Abstract
Background
In December 2018, a large, tertiary, university-affiliated hospital in the Philippines discovered that their legitimate supply chain was infiltrated with counterfeit rabies vaccines.
Methods
All vials suspected to be counterfeit were quarantined and surrendered to the Philippine Food and Drug Administration. Patients who may have received the counterfeit products were recalled, evaluated, and revaccinated accordingly. Vials of the counterfeit vaccines were sent to various laboratories for testing.
Results
Two batches of counterfeit rabies vaccines were found to have infiltrated the hospital’s supply chain between December 2017 and December 2018. Of the 1711 patients who may have received counterfeit vaccines, 1397 patients were successfully contacted, and 734 were revaccinated with at least 1 dose of authentic rabies vaccine. The counterfeit vials were sterile, contained no toxic substances, and both contained active antirabies ingredient. No report of rabies infection or other adverse events were noted.
Conclusions
Our experience demonstrates the need for strong intervention and collaborative response from all stakeholders—government and regulatory bodies, the pharmaceutical industry, and individual institutions and consumers—to effectively eradicate counterfeiting and protect our patients.
Keywords: counterfeit vaccines, rabies, supply chain
This article describes how counterfeit rabies vaccines infiltrated one hospital’s supply chain, details its comprehensive response, including investigation, patient recall and revaccination, and vaccine testing, and illustrates how governments, the pharmaceutical industry, and individual institutions can collaborate to prevent recurrences.
Rabies is an invariably fatal acute encephalitis acquired from the bite of an animal infected with the rabies virus. Approximately 99% of all infections worldwide are transmitted through the bite of infected dogs [1], but cats, bats, raccoons, skunks, and foxes are also able to transmit the virus. Once a patient becomes symptomatic, the virus has disseminated into most organs such that death follows almost immediately. In the United States, where canine rabies virus variants have been completely eliminated, human rabies is rare with only 1 or 2 cases occurring annually since the 1960s [2]. These cases were acquired domestically from bats or from rabid dogs during international travel.
In developing countries such as the Philippines, rabies is still a very significant concern with approximately 250 patients dying from the infection each year [3]. Because there is no effective treatment once symptoms develop, there must be strong emphasis on prevention strategies. In high-prevalence countries, the World Health Organization (WHO) recommends vaccination of all patients with significant exposure even for domestic pets, risk stratified by the presence of blood on intact skin or bites on mucous membranes [4]. When given using appropriate doses and schedule, postexposure prophylaxis using inactivated rabies vaccines (with or without rabies immunoglobulin, depending on risk) is universally effective.
The WHO maintains a short list of “prequalified” vaccines that have been thoroughly evaluated to be effective and safe [5]. Of the 4 WHO prequalified vaccines, 2 are available in the Philippines: (1) an inactivated purified chick embryo cell vaccine (PCEC) manufactured by Glaxo Smith Kline ([GSK] Rabipur) and (2) a purified vero cell rabies vaccine (PVRV) manufactured by Sanofi Pasteur (Verorab).
In February 2018, the Philippine Department of Health (DOH) announced a delay in the delivery of Rabipur due to a global shortage of supply. They reported that in April 2017, the Chinese government discovered the presence of bacterial residues in their allotted supply, prompting the manufacturer, GSK, to suspend the release of Rabipur worldwide [6, 7]. This resulted in the loss of 50% of the country’s supply of rabies vaccines, which in turn affected the demand for Verorab, resulting in a nationwide shortage of vaccines [8]. It was in this environment when the Philippine Food and Drug Administration (FDA) issued several advisories regarding counterfeit Rabipur and Verorab infiltrating the legitimate supply chains in the country [9, 10].
In December of 2018, The Medical City (TMC), one of the largest tertiary hospitals in the Philippines, discovered that their supply chain was among those infiltrated by counterfeit Verorab, and these had been administered to patients. This study’s objectives are to (1) describe a single-center experience regarding counterfeit vaccines, including the modes by which patients were recalled for revaccination and the process taken to determine the nature of the counterfeit products, (2) describe the role of government and international bodies in similar events, and (3) provide recommendations to minimize institutional risk of acquiring or dispensing counterfeit medical products.
METHODS
Study Setting
The Medical City is a 500-bed, tertiary, university-affiliated, Joint Commission International (JCI)-accredited hospital in Metro Manila, Philippines. Each year, more than 70 000 patients are seen in the emergency department (ED), 1700 (2.4%) of whom consult for animal bites requiring antirabies vaccination. Before the rabies vaccine shortage, TMC procured rabies vaccines from the manufacturer (ie, Sanofi Pasteur for Verorab, Company A) only through its primary/exclusive distributor in the Philippines (ie, Zuellig Pharma, Company B). Due to the shortage of rabies vaccine, TMC was constrained to source vaccines from secondary sources, such as other FDA-licensed establishments (wholesaler/distributor) to meet its substantial patient demand. These wholesalers purchased vaccines from the exclusive distributor and are allowed by the Philippine FDA to sell them to hospital pharmacies and other outlets around the country. In December 2018, we were informed by Company A that counterfeit vaccine had been given to a patient seen at our ED (index patient). The counterfeit vaccine status was determined by its lot number (ie, the product’s lot number was found to be invalid) and was traced back to a single FDA-licensed wholesaler/distributor (Company X) operating from a city north of Metro Manila.
Investigation
The Pharmacy and Purchasing departments reviewed the records of products purchased from Company X. All products found to have been received from Company X were immediately quarantined and voluntarily surrendered to the FDA. The lot (or batch) numbers were listed and submitted to both Company A and B for verification of authenticity by lot number comparison. There were 2 lot numbers in our supply chain that were found to be counterfeit: H1833 and N1E353M. Pharmacy records were then reviewed to generate a list of all patients who had received Verorab from our hospital and that corresponded to the time we were procuring products from Company X. The list of patients was triaged according to a risk stratification scheme, which took into account the severity of the bite (category III at higher risk versus category II bites), ability to observe the biting animal (strays at higher risk versus pets), and time when the bite occurred (within the latter 6 months at higher risk versus beyond 6 months from discovery). Patients whose biting animals were alive 2 weeks after the bite were considered to have negligible risk of acquiring rabies. Patients were then informed of the situation and recalled for evaluation and possible revaccination.
Vaccine Testing
The counterfeit vaccines were subjected to the following tests done locally: aerobic culture and sensitivity, lead and mercury assays, and mass spectrometry. With the help of the Philippine FDA and the WHO, vials belonging to lot H1833 were sent to the National Institute of Infectious Diseases, a WHO Collaborating Center in Japan, where the vaccines underwent endotoxin testing and inactivation and potency testing using standard published protocols [6]. One vial belonging to lot N1E353M was sent to the Anti-Counterfeiting Laboratory of Sanofi Pasteur in Tours, France where it underwent traceability and visual analysis of the packaging as well as testing for glycoprotein G, an antigenic protein derived from the envelope of the rabies virus that induces the production of virus-neutralizing antibodies [11]. The protocols used for analysis by Sanofi Pasteur are proprietary and are not available to the authors.
RESULTS
Investigation
Our investigation revealed that the initial rabies vaccine delivery from Company X were authentic Verorab vaccines that were purchased directly from Company B. However, subsequent deliveries from Company X were found to be a mixture of authentic rabies vaccines (purchased from Company B) and rabies vaccines sourced elsewhere. As soon as the hospital received information that possible counterfeits had been dispensed, the hospital proceeded to carry out both corrective and preventive steps towards ensuring patient safety and restoring the integrity of the supply chain.
The very first step taken by TMC was to quarantine all remaining rabies vaccines purchased from Company X. This decision was reached upon discovering how TMC’s supply chain could be compromised externally and how counterfeit vaccines could still be administered to patients despite compliance with existing regulatory and mandatory safeguards. A list of products purchased from Company X was generated and then sent to Company A to determine which products were authentic.
After a comparison of the product list with Company A’s global databases, it was determined that the first delivery of counterfeit Verorab vaccines were received in December 2017 (Lot N1E353M). A second wave of Verorab counterfeits were received in August 2018 (Lot H1833; this was the lot number of the product given to the index patient). A multidisciplinary taskforce headed by an infectious disease specialist (K.E.R.H.) and composed of leaders from nursing, procurement, operations, pharmacy (A.A.C.S.), and legal (S.S.N.) departments, was created to address all aspects of the issues surrounding the counterfeit vaccines. The approach of the taskforce was 3-pronged: patient safety (corrective and preventive), vaccine investigation and testing, and legal matters.
Patients
Based on information from Company A (ie, lot number as basis for determining authenticity of the vaccines), and preferring to err on the side of caution, the taskforce assumed that patients who received counterfeit vaccines were at risk for developing rabies after their postexposure prophylaxis. It was then incumbent upon the hospital to inform the patients of this and, if required, revaccinate them using authentic vaccines. Due to variable medical record keeping, the lot numbers of administered products were not consistently recorded. This constrained the taskforce, for the purpose of patient safety, to work on another assumption: that patients without definitive evidence that they had been given authentic product (ie, vaccine cards showing lot numbers found on the authentic vaccines list) were given counterfeits.
The taskforce followed WHO 2017 rabies vaccination guidelines, which indicate that the vaccine series do not need to be restarted when doses are missed [4]. Furthermore, and upon consultation with a national rabies vaccine expert, the taskforce assumed that patients who got bitten more than 6 months ago had a very small chance of developing rabies such that they can be safely given a pre-exposure prophylaxis regimen. As much as 4 doses (after the full postexposure prophylaxis schedule) were given to patients who were bitten within the last 6 months, whereas 2 doses (after the pre-exposure prophylaxis schedule) were given for bites sustained more than 6 months from the time of discovery. For example, a patient who got bitten 3 months ago and had received 2 counterfeits and 2 authentic vaccines as postexposure prophylaxis (based on lot number) was given 2 authentic vaccines by the taskforce to replace the counterfeit doses. Information packets, containing an explanatory letter from the hospital’s President and CEO and the FDA advisory on counterfeit vaccines, were sent to patients by courier. Patients were then contacted by phone to inform them of the need for re-evaluation and possible revaccination. A clinic staffed by specially trained nurses was set up to manage the affected patients.
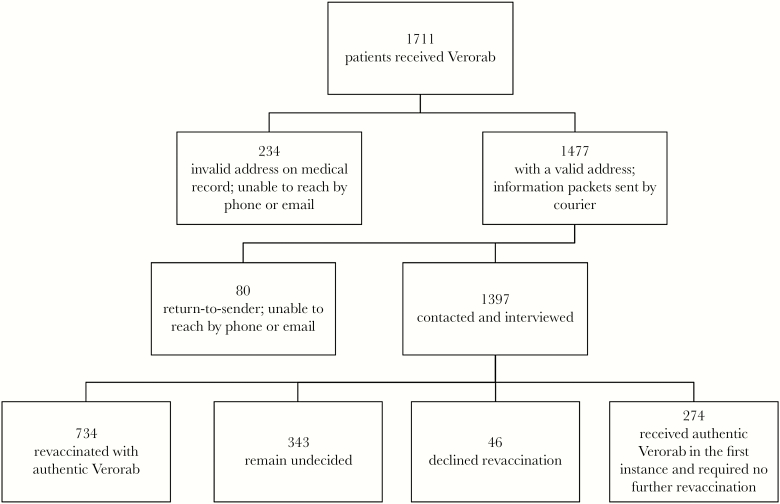
Of the 1711 patients who received Verorab vaccines from TMC between December 2017 and December 2018 when the counterfeits were discovered (Figure 1), 100% received the vaccines as postexposure prophylaxis. We sent an information packet to 1477 patients and were unable to contact 314 patients due to incorrect or outdated information in their medical record. Of the 1397 patients we informed, 734 patients were given at least 1 dose of authentic antirabies vaccine, 46 declined further doses, and 343 remain undecided or unable to schedule a visit. The rest of the patients were found to have received authentic vaccines and did not require further booster doses. None of the patients we were able to trace developed symptoms or signs of rabies. We also had no reports of any adverse events directly related to the products’ administration.
Figure 1.
Distribution of patients who received Verorab from The Medical City between December 2017 and December 2018.
Counterfeit Vaccine Testing
Aerobic cultures done on H1833 showed no bacterial growth. Lead and mercury assays showed negligible levels of these heavy metals. A comparison of the mass spectrometry of the counterfeit H1833 and an authentic vial of Verorab showed nonidentical peaks between the 2 products. When injected into suckling mice to check for inactivation of the rabies virus, the H1833 vial showed persistently inactive virus because none of the mice showed signs of clinical rabies after 21 days of observation [12]. Authentic antirabies vaccines are expected to have a minimal acceptable potency of 2.5 IU per single intramuscular dose (ie, 1 vial of vaccine = 1 dose), and no bacterial endotoxin is expected in these products [12]. The H1833 vial contained 239 IU of antigen (much higher than expected) and a negligible 0.0044 EU/vial of endotoxin was detected.
The N1E353M product had expiration dates (April 2019) consistent with other authentic rabies vaccines produced at Sanofi Pasteur Marcy L’Etoile (France) plant, but these were not marketed using its presented packaging and was never distributed in the Philippine market. Visual examination showed a fraudulent box and leaflet but genuine primary packaging (containing the actual syringe and vial), solvent, and drug product. Visual analysis concluded that only the box and leaflet were falsified and the actual drug product was genuine. Chemical analysis detected 1.9 IU of the antigen, and the report noted that the low value can be explained by the expired product (ie, expired at the time of testing), inappropriate storage conditions, and potential degradation.
Actions Taken by Hospital
One year after we discovered that counterfeit vaccines had infiltrated our supply chain, the Philippine FDA has released several other advisories about counterfeit rabies vaccines proliferating in the Philippine market [13, 14]. With very few exceptions, the hospital has since decided to no longer purchase any medical products from companies other than the exclusive distributor in the country. We have also filed the appropriate legal cases against Company X with the assistance of the Philippine FDA.
DISCUSSION
Most studies on counterfeit medical products differentiate between “substandard” and “counterfeit” drugs. Substandard drugs are “genuine products that do not meet quality specification set for them,” whereas counterfeits are “deliberately and fraudulently mislabeled with respect to identity, source, or both” [15]. Under Philippine law, substandard drugs are considered to be counterfeit pharmaceutical products. In our case, H1833 and N1E353M were counterfeit. Approximately 10% of all medical products worldwide are counterfeit, and a significant proportion of this is sold in Asia and Africa. Although most counterfeit drugs are sold in dubious online pharmacies and the “illicit/uncontrolled supply chain,” infiltration of these products in legitimate supply chains occur and should be a special cause for concern [16].
Counterfeit vaccines deserve special consideration. A counterfeit antibiotic might result in immediate worsening of an infection, possibly leading to an investigation and subsequent discovery of the counterfeit. Counterfeit vaccines, on the other hand, are more challenging to detect because development of the disease of interest, if it happens at all, may be attributed to simple vaccine failure or some other patient-related factor [17]. The long latency of rabies and almost 100% fatality once patients become symptomatic compound the challenges presented by counterfeit rabies vaccines.
Of the many methods outlined by Kelesidis and Falagas [18] on how to detect counterfeit drugs, we used the simplest method to assess and determine whether the products in our possession are counterfeit. One of the products had a lot number not used by the manufacturer (H1833), whereas the other product (N1E353M) had physical features different from a legitimate product. Comparing physical differences in packaging is unreliable especially when counterfeiter tools have become more sophisticated. Our entire investigation on the Verorab counterfeits revolved around how our products’ lot numbers were not on the list of lot numbers that were supposed to be in circulation in the Philippines.
In late 2019, a related investigation of the same FDA-licensed establishment (Company X) revealed that they were peddling another counterfeit rabies vaccine brand (Speeda) using the same valid lot number as a legitimate product [19]. It became very clear to us then that depending on lists of lot numbers and simple visual inspection of documentary evidence, which themselves can be easily counterfeited, will not suffice if we want to avoid a repeat of this mishap in the future.
In our institution’s attempt to discover what product was injected to our patients, we sought a full analysis of the contents of the vials. The H1833 vials showed a 95-fold higher antigen level compared with an authentic product. This reflects the lack of quality control in the production of the counterfeit products. It suggests that other vials in the same lot might have different levels of antigens. There are no upper limit specifications for rabies vaccine manufacturers, and untoward effects on patients from very high levels of antigen are not expected. It is notable that counterfeits containing an excess of active ingredient have been described before [20].
Sanofi Pasteur provided TMC with the final authenticity testing report, which showed that the N1E353M vaccine was genuine. It was unexpired at the time of administration to patients. However, the packaging was fraudulent, and the final report noted that the outbox and leaflet looked like “obsolete versions of packaging materials used for Verorab presentation market” in the Philippines. The counterfeit preparation certainly casts doubt on the integrity of the cold chain and, hence, product efficacy. Because the products were not intended for the Philippine market, it is possible that these genuine products, destined for another country, were smuggled in and repackaged to conform with packaging requirements set by the local FDA.
The counterfeit drug market is more profitable than even the illicit drug trade [21], and although it is more rampant in countries with less robust healthcare systems, counterfeit products can be found in every country and continent. Despite global and regional efforts to combat medical product counterfeiting, there is a pervasive attitude to keep silent about specific cases of counterfeiting among governments and pharmaceutical companies [22]. Despite an initial reluctance from Sanofi Pasteur to share the results of the vaccine testing with us, intervention by the Philippine FDA allowed us to obtain the results. Furthermore, the Philippine FDA and the WHO (through its representative in the Philippines) were instrumental in arranging for the H1833 vials to be tested in Japan. Our experience demonstrates how a collaboration between the pharmaceutical industry, government, and the private sector can result in stronger action to eradicate this global problem.
One of the most important questions we considered throughout this experience was this: how can individual institutions minimize the risk of counterfeit products infiltrating the supply chain? In a shortage environment, hospitals can be overwhelmed with evaluating suppliers in the interest of ensuring continuous supply of critically needed medications. Following the paper trail is not enough—Licenses to Operate, Certificates of Product Registration, and Lot Release Certificates can all be counterfeited by using simple, readily available software. The packaging of counterfeit medical products increasingly looks more like the original, and it might soon be impossible to tell a genuine product from a counterfeit without actual product testing. For these reasons, TMC decided to restrict procurement of medical products only from the exclusive country distributor. However, where does that leave legitimate wholesalers and distributors who can improve the access to authentic pharmaceutical products especially in remote areas? The complexity of counterfeit medical products leaves more questions than answers. However, we posit that the answers lie in more collaboration and cooperation, not less, among healthcare institutions, governments, and the pharmaceutical industry.
Our study is the first to comprehensively report on how healthcare institutions can address the problem of counterfeit vaccines. To our knowledge, this report is also the first to describe vaccine testing and correlate it with patient outcomes. Analogous to a case report, this is a single-center experience and other centers might be presented with different circumstances necessitating an entirely different strategy. Our report is a commentary on both the Philippine (and other lower- and middle-income countries’) healthcare system and the global healthcare structure. The fact that counterfeit vaccines remain a significant problem worldwide, even for industrialized nations, and despite massive intergovernmental efforts to eradicate it, shows how complex and deeply rooted the issue is. This experience has allowed TMC to further strengthen our supply chain management and record-keeping processes. However, hospitals like TMC must remain vigilant and should be ready to respond to similar situations in the future with transparency and a sense of justice.
CONCLUSIONS
Counterfeit medical products continue to proliferate worldwide and has remained a significant public health concern. This single-center experience reminds us of the need for strong intervention from all stakeholders to address this critically important global health issue. Governments must take the lead in providing strict regulatory measures as well as assistance to victims of this crime, both personal and institutional. Pharmaceutical companies must be willing partners of government and private institutions to ensure the integrity of supply chains. In the interest of patient safety, institutions affected by counterfeiting must be transparent and provide all the necessary medical and psychosocial support for patients. Our experience is a call for proactive collaboration and cooperation among suppliers, consumers, and regulatory bodies to ensure that our patients receive safe, effective, and authentic medical products.
Acknowledgments
The Medical City’s President and CEO, Dr. Eugenio Jose F. Ramos, gave his wholehearted support to the writing of this article and proofread the final draft before submission. We thank Maria Katrina Rayos for her indispensable assistance as well as all the members of the The Medical City Taskforce for Verorab for their selfless dedication to the pursuit of true patient partnership.
Author contributions. K. E. R. H. wrote most of the paper, proofread the manuscript, and prepared Figure 1. A. A. C. S. wrote part of the Methods, Results (vaccine testing), Discussion, and Conclusions. S. S. N. wrote parts of the Methods, Results, Discussion, and Conclusions.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization, Food and Agriculture Organization of the United Nations, World Organization for Animal Health, Global Alliance for Rabies Control. United Against Rabies Collaboration First annual progress report: global strategic plan to end human deaths from dog-mediated rabies by 2030. Geneva: World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE); 2019. [Google Scholar]
- 2. Rupprecht CE, Briggs D, Brown CM, et al. ; Centers for Disease Control and Prevention (CDC) Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep 2010; 59:1–9. [PubMed] [Google Scholar]
- 3. World Health Organization. Global health observatory data on rabies Available at: https://apps.who.int/neglected_diseases/ntddata/rabies/rabies.html. Accessed 16 June 2020.
- 4. World Health Organization. Rabies vaccines: WHO position paper, April 2018 – recommendations. Vaccine 2018; 36:5500–3. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. WHO prequalified vaccines Available at: https://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/. Accessed 16 June 2020.
- 6. National Institute of Infectious Diseases. Minimum Requirements for Biological Products Japan, 2006. Available at: https://www.niid.go.jp/niid/en/mrbp-e.html [Google Scholar]
- 7. Visperas E. Department of Health warns of rabies vaccine shortage The Philippine Star; 2018. Available at: https://www.philstar.com/nation/2018/03/28/1800887/department-health-warns-rabies-vaccine-shortage. Accessed 19 June 2020. [Google Scholar]
- 8. Cordova C. DOH runs out of anti-rabies vaccines Available at: https://mb.com.ph/2018/06/12/doh-runs-out-of-anti-rabies-vaccines/. Accessed 10 February 2020.
- 9. Food and Drug Administration Philippines. FDA Advisory No. 2018-138: public health warning against the purchase and use of the verified counterfeit drug product purified chick embryo cell rabies vaccine (Inactivated) (Rabipur) 2.5 I.U./mL lyophilized powder for injection (ID/IM). Manila Availabe at: https://ww2.fda.gov.ph/index.php/advisories-2/pharmaceutical-2/501819-fda-advisory-no-2018-138. Accessed 20 June 2020.
- 10. Food and Drug Administration Philippines. FDA Advisory No. 2018-334: public health warning against the purchase and use of the verified counterfeit drug product Verorab rabies vaccine. Manila Available at: https://ww2.fda.gov.ph/index.php/advisories-2/pharmaceutical-2/548944-fda-advisory-no-2018-334. Accessed 20 June 2020.
- 11. Cox JH, Dietzschold B, Schneider LG. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun 1977; 16:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization. WHO expert consultation on rabies. Third Report Available at: https://rabiesalliance.org/resource/who-2018-expert-consultation-rabies-who-trs-ndeg1012. Accessed 20 June 2020.
- 13. Food and Drug Administration Philippines. FDA Advisory No. 2019-153: public health warning against the purchase and use of the verified counterfeit version of drug Speeda rabies vaccine Available at: https://www.fda.gov.ph/fda-advisory-no-2019-153-public-health-warning-against-the-purchase-and-use-of-the-verified-counterfeit-version-of-drug-speeda-rabies-vaccine/. Accessed 20 June 2020.
- 14. Food and Drug Administration Philippines. FDA Advisory No. 2019-170: public health warning against the purchase and use of the counterfeit versions of Rabipur PCEC rabies vaccine for human use Available at: https://www.fda.gov.ph/fda-advisory-no-2019-170-public-health-warning-against-the-purchase-and-use-of-the-counterfeit-versions-of-rabipur-pcec-rabies-vaccine-for-human-use/Accessed on 20 June 2020.
- 15. Newton PN, Green MD, Fernández FM, et al. Counterfeit anti-infective drugs. Lancet Infect Dis 2006; 6:602–13. [DOI] [PubMed] [Google Scholar]
- 16. Mackey TK, Liang BA, York P, Kubic T. Counterfeit drug penetration into global legitimate medicine supply chains: a global assessment. Am J Trop Med Hyg 2015; 92:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blackstone EA, Fuhr JP Jr, Pociask S. The health and economic effects of counterfeit drugs. Am Health Drug Benefits 2014; 7:216–24. [PMC free article] [PubMed] [Google Scholar]
- 18. Kelesidis T, Falagas ME. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev 2015; 28:443–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Food and Drug Administration Philippines. FDA Advisory No. 2020-348: public health warning against the purchase and use of the counterfeit versions of purified rabies vaccine (Vero Cell) Speeda 2.5 I.U. and 0.5 mL of solvent freeze-dried powder for injection Available at: https://www.fda.gov.ph/fda-advisory-no-2020-348-public-health-warning-against-the-purchase-and-use-of-the-counterfeit-versions-of-purified-rabies-vaccine-vero-cell-speeda-2-5-i-u-and-0-5-ml-of-solvent-freeze-dried-po/. Accessed 20 June 2020.
- 20. Almuzaini T, Choonara I, Sammons H. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open 2013; 3:e002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delepierre A, Gayot A, Carpentier A. Update on counterfeit antibiotics worldwide; public health risks. Med Mal Infect 2012; 42:247–55. [DOI] [PubMed] [Google Scholar]
- 22. Cockburn R, Newton PN, Agyarko EK, et al. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med 2005; 2:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]