Abstract
Background
Risk factors and outcomes associated with carbapenem-resistant Enterobacteriaceae (CRE) acquisitions are derived primarily from cohorts consisting of carbapenemase-producing (CP) strains. Worldwide epidemiology of non-CP-CRE is evolving, but controlled epidemiological analyses are lacking.
Methods
A matched case-case-control investigation was conducted at Shamir (Assaf Harofeh) Medical Center, Israel, on November 2014–December 2016. Noncarbapenemase-producing CRE (as defined by the US Clinical and Laboratory Standards Institute Standards) carriers were matched to patients with non-CRE Enterobacterales and to uninfected controls (1:1:1 ratio). Matched and nonmatched multivariable regression models were constructed to analyze predictors for acquisition and the independent impact of carriage on multiple outcomes, respectively. Representative isolates were whole genome sequenced and analyzed for resistome and phylogeny.
Results
Noncarbapenemase-producing CRE carriers (n = 109) were matched to the 2 comparative groups (overall n = 327). Recent exposure to antibiotics (but not specifically to carbapenems), prior intensive care unit admission, and chronic skin ulcers were all independent predictors for non-CP-CRE acquisition. Acquisitions were almost exclusively associated with asymptomatic carriage (n = 104), and despite strong associations per univariable analyses, none were independently associated with worse outcomes. Genomic analyses of 13 representative isolates revealed polyclonality, confirmed the absence of carbapenemases, but confirmed the coexistence of multiple other genes contributing to carbapenem-resistance phenotype (multiple beta-lactamases and efflux pumps).
Conclusions
Noncarbapenemase-producing CRE acquisitions are primarily associated with asymptomatic carriage, specifically among prone populations with extensive recent exposures to antibiotics. The prevalent mode of acquisition is “emergence of resistance” (not “patient-to-patient transmission”), and therefore the role of stewardship interventions in reducing the spread of these therapeutically challenging pathogens should be further explored.
Keywords: antimicrobial resistance, case-case control, CRE, multidrug resistance, nosocomial infection
In case-case-control matched analyses, exposure to antibiotics was a modifiable independent predictor for non-CP-CRE acquisition, whereas genomic analyses revealed strains’ polyclonality. Therefore, the role of stewardship interventions in curbing the continued spread of non-CP-CRE in hospitals should be further explored.
Infections caused by resistant pathogens is a well established and frequently encountered global threat [1]. The World Health Organization and the Infectious Diseases Society of America listed groups of pathogens that pose the greatest epidemiological threat [2, 3]. Among these are Enterobacterales, the most common human offending pathogens in many regions [4], which are resistant to carbapenems (ie, carbapenem-resistant Enterobacteriaceae [CRE]).
Carbapenem-resistant Enterobacteriaceae created a massive global pandemic, resulting in devastating outcomes among millions of patients [5]. The initial emergence of CRE was marked by the clonal spread of a certain strain, a transposon Tn440-mediated blaKPC-producing sequence type (ST) 258 Klebsiella pneumoniae [5–7]. The epidemiology of CRE then rapidly evolved, with dissemination of the blaKPC to other Enterobacterales [8] and emergence of Enterobacterales harboring other carbapenemases [9]. However, our knowledge pertaining to the epidemiological features of CRE are derived primarily of cohorts consisting of carbapenemase-producing Enterobacteriaceae (CPE), specifically blaKPC [5, 9].
After the global spread of CRE, both the US Clinical and Laboratory Standards Institute Standards (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) revised their recommended diagnostic criteria for CRE and CPE, with considerable reductions (ie, 2–3 doubling dilutions) in the breakpoints for defining nonsusceptibility to carbapenems and enabling the diagnosis of CPE carriage solely on the presence of a carbapenemase [10–13]. This suddenly resulted in identification of a “new” group of CREs with no carbapenemases detected: noncarbapenemase-producing CRE (non-CP-CRE).
Resistance mechanisms to carbapenems among non-CP-CRE were attributed to the possible production of extended-spectrum beta lactamases (ESBLs) and/or blaAmpC cephalosporinases, coupled with outer membrane modifications such as loss of porin channels or up-regulation of efflux pumps [14]. However, molecular epidemiologically controlled analyses and explorative sequencing data are lacking. In addition, clinical epidemiological analyses of this clinical and microbiological entity are lacking: however, although the mode of acquisition and the transmission dynamics of CPE clearly demonstrated “patient-to-patient” horizontal spread [9, 15], the mode of acquisition for non-CP-CRE is still undetermined [15]. In a recent multicenter, retrospective, noncontrolled analysis from Singapore, recent carbapenem exposure was 3 times more common among patients with new acquisitions of non-CP-CRE compared with patients who acquired CPE [16]. Moreover, a small report suggested that non-CP-CRE new isolations are not commonly clustered in time nor space [17], and these 2 findings suggest that non-CP-CRE acquisition stems from endogenous emergence of carbapenem resistance among susceptible strains rather than “patient-to-patient transmission” [15], but controlled data are lacking. This issue is important, because the mode of acquisition should direct the implementation of appropriate preventive resources [18], ie, enhanced isolation and barrier precautions or stewardship initiatives to curb inappropriate usage of carbapenems or antimicrobials in general [19].
In 2 small epidemiological investigations of non-CP-CRE conducted at Johns Hopkins Hospital [20, 21], the overall 14-day mortality of monomicrobial CPE bloodstream infections ([BSIs] n = 37) was significantly worse compared with non-CP-CRE monomicrobial BSI (n = 46) [20], and non-CP-CRE asymptomatic carriage was significantly less associated with later active infection compared with CPE [21]. These data suggest that the epidemiology of non-CP-CRE differ considerably from that of CPE, and therefore well designed and well controlled epidemiological investigations of non-CP-CRE are warranted, to curb the continued emergence of these therapeutically challenging resistant organisms.
A matched case-case-control design is the preferred method to analyze the epidemiology for acquisitions of multidrug-resistant organisms (MDROs) [22–24]. It enables us to control for multiple confounders and to isolate the parameters that are independently associated with the resistance determinants [22, 23]. Our study aims were to study the clinical and molecular epidemiology of non-CP-CRE acquisitions while implementing matched case-case-control design.
METHODS
Ethics Approval
The institutional ethics committee followed the Helsinki declaration, approved the study before its initiation, and waved the need to obtain written informed consent from participants, due to the historical retrospective chart-based design.
Setting and Design
A retrospective matched case-case-control investigation was conducted among patients from all age groups at Shamir (Assaf Harofeh) Medical Center, central Israel (November 2014 to December 2016). The institutional ethics committee had approved the study before its initiation. The study comprised 3 groups: (1) “resistant case patients” with non-CP-CRE from either a screening or a clinical specimen; (2) “susceptible case patients” with an Enterobacterales isolate susceptible to carbapenems; and (3) “uninfected control patients,” ie, patients who were screened and had no documented isolation of Enterobacterales. Patients were included in the cohort only once. A susceptible case patient and an uninfected control patient were randomly selected (Excel; Microsoft) and matched to a resistant case in 1:1:1 ratio. Matching criteria (in established order of importance [22]) included the following: the infection versus asymptomatic colonization status [25]; the species, age group, and “time at risk”; and the calendar year. For both resistant and susceptible case patients, the time at risk was defined as the length of stay (LOS) from admission to culture [26]. For uninfected controls, the time at risk was adjusted by multiplying the total LOS by a random number (Excel; Microsoft) between 0 and 1 [26]. Patients who were colonized or infected with a nonenteric Gram-negative carbapenem-resistant organism were excluded (eg, Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia). Data were retrieved from all available records and included demographics, underlying conditions, recent exposures to healthcare and to antimicrobials, acute illness indices, and clinical and microbiological outcomes. Posthospitalization deaths were captured from a national registry governed by the Israeli Ministry of Interior.
Microbiological Analysis
Carbapenem-resistant Enterobacteriaceae screening policy at Shamir (Assaf Harofeh) Medical Center, as in every Israeli hospital, is mandated and tightly regulated by the Israeli Ministry of Health [27, 28]. Patients who meet certain criteria are screened upon admission and weekly thereafter [27]. Processing is conducted in accordance with established criteria [11–13] while using selective media and conducting thereafter confirmatory genotypic testing (Xpert Carba-R test; Cepheid) and full identification and susceptibility determination (VITEK 2; bioMérieux, Mercy l’Etoile, France). Non-CP-CRE were either Klebsiella species (K pneumoniae or Klebsiella oxytoca), Enterobacter species, or Escherichia coli, demonstrating a meropenem minimum inhibitory concentration ≥2 µg/dL, followed by confirmatory disk diffusion tests [13]. Isolates were tested by assays to determine the presence of blaKPC, blaVIM, blaOXA-48, blaNDM, and blaIMP (Xpert Carba-R test; Cepheid), and blaIMI [29].
Molecular Analyses
A sample of 13 K pneumoniae non-CP-CRE strains were sequenced using Illumina MiSeq (Illumina, San Diego, CA) after Nextera Flex library preparation. Bioinformatics analysis was performed as following: FASTQ files underwent quality control, filtering, trimming, and de novo assembly using fastQC (v0.11.8), Kraken (v1, with the DustMasked MiniKraken db 4GB – 2017) [30], and shovill (v1.0.0; with spades, v3.11; using the parameters “--trim” and “--opts ‘--sc’”) (https://github.com/tseemann/shovill). All assemblies were multilocus sequence typed (MLST) in silico using the tool MLST (v 2.10) (https://github.com/tseemann/mlst) based on the K pneumoniae pubMLST schema (updated April 2018) (https://bigsdb.pasteur.fr/cgi-bin/bigsdb/bigsdb.pl?db = pubmlst_klebsiella_seqdef and PubMLST website [https://pubmlst.org/]) [31]. An ad hoc core genome (cg)MLST schema was generated using chewBBACA (v2.0.9) [32], producing a schema of 3635 loci (at 95% loci presence). Results were visualized using GrapeTree (v1.5) [33]. The assemblies were searched for antimicrobial resistance genes using c-SSTAR (v1.2c, with the ResGANNOT_srst2.fasta db—07082018) (https://github.com/tomdeman-bio/Sequence-Search-Tool-for-Antimicrobial-Resistance-SSTAR-/blob/master/ARG-ANNOT.srst2_July-12-2016.fasta) [34, 35] and ABRicate (v0.8.13, with the CARD database from July 2019) [https://github.com/tseemann/abricate¡ [36].
Statistical Analyses
Analyses were all executed with IBM SPSS 25.0 (2018). Univariable matched analyses compared the characteristics of non-CP-CRE and susceptible Enterobacterales to the uninfected control group. Variables with P < .1 were incorporated into the stepwise backward selection to create 2 separated logistic regression models. In each step, the proposed model was tested for confounding. Subgroup analyses were performed when any interaction effect was identified. Time at risk was entered to both models [26]. The 2 final models of predictors associated with either non-CP-CRE or susceptible Enterobacterales were then contrasted to identify those predictors solely associated with non-CP-CRE [22, 23].
To determine whether non-CP-CRE impact hospitalization outcomes, we conducted univariable analyses pertaining to 8 different outcome parameters: in-hospital mortality, 30-days mortality, and 90-days mortality for the entire cohort and among survivors only; LOS from date of culture to discharge (or total LOS for uninfected controls); functional status deterioration [37]; additional hospitalizations (in 6 months); invasive procedures (in 3 months); and discharge to a long-term care facility (LTCF) after being admitted to the index hospitalization from home. Unmatched multivariable regression models for outcomes were constructed while enforcing the non-CP-CRE determinant into all models.
RESULTS
Initially, 111 unique patients with non-CP-CRE were enrolled. For 2 patients, no uninfected control patients could be matched due to extreme prolonged time at risk. The final cohort included 327 patients (3 groups of 109 patients). Of the non-CP-CRE, 89 were K pneumoniae, 17 were E coli, and 3 were Enterobacter spp. One-hundred four patients were asymptomatic carriers, 4 had active infection (intra-abdominal, skin and soft tissue, catheter-related bloodstream infection, and bloodstream infection with no determined focus), and for 1 patient, no sufficient data were available to determine whether she had had a catheter-associated urinary tract infection or asymptomatic bacteriuria. Among the 104 asymptomatic carriers, 4 patients (3.8%) subsequently developed a non-CP-CRE clinical infection. In the majority of susceptible case patients that were matched to the asymptomatic non-CP-CRE carriers, the susceptible Enterobacterales was isolated from patients with asymptomatic bacteriuria (n = 70), or from surveillance sputum (n = 28) or rectal (n = 6) cultures, obtained for infection control purposes. The median age of the cohort was 76 years (interquartile range, 64–85), 240 (74%) were elderly, 218 (68%) were already functionally dependent upon admission [37], 97 (30%) were cognitively impaired, and 191 (58%) had stayed in a healthcare facility in the preceding 6 months.
Table 1 summarizes the bivariable analyses conducted between the 3 groups of patients. There were no demographic differences between the various groups. Patients with Enterobacterales isolation (both non-CP-CRE or susceptible Enterobacterales) were more likely to be functionally dependent, with dementia, chronic renal failure, and recent MDRO isolation. Non-CP-CRE carriers had significant elevated Charlson’s scores [38], prior recent hospitalization(s), and/or intensive care unit (ICU) stay and were more often mechanically ventilated. The exposures to antimicrobials were more common among the non-CP-CRE carriers: namely, exposure overall (to any antibiotic) and specifically to cephalosporins, penicillins (with or without beta-lactamase inhibitors), carbapenems, metronidazole, fluoroquinolones, and vancomycin. Because the cohort primarily consisted of asymptomatic carriers, and the infection versus colonization state was the primary matching criteria, acute illness indices were indifferent between groups. However, the McCabe scores were lower among non-CP-CRE carriers [39].
Table 1.
Selected Bivariable Analyses Comparing Risk Factors and Outcomes of Patients With Non-CP-CRE, Shamir (Assaf Harofeh) Medical Center (November 2014–December 2016), (n = 109 in Each Group)
| Non-CP-CRE vs Uninfected | Susceptible Enterobacteriaceae vs Uninfected | CRE vs Susceptible Enterobacteriaceae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Non-CP-CRE No. (%)a |
Susceptible Enterobacteriaceae No. (%)a | Uninfected Controls No. (%)a | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Demographics | ||||||||||
| Age (years), median (IQR) | 76 (64–84) | 75 (63–84) | 76 (64–91) | .9 | .6 | .6 | ||||
| Age group | Pediatrics (<16 years) | 2 (1.8) | 2 (1.8) | 2 (1.8) | - | - | - | - | - | - |
| Elderly (>65 years) | 80 (73.4) | 80 (73.4) | 80 (73.4) | - | - | - | - | - | - | |
| Female gender | 49 (45.0) | 58 (53.2) | 53 (48.6) | 0.9 (0.5–1.5) | .6 | 1.2 (0.7–2.0) | >.99 | 0.7 (0.4–1.2) | .2 | |
| Background Conditions and Comorbidities Upon Admission | ||||||||||
| Partially or fully dependent in terms of functional status [37] | 91 (85.8) | 79 (73.8) | 48 (44.0) | 7.7 (4.0–15.0) | <.001 | 3.6 (2.0–6.4) | <.001 | 2.1 (1.1–4.3) | .03 | |
| Deteriorated consciousness level | 33 (31.1) | 38 (35.5) | 26 (23.9) | 1.4 (0.8–2.6) | .2 | 1.8 (1.0–3.2) | .06 | 0.8 (0.5–1.5) | .5 | |
| Ischemic heart disease | 40 (36.7) | 23 (21.1) | 39 (35.8) | 1.0 (0.6–1.8) | .9 | 0.5 (0.3–0.9) | .02 | 2.2 (1.2–4.0) | .01 | |
| Diabetes mellitus | 67 (61.5) | 57 (52.3) | 47 (43.1) | 2.1 (1.2–3.6) | .01 | 1.4 (0.8–2.5) | .17 | 1.5 (0.8–2.5) | .2 | |
| Chronic renal diseaseb | 34 (31.2) | 35 (32.1) | 20 (18.3) | 2.0 (1.1–3.8) | .03 | 2.1 (1.1–4.0) | .02 | 1.0 (0.5–1.7) | .9 | |
| Dementia | 32 (29.4) | 28 (25.7) | 14 (12.8) | 2.8 (1.4–5.7) | .003 | 2.3 (1.2–4.8) | .02 | 1.2 (0.7–2.2) | .5 | |
| Malignancy (active or in the past) | 18 (16.5) | 24 (22.0) | 21 (19.3) | 0.8 (0.4–1.7) | .6 | 1.2 (0.6–2.3) | .6 | 0.7 (0.4–1.4) | .3 | |
| Chronic skin ulcersc | 43 (39.4) | 10 (9.2) | 9 (8.3) | 7.2 (3.3–15.8) | <.001 | 1.1 (0.4–2.9) | .8 | 6.4 (3.0–13.8) | <.001 | |
| Charlson’s weighted index comorbidity [38], mean ± SD | 4.0 ± 2.3 | 3.2 ± 2.5 | 2.8 ± 2.4 | <.001 | .22 | .02 | ||||
| Overall immunosuppressiond | 22 (20.2) | 18 (16.5) | 23 (21.1) | 0.95 (0.5–1.8) | .9 | 0.7 (0.4–1.5) | .4 | 1.3 (0.6–2.5) | .5 | |
| Past MDROe in preceding 3 months before eventf | 63 (57.8) | 43 (39.4) | 2 (1.8) | 73.3 (17.2–312.2) | <.001 | 34.9 (8.2–148.7) | <.001 | 2.1 (1.2–3.6) | .01 | |
| Recent Exposures to Healthcare and to Antimicrobials | ||||||||||
| LCTF stay in the past 6 months | 32 (29.4) | 25 (22.9) | 25 (22.9) | 1.4 (0.8–2.6) | .3 | 1.0 (0.5–1.9) | >.99 | 1.4 (0.8–2.6) | .3 | |
| Hospitalized in the past 6 months | 70 (64.2) | 59 (54.1) | 46 (42.2) | 2.5 (1.4–4.2) | .001 | 1.6 (1.0–2.8) | .08 | 1.5 (0.9–2.6) | .11 | |
| ICU stay in the past 3 months | 54 (49.5) | 35 (32.1) | 28 (25.7) | 2.8 (1.6–5.0) | <.001 | 1.4 (0.8–2.5) | .3 | 2.0 (1.2–3.6) | .01 | |
| Invasive procedureg in the past 6 months before eventf | 84 (77.1) | 41 (37.6) | 74 (67.9) | 1.6 (0.9–2.9) | .1 | 0.3 (0.2–0.5) | <.001 | 5.6 (3.1–10.1) | <.001 | |
| Permanent devicesh for at least 48 hours before eventf,i | 84 (77.1) | 54 (49.5) | 74 (67.9) | 1.6 (0.9–2.9) | .1 | 0.5 (0.3–0.8) | .006 | 3.4 (1.9–6.1) | <.001 | |
| Exposures to antibioticsj in the 3 months before the eventf | Penicillins | 60 (55.0) | 26 (23.9) | 28 (25.7) | 3.5 (2.0–6.3) | <.001 | 0.9 (0.5–1.7) | .8 | 3.9 (2.2–7.0) | <.001 |
| Cephalosporins | 78 (71.6) | 43 (39.4) | 63 (57.8) | 1.8 (1.0–3.2) | .03 | 0.5 (0.3–0.8) | .007 | 3.9 (2.2–6.8) | <.001 | |
| Carbapenems | 50 (45.9) | 11 (10.1) | 7 (6.4) | 12.3 (5.3–29.0) | <.001 | 1.6 (0.6–4.4) | .3 | 7.5 (3.6–15.6) | <.001 | |
| Fluoroquinolones | 46 (42.2) | 16 (14.7) | 24 (22.2) | 2.6 (1.4–4.7) | .001 | 0.6 (0.3–1.2) | .2 | 4.2 (2.2–8.2) | <.001 | |
| Vancomycin | 34 (31.2) | 12 (11.0) | 12 (11.0) | 3.7 (1.8–7.6) | <.001 | 1.0 (0.4–2.3) | >.99 | 3.7 (1.8–7.6) | <.001 | |
| Metronidazole | 50 (45.0) | 6 (5.5) | 15 (13.8) | 5.1 (2. 6–9.9) | <.001 | 0.4 (0.1–1.0) | .04 | 14.0 (5.7–34.7) | <.001 | |
| Any antibiotic | 104 (95.4) | 61 (56.0) | 74 (67.9) | 9.8 (3.7–26.3) | <.001 | 0.6 (0.3–1.0) | .07 | 16.4 (6.2–43.3) | <.001 | |
| Conditions at the Date of Eventf | ||||||||||
| Mechanically ventilated | 38 (34.9) | 4 (3.7) | 18 (16.5) | 2.7 (1.4–5.1) | .002 | 0.2 (0.1–0.6) | .002 | 14.0 (4.8–41.1) | <.001 | |
| Rapidly fatal McCabe score [39] | 44 (41.1) | 27 (24.8) | 19 (17.4) | 3.2 (1.7–6.0) | <.001 | 1.6 (0.8–3.0) | .2 | 2.1 (1.2–3.7) | .01 | |
| Outcomes | ||||||||||
| In-hospital mortality | 37 (33.9) | 26 (23.9) | 19 (17.4) | 2.4 (1.3–4.6) | .005 | 1.5 (0.8–2.9) | .2 | 1.6 (0.9–3.0) | .1 | |
| 30-day mortality | 45 (41.3) | 35 (32.1) | 22 (20.2) | 2.8 (1.5–5.1) | .001 | 1.9 (1.0–3.5) | .04 | 1.5 (0.9–2.6) | .2 | |
| 90-day mortality | 52 (47.7) | 46 (42.2) | 28 (25.7) | 2.6 (1.5–4.7) | .001 | 2.1 (1.2–3.7) | .01 | 1.2 (0.7–2.13) | .4 | |
| Among patients who survived the index hospitalization | Length of stay from culture to dischargek, median )IQR(, n = 240 | 15 (6,32) | 5 (2,14) | 18 (10,29) | .22 | <.001 | <.001 | |||
| Functional deterioration [37], n = 238 | 34 (50.7) | 10 (12.3) | 50 (55.6) | 0.8 (0.4–1.5) | .5 | 0.1 (0.1–0.3) | <.001 | 7.3 (3.2–16.6) | <.001 | |
| Discharged to LTCF after being admitted from home, n = 220 | 28 (50.0) | 17 (22.7) | 42 (47.2) | 1.1 (0.6–2.2) | .7 | 0.3 (0.2–0.6) | .001 | 3.4 (1.6–7.2) | .001 | |
| Additional hospitalizations in the following 6 months, n = 239 | 36 (54.5) | 46 (55.4) | 38 (42.2) | 1.6 (0.9–3.1) | .1 | 1.7 (0.9–3.1) | .08 | 1.0 (0.5–1.8) | .9 | |
| An invasive procedureg in the following 3 months, n = 236 | 16 (24.6) | 23 (28.4) | 13 (14.4) | 1.9 (0.9–4.4) | .1 | 2.3 (1.1–5.0) | .02 | 0.8 (0.4–1.7) | .6 | |
Abbreviations: CI, confidence interval; CP, carbapenemase producing; CRE, carbapenem-resistant Enterobacteriaceae; CVA, cerebrovascular accident; ICU, intensive care unit; IQR, interquartile ratio; LTCF, long-term care facility; MDRO, multidrug-resistant organism; OR, odds ratio; SD, standard deviation.
NOTE: Significant associations are highlighted in bold.
aValid percentage: count divided by the total number of valid (ie, nonmissing) observations.
bSerum creatinine over 1.5 mg/dL or if patient was regularly undergoing hemodialysis or peritoneal dialysis.
cLower limb diabetic foot wounds, decubitus ulcers, dwelling wound surrounding PEG insertion, surgical-site wounds, surrounding catheters.
dImmunosuppression includes any of the following: neutropenia at culture date (<500 neutrophils/mm), exposure to glucocorticoids in the previous month, chemotherapy in the previous 3 months, radiotherapy, posttransplantation of any kind, or any immunomodulator.
eIncludes methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, extended-spectrum beta lactamase-producing Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa.
fEvent was defined as bacterial isolation for the patients who acquired non-CP-CRE or susceptible Enterobacteriaceae and as patients’ discharge date for uninfected patients.
gAny type of invasive procedure, including endoscopies, any percutaneous intervention, biopsies, and any type of surgery.
hTracheotomies, permanent central venous lines, permanent urinary catheters, orthopedic external fixators, gastrostomies, drains. Not included: internal stents, prosthetic heart valve, and prosthetic joints.
iFor uninfected patients, we considered patients to have had a permeant device if it was placed for more than 48 hours during their entire hospitalization.
jPatient received an antibiotic course of at least 48 hours.
kFor uninfected patients, we calculated the total length of stay.
In multivariable analyses (Table 2), the independent predictors associated with non-CP-CRE acquisition, but not with susceptible Enterobacterales, were as follows: (1) recent hospitalization in an ICU (or mechanically ventilated in an advanced-care room), (2) chronic skin ulcers (eg, decubitus ulcers, diabetic foot infection), and (3) recent exposure to (any) antibiotics (not specifically to carbapenems). Recent MDRO acquisition was an independent predictor for Enterobacterales acquisition in general: it was independently associated with both non-CP-CRE isolation and with susceptible Enterobacterales isolation.
Table 2.
Multivariable Models of Risk Factors for Non-CP-CRE and Carbapenem-Susceptible Enterobacteriaceae Acquisitions
| Non-CP-CRE vs Uninfected | Susceptible Enterobacteriaceae vs Uninfected | |||
|---|---|---|---|---|
| Parameter | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| ICU stay in the prior 3 months | 3.3 (1.4–7.7) | .006 | ||
| Received antibiotics in preceding 3 months before eventa | 3.1 (1.0–10.3) | .05 | ||
| Chronic skin ulcersb | 11.6 (4.3–30.8) | <.001 | ||
| Impaired functional status upon admission to hospital | 3.9 (1.7–9.2) | .002 | ||
| Past MDROc in preceding 3 months before eventa | 52.3 (11.7–233.9) | <.001 | 258.3 (32.4–2058.0) | <.001 |
| Time at riskd | 0.96 (0.93–1.0) | .04 | ||
| McCabe score [39] | 2.3 (1.2–4.4) | .008 | ||
| Received cephalosporins in preceding 3 months before eventa | 0.2 (0.1–0.5) | <.001 | ||
Abbreviations: CI, confidence interval; CP, carbapenemase producing; CRE, carbapenem-resistant Enterobacteriaceae; ICU, intensive care unit; MDRO, multidrug-resistant organism; OR, odds ratio.
aEvent was defined as bacterial isolation for the patients who acquired non-CP-CRE or susceptible Enterobacteriaceae and as patients’ discharge date for uninfected patients.
bLower limb diabetic foot wounds, decubitus ulcers, dwelling wound surrounding PEG insertion, surgical-site wounds, surrounding catheters.
cIncludes methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, extended-spectrum beta lactamase-producing Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa.
dFor both resistant and susceptible case patients, time at risk was defined as the length of stay from admission to culture. For uninfected control patients, the time at risk was adjusted by multiplying the total length of stay by a random number between 0 and 1 (Excel; Microsoft).
There were also multiple worse outcomes associated with non-CP-CRE isolation as per bivariable analyses (bottom of Table 1). For the outcomes that were captured among survivors of the index hospitalization (ie, LOS from isolation to discharge, functional deterioration [37], discharge to LTCF after being admitted from home), the non-CP-CRE determinant did not remain significantly associated with any of those adverse outcomes. For the mortality outcomes (ie, in-hospital, 30-days, and 90-days), the non-CP-CRE determinant that was enforced into the models (as depicted under Methods) became a “protecting predictor” to the outcome of interest, implying a strong confounding effect called the “Simpson’s paradox” [40].
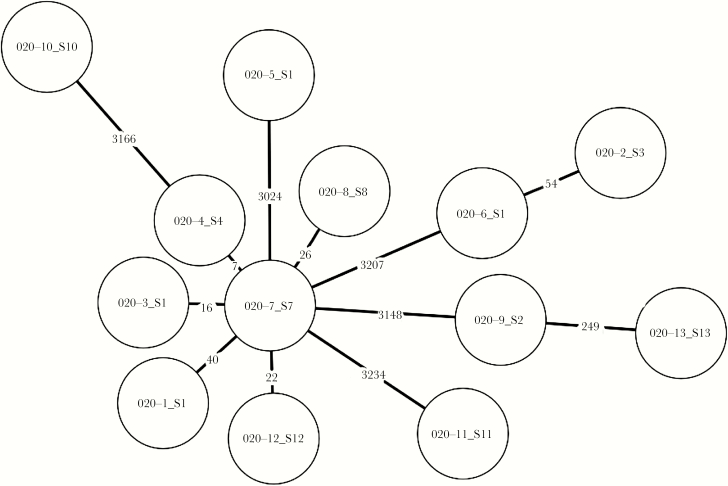
Phylogenetic analysis of the 13 isolates subjected to whole-genome sequencing is depicted in Figure 1. Six of the isolates belonged to ST-395, and others belonged to ST-101, ST-37, ST-147, ST-54, and ST-1. It is notable that the 13 K pneumoniae non-CP-CRE isolates displayed a wide range in the number of differing alleles (7–3234). Several isolates (n = 6) appeared to be clustered to some extent at a range of 7–40 alleles, which could coincide with theoretical epidemiological relatedness, at least for few of those isolates across time, but the majority of isolates appeared polyclonal and unrelated. None of the genomes harbored a known carbapenemase gene. All isolates had >1 mechanism of resistance. It is notable that each of the 13 strains harbored 4 distinct beta-lactamase genes including blaOXA-1, blaTEM-1, blaSHV (including ESBLs), and blaCTX-M-15. Typical RND-family efflux pump genes associated with beta-lactamase resistance such as acrB were found in all of the samples, in addition to efflux pumps of the oqxAB family, which mainly contribute to quinolone resistance.
Figure 1.
Phylogenetic analysis of representative study isolates. A minimum spanning tree was constructed based on an ad hoc core genome multilocus sequence typed schema of 3635 loci. Each node represents a Klebsiella pneumoniae isolate (n = 13), and the numerical labels over connecting lines indicate the number of different loci between the sequenced isolates.
DISCUSSION
The CRE healthcare-associated pandemic is one of the biggest challenges and threats in modern medicine [2]. Its epidemiology in recent years rapidly evolved, and along with (or partially due to) the CLSI and EUCAST breakpoints modifications [11–13], a new “epidemiological entity” of non-CP-CRE has emerged and has been reported in studies from around the world [15]. Despite the early reports displaying wide differences between the epidemiology of CPE and non-CP-CRE [20], controlled analyses pertaining to the predictors, outcomes, mode of acquisition, and transmission dynamics of non-CP-CRE are lacking. As far as we know, this is the first matched case-case-control investigation in this research field.
There are currently several definitions and recommendations pertaining to the diagnosis, treatment, and prevention of non-CP-CRE [10, 11]. Some have not even addressed the issue and use a broad definition of “CRE” to guide the management of both CPE and non-CP-CRE. Even the 2015 guidelines by the Centers for Disease Control and Prevention do not differentiate between the 2 entities in terms of infection control-suggested measures (although it does acknowledge the issue) [10]. As depicted in this study and by others [15, 16, 20, 21], the epidemiology of non-CP-CRE differ considerably from that of CPE, and therefore measures should be tailored based of controlled analyses.
The study included 327 patients (consisting of 3 groups of 109 patients), but only 8 had acute active infection (ie, 4 with non-CP-CRE and 4 with susceptible Enterobacterales). This might imply, as suggested by others, that current non-CP-CRE isolates display low virulence properties and reduced endemic profiles, compared with CPE isolates, but this necessitates directed controlled investigations [15, 16, 20, 21]. However, theoretically, the therapeutic challenges associated with non-CP-CRE active infection resemble those of CPE [41], and therefore the exploration of the clinical and molecular epidemiological features of non-CP-CRE, and the mode of their acquisition and spread in hospitals, is still of paramount importance. Moreover, as displayed in our cohort as well [21], some asymptomatic carriers (4%), eventually develop active infection, including BSIs.
In this matched case-case-control investigation, we were able to provide some genuine insights into the clinical and the molecular epidemiology of non-CP-CRE. The fact that the majority of our cohort consisted of asymptomatic carriers, and not patients with active infection (as studied in other cohorts [20]), is one of the study’s advantages, because this is the most common mode in which CRE is first diagnosed in most regions [42], and early prevention interventions could be applied. We discovered that the independent predictors for non-CP-CRE acquisitions were recent admission to an ICU, chronic skin ulcers (mostly decubitus ulcers, and/or chronic diabetic foot infections), and recent exposures to (any) antibiotics. Recent ICU admissions and chronic skin ulcers are somewhat nonmodifiable predictors that might serve as markers for exposures to (1) high selective pressures and/or colonization pressures in units [43], (2) severe burdens of active and/or chronic background conditions, and (3) deteriorated functional status in baseline. However, exposure to (any) antibiotics is a modifiable predictor and should therefore direct our future allocation of non-CP-CRE prevention resources.
Exposure to any antibiotics, not specifically to carbapenems, was a predictor for non-CP-CRE in this trial, as opposed to a previous investigation [16]. This again highlights the advantages associated with the case-case-control design, because it enables us to isolate the factors that “truly” impact, independently, the emergence of the resistance determinant(s) among susceptible strains. Moreover, this distinction of recent exposure to any antibiotic versus specific exposure to carbapenems was also illustrated in the past, among cohorts of CPE, that were analyzed by the matched case-case-control design, versus the “case-case design” [24]. In univariable analyses, exposures to many antibiotics were associated with non-CP-CRE acquisition, even recent exposures to vancomycin and metronidazole (Table 1), which have no established activity against Enterobacterales.
Whole-genome sequencing of 13 K pneumoniae non-CP-CRE strains (all obtained from asymptomatic carriers) displayed notable polyclonality (Figure 1). In addition, isolates displayed a coexistence of several mechanisms for beta-lactam resistance but not carbapenemases. These molecular features, along with the clinical epidemiology features as depicted above (ie, the independent association with recent exposure to any antibiotic), should lead to stewardship interventions that focus on reducing usage of antimicrobials consumption in general and not specifically focus solely on restriction of carbapenems.
Despite the fact that the cohort consisted mostly of asymptomatic carriers, non-CP-CRE was a strong marker for severe chronic and background conditions, with elevated Charlson’s scores [38]. In univariable analyses, non-CP-CRE was significantly associated with worse morbidity and mortality outcomes (Table 1). However, when we enforced this parameter into the outcomes’ regression models, along with other parameters associated with worse outcomes, it became a protecting factor, illustrating the Simpson’s paradox [40]: although non-CP-CRE was diagnosed among asymptomatic carriers, acute illness indices (eg, mechanical ventilation, McCabe score), which are naturally associated with worse outcomes of the acute condition, diluted the potential impact of being a non-CP-CRE asymptomatic carrier.
The study has several limitations. First, it is a retrospective, chart review-based, single-center analysis, with all its inherent limitations. However, the matched case-case-control design could overcome, at least in part, some of these nonmodifiable limitations, particularly regarding the predictors’ (for acquisition) analysis. Second, we sequenced the whole genome of only 13 isolates of non-CP-CRE. This might limit our conclusions pertaining to clonality, but in terms of representing the prevalent mechanisms of resistances to carbapenems and genetic relatedness, the conclusions are probably less affected by the low sample size of sequenced isolates. In addition, we did not perform molecular analysis of gene expression of porins, which could also contribute to carbapenem-resistant phenotypes. Third, there are inconsistencies in many clinical laboratories in non-CP-CRE diagnostic recommendations [11]. Therefore, some isolates that grow on the initial screening selective media and are directly subjected to a genotypic test of already known carbapenemases might not have been enrolled. Finally, because the majority of patients with non-CP-CRE were asymptomatic carriers, the impact of the resistance determinant on patients’ outcomes and efficacy analyses pertaining to the therapeutic management of non-CP-CRE active infection are limited.
CONCLUSIONS
This is the first study to explore, by using case-case-control design, the epidemiology of non-CP-CRE, which has become prevalent in many facilities around the world. Due to lack of controlled data, recommendations pertaining to non-CP-CRE diagnostics, prevention, and management are inconsistent. These analyses clearly illustrate that focusing on stewardship interventions, to limit usages of antibiotics in hospitals, could curb the continued emergence and spread of non-CP-CRE.
Acknowledgments
This work was performed in partial fulfillment of the MD thesis requirements of the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med 2010; 362:1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed Available at: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/. Accessed 14 November 2019.
- 3. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 4. Marchaim D, Zaidenstein R, Lazarovitch T, et al. Epidemiology of bacteremia episodes in a single center: increase in Gram-negative isolates, antibiotics resistance, and patient age. Eur J Clin Microbiol Infect Dis 2008; 27:1045–51. [DOI] [PubMed] [Google Scholar]
- 5. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navon-Venezia S, Leavitt A, Schwaber MJ, et al. ; Israeli KPC Kpn Study Group First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lazarovitch T, Amity K, Coyle JR, et al. The complex epidemiology of carbapenem-resistant enterobacter infections: a multicenter descriptive analysis. Infect Control Hosp Epidemiol 2015; 36:1283–91. [DOI] [PubMed] [Google Scholar]
- 9. van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 8:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE), November 2015 update - CRE toolkit Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed 15 November 2019.
- 11. Richter SS, Marchaim D. Screening for carbapenem-resistant Enterobacteriaceae: who, when, and how? Virulence 2017; 8:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters Available at: www.eucast.org/clinical_breakpoints/. Accessed 15 November 2019.
- 13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 22nd ed. Informational supplement. M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; ; 2012. [Google Scholar]
- 14. Iredell J, Brown J, Tagg K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications. BMJ 2016; 352:h6420. [DOI] [PubMed] [Google Scholar]
- 15. Goodman KE, Simner PJ, Tamma PD, Milstone AM. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev Anti Infect Ther 2016; 14:95–108. [DOI] [PubMed] [Google Scholar]
- 16. Marimuthu K, Ng OT, Cherng BPZ, et al. Antecedent carbapenem exposure as a risk factor for non-carbapenemase-producing carbapenem-resistant-enterobacteriaceae and carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2019; 63:e00845-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dykman L, Mor D, Fakeh O, et al. The Epidemiology of Non-carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae (CRE non-CP). Madrid, Spain; European Congress of Clinical Microbiology and Infectious Diseases, 2018. [Google Scholar]
- 18. Adler A, Friedman ND, Marchaim D. Multidrug-resistant Gram-negative bacilli: infection control implications. Infect Dis Clin North Am 2016; 30:967–97. [DOI] [PubMed] [Google Scholar]
- 19. Pogue JM, Kaye KS, Cohen DA, Marchaim D. Appropriate antimicrobial therapy in the era of multidrug-resistant human pathogens. Clin Microbiol Infect 2015; 21:302–12. [DOI] [PubMed] [Google Scholar]
- 20. Tamma PD, Goodman KE, Harris AD, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis 2017; 64:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamma PD, Kazmi A, Bergman Y, et al. The likelihood of developing a carbapenem-resistant Enterobacteriaceae infection during a hospital stay. Antimicrob Agents Chemother 2019; 63:e00757-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaye KS, Harris AD, Samore M, Carmeli Y. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol 2005; 26:346–51. [DOI] [PubMed] [Google Scholar]
- 23. Harris AD, Samore MH, Carmeli Y. Control group selection is an important but neglected issue in studies of antibiotic resistance. Ann Intern Med 2000; 133:159. [DOI] [PubMed] [Google Scholar]
- 24. Marchaim D, Chopra T, Bhargava A, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect Control Hosp Epidemiol 2012; 33:817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008; 52:1028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Center for Infection Control, Israel Ministry of Health. Guidelines for Labratory Screening, Reporting and Prevention of Infections Caused by Carbapenem-Resistant Enterobacteriaceae (CRE). MInistry of Health; 2016. Available at: https://www.health.gov.il/hozer/mr14_2016.pdf. Accessed 4 August 2020. [Google Scholar]
- 28. Schwaber MJ, Lev B, Israeli A, et al. ; Israel Carbapenem-Resistant Enterobacteriaceae Working Group Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011; 52:848–55. [DOI] [PubMed] [Google Scholar]
- 29. Babu Rajendran N, Gladstone BP, Rodríguez-Baño J, et al. EpideMiology and control measures of outBreaks due to Antibiotic-Resistant orGanisms in EurOpe (EMBARGO): a systematic review protocol. BMJ Open 2017; 7:e013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 2014; 15:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva M, Machado MP, Silva DN, et al. chewBBACA: a complete suite for gene-by-gene schema creation and strain identification. Microb Genom 2018; 4:e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou Z, Alikhan NF, Sergeant MJ, et al. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res 2018; 28:1395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cunningham SA, Limbago B, Traczewski M, et al. Multicenter performance assessment of carba NP test. J Clin Microbiol 2017; 55:1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Man TJB, Limbago BM. SSTAR, a stand-alone easy-to-use antimicrobial resistance gene predictor. mSphere 2016; 1:e00050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia B, Raphenya AR, Alcock B, et al. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 2017; 45:D566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA 1963; 185:914–9. [DOI] [PubMed] [Google Scholar]
- 38. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 39. Bion JF, Edlin SA, Ramsay G, et al. Validation of a prognostic score in critically ill patients undergoing transport. Br Med J (Clin Res Ed) 1985; 291:432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Julious SA, Mullee MA. Confounding and Simpson’s paradox. BMJ 1994; 309:1480–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 2013; 75:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhargava A, Hayakawa K, Silverman E, et al. Risk factors for colonization due to carbapenem-resistant Enterobacteriaceae among patients exposed to long-term acute care and acute care facilities. Infect Control Hosp Epidemiol 2014; 35:398–405. [DOI] [PubMed] [Google Scholar]
- 43. Bonten MJM. Colonization pressure: a critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care (London, England) 2012; 16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]