Abstract
Purpose
To evaluate the incidence, visual prognosis, and mortality in retinopathy of prematurity (ROP) in Korea.
Methods
We used the National Health Insurance and the Korean Disability Registry database, which covers the entire newborn population in 2006 to 2014 and includes information on all newborns diagnosed with ROP until 2016. Using these databases, we evaluated the incidence, rate of visual impairment (VI), and mortality in patients with ROP according to the birth weight categories and treatment modalities.
Results
The ROP incidence per 1000 newborns was 1.99, which broke down into 317.14 in the very low birth weight (VLBW) less than 1500 g population, 25.45 in the 1500 to 2499 g population, and 0.29 in the 2500 g or greater population. When assessed at age 10, the VI rate was 2.2 per 100 person-years, which was highest at 4.5 per 100 person-years in the VLBW population compared with the population in other birth weight categories. Among treated cases, the proportion of VI in patients undergoing laser photocoagulation or cryotherapy was 1.6% (42/2595), which was lower than the 2.9% (2/68) of patients treated with anti-vascular endothelial growth factor injection, and 32.2% (82/255) of patients undergoing vitrectomy or scleral buckling. The mortality rate was 4.8 per 1000 person-years, which was highest in the VLBW population, but similar across treatment modalities.
Conclusions
The ROP incidence in Korea was approximately 1 in 500 among all newborns, and 1 in 3 in the VLBW population. As the first nationwide population-based study of long-term visual prognosis in ROP, we report the higher VI rate in ROP than previously determined in other studies. Differences in visual outcomes and comparable mortality risks between treatment modalities require further verification.
Keywords: retinopathy of prematurity, incidence, visual prognosis, mortality
Retinopathy of prematurity (ROP) is a vascular proliferative disease of the developing retinal vasculature in premature infants, and one of the leading treatable causes of childhood blindness.1 Because the rate of premature births with higher risk of ROP is increasing,2 detection and possible treatment of acute phase ROP are becoming more important.
Low birth weight is one of the most important risk factor for ROP development.3 However, birth weight–specific incidence differs between studies including those conducted in the United States and Turkey, ranging from 19% to 42% in very low birth weight (VLBW) infants weighing less than 1500 g, and 26% to 56% in extremely low birth weight infants weighing more than 1000 g.4–6 Furthermore, these findings are from hospital-based studies, which could be prone to selection bias, and influenced by the quality of neonatal care in institutions. Although population-based studies are needed for incidence estimation of ROP, only a few have been done, most of them including the Swedish and UK studies were outdated.7,8
Although ROP is a major cause of blindness in premature infants,1,9 little is known about the rate of visual impairment (VI) in ROP. Most ROP studies were limited to the initial hospitalization period, thus making it difficult to identify the presence of VI through subsequent follow-ups. Because most children should reach a certain age for proper assessment of visual status, the precise estimation of the VI rate may require a long observation period.
To our knowledge, the proportion of treated ROP children and distribution of treatment modalities, such as cryotherapy, laser photocoagulation, and anti-vascular endothelial growth factor (VEGF) injection for acute phase disease, and surgery which is generally undertaken for retinal detachment, have not been well evaluated nationwide in Korea. Furthermore, recent studies reported the ophthalmologic outcomes and death risks according to the treatment methods in ROP.10,11 Therefore, we evaluated the nationwide distribution of treatment modalities in Korea, and investigated the possible associations of the type of ROP treatment with long-term visual prognosis and mortality.
In Korea, the National Health Insurance (NHI) run by the government covers the entire newborn population and records all medical status and procedures conducted through follow-ups. Through the NHI database, we could identify the diagnosis of ROP and related treatments in the entire Korean newborn population. The government also runs the Korean Disability Registry (KDR) program, where visually impaired persons are registered based on specific visual acuity (VA) and visual field criteria confirmed by ophthalmologists. Using the NHI-KDR database, we could observe all newborns diagnosed with ROP over time and evaluate the incidence, VI occurrence, and mortality in patients with ROP according to the birth weight categories and treatment modalities across the entire Korean newborn population.
Methods
Ethical Considerations
This study followed the tenets of the Declaration of Helsinki, and the study protocol was approved by the Research Ethics Committee of Korea University College of Medicine. All personal information was made anonymous by encryption.
Data Source
We assessed data from the NHI claims database and the KDR program. The NHI database of Korea is a repository of medical claims data collected through the reimbursement process for healthcare providers. Under the mandatory nationwide insurance system, the NHI claims database covers the entire population, including all newborns, and contains comprehensive information on healthcare services such as demographics; admission, discharge, and date of hospital visit; prescriptions, procedures, and surgeries; principal diagnosis and comorbidities based on the Korean Standard Classification of Disease, which is a modified version of the International Classification of Diseases, 10th edition (ICD-10). The NHI database also contains the results of the National Health Screening Program for Infants and Children, which is a government-run health screening program for all children under age 6, launched in 2007. The examinations in the health screening program are conducted seven times at prespecified intervals between 4 and 71 months of age and include birth weight information from structured questionnaires to children's parents or guardians.
The Korean government operates the KDR program for certain disabilities, including VI. The KDR is a registration program for persons with disabilities, and requires a verification executed by a physician specializing in the related field, thus ensuring diagnostic reliability. Patients in this program become eligible for financial support, including disability pensions, tax benefits, and reduction of public facility fees. The registration for the KDR program for VI is based on the specific criteria of best-corrected VA or visual field status defined by the Korean Ministry of Health and Welfare, which should be confirmed by an ophthalmologist who achieved board certification.12 VI is classified into the following 6 grades12:
Stage 1 (most severe): VA of 20/1000 or worse in the better-seeing eye
Stage 2: VA of 20/500 or worse in the better-seeing eye
Stage 3: (1) VA of 20/320 or worse in the better-seeing eye or (2) visual field of 5 degrees or less in each eye
Stage 4: (1) VA of 20/200 or worse in the better-seeing eye or (2) visual field of 10 degrees or less in each eye
Stage 5: (1) VA of 20/100 or worse in the better-seeing eye or (2) loss of 50% or more of the visual field in each eye
Stage 6 (mildest): VA of 20/1000 or worse in the worse-seeing eye
Using the NHI-KDR database, we could observe all newborns diagnosed with ROP until 2016, and evaluate the incidence, treatment performed, and visual outcomes in patients with ROP in Korea. We also assessed the data from Statistics Korea, which is a national statistics database on deaths in the entire Korean population, and is integrated into the eligibility database of the NHI, to evaluate the vital status for all patients with ROP.
Study Population
We studied all newborns during the 9-year period from 2006 to 2014, classifying them into three categories of birth weight: VLBW less than 1500 g, between 1500 g and 2499 g, and 2500 g or more. These categories were based on birth weight data from the health screening program and ICD-10 code (P0700 to P0711 for VLBW; P0712 to P0714 for a birth weight of 1500–2499 g) from the NHI database.
Identification of ROP
To identify patients with ROP from the NHI database, our research team, which included six experts in the fields of ophthalmology, epidemiology, and pediatrics, developed the following algorithms by considering how coding is done for ROP in Korea and by reviewing clinical information in medical records of patients with ROP in Korea University Medical Center:
-
(1)
Inpatient ICD-10 code H35.1;
-
(2)
Birth weight of less than 2500 g given inpatient ICD-10 code H35;
-
(3)
Inpatient or outpatient ICD-10 code H35.1 with evidence of treatment for ROP;
-
(4)
Birth weight of less than 2500 g given inpatient or outpatient ICD-10 code H35 with evidence of treatment for ROP.
Determination of Ocular Comorbidities
We determined the association of the following ocular comorbidities other than ROP based on ICD-10 code, which could influence the visual outcomes in patients with ROP:
-
(1)
Congenital ocular anomalies, such as anophthalmos, microphthalmos, macrophthalmos, congenital glaucoma, and congenital malformations of lens, anterior, or posterior segment of eye,
-
(2)
Retinal dystrophies, including retinitis pigmentosa, Leber congenital amaurosis, and Stargardt disease,
-
(3)
Ocular neoplasms, such as retinoblastoma, and optic nerve glioma,
-
(4)
Severe ocular traumas, including injuries of the optic nerve and pathways, rupture or penetrating wound of eyeball, and avulsion of eye.
Classification of Treatment Modalities
We obtained the information from the NHI database about the type and date of treatment performed. We divided treatment modalities into three categories:
-
(1)
Laser/Cryo: laser photocoagulation (NHI claims code S5160/S5161) or cryotherapy (S5140),
-
(2)
Anti-VEGF: intravitreal injection (S5070) of anti-VEGF with/without laser photocoagulation or cryotherapy, and
-
(3)
Surgery: vitrectomy (S5121/S5122) or scleral buckling (S5130), with or without laser photocoagulation, cryotherapy, or anti-VEGF injection.
According to this classification, patients undergoing anti-VEGF injection were assigned to the anti-VEGF category even if they had undergone Laser/Cryo, and surgically treated patients were assigned to the surgery category even if they had also been treated by nonsurgical modalities. Therefore, each ROP patient was allocated to a single category of treatment modalities.
Ascertainment of VI
We obtained the VI information by using the KDR database, where VI was graded from 1 (most severe; VA of 20/1000 or worse in the better-seeing eye) to 6 (mildest; VA of 20/1000 or worse in the worse-seeing eye). We classified the degree of VI into either severe or mild. A severe VI was grade 1 or 2, which was defined as a VA of 20/500 or worse in the better-seeing eye, corresponding to the World Health Organization criteria for blindness.13 A mild VI, of grades 3 to 6, was defined as (1) a VA of 20/100 or worse in the better-seeing eye, (2) a VA of 20/1000 or worse in the worse-seeing eye, or (3) a loss of 50% or more of the visual field in each eye. We observed newborns with ROP until 2016, and assessed the VI occurrence, degree of VI, and date of VI registration in the KDR database.
Assessing the Mortality Rate
We acquired the data from the Statistics Korea database, which includes physician-certified death information for all Koreans since 1981 and is integrated into the eligibility database of the NHI. Using this database, we identified the cases and times of death for all patients with ROP until 2016.
Statistical Analysis
We studied the incidence, visual outcomes, and mortality rates for patients with ROP in Korea. The incidence in each fiscal year was calculated as the number of incident ROP cases divided by the number of newborns in that year from 2006 to 2014. The newborn population in each fiscal year according to the data from the Korea Statistical Information Service was used to calculate the incidence (http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01; as of January 31, 2019). Birth weight-specific incidence rates were also calculated. We also performed linear regression analyses to determine the annual trends of the ROP incidence by birthweights. Moreover, the proportions of patients with ROP who underwent each treatment modality until 2016 were calculated.
We calculated the VI rate at each age-point until 2016 according to the birth weight, based on the registration age in the KDR program. Logistic regression analysis was conducted to examine the association of treatment modalities with VI. Variables for the analysis were sex, birth weight, birth year, and ocular comorbidities.
We estimated the mortality rate by calculating person-years at risk, based on the age at death in the Statistics Korea database. We also compared the mortality risk between treatment methods, after excluding the infantile deaths in which treatments for ROP might not be performed owing to poor general condition, thus possibly decreasing the influence of treatment on mortality in the analysis. We used Cox regression to calculate hazard ratios for mortality between treatments, adjusting for sex, birth weight, birth year, and ocular comorbidities; we applied logistic regression to analyze VI-associated factors, considering the gap between the VI occurrence and the time of registration for the KDR program as VI. We used Stata/MP2 (version 13.0; StataCorp, College Station, TX) in the analyses.
Results
Incidence
In 4,149,959 Korean newborn population between 2006 and 2014, we identified 8273 with ROP, of which 4352 (52.6%) were boys and 3921 (47.4%) were girls. The crude incidence during the 9-year period was 1.99 per 1000 newborns, which broke down into 2.04 per 1000 boys and 1.95 per 1000 girls. By birth weight, the incidence per 1000 newborns was highest at 317.14 in the VLBW population, 25.45 in the 1500 to 2499 g population, and lowest at 0.29 in the 2500 g or greater population (Table 1).
Table 1.
Incidence of ROP in Korea by Birthweights During 2006–2014
| Total | Birth Weight <1500 g | Birth Weight 1500–2499 g | Birth Weight ≥2500 g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | No. of Population | No. of ROP | Incidence* | No. of population | No. of ROP | Incidence* | No. of Population | No. of ROP | Incidence* | No. of Population | No. of ROP | Incidence* |
| Total | 4,149,959 | 8273 | 1.99 | 6795 | 2155 | 317.14 | 196,226 | 4993 | 25.45 | 3,946,938 | 1125 | 0.29 |
| Boys | 2,136,861 | 4352 | 2.04 | 3176 | 1003 | 315.81 | 92,541 | 2678 | 28.94 | 2,041,144 | 671 | 0.33 |
| Girls | 2,013,098 | 3921 | 1.95 | 3619 | 1152 | 318.32 | 103,685 | 2315 | 22.33 | 1,905,794 | 454 | 0.24 |
Per 1000 newborns.
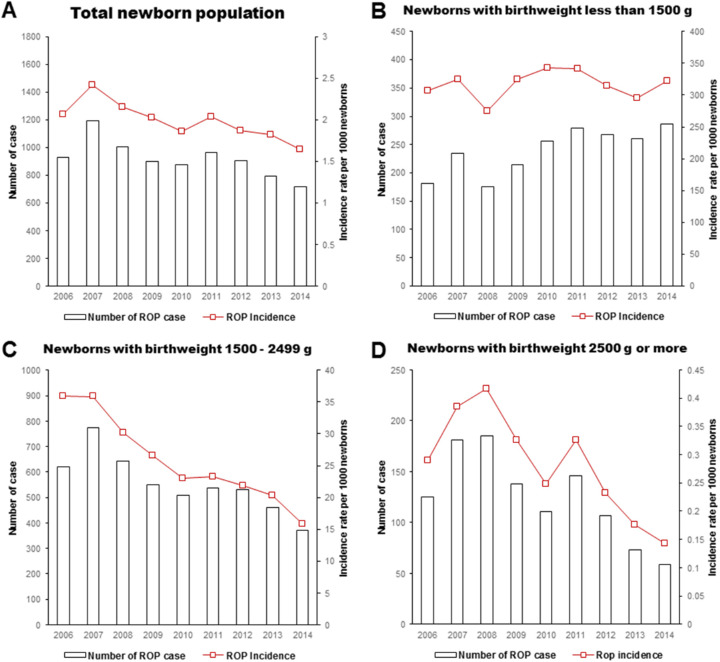
During the study period, the total number of ROP cases decreased from a peak of 1191 in 2007 to 715 in 2014, and the overall ROP incidence decreased from the peak at 2.41 per 1000 in 2007 to 1.64 per 1000 in 2014 (β coefficient = –0.07 per 1000 person; P = 0.006 by the linear regression model), particularly for those weighing 1500 to 2499 g (–2.45; P < 0.001) and those weighing 2500 g or greater (–0.03; P = 0.012). However, in the VLBW population, the number of ROP cases increased; therefore, the incidence did not considerably change between 274.57 and 342.70 per 1000 (1.21; P = 0.697) (Fig. 1).
Figure 1.
The incidence of ROP in the total newborn population (A), VLBW population less than 1500 g (B), 1500 to 2499 g population (C), and 2500 g or greater population (D) in Korea from 2006 to 2014.
Visual Impairment
A total of 138 had VI, of whom 87 (63.0%) had severe VI. The rate of VI was 1.8 per 100 person-years at age 6 and 2.2 per 100 person-years at age 10. By birth weight, the VI rate was highest in the VLBW population, 3.7 per 100 person-years at age 6 and 4.5 per 100 person-years at age 10 (odds ratio [OR], 1.32 for VI and 2.47 for severe VI when compared with ≥2500 g patients, Table 2). In 1500 to 2499 g and 2500 g or greater patients with ROP, the rate was 1.0 and 1.7 per 100 person-years at age 6, and 1.3 and 2.1 per 100 person-years at age 10, respectively (Fig. 2). Of the 138 patients with ROP with VI, 36 (26.1%) had ocular comorbidities, most of which were congenital ocular anomalies (Supplementary Table S1).
Table 2.
Logistic Regression Analysis of VI in ROP
| All VI | Severe VI | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Treatment | ||||
| No treatment | Reference | Reference | ||
| Laser/Cryo | 6.17 | 3.13–12.19 | 5.73 | 2.24–14.70 |
| anti-VEGF | 13.80 | 2.90–65.66 | 26.50 | 5.04–139.29 |
| Surgery | 132.10 | 67.14–259.92 | 208.75 | 84.72–514.39 |
| Female sex | 0.80 | 0.54–1.18 | 0.68 | 0.42–1.09 |
| Birth year | 0.76 | 0.69–0.83 | 0.77 | 0.70–0.86 |
| Birth weight (g) | ||||
| ≥2500 | Reference | Reference | ||
| 1500–2499 | 0.72 | 0.36–1.41 | 1.36 | 0.51–3.65 |
| <1500 | 1.32 | 0.66–2.67 | 2.47 | 0.91–6.73 |
| Ocular comorbidities* | 1.77 | 1.35–2.32 | 1.01 | 0.71–1.45 |
CI, confidence interval; OR, odds ratio.
Congenital ocular anomalies, retinal dystrophies, neoplasms, and severe ocular traumas.
Figure 2.
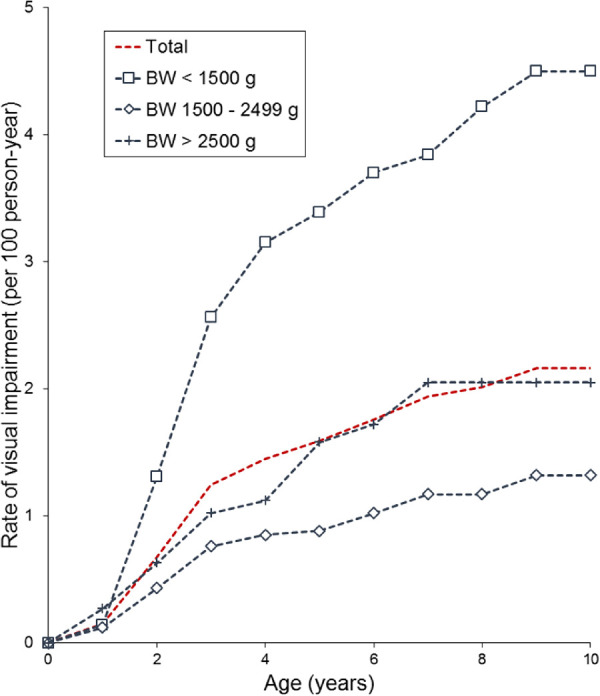
The rate of VI in patients with ROP at each age point. BW, birth weight.
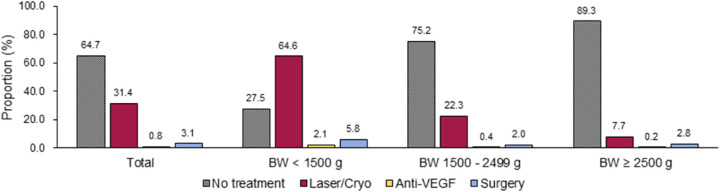
Among all the patients with ROP, 2918 received treatment until 2016; of these, 2595 (88.9%) underwent Laser/Cryo, 68 (2.3%) underwent anti-VEGF therapy, and 255 (8.7%) underwent surgery. By birth weight, the proportion of treatment was highest at 72.5% in the VLBW population, 24.8% in the 1500 to 2499 g population, and lowest at 10.7% in the 2500 g or greater population (Fig. 3). Compared with nontreated patients, patients undergoing treatment showed a higher proportion of VI (0.2% vs. 4.3%). By treatment modalities, the proportion of VI was 32.2% (severe VI rate of 22.7%) in the surgically treated patients, 2.9% (severe VI rate of 2.9%) in the patients undergoing anti-VEGF, and 1.6% (severe VI rate of 0.8%) in the patients treated with Laser/Cryo (Fig. 4). Logistic regression analysis showed the higher association with VI in surgically treated patients (OR, 132.10 for VI vs. OR, 208.75 for severe VI) compared with patients treated with other methods, and in patients undergoing anti-VEGF than in patients treated with Laser/Cryo (OR, 13.80 vs. Or, 6.17 for VI, 26.50 vs. 5.73 for severe VI). The presence of ocular comorbidities was associated to the greater OR for all VI (OR, 1.77), but not for severe VI (OR, 1.01) (Table 2).
Figure 3.
The proportion of newborns with ROP who underwent treatment. BW, birth weight.
Figure 4.
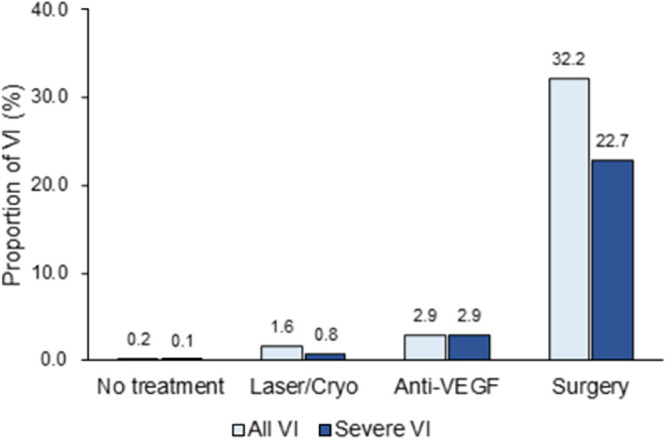
The proportion of VI in patients with ROP according to the type of treatment performed.
Mortality
Until 2016, a total of 260 mortalities were recorded in patients with ROP, with the overall mortality rate of 4.8 per 1000 person-years. By birth weight, the mortality rate was highest at 10.3 per 1000 person-years in the VLBW population, whereas 1500 to 2499 g, and 2500 g or greater patients demonstrated a mortality rate of 2.9 and 3.9 per 1000 person-years, respectively. In a Cox regression analysis, patients undergoing treatment had a higher risk of mortality than did nontreated patients. However, no significant difference in mortality risk was found between treatment modalities (Table 3).
Table 3.
Cox Regression Analysis of Mortality in ROP*
| Hazard Ratio | 95% CI | |
|---|---|---|
| Female sex | 0.86 | 0.55–1.36 |
| Ocular comorbidities† | 2.18 | 0.73–6.50 |
| Birth year | 1.04 | 0.94–1.15 |
| Birth weight (g) | ||
| <1500 (n = 2057) | Reference | |
| 1500–2499 (n = 4923) | 0.54 | 0.31–0.95 |
| ≥2500 (n = 1109) | 1.24 | 0.60–2.54 |
| Treatment | ||
| No treatment (n = 5240) | Reference | |
| Laser/Cryo (n = 2533) | 1.98 | 1.14–3.46 |
| anti-VEGF (n = 66) | 2.14 | 0.28–16.18 |
| Surgery (n = 250) | 2.84 | 1.06–7.62 |
CI, confidence interval.
The Cox regression analysis of mortality was based on 8089 patients with ROP after excluding 184 infantile deaths from the total 8273 patients.
Congenital ocular anomalies, retinal dystrophies, neoplasms, and severe ocular traumas.
Discussion
We reported the epidemiologic characteristics of ROP across the entire newborn population in Korea. To the best of our knowledge, this is the first nationwide study to investigate the visual outcomes and mortality rates for ROP through a longitudinal observation.
The incidence of ROP was 1 in 502 newborns in Korea. By birth weight, the incidence was approximately 1 in 3 in VLBW newborns, 1 in 40 in 1500 to 2499 g newborns, and 1 in 3500 in 2500 g or greater newborns. Although there were only a few population-based studies on ROP incidence covering the entire birth weight range, the incidences from two studies conducted in the US were 1 in 511 newborns and 1 in 579 newborns, numbers similar to those of this Korean study.4,5 The incidence in the VLBW population was higher in this study than the one in five of those US studies,4,5 and one in eight in a UK study.14
In this study, the total number of ROP cases and the overall incidence decreased over time, especially in the 1500 g or greater population. On the contrary, in the VLBW population, the number of ROP cases increased. Such an incidence pattern according to birth weight could also be found in previous studies.6,14,15 The decrease in the overall ROP incidence could be attributed to the improvements in neonatal care, preventing comorbidities such as ROP.16 In contrast, however, the better survival of extreme babies at a high risk of ROP may explain the increase in the number of ROP cases in the VLBW population. In Korea, neonatal survival rates of VLBW newborns increased from 74.5% in 2000 through 2004 to 84.8% in 2010 through 2014.17 In this study, the number of VLBW live births in Korea increased from 590 in 2006 to 886 in 2014.
The rate of VI in ROP was 2.2 per 100 person-years when assessed at age 10, which was highest at 4.5 per 100 person-years in the VLBW population compared with population in other birth weight categories. More important, two-thirds of all visually impaired children with ROP were blind according to the World Health Organization criteria. Therefore, the disease burden caused by ROP would be considerable. Among treated patients, the proportion of VI was 32.2%, 2.9%, and 1.6% in the patients undergoing surgery, anti-VEGF, and Laser/Cryo therapy. Previous hospital-based studies reported that 24.6% of patients with ROP treated with laser photocoagulation, and 44.4% of patients with ROP treated with cryotherapy had unfavorable visual outcomes being 20/200 or worse.18,19 Although it is difficult to directly compare the results of this study and those of the previous ones, in which base population characteristics, the criteria for visual outcomes, and the evaluation timing differed, the result of the VI analysis in the current population-based study covering the entire Korean newborns of all birth weight ranges has significance.
One comparable Danish population-based study reported that the VI incidence caused by ROP was 0.6%,20 which was lower than that of this study. This difference in the VI rate could be explained by the duration of observation period that the Danish study was limited to a 2-year period after birth. Because the enrollment of visually impaired children in the disability registration system could be delayed until the age at which ophthalmologic assessments can be performed or when socioeducational supports are needed for children because of their VI, a reliable analysis of VI requires a long observation period, as in our study.
The VI rate in ROP was higher in the VLBW population than in other birth weight categories. It is well-known that the severity of ROP was higher in lower-birth weight patients.21,22 Following this, our findings determined that lower birth weight eventually associates to poor long-term visual outcomes in patients with ROP.
We included potential confounders for VI into the analyses and found that accompanying severe ocular comorbidities increased the OR for VI in patients with ROP. Although this confounding effect could not be completely removed in the current study, the VI proportion in patients with ROP remained similar even when excluding the cases with ocular comorbidities from the ROP cohort (102 VI cases in 8128 patients with ROP; data not shown). Furthermore, those ocular comorbidities did not show additional impact in patients with ROP with severe VI. Therefore, it can be assumed that the VI in patients with ROP is mostly caused by ROP itself in this study.
In this study, approximately one-third of the overall patients with ROP and three-fourths of the VLBW patients with ROP underwent treatment, which was a higher rate than in previous studies that reported a 11% to 24% treatment rate.6,14,15,23,24 The high proportion of ROP treatment may be attributed to the relatively easier access to health care service, including ROP management under the social insurance system with low copayment. Furthermore, active management for patients with ROP is a commonly accepted practice behavior among ophthalmologists in Korea. This phenomenon raises the need for developing a national consensus or guideline for the appropriate management of ROP in Korea.
According to treatment modalities, surgically treated patients had the highest proportion of VI, as would be expected, because vitrectomy or scleral buckling is done for severe cases of retinal detachment.25 Of note, we found a greater association of anti-VEGF than of Laser/Cryo with VI, even when controlling age, sex, birth weight, and ocular comorbidities in the analyses. Given that both modalities can be performed for nonsurgical indications,10,26 this finding suggests a possible difference in long-term visual outcomes between anti-VEGF injection and Laser/Cryo. Nevertheless, because we could not analyze stages or involved zones of ROP, direct comparison of those nonsurgical modalities should be avoided. Moreover, further studies are needed to verify whether the high treatment rates in Korea result in more favorable outcomes.
The overall mortality rate in ROP was 4.8 per 1000 person-years in this study. This result suggests that clinicians should consider general medical conditions in babies with ROP, which may lead to death.
The mortality rate in patients with ROP was greater in the VLBW population than in that of the 1500 g or greater newborns. Although patients undergoing treatment had a higher mortality rate, when compared with nontreated patients, the risk of death was similar between treatment modalities. This may contradict the concern about death in a previous randomized, controlled study that compared the effects of bevacizumab and laser therapy in ROP.10 Further studies evaluating the systemic effects according to each anti-VEGF agent, laser, and cryotherapy are needed to verify the mortality issue.
To compare the mortality risk according to treatment methods, we excluded the cases of infant death who could not be treated for ROP because of their poor general condition. When infant death cases were included in the analyses, the influence of treatments on mortality would be underestimated, as in a subanalysis demonstrating that the hazard ratios for mortality compared with no treatment had decreased to 0.74 for Laser/Cryo, 0.85 for anti-VEGF, and 0.80 for surgery (data not shown).
The risk of mortality was comparable across the treatment modalities in this study. This result is contrary to the expectation that patients with severe ROP who require surgical treatment may also have a high degree of immaturity,3,27 resulting in a greater mortality risk. However, it is also assumed that the poor medical condition in patients with severe ROP, probably associated with fatal comorbidities, may not allow them to undergo surgical management. Therefore, the result of this study should not be interpreted as the causality between type of treatment modalities and mortality risk.
Although we analyzed the study population according to the birth weights, gestational age is also an important parameter when evaluating the patients with ROP. Therefore, we performed further analysis with dividing the study population according to the gestational age into three groups: extremely preterm, less than 28 weeks of gestation; between 28 and 37 weeks of gestation; and 37 weeks of gestation or greater (Supplementary Table S2). Although the information on gestational age was missing in 1.73% of the study population, we found that the ROP incidence and VI proportion in patients with ROP were similar among the subgroup of extremely preterm and VLBW population. However, the gestational age information in our database was calculated using the estimated date of delivery and actual delivery date, which were obtained from the children's parents or guardians. Therefore, the gestational age would be more vulnerable to recall bias than birth weight in our database. Moreover, among patients without gestational age data, a considerable number of cases with ROP and VI were included (Supplementary Table S2). For these reasons, we used birth weight instead of gestational age as a criterion for classifying the study population in this study.
It could be argued that the ROP screening practice varies among institutions, resulting in the incidence or other epidemiologic characteristics of ROP also differing among institutions. However, in Korea, most of premature infants or infants showing unstable clinical course are managed in neonatal intensive care units, in which essential diagnostic devices are well equipped and trained neonatologists provide specialized care.28 Therefore, it could be assumed that almost all patients with ROP had been screened and managed in a similar manner in neonatal intensive care unit settings in Korea.
A major strength of our study is that we evaluated a large number of patients with ROP based on nationwide population-based data with an 11-year period of data collection. As a nationwide cohort study covering the entire newborn population, our research is less likely to have a selection bias than have previous studies from hospitals or specific regions. Therefore, this national-level study with a large sample provides unbiased information on incidence, visual prognosis, and mortality for ROP in Korea.
Nevertheless, this study also has several limitations. First, the NHI-KDR database does not include detailed clinical information. Therefore, we could not clarify the outcomes according to the stage of ROP. Furthermore, because we could not distinguish the type of anti-VEGF agents in the database, the treatment effect of each type of anti-VEGF agent could not be identified. Second, one of the most important findings of our study, the results of the VI analysis, is limited to the small number of VI cases, even though the result was derived from a complete enumeration in the KDR database across the entire Korean population. Given the large socioeconomic support that comes with a KDR program, the probability of an ROP patient not being enrolled in the KDR database is low. Nevertheless, we cannot exclude the possibility that some VI cases were omitted from this study, perhaps because of delayed registration. Third, because our study used administrative data, one may question whether the diagnosis for ROP was unreliable. However, given the increased awareness among physicians and parents about ROP and high rate of health care use in Korea, the diagnostic algorithms based on inpatient/outpatient diagnosis codes, birth weight, and treatment codes can be considered reliable. Fourth, with the NHI-KDR database, we could not analyze the patients with ROP by stratifying them into smaller birth weight categories of less than 1500 g. Fifth, although we considered the association of the ocular comorbidities in the analyses as possible, which would confound the VI findings in this study, some comorbidities that could also affect the patients’ visual outcomes, but are not well-defined in the ICD-10, could not be evaluated in this study.
In this study, we reported the incidence, visual outcome, and mortality for ROP in Korea. The incidence rate was approximately 1 in 500 across the entire newborn population, and 1 in 3 in the VLBW population. The VI rate was 2.2 per 100 person-years when assessed at age 10, which was higher than previously reported, of which over 60% met the World Health Organization criteria for blindness. The VI rate in ROP was high at 4.6 per 100 person-years in the VLBW population, and the greater association with VI was shown in those undergoing scleral buckling or vitrectomy. The mortality rate for ROP was 4.8 per 1000 person-years in this study. Differences in association with VI and similar mortality risks between treatments require further evaluation.
Supplementary Material
Acknowledgments
Disclosure: K.-H. Na, None; K.H. Kim, None; T.U. Kang, None; H.J. Hann, None; H.S. Ahn, None; H.J. Kim, None
References
- 1. Chan-Ling T, Gole GA, Quinn GE, Adamson SJ, Darlow BA. Pathophysiology, screening and treatment of ROP: a multi-disciplinary perspective. Prog Retin Eye Res. 2018; 62: 77–119. [DOI] [PubMed] [Google Scholar]
- 2. Bae J, Park JH, Park YK, Kim JY, Lee SW, Park SW. Changes in the distribution of maternal age and parity and increasing trends in the low birth weight rate in Korea between 1995 and 2005. J Prev Med Public Health. 2011; 44: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darlow BA, Hutchinson JL, Henderson-Smart DJ, et al.. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005; 115: 990–996. [DOI] [PubMed] [Google Scholar]
- 4. Chiang MF, Arons RR, Flynn JT, Starren JB. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004; 111: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 5. Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. 2009; 148: 451–458. [DOI] [PubMed] [Google Scholar]
- 6. Bas AY, Koc E, Dilmen U; ROP Neonatal Study Group. Incidence and severity of retinopathy of prematurity in Turkey. Br J Ophthalmol. 2015; 99: 1311–1314. [DOI] [PubMed] [Google Scholar]
- 7. Larsson E, Carle-Petrelius B, Cernerud G, Ots L, Wallin A, Holmström G. Incidence of ROP in two consecutive Swedish population based studies. Br J Ophthalmol. 2002; 86: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haines L, Fielder AR, Baker H, Wilkinson AR. UK population based study of severe retinopathy of prematurity: screening, treatment, and outcome. Arch Dis Child Fetal Neonatal Ed. 2005; 90: F240–F244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Verdier K, Ulla E, Lofgren S, Fernell E. Children with blindness - major causes, developmental outcomes and implications for habilitation and educational support: a two-decade, Swedish population-based study. Acta Ophthalmol. 2018; 96: 295–300. [DOI] [PubMed] [Google Scholar]
- 10. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364: 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lepore D, Quinn GE, Molle F, et al.. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018; 125: 218–226. [DOI] [PubMed] [Google Scholar]
- 12. Korean Ministry of Health and Welfare. Disability grading criteria. Available at: http://www.mohw.go.kr/react/jb/sjb0406vw.jsp. Accessed August 19, 2016. [Google Scholar]
- 13. World Health Organization. Blindness and visual impairment. Available at: http://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment. Accessed November 18, 2007.
- 14. Painter SL, Wilkinson AR, Desai P, Goldacre MJ, Patel CK. Incidence and treatment of retinopathy of prematurity in England between 1990 and 2011: database study. Br J Ophthalmol. 2015; 99: 807–811. [DOI] [PubMed] [Google Scholar]
- 15. Bas AY, Demirel N, Koc E, et al.. Incidence, risk factors and severity of retinopathy of prematurity in Turkey (TR-ROP study): a prospective, multicentre study in 69 neonatal intensive care units. Br J Ophthalmol. 2018; 102: 1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SY. Changes in neonatal outcomes in Korea. J Korean Med Assoc. 2016; 59: 498–505. [Google Scholar]
- 17. Shim JW, Jin HS, Bae CW. Changes in survival rate for very-low-birth-weight infants in Korea: comparison with other countries. J Korean Med Sci. 2015; 30(Suppl 1): S25–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001; 119: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 19. Good WV, Hardy RJ, Dobson V, et al.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010; 128: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slidsborg C, Olesen HB, Jensen PK, et al.. Treatment for retinopathy of prematurity in Denmark in a ten-year period (1996 2005): is the incidence increasing? Pediatrics. 2008; 121: 97–105. [DOI] [PubMed] [Google Scholar]
- 21. Quinn GE, Ying GS, Bell EF, et al.; G-ROP Study Group. Incidence and early course of retinopathy of prematurity: secondary analysis of the Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study. JAMA Ophthalmol. 2018; 136: 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmer EA, Flynn JT, Hardy RJ, et al.. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991; 98: 1628–1640. [DOI] [PubMed] [Google Scholar]
- 23. Holmstrom G, Hellström A, Jakobsson P, Lundgren P, Tornqvist K, Wallin A. Five years of treatment for retinopathy of prematurity in Sweden: results from SWEDROP, a national quality register. Br J Ophthalmol. 2016; 100: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 24. Demir S, Yücel ÖE, Niyaz L, Karakuş G, Arıtürk N. Incidence of retinopathy of prematurity in the middle Black Sea region of Turkey over a 10-year period. J AAPOS. 2015; 19: 12–15. [DOI] [PubMed] [Google Scholar]
- 25. Repka MX, Tung B, Good WV, Capone A Jr., Shapiro MJ. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol. 2011; 129: 1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015; 122: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valentine PH, Jackson JC, Kalina RE, Woodrum DE. Increased survival of low birth weight infants: impact on the incidence of retinopathy of prematurity. Pediatrics. 1989; 84: 442–445. [PubMed] [Google Scholar]
- 28. Korean Ministry of Health and Welfare. Financial support program for medical care of premature infants or infants with congenital anomalies. Available at: http://www.g-health.kr/portal/bbs/selectBoardList.do?bbsId=U00245&cNttId=216&menuNo=200593. Accessed July 31, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.