Abstract
Asthma and obesity are among the most prevalent chronic health conditions in children. Although there has been compelling evidence of co-occurrence of asthma and obesity, it is uncertain whether asthma contributes to the development of obesity or obesity contributes to the onset of asthma or both. In this study, we used a joint transition modeling approach with cross-lagged structure to understand how asthma and obesity influence each other dynamically over time. Subjects for this study included 5,193 kindergarten and first-grade students enrolled from 13 communities in 2002–2003 in the Southern California Children’s Health Study, with up to 10 years of follow-up. We found that nonobese children with diagnosed asthma at a study visit were at 37% higher odds of becoming obese by the next annual visit compared with children without asthma (odds ratio = 1.38; 95% credible interval: 1.12, 1.71). However, the presence of obesity at the current visit was not statistically significantly associated with asthma onset in the next visit (odds ratio = 1.25; 95% credible interval: 0.94, 1.62). In conclusion, childhood asthma appears to drive an increase in the onset of obesity among schoolchildren, while the onset of obesity does not necessarily imply the future onset of asthma, at least in the short term.
Keywords: asthma, joint transition modeling, obesity, schoolchildren
Abbreviations
- BMI
body mass index
- CHS
Southern California Children’s Health Study
- CrI
credible interval
- OR
odds ratio
Asthma and obesity are among the most common chronic health conditions in children, showing a parallel rise in prevalence over the last several decades (1, 2). In the United States, the prevalence of asthma during childhood increased from the early 1980s, and doubled from 3.6% in 1980 to 7.5% in the mid-1990s (3). Although childhood asthma prevalence increased more slowly from 2001–2010, the latest estimates by the Centers for Disease Control and Prevention showed that asthma prevalence has increased to 8.4% and over 6 million children under age 18 have been diagnosed with asthma (4). In the past 3 decades, the increases in obesity prevalence have been reported among all age groups in children (5). Especially among children aged 6–11 years, the prevalence of obesity tripled from 4.2% in early 1960s to 15.3% in the late 1990s (5). Despite previously reported declines in the prevalence of obesity among preschool-aged children (aged 2–5 years), in 2011–2014, 17% of children aged 2–19 years (about 12.7 million children) were classified as obese based on the Centers for Disease Control and Prevention criteria, with obesity prevalence being highest at ages 12–19 years (20.5%), followed by ages 6–11 years (17.5%) (6). The most recent study found that children aged 2–5 years had a significant increase in obesity prevalence since 2013–2014 (7).
A large body of evidence has shown that asthma and obesity can affect the life of children physically, emotionally, and socially (8, 9). Children with asthma often have higher risk of hospitalization and disability, and are more likely to depend on medication, have limitations in activities, and face numerous hardships in school life. Compared with normal-weight children, obese children have a higher lifetime prevalence of anxiety disorders and eating-related pathology (e.g., anorexia), and they have lower self-esteem and body satisfaction (8). Childhood obesity has been linked with numerous adverse medical conditions, such as type 2 diabetes, sleep apnea, and cardiovascular disease. In addition, obese children are more likely to be teased or bullied, and they face a variety of hardships, including discrimination and marginalization.
Although there has been compelling evidence of co-occurrence of asthma and obesity in children (10), it is uncertain whether one condition contributes to the onset of the other and/or vice versa. Numerous studies have shown that being obese is associated not only with increased risk of new asthma onset (11–18) but also with greater asthma severity (19–23). In apparent contrast to these results, recent findings support an etiologic role for childhood asthma in the onset of obesity. For example, Fletcher et al. (24) found substantial long-term impacts of childhood asthma on increases in obesity. Hossain et al. (25) employed piecewise linear mixed-effect model and showed that the development of asthma in children might be associated with accelerated weight gain, which eventually leads to the onset of obesity. Chen et al. (26) found that children with asthma had higher risk of obesity. However, the limitation of the current literature is that most studies focused only on a unidirectional relationship between asthma and obesity. To our knowledge, no studies have been reported that simultaneously evaluated the potential bidirectional relationship between these 2 conditions. Green (27) explored the relationship between childhood obesity and asthma using 2 separate models; he found that onset of asthma was related to subsequent weight gain over time, but there was no association between onset of overweight and the subsequent asthma onset in separate modeling within the same study.
In this study, we used a novel dynamic joint modeling approach to examine the bidirectional relationship between asthma and obesity over time in a cohort of school-aged children who participated in the Southern California Children’s Health Study (CHS). In essence, we estimated the bidirectional effects of current asthma and obesity status on the probability of becoming obese and having new-onset asthma at a subsequent annual school visit.
METHODS
Study population
The study population consisted of children in the CHS, which is one of the largest and most detailed studies of air pollution and the respiratory health of schoolchildren. Specifically, the main analyses were conducted on the most recent CHS population, called cohort E, which included 5,193 kindergarten and first-grade students enrolled in 2002–2003 from 13 Southern California communities. To assess the reproducibility of the main result, we also conducted a replication analysis using 2 earlier CHS cohorts (cohorts C and D) consisting of fourth-grade children enrolled in 1992–1993 and 1995–1996 from 12 southern California communities, 9 of which overlapped with the 13 communities in Cohort E. The replication samples included a total of 3,498 schoolchildren, of whom 1,642 were in cohort C, and 1,856 were in cohort D. The details of CHS study design for each cohort have been described previously (28–30). Written informed consent was provided by a parent or legal guardian for all study participants. The research protocol was reviewed and approved by the University of Southern California Health Campus Institutional Review Board.
Definitions of asthma and obesity
Enrolled children were examined annually or biannually during the follow-up periods. Asthma was defined based on answers to questionnaires. More specifically, in cohorts C and D, when a parent or legal guardian answered “yes” to the question “Has a doctor diagnosed your child with asthma?” in the baseline questionnaire, or the child answered “yes” to the question “Has a doctor ever said you had asthma?” in the annual questionnaires during study follow-up, the child was classified from then on as having asthma. In cohort E, questionnaires were completed by parents or legal guardians from baseline to year 5, and by children thereafter. At each study visit, asthma history was classified based on the response to the question “Has a doctor ever diagnosed this child as having asthma?” In all analyses, once asthma status was confirmed in a study visit, the child was classified as an asthma case during all the follow-up visits.
Trained technicians measured children’s heights and weights at each school study visit following a standardized protocol (31). These measurements of height and weight were used to calculate body mass index (BMI, calculated as weight (kg)/height (m)2). Obesity was defined as BMI at or above 95th percentile for children based on the age- and sex-specific BMI growth curve from the Centers for Disease Control and Prevention (32). We classified obesity status for each child based on the BMI measurements at each study visit.
Other covariates
In each cohort, the questionnaire completed at study entry by a child’s parent or legal guardian was used to obtain information on the children’s characteristics, such as sex, race/ethnicity, children’s insurance status, level of parental education, annual household income, exposure to maternal smoking in utero, and environmental tobacco smoke. The choice of using a Spanish questionnaire was treated as a surrogate measure of acculturation. Time-varying covariates were also included in the analysis. Physical activity was characterized based on the reported number of team sports played in the last 12 months. Medication use was determined based on the response to questions about rescue, controller, and other medication use for asthma or wheezing in the past 12 months. Bronchitis symptoms over the past 12 months was defined as bronchitis, a daily cough for 3 months in a row, or congestion/phlegm other than when accompanied by a cold. The details of the questions in the questionnaire used for the CHS study can be found in previous publications (28–30).
Statistical analyses
Descriptive and exploratory data analyses were conducted to examine the characteristics of the study population according to the combination of asthma and obesity status at baseline. We then summarized the observed number of transitions among the combinations of asthma and obesity status over the study period. We used a joint transition modeling approach with cross-lagged structure (Figure 1) to evaluate how asthma and obesity influenced each other dynamically over time (33). This statistical approach consisted of 2 modeling components: a structural model at baseline and a structural model for the follow-up period.
Figure 1.

Conceptual graph for joint transition modeling with cross-lagged structure. The ellipses in the figure stand for the time points during follow-up, which follow the same transition pattern as that from t = 1 to t = 2.
Structural models at baseline: for the asthma status variable,  ,
,
 |
(1) |
For the obesity status at baseline variable,  ,
,
 |
(2) |
Structural models at follow-up: for the asthma status variable, 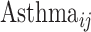 ,
,
 |
(3) |
For the obesity status variable at follow-up,  ,
,
 |
(4) |
where the vector  stands for the vector of adjusted confounders, and
stands for the vector of adjusted confounders, and  is subject-level random intercept to account for the within-subject correlation between asthma and obesity at baseline. Equations (1) and (2) model the cross-sectional association between asthma and obesity at baseline. The model given by equation (3) assesses the impact of obesity status at previous visit on the conditional probability of being asthmatic at current visit given asthma status at previous visit. The inclusion of the term
is subject-level random intercept to account for the within-subject correlation between asthma and obesity at baseline. Equations (1) and (2) model the cross-sectional association between asthma and obesity at baseline. The model given by equation (3) assesses the impact of obesity status at previous visit on the conditional probability of being asthmatic at current visit given asthma status at previous visit. The inclusion of the term  sets “asthma” as an absorbing status (i.e., once a child has physician-diagnosed asthma, he/she is assumed to be in an asthmatic state afterward). Equation (4) models the effect of asthma on obesity, where obesity status is not assumed to be an absorbing status. Both equations (3) and (4) are specified using first-order cross-lagged structure in order to estimate the associations with each other over time among 2 or more outcomes. The parameters of interest in the joint modeling framework are
sets “asthma” as an absorbing status (i.e., once a child has physician-diagnosed asthma, he/she is assumed to be in an asthmatic state afterward). Equation (4) models the effect of asthma on obesity, where obesity status is not assumed to be an absorbing status. Both equations (3) and (4) are specified using first-order cross-lagged structure in order to estimate the associations with each other over time among 2 or more outcomes. The parameters of interest in the joint modeling framework are  ,
,  , and
, and  . The parameter
. The parameter  captures the effect of obesity at previous visit on the probability of becoming asthmatic. The parameters
captures the effect of obesity at previous visit on the probability of becoming asthmatic. The parameters  and
and  measure the effect of asthma at the previous visit on the probabilities of remaining obese and becoming obese, respectively. The probability of remaining asthmatic is 1 because a child stays in the asthmatic category after the first report of physician-diagnosed asthma. Models adjusted for baseline child and home characteristics, such as age, sex, race/ethnicity, parent’s education, annual family income, child’s insurance status, exposure to maternal smoking in utero and environmental tobacco smoke, Spanish-language questionnaire indicator, and a fixed effect of the community of residence. Models additionally adjusted for time-dependent factors including physical activity and medication status. The missing-indicator approach was used to handle missing covariate information (34). We also tested for potential effect modification on the effect of asthma on obesity risk, which was found to be statistically significant in the main effects models. The potential effect modifiers we considered included sex, Hispanic ethnicity, medication use history, age at asthma diagnosis, bronchitis symptoms over the past 12 months, and physical activity. The statistical inference in the proposed joint modeling approach relied on Bayesian techniques using the Markov chain Monte Carlo algorithm for posterior computation. Following Garrett and Zeger’s recommendation (35), we assigned a normal prior with mean 0 and variance 2.25 for all regression coefficient parameters. The parameters in the models can be updated using a Metropolis-step or directly sampled from their posterior distributions. In the process of fitting all models, 2 chains of 100,000 iterations were run with every 50th iteration being saved after a burn-in of 50,000 iterations. The model convergence was assessed by the Gelman-Rubin statistic (36). The 95% credible intervals, using a highest posterior density approach, were reported to examine the null hypothesis on the regression coefficients. All of those computations can be implemented using WinBUGS (37) and statistical package R2Winbugs in statistical analysis software R (R Foundation for Statistical Computing, Vienna, Austria) (38). The main analyses were conducted using data from cohort E, and replication analyses were performed using data from combined cohorts C and D for examining the reproducibility of the results.
measure the effect of asthma at the previous visit on the probabilities of remaining obese and becoming obese, respectively. The probability of remaining asthmatic is 1 because a child stays in the asthmatic category after the first report of physician-diagnosed asthma. Models adjusted for baseline child and home characteristics, such as age, sex, race/ethnicity, parent’s education, annual family income, child’s insurance status, exposure to maternal smoking in utero and environmental tobacco smoke, Spanish-language questionnaire indicator, and a fixed effect of the community of residence. Models additionally adjusted for time-dependent factors including physical activity and medication status. The missing-indicator approach was used to handle missing covariate information (34). We also tested for potential effect modification on the effect of asthma on obesity risk, which was found to be statistically significant in the main effects models. The potential effect modifiers we considered included sex, Hispanic ethnicity, medication use history, age at asthma diagnosis, bronchitis symptoms over the past 12 months, and physical activity. The statistical inference in the proposed joint modeling approach relied on Bayesian techniques using the Markov chain Monte Carlo algorithm for posterior computation. Following Garrett and Zeger’s recommendation (35), we assigned a normal prior with mean 0 and variance 2.25 for all regression coefficient parameters. The parameters in the models can be updated using a Metropolis-step or directly sampled from their posterior distributions. In the process of fitting all models, 2 chains of 100,000 iterations were run with every 50th iteration being saved after a burn-in of 50,000 iterations. The model convergence was assessed by the Gelman-Rubin statistic (36). The 95% credible intervals, using a highest posterior density approach, were reported to examine the null hypothesis on the regression coefficients. All of those computations can be implemented using WinBUGS (37) and statistical package R2Winbugs in statistical analysis software R (R Foundation for Statistical Computing, Vienna, Austria) (38). The main analyses were conducted using data from cohort E, and replication analyses were performed using data from combined cohorts C and D for examining the reproducibility of the results.
RESULTS
The children’s characteristics at baseline in cohort E for main analyses are presented in Table 1. At study entry, 62.7% of the children were younger than 7 years old, sex was evenly distributed, and the majority of children were Hispanic white (55.6%). Of the 5,193 children at baseline, 72% had neither asthma nor obesity, while 2.9% had both conditions. Asthma only and obesity only were present among 12.9% and 12.2% of children, respectively. Children with both asthma and obesity had the highest prevalence of medication use (73.9%), secondhand-smoke exposure (8.5%), and in utero smoking exposure (12.4%). Children with obesity were more likely to be from a family with low income (65.5% and 64.1% for obese children without and with asthma, respectively) and have parental educational level at high school or below (79.8% and 80.4% for obese children without and with asthma, respectively). The lowest prevalence of child health insurance was observed among children with obesity only (77.9%). Physical activity and secondhand-smoke exposure were not statistically associated with asthma and obesity combined status at baseline.
Table 1.
Descriptive Summary for Covariates According to Asthma and Obesity Status at Baseline in Cohort E of the Children’s Health Study, California, 2003–2012
| Asthma and Obesity Status at Baseline | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total | Nonasthmatic, Nonobese | Asthmatic, Nonobese | Nonasthmatic, Obese | Asthmatic, Obese | P Value a | |||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Age, years | <0.0001 | ||||||||||
| <7 | 3,255 | 62.7 | 2,441 | 65.4 | 373 | 55.6 | 374 | 59 | 67 | 43.8 | |
| ≥7 | 1,938 | 37.3 | 1,294 | 34.6 | 298 | 44.4 | 260 | 41 | 86 | 56.2 | |
| Sex | <0.0001 | ||||||||||
| Female | 2,535 | 48.8 | 1,920 | 51.4 | 265 | 39.5 | 297 | 46.8 | 53 | 34.6 | |
| Male | 2,658 | 51.2 | 1,815 | 48.6 | 406 | 60.5 | 337 | 53.2 | 100 | 65.4 | |
| Ethnicity | <0.0001 | ||||||||||
| Non-Hispanic white | 1,642 | 31.6 | 1,266 | 33.9 | 236 | 35.2 | 107 | 16.9 | 33 | 21.6 | |
| Hispanic white | 2,885 | 55.6 | 2,019 | 54.1 | 316 | 47.1 | 456 | 71.9 | 94 | 61.4 | |
| Other | 666 | 12.8 | 450 | 12 | 119 | 17.7 | 71 | 11.2 | 26 | 17 | |
| No. of team sports | 0.611 | ||||||||||
| 0–1 | 4,063 | 78.2 | 2,945 | 78.8 | 510 | 76 | 495 | 78.1 | 113 | 73.9 | |
| >1 | 746 | 14.4 | 536 | 14.4 | 103 | 15.4 | 83 | 13.1 | 24 | 15.7 | |
| Missing | 384 | 7.4 | 254 | 6.8 | 58 | 8.6 | 56 | 8.8 | 16 | 10.5 | |
| Spanish questionnaire use | <0.0001 | ||||||||||
| Yes | 1,288 | 24.8 | 922 | 24.7 | 83 | 12.4 | 248 | 39.1 | 35 | 22.9 | |
| No | 3,905 | 75.2 | 2,813 | 75.3 | 588 | 87.6 | 386 | 60.9 | 118 | 77.1 | |
| Medication use | <0.0001 | ||||||||||
| Yes | 796 | 15.3 | 212 | 5.7 | 430 | 64.1 | 41 | 6.5 | 113 | 73.9 | |
| No | 4,397 | 84.7 | 3,523 | 94.3 | 241 | 35.9 | 593 | 93.5 | 40 | 26.1 | |
| Parental education | <0.0001 | ||||||||||
| High school or below | 3,932 | 75.7 | 2,805 | 75.1 | 498 | 74.2 | 506 | 79.8 | 123 | 80.4 | |
| Beyond high school | 996 | 19.2 | 756 | 20.2 | 139 | 20.7 | 80 | 12.6 | 21 | 13.7 | |
| Missing | 265 | 5.1 | 174 | 4.7 | 34 | 5.1 | 48 | 7.6 | 9 | 5.9 | |
| Annual family income, $ | <0.0001 | ||||||||||
| <50,000 | 3,123 | 60.1 | 2,221 | 59.5 | 389 | 58 | 415 | 65.5 | 98 | 64.1 | |
| ≥50,000 | 1,248 | 24 | 939 | 25.1 | 179 | 26.7 | 95 | 15 | 35 | 22.9 | |
| Missing | 822 | 15.8 | 575 | 15.4 | 103 | 15.4 | 124 | 19.6 | 20 | 13.1 | |
| Child had health insurance | <0.0001 | ||||||||||
| Yes | 4,329 | 87.5 | 3,110 | 83.3 | 593 | 88.4 | 494 | 77.9 | 132 | 86.3 | |
| No | 617 | 12.5 | 469 | 12.6 | 46 | 6.9 | 90 | 14.2 | 12 | 7.8 | |
| Secondhand smoke | 0.908 | ||||||||||
| Yes | 390 | 7.5 | 275 | 7.4 | 52 | 7.7 | 50 | 7.9 | 13 | 8.5 | |
| No | 4,735 | 91.2 | 3,414 | 91.4 | 609 | 90.8 | 574 | 90.5 | 138 | 90.2 | |
| Missing | 68 | 1.3 | 46 | 1.2 | 10 | 1.5 | 10 | 1.6 | 2 | 1.3 | |
| In utero smoking exposure | 0.002 | ||||||||||
| Yes | 397 | 8 | 282 | 7.6 | 64 | 9.5 | 32 | 5 | 19 | 12.4 | |
| No | 4,569 | 92 | 3,296 | 88.2 | 573 | 85.4 | 572 | 90.2 | 128 | 83.7 | |
a χ2 test.
The descriptive summary of children’s characteristics at baseline for cohorts C and D (used in replication analysis) is presented in Web Table 1, available at https://academic.oup.com/aje. Unlike cohort E, non-Hispanic white was the major ethnic group (56.4%), and the proportion of children using the Spanish questionnaire was therefore much lower. Sex, ethnicity, Spanish questionnaire use, medication use, annual family income, and child insurance status were significantly associated with asthma and obesity combined status at baseline. However, physical activity, secondhand-smoke exposure, age, in utero smoking exposure, and parental education were not statistically significantly associated with asthma and obesity combined status at baseline.
The observed number of transitions among the states defined jointly by asthma and obesity status through the study period in Cohort E are summarized in Table 2. We found that children with asthma only (but not obesity) at a given visit had the highest likelihood of staying in the same state in the next visit (93.7%), and those with obesity only had the lowest (80.8%). Children with neither asthma nor obesity were more likely to move to the obesity-only state in the next visit (4.9%). It was very rare for children to develop asthma and become obese simultaneously during the next visit (0.2%). Irrespective of the obesity status at current visit, 3.5% of children without asthma were estimated to develop asthma. Among children with obesity, 17.2% and 14.9% were estimated to become nonobese in the next visit among the children with and without asthma, respectively. In Web Table 2, the similar summary is shown for cohorts C and D. The overall transition pattern in these 2 cohorts was very similar to that in cohort E except that the children seemed to be more likely to stay in the same states from visit to visit.
Table 2.
Observed Number of Transitions in Asthma and Obesity Status During Follow-up of Cohort E in the Children’s Health Study, California, 2003–2012
| State in Current Visit | ||||||||
|---|---|---|---|---|---|---|---|---|
| State in Previous Visit | Nonasthmatic, Nonobese | Asthmatic, Nonobese | Nonasthmatic, Obese | Asthmatic, Obese | ||||
| No. | % a | No. | % a | No. | % a | No. | % a | |
| Nonasthmatic, nonobese | 8,539 | 91.4 | 330 | 3.5 | 459 | 4.9 | 19 | 0.2 |
| Asthmatic, nonobese | 2,153 | 93.7 | 144 | 6.3 | ||||
| Nonasthmatic, obese | 302 | 14.9 | 15 | 0.7 | 1,642 | 80.8 | 72 | 3.5 |
| Asthmatic, obese | 114 | 17.2 | 547 | 82.8 | ||||
a Row percentage.
Results from the joint transition modeling with cross-lagged structure, which allowed us to simultaneously examine the bidirectional effects of asthma and obesity, are shown in Table 3. In cohort E, we found that children with diagnosed asthma at a given study visit were at 38% higher odds of becoming obese by the next annual visit compared with children without asthma (odds ratio (OR) = 1.38, 95% credible interval (CrI): 1.12, 1.71). Although the presence of obesity at the current visit was positively associated with the probability of becoming asthmatic in the next visit, this observed association was not statistically significant (OR = 1.25, 95% CrI: 0.94, 1.62). Additionally, physician-diagnosed asthma at a given visit was not statistically significantly associated with the probability of remaining obese in the next visit (OR = 1.04, 95% CrI: 0.81, 1.37). Similar results were observed in cohorts C and D. We found that children with diagnosed asthma at a study visit were at 42% higher odds of becoming obese by the next annual visit compared with children without asthma (OR = 1.42, 95% CrI: 1.09, 1.84). Neither the obesity effect on the probability of becoming asthmatic (OR = 0.96, 95% CrI: 0.05, 17.31) nor the asthma effect on the probability of remaining obese (OR = 0.94, 95% CrI: 0.7, 1.29) were statistically significant.
Table 3.
Estimation of Dynamic Relationship Between Asthma and Obesity in Cohorts E (2003–2012) and C (1993–2002) and D (1995–2006) of the Children’s Health Study, California
| Bidirectional Effects of Asthma and Obesitya | ||||
|---|---|---|---|---|
| Probability of Interest | Asthma | Obesity | ||
| OR | 95% CrI | OR | 95% CrI | |
| Cohort E | ||||
| Becoming asthmatic | 1.25 | 0.94, 1.62 | ||
| Becoming obese | 1.38 | 1.12, 1.71 | ||
| Remaining obese | 1.04 | 0.81, 1.37 | ||
| Cohorts C and D | ||||
| Becoming asthmatic | 0.96 | 0.05, 17.31 | ||
| Becoming obese | 1.42 | 1.09, 1.84 | ||
| Remaining obese | 0.94 | 0.70, 1.29 | ||
Abbreviations: CrI, credible interval; OR, odds ratio.
a Adjustments included age, sex, race/ethnicity, parent’s education, annual family income, child’s insurance status, exposure to maternal smoking in utero and environmental tobacco smoke, Spanish-language questionnaire indicator, the community of residence, physical activity, and medication status.
We found little evidence to support any heterogeneity in the effects of asthma on the probability of becoming obese in the next visit (Table 4). The estimated interactions between asthma status and all potential effect modifiers (such as sex) were all statistically nonsignificant (i.e., all 95% CrIs included zero).
Table 4.
Estimated Effect of Potential Modifier on the Asthma Effect on the Probability of Becoming Obese in Cohort E of the Children’s Health Study, California, 2003–2012
| Modifier | Interaction Effects Between Asthma and Modifier on the Probability of Becoming Obese a | ||
|---|---|---|---|
| Estimate | Lower Limit | Upper Limit | |
| Sex | 0.01 | −0.35 | 0.39 |
| Hispanic | 0.13 | −0.22 | 0.48 |
| Team sports | −0.11 | −0.57 | 0.36 |
| Medication use | −0.13 | −0.55 | 0.30 |
| Bronchitis symptoms over the past 12 months | 0.3 | −0.13 | 0.73 |
| Age at asthma onset | 0.13 | −0.25 | 0.49 |
a Adjustments included age, sex, race/ethnicity, parent’s education, annual family income, child’s insurance status, exposure to maternal smoking in utero and environmental tobacco smoke, Spanish-language questionnaire indicator, the community of residence, physical activity, and medication status.
DISCUSSION
In this study, we applied a flexible joint transition modeling approach to understand the dynamic and mutual relationships between asthma and obesity during childhood. Our results using this approach provide evidence that childhood asthma drives obesity development, whereas obesity conferred no significantly increased risk for asthma development. We replicated these results in separate (but identically designed) cohorts (cohorts C and D) from the Children’s Health Study.
The joint transition modeling approach not only accounts for the temporal relationship between obesity and asthma observations in the longitudinal data set but also relaxes the strong unidirectional presumption by allowing a bidirectional relationship between asthma and obesity. Our results are consistent with findings from previous studies in children (26, 27) and adults (24, 39) showing that early-life asthma history is a strong risk factor for obesity development in later life. Hossain et al. (25) used novel linear piecewise mixed-effects models and detected differential weight-gain patterns before and after asthma diagnosis. Colak et al. (40) conducted a Mendelian randomization study and found causal effects of BMI on the risk of wheezing but not on the risk of asthma. Although the mechanism linking asthma history to later development of obesity is unclear, hypothesized pathways include asthma-related characteristics such as shared prenatal exposures (41), gestational age, reduced physical activity (42, 43), perturbation in sleep (44, 45), chronic medication use (46), imbalance of autonomic nervous system (47, 48), chronic inflammatory disorder (49, 50), and potential metabolic disorder (51–53) (e.g., insulin and leptin resistance). All these lifestyle and biological changes are well-known risk factors for obesity. Besides these factors, common genetic (e.g., β2-adreneregic receptor (ADRB2), leptin (LEP), and tumor necrosis factor-α (TNFα)) and environmental risk factors (e.g., stress and maternal smoking in utero) could also contribute to the causal effect of early-life asthma on the risk of developing obesity (54–58). Therefore, future studies are needed to identify the key pathways linking asthma to obesity onset.
Although we did not find significant association between obesity history and asthma development in later life, the association estimate in our study is positive in one cohort but not in the replication cohort. A large body of literature exists on the deleterious effect of obesity in the form of increased risk of developing asthma (11–23, 59, 60). However, there is limited evidence to draw causal relationship between obesity and asthma. Granell et al. (61) used Mendelian randomization and found causal effects of BMI, fat mass, and lean mass at age 7 years on current asthma at age 7.5 years. However, in this study, current asthma was based on parental report of asthma or symptoms of wheezing or treatment for asthma in the previous 12 months at age 7.5 years. Therefore, the temporal precedence criteria might have been because the observed current asthma could have occurred prior to BMI measurement. In our study, we used a novel joint modeling approach to investigate the dynamic relationship between asthma and obesity during childhood, and we found that asthma is a strong predictor for obesity development, while obesity might not be a significant indicator for asthma development. The nonsignificant association between early-life obesity and the risk of developing asthma in later childhood might be due to the relatively lower prevalence of asthma compared with obesity in the general population. It should be also noted that our study sample is school-aged children. Because the etiology of childhood-onset and adult-onset asthma could be different (62, 63), our results do not preclude the hypothesis that obesity increases the risk of asthma onset in adults.
The strengths of the present study include the population-based prospective cohort design, a large and diverse sample, and a large number of BMI and asthma measurements over a long follow-up period. Using a joint transition modeling approach with cross-lagged structure provided information about the dynamic bidirectional relationship between asthma and obesity, and it allowed us to simultaneously estimate which condition contributes to the onset of the other condition. Additionally, we observed that the effect of asthma status on the elevated risk of becoming obese was reproducible in a separate cohort.
Some potential limitations require attention. Asthma was defined based on self-report questionnaire data and might be prone to misclassification due to recall bias. This misclassification might be differential and might lead to bias toward the null (64). However, this misclassification should be limited based on our previous review of medical records (65). In addition, although we controlled for several previously identified confounders in the analysis, the observed associations might have been influenced by some unmeasured confounders, such as dietary information. Effects of such unmeasured confounders in our observational study setting limit our ability to reach a causal conclusion from our observed results. Finally, the lack of significant findings in some of the subgroups might be due to the lack of statistical power. A future cohort study using larger sample size is warranted.
In conclusion, childhood asthma drives an increase in the onset of obesity among schoolchildren, but the onset of obesity does not necessarily imply future onset of asthma, at least in the short term. This insight into the relationship between asthma and obesity suggests that early intervention among children with asthma for obesity prevention should be tested for use in the clinical practice. The joint transition modeling approach with cross-lagged structure disentangles the temporal relationship between asthma and obesity, and allows us to simultaneously explore how asthma and obesity influence each other dynamically over time. Our study illustrates the advantage of using this class of joint modeling approach in observational studies to provide novel insights into the dynamic relationship among multiple outcomes of interest.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Internal Medicine, Division of Epidemiology, School of Medicine, University of Utah, Salt Lake City, Utah (Yue Zhang); Department of Family and Preventive Medicine, School of Medicine, University of Utah, Salt Lake City, Utah (Yue Zhang); Veteran Affairs Salt Lake City Health Care System, Salt Lake City, Utah (Yue Zhang); and Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Zhanghua Chen, Kiros Berhane, Robert Urman, Vaia Lida Chatzi, Carrie Breton, and Frank D. Gilliland).
This work was supported by the Office of the Director of the National Institutes of Health (grants UG3OD023287 and UG3OD023249), the Southern California Environmental Health Sciences Center (grant P30ES007048) funded by the National Institute of Environmental Health Sciences, the Children’s Environmental Health Center (grants P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency, the Maternal and Developmental Risks from Environmental and Social Stressors Center (grants P50ES026086, 83615801-0) funded by the National Institute of Environmental Health Sciences, the National Institute for Minority Health and Health Disparities, and the Hastings Foundation.
We thank the school principals, teachers, students, and parents in each of the study communities for their cooperation and especially the members of the field team for their efforts. We also thank Ed Rappaport for excellent data management.
Conflict of interest: none declared.
REFERENCES
- 1. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. [DOI] [PubMed] [Google Scholar]
- 2. Perrin JM, Bloom SR, Gortmaker SL. The increase of childhood chronic conditions in the United States. JAMA. 2007;297(24):2755–2759. [DOI] [PubMed] [Google Scholar]
- 3. Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1):e20152354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moorman JF, Akinbami LJF, Bailey CM, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;35:1–11. [PubMed] [Google Scholar]
- 5. Cheung PC, Cunningham SA, Narayan KM, et al. Childhood obesity incidence in the United States: a systematic review. Child Obes. 2016;12(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skinner AC, Ravanbakht SN, Skelton JA, et al. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3):e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahoo K, Sahoo B, Choudhury AK, et al. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4(2):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Bemt L, Kooijman S, Linssen V, et al. How does asthma influence the daily life of children? Results of focus group interviews. Health Qual Life Outcomes. 2010;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med. 2013;107(9):1287–1300. [DOI] [PubMed] [Google Scholar]
- 11. Chen YC, Liou TH, Chen PC, et al. Growth trajectories and asthma/rhinitis in children: a longitudinal study in Taiwan. Eur Respir J. 2017;49:1600741. [DOI] [PubMed] [Google Scholar]
- 12. Gilliland FD, Berhane K, Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158(5):406–415. [DOI] [PubMed] [Google Scholar]
- 13. Gold DR, Damokosh AI, Dockery DW, et al. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36(6):514–521. [DOI] [PubMed] [Google Scholar]
- 14. Hancox RJ, Milne BJ, Poulton R, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005;171(5):440–445. [DOI] [PubMed] [Google Scholar]
- 15. Jeong Y, Jung-Choi K, Lee JH, et al. Body weight at birth and at age three and respiratory illness in preschool children. J Prev Med Public Health. 2010;43(5):369–376. [DOI] [PubMed] [Google Scholar]
- 16. Mannino DM, Mott J, Ferdinands JM, et al. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY). Int J Obes (Lond). 2006;30(1):6–13. [DOI] [PubMed] [Google Scholar]
- 17. Rzehak P, Wijga AH, Keil T, et al. Body mass index trajectory classes and incident asthma in childhood: results from 8 European birth cohorts—a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013;131(6):1528–1536. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z, Lai HJ, Roberg KA, et al. Early childhood weight status in relation to asthma development in high-risk children. J Allergy Clin Immunol. 2010;126(6):1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black MH, Zhou H, Takayanagi M, et al. Increased asthma risk and asthma-related health care complications associated with childhood obesity. Am J Epidemiol. 2013;178(7):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castro-Rodriguez JA, Holberg CJ, Morgan WJ, et al. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163(6):1344–1349. [DOI] [PubMed] [Google Scholar]
- 21. Mamun AA, Lawlor DA, Alati R, et al. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: evidence from a birth cohort study. Int J Obes (Lond). 2007;31(4):578–583. [DOI] [PubMed] [Google Scholar]
- 22. Taveras EM, Rifas-Shiman SL, Camargo CA Jr, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J Allergy Clin Immunol. 2008;121(5):1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tollefsen E, Langhammer A, Romundstad P, et al. Female gender is associated with higher incidence and more stable respiratory symptoms during adolescence. Respir Med. 2007;101(5):896–902. [DOI] [PubMed] [Google Scholar]
- 24. Fletcher JM, Green JC, Neidell MJ. Long term effects of childhood asthma on adult health. J Health Econ. 2010;29(3):377–387. [DOI] [PubMed] [Google Scholar]
- 25. Hossain MJ, Xie L, Lang JE, et al. Piecewise mixed effects model to compare the weight-gain patterns before and after diagnosis of asthma in children younger than 5 years. J Biom Biostat. 2015;6(4):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Z, Salam MT, Alderete TL, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. 2017;195(9):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green TL. Examining the temporal relationships between childhood obesity and asthma. Econ Hum Biol. 2014;14:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peters JM, Avol E, Gauderman WJ, et al. A study of twelve Southern California communities with differing levels and types of air pollution II. Effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–775. [DOI] [PubMed] [Google Scholar]
- 30. Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159(3):760–767. [DOI] [PubMed] [Google Scholar]
- 31. Jerrett M, McConnell R, Chang CC, et al. Automobile traffic around the home and attained body mass index: a longitudinal cohort study of children aged 10–18 years. Prev Med. 2010;50(suppl 1):S50–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuczmarski RJ, Ogden CLF, Guo SSF, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 3. 2002;246:1–190. [PubMed] [Google Scholar]
- 33. Zhang Y, Berhane K. Dynamic latent trait models with mixed hidden Markov structure for mixed longitudinal outcomes. J Appl Stat. 2016;43(4):704–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Groenwold RH, White IR, Donders AR, et al. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garrett ES, Zeger SL. Latent class model diagnosis. Biometrics. 2000;56(4):1055–1067. [DOI] [PubMed] [Google Scholar]
- 36. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7(4):457–472. [Google Scholar]
- 37. Spiegelhalter DJ, Thomas A, Best N, et al. WinBUGS User Manual, Version 1.4. Cambridge, UK: MRC Biostatistics Unit; 2003. [Google Scholar]
- 38. Team RDC R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation For Statistical Computing; 2014. [Google Scholar]
- 39. Hasler G, Gergen PJ, Ajdacic V, et al. Asthma and body weight change: a 20-year prospective community study of young adults. Int J Obes (Lond). 2006;30(7):1111–1118. [DOI] [PubMed] [Google Scholar]
- 40. Colak Y, Afzal S, Lange P, et al. Obese individuals experience wheezing without asthma but not asthma without wheezing: a Mendelian randomisation study of 85,437 adults from the Copenhagen General Population Study. Thorax. 2016;71(3):247–254. [DOI] [PubMed] [Google Scholar]
- 41. Perzanowski MS, Chew GL, Divjan A, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131(3):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ritz T, Rosenfield D, Steptoe A. Physical activity, lung function, and shortness of breath in the daily life of individuals with asthma. Chest. 2010;138(4):913–918. [DOI] [PubMed] [Google Scholar]
- 43. Firrincieli V, Keller A, Ehrensberger R, et al. Decreased physical activity among Head Start children with a history of wheezing: use of an accelerometer to measure activity. Pediatr Pulmonol. 2005;40(1):57–63. [DOI] [PubMed] [Google Scholar]
- 44. Ginis T, Akcan FA, Capanoglu M, et al. The frequency of sleep-disordered breathing in children with asthma and its effects on asthma control. J Asthma. 2017;54(4):403–410. [DOI] [PubMed] [Google Scholar]
- 45. van Maanen A, Wijga AH, Gehring U, et al. Sleep in children with asthma: results of the PIAMA study. Eur Respir J. 2013;41(4):832–837. [DOI] [PubMed] [Google Scholar]
- 46. Schwarzer G, Bassler D, Mitra A, et al. Ketotifen alone or as additional medication for long-term control of asthma and wheeze in children. Cochrane Database Syst Rev. 2004;1:CD001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gomes EL, Sampaio LM, Costa IP, et al. Analysis of autonomic modulation during maximal and submaximal work rate and functional capacity in asthmatic children. J Asthma. 2013;50(6):613–618. [DOI] [PubMed] [Google Scholar]
- 48. Lutfi MF. Patterns of heart rate variability and cardiac autonomic modulations in controlled and uncontrolled asthmatic patients. BMC Pulm Med. 2015;15:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wouters EF, Reynaert NL, Dentener MA, et al. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: is there a connection? Proc Am Thorac Soc. 2009;6(8):638–647. [DOI] [PubMed] [Google Scholar]
- 50. Jenni R, Ritter M, Vieli A, et al. Determination of the ratio of pulmonary blood flow to systemic blood flow by derivation of amplitude weighted mean velocity from continuous wave Doppler spectra. Br Heart J. 1989;61(2):167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arshi M, Cardinal J, Hill RJ, et al. Asthma and insulin resistance in children. Respirology. 2010;15(5):779–784. [DOI] [PubMed] [Google Scholar]
- 52. Forno E, Han YY, Muzumdar RH, et al. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsaroucha A, Daniil Z, Malli F, et al. Leptin, adiponectin, and ghrelin levels in female patients with asthma during stable and exacerbation periods. J Asthma. 2013;50(2):188–197. [DOI] [PubMed] [Google Scholar]
- 54. Danielewicz H. What the genetic background of individuals with asthma and obesity can reveal: is beta2-adrenergic receptor gene polymorphism important? Pediatr Allergy Immunol Pulmonol. 2014;27(3):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oren E, Gerald L, Stern DA, et al. Self-reported stressful life events during adolescence and subsequent asthma: a longitudinal study. J Allergy Clin Immunol Pract. 2017;5(2):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Research. 2016;2:00042-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riedel C, Schönberger K, Yang S, et al. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared with paternal smoking—a meta-analysis. Int J Epidemiol. 2014;43(5):1593–1606. [DOI] [PubMed] [Google Scholar]
- 58. Bose M, Oliván B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mohanan S, Tapp H, McWilliams A, et al. Obesity and asthma: pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med. 2014;239(11):1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scholtens S, Wijga AH, Seidell JC, et al. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. J Allergy Clin Immunol. 2009;123(6):1312–1318. [DOI] [PubMed] [Google Scholar]
- 61. Granell R, Henderson AJ, Evans DM, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11(7):e1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013;22(127):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ségala C, Priol G, Soussan D, et al. Asthma in adults: comparison of adult-onset asthma with childhood-onset asthma relapsing in adulthood. Allergy. 2000;55(7):634–640. [DOI] [PubMed] [Google Scholar]
- 64. Zhang Y, Berhane K. Bayesian mixed hidden Markov models: a multi-level approach to modeling categorical outcomes with differential misclassification. Stat Med. 2014;33(8):1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salam MT, Li YF, Langholz B, et al. Early-life environmental risk factors for asthma: findings from the Children's Health Study. Environ Health Perspect. 2004;112(6):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


