Abstract
Late presentation to care and antiretroviral therapy (ART) initiation with advanced human immunodeficiency virus (HIV) disease are common in Latin America. We estimated the impact of these conditions on mortality in the region. We included adults enrolled during 2001–2014 at HIV care clinics. We estimated the adjusted attributable risk (AR) and population attributable fraction (PAF) for all-cause mortality of presentation to care with advanced HIV disease (advanced LP), ART initiation with advanced HIV disease, and not initiating ART. Advanced HIV disease was defined as CD4 of <200 cells/μL or acquired immune deficiency syndrome. AR and PAF were derived using marginal structural models. Of 9,229 patients, 56% presented with advanced HIV disease. ARs of death for advanced LP were 86%, 71%, and 58%, and PAFs were 78%, 58%, and 43% at 1, 5, and 10 years after enrollment. Among people without advanced LP, ARs of death for delaying ART were 39%, 32%, and 37% at 1, 5, and 10 years post-enrollment and PAFs were 20%, 14%, and 15%. Among people with advanced LP, ART decreased the hazard of death by 63% in the first year after enrollment, but 93% of these started ART; thus universal ART among them would reduce mortality by only 10%. Earlier presentation to care and earlier ART initiation would prevent most HIV deaths in Latin America.
Keywords: acquired immune deficiency syndrome, adult, early diagnosis, HIV infection, Latin America, retrospective studies
Abbreviations
- advanced LP
advanced disease with late presentation to care
- AIDS
acquired immune deficiency syndrome
- AR
attributable risk
- ART
antiretroviral therapy
- CCASAnet
Caribbean, Central and South America network for HIV epidemiology
- HIV
human immunodeficiency virus
- IPW
inverse-probability weighting
- LI
late initiation of ART
- LP
late presentation to care
- LTFU
lost to follow-up
- NI
no ART initiation
- PAF
population attributable fraction
- PLWHIV
people living with human immunodeficiency virus
The use of combined antiretroviral therapy (ART) after 1996 was followed by an abrupt reduction in acquired immune deficiency syndrome (AIDS) mortality (1), although this was less dramatic in low- and middle-income countries after the expansion of access to ART (2). Particularly, mortality remains high in most of Latin America (3–6). While the lower-than-expected impact of improved ART access on mortality in our region has been attributed to the frequency of late human immunodeficiency virus (HIV) diagnosis and ART initiation (7), to our knowledge, no formal assessment of the population impact of these factors on mortality has been performed.
People living with HIV (PLWHIV) who are starting ART in resource-poor settings have higher mortality during the first months on therapy than do those in high-income countries. Advanced immunosuppression when starting ART is the main factor associated with these differences (8–10). In Latin America, 37%–76% of patients initiate ART with CD4 counts of <200 cells/μL (11, 12), and late ART initiation is thought to be the main determinant of mortality (13). Previous analyses, however, only considered patients starting ART, although a high proportion of deaths could occur before ART initiation (14). Here, we aimed to estimate the potential impact that eliminating the combination of presentation to care and starting ART with advanced HIV disease would have on mortality among adults with HIV in Latin America. Using the Caribbean, Central and South America network for HIV epidemiology (CCASAnet) cohort, we estimated the proportion of deaths attributable to late presentation to care with advanced HIV disease (advanced LP), initiating ART in this (late) stage (LI), and the impact of not initiating ART (NI) in people with advanced LP. As measures of impact, we estimated the proportion of all deaths among people with advanced LP and among the entire study population that could have been prevented by earlier presentation (i.e., the attributable risk (AR) and population attributable fraction (PAF), respectively, of advanced LP on mortality). Similar measures were used to estimate the impact of LI and NI. Both the AR and the PAF are relevant to public health and consider not only the magnitude of association between exposure and outcome, but also the frequency of the exposure in the population. The rationale for this study is to quantify the potential public health benefit of implementing strategies that reduce late diagnosis/linkage to care and reduce time to ART initiation.
METHODS
Study design, cohort description, and analytical approach
We used clinical data from PLWHIV receiving care in 6 centers of CCASAnet: Fundación Huésped in Buenos Aires (FH-Argentina); Instituto de Pesquisa Clínica Evandro Chagas, Fundacão Oswaldo Cruz in Rio de Janeiro (FC-Brazil); Fundación Arriarán in Santiago (FA-Chile); Instituto Hondureño de Seguridad Social and Hospital Escuela in Tegucigalpa (IHSS/HE-Honduras); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City (INCMNSZ-Mexico); and Instituto de Medicina Tropical Alexander von Humboldt in Lima (IMTAvH- Peru) (15). We included all ART-naive adults (aged ≥18 years), enrolled in care between January 1, 2001, and December 31, 2014, who had a CD4 count recorded between 360 days before and 90 days after enrollment.
We performed 3 sets of analyses. First, we estimated the impact of advanced LP on mortality in the whole cohort, regardless of ART initiation; second, we estimated the impact of LI among those who presented without advanced LP; and third, we estimated the impact of NI among people with advanced LP.
Outcomes and definitions
The primary endpoint was all-cause mortality. Methods for mortality ascertainment differed by cohort site, with varying levels of patient tracing and/or linkage to national death registries (13). We defined LP as enrollment in participating centers and having AIDS or a CD4 count of <350 cells/μL, and we defined advanced HIV disease as having AIDS or a CD4 count of <200 cells/μL (16). Patients were classified accordingly as LP with advanced HIV disease (advanced LP) and as LI if starting ART with advanced HIV disease. Patients enrolled with advanced LP that did not start ART were defined as NI. We did our primary analysis based on the impact of advanced LP and LI. In secondary analysis, we assessed the impact of LP and late ART initiation (16). AIDS was defined using Centers for Disease Control and Prevention (category C) or World Health Organization (stage 4) criteria. Patients whose last visit occurred more than 1 year before cohort closure were considered lost to follow-up (LTFU), and their data were censored at their last visit date. Otherwise, individuals were followed until death or administrative censoring. Analyses assessed the impact of starting ART on mortality; any information on nonadherence or discontinuation of ART after ART initiation was not included.
Statistical analysis
The AR of mortality due to exposure (advanced LP, advanced LI among patients without advanced LP, and NI among those with advanced LP) was computed as the difference in the estimated probabilities of death between being exposed and being nonexposed divided by the probability of death in the exposed. Hence, the AR estimates can be interpreted as the proportion of deaths among people with the exposure that can be attributed to the exposure itself, or as the proportion of deaths that could had been averted had there been no exposure (17). The PAF of exposure on mortality was estimated using Miettinen’s formula (18). The PAF can be interpreted as the proportion of deaths in the entire study population that would have been prevented had exposed patients not been exposed. We used inverse-probability weighting (IPW) methods to correct for potential selection biases (19).
Specifically, to assess the impact of advanced LP on mortality, we estimated probabilities of mortality after enrollment for all participants in the cohort using IPW Kaplan-Meier estimates. IPW for advanced LP was constructed using logistic regression and including study site, enrollment date, sex, and age. Age and enrollment date were included in the model using restricted cubic splines with 5 knots, with the number of knots selected as explained in Web Appendix 1 (available at https://academic.oup.com/aje) (20). We computed the adjusted AR and PAF of death due to advanced LP from the IPW Kaplan-Meier estimates of survival comparing patients with and without advanced LP. We computed confidence intervals using bootstrap techniques with 200 replications.
In the second analysis, among patients without advanced LP, we estimated the impact of failing to start ART before having advanced HIV disease; therefore, we included only patients who presented to care with a CD4 of ≥200 cells/μL and without AIDS. We used a dynamic marginal structural model to estimate probabilities of mortality over time based on different treatment initiation conditions to correct selection biases arising from lack of randomization to time of treatment initiation (19, 21). We compared the following treatment initiation conditions: 1) start ART at a nonadvanced disease stage (CD4 of ≥200 cells/μL and no AIDS) versus 2) start ART at an advanced HIV disease stage (CD4 of <200 cells/μL or AIDS) (advanced LI)). This analysis was conducted using exposure time in each condition, such that patients contributed follow-up time to the treatment initiation condition with which their data were consistent, and patients were artificially censored from specific treatment conditions when their data no longer were consistent with the condition (e.g., if someone started ART prior to being in an advanced HIV disease stage, the patient would be censored from condition 2 at the time of ART initiation; when someone entered an advanced stage without having started ART, they were censored from condition 1). Pooled logistic regression (i.e., a discrete-time survival model) was used to estimate the mortality hazard ratio of deferring therapy. The pooled logistic regression model included time (expanded using restricted cubic splines with 4 knots) and was weighted by the inverse probability of not being censored (artificially or otherwise) at a given time point. Prior to ART initiation or progression to advanced HIV disease, the probability of not being artificially censored was set as the inverse of the estimated cumulative probability of not starting ART for patients in the advanced LI group. For patients in the not-advanced LI group who had not yet started ART, this was set as the inverse of the estimated cumulative probability of not entering advanced HIV disease. The probability of starting ART was estimated for those who had not yet started ART using pooled logistic regression and including time from enrollment, year of enrollment, most recent CD4, time since most recent CD4 measurement, age at enrollment, study site, and sex; continuous covariates were expanded using restricted cubic splines with 4 knots. The probability of progressing to advanced HIV disease was estimated among those who had not started ART. The predicted survival probability for those in the advanced LI and not-advanced LI groups were estimated using a similar model, except including an interaction between time from enrollment and treatment condition. In all models, we used log (time) and selected the number of knots as described above (20). ARs and PAFs of mortality for LI and their corresponding 95% confidence intervals were calculated as previously explained, from the estimated probabilities of death by treatment initiation condition, derived from the dynamic marginal structural model.
In the third analysis, we assessed the impact of NI among the advanced LP group. The goal was to compare the probability of mortality over time for advanced LP for immediately starting ART versus never starting ART. We fitted a standard marginal structural model (22), where the exposure was starting ART. We adjusted for the same covariates described above in our second analysis. The AR and PAF were computed using estimates of the probabilities of mortality predicted from the fitted marginal structural model, and confidence intervals were computed using the bootstrap with 200 replications. All primary analyses winsorized IPW at the 2.5th and 97.5th percentiles; sensitivity analyses considered other percentiles.
In secondary analyses, we computed the population impact of advanced LP on mortality under a more plausible scenario of reducing advanced LP by approximately 50%. In this calculation, we used the distribution of advanced LP in the study population, the magnitude of the association between advanced LP and death derived from the weighted Kaplan-Meier estimates, and the expected attainable reduction of advanced LP under normal programmatic conditions (23). We chose an attainable 50% reduction to achieve a prevalence of advanced LP of approximately 30%, because this is close to the overall prevalence reported in Europe (24). We also repeated each set of analyses using a CD4 count of <350 cells/μL or AIDS to define late presentation to care (LP) and late treatment initiation (LI) in people presenting early to care. We computed E values to assess sensitivity of results to unmeasured confounding (25). We also repeated analyses excluding data from INCMNSZ-Mexico because for most of the analysis period, this site captured data only after patients were started on ART. Analyses were performed in Mexico and the Data Coordinating Center in the United States using R, version 1.2.1335 (R Foundation for Statistical Computing, Vienna, Austria). Analysis scripts are available online (http://biostat.mc.vanderbilt.edu/ArchivedAnalyses).
Ethical considerations
Institutional ethics review boards from each participating site and the data coordinating center reviewed and approved procedures. We followed the principles outlined in the Declaration of Helsinki by the 41st World Medical Assembly.
RESULTS
Baseline characteristics
We included 9,229 patients enrolled in care in 6 CCASAnet centers between 2001 and 2014 (Table 1). Median age at enrollment was 34 years (interquartile range, 28–42) and 75% were men. Median CD4 at enrollment was 198 cells/μL (interquartile range, 68–381). Patients were followed for a median of 4 years (interquartile range, 1–7). Sociodemographic and clinical characteristics at enrollment by exposure group are summarized in Web Table 1.
Table 1.
Demographic and Clinical Characteristics of 9,229 Patients Enrolled in Care in 6 Clinical Care Centersa Participating in the Caribbean, Central and South America Network for HIV Epidemiology, 2000–2014
| Characteristic | Argentina (n = 1,636) |
Brazil (n = 2,008) |
Chile (n = 1,415) |
Honduras (n = 777) |
Mexico (n = 786) |
Peru (n = 2,607) |
All Sites (n = 9,229) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, yearsb | 36 (30–44) | 34 (28–42) | 34 (28–41) | 36 (30–43) | 33(28–41) | 33 (27–41) | 34 (28–42) | |||||||
| Male sex | 1,175 | 72 | 1,435 | 71 | 1,281 | 91 | 433 | 56 | 702 | 89 | 1,926 | 74 | 6,952 | 75 |
| CD4 at enrollment, cells/uLb | 214 (81–366) | 292 (104–507) | 267 (96–453) | 123 (58–205) | 142 (43–309) | 156 (54–324) | 198 (68–81) | |||||||
| AIDSc | 353 | 22 | 562 | 28 | 355 | 25 | 291 | 37 | 432 | 55 | 948 | 36 | 2,941 | 32 |
| AIDS or CD4 of <200 | 816 | 50 | 888 | 44 | 625 | 44 | 621 | 80 | 560 | 71 | 1,652 | 63 | 5,162 | 56 |
| AIDS or CD4 of <350 | 1,197 | 73 | 1,215 | 61 | 896 | 63 | 738 | 95 | 664 | 84 | 2,087 | 80 | 6,797 | 74 |
| Transmission route | ||||||||||||||
| Heterosexual | 647 | 40 | 920 | 46 | 322 | 23 | 456 | 59 | 225 | 29 | 1,590 | 61 | 4,160 | 45 |
| Homosexual | 486 | 30 | 772 | 38 | 1,083 | 77 | 64 | 8 | 523 | 67 | 987 | 38 | 3,915 | 42 |
| Other | 115 | 7 | 33 | 2 | 5 | <1 | 2 | <1 | 17 | 2 | 23 | 1 | 195 | 2 |
| Unknown | 388 | 24 | 283 | 14 | 5 | <1 | 255 | 33 | 21 | 3 | 7 | <1 | 959 | 10 |
| Lost to follow-up | 625 | 38 | 174 | 9 | 187 | 13 | 152 | 19 | 106 | 13 | 473 | 18 | 1,717 | 19 |
Abbreviations: AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus; IQR, interquartile range.
a Characteristics for patients included in the analysis according to site of enrollment in the Caribbean, Central and South America network for HIV epidemiology cohort.
b Values are expressed as median (IQR).
c AIDS was defined using Centers for Disease Control and Prevention (category C) or World Health Organization (stage 4) criteria.
Frequency of advanced LP, advanced LI, and NI
There were 5,162 (56%) patients with advanced LP, of whom 93% started ART. Among 4,067 patients without advanced LP, 3,119 (77%) started ART, 82% (n = 2,571) while they were asymptomatic, with a CD4 of ≥200 cells/μL (Figure 1). Sex, age, site, and year at enrollment were all independently associated with advanced LP (Web Table 2).
Figure 1.
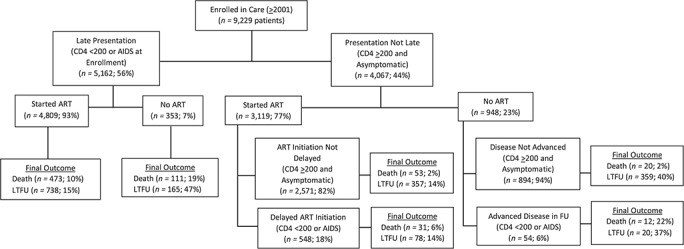
Distribution of patients enrolled in 6 human immunodeficiency virus (HIV) care centers in Latin America, according to stage of HIV disease at enrollment and at antiretroviral treatment initiation, in the Caribbean, Central and South America network for HIV epidemiology cohort, 2001–2014. AIDS, acquired immune deficiency syndrome; ART, combined antiretroviral therapy; FU, follow-up; LTFU, lost to follow-up.
Mortality
Seven hundred (7.6%) patients died during the study period, and 1,717 (19%) were LTFU. Figure 1 shows the proportion of deaths according to stage at enrollment and ART initiation. Figure 2 shows the weighted survival probabilities and hazard ratios after enrollment for people with advanced LP compared with those without advanced LP (Figure 2A), for advanced LI versus nonadvanced LI among those without advanced LP (Figure 2B), and NI in people with advanced LP compared with those that initiated ART (Figure 2C).
Figure 2.
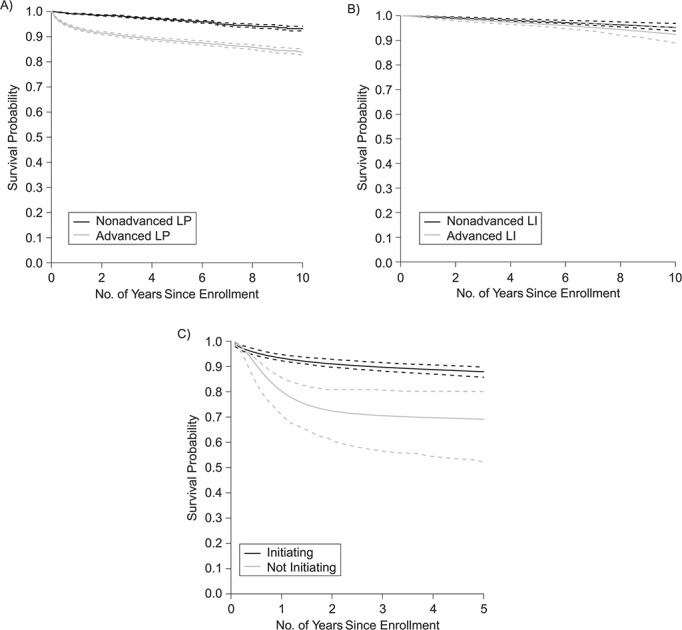
Adjusted survival probability after enrollment in care in 6 HIV care centers, according to human immunodeficiency virus (HIV) disease stage at enrollment and at treatment initiation, for participants in the Caribbean, Central and South America network for HIV epidemiology cohort, 2001–2014. A) Advanced late presentation (LP) versus LP without advanced disease (n = 5,162 patients vs. n = 4,067 patients). B) Advanced late initiation of antiretroviral therapy (ART) (LI) versus LI without advanced disease among LP patients (n = 602 patients vs n = 3,465 patients). C) Initiating versus not initiating ART among advanced LP patients (n = 4,809 patients vs n = 353 patients). Advanced HIV disease was defined as having acquired immune deficiency syndrome (AIDS) or a CD4 count of <200 cells/μL. AIDS was defined using Centers for Disease Control and Prevention (category C) or World Health Organization (stage 4) criteria. Presentation with advanced HIV disease was defined as having advanced HIV disease at enrollment in care (defined as the first visit in each center).
Potentially averted deaths if advanced LP, advanced LI, and NI were eliminated
The AR and PAF of mortality for advanced LP, advanced LI among people without advanced LP, and NI among advanced LP are shown in Table 2. We estimated that eliminating advanced LP would have prevented 86%, 71%, and 58% of deaths at 1, 5, and 10 years after enrollment, respectively, among those enrolled with advanced HIV disease and 78%, 58%, and 43% of all deaths. In secondary analysis, we estimated that reducing advanced LP from 56% to 30% would had prevented 66% (95% confidence interval: 54, 77) of all deaths occurring in the first year after enrollment, 42% (95% confidence interval: 34, 51) of deaths in the first 5 years, and 29% (95% confidence interval: 20, 38) of deaths in the first 10 years.
Table 2.
Attributable Risk of Deaths and Population Attributable Fraction of Deathsa Due to Late Presentation to Care, Delayed Antiretroviral Initiation, and Not Initiating Antiretrovial Therapy, Among Those Presenting to Care With Advanced Diseaseb at 1, 5, and 10 Years After Enrollment in Care, Central and South America Network for HIV Epidemiology, 2000–2014
| Time After Enrollment, years | Attributable Deaths | Population Attributable Fraction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced LP | Advanced LI in People Without Advanced LP | NI in People With Advanced LP | Advanced LP | Advanced LI in People Without Advanced LP | NI in People With Advanced LP | |||||||
| Proportion | 95%CI | Proportion | 95%CI | Proportion | 95%CI | Proportion | 95%CI | Proportion | 95%CI | Proportion | 95%CI | |
| 1 | 0.86 | 0.81–0.92 | 0.39 | 0.01–0.67 | 0.66 | 0.54–0.80 | 0.78 | 0.7–0.87 | 0.20 | 0.00–0.44 | 0.1 | 0.06–0.17 |
| 5 | 0.71 | 0.64–0.78 | 0.32 | 0.04–0.54 | 0.61 | 0.43–0.75 | 0.58 | 0.49–0.67 | 0.14 | 0.01–0.28 | 0.02 | 0.01–0.04 |
| 10 | 0.58 | 0.47–0.69 | 0.37 | −0.02-0.67 | N/Ac | N/Ac | 0.43 | 0.33–0.54 | 0.15 | 0.00–0.39 | N/Ac | N/Ac |
Abbreviations: advanced LI, advanced disease and late initiation of antiretroviral therapy; advanced LP, advanced disease and late presentation; AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus; N/A, not applicable.
a Estimations in 6 clinical care centers participating in the Caribbean, Central and South America network for HIV epidemiology.
b Advanced HIV disease was defined as having AIDS or a CD4 count of <200 cells/μL. AIDS was defined using Centers for Disease Control and Prevention (category C) or World Health Organization (stage 4) criteria. Presentation with advanced HIV disease was defined as having advanced HIV disease at enrollment in care (defined as the first visit in each center).
c Not estimated because most patients presenting to care with advanced HIV disease not initiating ART did not reach 10 years of follow-up.
Among people without advanced LP, ARs of death for delaying ART were 39%, 32%, and 37% at 1, 5, and 10 years after enrollment, and PAFs were 20%, 14%, and 15%, respectively. Among people with advanced LP, ART decreased the hazard of death by 63% in the first year after enrollment, but 93% of these started ART; thus universal ART among them would reduce mortality by only 10%.
Secondary analysis and sensitivity analysis
We observed very similar results when estimating the impact of LP to care (CD4 of <350 cells/μL or AIDS at enrollment), late ART initiation among non-LP, and not initiating ART among LP (Web Table 3). These results are presented in Web Figures 1–4. Our results were robust to the choice of winsorization level for the IPW. We observed no changes when excluding INCMNSZ-Mexico from analyses. The estimated E value for our advanced LP analysis was 6.12, meaning that an unmeasured confounder with an association with both advanced LP and death with a risk ratio of at least 6.12 (for the point estimate to be 1) or 5.33 (for the upper limit of the 95% confidence interval to include 1) would be needed to explain away the observed hazard ratio. The E values for the association between advanced LI and death were 2.5 for the point estimate and 1.36 for the upper limit of the 95% confidence interval. E values for the association between NI and death were 4.84 for the point estimate and 3.18 for the 95% confidence interval to include 1.
DISCUSSION
Using model-based ARs and PAFs of mortality in a multinational observational cohort in Latin America, we found that during the first year after enrollment, 86% of deaths occurring among people with advanced LP and 78% of deaths in the entire cohort could have been prevented by eliminating advanced LP, which occurred in 56% of patients. Even reducing advanced LP to the more plausibly obtainable goal of 30%, would eliminate approximately two-thirds of all deaths occurring during the first year of enrollment. Starting ART before advanced disease in all patients presenting with nonadvanced HIV disease would have prevented a lower proportion of deaths (39% among advanced LI, and 20% among all non-LP the first year in care). Finally, starting ART in all patients with advanced LP would have prevented 66% of deaths during the first year after enrollment for NI but only 10% of deaths among all patients with advanced LP.
The implications of our results are better understood when considering that around 850,000 people died due to AIDS in Latin America during this period (26). Our results suggest that the absolute number of HIV-related deaths that could have been prevented in Latin America by reducing advanced LP is likely in the hundreds of thousands, and more importantly, tens of thousands of annual deaths could be prevented in the next decade by reducing advanced LP and by initiating ART in all adults with HIV. The estimated PAFs for mortality of advanced LP were particularly high due to the high frequency of this condition. The benefits of starting ART earlier for people who enroll in care without advanced disease are also apparent, although not as striking, due in part to the low frequency of early presentation, an already relatively high frequency of ART initiation, and lower mortality risk in these patients. Even so, a quarter of all deaths over 10 years among people without advanced LP would have been averted had they all started ART before progressing to advanced HIV disease. In contrast, not starting ART for people with advanced LP entailed a very high short- and long-term mortality, but the estimated PAF was low because most of them did start ART. In the present context of guidelines to treat all adults with HIV (27), the goals of eliminating all LI in nonadvanced LP and NI in all advanced LP should by all rights be attained immediately, although the goal of rapid ART initiation in all populations faces challenges.
These findings contribute to the knowledge of a previously well-documented problem in our region, namely presentation with advanced HIV disease (11, 12, 28) and late ART initiation (11, 29, 30), by quantifying their population impact on AIDS-related mortality in Latin America. A skeptic might suggest that presentation to care and ART initiation with advanced HIV disease are already known to affect patient mortality, and that these results are largely confirmatory. However, quantification of the population impact of these phenomena in Latin America is important to demonstrate the magnitude of the problem. Our results also help to identify the potential impact of reducing gaps in the continuum of care for PLWHIV in the region. While previous studies by our group and others had focused on the survival of PLWHIV starting ART (13), measures of association by themselves are insufficient to determine public health impacts.
Our results show that most deaths among PLWHIV in our region can be prevented with the implementation of evidence-based, effective, and available strategies to reduce the proportions of undiagnosed people, improve linkage to care, and increase ART use. In British Columbia, widespread HIV screening increased the proportion of PLWHIV diagnosed at earlier stages and effectively reduced the incidence of AIDS-defining events, all-cause mortality, and the proportion of deaths from AIDS-related causes (1, 31). Expansion of testing services—including routine HIV-care testing for all patients receiving emergency care (32), community-based and home-based testing (33, 34), supervised or unsupervised self-testing (35), and targeted testing of high-risk groups (36)—have all improved early diagnosis and ART initiation in regions with concentrated epidemics, such as ours. Similarly, large-scale interventions to reduce time between diagnosis and enrollment in care, such as point-of-care technology (37), peer-navigator programs (38), and home-based initiation of care (39), have successfully increased enrollment in care and reduced time to ART initiation in other settings. These same interventions would help identify people who die before HIV diagnosis or enrollment in care, which are not included in this cohort and suggest that we might be underestimating the impact of eliminating advanced LP.
Of note, we employed modern causal inference techniques incorporating IPW to obtain properly adjusted estimates of the AR and PAF. Despite the propriety of AR and PAF for measuring the population impact of a risk factor and recent developments in their use (40–42), we have not seen these measures used in conjunction with methods that permit the inclusion of time-varying confounders or the comparison of dynamic treatment strategies. Our application of dynamic marginal structural modeling to estimate the AR and PAF of late ART initiation on mortality is methodologically innovative. That said, the limited number of centers contributing information and the characteristics of these centers might limit the generalizability of our estimates. Nonetheless, our results are in agreement with previous studies about late HIV diagnosis (7, 11, 28), LP (11, 12, 28), and LI (29, 30) in Latin America.
Among potential limitations of the study is that the aggregated estimates might obscure heterogeneity across the region. In addition, because 75% of the study population was enrolled before 2011, our results might not be applicable to people enrolled in care recently, particularly in an era of universal-treatment guidelines. However, quantification of the potential impact of earlier enrollment in care and earlier ART initiation in the past can be used to motivate better policy and implementation in the future, in particular in our region, where the frequency of late diagnosis and mortality has overall remained constant. Also, we did not have cause-of-death information, and as with all observational studies, there could be confounding variables that were not accounted for in our analyses. High E values obtained in sensitivity analyses suggest that important unmeasured confounders (e.g., drug use) are unlikely to explain away the associations between exposures and mortality (43), but they could alter AR and PAF estimates. Finally, some of our study sites had high rates of LTFU that might have biased our estimates toward a reduction in the magnitude of the impact of advanced LP and advanced LI, considering that these are associated with higher LTFU rates (13) and people who are LTFU are typically more likely to die than those remaining in care (44).
In conclusion, the majority of deaths among PLWHIV in Latin America are attributable to advanced LP, and to a lesser extent to delaying ART initiation. Earlier presentation to care, and earlier initiation of ART after enrollment would substantially reduce mortality among PLWHIV in Latin America, mainly during the 5 years after enrollment in care but even 10 years later. Our results show that most deaths among PLWHIV in our region are preventable using existing, evidence-based, effective interventions to improve early diagnosis, linkage to care, and early ART initiation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Departamento de Infectología, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico (Pablo F. Belaunzarán-Zamudio, Yanink N. Caro-Vega, Brenda E. Crabtree-Ramírez, Juan G. Sierra-Madero); Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, Tennessee (Bryan E. Shepherd, Peter F. Rebeiro); Department of Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee (Peter F. Rebeiro, Catherine C. McGowan); Fundación Arriarán, Universidad de Chile, Santiago, Chile (Claudia P. Cortes); Instituto de Pesquisa Clínica Evandro Chagas, Fundacão Oswaldo Cruz, Rio de Janeiro, Brazil (Beatriz Grinsztejn, Valdilea Veloso, Sandra Wagner Cardoso); Instituto de Medicina Tropical Alexander von Humboldt, Lima, Peru (Eduardo Gotuzzo, Fernando Mejia); Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras (Denis Padgett); and Le Groupe Haitien d’Etude du Sarcome de Kaposi et des Infections Opportunistes, Port-au-Prince, Haiti (Jean W. Pape, Vanessa Rouzier).
This work was supported by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (grant U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Cancer Institute, National Institute of Allergy and Infectious Diseases, National Institute of Mental Health, and the Office of the Director, National Institutes of Health.
The Caribbean, Central and South America Network for HIV Epidemiology includes the following sites and staff: Fundación Huesped, Argentina (Pedro Cahn, Carina Cesar, Valeria Fink, Omar Sued, Patricia Patterson, Emanuel Dell’Isola, Hector Perez, Jose Valiente, Cleyton Yamamoto); Instituto Nacional de Infectologia-Fiocruz, Brazil (Beatriz Grinsztejn, Valdilea Veloso, Paula Luz, Raquel de Boni, Sandra Wagner Cardoso, Ruth Friedman, Ronaldo Moreira); Universidade Federal de Minas Gerais, Brazil (Jorge Pinto, Flavia Ferreira, Marcelle Maia); Universidade Federal de São Paulo, Brazil (Regina Célia de Menezes Succi, Daisy Maria Machado, Aida de Fátima Barbosa Gouvêa); Fundación Arriarán, Chile (Marcelo Wolff, Claudia P. Cortes, Maria Fernanda Rodriguez, Gladys Allende); Les Centres GHESKIO, Haiti (Jean William Pape, Vanessa Rouzier, Adias Marcelin, Christian Perodin); Hospital Escuela Universitario, Honduras (Marco Tulio Luque); Instituto Hondureño de Seguridad Social, Honduras (Denis Padgett); Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México (Juan Sierra Madero, Brenda E. Crabtree-Ramirez, Pablo F. Belaunzarán-Zamudio, Yanink N. Caro-Vega, Rocío Velázquez Pastrana); Instituto de Medicina Tropical Alexander von Humboldt, Peru (Eduardo Gotuzzo, Fernando Mejia, Gabriela Carriquiry); Vanderbilt University Medical Center, Tennessee (Catherine C McGowan, Bryan E. Shepherd, Timothy Sterling, Karu Jayathilake, Anna K. Person, Peter F. Rebeiro, Jessica Castilho, Stephany N. Duda, Fernanda Maruri, Hilary Vansell, Sally Bebawy, James Logan).
Preliminary results presented at HIV Drug Therapy 2016 in October 23–27, 2016, Glasgow, United Kingdom (abstract P340), and at the 22nd International Workshop on HIV and Hepatitis Observational Databases, March 22–24, 2018, Fuengirola, Spain.
The sponsors had no role in the design and conduct of the study; no role in the collection, analysis, and interpretation of the data; nor in the preparation, review, or approval of the manuscript or decision to submit.
J.G.S.-M. reports personal fees and nonfinancial support from Gilead, nonfinancial support from MSD, grants from BMS, grants from Pfizer, and personal fees from Janssen, all outside the submitted work. The other authors report no conflicts.
REFERENCES
- 1. Lima VD, Lourenço L, Yip B, et al.. AIDS incidence and AIDS-related mortality in British Columbia, Canada, between 1981 and 2013: a retrospective study. Lancet HIV. 2015;2(3):e92–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS AIDSinfo global AIDS monitoring database. http://www.aidsinfoonline.org/devinfo/libraries/aspx/dataview.aspx. Accessed August 15, 2017.
- 3. Hernández-Ávila JE, Palacio-Mejía LS, Hernández-Romieu A, et al.. Effect of universal access to antiretroviral therapy on HIV/AIDS mortality in Mexico 1990–2011. J Acquir Immune Defic Syndr. 2015;69(3):e100–e108. [DOI] [PubMed] [Google Scholar]
- 4. Joint United Nations Programme on HIV/AIDS The Gap report 2014. http://www.unaids.org/en/resources/campaigns/2014/2014gapreport/slides/. Accessed August 15, 2017.
- 5. Ministerio de Salud y Desarrollo Social Boletín epidemiológico sobre VIH, sida e ITS en la Argentina, no. 31, año XXII, diciembre de 2019. http://www.msal.gob.ar/images/stories/bes/graficos/0000001754cnt-boletin-epidemiologico-2019_vih-sida-its.pdf. Accessed October 9, 2019.
- 6. Ministerio de Salud y Protección Social, República de Colombia Informe GARPR—2014. Bogota, Colombia: Ministerio de Salud y Protección Social; 2014. [Google Scholar]
- 7. Crabtree-Ramírez B, Caro-Vega Y, Belaunzarán-Zamudio F, et al.. High prevalence of late diagnosis of HIV in Mexico during the HAART era. Salud Publica Mex. 2012;54(5):506–514. [DOI] [PubMed] [Google Scholar]
- 8. Braitstejn P, Brinkhof MW, Dabis F, et al.. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. [DOI] [PubMed] [Google Scholar]
- 9. Grinsztejn B, Veloso VG, Friedman RK, et al.. Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS. 2009;23(16):2107–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luz PM, Bruyand M, Ribeiro S, et al.. AIDS and non-AIDS severe morbidity associated with hospitalizations among HIV-infected patients in two regions with universal access to care and antiretroviral therapy, France and Brazil, 2000–2008: hospital-based cohort studies. BMC Infect Dis. 2014;14:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crabtree-Ramírez B, Caro-Vega Y, Shepherd BE, et al.. Cross-sectional analysis of late HAART initiation in Latin America and the Caribbean: late testers and late presenters. PLoS One. 2011;6(5):e20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belaunzarán-Zamudio P, Zitko P, Araúz Rodríguez A, et al.. Modest progress in early linkage to care in Latin American HIV-care centres from the HIV Latin American Workshop Group (2013-2017) [abstract P044] Presented at HIV & Hepatitis in the Americas 2019, Bogotá, Colombia, April 4–6, 2019. [Google Scholar]
- 13. Carriquiry G, Fink V, Koethe JR, et al.. Mortality and loss to follow-up among HIV-infected persons on long-term antiretroviral therapy in Latin America and the Caribbean. J Int AIDS Soc. 2015;18:20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martín-Onraet A, Piñeirúa-Menéndez A, Perales-Martínez D, et al.. Mortalidad hospitalaria en pacientes con infección por VIH: a diez años del acceso universal a TARAA en México. Salud Pub Mex. 2015;57(suppl 2):S163–S170. [PubMed] [Google Scholar]
- 15. McGowan CC, Cahn P, Gotuzzo E, et al.. Cohort profile: Caribbean, Central and South America network for HIV research (CCASAnet) collaboration within the international epidemiologic databases to evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007;36(5):969–976. [DOI] [PubMed] [Google Scholar]
- 16. Antinori A, Coenen T, Costagiola D, et al.. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61–64. [DOI] [PubMed] [Google Scholar]
- 17. Walter SD. The estimation and interpretation of attributable risk in Health Research. Biometrics. 1976;32(4):829–849. [PubMed] [Google Scholar]
- 18. Miettinen O. Proportion of disease caused or prevented by a given exposure, trait or intervention? Am J Epidemiol. 1974;99(5):325–332. [DOI] [PubMed] [Google Scholar]
- 19. Hernán MA, Lanoy E, Costagliola D, et al.. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;98(3):237–242. [DOI] [PubMed] [Google Scholar]
- 20. Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed New York, NY: Springer International Publishing; 2015.
- 21. Cain LE, Robins JM, Lanoy E, et al.. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat. 2010;6(2):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. [DOI] [PubMed] [Google Scholar]
- 23. Morgenstern H, Bursic ES. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7(4):292–309. [DOI] [PubMed] [Google Scholar]
- 24. Late presenters working group in COHERE in EuroCoord, Mocroft A, Lundgren J, et al.. Late presentation for HIV care across Europe: update from the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study, 2010 to 2013. Euro Surveill. 2015;20(47):30070. [DOI] [PubMed] [Google Scholar]
- 25. Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–603. [DOI] [PubMed] [Google Scholar]
- 26. Joint United Nations Programme on HIV/AIDS Indicator: AIDS-related deaths, Latin America (AIDSinfo database). Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2019. http://aidsinfo.unaids.org/#. Accessed October 9, 2019. [Google Scholar]
- 27. World Health Organization Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2nd ed. Geneva, Switzerland: World Health Organization; 2016. [PubMed] [Google Scholar]
- 28. Valentini MB, Guerra de Toledo ML, Oliveira Fonseca M, et al.. Evaluation of late presentation for HIV treatment in a reference center in Belo Horizonte, southeastern Brazil, from 2008 to 2010. Braz J Infect Dis. 2015;19(3):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warley E, Fernández-Galimberti G, Vieni MI, et al.. Factores asociados al estadio clínico avanzado en el inicio de la terapia antirretroviral. Medicina. 2012;72(5):367–370. [PubMed] [Google Scholar]
- 30. Magis-Rodríguez CL, Villafuerte-García A, Cruz-Flores RA, et al.. Inicio tardío de terapia antirretroviral en México. Salud Pub Mex. 2015;57(suppl 2):S127–S134. [PubMed] [Google Scholar]
- 31. Cheung CC, Ding E, Sereda P, et al.. Reductions in all-cause and cause-specific mortality among HIV-infected individuals receiving antiretroviral therapy in British Columbia, Canada: 2001–2012. HIV Med. 2016;17(9):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mosqueda JL, León-Guerrero EM, Álvarez J, et al.. Improving early HIV diagnosis by increasing HIV testing: experience in León, Guanajuato, Mexico (abstract P014). J Int AIDS Soc. 2015;18(suppl 2):20177. [Google Scholar]
- 33. Suthar AB, Ford N, Bachanas PJ, et al.. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8):e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bigogo G, Amolloh M, Laserson KF, et al.. The impact of home-based HIV counseling and testing on care-seeking and incidence of common infectious disease syndromes in rural western Kenya. BMC Infect Dis. 2014;14:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pant Pai N, Sharma J, Shivkumar S, et al.. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10(4):e1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz V, Medina Y, Iracheta P, et al.. HIV/STI testing among MARPs in Mexico City: a key first step to improve the cascade of care. J Int AIDS Soc. 2016;19(suppl 1):21083. [Google Scholar]
- 37. Jani IV, Sitoe NE, Alfai ER, et al.. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–1579. [DOI] [PubMed] [Google Scholar]
- 38. Govindasamy D, Meghij J, Kebede Negussi E, et al.. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings—a systematic review. J Int AIDS Soc. 2014;17(1):19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacPherson P, Lalloo DG, Webb EL, et al.. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: a randomized clinical trial. JAMA. 2014;312(4):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cox C. Model-based estimation of the attributable risk in case-control and cohort studies. Stat Methods Med Res. 2006;15(6):611–625. [DOI] [PubMed] [Google Scholar]
- 41. Chen YQ, Hu C, Wang Y. Attributable risk function in the proportional hazards model for censored time-to-event. Biostatistics. 2006;7(4):515–529. [DOI] [PubMed] [Google Scholar]
- 42. Lin H, Allore HG, McAvay G, et al.. A method for partitioning the attributable fraction of multiple time-dependent coexisting risk factors for an adverse health outcome. Am J Pub Health. 2013;103(1):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Op de Coul EL, van Sighem A, Brinkman K, et al.. Factors associated with presenting late or with advanced HIV disease in the Netherlands, 1996-2014: results from a national observational cohort. BMJ Open. 2016;6(1):e009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teixeira da Silva DS, Luz PM, Lake JE, et al.. Poor retention in early care increases risk of mortality in a Brazilian HIV-infected clinical cohort. AIDS Care. 2017;29(2):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


