Abstract
We present a case report of a 54-year-old male with metastasized nasopharyngeal carcinoma presenting to the hospital with dyspnea, anorexia and fever. Examination revealed chemotherapy-induced pancytopenia. The patient tested positive for SARSCoV-2, but respiratory complications were mild. The patient was treated with granulocyte-colony stimulating factor (G-CSF) leading to amelioration of the neutropenia. However, severe acute respiratory distress syndrome (ARDS) occurred, prompting the diagnosis of immune reconstitution inflammatory syndrome (IRIS). GCSF is currently investigated as additional therapy in ARDS, but this case report emphasizes that risks and benefits must be carefully assessed. To our knowledge, this is the first case report of IRIS-induced ARDS in a COVID-19 patient.
Keywords: ARDS, COVID-19, granulocyte-colony stimulating factor, immune reconstitution inflammatory syndrome, IRIS, SARS-CoV-2
CASE REPORT
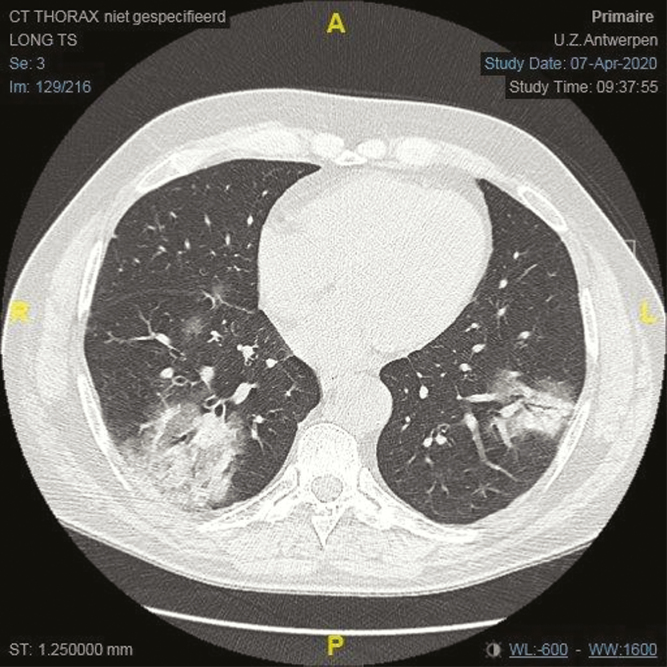
A 54-year-old male, known to have nasopharyngeal carcinoma with bone and lung metastasis, presented to the hospital with dyspnea, anorexia, and fever up to 39°C for 1 day. His last chemotherapy with cisplatinand gemcitabine was administered 4 days before presentation. As additional risk factors, he had type 2 diabetes mellitus, hypertension, and rheumatoid arthritis. His chronic medications were azathioprine, metformin, amlodipine, and pantoprazole. On presentation, he was hemodynamically stable and afebrile; respiration was stable. Arterial blood gas showed a pH within reference range with normal oxygenation levels. Blood tests revealed a profound neutropenia (0.51*109/L), a hemoglobin level of 7.1 g/dL, and thrombocytopenia of 24*109/l. His creatinine was 0.76 mg/dL (0.6–1.1 mg/dL), and his C-reactive protein (CRP) was 138.2 mg/dL (<0.10 mg/dL). Both ferritin (5452 µg/L: normal values, 22–322 µg/L) and D-dimers (3.7 µg/mL; normal values, <0.48 µg/mL) were elevated. A pulmonary computed tomography (CT) scan showed mild typical ground-glass opacities suggestive of COVID-19 (Figure 1). A positive polymerase chain reaction for SARS-CoV-2 on nasopharyngeal swab confirmed the diagnosis of COVID-19. A diagnosis of pancytopenia/neutropenic fever was made, and piperacillin/tazobactam and hydroxychloroquine were started after collection of blood and urine cultures.
Figure 1. .
Axial computed tomography thorax image showing predominantly peripheral mild ground-glass opacities compatible with COVID-19 lung injury.
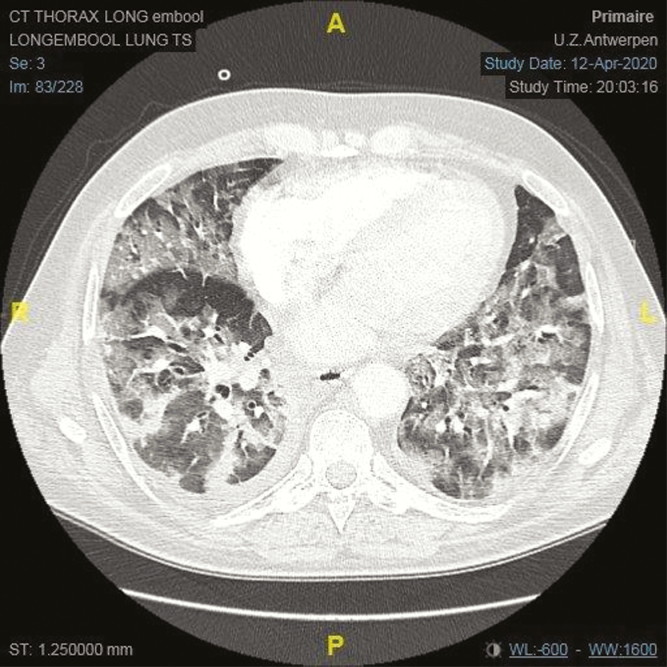
Out of concern that the pancytopenia was chemotherapy-induced, granulocyte colony-stimulating factor (G-CSF) was administered on day 2 postadmission. Leukopenia and thrombopenia are described in COVID-19 [1], but anemia is not commonly present; therefore, chemotherapy-induced pancytopenia remained the preferable diagnosis. The neutropenia resolved on day 6 postadmission, but 1 day later the patient developed progressive respiratory failure with rapidly increasing oxygen demand up to 15 L/min with a nonrebreathing mask. A new pulmonary CT scan showed severe acute respiratory distress syndrome (ARDS) with worsening of the ground-glass opacities and multiple consolidations (Figure 2). His CRP levels increased to 315.6 mg/L, and his neutrophils to 21.68*109/L. The temporal association between the G-CSF, subsequent increase in neutrophil count, and concurrent worsening of respiratory failure/pulmonary infiltrates was suggestive of a paradoxical immune reconstitution inflammatory syndrome (IRIS) to the already present SARS-CoV-2 infection. To treat this presumed IRIS, high-dose corticosteroid therapy was started. The patient recovered rapidly over the subsequent 8 days. He was no longer oxygen dependent within 14 days, his CRP declined to 50.9 mg/L, and a repeat CT scan on day 19 revealed considerable resolution of pulmonary consolidation (Figure 3).
Figure 2.
Axial computed tomography thorax with contrast image showing acute respiratory distress syndrome with massive worsening of preexisting lesions.
Figure 3.
Axial computed tomography thorax image showing residual lung injury 12 days after initial acute respiratory distress syndrome. All images illustrated were taken at approximately the same level.
DISCUSSION
Severe COVID-19 is frequently characterized as a biphasic illness, with a number of patients deteriorating ~7–14 days after initial presentation. Viral replication is thought to play an important role in the pathophysiology during the first phase, whereas systemic inflammation is considered to predominate in the second phase [2]. The evolution of the clinical presentation in this patient is most suggestive of a G-CSF-induced IRIS, but we cannot exclude the possibility that he coincidentally entered the second hyperinflammatory phase of COVID-19 soon after receiving G-CSF. The temporal association between his receipt of the G-CSF, subsequent large increase in neutrophil count, and rapid increase in pulmonary infiltrates and rapid response to corticosteroid therapy are, however, most parsimoniously explained by an IRIS-type reaction. Until very recently, there was no compelling evidence advocating for the use of corticosteroids in COVID-19 [3–5]. This situation has changed with the release of the preliminary results of the RECOVERY trial. This large open-label randomized controlled trial found that the use of 6 mg of dexamethasone for 10 days was associated with a 20%/35% reduction in mortality rate in hospitalized COVID-19 patients on noninvasive oxygen therapy/on invasive mechanical ventilation [6]. Although unpublished, the results of this trial have been considered sufficiently robust to have resulted in changes to treatment guidelines in countries like the United Kingdom. These results were not available when our patient presented to us. This new evidence of the efficacy of corticosteroid therapy does, however, reinforce the notion that our patient’s deterioration and subsequent recovery may have been due to the second phase of COVID-19 and not IRIS.
IRIS is a condition commonly seen in patients with severe immunosuppression in response to rapid immune reconstitution. It has been most commonly observed with HIV-infected individuals after starting antiretroviral therapy. Two forms have been described [3–5]. In unmasking IRIS, the immune reconstitution unmasks a previously undiagnosed infection. In paradoxical IRIS, the patient has recovered symptomatically from an infection such as pulmonary tuberculosis but then has a recurrence of symptoms after the commencement of antiretroviral therapy. A typical feature of this syndrome is that the pulmonary consolidations occur in the same location but as more pronounced versions of the original lesions [7]. Furthermore, rapid clinical improvement typically follows corticosteroid therapy [8]. These radiological and clinical features of paradoxical IRIS were both present in our patient’s presentation.
Two salient points to emerge from this case are the need for caution in the use of G-CSF in patients with COVID-19 and the rapid response to corticosteroids. Colony-stimulating factors are glycoproteins that promote production of white blood cells, mainly granulocytes, in response to infection. The most used types are macrophage colony-stimulating factor (M-CSF), G-CSF, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [9]. They are useful to reduce time spent in neutropenia in order to decrease the risk of infections and shorten the interval between chemotherapy [10, 11]. Meta-analyses have suggested that the combination of G-CSF with chemotherapy is associated with better survival [12, 13]. A number of studies are evaluating the use of CSFs in therapy for ARDS. Local therapeutic application of GM-CSF, for example, via inhalation, has been shown to increase host defense and accelerate epithelial repair processes in preclinical models [14]. It might thus be a powerful therapy in viral pneumonia and associated ARDS [14]. Animal models have suggested a benefit of combined GM-CSF in neutropenic rats with experimentally induced lung injury [15]. A randomized controlled phase II trial of GM-CSF in 10 humans with lung injury found an improvement in oxygenation over a 5-day period [16]. Furthermore, the use of inhaled GM-CSF in a small group of patients with pneumonia-associated ARDS demonstrated improvement in oxygenation, lung compliance, and severity of illness scores [17]. On the other hand, a randomized phase II trial with 130 subjects with acute lung injury and/or ARDS given intravenous GM-CSF treatment did not find any evidence of benefit [18]. A randomized controlled trial is ongoing now at the University of Michigan to determine whether a 14-day course of G-CSF improves clinical outcomes, including ventilator-free days and mortality, in patients with ARDS from all causes (https://clinicaltrials.gov/ct2/show/NCT00201409). Another trial is ongoing evaluating the efficacy and safety of inhaled GM-CSF in 45 patients with pneumonia-associated ARDS (https://clinicaltrials.gov/ct2/show/NCT02595060).
An ongoing randomized open-label trial is investigating the effectiveness of additional inhaled sargramostim (GM-CSF) vs standard of care for blood oxygenation in patients with COVID-19 and acute hypoxic respiratory failure (https://clinicaltrials.gov/ct2/show/NCT04326920). While we await the results of this RCT, our case is a reminder to carefully weigh the risks and benefits of G-CSF therapy in patients presenting with COVID-19 and neutropenia. If patients are treated with G-CSF and then deteriorate, the diagnosis of paradoxical IRIS should be considered, as corticosteroid therapy can be highly efficacious. To our knowledge, this is the first case report of a possible IRIS-induced ARDS in COVID-19.
Acknowledgments
Potential conflicts of interest. The 3 authors declare that there is no potential conflict of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Written consent of the patient was obtained by the authors. Since this paper discusses a case report, no approval of a local ethical committee was necessary.
References
- 1. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80:e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meintjes G, Lawn SD, Scano F, et al. ; International Network for the Study of HIV-associated IRIS Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med 2009; 30:797–810, x. [DOI] [PubMed] [Google Scholar]
- 5. Meintjes G, Lynen L. Prevention and treatment of the immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS 2008; 3:468–76. [DOI] [PubMed] [Google Scholar]
- 6. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv 20137273 [Preprint]. 22 June 2020. Available at: 10.1101/2020.06.22.20137273. Accessed 5 July 2020 [DOI] [Google Scholar]
- 7. Calligaro G, Meintjes G, Mendelson M. Pulmonary manifestations of the immune reconstitution inflammatory syndrome. Curr Opin Pulm Med 2011; 17:180–8. [DOI] [PubMed] [Google Scholar]
- 8. Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010; 24:2381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nemunaitis J. A comparative review of colony-stimulating factors. Drugs 1997; 54:709–29. [DOI] [PubMed] [Google Scholar]
- 10. Heuser M, Ganser A, Bokemeyer C; American Society of Clinical Oncology; National Comprehensive Cancer Network; European Organization for Research and Treatment of Cancer Use of colony-stimulating factors for chemotherapy-associated neutropenia: review of current guidelines. Semin Hematol 2007; 44:148–56. [DOI] [PubMed] [Google Scholar]
- 11. Mhaskar R, Clark OA, Lyman G, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev 2014; 2014:CD003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyman GH, Yau L, Nakov R, Krendyukov A. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol 2018; 29:1903–10. [DOI] [PubMed] [Google Scholar]
- 13. Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 2013; 24:2475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rösler B, Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol Cell Pediatr 2016; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lechner AJ, Lamprech KE, Potthoff LH, et al. Recombinant GM-CSF reduces lung injury and mortality during neutropenic Candida sepsis. Am J Physiol 1994; 266:L561–8. [DOI] [PubMed] [Google Scholar]
- 16. Presneill JJ, Harris T, Stewart AG, et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 2002; 166:138–43. [DOI] [PubMed] [Google Scholar]
- 17. Herold S, Hoegner K, Vadász I, et al. Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2014; 189:609–11. [DOI] [PubMed] [Google Scholar]
- 18. Paine R 3rd, Standiford TJ, Dechert RE, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med 2012; 40:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]