Graphical abstract
Keywords: Carfentanil, Naloxone, Opioid, Respiratory depression, Cardiac
Highlights
-
•
Carfentanil aerosol induced profound cardiorespiratory and physiological effects.
-
•
Carfentanil was bioavailable approximately 3 times longer than naloxone.
-
•
Naloxone was able to rapidly reverse the effects of a lethal exposure.
Abstract
Carfentanil is a powerful synthetic opioid that is approximately 100 times more potent than fentanyl and 10,000 times more potent than morphine. Carfentanil was originally intended to be used as a sedative for big game animals in a veterinary setting, but it is becoming increasingly recognized as a public health concern. We set out to investigate the effectiveness of naloxone against a potentially lethal dose of inhaled carfentanil in male ferrets. Ferrets were implanted with telemetry devices to study cardiac parameters and exposed to aerosolized carfentanil in a whole-body plethysmography chamber to record respiratory parameters. We observed profound respiratory depression in exposed animals, which led to apneic periods constituting 24–31 % of the exposure period. Concomitant with these apneic periods, we also observed cardiac abnormalities in the form of premature junctional contractions (PJCs). At our acute exposure dose, lethal in 3 % of our animals, naïve ferrets were unresponsive and incapacitated for a total of 126.1 ± 24.6 min. When administered intramuscularly at human equivalent doses (HEDs) of either 5 mg or 10 mg, naloxone significantly reduced the time that ferrets were incapacitated following exposure, although we observed no significant difference in the reduction of time that the animals were incapacitated between the treatment groups. Naloxone was able to quickly resolve the respiratory depression, significantly reducing the frequency of apneic periods in carfentanil-exposed ferrets. Our results suggest that naloxone, when administered via intramuscular injection following incapacitation, is a viable treatment against the effects of a potentially lethal dose of inhaled carfentanil.
1. Introduction
Carfentanil is a powerful synthetic opioid that is approximately 100 times more potent than fentanyl and 10,000 times more potent than morphine [1,2]. Carfentanil, like most natural and synthetic opioids, elicits its main analgesic effect through binding to μ-opioid receptors in the central nervous system [2]. Intoxication from carfentanil, both from intravenous and inhaled administration, results in severe respiratory depression and the development of cardiac abnormalities [[3], [4], [5]], although case reports from fatal overdoses have typically shown no gross tissue damage [6]. The pharmacokinetics of carfentanil and current countermeasures make renarcotization (the return of respiratory depression and general opioid effects following treatment) a potential threat and a concern when treating potential acute exposure casualties or overdose patients [7,8]. Carfentanil was originally intended to be used as a sedative for big game animals in a veterinary setting (under the brand Wildnil®), but is becoming increasingly common in the illicit drug trade [9]. According to the National Center for Health Statistics’ provisional counts for 2016 and 2017, drug overdose deaths involving synthetic opioids (excluding methadone) in the United States increased approximately 202 % from 2016 to 2017 (9,945 to 20,145) (10). Carfentanil’s increasingly widespread use and abuse has put a strain on first responders, who need to administer larger and larger doses of countermeasures to counteract the effects and simultaneously concern themselves with potential self-exposure through inhalation of or dermal contact with carfentanil [11].
Synthetic opioids also have the potential to be exploited for use as weapons of incapacitation. On October 26, 2002, Russian military special forces introduced a “poison” gas to incapacitate Chechen rebels who had taken more than 800 people hostage in the Moscow Dubrovka Theater Center [12]. The composition of the “poison” gas was not immediately released to first responders, a factor that delayed or prevented accurate treatment and likely played a large role in the death of more than 120 of the hostages. However, the Russian health minister announced 4 days after the event that “a fentanyl derivative was used to neutralize the terrorists” [12]. Further analysis of the clothing and urine of British nationals who were in the theater at the time of the siege revealed that a mixture of carfentanil and remifentanil was most likely used [13].
Naloxone is a competitive antagonist for opioid receptors and is FDA-approved for the rapid reversal of opioid overdose [14,15]. Naloxone is non-selective but binds the μ opioid receptor with a binding affinity of 7 nM [16] and is able to reverse respiratory depression by displacing opioids at the binding site [17,18]. Naloxone is also an antagonist, with lower affinity for the κ- and δ-opioid receptors, but does not possess the ‘agonistic’ or morphine-like characteristics of other opioid antagonists [19]. While naloxone is well-tolerated and is lifesaving in cases of opioid overdose, it has a relatively short half-life compared to some synthetic opioids which increases the risk of renarcotization [[20], [21], [22]]. Globally, the increase in opioid overdoses due to synthetic opioids [23] has given rise to the fear of “naloxone resistant” opioids and potential depletion of current stocks of countermeasures [[24], [25], [26]]. While the body of literature from the veterinary use of carfentanil and naloxone [[27], [28], [29]] effectively refutes claims of actual naloxone resistance, dedicated studies of opioid countermeasure efficacy against acute lethal doses of synthetic opioids are few.
As evidenced by the Moscow theater incident [12] and the increasing concern among law enforcement and first responders [11], acute inhalation exposures to carfentanil are a potential danger; we therefore assessed the efficacy of naloxone against a potentially lethal inhaled dose in ferrets. The ferret has many advantages as a research model compared to other animal models: their relative small size and similarities to human anatomy, physiology, and metabolism [[30], [31], [32]]. Ferrets have been utilized in medical research as models of respiratory function and used to investigate respiratory pathogens like influenza and severe acute respiratory syndrome-associated corona virus, mainly because of similarities between ferret and human upper and lower respiratory tracts, pathogenesis, and symptoms [[33], [34], [35]]. We observed that ferrets were more sensitive to the effects of carfentanil than previously studied rodent models [3,36] and that exposure to carfentanil produced severe apnea and prolonged incapacitation in ferrets, which suggests that ferrets have a closer approximation of opioid intoxication in humans when compared to mice. Treating with naloxone following exposure significantly reduced the total time the animals were incapacitated and reduced the apneic periods.
2. Materials and methods
2.1. Animals
Adult male ferrets (1.0–1.5 kg) were obtained from Marshall BioResources (North Rose, NY, USA) through Data Sciences International (St. Paul, MN), where animals were surgically implanted with telemetry probes (HD-S11 PhysioTel Hybrid Digital, Data Sciences International, Inc.) in the ventral abdomen and shipped to the USAMRICD after one week of recovery. Ferrets were group housed until exposure and individually housed afterward. For the pharmacokinetic studies 30 animals were used for the naloxone study and 30 animals were used for the carfentanil study. The numbers of animals utilized were 8 naïve-exposed, 8 sham control, 8 sham-exposed, 8 naloxone 5 mg control, 8 naloxone 5 mg exposed, 8 naloxone 10 mg control, and 8 naloxone 10 mg exposed, for a total of 56 animals. In total 116 animals were used. One animal from the 10 mg naloxone-exposed cohort died from the effects of carfentanil prior to treatment. The study protocol was approved by the Institutional Animal Care and Use Committee, USAMRICD, Aberdeen Proving Ground, Maryland, and this research complied with the Animal Welfare Act and implementing Animal Welfare Regulations, as well as the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and adhered to the principles noted in The Guide for the Care and Use of Laboratory Animals (NRC, 2011).
2.2. Chemicals
Carfentanil, methyl 4-[(1-oxopropyl) phenylaminol-1-(2-phenylethyl)-4-piperdine carboxylate, >95 % pure (C24H30N2O3; MW 394.51), was obtained in crystalline form as a citrate salt from the chemical synthesis laboratory at the Combat Capabilities Development Command Chemical Biological Center, APG, MD. From this, stock solutions of 4.5 mg/mL were made in sterile water and stored at −80 °C until needed. Xylazine and ketamine were purchased from Webster Veterinary Supplies (Devens, MA). Naloxone (NX), 4,5-epoxy-3,14-dihydroxy-17-(2-propenyl) morphine-6-one HCl, was obtained from Sigma Chemical Co. (St. Louis, MO). Isotopically labeled carfentanil (carfentanil-D5), isotopically labeled naloxone (naloxone-D5), and standardized naloxone were obtained from Cerilliant (Round Rock, TX)
2.3. Pharmacokinetic profiling of carfentanil and naloxone
Male ferrets were administered either 25 μg/kg of carfentanil citrate via subcutaneous (s.c.) injection or 0.75 mg of naloxone via intramuscular (i.m.) injection. Serial blood draws were obtained at 0, 1, 2, 4, 8, 16, 30, 32, 60, 120, 180, 240, 360, or 1440 min via the cephalic or saphenous vein. Each animal contributed no more than two time points for each carfentanil or naloxone administration. A two week “wash out period” was utilized in order to obtain additional time points from the same animal. This resulted in an n of 4–8 for each time point. Blood was collected in a heparinized collection tube and spun, and plasma was removed and stored at −80 °C for analysis. No more than two blood draws were taken from an animal in a single day. If an animal needed to be reused for additional collections, there was at least a two week “wash out” period before re-administration.
Two LC–MS/MS assays were developed for the analysis of carfentanil-exposed and naloxone-administered ferret plasma samples. These developed assays were validated using blank, heparinized ferret plasma (BioIVT, Chestertown, MD, USA) to prepare calibration curves and quality control samples. For carfentanil, plasma was spiked at 400 ng/mL with standardized carfentanil (100 μg/mL carfentanil) and serially diluted with plasma to produce the following concentrations, which served as calibrators: 100, 25, 6.25, 1.56, 0.391, 0.098 ng/mL. Isotopically labeled carfentanil (100 μg/mL Carfentanil-D5) was spiked into each sample to produce a final concentration of 10 ng/mL in each calibrator and control. For naloxone, plasma was spiked at 400 ng/mL with standardized naloxone (1.0 mg/mL Naloxone) and serially diluted with plasma to produce the following concentrations, which served as calibrators: 100, 25, 6.25, 1.56, 0.391 ng/mL. Isotopically labeled naloxone (100 μg/mL naloxone –D5 was spiked into each sample to produce a final concentration of 5 ng/mL in each calibrator and control. These assays were validated according to the FDA guidelines regarding bioanalytical method development. Calibration curves were generated in duplicate and analyzed in triplicate, and a total of 6 sets of calibration curves were prepared over non-consecutive days (five inter-day and 1 intra-day). Quality control (QC) samples were prepared at 100, 10 and 1.0 ng/mL. QC samples were used to determine intra- and inter-day variability. Quantification of the QC samples was accomplished by running a calibration curve on each day. A linear least squares analysis with a 1/y weighting scheme was used to calculate the calibration parameters. The precision (%CV) was calculated using the formula %CV = (SD/mean) x 100 %, and the accuracy (%error) was calculated using the formula % error = ((calculated concentration – actual concentration)/actual concentration) x 100 %. Precision and accuracy were below 15 % for all validation samples and QCs.
Prior to processing, animal samples were stored at −80 °C. Samples were thawed and 200 μL was transferred to clean microcentrifuge tubes. Isotopically labeled carfentanil (100 μg/mL carfentanil-D5,) or naloxone (100 μg/mL naloxone –D5, Cerilliant) was spiked into each sample to produce a final concentration of 10 or 5 ng/mL in each sample, respectively. A calibration curve was prepared each day that samples were processed. All calibrators, QCs and samples were extracted by solid-phase extraction (SPE) using Oasis 1cc HLB cartridges with 30 mg sorbent (Waters Corporation, Milford, MA). The SPE procedure was as follows: 1) wash with 2 mL methanol, 2) wash with 2 mL water with 20 mM ammonium formate, 3) load 100 μL sample, 4) wash with 2 mL water with 20 mM ammonium formate, and 5) elute with 2 mL methanol containing 0.2 % formic acid. The eluent for all calibrators, QCs and samples was evaporated under a dry nitrogen stream at 40 °C. Samples were reconstituted in 90 μL of 10 % methanol in 0.1 % formic acid in water. Extraction was performed in duplicate, and the replicates were analyzed via LC–MS/MS in triplicate.
Liquid chromatography was performed using an Agilent 1290 Infinity liquid chromatograph (Agilent Technologies, Santa Clara, CA). Separation was performed on a Halo C18 column (2.7 μm, 2.1 mm x 50 mm) (Advanced Materials Technology, Wilmington, DE) with a chromatographic ramp with mobile phase B = 0.2 % formic acid in methanol and mobile phase A = 0.2 % formic acid, consisting of the following schedule: 0 min – 3 min (10 % mobile phase A -95 % mobile phase A), 3 min–4 min (95 % mobile phase A), 4.0 min–4.1 min (95 % mobile phase A - 10 % mobile phase A), and 4.1 −7 min (10 % mobile phase A). The flow rate was 500 μL/min and an injection volume of 5 μL was used. A retention time of 1.1 min was observed.
Tandem mass spectrometry was accomplished using a Sciex 6500 QTrap triple quadrupole mass spectrometer (Sciex, Ottawa, CA). It was operated in electrospray mode using multiple reaction monitoring (MRM). The ion source temperature was 700 °C. Capillary voltage was +5500 V, curtain gas was 30 and the collision assisted dissociation gas was medium. Ion source gas 1 and 2 were 50 and 70. Declustering potential was 50 V and entrance potential was 10 V. For carfentanil, the quantifier ion transition was 395.2 Da to 246.1 Da with collision energy of 27 eV and collision exit potential of 11 V, while the qualifier ion transition was 395.2 Da to 335.1 Da with collision energy of 15 eV and collision exit potential of 8 V. For carfentanil-D5, the quantifier ion transition was 400.2 Da to 284.0 Da with collision energy of 26 eV and collision exit potential of 12 V, while the qualifier ion transition was 400.2 Da to 340.2 Da with collision energy of 15 eV and collision exit potential of 9 V. For naloxone, the quantifier ion transition was 328.2 Da to 212.1 Da with collision energy of 61 eV and collision exit potential of 11 V, while the qualifier ion transition was 328.2 Da to 253.0 Da with collision energy of 35 eV and collision exit potential of 8 V. For naloxone-D5, the quantifier ion transition was 333.2 Da to 212.1 Da with collision energy of 62 eV and collision exit potential of 12 V, while the qualifier ion transition was 333.2 Da to 258.0 Da with collision energy of 36 eV and collision exit potential of 9 V. Peak areas were integrated using Analyst software (Sciex, Ottawa, Ontario).
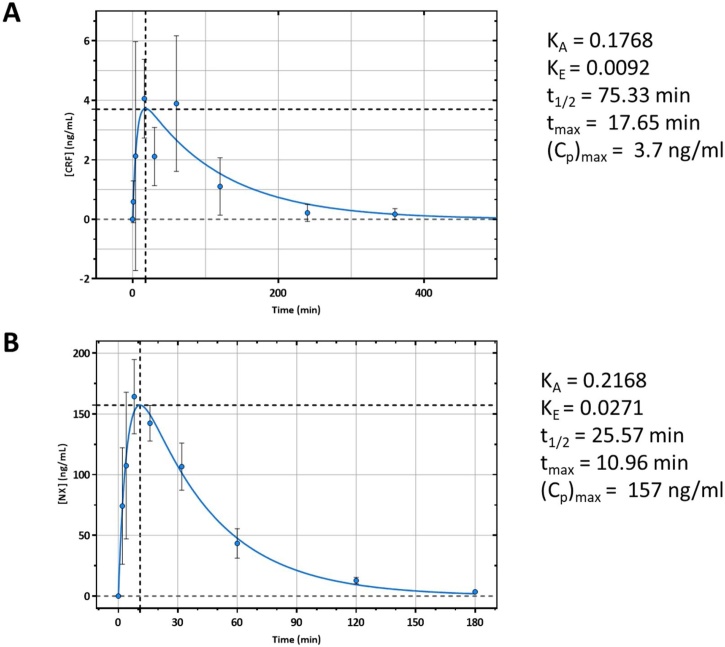
The plasma concentrations of carfentanil and naloxone were then plotted versus time and fitted by a one-compartment absorption and elimination model (GraphPad Prism v.7.05), from which the (Cp)max absorption (KA) and elimination (KE) constants were determined. The t1/2 and tmax were then calculated using KA, KE, and the equations t1/2 = 0.693/KE and .
2.4. Inhalation exposures
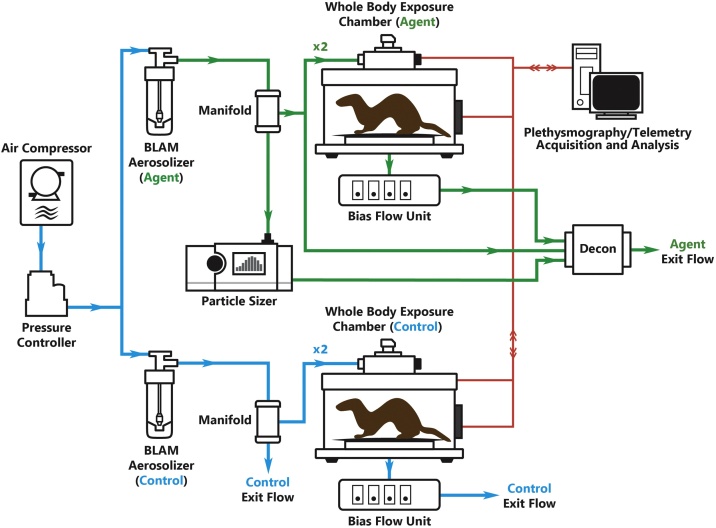
Exposures to carfentanil and controls were conducted within a custom inhalation system as shown in Fig. 1. Carfentanil- and control-exposed animals were contained within whole-body plethysmograph (WBP) chambers (Data Sciences International, St. Paul, MN). Aerosols were generated by placing the entire contents of the exposure aliquot into a Blaustein atomizing module (BLAM) (CH Technologies, Westwood, NJ) and operating the unit according to the manufacturer’s instructions. A carfentanil concentration-time product (Ct) of 0.72 mg/m3 for 20 min (equating to 14.4 mg × min/m3 or an estimated exposure of 70 μg in humans based on allometric scaling to the ferret) was used throughout the study and chosen for its profound and deep sedative effects. Mass median aerodynamic diameter (MMAD) and particle concentration were determined to be 3−4 μm and 130 mg/m3, respectively, using an Aerodynamic Particle System (TSI Model 3321, Shoreview, MN). Nebulizers were pressurized using a medical air compressor (Jun-Air, Benton Harbor, MI) to 15−20 psi to generate carfentanil aerosol, which was then fed into a custom-designed air manifold to which two WBP chambers were connected. Exposure chambers were modified WBP units (FinePointe Series Whole Body Plethysmography Rabbit Chamber; Data Sciences International; St. Paul, MN, USA) capable of collecting respiratory parameters before, during, and after exposure. Bias flow generators (Bias Flow Fresh Air Pump; Data Sciences International; St. Paul, MN, USA) connected to the WBPs generated a slight vacuum to pull aerosolized carfentanil from the manifold into the chamber, ensuring agent flow to each exposure unit at physiologically compatible and sufficient rates. Exit flows from the manifolds and bias flow units passed through a custom-built activated charcoal decontamination unit.
Fig. 1.
Schematic of Ferret Exposure System.
Immediately before exposure, all animals were allowed to acclimate to the exposure chamber for 10 min, and baseline plethysmography recordings were then collected for 30 min. A 20 min aerosolized carfentanil exposure followed baseline respiratory data collection. Concurrently, control animals were exposed to aerosolized, sterile H2O for the same duration using a separate but identical exposure setup. All exposures were conducted within a custom-designed certified glovebox (Baker Co., Sanford, Maine).
2.5. Clinical observations
Clinical observations were collected for all carfentanil-exposed (14.4 mg × min/m3) and control-exposed animals during exposure. Events recorded during the 20 min exposure period included the onset of loss of responsiveness (defined as the inability to respond to external stimuli) and incapacitation (defined as animals having their heads down, lacking responsiveness as described previously, and demonstrating no controlled movements). Additional clinical observations included general signs (dyspnea or enophthalmos) and movement (ataxia, restlessness, tonus, or tremors).
2.6. Respiratory dynamics
Respiratory dynamics measured included minute volume (MV, mL/min), respiratory frequency (f, breaths/minute), and duty cycle (DC, unitless), which was calculated as inspiratory time divided by the sum of inspiratory and expiratory time. Data collection occurred at 15-second intervals, and the acquisition software (FinePointe, DSI) utilized a rejection index to exclude statistical inaccuracies and external noise. Due in part to both the difficulty of observing breaths (depending on the positioning of the animals) and the scoring of the acquisition software, bradypnea was quantified as a reduction in f of greater than 50 % from baseline, and apnea was quantified as a reduction in f of greater than 85 % from baseline. Ferrets remained in the WBP for continuous recording of respiratory dynamics for up to 4 h.
2.7. Telemetry
The implanted transmitter broadcasted digitized data in the radio frequency range to receivers located beneath the WBPs. The biopotential signals collected from the telemetry units for all experimental animals were verified post-implantation by both DSI and USAMRICD staff. Parameters such as heart rate (HR), core body temperature (Tc) and mean arterial blood pressure (MAP) were collected continuously during baseline (30 min) and exposure (20 min), and through to the 24 h endpoint.
2.8. Treatment protocol
Animals in this section were divided into several groups based on their exposure to (1) aerosolized carfentanil, (2) aerosolized carfentanil followed by injection with water (sham group), (3) aerosolized carfentanil followed by treatment with 0.2 mg (5 mg HED) naloxone, (4) aerosolized carfentanil followed by treatment with 0.75 mg (10 mg HED) naloxone, and corresponding controls for groups 2–4 which were exposed to aerosolized water (Table 1). At 26 min post-incapacitation, ferrets in the treatment groups were given a single i.m. administration of naloxone (or water) and immediately returned to the WBP chamber for data collection. The HED was estimated using the equation HED = animal dose [mg/kg] x (animal weight [kg]/human weight [kg])0.33 [37,38]. Clinical observations were collected up to 24 h following exposure from animals that received post-exposure administration of NX.
Table 1.
Inhalation Exposure Cohorts.
| Treatment | Exposure |
|
|---|---|---|
| Water | Carfentanil | |
| None | – | naïve-exposed (n = 8) |
| Water | sham control (n = 8) | sham-exposed (n = 8) |
| 5 mg HED NX | naloxone 5 mg control (n = 8) | naloxone 5 mg exposed (n = 8) |
| 10 mg HED NX | naloxone 10 mg control (n = 8) | naloxone 10 mg exposed (n = 8) |
2.9. Data analysis
Data are presented as the mean ± the standard deviation, unless otherwise noted. Where appropriate, the multiple exposure groups were compared utilizing either a one-way or two-way ANOVA (with appropriate multiple comparison test) with significance set to p < 0.05. Specialized software and customized routines were used to collect respiratory dynamics (FinePointe Software v2.3.1.16, DSI) and cardiac (Ponemah Software v5.2, DSI) data, and all raw data were exported and analyzed using custom-designed programs (Microsoft Visual Basic for Applications v7.0.1639; Microsoft Corporation; Redmond, WA, USA), spreadsheet software (Microsoft Excel v14.1.7166.5000 [32-bit]; Microsoft Corporation; Redmond, WA, USA), and statistical and graphing software (GraphPad Prism v5.04, v7.04; GraphPad Software, Inc.; La Jolla, CA, USA).
3. Results
3.1. Pharmacokinetic profiling of carfentanil and naloxone
We observed that s.c. injected carfentanil entered the blood stream slower than i.m. injected naloxone, with tmax achieved at approximately 18 min for carfentanil and at approximately 11 min for naloxone (Fig. 2A and B). From a 25 μg/kg injection of carfentanil, peak plasma concentration was 3.7 ng/mL, and from a 0.75 mg injection of naloxone, peak plasma concentration was 157 ng/mL. Carfentanil had a relatively long elimination half-life, with t1/2 equaling 75.33 min. When compared to the pharmacokinetics of the i.m. injected naloxone, naloxone was eliminated quicker than carfentanil with a biological half-life equaling 25.57 min.
Fig. 2.
Pharmacokinetic Profile of Injected Carfentanil and Naloxone. Male ferrets were injected with either 25 μg/kg of carfentanil subcutaneously (A) or 0.75 mg of naloxone intramuscularly (B). Serial blood draws were then obtained from the cephalic or saphenous vein post-injection. Plasma concentrations of carfentanil and naloxone were determined via LCMS methodology. Analyte concentrations were then plotted vs. time and fit with a one-compartment absorption and elimination nonlinear curve. From the fit the (Cp)max, absorption constant (KA) and elimination constant (KE) were determined. The t1/2 and tmax were then calculated using the KA and KE. As expected both carfentanil (A) and naloxone (B) were rapidly absorbed with carfentanil having an approximately 3 times longer t1/2 than naloxone. n = 4-8.
3.2. Clinical observations
In an inhaled carfentanil exposure model, ferrets exhibit signs of severe respiratory depression, cardiac abnormalities likely due to hypoxia, and lethality. An exposure to aerosolized carfentanil at 0.72 mg/m3 for 20 min (14.4 mg × min/m3) caused all animals to become unresponsive and produced 100 % incapacitation with 3% mortality. The average time to incapacitation was 4.1 ± 1.3 min. In exposed animals, tremors were observed in 97 %, gasping or paradoxical breathing was observed in 63 %, enophthalmos was observed in 57 %, and convulsions were observed in 29 %. If left untreated, naïve-exposed animals were incapacitated for an average of 126.1 ± 24.6 min.
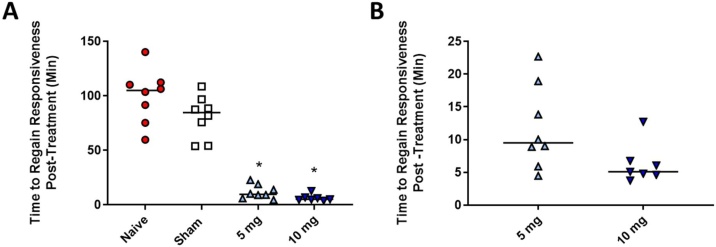
To assess the efficacy of naloxone to reverse the effects of carfentanil, we treated exposed ferrets with either a single 5 mg HED of naloxone or a single 10 mg HED administered i.m. at approximately 26 min post-incapacitation. We recorded the time until the animals regained responsiveness, qualified as an animal with its head up that was responsive to external stimuli and that demonstrated controlled movements. We observed that both of the treatment groups showed a significant reduction in the time the animals were incapacitated (Fig. 3A) and that sham treatment showed no statistical difference from naïve-exposed animals. Naïve animals remained incapacitated for an average of 99.7 ± 24.6 min following average treatment time (26.3 min post-incapacitation), and sham animals remained incapacitated for 89.7 ± 11.6 min following injection. In the treated groups, incapacitation was 11.7 ± 2.9 min following treatment for the 5 mg HED group and 7.0 ± 2.9 min following treatment for the 10 mg HED group (Fig. 3B). When the two treatment groups were compared to one another, there was no statistical difference between the two groups.
Fig. 3.
Efficacy of Naloxone in Reversing Incapacitation Resulting from Inhaling Aerosolized Carfentanil. Male ferrets were exposed to aerosolized carfentanil for 20 min and then either left untreated (Naïve) or treated with a single i.m. injection of water (sham) or naloxone at 26 min post-incapacitation. The time that it took the animal to regain responsiveness (head up, responsive to external stimuli, and having controlled movements) was recorded for each animal. We observed that both treatment doses of naloxone (5 and 10 mg HED) were able to significantly reduce the amount of time an exposed animal was incapacitated (A), but there was no significant difference between Naïve and Sham. When compared to one another, there was no significant difference between the 5 mg and 10 mg dose. n = 7-8, solid bar = mean, A) * p < 0.05 one-way ANOVA vs. Naïve with Dunnett’s multiple comparison test, B) Welch’s t-test.
3.3. Respiratory function
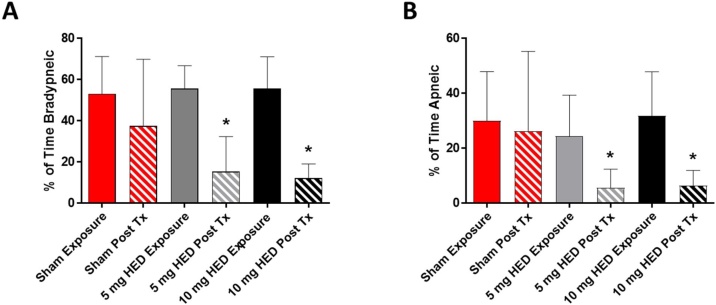
We observed a marked reduction in respiratory frequency (f) during exposure, with all exposed and untreated animals becoming either bradypneic or apneic for a portion of the exposure. There was no statistical difference in baseline f between any of the experimental groups with the average equaling 48.2 ± 13.3 bpm. We observed that exposure to carfentanil had a profound effect on respiratory function, with bradypneic events occurring over 50 % of the time (Sham – 53 % ± 18 %, 5 mg HED naloxone – 56 % ± 11 %, 10 mg naloxone - 56 % ± 15 %) during exposure and apneic events occurring from 24 to 31% of the time (Sham – 30 % ± 18 %, 5 mg HED naloxone – 24 % ± 15 %, 10 mg naloxone - 32 % ± 16 %) (Fig. 4A and B). Treatment with naloxone significantly reduced the bradypneic and apneic events in the 20 min period post-treatment (Fig. 4A and B) at both the 5 mg and 10 mg HEDs, while sham injection had no significant effect on bradypnea or apnea post-treatment.
Fig. 4.
Bradypnea and Apnea in Ferrets Exposed to Carfentanil. Male ferrets were exposed to aerosolized carfentanil for 20 min and then treated; respiratory dynamic measurements were recorded for the exposure time period (solid bars) and 20 min following treatment (striped bars). We observed a significant increase in both bradypneic (A) and apneic (B) incidents in the animals exposed to carfentanil. Sham treatment (red bars) had no effect on either bradypnea or apnea post-treatment (Post Tx), while both 5 mg HED (gray bars) and 10 mg HED (black bars) naloxone significantly reduced the percentages of bradypneic and apneic periods following treatment. n = 7-8 *p < 0.05, one-way ANOVA vs. Sham Exposure with Dunnett’s multiple comparison test, error bars = standard deviation.
A marked and sustained decrease in duty cycle (DC), the ratio of inspiratory time to total breath time, was observed in animals that were exposed to carfentanil (Fig. 5). The decrease in DC began at the onset of exposure and lasted for an additional 25 min following the end of exposure. Ferrets treated with i.m. injections of either 5 mg HED or 10 mg HED of naloxone at approximately 30 min from the start of exposure saw a recovery of DC to control levels with in 15 min. A high dosage of naloxone resulted in a quicker recovery of DC, with recovery observed at 10 min post-treatment in animals administered the 10 mg HED. Ferrets treated with the 5 mg HED of naloxone had a slower recovery in DC, which occurred at 15 min post-treatment.
Fig. 5.
Duty Cycle with Treatment. Male ferrets were exposed to aerosolized carfentanil for 20 min (red shaded region, A. and B.), during which respiratory dynamic measurements were recorded. We observed a marked and sustained decrease in duty cycle (ratio of inspiratory time to total breath time) that began at the onset of exposure and lasted for an additional 25 min following the end of exposure (open circles, A and B). Ferrets were treated with i.m. injections of either 5 mg HED (A) or 10 mg HED of naloxone at approximately 30 min from the start of exposure (approx. 25 min after incapacitation, green shaded region A and B). In both A and B, the duty cycle of treated controls (closed squares) was significantly higher than for sham-exposed from 5 through 45 min (p < 0.05). We observed a reversal in the duty cycle depression in animals treated with both 5 mg and 10 mg HED of naloxone, with 10 mg HED returning duty cycle to normal levels sooner (B). n = 7-8, *p < 0.05 two-way ANOVA with Dunnett’s multiple comparison test (* for treated exposed vs. sham exposed only, treated control vs. exposed control not shown).
3.4. Cardiac function
The prolonged apneic periods coincided with increased instances of cardiac abnormalities and dysrhythmias in the form of premature junction contractions (PJCs) that developed (Fig. 6) as exposure progressed. PJCs were observed on the electrocardiograph during exposure to carfentanil and were confirmed by independent veterinary and clinical subject matter experts. Shortly after the end of exposure, PJCs resolved without the aid of a countermeasure, and this recovery corresponded with the gradual increases in f. Although cardiac abnormalities were observed during aerosolized exposure to carfentanil, neither gross histopathological cardiac damage nor elevation of circulating troponin levels (not shown) was observed 24 h post-exposure.
Fig. 6.
Electrocardiograph of Premature Junction Contractions in Carfentanil-Exposed Ferrets. Telemetered male ferrets were exposed to aerosolized carfentanil for 20 min, during which time cardiac parameters were recorded. We observed cardiac abnormalities in the pressure traces (pink) and electrocardiograph (green) in animals exposed to carfentanil. Premature junction contractions (PJCs) (circled in red) were seen throughout exposure timeframe. The PJCs were present in all exposed animals, and their appearance during times of respiratory depression indicates that they are most likely linked hypoxic conditions.
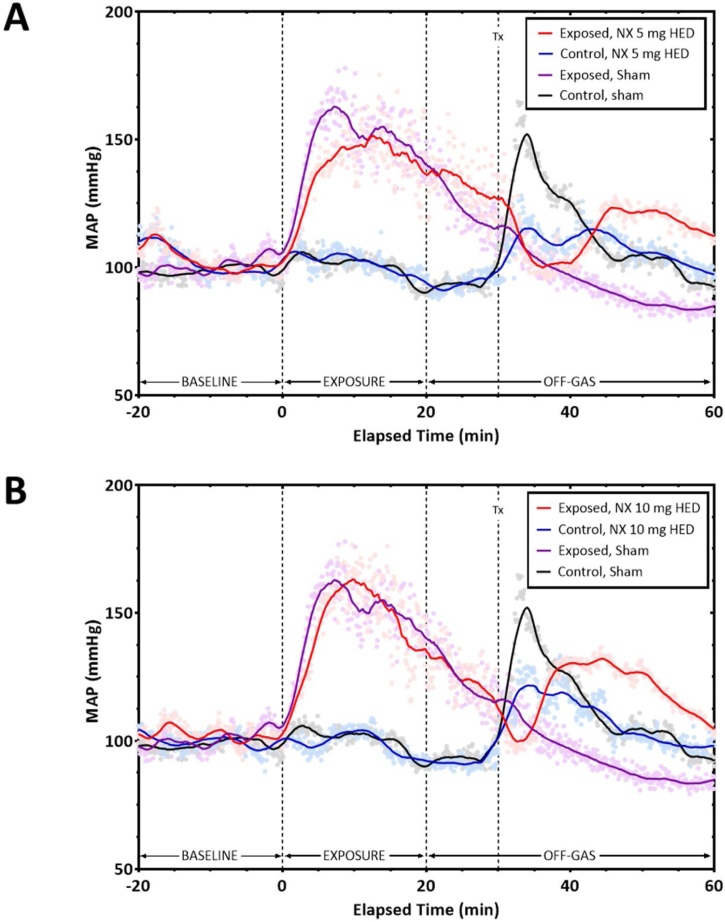
We also observed a marked increase in mean arterial pressure (MAP) (Fig. 7A and B) during the exposure period, which gradually decreased after exposure, in animals exposed to carfentanil. Animals, both control and exposed, that received treatment with i.m. naloxone displayed an increase in MAP following the injection. Sham control animals also exhibited a rise in MAP following handling and exposure, while the sham-exposed animals had no rise in MAP. Increasing the dose of naloxone appeared to lead to a quicker and more pronounced rise in pressure, although there was no statistical difference between times at which each group regained responsiveness (Fig. 3). The data may indicate that the rise in arterial pressure is due to a combination of the effects of naloxone and the physical effects of the i.m. injection itself.
Fig. 7.
Mean Arterial Pressure during the Carfentanil Exposure Timeframe and after Treatment with Naloxone. Telemetered male ferrets were exposed to aerosolized carfentanil for 20 min, with mean arterial pressure (MAP) recorded before, during, and post-exposure. We observed a pronounced increase in MAP in animals exposed to carfentanil (red and purple line, A. and B.) within 5 min after the start of the exposure period. After the initial peak, the MAP slowly decreased towards baseline levels. Animals, both control and exposed, that received treatment with i.m. naloxone (blue and red line, A and B) displayed an increase in mean arterial pressure following the injection. Sham control animals also exhibited a rise in arterial pressure following handling and exposure (black line A and B), while the animals exposed to carfentinal and treated with water had no rise in arterial pressure (sham exposed, purple). The 10 mg dose of naloxone appears to cause a greater increase in mean arterial pressure (B) after administration compared to the 5 mg dose (A). n = 7-8.
4. Discussion
Carfentanil is a powerful synthetic opioid [1,2] that is a current concern, both inside [9,10] and outside the realm of public health [12,13]. Although naloxone is FDA approved to counteract the effects of opioids [15], its use as a treatment for an acute exposure to synthetic fentanyl derivatives may have some limitations [11,24,25]. We developed a whole-body exposure model for aerosolized carfentanil and utilized a ferret model to test the effectiveness of intramuscularly administered naloxone post-exposure. A whole body exposure system offers some benefits over a traditional “head or nose-only” exposure systems allowing the animals to be unrestrained before, during, and post exposure. While there is a potential for exposure through dermal or ocular routes in our system, we believe that the effects of exposure are largely driven by the inhalation of carfentanil as recent studies have noted that skin exposures of carfentanil may not result in significant rapid toxicity as first indicated [39]. Our model produced profound incapacitation, severe apnea, cardiac abnormalities, tremors, convulsions, and lethality in male ferrets. The signs of exposure to carfentanil in our model were consistent with human signs and symptoms of opioid intoxication.
We were able to determine the pharmacokinetic profiles of both subcutaneously injected carfentanil and intramuscularly injected naloxone. We observed that both compounds were rapidly absorbed, with a tmax for carfentanil of 17.65 min and a tmax for naloxone of 10.96 min. Carfentanil was bioavailable much longer than naloxone, with a circulating half-life approximately 3 times longer (t1/2 = 75.33 min vs. 25.57 min). Our data are in agreement with that obtained from other animal models and human case reports, indicating that for some exposures to carfentanil, repeated administration of naloxone may be necessary given its shorter half-life or a large bolus of naloxone may be needed at the time of treatment [22,[40], [41], [42]].
To assess the efficacy of naloxone as a therapeutic for a potentially lethal exposure to aerosolized carfentanil, we utilized our whole-body exposure system and a treatment paradigm of intramuscularly administered naloxone, at 5 mg and 10 mg HEDs, given at the first instance where we could safely access the animal (approximately 26 min post-incapacitation). We observed that both treatment doses of naloxone were able to significantly reduce the amount of time ferrets were incapacitated from carfentanil. We did not observe any significant difference between the 5 mg and 10 mg HEDs in the recovery of incapacitated animals. Our model was also unable to distinguish any potential renarcotization following treatment. While renarcotization is a concern given the short half-life of opioid treatments and the longer half-life of synthetic opioids [[20], [21], [22]], the ferret model presents challenges in properly assessing renarcotization. Due to the ferret’s propensity to become disinterested with external stimuli and sleep [43], it is difficult to distinguish between a normally behaving ferret that is sleepy and one that is becoming “drowsy” from the effects of opioids. Higher order animal models will be needed to properly assess this portion of treatment against synthetic opioids, such as carfentanil.
During and following exposure to aerosolized carfentanil, we observed prolong periods of both bradypnea and apnea in our animal model, as well as reductions in duty cycle which is consistent with the respiratory depression seen in both humans and other animal models [3,22,44]. Naloxone treatment was able to significantly reduce the number of both bradypneic and apneic events following administration. The 10 mg HED of naloxone was also quicker than the 5 mg HED in returning duty cycle to normal levels. Since respiratory depression is the primary mechanism of mortality in humans following opioid overdose, our data are encouraging given that intramuscularly administered naloxone following a potentially lethal exposure to aerosolized carfentanil is able to rapidly return respiration to normal.
Cardiac abnormalities were present in our animals during exposure to carfentanil and manifested as cardiac dysrhythmias in the form of PJCs. The PJCs coincided with prolonged periods of both bradypnea and apnea during the exposure period. PJCs, which may warn of progression to more serious dysrhythmias, occur when the atrioventricular tissue becomes irritated or, in cases of hypoxia, as a result of excessive vagal tone/parasympathetic stimulation, chronic lung disease, some cardiac diseases, and from some drugs (e.g., catecholamine, epinephrine, norepinephrine) [45]. The dysrhythmia is characterized by a normal QRS complex with inverted or abnormal P wave, absent P wave, a P wave that appears after the QRS complex, and/or a short PR interval [46]. Although the PJCs may indicate more serious cardiac issues, we observed no gross cardiac damage or presence of elevated plasma troponin. While opioid use may be associated with increased risk of heart dysrhythmias [47], in our model the PJCs were transient, and following the exposure period their appearance on the electrocardiogram decreased in all exposed groups including naïve-exposed animals.
We observed a pronounced increase in mean arterial pressure (MAP) in ferrets during exposure to carfentanil. In other animal species, carfentanil has caused both increases [5] and decreases [3] in MAP, though in a human carfentanil exposure case report, the patient was reportedly found unconscious and hypotensive [48]. Following treatment with naloxone, we observed a second, smaller, increase in MAP. While a portion of this increase is likely due to stress of physical manipulation and discomfort from injection, which was observed in our sham control animals, naloxone is known to increase arterial pressure [49,50]. In our study, the increase in arterial pressure following treatment with naloxone is most likely due to a combination of both physical stress and the effects of naloxone on blood pressure.
In our study we have developed a model of potentially lethal acute exposure to aerosolized carfentanil and utilized male ferrets to assess the efficacy of naloxone to reverse the deleterious effects. Unfortunately, our study was not able to fully answer questions regarding potential renarcotization following treatment or potential increases in mean arterial pressure due to naloxone administration. Future studies in higher order species will be needed to properly assess renarcotization and the limitations or adverse side effects of higher doses of naloxone. The data we presented continue to support the use of naloxone as a first-line treatment for the accidental exposure to highly potent synthetic opioids. We can unequivocally say that, in our model, naloxone intramuscularly administered post-exposure is a viable treatment for reversing the incapacitating and deleterious effects of exposure to aerosolized carfentanil.
CRediT authorship contribution statement
Bryan J. McCranor: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft, Visualization, Supervision, Writing - review & editing, Funding acquisition. Laura Jennings: Validation, Investigation, Resources, Data curation, Formal analysis, Writing - review & editing. Justin Tressler: Validation, Investigation, Resources, Data curation, Writing - review & editing. Wing Y. Tuet: Validation, Investigation, Formal analysis, Data curation, Writing - review & editing. Vanessa E. DeLey Cox: Validation, Investigation, Data curation, Writing - review & editing. Michelle Racine: Validation, Investigation, Data curation. Samuel Stone: Validation, Investigation, Data curation. Samuel Pierce: Validation, Investigation, Data curation. Erin Pueblo: Validation, Investigation, Data curation. Aliyah Dukes: Validation, Investigation, Data curation. Samantha R. Litvin: Validation, Investigation, Data curation. Melissa R. Leyden: Validation, Investigation, Data curation. Justin N. Vignola: Validation, Investigation, Data curation. M. Ross Pennington: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Benjamin Wong: Conceptualization, Methodology, Formal analysis, Investigation, Writing - review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The views expressed herein are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government. This work was supported by the Defense Threat Reduction Agency (DTRA CB, DB3950). The experimental protocol was approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), and the Animal Welfare Act of 1966 (P.L. 89-544), as amended. W.Y.T., V.D.C., M.R., S.A.P., E.P., A.D., S.R.L., and M.R.L were supported in part by an appointment to the Research Participation Program for the U.S. Army Medical Research and Development Command administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and U.S. Army Medical Research and Development Command.
References
- 1.George A.V., Lu J.J., Pisano M.V., Metz J., Erickson T.B. Carfentanil—an ultra potent opioid. Am. J. Emerg. Med. 2010;28(4):530–532. doi: 10.1016/j.ajem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Pasternak G.W., Pan Y.-X. Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev. 2013;64(4):1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong B., Perkins M.W., Tressler J., Rodriguez A., Devorak J., Scuito A.M. Effects of inhaled aerosolized carfentanil on real-time physiological responses in mice: a preliminary evaluation of naloxone. Inhal. Toxicol. 2017;29(2):65–74. doi: 10.1080/08958378.2017.1282065. [DOI] [PubMed] [Google Scholar]
- 4.Villemagne P.S., Dannals R.F., Ravert H.T., Frost J.J. PET imaging of human cardiac opioid receptors. Eur. J. Nucl. Med. Mol. Imaging. 2002;29(10):1385–1388. doi: 10.1007/s00259-002-0897-z. [DOI] [PubMed] [Google Scholar]
- 5.Heard D.J., Killias G.V., Buss D., Caligiuri R., Coniglario J. Comparative cardiovascular effects of intravenous etorphine and carfentanil in domestic goats. J. Zoo Wildl. Med. 1990;21(2):166–170. [Google Scholar]
- 6.Swanson D.M., Hair L.S., Rivers S.R.S., Smyth B.C., Brogan S.C., Ventoso A.D., Vaccaro S.L., Pearson J.M. Fatalities involving carfentanil and furanyl fentanyl: two case reports. J. Anal. Toxicol. 2017;41(6):498–502. doi: 10.1093/jat/bkx037. [DOI] [PubMed] [Google Scholar]
- 7.WHO . 2017. WHO Model List of Essential Medications: 20th List.http://www.who.int/medicines/publications/essentialmedicines/en/ Available from: [Google Scholar]
- 8.Dahan A., Aarts L., Smith T.W. Incidence, reversal, and prevention or opioid-induced respiratory depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 9.W.H.O . 2017. Carfentanil: Critical Review Report Agenda Item 4.8.http://www.who.int/medicines/access/controlled-substances/Critical_Review_Carfentanil.pdf Available from: [Google Scholar]
- 10.N.C.H.S . 2017. Provisional Counts of Drug Overdose Deaths, as of 8/6/2017.https://www.cdc.gov/nchs/data/health_policy/monthly-drug-overdose-death-estimates.pdf Available from: [Google Scholar]
- 11.D.E.A . 2016. DEA Issues Carfentanil Warning to Police and Public.https://www.dea.gov/divisions/hq/2016/hq092216.shtml [updated September 22, 2016]. Available from: [Google Scholar]
- 12.Wax P.M., Becker C.E., Curry S.C. Unexpected “gas” casualties in Moscow: a medical toxicology perspective. Ann. Emerg. Med. 2003;41(5):700–705. doi: 10.1067/mem.2003.148. [DOI] [PubMed] [Google Scholar]
- 13.Riches J.R., Read R.W., Black R.M., Cooper N.J., Timperley C.M. Analysis of clothing and urine from moscow theatre siege casualties reveals carfentanil and remifentanil use. J. Anal. Toxicol. 2012;36(9):647–656. doi: 10.1093/jat/bks078. [DOI] [PubMed] [Google Scholar]
- 14.Sawynok J., Pinsky C., LaBella F.S. On the specificity of naloxone as an opiate antagonist. Life Sci. 1979;25(19):1621–1631. doi: 10.1016/0024-3205(79)90403-x. [DOI] [PubMed] [Google Scholar]
- 15.F.D.A . 2018. Information About Naloxone.https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm472923.htm [updated 0423/2018]. Available from: [Google Scholar]
- 16.Wang D., Sun X., Sadee W. Different effects of opioid antagonists on mu, delta, and kappa opioid receptors with and without agonist pretreatment. J. Pharmacol. Exp. Ther. 2007;321(2):544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- 17.Shaw L.V., Moe J., Purssell R., Buxton J.A., Godwin J., Doyle-Waters M.M., Brasher P.M.A., Hau J.P., Curran J., Hohl C.M. Naloxone interventions in opioid overdose: a systematic review protocol. Syst. Rev. 2019;8:138. doi: 10.1186/s13643-019-1048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S. Historical review: opiate addiction and opioid receptors. Cell Transplant. 2019;28(3):233–238. doi: 10.1177/0963689718811060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzatzarakis M.N., Vakonaki E., Kovatsi L., Belivanis S., Mantsi M., Alegakis A., Liesivuori J., Tsataskis A.M. Determination of buprenorphine, norbuprenorphine and naloxone in fingernail clippings and urine of patients under opioid substitution therapy. J. Anal. Toxicol. 2015;39(4):313–320. doi: 10.1093/jat/bkv003. [DOI] [PubMed] [Google Scholar]
- 20.Ryan S.A., Dunne R.B. Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systematic reveiw. Pain Manag. 2018;8(3):231–245. doi: 10.2217/pmt-2017-0060. [DOI] [PubMed] [Google Scholar]
- 21.Armenian P., Vo K.T., Barr-Walker J., Lynch K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology. 2018;134(Part A):121–132. doi: 10.1016/j.neuropharm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Uddayasankar U., Lee C., Oleschuk C., Eschun G., Ariano R.E. The pharmacokinetics and pharmacodynamics of carfentanil after recreational exposure: a case report. Pharmacotherapy. 2018;38(6) doi: 10.1002/phar.2117. e41-e5. [DOI] [PubMed] [Google Scholar]
- 23.Melton S.H., Melton S.T. Current state of the problem: opioid overdose rates and deaths. Curr. Treat. Opt. Psychiatry. 2019;6(2):164–177. [Google Scholar]
- 24.Sutter M.E., Gerona R.R., Davis M.T., Roche B.M., Colby D.K., Chenoweth J.A., Adams A.J., Owen K.P., Ford J.B., Black H.B., Albertson T.E. Fatal fentanyl: one pill can kill. Acad. Emerg. Med. 2017;24(106–113) doi: 10.1111/acem.13034. [DOI] [PubMed] [Google Scholar]
- 25.Firger J. Newsweek; 2017. Fentanyl Found in Georgia Resists Life-Saving Naloxone Antidote. June 28, 2017;Sect. Tech and Science. [Google Scholar]
- 26.Moss R.B., Carlo D.J. Higher doses of naloxone are needed in the synthetic opioid era. Subst. Abuse Treat. Prev. Policy. 2019;14(6) doi: 10.1186/s13011-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moresco A., Larsen R.S., Sleeman J.M., Wild M.A., Gaynor J.S. Use of naloxone to reverse carfentanil citrate-induced hypoxemia and cardiopulmonary depression in rocky mountain wapiti (cervus elaphus nelsoni) J. Zoo Wildl. Med. 2001;32(1):81–89. doi: 10.1638/1042-7260(2001)032[0081:UONTRC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Haigh J.C. Immobilization of wapiti with carfentanil and xylazine and opioid antagonism with diprenorphine, naloxone, and naltrexone. J. Zoo Wildl. Med. 1991;22(3):318–323. [Google Scholar]
- 29.Williams D.E., Riedesel D.H. Chemical immobilization of wild ruminants. Iowa State Univ. Vet. 1987;49(1) [Google Scholar]
- 30.Enkirch T., Messling V.V. Ferret models of viral paathogenesis. Virology. 2015;479–480:259–270. doi: 10.1016/j.virol.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clingerman K.J., Fox J.G., Walke M. US Department of Agriculture, National Agriculture Library; Bettsville, MD: 1991. Ferrets as Laboratory Animals: A Bibliography. [Google Scholar]
- 32.Gad S.C. Pigs and ferrets as models in toxicology and biological safety assessment*. Int. J. Toxicol. 2000;19:149–168. [Google Scholar]
- 33.Maher J.A., DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim. 2004;33(9):50–53. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 34.Ball R.S. Issues to consider for preparing ferrets as research subjects in the laboratory. ILAR J. 2006;47(4):348–357. doi: 10.1093/ilar.47.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson-Delaney C.A., Orosz S.E. Ferret respiratory system: clinical anatomy, physiology, and disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011;14(2):357–367. doi: 10.1016/j.cvex.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Tuet W.Y., Pierce S.A., Racine M.C., Tressler J., McCranor B.J., Sciuto A.M., Wong B. Changes in murine respiratory dynamics induced by aerosolized carfentanil inhalation: efficacy of naloxone and naltrexone. Toxicol. Lett. 2019;316:127–135. doi: 10.1016/j.toxlet.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 37.C.D.E.R . U.S. Department of Health and Human Services; Rockville, MD: 2005. Guianance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. [Google Scholar]
- 38.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lent E.M., Maistros K.J., Oyler J.M. In vitro dermal absorption of carfentanil. Toxicol. Vitro. 2020;62 doi: 10.1016/j.tiv.2019.104696. [DOI] [PubMed] [Google Scholar]
- 40.Cole A., Mutlow A., Isaza R., Carpenter J.W., Koch D.E., Hunter R.P., Dresser B.L. Pharmacokinetics and pharmacodynamics of carfentanil and naltrexone in female common eland (taurotragus oryx) J. Zoo Wildl. Med. 2006;37(3):318–326. doi: 10.1638/05-070.1. [DOI] [PubMed] [Google Scholar]
- 41.MS-S Bergh, Bogen I.L., Garibay N., Baumann M.H. Evidence for nonlinear accumulation of the ultrapotent fentanyl analog, carfentanil, after systemic administration to male rats. Neuropharmacology. 2019;158 doi: 10.1016/j.neuropharm.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang P., Li Y., Li W., Zhang H., Gao J., Sun J., Yin X., Zheng A. Preparation and evaluation of carfentanil nasal spray employing cyclodextrin inclusion technology. Drug Dev. Ind. Pharm. 2018;44(6):953–960. doi: 10.1080/03639045.2018.1425426. [DOI] [PubMed] [Google Scholar]
- 43.Marks G.A., Shaffery J.P. A preliminary study of sleep in the ferret, mustela putorius furo: a carnivore with an extremely high proportion of REM sleep. Sleep. 1996;19(2):83–93. doi: 10.1093/sleep/19.2.83. [DOI] [PubMed] [Google Scholar]
- 44.Paterson J.M., Caulkett N.A., Woodbury M.R. Physiological effects of nasal oxygen or medical air administered prior to and during carfentanil-xylazine anesthesia in north american elk (cervus canadensis manitobensis) J. Zoo Wildl. Med. 2009;40(1):39–50. doi: 10.1638/2007-0107.1. [DOI] [PubMed] [Google Scholar]
- 45.UNM . 2016. University of New Mexico Basic Arrhythmia Course-Self Study: Junctional Rhythms.https://learningcentral.health.unm.edu/learning/user/onlineaccess/CE/bac_online/junc/pjc_interpret.html [8 August 2018]. Available from: [Google Scholar]
- 46.Golchha S.K., Bachani N., Lokhandwala Y. Premature complexes and pauses. Indian Pacing Electrophysiol. J. 2017;17(1):20–22. doi: 10.1016/j.ipej.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stock J.D., Chui P., Rosman L., Malm B.J., Bastian L., Burg M.M. Association of opioid use with atrial fibrillation in a post-9/11 veteran population. Circulation. 2018;1380(Suppl_1) [Google Scholar]
- 48.Muller S., Nussbaumer S., Plitzko G., Ludwing R., Weinmann W., Krahenbuhl S., Liakoni E. Recreational use of carfentanil - a case report with laboratory confirmation. Clin. Toxicol. 2018;56(2):151–152. doi: 10.1080/15563650.2017.1355464. [DOI] [PubMed] [Google Scholar]
- 49.Levin E.R., Sharp B., Drayer J.I.M., Wber M.A. Sevre hypertension induced by naloxone. Am. J. Med. Sci. 1985;290(2):70–72. doi: 10.1097/00000441-198508000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Sun S.-Y., Liu Z., Li P., Ingenito A.J. Central effects of opioid agonists and naloxone on blood pressure and heart rate in normotensive and hypertensive rats. Gen. Pharmacol. 1996;27(7):1187–1194. doi: 10.1016/s0306-3623(96)00055-9. [DOI] [PubMed] [Google Scholar]