Abstract
Marine bacterial strains are of great interest for their ability to produce secondary metabolites with anticancer potentials. Isolation, identification, characterization and anticancer activities of isolated bacteria from El-Hamra Lake, Wadi El-Natrun (Egypt) were the objectives of this study. The isolated bacteria were identified as a moderately halophilic alkaliphilic strain. Ethyl acetate extraction was performed and identified by liquid chromatography-mass spectrophotometry (LC–MS–MS) and nuclear magnetic resonance analysis (NMR). Cytotoxicity of the extract was assessed on the HepG2 cell line and normal human peripheral lymphocytes (HPBL) in vitro. Halomonas sp. HA1 extract analyses revealed anticancer potential. Many compounds have been identified including cyclo-(Leu-Leu), cyclo-(Pro-Phe), C17-sphinganine, hexanedioic acid, bis (2-ethylhexyl) ester, surfactin C14 and C15. The extract exhibited an IC50 of 68 ± 1.8 μg/mL and caused marked morphological changes in treated HepG2 cells. For mechanistic anticancer evaluation, 20 and 40 µg/mL of bacterial extract were examined. The up-regulation of apoptosis-related genes' expression, P53, CASP-3, and BAX/BCL-2 at mRNA and protein levels proved the involvement of P53-dependant mitochondrial apoptotic pathway. The anti-proliferative properties were confirmed by significant G2/M cell cycle arrest and PCNA down-regulation in the treated cells. Low cytotoxicity was observed in HPBL compared to HepG2 cells. In conclusion, results suggest that the apoptotic and anti-proliferative effects of Halomonas sp. HA1 extract on HepG2 cells can provide it as a candidate for future pharmaceutical industries.
Subject terms: Biochemistry, Microbiology, Chemistry
Introduction
Hepatocellular carcinoma (HCC) is the fifth type of cancer in patients with chronic liver disease and cirrhosis and the second cause of death in cancer patients1. There are nearly 800,000 deaths annually due to HCC2, and the HCC's prevalence has gradually increased over the past decade3. Cancer treatment can involve surgical intervention, radiation, chemotherapy, targeted therapy and/or other therapies4. Despite the extreme efforts to treat HCC effectively, side effects are involved, such as immunodeficiency, cell damage, and neurological, renal, and cardiac toxicity5–7. Currently, the pharmaceutical industry and research are focused on developing and applying new strategies to lower the side effects and enhance the economic value8,9. Therefore, finding and developing natural products-derived anticancer drugs with good efficacy and low toxicity10,11 from different sources such as essential oils12,13, certain types of mushrooms14 or even plant crude extracts15,16 are very crucial.
Microbial metabolites are among the most important natural cancer chemotherapeutics17. With actinomycin discovery, they began to appear around 1940, and since then, most anticancer compounds have been derived from natural sources18. More than 60 percent of the existing compounds with antineoplastic activities are initially either identified from or inspired by natural compounds and their derivatives19. Bacteria are the greatest producers of bioactive products and thus of immense importance for the drugs discovery20. Marine pharmacology is a new discipline that explores the potential pharmaceutical products that originated in the marine world. In the last two decades, extensive screening of marine compounds and their antivirals21, antibacterial22, antifungal23, antiparasitic24, antitumor25, and anti-inflammatory26 activities have been reported. Systematic studies of various soda lakes have shown that microorganisms, which thrive in these areas, are adapted to the extreme pH and salt conditions, many of which are alkaliphilic and halophilic or extremely halotolerant, and many represent separate alkaliphilic lines within the recognized taxa. El-Hamra Lake, Wadi El-Natrun (Egypt) has a high water salinity (300 g/L) and pH 10.0. In both saline and alkaline conditions, haloalkaliphilic bacteria can be present and the preliminary results prompted the identification of the metabolites produced by alkaliphilic bacteria27. Marine bacteria have genes such as PKS (polyketides) and NRPS (natural products) that are characteristic of secondary metabolite production. Halomonas strains from a marine origin may generate siderophores (low molecular weight Fe3+ chelating molecules) and loihichelins28. The progression of gastric adenocarcinoma cell lines (HM02), hepatocellular carcinoma (HepG2) and breast cancer (MCF-7) has been inhibited by apoptosis initiation and cell cycle arrest when treated with marine-derived Halomonas sp. (GWS-BW-H8hM strain)29,30. However, to the best of our knowledge, the novel strain has not been studied yet. Consequently, the characterization of bioactive compounds and evaluation of the possible anticancer potential of the bacterial extract against the HepG2 cell line were warranted.
Results
Identification of bacterial strain
Morphological investigations showed two isolates of gram-negative short motile rods. Full-length 16S rRNA (about 1,500 bp) was sequenced and deposited under GenBank accession numbers (KT223026) for H. HA1 isolate. The isolated H. HA1 16S rRNA sequence showed 99% similarity with the 16S rRNA gene sequence of Halomonas denitrificans BC7. However, the isolated strain is closer to Halomonas nitroreducens evolutionary group (Fig. 1).
Figure 1.
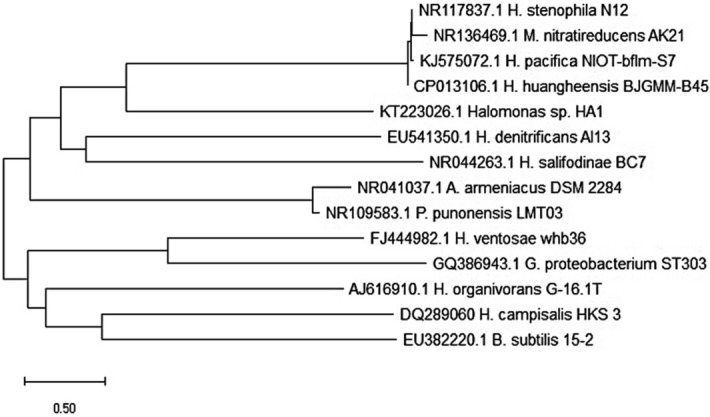
The phylogenetic tree based on 16 s rRNA sequences constructed by the neighbor-joining method, showing the position of strain HA1 and representatives of some related taxa.
LC–MS-MS and NMR fraction analyses of H. HA1 extract
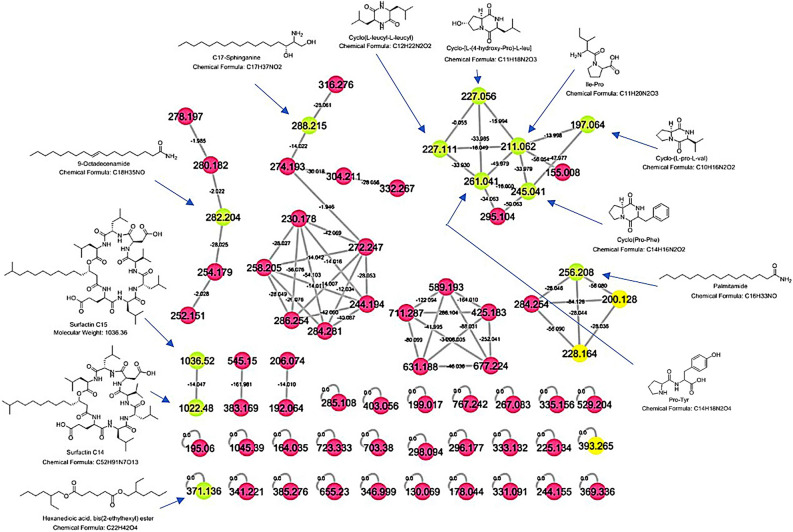
The molecular networking of metabolome mass profile for the species Halomonas sp.HA1 was screened and exhibited in 67 parent ions (nodes) (Fig. 2).
Figure 2.
Molecular network of 67 parent ions produced from Halomonas sp. HA1. The fuchsia nodes indicate to the whole molecular weights that have unique detected peaks in the molecular network. The green Nodes represent parent ions that are identified from the molecular networking database as they have been already isolated before. The yellow nodes indicate some identified compounds but with wrong precursor parent ions so they should not be recognized.
The number of nodes was considered an indication of the unique peaks. Compounds with related molecular weight and shared class grouped together to form a cluster. Four clusters are noticed with some chemically related identified compounds. From the 67 nodes only 15 parent ions within the molecular network matched 15 known standards from the molecular networking database but three of them in the yellow nodes [M+H]+ (m/z; 200.128, 228.164 and 393.265) have wrong precursor parent ions, so their identification can’t be considered (Fig. 2 and Table 1).
Table 1.
The parent and the fragments’ masses of the identified compounds compared with that of the standards from the molecular networking database.
| Compound name | Precursor mass (m/z) | Database fragments | Identified fragments |
|---|---|---|---|
| Cyclo-(L-pro-L-val) | 197.064 | 179.12, 169.14, 152.11, 141.14, 124.12 and 70.07 | – |
| Ile-Pro | 211.06 | – | 183.128, 138.125, 114.102, 98.065, 86.105 and 70.064 |
| Cyclo-[L-(4-hydroxy-Pro)-L-leu] | 227.058 | 199.15, 181.14, 153.15, 136.12 and 86.08 | – |
| Cyclo-(Leu-Leu) | 227.115 | – | – |
| Cyclo (Pro-Phe) | 245.042 | 217.69, 120.03 and 70.79 | 154.100, 120.10 and 70.064 |
| Palmitamide | 256.21 | – | – |
| Pro-Tyr | 261.038 | 243.06, 233.09, 215.04, 135.92, 119.94 and 85.94 | 187.183, 120.085 and 86.056 |
| 9-Octadecenamide | 282.205 | 265.27, 247.30, 149.10 and 135.13 | – |
| C17-Sphinganine | 288.218 | – | – |
| Hexanedioic acid, bis(2-ethylhexyl) ester | 371.135 | – | – |
| Surfactin C14 | 1,022.48 | 909.46, 891.41, 86e3.38, 685.37, 667.44, 582.31, 554.33 and 441.23 | - |
| Surfactin C15 | 1,036.52 | 1,018.47, 923.58, 905.42, 877.39, 808.44, 792.44, 695.38, 685.37, 667.35, 596.41, 554.27 and 441.24 | 923.574, 685.500, 596.445, 483.382, 441.298, 326.238, 272.167 and 86.088 |
Six identified dipeptides (Ile-Pro, cyclo-(L-pro-L-val), cyclo-(Pro-Phe), Pro-Tyr, cyclo-(Leu-Leu) and cyclo-[L-(4-hydroxy-Pro)-L-leu]) with [M + H]+ (211.06, 197.064, 245.042, 261.038, 227.115 and 227.058) respectively are clustered together (Fig. 2 and Table 1). Palmitamide and 9-octadecenamide with [M + H]+ (256.21 and 282.205) (Fig. 2 and Table 1), are two identified fatty acid amides produced as endogenous substances. One sphingolipid (aminodiol) that is C17-sphinganine with m/z 288.218 [M+H]+ (Fig. 2 and Table 1), was isolated from different fungus species31. C17-sphingolipid identified as mycotoxin (C17-SAMT) analog exerted potent toxicity in different assays32,33. The most potent identified compounds were two biosurfactants (Surfactin C14 and surfactin C15) with m/z 1,022.48 and 1,036.52 [M+H]+ (Fig. S1).
The parent ion masses fragmentation data for Surfactin C15 and Surfactin C14 from the molecular networking database were compared (Figs. S1,S2).
Surfactin C15 parent ion fragmentations are recognized from MS/MS chromatogram and the fragment with [M+H]+ m/z 685.500 represents the base peak ion (Fig. S3).
Surfactin C15 fragmentation mechanism in the positive ion mode starts by loss of fragment ion [M+]+ m/z 113.13 and C8H17• then continuous cleavage of different peptide bonds and loss of different amino acid fragments to reach the last fragment with [M+H]+ (m/z 85.09) and molecular formula C5H11N2· (Fig. S4).
Anticancer activities
Cytotoxicity on HepG2 cells
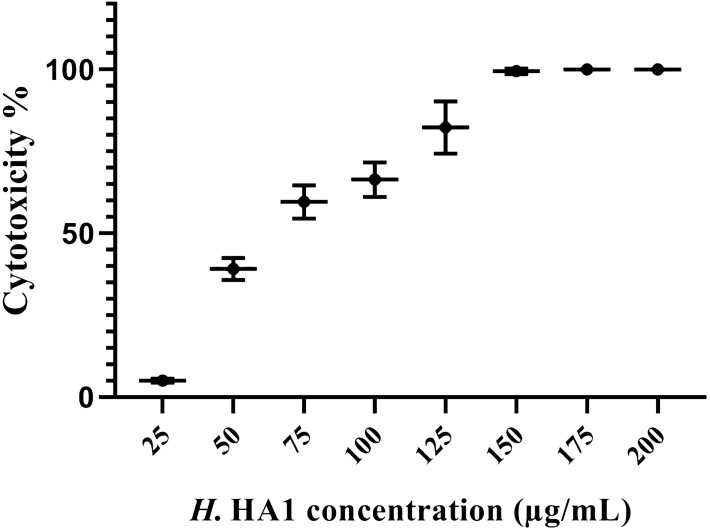
The bacterial extract has been examined for their anticancer activity against HepG2 cell line using MTT assay. Results, as shown in Fig. 3, reflected a strong cytotoxic potential of the extract with 68 ± 1.8 μg/mL maximal inhibitory concentration (IC50).
Figure 3.
The cytotoxic effect of the H. HA1 extract on HepG2 using MTT assay after 24 h. Incubation with serial concentrations of the extract showed a potent anticancer effect with an IC50: 68 ± 1.8 µg/mL. Data were represented as (Mean ± SD) of three different experiments (n = 3). H. HA1: Halomonas. HA1.
Assessment of morphological changes in HepG2
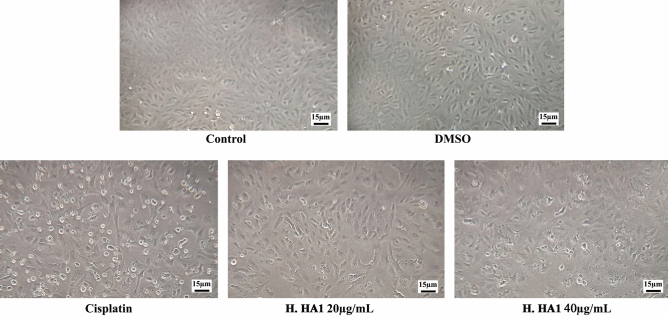
Results of the inverted phase-contrast examination demonstrated the remarkable anticancer potentials as evidenced by cellular shrinkage and irregular cell shapes following bacterial extract treatments in a dose-dependent manner when compared with control groups (Fig. 4).
Figure 4.
Representative photomicrographs of control and treated HepG2 cells after 24 h. Cellular morphological alterations and detached cells were observed by phase contrast light inverted microscope (Olympus IMT-2, Japan). H. HA1: Halomonas. HA1; Cisplatin (3 µg/mL).
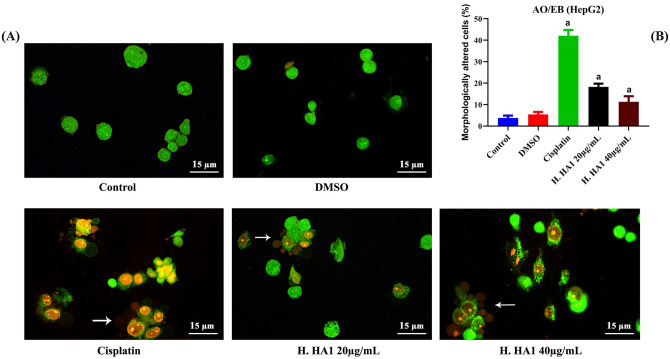
Furthermore, AO/EB staining revealed cytoplasmic vacuolation, membrane blebbing and irregular nuclear morphology as hallmarks of apoptosis and cell death. The records of abnormal morphology were significant (P < 0.05) with H. HA1 extract incubations compared to control HepG2 cells (Fig. 5).
Figure 5.
Representative photomicrographs of cellular alterations in untreated and treated HepG2 cells after 24 h of different incubations. Observable decrease in viability of cells and increased morphological alterations towards apoptosis induction such as membrane blebbing (white arrows) were noticed after acridine orange/ethidium bromide (AO/EB) double fluorescent labeling (A). Data represent the means of three independent experiments; bars, standard deviation and a: significant (P < 0.05) with respect to the control (B). H. HA1: Halomonas. HA1; Cisplatin (3 µg/mL).
Flow cytometric analysis of cell cycle distribution
Cell cycle analysis revealed significant (P < 0.05) G2/M cell cycle arrest in H. HA1 crude extract-treated HepG2 cells (Fig. 6A). Results showed remarkable accumulation of G2/M populations in treated cells with 18.42 and 24.18% for 20 and 40 µg/mL respectively when compared to 13.45% in untreated cells. However, cisplatin induced significant (P < 0.05) apoptosis particularly in S phase when compared with untreated cells (Fig. 6B).
Figure 6.
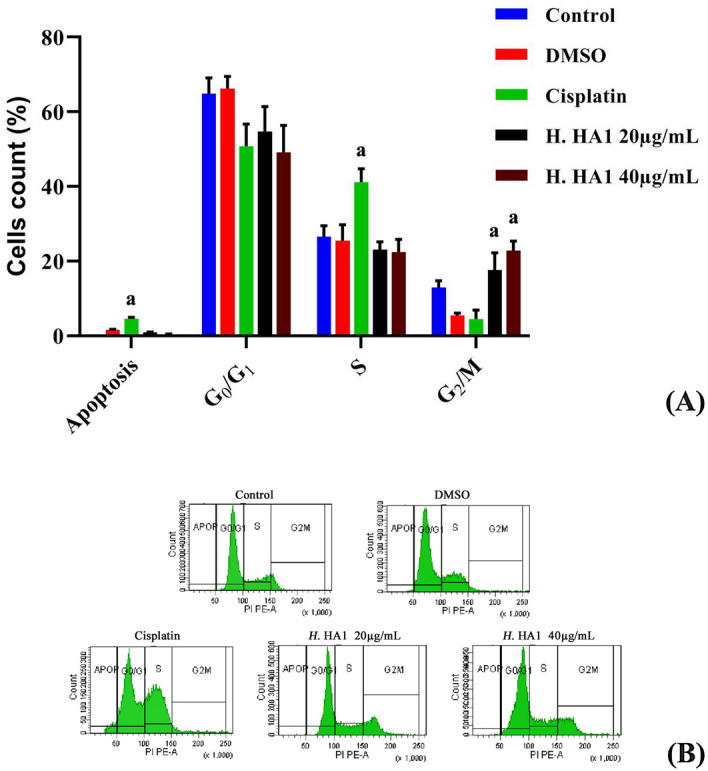
Effect of different concentrations of H. HA1 crude extract incubation for 24 h on cell cycle distribution of treated and control HepG2 cells. The cell cycle phases were analyzed according to DNA content as evaluated after propidium iodide (PI) labeling (B). Data represent means of three different experiments; bars, standard deviation; and a, significant increase (P < 0.05) relative to untreated cells (A). H. HA1: Halomonas. HA1; Cisplatin (3 µg/mL).
Immunocytochemical assessment of apoptosis and proliferation-related proteins
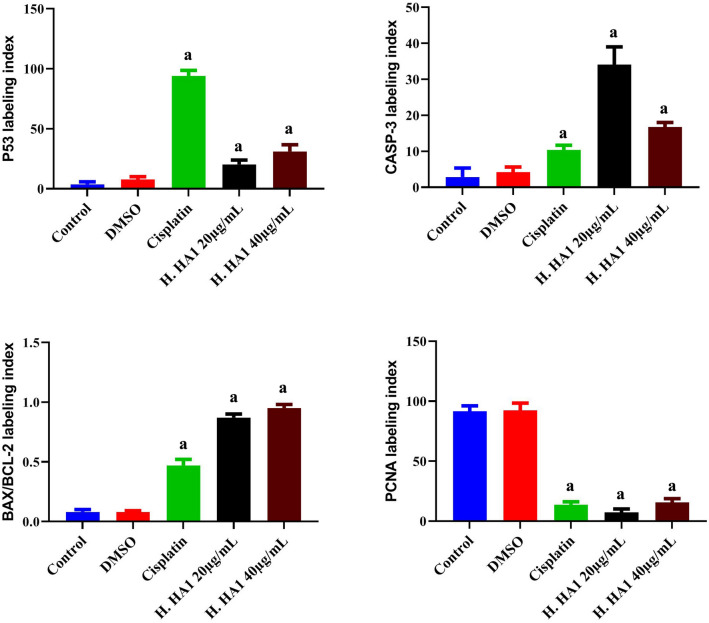
Changes in protein expression of P53, CASP-3, and BAX/BCL-2 ratio were examined in treated cells and controls. Results revealed that H. HA1 extract induced apoptosis as confirmed by P53 and CASP-3 significant (P < 0.05) up-regulation of protein expression. The evaluated immunocytochemical reactivities showed an increase of ~ 5 and 8 folds, in P53 protein level, for 20 and 40 µg/mL respectively, relative to the control. The level of CASP-3 (procaspase-3) protein was increased by ~ 11 and 6 folds, for 20 and 40 µg/mL respectively, when compared to untreated cells. Moreover, BAX/BCL-2 ratio exhibited a significant (P < 0.05) concentration-dependent elevation when compared to the control confirming that the induction of apoptosis was via the intrinsic mitochondrial pathway (Fig. 7). However, PCNA, a nuclear proliferating marker, showed a significant (P < 0.05) down-regulation in H. HA1 (7.3 and 15.7% for 20 and 40 µg/mL, respectively) and cisplatin-treated cells (13.7%) when compared with the untreated group (91.7%).
Figure 7.
Effects of crude extract incubation for 24 h on the positively stained cells in control and treated HepG2 cells. Increased percentage of P53, CASP-3 positive immuno-reactive cells and elevated BAX/BCL-2 ratio as well as decreased levels of PCNA were noticed in treated cells. Data represent means of five different fields; bars, standard deviation; and a, significant (P < 0.05) relative to untreated cells. H. HA1: Halomonas. HA1; Cisplatin (3 µg/mL).
Altered mRNA expression of apoptosis markers
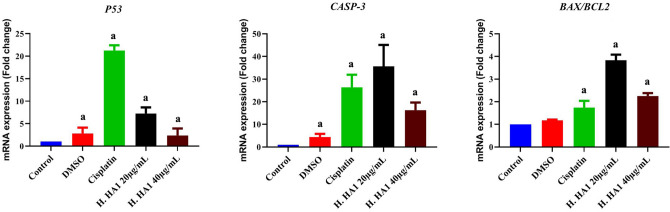
The apoptotic effect of H. HA1 crude extract on HepG2 was confirmed by apoptosis-related genes expression. The mRNA expression was quantified by qRT-PCR for P53, CASP-3, BAX and BCL-2 genes in the control and treated cells. Results of P53 and CASP-3 showed significant up-regulation among H. HA1 treated cells (~ 6 and 2.5 folds, for 20 and 40 µg/mL respectively). BAX/BCL-2 ratio exhibited a significant (P < 0.05) elevation with H. HA1 crude extract treatment (~ 0.95 and 1.2 folds, for 20 and 40 µg/mL, respectively) compared to the untreated control. These results suggest that H. HA1 extract induced apoptosis in HepG2 cells via the intrinsic mitochondrial pathway. The lower concentration of H. HA1 (20 µg/mL) was more potent in apoptosis induction than the higher one (Fig. 8).
Figure 8.
Effect of H. HA1 crude extract on relative mRNA alterations of P53, CASP-3 and BAX/BCL-2. Data (means of three experiments) were standardized to the level of GAPDH mRNA and expressed as fold-induction relative to the control mRNA levels. Bars, standard deviation and a: significant (P < 0.05) compared to untreated cells. H. HA1: Halomonas. HA1; Cisplatin (3 µg/mL).
Effect of the extract on normal human peripheral lymphocytes
Assessment of cytotoxicity on normal HPBL
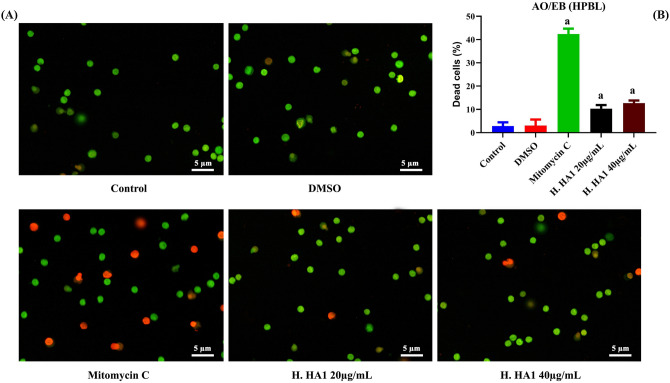
HPBLs were treated with H. HA1 crude extract for 24 h. Trypan blue exclusion method (Fig. 9) and AO/EB dual fluorescent staining (Fig. 10) were performed. Results revealed low cytotoxicity and morphological changes among extract-treated groups compared to higher toxicity of mitomycin C-treated cells (Figs. 9, 10B).
Figure 9.
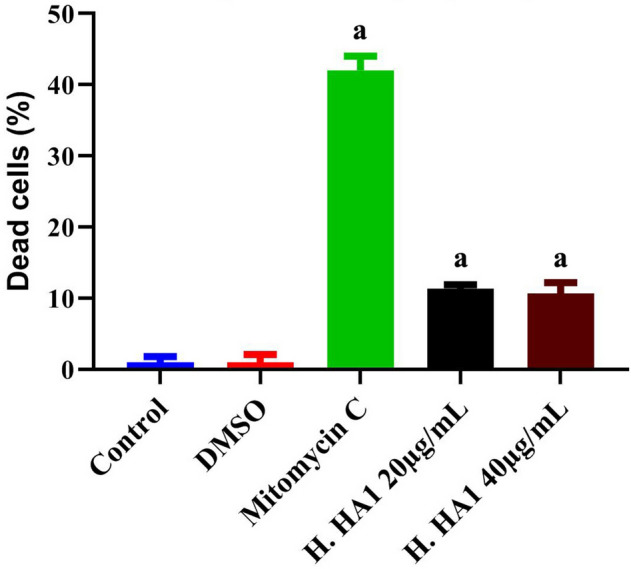
Effect on HPBL after incubation with various concentrations of H. HA1 extract, for 24 h. Cells were stained with trypan blue (TB) for cytotoxicity assessment. Data were represented as Mean ± SD of three independent experiments (n = 3). Bars, standard deviation and a: significant (P < 0.05) compared to the untreated cells. H. HA1: Halomonas. HA1; Mitomycin C (0.5 µg/mL).
Figure 10.
Effect on HPBL after incubation with various concentrations of HL extract, for 24 h. Cells were labeled with AO/EB fluorescent staining for cell death (A). Data were represented as Mean ± SD of three independent experiments (B). Bars, standard deviation and a: significant (P < 0.05) compared to the untreated cells (n = 3). H. HA1: Halomonas. HA1; Mitomycin C (0.5 µg/mL).
Assessment of DNA single-strand breaks (comet assay)
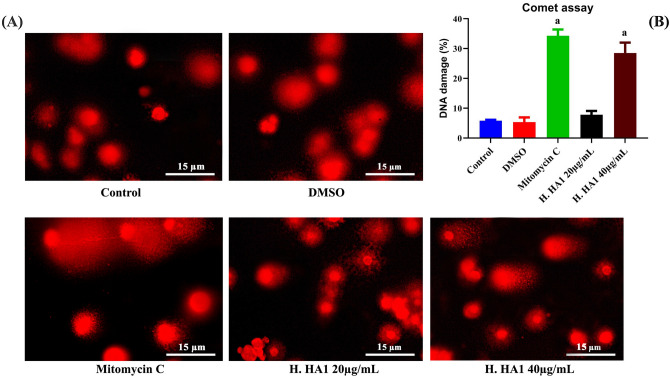
For DNA single-strand breaks assessment in normal cells, HPBLs were treated with H. HA1 crude extract for 24 h. The comet assay was carried out (Fig. 11A). Results revealed significant (P < 0.05) genotoxic effect with high concentration (40 µg/mL) of H. HA1 extract as well as mitomycin C-treated group when compared to control cells (Fig. 11B).
Figure 11.
Effect of H. HA1 extract on DNA single-strand breaks (alkaline comet assay) in HepG2 cells stained with ethidium bromide (A). Data were represented as Mean ± SD of three independent experiments (B). Bars, standard deviation and a: significant (P < 0.05) compared to the untreated cells (n = 3). H. HA1: Halomonas. HA1; Mitomycin C (0.5 µg/mL).
Discussion
Currently, natural product extracts are considered to be the most promising source of new drugs for cancer34. Several studies have indicated the advantages of marine flora and fauna extracts in combating cancer and a number of other diseases35–37. Bacteria are the greatest producers of bioactive natural products and are of immense importance for drug discovery20. In this study, the newly identified strain of bacteria Halomonas sp. HA1 was investigated for its anticancer potential against HepG2 cells. In agreement with some earlier studies on some microbial extracts, the results clearly indicated that the crude extract of Halomonas sp. HA1 showed a significant anticancer effect on HepG2 cells associated with decreased viability and morphological alterations of the treated cells leading eventually to cell death29,30,38. To investigate the pro-apoptotic pathway of H. HA1 bacterial extract, the changes in apoptosis regulatory genes upon applying the extract on HepG2 cells were evaluated.
Apoptosis is a process involving alterations in the expression of an array of genes. BCL-2 family plays a crucial role in apoptosis regulation39. However, one of the early apoptosis hallmark changes is a reduction in mitochondrial membrane potential. The potential decrease of the mitochondrial membrane found as a result of mitochondrial membrane depolarization indicated the mitochondrial damage. Another factor in the process of mitochondrial apoptosis pathway after mitochondrial membrane destruction is the release of pro-apoptotic protein factors from the mitochondria40.
In the present study, BAX/BCL-2, CASP-3 (as a procaspase-3) and P53 were assessed at the transcriptional and protein levels. The ratio of BAX/BCL-2 mRNA expression recorded a significant increase as well as CASP-3 and P53 up-regulation in treated HepG2 cells. It is well-known that BAX is up-regulated by P53 protein while BCL-2 protein expression is down-regulated by P5341. P53 is a nuclear transcriptional factor that usually is activated in apoptosis by regulating numerous down-stream effectors42. Over-expression of BAX (pro-apoptotic protein)43 and inhibition of BCL-2 protein expression can speed up cell apoptosis. Apoptosis can be induced by P53-mitochondrial localization in a transcription-independent manner44,45 or by induction of endoplasmic reticulum stress to prevent P53-dependent apoptosis via the glycogen synthase kinase-3β pathway46. The expression profile of H. HA1 extract indicated the P53-dependant mitochondrial apoptotic pathway. These results are in agreement with Ruiz-Ruiz et al.47, suggesting the anti-proliferative effect of the novel halophilic Halomonas sp. extract. The results of CASP-3 expression profile exhibted caspase-dependent apoptosis. However, due to the elevated toxicity and stress of the higher concentration of the extract, treated cells may tend to undergo apoptosis via caspase-independent pathway48 owned to the processes of autophagy, endoplasmic reticulum stress or mitotic catastrophe49. Further, the different biological and technical factors, such as transcriptional or post-translational alterations as well as a long half-life of some proteins, can affect the association between mRNA expression and the level of proteins50. The treatments may cause internal cellular dysfunctions which lead to the degradation of mRNA molecules faster than the protein ones51. Although the cleaved caspase-3 has not been assessed in this study, the morphological apoptotic features such as membrane blebbing, loss of membrane integrity, nuclear fragmentation and elevated BAX/BCL-2 ratio suggested the occurrence of apoptosis52. Moreover, the loss of membrane integrity, confirmed by AO/EB labeling, is a late event in apoptosis. Caspase-3 is one of the death substrates that serve as an effector in apoptosis inducing phosphatidylserine (PS) externalization, shrinkage of cell, membrane blebbing, and DNA fragmentation by cleaving53,54. In the absence of caspase-3, the cells morphological features were impaired55 and cytochrome c release was also delayed56. Due to apoptosis induction, the decreased value of CASP-3 protein in the higher concentration of the extract may be due to the increase of the expected cleavage process occurred in the procaspase-3 protein. However, the CASP-3 cleavage assessment is needed in the future studies. Collectively, the explanation of the different transcriptional level of CASP-3 in comparison to its protein level as well as the decreased levels of up-regulated P53 and CASP-3 in the higher concentration-treated cells could be proved.
Moreover, the analysis of cell cycle distribution revealed accumulations of sub-G0 (apoptotic cells) and significant G2/M arrest in addition to the down-expression of PCNA (proliferating-cell nuclear antigen) protein. The expression of PCNA protein regulates the cell cycle and promotes apoptosis in fetal and neonatal mouse ovaries57. Further, the arrest of G2/M phase may enable damaged cells to undergo apoptosis58. However, cisplatin-induced arrest in sub-G0 and S phases (DNA synthesis) eventually led to DNA damage and apoptosis59. The down-regulation of PCNA, the anti-proliferative effects and the cell cycle arrest are key players of the apoptotic cascade.
The results suggested the anti-proliferative and apoptotic effects of Halomonas sp. HA1 extract can be attributed to the secondary metabolites identified by LC–MS-MS and NMR analysis of Halomonas sp. HA1 extract. However, sphinganine is the backbone precursor of sphingolipids60. Sphingolipids are produced in most eukaryotic cells but rarely in prokaryotes, which possess only glycerol-based phospholipids in their membrane61. However, a few isolated bacterial species produce sphingolipids62–64. A previous study reported that, both cyclic dipeptides cyclo-(prolyltyrosyl) and cyclo-(prolylphenylalanyl) isolated from Bacillus spp. showed potential anticancer activity65. Another study showed that a CDP mix composed of cyclo-(L-Pro-L-Tyr), cyclo-(L-Pro-L-Val), and cyclo-(L-Pro-L-Phe), isolated from the P. aeruginosa PAO1 strain, exhibited anticancer activity in HeLa and Caco-2 cell lines66. In another study cyclo-(-Pro-Tyr) exhibited anticancer and anti-proliferative activity against HepG2 cell lines67. A previous study has proven the anticancer activity of 1, 2-Benzenedicarboxylic acid, bis (2-ethylhexyl) ester on PC3, MCF, HCT-116, A549, and MIAPACA cell lines68. Surfactin biosynthesis occurs in many natural microorganisms i.e. Bacillus pumilus, B. mojavensis, B. circulans, B. natto, and B. amyloliquefaciens69; B. safensis F4 and B. subtilis strains70,71. It exhibits powerful therapeutic activities i.e. antimicrobial, antimycoplasma, antiviral, anti-inflammatory, antibacterial and antitumor10,70,72–75. Additionally, it is widely applicable environmentally in biopesticides, food processing, pharmaceuticals, cosmetics and oil recovery76–78.
In fact, surfactin mediated a forceful anticancer activity on various cancer cell lines. It showed anti-proliferative potentials on human breast cancer cells (MCF-7)79, cervical cancer (HeLa) and hepatoma (Bel-7402)80. An in vivo anticancer activities of surfactin on mice ascites tumors were also reported81. It also inhibited the growth of LoVo colon cancer cells, with IC50 of 26 µM at 48 h82. The anticancer activity of surfactin is attributed to its amphiphilic nature. Lipopeptides including surfactin exert cytotoxicity on cancer cells with different IC50 depending on the length of fatty acid chains80. Therefore, surfactin C15 should exhibit higher anticancer activity than surfactin C14 and it is supposed to be responsible for the anticancer activity of the extract.
In conclusion, we reported for the first time that, Halomonas sp. HA1 extract has a potent anticancer activity on HepG2 cells via anti-proliferative and apoptotic potentials. Surfactin C14 and Surfactin C15, biosurfactants, were identified in Halomonas sp. HA1 extract by LC–MS-MS and NMR analyses. However, further in vivo studies are needed to investigate the metabolism and the impact of biotransformation on the efficacy of H. HA1 extract's active constituents as anticancer agents.
Methods
Isolation of haloalkaliphilic bacteria
Water samples were collected from El-Hamra Lake in Wadi El-Natrun, Egypt where the pH was 10.0 and water salinity was 300 g/L. Halophile growth medium (HGM) was prepared according to Dorn et al.83, and pH 9 was adjusted using NaHCO3. The media were supplemented with 3 M NaCl and 2.0 mL 10% (w/v) yeast extract per liter. The water sample was added to HGM media with 1:10 (v/v) and incubated at 30 °C for 48 h. Two more successive culturing were carried out using 50 µL of diluted culture spread on an agar plate of the same media. Single colonies were plated on new agar plates with the same media for biochemical characterization.
Phylogenetic analysis of the 16S ribosomal RNA gene
Genomic DNA was extracted from the pure culture using GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific, US). PCR amplification of the 16S rRNA gene carried out using Bact 27f. (5′-AGAGTTTGATC (A/C)-TGGCTCAG-3′), Bact 1492r (5′-TACGG(C/T)-ACCTTGTTACGACTT-3′), and Bact 1098r (5′-AAGGGTTGCGCTCGTTGCG-3′)84. Using a 3,100 Genetic Analyzer (Applied Biosystems, US), the amplified products were sequenced according to the manufacturer’s protocols. The obtained sequence of 16S rRNA gene was compared using the BLASTN program against the nucleotide sequences collection (nr/nt) database, available at the National Center for Biotechnology Information website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic tree relative to high scoring BLASTP hits was performed using the (MEGA 7.0.26) software.
The complete sequence of 1, 2 CDG of Halomonas was isolated using universal primer UDOG: (5′-ATGACTGTTAAAATTTATGACACCCCTGAAG-3′) and reversal primer RDOG: (5′-TTATGGACGCGCTTGCAGCTC-3′). Primers were designed according to sequence alignment of 1, 2 DOG NCBI database sequences. The PCR conditions were 94 °C for 30 s, 60 °C 30 s and 72 °C for 1 min. Using a 3100 Genetic Analyzer (Applied Biosystems, US), PCR products were sequenced following the manufacturer’s protocols. The obtained sequence of 16S rRNA gene was compared using the BLASTN program against the nucleotide sequences collection database, available at the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The phylogenetic tree relative to high scoring BLASTP hits was performed using the (MEGA 7.0.26) software.
Preparation of crude extract
The bacterial isolate was cultured in (HGM) medium and incubated in shaker incubator 100 rpm at 30 °C for 72 h. The culture medium was centrifuged at 6,500 rpm for 20 min. The supernatant was collected and filtered through a 0.45 µm sterile membrane. Ethyl acetate was added to culture filtrate (1:1) and stirred at 130 rpm for 12 h. the ethyl acetate phase on the upper part of the culture was removed and followed by vacuum evaporation to obtain the dry extracts by vacuum rotary evaporator 40 °C85. Crude extracts produced were stored at − 20 °C for further investigations.
Characterization of bacterial extract using NMR and LC–MS-MS
Generally, 1H and 13C NMR spectra were recorded on a Bruker DRX 600 spectrometer at 600.1 and at 150.9 MHz, respectively, at 25 °C using CD3OD as the solvent. All chemical shifts are expressed relative to TMS86.
For the LC–MS analysis the crude extract was dissolved in 50% acetonitrile (ACN), 0.1% formic acid (FA). The samples were injected by syringe through a PicoTip emittor at 0.3 µL/min connected to a Q-Tof Micro (Waters, Milford, Massachusetts, US) with the voltage set at 1.4 kV. The analysis was carried out in positive ion mode and linear gradient from 10% (v/v) H2O to 99% (v/v) ACN in 0.1% (v/v) FA at a flow rate of 0.3 µL/min over 75 min. The analysis was followed by LC/MS–MS via dissolved 0.1 mg/mL in LC–MS solvent: 60% MeCN in 0.1% FA with a linear gradient ranging from 10–60% (v/v) MeCN in 0.1% (v/v) FA at a flow rate of 0.3 mL/min over 75 min. The capillary temperature was set at 220 °C and the spray voltage at 4 kV87.
Biological investigations
The effect of the extract was evaluated against hepatocellular carcinoma (HepG2) cell line as well as the normal human peripheral lymphocytes (HPBL) in vitro. The study was approved and followed the Institutional Ethical Committee guidelines at Faculty of Science, Menoufia University, Egypt (MUFS-F-GE-4-20).
Anticancer activities
Maintenance of HepG2 cell line
Hepatocellular carcinoma (HepG2) cell line was acquired from VACSERA, Giza, Egypt. The concentration of cells per milliliter was determined using a hemocytometer and calculated using the following equation:
The cell line was maintained and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in a humidified 5% CO2 atmosphere at 37 °C in T25 culture flasks at a density of 2 × 104 cells/cm2. The medium in flasks was changed every 48 h. The confluency of cells was confirmed by an inverted microscope until reaching 75%. Cells were harvested after trypsinization (0.025% trypsin and 0.02% EDTA) then washed twice with phosphate-buffered saline (PBS). All experiments were done in triplicates. All reagents and media were purchased from Lonza supplier, Egypt.
Cytotoxicity on HepG2 cell line
To evaluate the maximal half inhibitory concentration (IC50), cytotoxicity of H. HA1 bacterial extract was carried out by microculture tetrazolium (MTT) assay method, 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide, against HepG2 cell line. Cells were seeded into 96-well plates at a plating density of 1 × 104 cells/well and incubated to allow the attachment of cells prior to the addition of treatments. The bacterial extract was dissolved in dimethyl sulfoxide (DMSO) and diluted in serum-free medium. After reaching the confluency of 75%, various concentrations (0–1,000 μg/mL) of the extract were added to the cultures then incubated for 24 h. Cisplatin (3 μg/mL) was used as a positive control46 and DMSO (8 μL/ mL) as negative solvent control. After 24 h, 100 μL of MTT in PBS were added to each well and incubated at 37 °C for 4 h. The formazan crystals were formed then solubilized in 100μL of acidified isopropanol and then measured the absorbance at 630 nm by using enzyme-linked immunosorbent assay (ELISA) microplate reader (Bio-RAD microplate reader, Japan). The percentages of cell inhibition were determined using the following formula:
This assay was carried out in triplicate. The IC50 value was determined from % cell inhibition and concentration curve88. For further anticancer mechanistic investigations, HepG2 cells were incubated with 20 and 40 μg/mL of bacterial extract for 24 h.
Morphological changes in HepG2
Phase contrast inverted microscopy
HepG2 cells were examined under the inverted Olympus BX41 microscope (Tokyo, Japan) at × 400 magnification to observe morphological alterations among treated groups.
Acridine orange/ethidium bromide (AO/EB) double fluorescent labeling
In order to investigate the altered cell structure towards apoptosis or necrosis, AO/EB staining was carried out89. Briefly, 4 μL of treated and control cell suspensions were transferred to glass slides then they were stained with 1μL AO/ EB staining solution (100 μg/mL AO and 100 μg/mL EB). Cells were examined immediately by a fluorescent microscope (Olympus BX 41, Japan) at 400 × magnification. One hundred cells were counted per each field of randomly selected five fields.
Flow cytometric analysis of cell cycle distribution
Cell cycle distribution and DNA content of treated cells and controls were evaluated by flow cytometry after propidium iodide (PI) labeling. After various treatments period, cells were trypsinized and washed with PBS twice and then fixed with cold ethanol (70%) for 2 h. The alcohol was entirely removed and cells were collected and washed with PBS at 1,200 rpm for 5 min. Cells were kept in PBS containing 50 μg/mL of RNase A for 2 h at 37 °C, mixed with 25 μg/mL of PI stain90,91 and analyzed on a FACS flow cytometer (Becton Dickinson, US) according to the manufacturer’s instructions. The analyses of cell cycle phases were performed using BD FACS Diva software. All reagents used were Sigma-Aldrich, Germany.
Immunocytochemical staining
The immunocytochemical reactions were performed using the avidin–biotin complex immunoperoxidase technique92. Control and treated cells were harvested and smeared on positive-charged slides13. Slides were then processed for P53 (tumor suppressor protein), BCL-2 (anti-apoptotic protein), BAX (BCL-2 associated X protein, apoptosis regulator) and caspase-3 (pro-apoptotic protein) immune-reaction. Moreover, PCNA (proliferating-cell nuclear antigen) were also used as proliferation markers. Across five randomly selected fields, two hundred HepG2 cells were studied. As the immunocytochemical reactivity, the mean percentage of positive cells was calculated (positive cells/total number of counted cells) × 100. Cells were counted under a light microscope (Olympus BX41, Japan) at × 400 magnification. All antibodies used were Invitrogen, CA, US.
Quantitative real-time polymerase chain reaction (qPCR)
The qPCR was carried out in treated and control cells to assess BCL-2, BAX, P53, and CASP-3 mRNA expressions. Briefly, total RNA was extracted using RNeasy Plus Minikit (Qiagen, Hilden, Germany) following the manufacturer's protocol. The quality of RNA was assessed by agarose gel and quantified by nanodrop (Quawell Q5000 UV–Vis Spectrophotometer, UK). For cDNA synthesis, total RNA was reverse transcribed following the manufacturer's instructions RevertAid H Minus Reverse Transcriptase (Thermo Fisher Scientific, US). Real-time PCR was performed using Power SYBR Master Mix (Thermo Fisher Scientific, US) on an Applied Biosystems 7500 system (Foster City, US) following the standard program of 40 cycles with 58 °C for primer annealing. Samples of cDNA were run in triplicate. All data were then normalized to the endogenous control, GAPDH, a housekeeping gene. Fold change in gene expression was calculated using the comparative threshold cycle (ΔΔCT) method. Primer sequences and accession numbers of genes used in this study are provided in Table 2.
Table 2.
Primer sequences of genes analyzed by real-time PCR.
| Name | Accession number | Sense (5′–3′) | Antisense (5′–3′) |
|---|---|---|---|
| GAPDH | NM_001289745.2 | GGATTTGGTCGTATTGGG | GGAAGATGGTGATGGGATT |
| BCL-2 | NM_001114735.1 | TACAGGCTGGCTCAGGACTAT | CGCAACATTTTGTAGCACTCTG |
| BAX | NM_001291431.1 | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT |
| CASP-3 | NM_001354777.1 | GGCGCTCTGGTTTTCGTTAAT | CAGTTCTGTACCACGGCAGG |
| P53 | NM_001126112.2 | TTCCCTGGATTGGCCAGACT | GCAGGCCAACTTGTTCAGTG |
GAPDH Glyceraldehyde-3-phosphate dehydrogenase; BCL-2 B-cell lymphoma 2; BAX BCL-2-associated X protein; CASP-3 Caspase-3.
Cytotoxicity of the bacterial extract on normal human peripheral blood lymphocytes (HPBL)
Cells’ culture and isolation
The extract toxicity was done on HPBL isolated from three male volunteers (healthy and non-smoker). Written informed consent was obtained from the three involved volunteers. Peripheral venous blood samples were collected using sterile syringes and then transferred into sterile tubes (KEMICO vacutainer, Egypt) containing K2-EDTA. Samples were processed for culturing in RPMI-1640 medium supplemented with 15% fetal calf serum, phytohemagglutinin (2%) and 1% (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C and humidified 5% CO2 atmosphere. After 48 h of culture setup, various treatments were applied and the cultures extended for 24 h. For HPBL isolation after the treatment period, cultures were incubated with five folds of erythrocyte lysing buffer (0.015 M NH4C1, 1 mM NaHCO3, 0.l mM EDTA) for 15 min. Then, centrifugation was done for 5 min at 1,500 rpm in a cooling centrifuge (Sigma 3 K 30, Germany). The incubation was repeated until a white pellet of lymphocytes appeared93. All reagents were from Lonza, Switzerland.
Trypan blue exclusion assay
Using the 0.4 percent trypan blue (Sigma-Aldrich, Germany) solution, the HPBL cells were stained and examined immediately under a light microscope at × 200 magnification (Olympus BX41, Japan). Randomly five fields were selected and 200 cells/field were scored. Finally, cytotoxicity was calculated as follows:
Acridine orange/ethidium bromide (AO/EB) dual fluorescent staining
The AO/EB staining was performed to investigate the viability of cells. Briefly, 4 μL of treated and control cells were stained with 1 μL stain solution AO/EB (100 μg/mL AO and 100 μg/mL EB) on glass slides and examined immediately by a fluorescent microscope (Olympus BX 41, Japan) at × 400 magnification. Randomly five fields were observed and 200 cells were counted from each. Two types of cells were observed, based on the emitted fluorescence: viable cells were green-colored cells with intact structures and late apoptotic or dead cells showed an orange-to-red color89.
DNA single-strand breaks (comet assay)
The alkaline single-cell gel electrophoresis (comet assay) method94 was carried out to evaluate the genotoxicity on normal HPBL. Briefly, cells were suspended in low melting point agarose gel (Sigma-Aldrich, Germany) on a microscopic glass slide between two layers of ultra-pure normal melting agarose (Sigma-Aldrich, Germany). Then, slides were immersed in lysis buffer (2.5 M NaCl, 100 mM EDTA and 10 mM Tris, pH 10.0) with freshly added 1% Triton X-100 (Sigma-Aldrich, Germany) and 10% DMSO for 1 h at 4 °C. Subsequently, slides were kept in the freshly prepared alkaline buffer (300 mM NaOH and 1 mM EDTA, pH > 13) for 20 min at 4 °C. Then, slides were electrophoresed in electric current of 25 V and 300 mA for 10 min. The slides were then immersed for 3 min in neutralizing buffer (0.4 M Tris–HCl, pH 7.5). They were stained with ethidium bromide (Sigma-Aldrich, Germany). Visualization of cells was carried out by a fluorescence microscope (Olympus BX 41, Japan), and representative images were taken. About 100 randomly selected cells were examined per one field of the total examined five fields. The results were divided as normal nuclei with no migrated tails and damaged with a migrated tail or with no distinct nucleus.
Statistical analysis
Data are reported as the mean ± standard deviation (SD). Data for multiple variable comparisons were analyzed by one-way analysis of variance. Duncan's test was used as a post-hoc test using the statistical package SPSS version 17.0 software to compare the significance of differences between groups (SPSS, Inc., Chicago, IL, US) and significance was considered at a probability level of P < 0.05.
Supplementary information
Acknowledgements
We are very grateful to the Swedish Research links grant VR (Stockholm, Sweden), Grant number 2016-05908 for generous financial support. Dr. S.A.M. Khalifa thanks the Department of Molecular Biosciences, Wenner-Grens Institute, Stockholm University, Sweden.
Abbreviations
- NMR
Nuclear magnetic resonance
- LC–MS–MS
Liquid chromatography-mass spectrophotometry
- MeOH
Methanol
- DMSO
Dimethyl sulphoxide
- HPBL
Normal human peripheral lymphocytes
- BCL-2
B-cell lymphoma-2 proteins
- BAX
BCL-2-associated X protein
Author contributions
H.S., S.S. and I.EL-G. planned and designed the experiments; I.El-G. and N.A. performed analyzed and wrote the biological and molecular experiments; H.S. and D.M. performed, analyzed and wrote the phytochemical experiments; H.A. performed the microbiological experiments; H.A. and I.El-G. wrote the manuscript; I.El-G., S.K. and O.E. revised the manuscript. All authors read and approved the final manuscript.
Data availability
All data of this study are introduced in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-73028-0"
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/30/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-024-73028-0
Contributor Information
Islam M. El-Garawani, Email: dr.garawani@yahoo.com
Hesham R. El-Seedi, Email: hesham.el-seedi@ilk.uu.se
Supplementary information
is available for this paper at 10.1038/s41598-020-70945-8.
References
- 1.Zhu, R. X., Seto, W. K., Lai, C. L. & Yuen, M. F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver10, 332–339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinyemiju, T. et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol.3, 1683–1691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang, J. D. & Roberts, L. R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol.7, 448–458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreidieh, M., Zeidan, Y. H. & Shamseddine, A. The Combination of Stereotactic Body Radiation Therapy and Immunotherapy in Primary Liver Tumors. J. Oncol.2019, (2019). [DOI] [PMC free article] [PubMed]
- 5.Florea, A.-M.M. & Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel).3, 1351–1371 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanif, R. et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol.52, 237–245 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Kamesaki, H. Mechanisms involved in chemotherapy-induced apoptosis and their implications in cancer chemotherapy. Int. J. Hematol.68, 29–43 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Tohamy, A. A., El-Garawani, I. M., Ibrahim, S. R. & Abdel Moneim, A. E. The apoptotic properties of Salvia aegyptiaca and Trigonella foenum-graecum extracts on Ehrlich ascites carcinoma cells: The effectiveness of combined treatment. Res. J. Pharm. Biol. Chem. Sci. 29:710–722 (2016).
- 9.Prada-Gracia, D., Huerta-Yépez, S. & Moreno-Vargas, L. M. Application of computational methods for anticancer drug discovery, design, and optimization. Bol. Méd. Hosp. Infant. México73, 411–423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai, S. Q., Li, J. & Shi, Q. C. Composition characterization, antioxidant capacities and anti-proliferative effects of the polysaccharides isolated from Trametes lactinea (Berk.) Pat. Int. J. Biol. Macromol.115, 114–123 (2018). [DOI] [PubMed] [Google Scholar]
- 11.El-Garawani, I. et al. In vitro antigenotoxic, antihelminthic and antioxidant potentials based on the extracted metabolites from lichen, candelariella vitellina. Pharmaceutics10.3390/pharmaceutics12050477 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Garawani, I., El Nabi, S. H., Nafie, E. & Almeldin, S. Foeniculum Vulgare and Pelargonium Graveolens essential oil mixture triggers the cell cycle arrest and apoptosis in MCF-7 cells. Anticancer. Agents Med. Chem.10.2174/1573399815666190326115116 (2019). [DOI] [PubMed] [Google Scholar]
- 13.El-Garawani, I. M., El-Nabi, S. H., Dawoud, G. T., Esmail, S. M. & Abdel Moneim, A. E. Triggering of apoptosis and cell cycle arrest by fennel and clove oils in Caco-2 cells: the role of combination. Toxicol. Mech. Methods29, 710–722 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Elkhateeb, W. A. et al. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother.101, 264–277 (2018). [DOI] [PubMed] [Google Scholar]
- 15.El-Nabi, S. H., Dawoud, G., El-Garawani, I. & El-Shafey, S. HPLC analysis of phenolic acids, antioxidant activity and in vitro effectiveness of green and roasted Caffea arabica bean extracts: a comparative study. Anti-Cancer Agents Med. Chem.18, 1281–1288 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Ahmed, A., Ali, M. & Sherif, N. Anticancer activity of Morus nigra on human breast cancer cell line ( MCF-7): the role of fresh and dry fruit extracts. J. Biosci. Appl. Res2, 352–361 (2016). [Google Scholar]
- 17.El-Garawani, I. M. et al. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: A novel anticancer agent. Food Chem. Toxicol.127, 110–119 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Demain, A. L. & Sanchez, S. Microbial drug discovery: 80 Years of progress. J. Antibiot. (Tokyo)62, 5–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charu, G., Dhan, P., Garg, A. P. & Sneh, G. Nutraceuticals from microbes. In Phytochemicals of Nutraceutical Importance 79–102 (CABI, Wallingford, 2014). [Google Scholar]
- 20.Berdy, J. Bioactive microbial metabolites. J. Antibiot. (Tokyo)58, 1–26 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Dang, V. T., Benkendorff, K., Green, T. & Speck, P. Marine snails and slugs: a great place to look for antiviral drugs. J. Virol.89, 8114–8118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desbois, A. P., Mearns-Spragg, A. & Smith, V. J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol.11, 45–52 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Plaza, A. et al. Celebesides A-C and theopapuamides B-D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem.74, 504–512 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei, X., Nieves, K. & Rodríguez, A. D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the Caribbean sea sponge Neopetrosia proxima. Bioorgan. Med. Chem. Lett.20, 5905–5908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuijen, B. et al. Pharmaceutical development of anticancer agents derived from marine sources. Anticancer. Drugs11, 793–811 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Asolkar, R. N. et al. Arenamides A-C, cytotoxic NFkappaB inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod.72, 396–402 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eltem, R. & Ucar, F. The determination of antimicrobial activity spectrum of 23 Bacillus strains isolated from Denizli-Acigol (Bitter Lake) which is soda lake (Na2SO4). J. KUKEM21, 57–64 (1998). [Google Scholar]
- 28.Giddings, L.-A. & Newman, D. J. Bioactive compounds from marine extremophiles. In Bioactive Compounds from Marine Extremophiles 1–124 (Springer, Cham, 2015). [Google Scholar]
- 29.Bitzer, J. et al. New aminophenoxazinones from a marine Halomonas sp.: fermentation, structure elucidation, and biological activity. J. Antibiot. (Tokyo)59, 86–92 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Sagar, S. et al. Induction of apoptosis in cancer cell lines by the Red Sea brine pool bacterial extracts. BMC Complement. Altern. Med.13, 344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mi, J. N., Wang, J. R. & Jiang, Z. H. Quantitative profiling of sphingolipids in wild Cordyceps and its mycelia by using UHPLC-MS. Sci. Rep.6, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrouchi, R. et al. Toxic C17-sphinganine analogue mycotoxin, contaminating tunisian mussels, causes flaccid paralysis in rodents. Mar. Drugs11, 4724–4740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kharrat, R. et al. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem.107, 952–963 (2008). [DOI] [PubMed] [Google Scholar]
- 34.El-Gendy, M. M. A., Shaaban, M., El-Bondkly, A. M. & Shaaban, K. A. Bioactive benzopyrone derivatives from new recombinant fusant of marine streptomyces. Appl. Biochem. Biotechnol.150, 85–96 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Cooper, E. L. & Albert, R. Tunicates: A vertebrate ancestral source of antitumor compounds. In Handbook of Anticancer Drugs from Marine Origin 383–395 (Springer, Cham, 2015). [Google Scholar]
- 36.Lee, J.-C. et al. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int.13, 55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sithranga Boopathy, N. & Kathiresan, K. Anticancer drugs from marine flora: an overview. J. Oncol.10.1155/2010/214186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganguly, R. K., Midya, S. & Chakraborty, S. K. Antioxidant and anticancer roles of a novel strain of Bacillus anthracis isolated from vermicompost prepared from paper mill sludge. Biomed Res. Int.10.1155/2018/1073687 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med.7, 249–257 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu, L., Wang, P., Qin, Q. L., Zhang, H. & Wu, Y. J. Protective effect of polypeptides from larva of housefly (Musca domestica) on hydrogen peroxide-induced oxidative damage in HepG2 cells. Food Chem. Toxicol.60, 385–390 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Kumar, S., Sharma, V. K., Yadav, S. & Dey, S. Antiproliferative and apoptotic effects of black turtle bean extracts on human breast cancer cell line through extrinsic and intrinsic pathway. Chem. Cent. J.11, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan, J., Wang, B. & Zhu, L. Regulation of survivin and Bcl-2 in HepG2 cell apoptosis induced by quercetin. Chem. Biodivers.6, 1101–1110 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Abdel Moneim, A. E. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE11, e158965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchenko, N. D., Zaika, A. & Moll, U. M. Death signal-induced localization of p53 protein to mitochondria a potential role in apoptotic signaling. J. Biol. Chem.275, 16202–16212 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell11, 577–590 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Qu, L. et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev.18, 261–277 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Ruiz, C. et al. An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl. Microbiol. Biotechnol.89, 345–355 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Liu, T., Brouha, B. & Grossman, D. Rapid induction of mitochondrial events and caspase-independent apoptosis in Survivin-targeted melanoma cells. Oncogene23, 39–48 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bröker, L. E., Kruyt, F. A. E. & Giaccone, G. Cell death independent of caspases: a review. Clin. Cancer Res.11, 3155–3162 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Maier, T., Güell, M. & Serrano, L. Correlation of mRNA and protein in complex biological samples. FEBS Lett.10.1016/j.febslet.2009.10.036 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Wu, G., Nie, L. & Zhang, W. Integrative analyses of posttranscriptional regulation in the yeast Saccharomyces cerevisiae using transcriptomic and proteomic data. Curr. Microbiol.10.1007/s00284-008-9145-5 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Elmore, S. Apoptosis: a review of programmed cell death. Toxicol. Pathol.10.1080/01926230701320337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shalini, S., Dorstyn, L., Dawar, S. & Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ.10.1038/cdd.2014.216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galluzzi, L. et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ.10.1038/s41418-017-0012-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jänicke, R. U., Sprengart, M. L., Wati, M. R. & Porter, A. G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem.10.1074/jbc.273.16.9357 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Blanc, C. et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res.60, 4386–4390 (2000). [PubMed] [Google Scholar]
- 57.Xu, B. et al. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE6, e16046 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DiPaola, R. S. To arrest or not to G(2)-M Cell-cycle arrest. Clin. Cancer Res.8, 3311–3314 (2002). [PubMed] [Google Scholar]
- 59.Bennukul, K., Numkliang, S. & Leardkamolkarn, V. Melatonin attenuates cisplatin-induced HepG2 cell death via the regulation of mTOR and ERCC1 expressions. World J. Hepatol.6, 230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandwagt, B. F. et al. A longevity assurance gene homolog of tomato mediates resistance to Altemaria alternata f sp lycopersici toxins and fumonisin B1. Proc. Natl. Acad. Sci. USA97, 4961–4966 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Meer, G. & De Kroon, A. I. P. M. Lipid map of the mammalian cell. J. Cell Sci.124, 5–8 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Olsen, I. & Jantzen, E. Sphingolipids in bacteria and fungi. Anaerobe7, 103–112 (2001). [Google Scholar]
- 63.Batrakov, S. G., Mosezhnyi, A. E., Ruzhitsky, A. O., Sheichenko, V. I. & Nikitin, D. I. The polar-lipid composition of the sphingolipid-producing bacterium Flectobacillus major. Biochim. Biophys. Acta1484, 225–240 (2000). [DOI] [PubMed] [Google Scholar]
- 64.An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell156, 123–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong, S. et al. Inhibitory effect against Akt by cyclic dipeptides isolated from Bacillus sp. J. Microbiol. Biotechnol.18, 682–685 (2008). [PubMed] [Google Scholar]
- 66.Vázquez-Rivera, D. et al. Cytotoxicity of cyclodipeptides from pseudomonas aeruginosa PAO1 leads to apoptosis in human cancer cell lines. Biomed Res. Int.20, 15. 10.1155/2015/197608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karanam, G., Arumugam, M. K. & Sirpu Natesh, N. Anticancer effect of marine sponge-associated Bacillus pumilus AMK1 derived dipeptide Cyclo (-Pro-Tyr) in human liver cancer cell line through apoptosis and G2/M phase arrest. Int. J. Pept. Res. Ther.26, 445–457 (2020). [Google Scholar]
- 68.Save, S. A., Lokhande, R. S. & Chowdhary, A. S. Determination of 1, 2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester from the twigs of Thevetia peruviana as a colwell biomarker. JIPBS2, 349–362 (2015). [Google Scholar]
- 69.Wu, Y. S. et al. Anticancer activities of surfactin potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol.8, 761 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdelli, F. et al. Antibacterial, anti-adherent and cytotoxic activities of surfactin(s) from a lipolytic strain Bacillus safensis F4. Biodegradation30, 287–300 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Alvionita, M. & Hertadi, R. Bioconversion of glycerol to biosurfactant by halophilic bacteria Halomonas elongata BK-AG18. Indones. J. Chem.19, 48–57 (2019). [Google Scholar]
- 72.Kim, S. D. et al. A comparison of the anti-inflammatory activity of surfactin A, B, C, and D from Bacillus subtilis. J. Microbiol. Biotechnol.16, 1656–1659 (2006). [Google Scholar]
- 73.Meena, K. R., Sharma, A. & Kanwar, S. S. Antitumoral and antimicrobial activity of surfactin extracted from Bacillus subtilis KLP2015. Int. J. Pept. Res. Ther.26, 423–433 (2020). [Google Scholar]
- 74.Vollenbroich, D., Pauli, G., Özel, M. & Vater, J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl. Environ. Microbiol.63, 44–49 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kracht, M. et al. Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivatives. J. Antibiot.52, 613–619 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Sen, R. Surfactin: Biosynthesis, Genetics and Potential Applications 316–323 (Springer, New York, 2010). [DOI] [PubMed] [Google Scholar]
- 77.Jacques, P. Surfactin and Other Lipopeptides from Bacillus spp 57–91 (Springer, Berlin, 2011). [Google Scholar]
- 78.Heerklotz, H. & Seelig, J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys. J.81, 1547–1554 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee, J. H. et al. The production of surfactin during the fermentation of cheonggukjang by potential probiotic Bacillus subtilis CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food Chem.131, 1347–1354 (2012). [Google Scholar]
- 80.Liu, X. et al. Effect of themicrobial lipopeptide on tumor cell lines: apoptosis induced by disturbing the fatty acid composition of cell membrane. Protein Cell1, 584–594 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kameda, Y. & Kanatomo, S. Abstracts papers, The 88th Annual Meeting of Pharmaceutical Society of Japan. In The 88th Annual Meeting of Pharmaceutical Society of Japan (p. 431) (1968).
- 82.Kim, S. Y. et al. Surfactin from Bacillus subtilis displays anti-proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Lett.581, 865–871 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Dorn, E., Hellwig, M., Reineke, W. & Knackmuss, H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch. Microbiol.99, 61–70 (1974). [DOI] [PubMed] [Google Scholar]
- 84.Chang, Y.-J. et al. Phylogenetic analysis of aerobic freshwater and marine enrichment cultures efficient in hydrocarbon degradation: effect of profiling method. J. Microbiol. Methods40, 19–31 (2000). [DOI] [PubMed] [Google Scholar]
- 85.Thomas, A. T. et al. In vitro anticancer activity of microbial isolates from diverse habitats. Braz. J. Pharm. Sci.47, 279–287 (2011). [Google Scholar]
- 86.Miao, L. et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem.290, 239–245 (2019). [DOI] [PubMed] [Google Scholar]
- 87.El-Seedi, H. R. et al. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol.145, 746–757 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Thakur, A. N. et al. Antiangiogenic, antimicrobial, and cytotoxic potential of sponge-associated bacteria. Mar. Biotechnol.7, 245–252 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Liu, K., Liu, P. C., Li, R. & Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res.21, 15–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dassonneville, L. et al. Cytotoxicity and cell cycle effects of the plant alkaloids cryptolepine and neocryptolepine: relation to drug-induced apoptosis. Eur. J. Pharmacol.409, 9–18 (2000). [DOI] [PubMed] [Google Scholar]
- 91.Dengler, R. et al. Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br. J. Haematol.89, 250–257 (1995). [DOI] [PubMed] [Google Scholar]
- 92.Hsu, S.-M., Raine, L. & Fanger, H. X. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem.29, 577–580 (1981). [DOI] [PubMed] [Google Scholar]
- 93.El-Garawani, I. M. Ameliorative effect of Cymbopogon citratus extract on cisplatin-induced genotoxicity in human leukocytes. J. Biosci. Appl. Res.1, 304–310 (2015). [Google Scholar]
- 94.Singh, N. P., McCoy, M. T., Tice, R. R. & Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.175, 184–191 (1988). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this study are introduced in this published article.