Abstract
The coronavirus disease-2019 pandemic has had a significant impact on the delivery of surgical services, particularly reconstructive surgery. This article examines the current evidence to assess the feasibility of recommencing immediate breast reconstruction services during the pandemic and highlights considerations required to ensure patient safety.
Keywords: Immediate, Breast, Reconstruction, COVID 19, Implant, Autologous
Introduction
The coronavirus disease-2019 (COVID-19) pandemic has had a significant impact on healthcare provision worldwide, particularly in the delivery of surgical services. Although decision-making has been less difficult for urgent procedures, it is proving more of a challenge for elective operations, particularly reconstructive surgery. Whilst reconstructive surgery is not life prolonging, it has proven health-related quality of life benefits.1 , 2 Immediate breast reconstruction (IBR), therefore, poses a unique surgical challenge as it fuses the urgency of surgical oncology (the mastectomy) with a functional and aesthetic procedure (the reconstruction). IBR, therefore, straddles oncological, functional and cosmetic aspects of surgery and poses a unique dilemma in the COVID-19 era. This article has been prepared in the light of aforementioned challenges and is particularly pertinent as we move towards the recovery phase of surgical services. The aims of this paper are threefold: 1) to examine the current evidence on the impact of oncological surgery on patients with COVID-19, 2) to describe our institution's processes to mitigate the risk of COVID-19 transmission and lastly 3) to consider complications that may arise post-operatively in IBR patients who contract COVID-19. Whilst the current COVID-19 pandemic appears to be resolving, it is likely that further pandemics, with second and even third waves can occur over the coming months and potentially years. Hence, it is imperative that robust protocols are now conceived, to avoid undue delay in decision-making in the future. This article represents the consensus of opinion of the Imperial College/Northwest-London Oncoplastic Breast multidisciplinary unit.
The impact of oncological surgery on patient outcomes in the COVID-19 era
Unfortunately, well-designed prospective studies in this arena remain elusive. Most of the studies that have reported their experience in patients with COVID-19, suffer from heterogeneity at multiple levels, including cancer biology, treatment protocols and COVID-19 targeted therapies. To date, there is very little literature published on peri-operative COVID-19 risks and outcomes following IBR. Indeed, breast cancer patients only comprise between 10% and 20% of the cohorts reported in these studies.3, 4, 5, 6, 7, 8, 9 This notwithstanding, the current literature from Wuhan, China3, 4, 5, 6 and New York,7, 8, 9 USA, suggest that cancer patients may be at increased risk of contracting COVID-19. Furthermore, if a cancer patient contracts COVID-19, they appear to suffer a more severe disease course with greater risk of adverse events defined as intensive care admissions, mechanical ventilation and death.5, 6, 7 Perhaps unsurprisingly, there is evidence that older age (>65 years) and multiple comorbidities are independent predictors for death in cancer patients with COVID-19.7 Whilst the majority of published studies failed to assess the impact of surgery on outcomes in COVID-19+ cancer patients, some have observed that surgery is a risk factor for severe events.3 , 5 , 6 Liang et al.5 reporting on the outcomes of 2007 patients from 575 hospitals across China identified that cancer patients who received treatment within the last month had significantly higher odds of severe events (OR=5.34, 95%CI 1.80–16.18 and p=0.0026). However, the article failed to distinguish between chemotherapy and surgery, making it difficult to interpret whether surgery or chemotherapy is implicated in this observed increased risk. Similarly, Dai et al.6 compared outcomes between 105 COVID-19+ cancer patients and 536 age-matched control non-cancer COVID-19+ patients, and observed that patients receiving surgery had a higher risk of adverse events. Finally, in a retrospective review of 28 patients conducted by Zhang et al.3, whilst the ‘anti-tumour therapy’ within the prior 14 days was associated with a greater risk of severe events (HR:4.079, 95%CI 1.086–15.322, p=0.037), the therapy did not include surgery. It should also be acknowledged that the number of COVID-19+ cancer patients with subsequent adverse events in these studies are extremely low, hence, making it difficult to interpret these findings. Liang et al.5 reported on 18 cancer patients, of which only four patients had received chemotherapy or surgery. Similarly, whilst Dai et al. reported on a substantial cohort (n=105), only 10% of the patients had cancer, and of the eight patients undergoing surgery, only one patient had a diagnosis of breast cancer.6 Similarly, of the 28 cancer patients reported by Zhang et al.3, only three had a breast cancer diagnosis. Unfortunately, none of these studies3 , 5 , 6 include sufficient operative details, to verify if any of the patients underwent immediate breast reconstruction. These studies describe outcomes during the peak transmission of the virus rather than in the recovery phase. Data from our oncoplastic MDT are more encouraging (unpublished). We audited patients who underwent autologous breast reconstruction six weeks prior to the UK lockdown (n=13) and no COVID-19-related complications were reported. Despite our reassuring local data, the published evidence makes us more cautious and selective when recommending reconstruction and on balance, the paucity of high quality prospective data has lead our oncoplastic MDT to conclude that there is insufficient evidence to continue to deny patients the benefits of immediate breast reconstruction.1 , 2
Reducing the risk and impact of COVID-19 in immediate breast reconstruction
One of the most challenging aspects of the current pandemic has been the impact on intensive care provisions. It is estimated that 3% of patients with COVID-19 will require admission to an intensive care unit for supportive therapy.10 In the UK, the majority of surgical and recovery facilities were converted into ‘makeshift’ intensive care units to increase the ventilatory capacity. Whilst the lack of intensive care facilities may be circumnavigated in future COVID-19 peaks, it is likely that surgical practice will again be significantly affected due to the shortage of anaesthetic and ancillary staff. Therefore, in future COVID-19 peaks, IBR services may have to be reduced or even halted until there is sufficient capacity in the system.
Timing of IBR
It may be argued that IBR may not be in the patient's or the health care system's best interest during the peak of the pandemic, and the Association of Breast Surgeons and the British Association of Plastic, Reconstructive and Aesthetic Surgeons supported this notion. Guidance on the reintroduction of services is less clear and perhaps should reflect on local safeguarding and the availability of facilities. As the peak of COVID-19 transmission has passed, our consensus of opinion is that the IBR service should be recommenced. We believe that this pathway is preferable to a delayed reconstruction for a number of reasons; first and foremost, an immediate reconstruction is completed in one hospital episode versus the mandatory two admissions for a delayed reconstruction, potentially reducing the risk of exposure to the virus. Secondly, we believe that the functional, psychological and aesthetic advantages of an immediate reconstruction over a delayed reconstruction are still pertinent in this setting.11 , 12 For health care systems such as the UK, it is difficult to gauge the deleterious impact of the recent COVID-19 pandemic on waiting lists. However, it is likely that many elective procedures will face significant delays with major functional and psychological consequences for these patients. It is important to consider that even in the ‘“pre-COVID-19 world’, delays of up to 2 years for an autologous reconstruction (Imperial College NHS Trust retrospective audit) were not uncommon. It is perhaps inevitable that this mismatch between demand and capacity will increase as we return to the ‘new normal’. Lastly, from a purely health economics perspective, the cost-benefit of immediate reconstruction over a delayed pathway is well documented.13 It has been shown that IBR is significantly less expensive than mastectomy, followed by a delayed reconstruction and will thus conserve NHS resources. We would, therefore, argue that on balance, IBR, is superior to delayed reconstruction financially, psychologically and aesthetically and should be the first choice pathway for patients seeking a reconstruction.
Type of IBR
It is important to consider the type of reconstructive techniques being offered to suitable candidates. Specifically, the relative merits and drawbacks of implant-based versus autologous techniques, and their impact on patient outcome following a mastectomy. Implant-based reconstructions have the advantage of shorter operative times, reduced inpatient stay 14 and the avoidance of additional morbidity in the form of scars. However, this must be balanced against the fact that implant-based reconstructions have higher revision rates,15 , 16 and some patients may either wish to avoid implants or are unsuitable candidates for an implant-based technique.
We believe that immediate autologous breast reconstruction is feasible and safe during a pandemic in high-volume microsurgical units that have tolerable operative times, low re-exploration rates and acceptable post-operative length of stay.14 In such units, autologous breast reconstructions have become routine low-risk procedures. Indeed, prior to COVID19, we offered autologous IBR to the majority of patients and did not discriminate on the basis of age, BMI or smoking status. However, we believe that during the current pandemic, changes to our standard protocol are required to enhance patient safety. Whilst we do not advocate the use of two consultants for autologous breast reconstruction in the pre-COVID-19 era, we believe that during the pandemic, this approach will improve operative times and reduce the risk of post-operative complications.17 Whilst the effect of the abdominal scar as a result of DIEP reconstruction on the patient's ability to cough remains scientifically untested, we believe that other autologous sites, such as the thighs and buttock, may be better ‘primary’ donor sites. Whenever these sites are not deemed suitable, then the abdomen can still be considered in low-risk patients, following the protocol set out in Figure 1 . Lastly, we propose that bilateral autologous breast reconstruction should be offered where clinically indicated, as the operative time and length of stay is not significantly different to patients receiving a unilateral reconstruction.18 This is particularly important in abdominal-based reconstructions, where there is only one opportunity to use the available tissue.
Figure 1.
Flow diagram of the patient's journey who is being considered and prepared for IBR.
Risk stratification for IBR and patient selection
We have considered the patients who may benefit from IBR, and of these, who would be most suitable for an autologous reconstruction (Table 1 ). It is imperative that patients being considered for IBR are risk-stratified and discussed at a local oncoplastic MDT. To guide these discussions, we have outlined factors rendering patients vulnerable to severe disease19 , 20 and factors that may increase wound complications necessitating a prolonged length of stay and/or hospital visits (thus increasing possible COVID-19 exposure) (Table 2 ). The CovidSurg Collaborative reported 30-day mortality in 1128 adults with COVID-19 who had surgery. Although overall mortality was high (23.8%), the major risk factors identified were advancing age, male sex and emergency surgery, which are not pertinent in the IBR setting.20 Taking these results together, we propose that patients over the age of 70 years, those with co-morbidities such as class II or III obesity (BMI >35), diabetes, chronic cardiac or respiratory disease should undergo a delayed breast reconstruction as they have risks, which straddle both columns in Table 2.21 , 22 Likewise, patients with more than two comorbidities, e.g. cancer and hypertension should be dissuaded from having IBR.20 Immunosuppressed patients must be given careful consideration, and patients who have completed neoadjuvant chemotherapy are a particular concern. On balance, these patients are often young, have fewer other comorbidities and will only have surgery after a four- to six-week period of recovery following their final treatment, reducing their risk to what we believe is an acceptable level. Thus, we propose that this cohort should not be excluded from being considered for IBR.
Table 1.
Patients considered as ideal candidates for IBR.
| Ideal candidate |
|---|
| Requires Chest wall resurfacing* |
| Adjuvant DXT/Previous DXT* |
| Psychological morbidity |
| Gross asymmetry leading to functional problems |
May be better suited to autologous forms of reconstruction.
Table 2.
Demonstrating risk stratification of patients being considered for IBR.
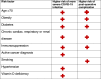 |
A highly contentious and potentially divisive risk factor that has emerged from worldwide studies is ethnicity.23 , 24 These concerns have also been highlighted in the UK in the recent observational data from the Intensive Care National Audit and Research Centre25 and the Opensafely Collaborative.19 The underlying predisposing factor is yet to be delineated, and genetic differences in the expression of angiotensin-converting enzyme 2 have been suggested as a possible mechanism.26 We propose that ethnicity should be considered in the risk assessment of patients and discussed with patients to allow for informed decision-making. A further mechanism that may explain these ethnic differences in the outcome is vitamin D levels. It appears that patients who are vitamin D deficient are at a greater risk of contracting COVID-19 and deficiency appears to be associated with a poorer outcome.27 Fortunately, vitamin D supplementation may reduce these risks.28 Vitamin D-deficient patients should take 10,000 IU of vitamin D3 per day to rapidly raise serum concentrations to 40–60 ng/mL (100–150 nmol/L). The risk of vitamin D deficiency must always be considered in the non-caucasian sub-population,29 but we propose that all patients being considered for surgery, have a vitamin D level measured preoperatively and deficiencies be corrected. We, therefore, advocate that patients being considered for IBR are risk-stratified at an oncoplastic MDT using these principles to guide decision-making. Prospective data must be collected as experience of operating during these conditions grows and criteria may have to be revised as we emerge through the various phases of the pandemic. Dynamic risk prediction tools, such as the PanSurg PREDICT study, may help to assess outcomes and complications during the COVID-19 pandemic and subsequent peaks, and facilitate in future decision-making (www.pansurg.org/predict/ ).
COVID-19 security
It is imperative to identify COVID-19+ patients preoperatively and also to reduce the risk of in hospital transmission of COVID-19 for patients being considered for IBR.20 In the UK, such procedures should only take place in hospitals designated as ‘green’ sites (www.rcseng.ac.uk). The patients should self-isolate for 14 days pre-operatively and a routine COVID-19 test should be carried out 48 h prior to admission. For autologous breast reconstructions, CT angiography should be requested because of the established benefits30 and ideally arranged on the same day as the face to face consultation. Pre-operative blood tests should also be performed on this visit. Pre-assessment and the second outpatient should then be conducted virtually where feasible. The length of inpatient stay should be reduced as much as possible, and we propose that a swab should be obtained prior to leaving the hospital to confirm that the patient does not have COVID-19 on discharge. This will have significant implications for patients as well as their caretakers. Patients should have urgent access to the surgical team and a specialist dressing clinic, but routine postoperative visits should be replaced with telephone calls and video consultations where appropriate (Figure 1).
Potential considerations in post-operative patients who contract COVID-19
We have examined the potential consequences of COVID-19 infection in post-operative patients earlier in this article. However, certain risks remain untested particularly for autologous forms of reconstruction and hence, it is important to consider some of these potential issues.
Respiratory complications
The major concern with immediate breast reconstruction and COVID-19 infection is respiratory complications. This is particularly important for autologous abdominal-based reconstructions that may affect patients’ ability to cough. It is well known that dysfunction of the respiratory muscles due to surgery may lead to a reduction in the vital capacity and thus, insufficient cough.31 , 32 Whilst the incidence of significant respiratory muscle dysfunction after upper abdominal surgery may approach 20%–40%, the figure appears much lower for lower abdominal surgery.33 We infer, therefore, that abdominal-based reconstruction is likely to be relatively safe as it will not compromise the patient's lung function significantly. To further mitigate the risk of pulmonary complications, we propose intensive post-operative physiotherapy for patients undergoing reconstruction.34 There is also the potential risk of post-operative wound dehiscence, should the patient develop a cough. We suggest that patients should be carefully selected to avoid tight abdominal closures and also to avoid patients who may have wound healing issues, such as those with a high BMI. It is imperative, however, to educate all the patients of this potential risk and a possible increased risk of hernias due to fascial dehiscence.35
Clotting complications
Hospitalised patients have an increased risk of venous thromboembolic events (VTE) and the use of chemoprophylaxis in patients undergoing autologous reconstruction is well established.36 The risk of a VTE for abdominal-based reconstructions is estimated to be around 2%-5%37 but appears much lower for implant-based reconstructions.38 COVID-19 appears paradoxically both to increase the risk of VTE and also of bleeding due to hepatic dysfunction.42 The increased risk of VTE with COVID-19 has led some to recommend high-dose anti-coagulation regimens even in the prophylactic setting.41 This will have significant implications in post-operative patients, who will be at a greater risk of developing haematomas at their surgical sites. Careful consideration must be given to this cohort of patients, and a close liaison with the haematology team is recommended. The exact mechanism of an increased risk of arterial and venous thrombosis with COVID-19 infections remains unclear. It is not yet known whether this pro-coagulant effect will also alter the microcirculation with obvious consequences for microsurgical anastomosis. This clearly is an area that requires auditing as microsurgical services resume.
Gastrointestinal and neurological complications
COVID-19+ patients may also develop gastrointestinal symptoms, such as abdominal pain, diarrhoea and vomiting. The incidence of these symptoms has been reported to be as high as 35%.40 Vomiting will need to be controlled to avoid surgical site dehiscence, and patients must be adequately resuscitated to avoid hypovolaemia that may affect flap perfusion. COVID-19+ patients can also develop acute hepatic injury leading to raised liver enzymes. Current data indicate that up to 50% develop abnormal levels of liver enzymes.40 This acute liver injury could be of consequence early in the post-operative recovery due to the risk of clotting abnormalities, which may lead to surgical site haematomas. While coronaviruses, such as COVID-19, are not usually associated with neurological disease, direct central nervous system (CNS) infection, and para-infectious disorders have been reported.39 Fortunately, most CNS symptomatology appears mild, i.e. headache and anosmia, and unlikely to have significant implications for patients recovering following IBR.
Discussion
The COVID-19 pandemic has had a significant impact on surgical services provision. IBR poses a unique surgical dilemma during such pandemics as it straddles both urgent and elective forms of surgery. The current data suggest that surgery may have a negative impact on the outcome of COVID-19+ patients; however, there is currently insufficient evidence to deny patients the benefits of an immediate reconstruction. Individual risk assessment within an oncoplastic MDT should help to risk stratify patients based on current data. We propose that IBR services be recommenced safely once the peak of transmission has passed, as long as patients are suitably selected and appropriate modifications outlined in this article are considered.
Declaration of Competing Interest
None.
Acknowledgments
Funding
None.
Ethical approval
Not applicable.
References
- 1.Zhong T, Hu J, Bagher S, et al. A comparison of psychological response, body image, sexuality, and quality of life between immediate and delayed autologous tissue breast reconstruction: a prospective long-term outcome study. Plast Reconstr Surg. 2016;138:772–780. doi: 10.1097/PRS.0000000000002536. [DOI] [PubMed] [Google Scholar]
- 2.Billig J, Jagsi R, Qi J, et al. Should immediate autologous breast reconstruction be considered in women who require postmastectomy radiation therapy? A prospective analysis of Outcomes. Plast Reconstr Surg. 2017;139 doi: 10.1097/PRS.0000000000003331. 1279-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Yin J, Qian Y, et al. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020;14:S0163–S0164. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai M, Liu D, Liu M. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0422. Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.0980. Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta V, Goel S, Kabarriti R, Cole D, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;1 doi: 10.1158/2159-8290.CD-20-0516. CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyashita H, Mikami T, Chopra N, Yamada T, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;21:S0923. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng L, Qiu H, Wan L, Ai Y, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mennie JC, Mohanna PN, O'Donoghue JM, et al. National trends in immediate and delayed post-mastectomy reconstruction procedures in England: a seven-year population-based cohort study. Eur J Surg Oncol. 2017;43:52–61. doi: 10.1016/j.ejso.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Wellisch DK, Schain WS, Noone RB, et al. Psychosocial correlates of immediate versus delayed reconstruction of the breast. Plast Reconstr Surg. 1985;76:713–718. doi: 10.1097/00006534-198511000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Khoo A, Kroll SS, Reece GP, et al. A comparison of resource costs of immediate and delayed breast reconstruction. Plast Reconstr Surg. 1998;101:964–968. doi: 10.1097/00006534-199804040-00011. [DOI] [PubMed] [Google Scholar]
- 14.Jeevan R, Cromwell DA, Browne JP, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthet Surg. 2014;67:1333–1344. doi: 10.1016/j.bjps.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Fischer JP, Fox JP, Nelson JA, et al. A Longitudinal assessment of outcomes and healthcare resource utilization after immediate breast reconstruction-comparing implant- and autologous-based breast reconstruction. Ann Surg. 2015;262:692–699. doi: 10.1097/SLA.0000000000001457. [DOI] [PubMed] [Google Scholar]
- 16.Atherton DD, Hills AJ, Moradi P, et al. The economic viability of breast reconstruction in the UK: comparison of a single surgeon's experience of implant; LD; TRAM and DIEP based reconstructions in 274 patients. J Plast Reconstr Aesthet Surg. 2011;64:710–715. doi: 10.1016/j.bjps.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Haddock NT, Kayfan S, Pezeshk RA, et al. Co-surgeons in breast reconstructive microsurgery: what do they bring to the table? Microsurgery. 2018;38:14–20. doi: 10.1002/micr.30191. [DOI] [PubMed] [Google Scholar]
- 18.de Silva TS, Russell VR, Henry FP, et al. Streamlining decision making in contralateral risk-reducing mastectomy: impact of PREDICT and BOADICEA computations. Ann Surg Oncol. 2018;25:3057–3063. doi: 10.1245/s10434-018-6593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.OpenSAFELY Collaborative. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. Ahead of print.
- 20.COVIDSurg Collaborative Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;29:S0140–S0167. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butz DR, Lapin B, Yao K, et al. Advanced age is a predictor of 30-day complications after autologous but not implant-based postmastectomy breast reconstruction. Plast Reconstr Surg. 2015;135 doi: 10.1097/PRS.0000000000000988. 253e-61e. [DOI] [PubMed] [Google Scholar]
- 22.Xue DQ, Qian C, Yang L, et al. Risk factors for surgical site infection after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 23.Khunti K, Singh AK, Pareek M, et al. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369 doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 24.Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;29:e207. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intensive Care National Audit and Research Centre. Covid-19 study case mix programme. 2020.
- 26.Liu M, Wang T, Zhou Y, et al. Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management. J Transl Int Med. 2020;8:9–19. doi: 10.2478/jtim-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhodes JM, Subramanian S, Laird E, et al. Editorial: low population mortality from COVID‐19 in countries south of latitude 35 degrees North – supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15777. Accepted Author Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant WB, Lahore H, McDonnell SL, et al. Evidence that Vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:E988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weishaar T, Rajan S, Keller B. Probability of Vitamin D deficiency by body weight and race/ethnicity. J Am Board Fam Med. 2016;29:226–232. doi: 10.3122/jabfm.2016.02.150251. [DOI] [PubMed] [Google Scholar]
- 30.Ghattaura A, Henton J, Jallali N, et al. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J Plast Reconstr Aesthet Surg. 2010;63:1597–1601. doi: 10.1016/j.bjps.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Neely WA, Robinson WT, McMullan MH, et al. Postoperative respiratory insufficiency: physiological studies with therapeutic implications. Ann Surg. 1970;171:679–685. doi: 10.1097/00000658-197005000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmo AF, Drummond GB. Respiratory mechanics after abdominal surgery measured with continuous analysis of pressure, flow and volume signals. Br J Anaesth. 1996;77:317–326. doi: 10.1093/bja/77.3.317. [DOI] [PubMed] [Google Scholar]
- 33.Celli B. Respiratory muscle strength after upper abdominal surgery. Thorax. 1993;48:683–684. doi: 10.1136/thx.48.7.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celli BR, Rodriguez K, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry and deep breathing exercise in preventing pulmonary complication after abdominal surgery. Am Rev Respir Dis. 1984;130:12–15. doi: 10.1164/arrd.1984.130.1.12. [DOI] [PubMed] [Google Scholar]
- 35.Butler DP, Plonczak AM, Reissis D, et al. Factors that predict deep inferior epigastric perforator flap donor site hernia and bulge. J Plast Surg Hand Surg. 2018;52:338–342. doi: 10.1080/2000656X.2018.1498790. [DOI] [PubMed] [Google Scholar]
- 36.Vamadeva SV, Henry FP, Hunter JE, et al. Thromboprophylaxis in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2018;71:434–435. doi: 10.1016/j.bjps.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Modarressi A, Schettini AV, Rüegg EM, et al. Venous thromboembolism events after breast reconstructions with DIEP free flaps in 192 consecutive case. Ann Chir Plast Esthet. 2018;63:11–19. doi: 10.1016/j.anplas.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Momeni A, Fox JP. Venous thromboembolism after surgical treatment of breast cancer. Ann Plast Surg. 2018;80:188–192. doi: 10.1097/SAP.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 39.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14888. May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhai Z, Li C, Chen Y, Gerotziafas G, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020 doi: 10.1055/s-0040-1710019. Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70:311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]



