Abstract
Hepatitis C virus (HCV) infection is common among people living with human immunodeficiency virus (PLWH). Extrahepatic manifestations of HCV, including myocardial infarction (MI), are a topic of active research. MI is classified into types, predominantly atheroembolic type 1 MI (T1MI) and supply-demand mismatch type 2 MI (T2MI). We examined the association between HCV and MI among patients in the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems, a US multicenter clinical cohort of PLWH. MIs were centrally adjudicated and categorized by type using the Third Universal Definition of Myocardial Infarction. We estimated the association between chronic HCV (RNA+) and time to MI while adjusting for demographic characteristics, cardiovascular risk factors, clinical characteristics, and history of injecting drug use. Among 23,407 PLWH aged ≥18 years, there were 336 T1MIs and 330 T2MIs during a median of 4.7 years of follow-up between 1998 and 2016. HCV was associated with a 46% greater risk of T2MI (adjusted hazard ratio (aHR) = 1.46, 95% confidence interval (CI): 1.09, 1.97) but not T1MI (aHR = 0.87, 95% CI: 0.58, 1.29). In an exploratory cause-specific analysis of T2MI, HCV was associated with a 2-fold greater risk of T2MI attributed to sepsis (aHR = 2.01, 95% CI: 1.25, 3.24). Extrahepatic manifestations of HCV in this high-risk population are an important area for continued research.
Keywords: chronic hepatitis C infection, hepatitis C virus, HIV, HIV coinfection, myocardial infarction, people living with HIV, type 2 myocardial infarction
Abbreviations
- aHR
adjusted hazard ratio
- AIDS
acquired immunodeficiency syndrome
- CFAR
Centers for AIDS Research
- CI
confidence interval
- CNICS
CFAR Network of Integrated Clinical Systems
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- MI
myocardial infarction
- PLWH
people living with HIV
- T1MI
type 1 myocardial infarction
- T2MI
type 2 myocardial infarction
Survival of people living with human immunodeficiency virus (HIV) (PLWH) has improved dramatically in the last 20 years, owing to a decline in mortality associated with antiretroviral therapy (1–5). During the same period, morbidity and mortality from illnesses not defining acquired immunodeficiency syndrome (AIDS), such as cardiovascular disease, have increased in this group (6–9). PLWH are at higher risk of myocardial infarction (MI) than the general population (10). Recent work has shown that among PLWH, approximately half of incident MIs are type 2 MI (T2MI) (11), whereas in the general population T2MI accounts for a much smaller proportion of MIs (12–20). In contrast to classical atheroembolic type 1 MI (T1MI), T2MI results from myocardial oxygen demand-supply mismatch, a scenario that may result from a variety of conditions, including sepsis, illegal stimulant- or drug-induced vasospasm, decompensated heart failure, and hypotension (21) (Table 1).
Table 1.
Types of Myocardial Infarction as Classified by the Third Universal Definition of Myocardial Infarctiona
| Type | Description |
|---|---|
| 1 | Spontaneous MI related to atherosclerotic plaque rupture resulting in coronary thromboembolism |
| 2 | MI secondary to an ischemic imbalance wherein a condition other than coronary artery disease contributes to the imbalance |
| 3 | Cardiac death which had symptoms and cardiac electrophysiological alterations suggestive of MI but which occurred before cardiac biomarker levels could be measured or before levels could rise |
| 4 | MI related to percutaneous coronary intervention or stent thrombosis |
| 5 | MI related to coronary artery bypass grafting |
Abbreviation: MI, myocardial infarction.
a See Thygesen et al. (21).
A large body of literature has linked cardiovascular disease to chronic infection with hepatitis C virus (HCV), a common viral infection affecting an estimated 10%–30% of PLWH in the United States (22–26). Observational evidence supports an association between HCV and clinically evident cardiovascular disease, both in the general population (27, 28) and in PLWH (29); yet general-population studies that have focused on the role of HCV in coronary artery disease risk have produced inconclusive results (27) and, when restricted to MI only, have generally not supported an association (30–33). One recent study found a higher risk of MI among persons with HCV, but only in the subgroup with high levels of total and low-density lipoprotein cholesterol (34). Among PLWH, very little research has assessed the association of HCV coinfection with MI, and to our knowledge none has examined whether such an association may differ by MI type.
Because of the higher prevalence of MI and HCV infection among PLWH, unclear associations between HCV and MI in the general population, different patterns of MI types among PLWH, and an increasing availability of highly effective treatments for HCV infection, it is important to establish whether HCV increases the risk of MI for PLWH. In this study, we sought to estimate the relative risks of incident T1MI and T2MI among PLWH who are coinfected with HCV as compared with those who are not.
METHODS
Data repository and study population
The Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort is a population of more than 32,000 PLWH who have received HIV care at one of 8 sites in the United States from 1995 to the present and have consented to participation in CNICS research activities (35). Investigators at each CNICS study site capture demographic, clinical, medication, and laboratory data from all outpatient and inpatient encounters, including data on cardiac biomarkers, medications, and diagnoses and historical clinical information. Included in this study were all participants aged ≥18 years from 6 CNICS sites with comprehensive access to inpatient and outpatient electronic medical records: Johns Hopkins University (Baltimore, Maryland); the University of Alabama at Birmingham (Birmingham, Alabama); the University of California, San Diego (La Jolla, California); the University of California, San Francisco (San Francisco, California); the University of North Carolina at Chapel Hill (Chapel Hill, North Carolina); and the University of Washington, Seattle (Seattle, Washington). This analysis includes participants who contributed person-time during the years 1998–2016; the majority of follow-up occurred from 2010 to 2016. Time of entry into the study cohort was defined as 6 months after entry into the CNICS cohort or the first date of surveillance for MI events at the study site—whichever was later. Participants were followed until 9 months after the date of the last clinic visit or laboratory result (36), death, or administrative censoring for the study site. Institutional review boards at each institution have approved CNICS research activities.
Variables of interest
Ascertainment and adjudication of MI events in this cohort have been described in detail elsewhere (37). Briefly, identification of potential events was done centrally using multiple criteria, including MI-associated diagnostic codes (International Classification of Diseases, Ninth Revision, codes 410.00, 410.01, and 410.10), a record of undergoing an invasive cardiac procedure (percutaneous coronary intervention or bypass grafting), and elevated levels of cardiac biomarkers. Researchers at the study sites then generated clinical data packets including primary data for all potential MI events. The packets contained chart notes, electrocardiograms, imaging and procedure reports, and laboratory values, but data on use of antiretroviral medication were redacted. Packets were reviewed centrally by 2 physician experts to adjudicate MIs as definite or probable and to classify them as T1MI or T2MI, based on the Third Universal Definition of Myocardial Infarction (11, 21, 37). While the Third Universal Definition includes other types of MI (e.g., cardiac procedure-related type 4 MI), these were uncommon (<10 cases) and so are not further discussed here. In cases of discrepant findings, the assessment of a third reviewer was used to break ties (37). For events adjudicated as T2MI, a probable cause to which the event could be attributed was determined on the basis of information available in the clinical data packet. “Stimulant-induced vasospasm” refers to coronary arterial spasm secondary to use of illegal stimulant drugs, such as cocaine or methamphetamine.
Laboratory test results obtained in the course of routine care were used to determine HCV status. Chronic HCV infection was defined as a detectable result (i.e., above the lower limit of detection) for the most recent HCV RNA test before entry into the study cohort, and all remaining participants in the study cohort were classified as not chronically infected with HCV.
Covariates
Covariate data were taken from measurements available at study entry, except for height used to calculate body mass index, which could have been measured at any time. Diabetes was defined as any one of the following: hemoglobin A1c concentration greater than or equal to 6.5%, a clinical diagnosis of diabetes mellitus type 1 or type 2 and prescription of diabetes-related medication, or prescription of a diabetes-specific medication. Hypertension was defined as systolic blood pressure greater than or equal to 140 mm Hg or diastolic blood pressure greater than or equal to 90 mm Hg. Documentation of antihypertensive medication use was also included as a separate covariate. Hepatitis B virus infection was defined on the basis of a positive result for hepatitis B e-antigen or surface antigen or DNA. Fibrosis-4 index, a noninvasive measure of predicted liver fibrosis, was calculated as [age (years) × aspartate aminotransferase (AST)]/[platelet count × alanine aminotransferase (ALT)1/2] (38) and categorized as >3.25 (severe fibrosis), 1.45–3.25 (indeterminate), or <1.45 (no severe fibrosis).
History of injecting drug use was derived from the HIV transmission risk factor reported at CNICS cohort entry and was coded as 1 if the participant reported membership in a transmission risk group that included injecting drug use. Ever smoking was defined as the presence of a physician’s diagnosis of tobacco use or self-reported tobacco use.
Statistical analysis
Missing values for all analytical variables were multiply imputed using chained equations with fully conditional specification in the R package “mice” (39) (R Foundation for Statistical Computing, Vienna, Austria) on the basis of all other covariates, yielding 100 complete data sets. At the beginning of follow-up, demographic and clinical covariates were missing for 1%–33% of participants (see Web Table 1, available at https://academic.oup.com/aje). For each complete data set, the association between chronic HCV infection and time to the patient’s first MI event was estimated using Cox proportional hazards regression models. Resulting inferences were pooled using Rubin’s rules (40). In separate models, we examined T1MI, T2MI, cause-specific T2MI outcomes, and a composite outcome of all MIs. The models adjusted for potentially confounding factors. Minimally adjusted models considered only age and sex, while fully adjusted models included study site, demographic characteristics (age, sex, race/ethnicity, men who have sex with men), clinical characteristics (diabetes, hypertension, antihypertensive medication use, statin use, body mass index, lipid profile, lowest CD4-positive cell count, hepatitis B virus infection, HIV viral load, antiretroviral therapy), ever smoking, and history of injecting drug use. Proportionality of hazards was assessed using Schoenfeld residuals for a random subset of imputations; no consistent deviations were discovered.
Because liver fibrosis was considered a potential mediator of the relationship between HCV and MI, it was not included in the regression models for the main analyses. We conducted post hoc assessment of potential mediation by liver fibrosis using the potential outcomes approach implemented with natural effect models (41) and adapted to the Cox proportional hazards regression setting as described by Lange et al. (42). Analysis was conducted on the multiply imputed data sets described above. Standard errors for model parameters were computed using 1,000 bootstrapped samples of each imputed data set (43). Inferences were then pooled across imputations as described above. The mediator variable was fibrosis-4 index, handled as a continuous variable.
Statistical analyses were conducted in R, version 3.4.3.
Sensitivity analysis
We repeated the analyses to estimate the risks of MI outcomes associated with prior HCV infection in the absence of chronic infection at the time follow-up began. The exposed group for that analysis was persons with a positive finding on the most recent HCV antibody test coupled with a negative finding for the most recent RNA test prior to baseline. The association between history of injecting drug use and time to a T2MI event was assessed using Cox proportional hazards regression, adjusting for age, sex, and race/ethnicity in the multiple imputation setting as described above.
RESULTS
Among 23,407 PLWH, we observed 336 T1MIs and 330 T2MIs (666 MIs in total) during a median of 4.7 years of follow-up. In all, 2,280 participants (9.7%) had evidence of chronic HCV infection at the beginning of MI follow-up. The median age of participants was 40 years, 81% were male, and approximately half were nonwhite (Table 2). Compared with participants without chronic HCV infection, those with HCV tended to be older and were less likely to report being men who had sex with men. Unsurprisingly, a much larger proportion (61.5% vs. 15.0%) had a history of injecting drug use (Table 2). The crude incidence rate for all MI events was 5.0 per 1,000 person-years (95% confidence interval (CI): 4.7, 5.2); the rate was 4.6 per 1,000 person-years (95% CI: 4.4, 4.9) among participants without chronic HCV and 8.2 per 1,000 person-years (95% CI: 7.1, 9.4) among those with chronic HCV. T2MI events were attributed to a variety of causes (Table 3), with sepsis/bacteremia and stimulant-induced vasospasm being the most common.
Table 2.
Baseline Clinical and Demographic Characteristics of Adults Living With HIV Who Were in Clinical Care at 6 CNICS Sites, by Chronic Hepatitis C Virus Infection Status, United States, 1998–2016
| Characteristic | Chronic HCV Infection Status | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chronic HCV Infection (n = 2,280) |
No Chronic HCV Infection
(n = 21,127) |
Total
(n = 23,407) |
|||||||
| No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | No. of Persons | % | Mean (SD) | |
| Demographic factors | |||||||||
| Male sex | 1,779 | 78.0 | 17,117 | 81.0 | 18,896 | 80.7 | |||
| Age, years | |||||||||
| <30 | 108 | 4.7 | 3,666 | 17.4 | 3,774 | 16.1 | |||
| 30–39 | 511 | 22.4 | 6,626 | 31.4 | 7,137 | 30.5 | |||
| 40–49 | 931 | 40.8 | 7,072 | 33.5 | 8,003 | 34.2 | |||
| 50–59 | 619 | 27.1 | 3,062 | 14.5 | 3,681 | 15.7 | |||
| ≥60 | 111 | 4.9 | 701 | 3.3 | 812 | 3.5 | |||
| Race/ethnicity | |||||||||
| Non-Hispanic white | 981 | 43.0 | 9,096 | 43.1 | 10,077 | 43.1 | |||
| Non-Hispanic black | 984 | 43.2 | 8,390 | 39.7 | 9,374 | 40.0 | |||
| Non-Hispanic other race | 119 | 5.2 | 1,036 | 4.9 | 1,155 | 4.9 | |||
| Hispanic (all races) | 196 | 8.6 | 2,605 | 12.3 | 2,801 | 12.0 | |||
| Man who had sex with men | 877 | 39.0 | 12,897 | 62.2 | 13,774 | 59.9 | |||
| Ever smoking | 873 | 38.3 | 5,160 | 24.4 | 6,033 | 25.8 | |||
| History of injecting drug use | 1,384 | 61.5 | 3,103 | 15.0 | 4,487 | 19.5 | |||
| Clinical characteristics | |||||||||
| Hepatitis B virus infection | 101 | 4.4 | 1,030 | 4.9 | 1,131 | 4.8 | |||
| Diabetes mellitus | 163 | 7.1 | 1,030 | 4.9 | 1,193 | 5.1 | |||
| Hypertensiona | 370 | 24.2 | 3,450 | 23.2 | 3,820 | 23.3 | |||
| Statin use | 97 | 4.3 | 1,422 | 6.7 | 1,519 | 6.5 | |||
| Body mass indexa,b | 25.6 (5) | 25.9 (6) | 25.9 (5) | ||||||
| Total cholesterol levela | 159.5 (41) | 174.9 (46) | 173.4 (45) | ||||||
| HDL cholesterol level | 43.5 (17) | 42.1 (15) | 42.2 (15) | ||||||
| Triglyceride levela | 157.2 (124) | 170.2 (154) | 168.9 (151) | ||||||
| Fibrosis-4 index | |||||||||
| <1.45 | 1,113 | 49.5 | 16,420 | 80.5 | 17,533 | 77.5 | |||
| 1.45–3.25 | 765 | 34.0 | 3,314 | 16.3 | 4,079 | 18.0 | |||
| >3.25 | 370 | 16.5 | 654 | 3.2 | 1,024 | 4.5 | |||
| HIV-related factors | |||||||||
| CD4+ cell count nadir,cells/μL | |||||||||
| <100 | 556 | 24.4 | 5,330 | 25.2 | 5,886 | 25.1 | |||
| 100–199 | 421 | 18.5 | 2,972 | 14.1 | 3,393 | 14.5 | |||
| 200–349 | 559 | 24.5 | 4,956 | 23.5 | 5,515 | 23.6 | |||
| 350–499 | 389 | 17.1 | 3,732 | 17.7 | 4,121 | 17.6 | |||
| ≥500 | 355 | 15.6 | 4,137 | 19.6 | 4,492 | 19.2 | |||
| CD4+ cell count, cells/μL | 416.2 (274) | 439.1 (285) | 436.8 (284) | ||||||
| CD8+ cell count, cells/μLa | 907.8 (504) | 925.5 (509) | 923.8 (508) | ||||||
| CD4+:CD8+ cell count ratioa | |||||||||
| <0.4 | 716 | 44.5 | 6,396 | 41.7 | 7,112 | 42.0 | |||
| 0.4–1.0 | 699 | 43.4 | 6,914 | 45.1 | 7,613 | 44.9 | |||
| >1.0 | 194 | 12.1 | 2,031 | 13.2 | 2,225 | 13.1 | |||
| HIV viral load ≤400 copies/mLc | 1,321 | 58.1 | 12,607 | 60.4 | 13,928 | 60.2 | |||
| Receiving ARTd | 1,911 | 83.8 | 17,954 | 85.0 | 19,865 | 84.9 | |||
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CD4+, CD4-positive; CD8+, CD8-positive; CFAR, Centers for AIDS Research; CNICS, CFAR Network of Integrated Clinical Systems; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; SD, standard deviation.
a Using information from the 6 months before initiation of study follow-up for myocardial infarction, values for this variable were unknown for more than 5% of participants. Percentages shown are percentages of nonmissing values.
b Weight (kg)/height (m)2.
c Number of copies of HIV RNA per mL.
d Refers to ART at initiation of study follow-up for myocardial infarction.
Table 3.
Numbers of Type 2 Myocardial Infarctions Attributed to Various Causes Among Adults Living With HIV Who Were in Clinical Care at 6 CNICS Sites, United States, 1998–2016
| Attributed Cause of T2MI |
No. of T2MI Events
(n = 330) |
% |
|---|---|---|
| Sepsis/bacteremia | 118 | 35.8 |
| Stimulant-induced vasospasma | 37 | 11.2 |
| Hypertensive urgency/ emergency | 35 | 10.6 |
| Hypoxia or respiratory failure | 26 | 7.8 |
| Hypotensionb | 20 | 6.1 |
| Arrhythmia | 17 | 5.2 |
| Procedure-relatedc | 14 | 4.2 |
| Gastrointestinal bleeding | 11 | 3.3 |
| Seizure | 6 | 1.8 |
| Anemia | 5 | 1.5 |
| Malignancy/chemotherapy | 5 | 1.5 |
| Pneumonia | 5 | 1.5 |
| Heart failure | 4 | 1.2 |
| Rhabdomyolysis | 4 | 1.2 |
| Other rare causesd | 23 | 7.0 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CFAR, Centers for AIDS Research; CNICS, CFAR Network of Integrated Clinical Systems; HIV, human immunodeficiency virus; T2MI, type 2 myocardial infarction.
a Coronary arterial spasm secondary to the use of an illegal stimulant.
b Hypotension not attributed to sepsis, gastrointestinal bleeding, drug overdose, or other listed causes.
c Events occurring in the setting of noncardiac procedures.
d Causes to which 3 or fewer events were attributed, including overdose, pulmonary embolism, intracranial hemorrhage, pulmonary edema, anaphylaxis, disseminated intravascular coagulation, pericarditis, and diabetic ketoacidosis.
Using a composite MI outcome, chronic HCV infection was not associated with MI after adjustment for demographic characteristics, traditional cardiovascular disease risk factors, clinical characteristics, and history of injecting drug use (adjusted hazard ratio (aHR) = 1.20, 95% CI: 0.95, 1.51) (Figure 1). When considering each type of MI separately, chronic HCV infection was associated with a 46% higher risk of T2MI (aHR = 1.46, 95% CI: 1.09, 1.97) after adjustment for potential confounders, but HCV was not associated with T1MI (aHR = 0.87, 95% CI: 0.58, 1.29). In exploratory analyses examining adjudicated causes of T2MI, HCV was found to be associated with an approximately 2-fold greater risk of T2MI attributed to sepsis (aHR = 2.01, 95% CI: 1.25, 3.24) but was not associated with T2MI attributed to use of a stimulant (aHR = 1.03, 95% CI: 0.43, 2.45) or other causes (aHR = 1.26, 95% CI: 0.82, 1.94) after covariate adjustment (Figure 1). Because there were few T2MI events attributed to use of a stimulant, we assessed the stability of the model for this outcome by fitting a model that adjusted for age, sex, and history of injecting drug use; that model produced relatively consistent results (Web Table 2). The evidence did not support mediation of the HCV-T2MI association by liver fibrosis (assessed using the fibrosis-4 index) (Table 4).
Figure 1.
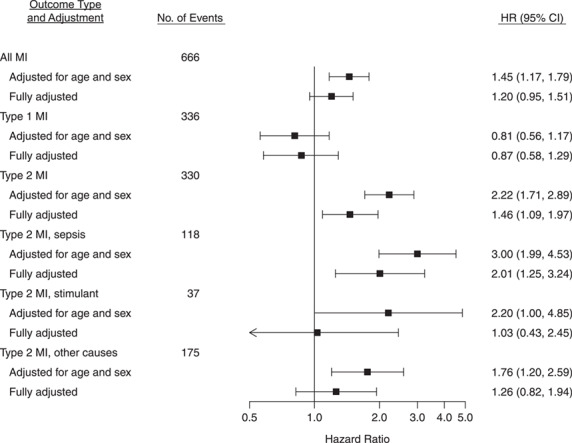
Hazard ratios (HRs) for the association between chronic hepatitis C virus infection and myocardial infarction (MI) outcomes among adults living with human immunodeficiency virus (HIV) at 6 sites in the CFAR Network of Integrated Clinical Systems, United States, 1998–2016. Fully adjusted models took into account age, sex at birth, race/ethnicity, study site, men who had sex with men, ever smoking, history of injecting drug use, diabetes status, statin use, hypertension, antihypertensive medication use, hepatitis B virus infection, antiretroviral therapy, CD4-positive cell count nadir, HIV viral load, body mass index, total cholesterol level, high-density lipoprotein cholesterol level, and triglyceride level. Bars, 95% confidence intervals (CIs). AIDS, acquired immunodeficiency syndrome; CFAR, Centers for AIDS Research.
Table 4.
Analysis of Mediation by Liver Fibrosisa of the Association Between Chronic Hepatitis C Virus Infection and Type 2 Myocardial Infarction Among Adults Living With HIV, United States, 1998–2016b
|
Association
Characteristic |
aHR c | 95% CI |
|---|---|---|
| Type of association | ||
| Natural indirect | 1.03 | 0.93, 1.18 |
| Natural direct | 1.40 | 1.32, 1.69 |
| Total | 1.44 | 1.23, 1.74 |
| % mediatedd | 10.3 | −16.7, 37.4 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus.
a The fibrosis-4 index (38) was used to determine liver fibrosis.
b Hazard ratios were estimated with a natural effects model (41) and adapted to the Cox proportional hazards regression setting as described by Lange et al. (42), considering fibrosis-4 index (as a continuous variable) a mediator. Multiple imputation was used, and parameters were pooled using Rubin’s rules (40).
c Adjusted hazard ratio for incident type 2 myocardial infarction among persons with chronic hepatitis C virus infection compared with those without evidence of chronic hepatitis C virus infection. Fully adjusted models took into account age, sex at birth, race/ethnicity, study site, men who had sex with men, ever smoking, history of injecting drug use, diabetes, statin use, hypertension, antihypertensive medication use, hepatitis B virus infection, antiretroviral therapy, CD4-positive cell count nadir, body mass index, total cholesterol level, high-density lipoprotein cholesterol level, and triglyceride level.
d Percentage of the association mediated by liver fibrosis.
In a sensitivity analysis, we found that there was no association between prior HCV infection without chronic HCV infection (antibody-positive, RNA-negative) and any MI outcome (Web Figure 1). We also found that history of injecting drug use was associated with an increased risk of T2MI after adjustment for age, sex, and race/ethnicity (aHR = 1.92, 95% CI: 1.53, 2.40).
DISCUSSION
In this cohort of PLWH selected from 6 CNICS sites across the United States, we found that HCV/HIV-coinfected persons experienced a greater risk of T2MI. On the basis of post hoc exploratory analysis, this finding appeared to be driven principally by sepsis-associated MI events. In contrast, there was no significant increase in the risk of having T1MI among coinfected persons.
In general, it is our findings with respect to T1MI that are likely to be most comparable to those of prior studies of this question, which have not found an association between chronic HCV infection and MI in the general population (30–33). Results of 2 prior studies carried out among PLWH were inconclusive. Bedimo et al. (44) reported a 25% higher risk of acute MI in US veterans coinfected with HCV and HIV than in those infected with HIV alone, but the association was not statistically significant, while investigators in the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study found no association between HCV seropositivity and MI (45). Although both of these studies ascertained composite MIs without reference to type, both defined MIs using administrative diagnosis codes. The proportion of MIs detected in prior studies that were T1MI versus T2MI is not known; however, the ability of administrative codes to detect T2MI seems generally poor (46). Therefore, prior evidence appears to concur with our finding that chronic HCV is not associated with classical, atheroembolic T1MI.
We observed a greater risk of T2MI associated with HCV and, more specifically, an approximately 2-fold greater risk of T2MI due to sepsis in persons coinfected with HCV and HIV than in those infected with HIV alone. This may be related to consequences of HCV infection, such as chronic inflammation, or to differences in sepsis risk factors between HCV-infected and -uninfected participants. While our results suggest a higher risk of T2MI attributed to sepsis in persons with evidence of chronic HCV infection, we did not detect a similar relationship when considering prior HCV infection without evidence of viremia. This lends credence to the hypothesis that ongoing chronic infection or associated biological factors (in addition to risk behaviors associated with HCV exposure) may play a role in the risk of T2MI resulting from sepsis or of sepsis, itself. A large US study of hemodialysis patients which found that the risk of bacteremia was greater in persons with HCV infection provides support for HCV as a risk factor for sepsis (47); and a landmark study, the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HCV (REVEAL-HCV) Study, found that HCV seropositivity was associated with a 50% higher risk of mortality due to circulatory diseases (48).
We did not find statistical support for mediation of the relationship between chronic HCV and T2MI by predicted severe liver fibrosis, suggesting that the impact of HCV on liver fibrosis plays, at most, a small role in the HCV-T2MI relationship observed here. We note, however, that patients with predicted severe fibrosis were uncommon in this cohort, and there were few MI events in that subgroup. In addition to overall replication of our findings, an ideal future study in this area would more comprehensively investigate the role of liver fibrosis in the relationship between HCV and cardiovascular disease outcomes, particularly in persons with advanced fibrosis. Bacterial infections and sepsis are known complications and major sources of morbidity for patients with cirrhosis (49).
Strengths of this study include adjudicated ascertainment of MI events, including typing, and extensive follow-up of participants, providing a wide range of information about potential confounders. HCV infection status in most prior studies of HCV and MI was defined on the basis of either seropositivity for anti-HCV antibodies or administrative diagnosis codes for HCV (30–33, 44, 45). These measures are likely to be less specific for chronic HCV infection than ascertainment based on an RNA test, as we performed here. One study of the general population in an Arkansas medical system found that participants with detectable HCV RNA had a greater incidence of coronary heart disease than those who tested positive for antibodies against HCV but did not have detectable HCV RNA (50). In combination with the findings presented here, this supports the use of HCV RNA testing to identify chronic HCV infection, something that should be highly feasible with the increasing uptake of nucleic acid–based tests for HCV in clinical practice (51).
We were unable to determine whether the observed relationship represents an association between HCV and sepsis incidence or rather an association between HCV and T2MI incidence in the setting of sepsis. This is an important area for future work. Because adjudication of causes of T2MI events must necessarily rely on expert judgment, some measurement error may have occurred when categorizing these complex medical events. Our study was also underpowered for assessment of causes of T2MI. We used HCV RNA testing performed in the course of routine clinical care, and therefore we potentially missed some cases of chronic HCV in persons who did not undergo RNA testing in that setting. Finally, because any study seeking to characterize the effects of HCV infection must be observational, the possibility of unmeasured and residual confounding cannot be completely excluded. In particular, we note that although information on history of injecting drug use was available for nearly all participants in this analysis, we did not have information on injecting drug use during follow-up. History of injecting drug use was associated with an increased risk of T2MI, highlighting the possibility that differences in substance use between participants with and without chronic HCV could explain some or all of the observed HCV-T2MI relationship. In addition, socioeconomic status and associated factors are important potential confounders that are not currently captured for participants in this cohort. Despite this, a rich database of clinical and patient-reported information, combined with analytical techniques accommodating missingness in covariates, enabled us to adjust for many important potential confounders.
In this large and diverse multicenter cohort of PLWH in the United States, we found that chronic HCV infection at the beginning of follow-up was not associated with incident classical atheroembolic MI (T1MI) but was associated with supply-demand mismatch MI (T2MI). These findings demonstrate the importance of examining MIs by type among PLWH and support increasing calls for broad-scale efforts to better understand the biological underpinnings of T2MI and ultimately identify effective management approaches (52). HCV care has been transformed by the recent development of safer, more effective direct-acting antiviral agents for HCV treatment (53); however, these agents are not yet uniformly available for all chronically infected patients (54). It is therefore important for the research community to establish the health risks associated with chronic HCV infection so that medical-care providers and payers can accurately weigh the costs and benefits of early treatment. Further research is needed to replicate these findings, elucidate biological mechanisms, and determine whether risk of sepsis and/or T2MI might be an important consideration for HCV treatment decisions in this high-burden population.
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Jessica Williams-Nguyen, Stephen E. Hawes, Sara Lindström, Susan R. Heckbert, Joseph A. Delaney); Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, Washington (Jessica Williams-Nguyen, Sara Lindström); Department of Medicine, School of Medicine, University of Washington, Seattle, Washington (Robin M. Nance, H. Nina Kim, Mari M. Kitahata, Heidi M. Crane); Center for AIDS Research, University of Washington, Seattle, Washington (H. Nina Kim, Mari M. Kitahata, Heidi M. Crane); Cardiovascular Health Research Unit, University of Washington, Seattle, Washington (Susan R. Heckbert, Joseph A. Delaney); Department of Medicine, School of Medicine, University of California, San Diego, La Jolla, California (W. Chris Mathews, Edward R. Cachay); Department of Medicine, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California (Matt Budoff); Institute for Global Health and Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Christopher B. Hurt); Division of Experimental Medicine, Department of Medicine, School of Medicine, University of California, San Francisco, San Francisco, California (Peter W. Hunt, Elvin Geng); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Richard D. Moore); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Richard D. Moore); Division of Infectious Diseases, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama (Michael J. Mugavero, Michael S. Saag); and Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, New York (Inga Peter).
This work was supported by the National Institutes of Health (grants R24 AI067039 (CNICS), R24S AI067039 (CNICS MI supplement), R01 HL126538, R01HL125027, U01AA020793, P30 AI027757 (University of Washington Center for AIDS Research), P30AI117943 (Third Coast Center for AIDS Research), U01DA037702, and U01AA020793) and the American Heart Association (grant 16FTF31200010).
Conflict of interest: none declared.
REFERENCES
- 1. Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antiretroviral Therapy Cohort Collaboration Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palella FJJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. [DOI] [PubMed] [Google Scholar]
- 5. Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366(9483):378–384. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan RC, Hanna DB, Kizer JR. Recent insights into cardiovascular disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep. 2016;13(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 8. Trickey A, May MT, Vehreschild J, et al. Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One. 2016;11(8):e0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer KH, Loo S, Crawford PM, et al. Excess clinical comorbidity among HIV-infected patients accessing primary care in US community health centers. Public Health Rep. 2018;133(1):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drozd DR, Kitahata MM, Althoff KN, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75(5):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crane HM, Paramsothy P, Drozd DR, et al. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol. 2017;2(3):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013;126(9):789–797. [DOI] [PubMed] [Google Scholar]
- 13. Javed U, Aftab W, Ambrose JA, et al. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol. 2009;104(1):9–13. [DOI] [PubMed] [Google Scholar]
- 14. Morrow DA, Wiviott SD, White HD, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009;119(21):2758–2764. [DOI] [PubMed] [Google Scholar]
- 15. Melberg T, Burman R, Dickstein K. The impact of the 2007 ESC-ACC-AHA-WHF Universal definition on the incidence and classification of acute myocardial infarction: a retrospective cohort study. Int J Cardiol. 2010;139(3):228–233. [DOI] [PubMed] [Google Scholar]
- 16. Stein GY, Herscovici G, Korenfeld R, et al. Type-II myocardial infarction—patient characteristics, management and outcomes. PLoS One. 2014;9(1):e84285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paiva LM, Providência R, Craveiro Barra SN, et al. Universal definition of myocardial infarction: clinical insights. Cardiology. 2015;131(1):13–21. [DOI] [PubMed] [Google Scholar]
- 18. Baron T, Hambraeus K, Sundstrom J, et al. Type 2 myocardial infarction in clinical practice. Heart. 2015;101(2):101–106. [DOI] [PubMed] [Google Scholar]
- 19. Meigher S, Thode HC, Peacock WF, et al. Causes of elevated cardiac troponins in the emergency department and their associated mortality. Acad Emerg Med. 2016;23(11):1267–1273. [DOI] [PubMed] [Google Scholar]
- 20. Szymanski FM, Karpinski G, Platek AE, et al. Clinical characteristics, aetiology and occurrence of type 2 acute myocardial infarction. Kardiol Pol. 2014;72(4):339–344. [DOI] [PubMed] [Google Scholar]
- 21. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275–295. [DOI] [PubMed] [Google Scholar]
- 22. Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–837. [DOI] [PubMed] [Google Scholar]
- 23. Tedaldi EM, Hullsiek KH, Malvestutto CD, et al. Prevalence and characteristics of hepatitis C virus coinfection in a human immunodeficiency virus clinical trials group: the Terry Beirn community programs for clinical research on AIDS. Clin Infect Dis. 2003;36(10):1313–1317. [DOI] [PubMed] [Google Scholar]
- 24. Kim HN, Crane HM, Rodriguez CV, et al. The role of current and historical alcohol use in hepatic fibrosis among HIV-infected individuals. AIDS Behav. 2017;21(7):1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. [DOI] [PubMed] [Google Scholar]
- 26. Spradling PR, Richardson JT, Buchacz K, et al. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996–2007. J Acquir Immune Defic Syndr. 2010;53(3):388–396. [DOI] [PubMed] [Google Scholar]
- 27. Babiker A, Jeudy J, Kligerman S, et al. Risk of cardiovascular disease due to chronic hepatitis C infection: a review. J Clin Transl Hepatol. 2017;5(4):343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambrosino P, Lupoli R, Di Minno A, et al. The risk of coronary artery disease and cerebrovascular disease in patients with hepatitis C: a systematic review and meta-analysis. Int J Cardiol. 2016;221:746–754. [DOI] [PubMed] [Google Scholar]
- 29. Osibogun O, Ogunmoroti O, Michos ED, et al. HIV/HCV co-infection and the risk of cardiovascular disease: a meta-analysis. J Viral Hepat. 2017;24(11):998–1004. [DOI] [PubMed] [Google Scholar]
- 30. Forde KA, Haynes K, Troxel AB, et al. Risk of myocardial infarction associated with chronic hepatitis C virus infection: a population-based cohort study. J Viral Hepat. 2012;19(4):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arcari CM, Nelson KE, Netski DM, et al. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43(6):e53–e56. [DOI] [PubMed] [Google Scholar]
- 32. Enger C, Forssen UM, Bennett D, et al. Thromboembolic events among patients with hepatitis C virus infection and cirrhosis: a matched-cohort study. Adv Ther. 2014;31(8):891–903. [DOI] [PubMed] [Google Scholar]
- 33. Momiyama Y, Ohmori R, Kato R, et al. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181(1):211–213. [DOI] [PubMed] [Google Scholar]
- 34. Butt AA, Yan P, Chew KW, et al. Risk of acute myocardial infarction among hepatitis C virus (HCV)-positive and HCV-negative men at various lipid levels: results from ERCHIVES. Clin Infect Dis. 2017;65(4):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lesko CR, Edwards JK, Cole SR, et al. When to censor? Am J Epidemiol. 2018;187(3):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crane HM, Heckbert SR, Drozd DR, et al. Lessons learned from the design and implementation of myocardial infarction adjudication tailored for HIV clinical cohorts. Am J Epidemiol. 2014;179(8):996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 39. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 40. Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 41. Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–195. [DOI] [PubMed] [Google Scholar]
- 42. Lange T, Hansen KW, Sørensen R, et al. Applied mediation analyses: a review and tutorial. Epidemiol Health. 2017;39:e2017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37(14):2252–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bedimo R, Westfall AO, Mugavero M, et al. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11(7):462–468. [DOI] [PubMed] [Google Scholar]
- 45. Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group, Weber R, Sabin C, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antivir Ther. 2010;15(8):1077–1086. [DOI] [PubMed] [Google Scholar]
- 46. Díaz-Garzón J, Sandoval Y, Smith SW, et al. Discordance between ICD-coded myocardial infarction and diagnosis according to the Universal Definition of Myocardial Infarction. Clin Chem. 2017;63(1):415–419. [DOI] [PubMed] [Google Scholar]
- 47. Chebrolu P, Colombo RE, Baer S, et al. Bacteremia in hemodialysis patients with hepatitis C. Am J Med Sci. 2015;349(3):217–221. [DOI] [PubMed] [Google Scholar]
- 48. Lee MH, Yang HI, Lu SN, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis. 2012;206(4):469–477. [DOI] [PubMed] [Google Scholar]
- 49. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28(1):26–42. [DOI] [PubMed] [Google Scholar]
- 50. Pothineni NV, Delongchamp R, Vallurupalli S, et al. Impact of hepatitis C seropositivity on the risk of coronary heart disease events. Am J Cardiol. 2014;114(12):1841–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brady JE, Vellozzi C, Hariri S, et al. Hepatitis C care cascade among persons born 1945–1965: 3 medical centers. Am J Manag Care. 2018;24(9):421–427. [PubMed] [Google Scholar]
- 52. McCarthy CP, Vaduganathan M, Januzzi JL Jr. Type 2 myocardial infarction—diagnosis, prognosis, and treatment. JAMA. 2018;320(5):433–434. [DOI] [PubMed] [Google Scholar]
- 53. Ahmed M. Era of direct acting anti-viral agents for the treatment of hepatitis C. World J Hepatol. 2018;10(10):670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong RJ, Jain MK, Therapondos G, et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol. 2018;113(9):1329–1338. [DOI] [PubMed] [Google Scholar]


