprisingly caught the global community as of August 10, 2020, the number of COVID-19 cases going to reach twenty million (20,055,196) with over seven hundred thousands (734,536) deaths worldwide (https://www.worldometers.info/coronavirus/). . The virus swiftly blowout from China to over >200 countries worldwide already exceeding the 2003 SARS CoV1 epidemic (Fischer et al., 2020). Currently, there are no effective medications against COVID-19/SARS-CoV2 although several research groups throughout the world are working to develop the vaccine. There is an urgent need for the development of effective prevention and treatment strategies for SARS-CoV2 outbreak. Drug repositioning or reprofiling is a promising strategy to conquer the battle against COVID-19 in a time-critical manner.
The newly identified SARS-CoV2 belongs to β-coronavirus. It has more than 96% sequence similarity with SARS-CoV1 (Fischer et al., 2020). SARS-CoV is a complex of structural and non-structural proteins (NSPs) (NSP-2-NSP-16) which are produced as cleavage products of the ORF1a and ORF1ab viral polyproteins (Ziebuhr, 2005). Among these NSP-12 along with co-factors, NSP-7 and NSP-8 possess RNA-dependent RNA polymerase activity (Ahn et al., 2012; Subissi et al., 2014). Thus, Nsp12, a conserved protein in coronavirus, is an RNA-dependent RNA polymerase (RdRp) and the vital enzyme of coronavirus replication/transcription complex.
All viruses (including SARS-CoV2) need host receptor proteins on the cell surface for successful entry and infection. Angiotensin-converting enzyme 2 (ACE2), located in the human lower respiratory tract, is recognized as a cell receptor for SARS-CoV (Jia et al., 2005) and controls equally human-to-human and cross-species transmission (Wan et al., 2020). Zhou et al (2020) confirmed that SARS-CoV2 uses the same receptor (ACE2) as SARS-CoV, after isolating the BALF (bronchoalveolar lavage fluid) of a COVID-19 patients.et alIn the war against corona, scientists are working on following possible therapeutics measures: one is against the human cell to prevent virus entry or block the signaling pathways required for viral infection and other therapeutics against the virus itself by preventing the virus replication (non-structural proteins) and self-assembly (structural proteins).
Broad-spectrum antiviral agents (BSAAs) estimated ‘safe-in-man’ tested on early phase clinical trials have been advertised as good drug repurposing candidates (Andersen et al., 2020). Chloroquine, an anti-malarial drug has been shown to have antiviral activity at entry and post-entry stages of the COVID-19 infection (Gao et al., 2020). It can boost the antiviral activity of remdesivir and possibly aid as a synergizer of BSAAs (Wang et al., 2020). Favipiravir the RNA polymerase inhibitor is also on Phase II clinical trial for SARS-CoV2 associated pneumonia (Chinese Clinical Trial Registry Identifier: ChiCTR2000029544). In February 2020 Thailand health ministry announced marked progress in COVID-19 treatment with successful use of a cocktail (combined anti-flu drug oseltamivir with anti-HIV lopinavir and ritonavir) against coronavirus (https://www.dawn.com/news/1532081). Adedeji et al., in 2013 used small-molecule inhibitor, SAA09E2 of SARS-CoV replication that blocks viral entry by three different mechanisms. Using, immunoblotting and immune precipitation it was confirmed that SSAA09E2 is the first small molecule inhibitor to block the interaction of the receptor-binding-domain (RBD) SARS-CoV spike with human ACE2 receptor enzyme (Adedeji et al., 2013).
In the present study, we have used the integrated structural informatics approach to explore the pharmacological interactions and inhibitory mechanism of chloroquine, remdesivir, favipiravir and SSAA09E2at the entry and virus replication stage of SARS-CoV2. Using molecular docking, dynamic simulations and structural proteomics techniques important structural crunch of ACE2 (Human receptor) enzyme, SARS-CoV2 spike protein and RNA-dependent RNA polymerase (RdRp) enzyme of SARS-CoV2 were identified and the inhibitory mechanism of existing drugs was uncovered through drug-target interactions. So, the existing drugs against other viruses are an attractive and credible approach to treat unexplained viruses, as rather than conventional one bug, one drug method one drug more than one viruses are a debate of the day.
2. Materials and methods
2.1. Protein and ligand structure
The 3-dimensional structure of the ACE2 (PDB ID:1R4L), SARS-CoV2 spike ectodomain structure (PDB ID:6vyB) and RdRp (PDB ID:6M71) were retrieved from Protein Data Bank (PDB) (Berman et al., 2000). Because of unavailability of the three-dimensional structure of RNA-dependent RNA polymerase (RdRp) (at the time when the study was designed) the I-Tasser server (Zhang, 2008) was used to build the model for downward structure analysis. 6nur was used as a template by I-Tasser with a TM score of 0.63 and having a sequence identity of 96.36%. Ramachandran plot was used to assess the stereochemistry and validity of the built three dimensional protein model (Laskowski et al., 2018). The structure of the ligand; remdesivir (CID: 121304016), favipiravir (CID: 492405), chloroquine (CID: 83818) and SSAA09E2 (CID: 2738575) were retrieved from the PubChem database (https://pusbchem.ncbi.nlm.nih.gov/).
2.2. Molecular docking
To find the appropriate binding orientations of the ligand dataset with the respective target automated dockings were performed using AutoDock 4.2 (Norgan et al., 2011) tool following standard protocol. Polar hydrogen atoms and Kollman charges were assigned to the receptor protein molecule. Energy minimization was performed using a Lamarckian genetic algorithm with default parameters. An energy-based clustering of docked complexes was performed with the RMSF tolerance of 1.0 Å. The resulting clusters were classified through the lowest energy representative of the individual cluster. Resulting complexes were evaluated for molecular interactions using UCSF Chimera (Pettersen et al., 2004) and Discovery Studio (https://accelrys-discovery-studio-visualizer.software.informer.com/3.0/).
2.3. Molecular dynamic simulations
Molecular dynamic (MD) simulation studies of attractive drug targets of COVID-19 illness, spike protein, RdRp enzyme (Nsp12) and human receptor ACE2 enzyme in the apo and bound state were performed to assess the folding, stability, conformational changes and dynamic behaviors of all the systems. Amber03 force field embedded in GROningen MAchine for Chemical Simulations (GROMACS) 4.5 package running on the high performance OpenSuse linux system was used to perform simulations (Hess et al, 2008). Throughout simulation experiments, SARS-CoV2 spike protein, RdRP, and ACE2 (human receptor) systems were solvated by transferable intermolecular potential 4 points (TIP4P) water model in a periodic box (Zlenko, 2012). The system was neutralized by the addition of Na+ and Clˉ counter ions. Energy minimization (steepest descent algorithm for 500 steps) was executed by the tolerance of 1000 kJ/mol Å to eliminate initial steric clashes. After completing the minimization step systems were subjected to simulations for 50 ns time scale under constant temperature (300 K) and pressure (1 atm). To this end, electrostatic interactions were calculated using Particle Mesh Ewald (PME) algorithm. To investigate the stability behavior of spike protein, RdRp enzyme, and ACE2 receptor systems, visual molecular dynamics (VMD) (Humphrey et al., 1996), PyMol (http://www.pymol.org) and GROMACS tools were used.
3. Results
3.1. The tertiary structure of target protein of COVID-19
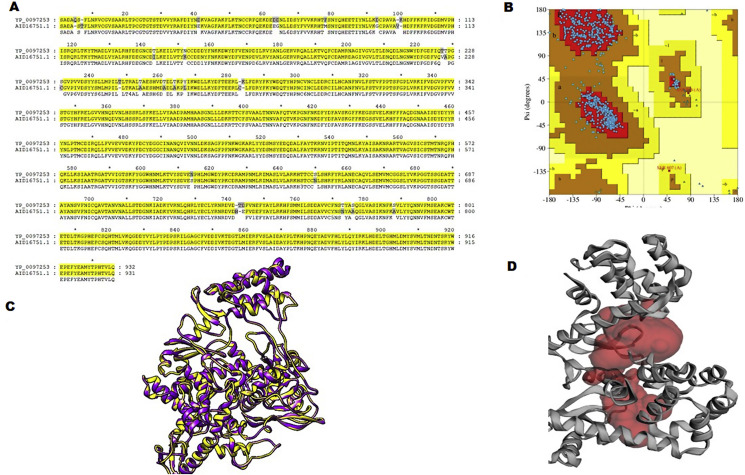
Because of the lack of tertiary structure of the RdRp, the homology modeling technique was used to build the three-dimensional structure of RdRp. Ramachandran plot of polymerase indicated the presence of more than 98% residues in sterically allowed regions (Fig. 1 A, B and C). Three dimensional structure of RdRp protein predicted through the I-Tasser server with TM score of 0.632 indicates the correct topology of the model for further studies. C-score (confidence score for predicting the quality of predicted model) of RdRp was 0.95 showed the fidelity of the model for further computational analysis. Pore analysis was also performed with the MOLE server (mole.upol.cz) (Fig. 1 D).
Fig. 1.
Pairwise sequence-structure alignment of RdRp of SARS-CoV2 with template (6nur). (A) Protein sequence alignments of SARS-CoV2-RdRp with its template 6nur protein. (B) Ramachandran plot. (C) Structure alignment of SARS-CoV2-RdRp (yellow ribbon) with template three-dimensional model (purple ribbon). (D) Pore analysis of SARS-CoV2-RdRp.
Viral spike protein, RdRp, and human receptor ACE2 were selected as the target for the present study to combat the COVID-19 disease at entry and replication stages.
3.2. Docking analysis
The effective therapeutic approach is to interfere with viral entry and replication for preventing COVID-19 pneumonia disease caused by SARS-CoV2. Recent, multi clinical trail of existing antiviral and malarial drugs possibly show their therapeutics value against COVID-19 disease but their exact mechanism of interaction with target molecules yet need to be explored. To understand the binding mode and critical pharmacophore features existing drugs for other diseases, with spike protein, RdRp enzyme, and human ACE2 receptor protein, a comprehensive interaction analysis was performed.
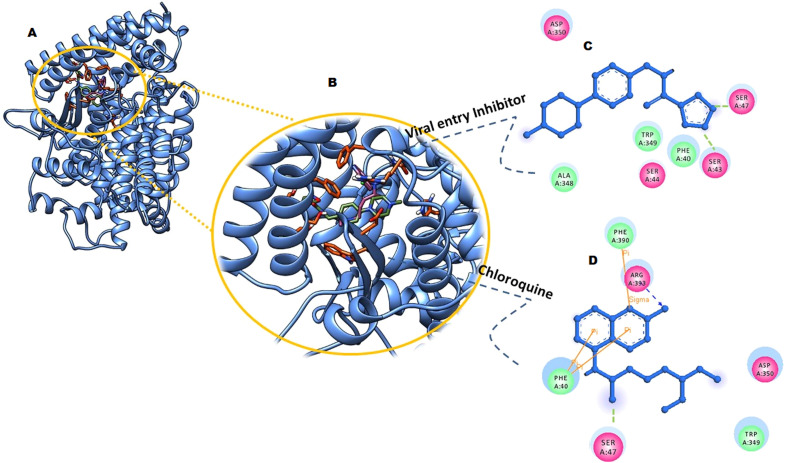
Detailed interaction analysis of chloroquine (4-N-(7-chloroquinolin-4-yl)-1-N,1-N-diethylpentane-1,4-diamine; phosphoric acid) in complex with ACE2 receptor (binding energy −9.34 kcal/mol) revealed strong binding in and around the region involved in binding with receptor binding domain (RBD) of SARS-CoV2-spike protein. Phe40 and Ser47 formed H-bonding and Pi-Pi stacking interactions with the chloroquine molecule. Asp350, Phe390, and Arg393 were involved in hydrophobic interaction with the chloroquine inhibitor (Fig. 2 A, B and C). Adedeji et al., in 2013 reported that SSAA09E2 an oxazole-carboxamide could block the viral entry of SARS-CoV and reported as promising SARS therapeutics. In the present study, we repurposed SSAA09E2 to block the entry of SARS-CoV2 due to close similarity with SARS-CoV. Docking interaction analysis of SSAA09E2 with ACE2 receptor (binding energy −10.34 kcal/mol) revealed an interaction with the residues at the binding interface with spike-RBD. Ser43 and Ser47 formed H-bonding with the phenyl moiety of entry inhibitor. Ser44 formed coordinate covalent interaction apart from hydrophobic interactions made by Phe40, Trp349, and Asp350 (Fig. 2A, B, and D).
Fig. 2.
Docked conformations, and interactions analysis of SSAA09E2and chloroquine with ACE2 human receptor. (A) and (B) represents the inhibitors (yellow and blue ball and stick model) bound within the cavity of ACE2 (blue ribbon) with interacting residues shown in orange sticks. (C) and (D) represent 2D interactions of viral entry inhibitor and chloroquine with ACE2 protein. Ligands are shown in blue ball and stick models with interacting residues shown in their respective colors.
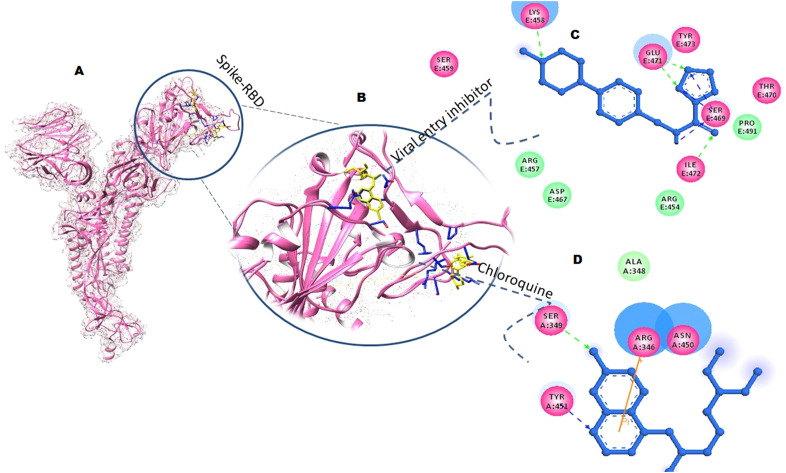
Similarly, to repurpose the dual action of chloroquine it was also docked with the spike-RBD and interaction analysis revealed binding of critical residues of spike-RBD with the different moieties of chloroquine. Ser349 and Tyr451 were involved in H-bonding and coordinate covalent interactions with the aromatic ring of chloroquine. While Arg346 formed Pi-Pi stacking interaction apart from the hydrophobic interaction of Asn450 (Fig. 3 A B, and C).
Fig. 3.
Molecular conformations analysis of SSAA09E2eand chloroquine with spike-SARS-CoV2. (A) and (B) represents the inhibitors (yellow ball and stick model) bound within the cavity of spike-SARS-CoV2 (pink ribbon) with interacting residues shown in blue sticks. (C) and (D) represent 2D interactions of viral entry inhibitor and chloroquine with spike-SARS-CoV2. Ligands are shown in blue ball and stick models with interacting residues shown in their respective colors.
SSAA09E2 showed strong binding with the RBD domain of spike protein with the binding free energy of −8.67 kcal/mol. Glu471, Ile472, and Lys458 formed H-bond with the 2-oxazole-5-carboxamide and phenylmethyl moiety of SSAA09E2, respectively. Ser469 formed coordinate covalent interaction while Arg454, Pro491 formed hydrophobic interaction to further strengthen the binding (Fig. 3 A, B and D).
Through viroinformatics approach, hloroquine and SSAA09E2 (SARS-CoV entry inhibitor) are repurposed as dual-action inhibitors of viral entry by interaction with the spike-RBD and ACE2 (receptor) proteins, simultaneously. While the binding energy of SSAA09E2 is high as compared to chloroquine.
Nsp12, a conserved protein in coronavirus, is an RNA-dependent RNA polymerase (RdRp) and the vital enzyme of coronavirus replication/transcription complex. The RdRp domain of polymerase is located at the C-terminus and has a conserved Ser-Asp-Asp motif (Subissi et al., 2014). Predominantly, RdRp targeted inhibition possibly not cause substantial harm to the host cells, but specific inhibitor against RdRp is still lacking (Chu et al., 2006).
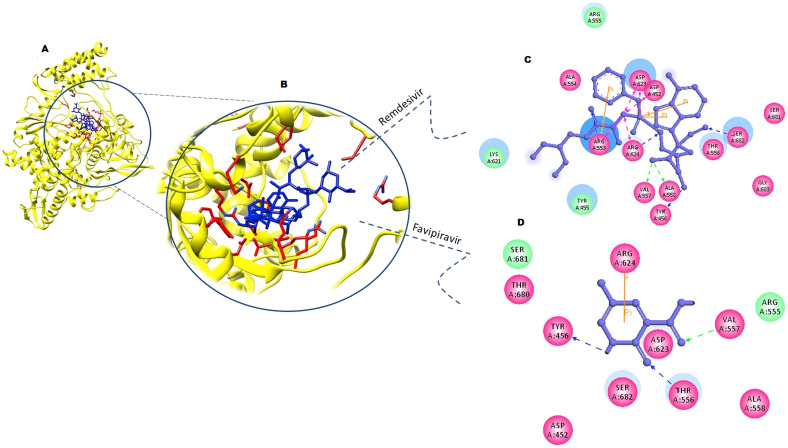
The computational targeting of RdRp with remdesivir and favipiravir showed both drugs were very well placed within the catalytic pocket. Interaction analysis of remdesivir within the pocket of RdRp (−12.67 kcal/mol) revealed Val557 and Ala558 formed H-bond where, Try456, Arg624 Arg553, and Ser682 formed coordinate covalent bond with remdesivir in addition to pi-pi interactions. A number of residues (Tyr455, Ala554, Arg555, Thr556, Lys621, Ser681, and Gly683) were observed in hydrophobic interactions with different chemical moieties remdesivir to keep it in RdRp pocket (Fig. 4 A, B and C). Our results are consistent with Wu et al. (2020).
Fig. 4.
Molecular interactions analysis of remdesivir and favipiravir with SARS-CoV2-RdRp. (A) and (B) represents the remdesivir and favipiravir (blue stick models) bound within the cavity of SARS-CoV2-RdRp (yellow ribbon) with interacting residues shown in red sticks. (C) and (D) represent 2D interactions of remdesivir and favipiravir with SARS-CoV2-RdRp. Ligands are shown in purple ball and stick models with interacting residues shown in their respective colors.
Similarly, detailed docking analysis of antiviral drug favipiravir (6-fluoro-3-oxo-3,4-dihydropyrazine-2-carboxamide) revealed strong binding to the binding cavity of RdRp with the binding free energy of −10.34 kcal/mol. Tyr456, Arg555 and Val557 formed H-bond and coordinate covalent interactions dihydropyrazine moiety of favipiravir. Ser624 formed pi-pi stacking interaction in addition to hydrophobic interactions formed by critical residues (Asp452, Arg555, Asp623, Thr680, Ser681, and Ser682) of catalytic site (Fig. 4 A, B and D). The strong interaction of both remdesivir and favipiravir with RdRp of SARS-CoV2 suggests a worthy option to treat COVID-19 disease with these existing antiviral medicines.
3.3. Molecular dynamics simulation analysis
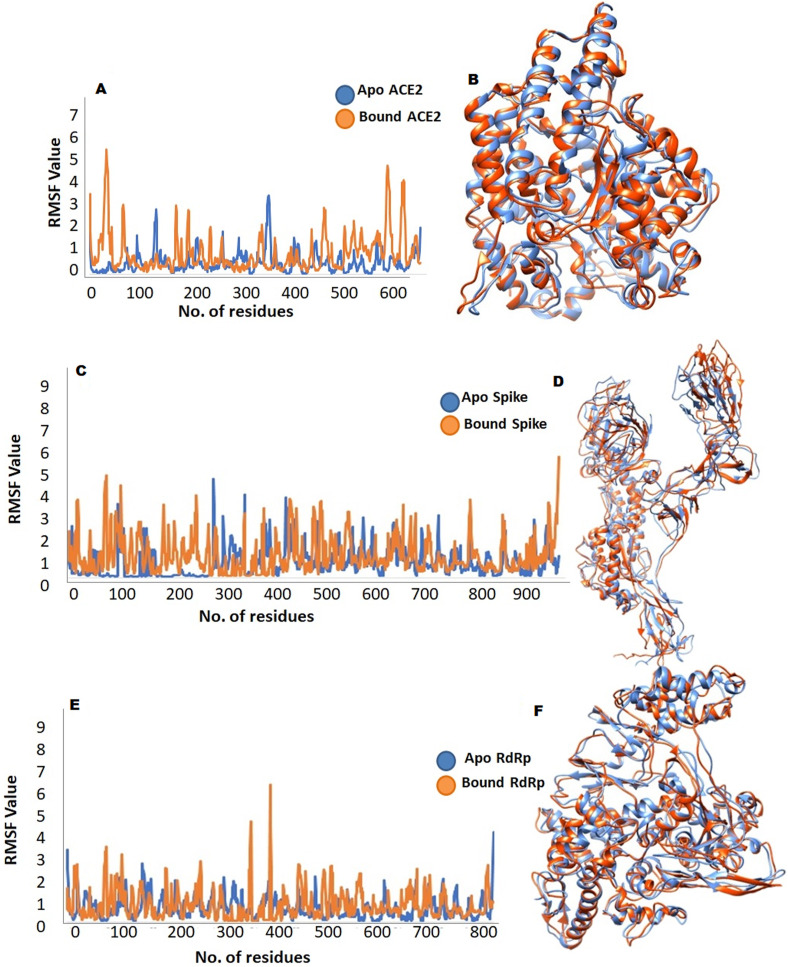
To measure the stability and structural behavior of ACE2 receptor, spike protein, and RdRp, Molecular Dynamics simulations were performed. Time series of root mean square deviation (RMSD) and root means square fluctuation (RMSF) was estimated to measure the stability and fluctuations of protein C-alpha atoms of ACE2 receptor, spike and RdRp bound and the apostates. An overall convergence of energies indicated well behaved systems. RMSF and RMSD for each system were calculated using Apo stat as a reference at 50 ns? The average RMSD value for the bound and apo state of ACE2, spike and RdRp showed stability in the overall system.
Detailed dynamic trajectories analysis of ACE2 apo, and ACE2SAA09E2 complex, showed fluctuation in and around the binding interface residues. Interestingly, all the critical residues (Phe40, Ser43, Ser44, Ser47, Trp349, Phe390, and Arg393) involved in binding showed slight changes to facilitate binding with inhibitors. Comparative analysis of the trajectories generated at different ns (ACE2-SAA09E2) with apo state of ACE2 receptor showed important structural twists. Phe40 and Ser44 bend towards the inhibitor molecule to get in pose favorable for binding. Ser43 and Ser47 were also tilted to attain binding conformation. The number of conformational changes was also observed in the loop region close to and far from the binding interface (Fig. 5 A and B).
Fig. 5.
Plots, to investigate the stability and fluctuation of molecular dynamics trajectories for the apo and bound state. ACE2 apo and bound state (A, B), spike-SARS-CoV2 (C, D) and SARS-CoV2-RdRp (e, f). Plots computed through each system trajectory apo (blue) and bound (orange) peaks. Ribbon models for fluctuation of all trajectories on the 50 ns time scale.
Similarly, the spike protein of SARS-CoV2 showed overall stability in the RMSF values in the bound (SSAA09E2) state in comparison to theapo state. Fluctuations were observed in the loop region near the binding site while the critical binding site residues (Lys458, Thr470, Glu471, Ile472, Try473, Phe497, Gly485, and Pro491) showed overall stability. Next, through analysis of dynamic trajectories of spike protein at different ns upon binding of SSAA09E2 significant structural changes were captured. Overall loops region closes to the binding residues showed more fluctuations as compared to critical binding site residues (Arg346, Ser349, Lys458, Thr470, Glu471, Ile472, Try473, Phe497, Gly485, and Pro491). Where Glu471 and Ile472 showed substantial movement to make H-bond with the SSAA09E2. Lys458 and Ser469 showed slanting towards the bound small molecule to make important interactions. In addition to these number of fluctuations were also observed in the loop region and secondary structure elements of spike protein of SARS-CoV2 (Fig. 5 C and D).
Next the analysis of RMSF fluctuation of apo and RdRp-remdesivir complex revealed more fluctuations in loop region close to the binding cavity where all the key binding residues (Val557, Ala558, Try456, Arg553, Ser683, Asp452, Tyr455, Ala554, Thr556, and Lys621) were stable with small changes to aid in binding with remdesivir.
Through comparative analysis, significant conformational changes were observed. Tyr546 to Arg555 loop region was tilted towards the center to make close contact with the drugs (remdesivir and favipiravir), Pro620 to Ala625 also showed fluctuation with the significant inward push towards the cavity. Ser681 to Gly683 region pf RdRp also tilted inwards to aid the Ser682 in the binding pose with drug molecule. In addition to these critical conformational changes, these a number of fluctuations was also detected in the loop regions and secondary structure lying far from the RdRp binding cavity (Fig. 5 E and F).
4. Discussion
The current COVID-19 epidemic painfully realizes us that our existing treatment options for hard-to-treat-disease are limited. Despite the extensive research conducted after the breakout of SARS-2003 and MERS-CoV in 2012, at present, there is no explicit drug to treat these coronaviruses. With the evident cost-and time-consuming nature of de novo drug development, the fastest and correct option is to use existing medicines for other diseases to conquer this war against COVID-19. So, drug repurposition also called drug reprofiling is the most promising strategy to identify drugs against SAR-CoV2 in a time-dependent manner.
SARS-CoV2 spike protein helps entry into cells with human receptor facilitator (ACE2). A number of studies were also confirmed that SARS-CoV2 efficiently spreads among humans with a high affinity for human ACE2 receptor protein (Walls et al., 2020). The interaction of virus spike protein with host cell surface receptor, ACE2 is appealing since it recruits the infection process of the virus. RdRp synthesizes a full-length negative-strand RNA template that makes more viral genomic RNA (Liu et al., 2020). Thus, RdRp could be an attractive target to stop the COVID-19 illness caused by SARS-CoV2.
The outbreak of this COVID-19 epidemic, triggered the researchers all over the world to explore drugs for potential therapeutics of this SARS-CoV2-induced respiratory disease. Scientists are struggling to obvious drug discovery against this virus. Several drugs such as chloroquine, arbidol, remdesivir, and favipiravir are currently undergoing clinical studies to test their efficacy and safety in the treatment of COVID-19 in China and all across the world; some promising results was achieved in March 2020 and anti-malarial drug chloroquine was confirmed as an active drug in treating coronavirus and FDA has approved it on March 28, 2020 (Wang et al., 2020). an the On June 5, 2020 the investigators from United kingdom recovery trial announced that chloroquine and hydroxychloroquine do not prove promising to reduce the mortality rate of hospitalized COVID-19 patients and results were supported by another solidarity trial, the french discovery trial (https://www.sciencemag.org/news/2020). There is little hope that chloroquine could be beneficial at the early onset of disease before getting into the hospital. Many other trials are still paused to get-go signal from the safety committee to make sure that these trials will not harm the patients under study (Kupferschmidt, June 9, 2020). So, the controversy of chloroquine at present is the dispute of the day. remdesivir and ravipiravir, are just approved for a clinical trial as a drug to treat COVID-19 (Maxmen, 2020). Although promising results were observed with remdesivir and favipiravir and many pharmaceutical companies are launching generic names for these drugs as COVID-19 treatment but to date, we are lacking sufficient pieces of evidence to call any drug as “game-changer”.
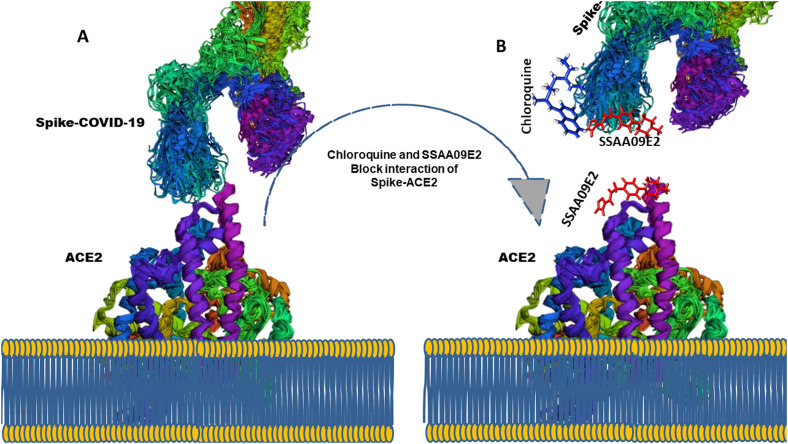
The present study was performed to identify the exact mechanistic action of repurposed drugs at a structural level using viroinformatics approach. Through molecular docking and dynamic simulation studies, we can speculate that chloroquine and SAA09E2 (Viral entry inhibitors) can block the viral entry into the human cell by blocking the binding interface of spike-RBD (virus) and human ACE2 receptor protein. So, the binding of these inhibitors with the spike-RBD domain may affect the key conformational switches required for the infection process. Similarly, binding of these inhibitors with the ACE2 receptor protein is of significant interest and it is speculated based on these results that virus entry could be stopped by blocking the viral host interaction to cure this COVID-19 illness (Fig. 6 A and B). So, these can be proposed as dual-action inhibitors instead of one bug one drug notion.
Fig. 6.
Models, of SARS-CoV2 entry into human cells through ACE2 receptor at the respiratory tract of humans. (A) Normal entry through the interaction of spike-SARS-CoV2 with ACE2 receptor (B) Chloroquine and SSAA09E2 block the interaction between two proteins to stop viral entry.
Our in-silico targeting of RdRp, via drug repurposition revealed important structural insights and important structural features of remdesivir and favipiravir to block the catalytic site so these drugs can block the synthesis of viral RNA. Docking analysis of remdesivir and favipiravir with RdRp showed strong binding with high binding free energy of −12.67 kcal/mol and −10.34 kcal/mol, respectively. Based on our computational analysis, we propose these antiviral drugs in combination with viral entry inhibitors as a good option to treat SARS-CoV2 associated respiratory disease. Likewise, our results support the computational (Wuet al, 2020) and muticlinical studies (Maxmen, 2020) conducted to find potential therapeutics measures in the war against COVID-19.
The limitation of the present study is as it was designed in march 2020, FDA approved the use of chloroquine as a treatment option for COVID-19. But there is such a rush to find the specific treatment for this rapidly spreading virus that is creating problems for the scientist to make decisions. As the controversial use of chloroquine and other drugs in more than 200 solidarity trials running in the world, so the limitation of the study is the immense pressure and controversy in the scientific community about the use of chloroquine. Secondly, at the time of methodology design, crystal structure of the RdRp enzyme of SARS-CoV2 was not available, so we predicted computationally and now the crystal structure is available in Protein Data Bank (https://www.rcsb.org/). Another limitation is we opted to run our simulations for 50 ns it would be good to perform multiple of 100 ns-scale molecular dynamics simulation with MM-GBSA/PBSA as well as detailed energy analysis.
Overall, by integrated molecular docking and dynamics simulations experiments we get confidence in our virorinformatic approach and binding behavior of the existing drugs repurposed against COVID-19 treatment. In this time of lockdown computational strategies as molecular modeling in addition to molecular dynamics simulation of receptor-drug molecules can facilitate and speed up the finding of specific and appropriate antiviral drugs for SARS-CoV2. Repurposition of existing viral entry and viral replication inhibitor in combination can be explicit therapeutic measures in the battle against COVID-19 in time-critical fashion by reducing the cost and time of de novo drug discovery.
Funding
None.
Availability of data and material
The data supporting the findings of the article are available in the Protein Data Bank (PDB) at http://www.rcsb.org and PubChem database at https://pubchem.ncbi.nlm.nih.gov/.
CRediT authorship contribution statement
Nousheen Bibi: Conceived and designed the experiments, performed experiments and manuscript writing, Sana Gul: made a significant contribution by performing molecular docking experiments and references editing, Mohammad Amjad Kamal and Johar Ali: made a substantial contribution in revising the manuscript for intellectual content.
Declaration of competing interest
The authors declare no conflict of interest, financial or otherwise.
References
- Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 2013;87(14):8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D.-G., Choi J.-K., Taylor D.R., Oh J.-W. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Arch. Virol. 2012;157(11):2095–2104. doi: 10.1007/s00705-012-1404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P.I., Ianevski A., Lysvand H., Vitkauskiene A., Oksenych V., Bjoras M., Kainov D.E. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H., Bourne P.E. The protein Data Bank. J.Nucleic Acids. Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235.%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.K., Gadthula S., Chen X., Choo H., Olgen S., Barnard D.L., Sidwell R.W. Antiviral activity of nucleoside analogues against SARS-coronavirus (SARS-CoV) Antivir. Chem. Chemother. 2006;17(5):285–289. doi: 10.1177/095632020601700506. [DOI] [PubMed] [Google Scholar]
- Fischer André, Sellner Manuel, Neranjan Santhosh, Lill Markus A., Smieško Martin. Potential inhibitors for novel coronavirus protease identified by virtual screening of 606 million compounds. ChemRxiv. Preprint. 2020 doi: 10.26434/chemrxiv.11923239.v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Hess B., Kutzner C., Van Der Spoel D., Lindahl GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4(3):435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K.J. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., McCray P.B. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79(23):14614–14621. doi: 10.1128/JVI.79.23.14614-14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt Kai. 2020. Three Big Studies Dim Hopes that Hydroxychloroquine Can Treat or Prevent COVID-19.https://www.sciencemag.org/news/2020 June. 9. [DOI] [PubMed] [Google Scholar]
- Laskowski R.A., Jablonska J., Pravda L., Varekova R.S., Thornton J.M. PDBsum: structural summaries of PDB entries. Protein Sci. 2018;27(1):129–134. doi: 10.1002/pro.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578(7795):347–348. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- Norgan A.P., Coffman P.K., Kocher J.P., Katzmann D.J., Sosa C.P. Multilevel parallelization of autoDock 4.2. J.Cheminformatics. 2011;3(1):12. doi: 10.1186/1758-2946-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Subissi L., Imbert I., Ferron F., Collet A., Coutard B., Decroly E., Canard B. SARS-CoV ORF1b-encoded nonstructural proteins 12-16: replicative enzymes as antiviral targets. J.Antiviral Res. 2014;101:122–130. doi: 10.1016/j.antiviral.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D.J.C. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127. doi: 10.1128/JVI.00127-20. 00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinf. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J. In: Coronavirus Replication and Reverse Genetics. Current Topics in Microbiology and Immunology. Enjuanes L., editor. vol. 287. Springer; Berlin, Heidelberg: 2005. The coronavirus replicase; pp. p57–94. [DOI] [Google Scholar]
- Zlenko D.V. Computing the self-diffusion coefficient for TIP4P water. J.Biophys. 2012;57:127–132. doi: 10.1134/S0006350912020273. [DOI] [Google Scholar]
Further reading
- Thailand Sees Apparent Success Treating Coronavirus with Drug Cocktail. DAWN; 2020. https://www.dawn.com/news/1532081 February 02, 2020. [Google Scholar]
- Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of the article are available in the Protein Data Bank (PDB) at http://www.rcsb.org and PubChem database at https://pubchem.ncbi.nlm.nih.gov/.