Abstract
Ethnopharmacological relevance
Herba Patriniae has been used for thousands of years in China as a traditional Chinese medicine with heat-clearing and detoxicating effects. It is applied widly for the treatment of rheumatoid arthritis, diarrhea, acute hepatitis, pelvic inflammatory disease and ulcerative colitis in clinic. Two species, namely Patrinia scabiosaefolia Fisch. (PS) and Patrinia villosa Juss. (PV) from the Caprifoliaceae family, are considered as Herba Patriniae in the pharmaceutical industry.
Aim of the review
This paper aims to comprehensively outline the traditional uses, botanical description, phytochemistry, pharmacology, toxicology, quality control, pharmacokinetics and patents of Herba Patriniae, and elaborate the same/different characteristics between PS and PV.
Materials and methods
Detailed information of Herba Patriniae was collected from various online databases (Pubmed, Web of Science, Google Schola, China National Knowledge Infrastructure Database, National Intellectual Property Administration, PRC National Medical Products Administration), and those published resources (M.Sc. Thesis and books).
Results
A total of 233 compounds have been identified in Herba Patriniae, including triterpenoid saponins, flavonoids, organic acids, iridoids, and volatiles. A very distinct difference was observed, that PS is rich in triterpenoid saponins and volatiles, while PV contains more flavonoids. Two source species of Herba Patriniae gave similar pharmacological effects on anti-cancer, anti-inflammatory, antioxidant, antimicrobial, sedative and hypnotic effects. But there were no reports were on antipruritic, proangiogenic and anti-diarrheal effects for PS, and no studies on anti-diabetic effects for PV. Generally, Herba Patriniae showed non-toxic in the clinical dose, but mild side effects, such as temporary leukopenia, dizziness and nausea, could be found when large and excessive dosage is used. A variety of compounds have been quantified for the quality control of PS and PV. The variety, growth environment, growth time, and harvest time not only affected the contents but also the pharmacological activities of the bioactive compounds. In the past year, patents for compositions containing PV and PS have been filed, mainly involving human health, hygiene, agriculture, and animal husbandry. Unfortunately, the research on pharmacokinetics is insufficient. Only the prototype components and metabolites were repored after intragastric administration of total flavonoids extract from PV in rats.
Conclusion
Herba Patriniae has displayed a significant medicinal value in clinic, but the differences in phytochemistry, pharmacological effects and the content of compounds have been found between two official recorded species. About side effects and pharmacokinetic characteristics, the differences between two species have not been well studied. For a better clinical use of Herba Patriniae, it is urgent to establish systematic pharmacology, quality control, pharmacokinetics, and clinical researches on the same/different characteristics between PS and PV.
Keywords: Herba patriniae, Traditional uses, Phytochemistry, Pharmacology, Quality control
Graphical abstract
List of abbreviations
- 3T3-L1
preadipocytes
- 5-FU/HCT-8
human ileocecal adenocarcinoma cells
- A2780
human ovarian cancer cells
- A375-S2
human melanoma cells
- A498
human renal carcinoma cells
- A549
human lung cancer cells
- ABTS+
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- AGS
human gastric cancer cells
- AKT
protein kinase B
- ALT
alanine aminotransferase
- AP
acute pancreatitis
- AP3
polysaccharide mixture
- AST
aspartate aminotransferase
- BAX
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma-2
- Bcl-xL
B cell lymphoma factor XL
- BEL-7402
human hepatoma cells
- BV-2
mouse microglia cells
- Caco2
human colon cancer cells
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- DAI
disease activity index
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- EC50
half maximal effective concentration
- EMT
epithelial-mesenchymal transition
- FAK
focal adhesion kinase
- GC-MS
gas chromatography-mass spectrometer
- GLUT4
glucose transporter 4
- GSH
glutathione
- H2O2
hydrogen peroxide
- HeLa
human cervical cancer cells
- HepG2
human hepatoma cells
- HL-60
human promyelocytic leukemia cells
- HO-1
heme oxygenase-1
- HPLC
high performance liquid chromatography
- HSP 60
heat shock proteins 60
- HSP 72
heat shock proteins 72
- HT1080
human fibrosarcoma cells
- HT-29
human colon carcinoma cells
- HUVECs
human umbilical vein endothelial cells
- IC50
50% inhibitory concentration
- ICAM-1
intercellular adhesion molecule 1
- ICR
institute of cancer research
- IL-1β
interleukin-1 beta
- IL-6
interleukin 6
- IL-8
interleukin 8
- iNOS
inducible nitric oxide synthase
- IRS
insulin receptor substrate
- K562
human malignant myeloid cells
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharides
- MCF-7
human breast cancer cells
- MDA
malondialdehyde
- MDA-MB-231
human breast cancer cells
- MPO
myeloperoxidase
- mRNA
messenger ribonucleic acid
- NF-κB
nuclear factor κB
- NGF
nerve growth factor
- NO
nitric oxide
- NQO-1
quinine oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- O2
superoxide anion
- OH
hydroxyl radical
- PCNA
proliferating cell nuclear antigen
- PID
pelvic inflammatory disease
- PS
Patrinia scabiosaefolia Fisch
- PV
Patrinia villosa Juss
- RAW264.7
mouse leukemic monocyte macrophage
- ROS
reactive oxygen species
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome
- SD
sprague dawley
- SGC-7901
human gastric cancer cells
- SMMC-7721
hepatocellular carcinoma cells
- STAT3
signal transducer and activator of transcription 3
- SW480
human colon carcinoma cells
- TC50
half toxic concentration
- TCM
traditional Chinese medicine
- TGF-β
transforming growth factor beta
- TI
drug treatment index
- TNF-α
tumor necrosis factor alpha
- T-AOC
total antioxidant capacity
- T-SOD
total superoxide dismutase
- U14
mice cervical cancer cells
- U266
human multiple myeloma cancer cells
- U937
human lymphoma cells
- UC
ulcerative colitis
- UV
ultraviolet.
1. Introduction
Herba Patriniae, as known as “Bai Jiang Cao” in Chinese, is a traditional Chinese medicine (TCM) originally recorded in “Shen Nong's Herbal Classic” as a “middle grade” medicinal material, which has been used for thousands years. Besides, Korean ancient pharmacopaea “Donguibogam” also record its medical value, and it has been used for more than 400 years in Korea (Jeon et al., 2010). It possesses the TCM properties of pungent and bitter in flavor and slightly cold in nature, and has been classified to the stomach, large intestine, and liver meridians (Xiao, 1995). Two official species of Patrinia scabiosaefolia Fisch. (PS) and Patrinia villosa Juss. (PV) (Fig. 1 ) were considered as Herba Patriniae in Chinese Pharmacopoeia (1977 edition) and Chinese provincial pharmacopoeias. These two plants have been widely used for more than 2000 years with good biological activities of clearing heat and detoxification, eliminating carbuncle and expelling pus, dispelling blood stasis, and relieving pain. Through an analysis of ancient and modern literatures, Herba Patriniae was mostly used in intestinal carbuncle, lung carbuncle, gynecological epigastric pain, postpartum blood stasis, and eczema in ancient times (Chen and Han, 2017). Modern pharmacological studies have found that it has effects of anti-cancer, anti-inflammation, anti-pathogenic microorganisms, anti-oxidation, sedation, and hypnosis (Wang et al., 2019a). Nowadays, Herba Patriniae is widely used in the respiratory system, digestive system, genitourinary system, gynecology, dermatology and other multi-disciplinary diseases in clinical practice (Zhu and Jiang, 2015), and the number of applied patents increases every year (http://pss-system.cnipa.gov.cn). In view of its high content of amino acids, vitamins, minerals and other nutrients, Herba Patriniae is not only regarded as a potherb with healthy value, but also processed into tea products (Su et al., 1999; Zeng et al., 2019; Zhong et al., 2001).
Fig. 1.
Two species of Herba Patriniae. (A) Patrinia villosa Juss.; (B) Patrinia scabiosaefolia Fisch (A: http://www.cvh.ac.cn/spm/CSH/CSH0005548; B: http://www.cvh.ac.cn/spm/SYAUF/SYAUF010108).
In the past decades, an increasing number of scholars have studied the chemical constituents and pharmacological effects of Herba Patriniae. Interestingly, based on these studies, we found that there are many different/same characteristics between PS and PV. Both of them are official species for Herba Patriniae, but differentiated clinical uses of them in different diseases may be better for the clinical outcome. Unfortunately, we cannot found a comprehensive and updated review on the same/different characteristics of the two sources of Herba Patriniae, and actually, these two species also have not been differentiated in clinical uses. Therefore, this review aims to systematically summarize the similarities and differences from the aspects of the traditional uses, botanical description, phytochemistry, pharmacology, and quality control of these two species of Herba Patriniae, as well as being evidences for their clinical application and further research.
2. Traditional uses
Herba Patriniae has a wide geographical distribution, mainly in East Asia and North America (He et al., 2017). Some plants, such as Sonchus Arvensis L., Sonchus Asper Vill, Sonchus oleraceus L. etc, may be confused as Herba Patriniae (Lu, 1996), and hence, these adulterants of Herba Patriniae should be exclude when clinical use. Traditionally, according to records of “Shen Nong's Herbal Classic” (神农本草经), “Compendium of Materia Medica” (本草纲目), and “Synopsis of the Golden Chamber” (金匮要略), “Tai Ping Sheng Hui Fang” (太平圣惠方), “Pu Ji Fang” (普济方), “Sheng Ji Zong Lu” (圣济总录), “Qian Jin Yi Fang” (千金翼方) and “Qian Jin Fang” (千金方), ancient doctors have used the whole herbs and roots of Herba Patriniae for disease treatment, such as the stomach, intestine, liver, gallbladder, and gynecological diseases (Tian and Tian, 2003; Zhu and Jiang, 2015). Herba Patriniae was recorded in Chinese Pharmacopoeia (1977 edition) for the treatments of appendicitis, dysentery, enteritis, hepatitis, conjunctivitis, postpartum blood stasis abdominal pain, swollen welling-abscess, and clove sores (Pharmacopoeia Committee of the Ministry of Health of P. R. China, 1978). In addition, Herba Patriniae is also recorded in the standards of traditional Chinese medicine in many provinces of China (Table 1 ). In Miao nationality, Herba Patriniae is also called “Jia jiang le” and used to treat rheumatoid arthritis, colds, and diarrheal (Qiu, 2005; Wang, 2002). In Dong medicine (Lu, 1992), Yi medicine (Drug Control Institute of Yunnan Chuxiong Health Bureau, 1983), and Dai medicine (Shi, 1983), PS is called “Nyangt ngeec liongc bail jangl”, “She wei long”, and “Pa hong”, respectively. Its whole herb is used to treat infantile diarrhea, schizophrenia, and infantile tinea capitis, respectively. PS is also called “Ba gai bao” in Zhuang medicine, and its root is used to treat icteric hepatitis, furuncles, and snakebites (Shi, 1983). PV is called “Bitter vegetable” by She nationality (Biological Products Identification Institute of the Ministry of Health, 1990) and “Pao zi tong” by Tujia nationality (Peng and Guan, 1994). Its whole herb can be used to treat appendicitis, intestinal febrile symptoms constipation, mammary abscess, blister carbuncle, and Qi stagnation. PV is also called “Ba gai lan” and “Hong pa” in Zhuang medicine (Biological Products Identification Institute of the Ministry of Health, 1990) and Dai medicine (Shi, 1983), respectively, and its root is used to treat jaundice hepatitis, furuncle, local ulceration caused by snake injury, and infantile convulsion. Moreover, in Korea, people usually use the roots or whole plants of PS as a traditional herbal medicine to treat appendicitis, inflammation, wound healing, edema, abscesses, endometritis, and abdominal pain after childbirth (Kang et al., 1997; Yang et al., 2001).
Table 1.
The information of Herba Patriniae in national and local standards in China.
| Standards | Application | Dosage | Standard-setting Department |
|---|---|---|---|
| Standard of traditional Chinese medicine in Hunan Province | Acute appendicitis, diarrhea, enteritis, hemorrhagic leucorrhea, red eye, pterygium, postpartum abdominal pain, boils and carbuncles | 9–15 g | Hunan Food and Drug Administration (2010) |
| Standard of traditional Chinese medicine in Shandong Province | Appendicitis, dysentery, enteritis, hepatitis, conjunctivitis, postpartum blood stasis abdominal pain, boils and carbuncles | 9–15 g | Shandong Medical Products Administration (2002) |
| Standard of traditional Chinese medicine in Heilongjiang Province | Acute appendicitis, diarrhea, hemorrhagic leucorrhea, postpartum blood stasis abdominal pain, swelling and pain of eye, hepatitis, boils and carbuncles | 9–15 g | Heilongjiang Medical Products Administration (2001) |
| Standard of traditional Chinese medicine in Liaoning Province | Acute appendicitis, diarrhea, dysentery, postpartum blood stasis abdominal pain, conjunctivitis, boils and carbuncles | 9–15 g | Liaoning Food and Drug Administration (2009) |
| Standard of traditional Chinese medicine in Sichuan Province | Acute appendicitis and its abdominal pain, postpartum blood stasis abdominal pain, boils and carbuncles | 9–15 g | Sichuan Food and Drug Administration (2011) |
| Standard of traditional Chinese medicine in Guizhou Province | Appendicitis, dysentery, enteritis, hepatitis, conjunctivitis, postpartum blood stasis abdominal pain, boils and carbuncles | 9–15 g | Guizhou Medical Products Administration (2003) |
| Chinese Pharmacopoeia (1977 edition) | Appendicitis, dysentery, enteritis, hepatitis, conjunctivitis, postpartum blood stasis abdominal pain, boils and carbuncles | 9–15 g | Pharmacopoeia Committee of the Ministry of Health of P. R. China (1978) |
In recent years, Herba Patriniae has been extensively applied in clinical practice in China, especially in gynecology, such as postpartum pain, mastitis, dysmenorrhea, and tubal obstructive infertility (Liu, 2019a). It is noteworthy that Herba Patriniae is one of the most important ingredients in many prescriptions of TCM which is effective in diarrhea (He, 1991), acute hepatitis (Song, 1987), pelvic inflammatory disease (Zhang, 1997a), typhoid fever, paratyphoid fever (Sun, 2000), ulcerative colitis (Liu, 2011), anal cryptitis (Shi, 2012), pelvic endometriosis (Yan and Qiu, 2013), acute pancreatitis (He et al., 2019b), itching (Wang and Wang, 2002), gastroesophageal reflux disease, benign prostatic hyperplasia, rhinosinusitis, mumps, and phlebitis (Kong and Zhao, 2008; Zhu and Jiang, 2015). A powder composed of Coicis Semen, Radix Aconiti Lateralis Preparata, and Herba Patriniae, is a classic prescription for treating intestinal carbuncle in the “Synopsis of the Golden Chamber”, which is clinically used to treat chronic appendicitis, chronic pelvic inflammatory disease, and chronic prostatitis (Ji, 2006). In addition, a powder containing Herba Patriniae in the prescription is also used to treat sinusitis, acute purulent tonsillitis, and recurrent upper respiratory tract infection (Qin and Diao, 2018; Zhu and Jiang, 2015). Moreover, it showed significant efficacy in the treatment of psoriasis vulgaris (Yan et al., 2015), Keshan disease (Scientific Research Cooperation Group of Herba Patriniae in Yan'an City for the Prevention and Control of Keshan Disease, 1979), and chronic pelvic inflammation (Jia, 2010) in the form of tablets. In the Chinese Pharmacopoeia (2015 edition), there are 6 Chinese herbal medicine prescriptions containing Herba Patriniae, among which Kangfu Xiaoyan Shuan and Yifei Qinghua Gao are used to treat gynecological diseases and respiratory diseases, respectively, while Longqing Pian, Nankang Pian, Niaosaitong Pian and Qianliexin Jiaonang are used to treat genitourinary diseases (State Pharmacopoeia Commission of P. R. China, 2015). A summary of the traditional and Traditional and clinical preparation of Herba Patriniae in China is given in Table 2 .
Table 2.
Traditional and clinical preparation of Herba Patriniae in China.
| Preparation name | Formulation | Main compositions | Reference |
|---|---|---|---|
| Yiyi Fuzi Baijiang San (薏苡附子败酱散) |
Powder | Coicis Semen, Aconiti Lateralis Radix Praeparata, Herba Patriniae | Jin Gui Yao Lüe ⟪金匮要略⟫ (Zhang, 1997b) |
| Machixian Heji (马齿苋合剂) |
Decoction | Portulacae Herba, Isatidis Folium, Arnebiae Radix, Herba Patriniae, Persicae Semen, Carthami Flos, Paeoniae Radix Rubar | Surgery of Chinese medicine ⟪中医外科学⟫ (Beijing Traditional Chinese Medicine Hospital, 1982) |
| Aiye San (艾叶散) |
Powder | Artemisiae Argyi Folium, Angelicae Sinensis Radix, Paeoniae Radix Alba, Dipsaci Radix, Achyranthis Bidentatae Radix, Herba Patriniae | Tai Ping Sheng Hui Fang ⟪太平圣惠方⟫ Volume 80 (Wang, 1958) |
| Baijiang San (败酱散) |
Powder | Herba Patriniae, Angelicae Sinensis Radix, Chuanxiong Rhizoma, Paeoniae Radix Alba, Cinnamomi Cortex | Pu Ji Fang ⟪普济方⟫ Volume 351 (Zhu, 1959) |
| Baijiang San (败酱散) |
Powder | Herba Patriniae, Moutan Cortex, Cinnamomi Cortex, Siphonostegiae Herba, Aucklandiae Radix | Tai Ping Sheng Hui Fang ⟪太平圣惠方⟫ Volume 80 (Wang, 1958) |
| Baijiang Tang (败酱汤) |
Decoction | Herba Patriniae, Notopterygii Rhizoma et Radix, Dianthi Herba, Aurantii Fructus, Cinnamomi Cortex, Persicae Semen | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 160 (Zhao, 1982) |
| Baijiang Tang (败酱汤) |
Decoction | Herba Patriniae | Qian Jin Yi Fang ⟪千金翼方⟫ Volume 6 (Sun, 1955) |
| Baijiang Tang (败酱汤) |
Decoction | Herba Patriniae, Rhei Radix et Rhizoma, Persicae Semen | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 129 (Zhao, 1982) |
| Baijiang Tang (败酱汤) |
Decoction | Herba Patriniae, Cinnamomi Cortex, Siphonostegiae Herba, Moutan Cortex, Aucklandiae Radix | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 161 (Zhao, 1982) |
| Baijiang Yin (败酱饮) |
Decoction | Herba Patriniae, Angelicae Sinensis Radix, Bambusae Caulis in Taenias, Rehmanniae Radix | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 161 (Zhao, 1982) |
| Changyong Tang (肠痈汤) |
Decoction | Moutan Cortex, Glycyrrhizae Radix et Rhizoma, Herba Patriniae, Zingiberis Rhizoma Recens, Poria, Coicis Semen, Platycodonis Radix, Liriopes Radix, Salviae Miltiorrhizae Radix et Rhizoma, Paeoniae Radix Alba, Rehmanniae Radix | Qian Jin Fang ⟪千金方⟫ Volume 23 (Sun, 1998) |
| Chenzhou Sheyao Pian (郴州蛇药片) |
Tablets | PV | Gu Jin Ming Fang ⟪古今名方⟫ (Yan and Liu, 1983) |
| Chure Jili Wan (除热蒺藜丸) |
Pills | Tribuli Fructus, Rhei Radix et Rhizoma, Herba Patriniae, Cinnamomi Cortex, Ginseng Radix et Rhizoma, Aconiti Lateralis Radix Praeparata, Coicis Semen, Coptidis Rhizoma, Astragali Radix, Abri Herba, Angelicae Sinensis Radix, Aurantii Fructus Immaturus, Paeoniae Radix Alba, Tetrapanacis Medulla | Qian Jin Fang ⟪千金方⟫ Volume 23 (Sun, 1998) |
| Danggui Xi Tang (当归洗汤) |
Decoction | Angelicae Sinensis Radix, Angelicae Pubescentis Radix, Angelicae Dahuricae Radix, Sanguisorbae Radix, Herba Patriniae, | Qian Jin Fang ⟪千金方⟫ Volume 3 (Sun, 1998) |
| Danggui Yin (当归饮) |
Decoction | Angelicae Sinensis Radix, Herba Patriniae, Dipsaci Radix, Paeoniae Radix Alba, Rehmanniae Radix, Bambusae Caulis in Taenias, | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 161 (Zhao, 1982) |
| Ganyan Chongji (肝炎冲剂) |
Electuary | Bupleuri Radix, Angelicae Sinensis Radix, Paeoniae Radix Alba, Paeoniae Radix Rubra, Citri Reticulatae Pericarpium, Aurantii Fructus, Curcumae Radix, Cyperi Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Scrophulariae Radix, Artemisiae Scopariae Herba, Isatidis Radix, Herba Patriniae | Study on the Treatment of Common Diseases with Traditional Chinese Medicine ⟪常见病的中医治疗研究⟫ (Teaching and Research Group of Traditional Chinese Medicine, the First Affiliated Hospital of Xi'an Medical College, 1975) |
| Huangdan Tang (黄疸汤) |
Decoction | Artemisiae Scopariae Herba, Gardeniae Fructus, Lonicerae Japonicae Flos, Forsythiae Fructus, Herba Patriniae, Isatidis Radix, Paeoniae Radix Rubra, Paeoniae Radix Alba, Bupleuri Radix, Perillae Caulis, Platycodonis Radix, Sojae Semen Germinatum | Lin Zheng Yi An Yi Fang ⟪临证医案医方⟫ (Sun, 1981) |
| Jiedu Dihuang Wan (解毒地黄丸) |
Pills | Rehmanniae Radix, Astragali Radix, Trichosanthis Radix, Scutellariae Radix, Liriopes Radix, Mantidis Ootheca, Rhei Radix et Rhizoma, Ginseng Radix et Rhizoma, Gardeniae Fructus, Cistanches Herba, Peucedani Radix, Cimicifugae Rhizoma, Paeoniae Radix Alba, Anemarrhenae Rhizoma, Vaccariae Semen, Polygalae Radix, Herba Patriniae, Jujubae Fructus | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 131 (Zhao, 1982) |
| Gualou San (栝蒌散) |
Powder | Trichosanthis Semen, Herba Patriniae, Asari Radix et Rhizoma, Zingiberis Rhizoma, Magnoliae Officinalis Cortex, Platycodonis Radix, Ginseng Radix et Rhizoma, Saposhnikoviae Radix | Sheng Ji Zong Lu ⟪圣济总录⟫ Volume 166 (Zhao, 1982) |
| Lanwei Xiaoyan Wan (阑尾消炎丸) |
Pills | Lonicerae Japonicae Flos, Isatidis Folium, Herba Patriniae, Taraxaci Herba, Spatholobi Caulis, Toosendan Fructus, Rhei Radix et Rhizoma, Aucklandiae Radix, Persicae Semen, Paeoniae Radix Rubra, Scutellariae Radix | Beijing Chinese Traditional Patent Medicine specification ⟪北京市中成药规范⟫ Volume 2 (Beijing Municipal Bureau of Health, 1974) |
| Lanweiyan Heji (阑尾炎合剂) |
Decoction | Lonicerae Japonicae Flos, Taraxaci Herba, Herba Patriniae, Forsythiae Fructus, Rhei Radix et Rhizoma, Paeoniae Radix Rubra, Toosendan Fructus, Aucklandiae Radix, Persicae Semen, | Selected Data of Acute Abdomen Treated by Integrated Traditional Chinese and Western Medicine ⟪中西医结合治疗急腹症资料选编⟫ (Affiliated Hospital of Guangzhou College of Traditional Chinese Medicine, 1978) |
| Lanweiyan Tang (阑尾炎汤) |
Decoction | Rhei Radix et Rhizoma, Moutan Cortex, Persicae Semen, Paeoniae Radix Alba, Salviae Miltiorrhizae Radix et Rhizoma, Bupleuri Radix, Lonicerae Japonicae Flos, Forsythiae Fructus, Herba Patriniae, Coicis Semen | Lin Zheng Yi An Yi Fang ⟪临证医案医方⟫ (Sun, 1981) |
| Lanwei Yihao Xiaoyan Wan (阑尾一号消炎片) |
Pills | Lonicerae Japonicae Flos, Isatidis Folium, Herba Patriniae, Taraxaci Herba, Toosendan Fructus, Rhei Radix et Rhizoma, Aucklandiae Radix, Persicae Semen, Paeoniae Radix Rubra, Scutellariae Radix, Talci Pulvis, Sargentodoxae Caulis | Compilation of Traditional Chinese Medicine Preparations ⟪中药制剂汇编⟫ (Cao, 1983) |
| Lenge Xiaoji Tang (棱莪消积汤) |
Decoction | Sparganii Rhizoma, Curcumae Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Paeoniae Radix Rubra, Corydalis Rhizoma, Moutan Cortex, Persicae Semen, Coicis Semen, Sargentodoxae Caulis Herba Patriniae, | Obstetrics and Gynecology⟪妇产科学⟫ (Shanghai College of Traditional Chinese Medicine, 1973) |
| Lishi Zhiyang Pu Yao (理湿止痒扑药) |
Powder | Kochiae Fructus, Bombyx Batryticatus, Dictamni Cortex, Angelicae Dahuricae Radix, Schizonepetae Spica, Artemisiae Scopariae Herba, Herba Patriniae, Alumen, Glycyrrhizae Radix et Rhizoma, Talcum, Cinnabaris | Selection of Medical Prescriptions of Cixi and Guangxu ⟪慈禧光绪医方选议⟫ (Chen, 1981) |
| Lidan Tuihuang Tang (利胆退黄汤) |
Decoction | Artemisiae Scopariae Herba, Herba Patriniae, Isatidis Radix, Curcumae Radix, Gardeniae Fructus | Gu Jin Ming Fang ⟪古今名方⟫ (Yan and Liu, 1983) |
| Neibu Wuxiang Wan (内补五香丸) |
Pills | Aquilariae Lignum Resinatum, Olibanum, Aucklandiae Radix, Caryophylli Flos, Dipsaci Radix, Rehmanniae Radix Praeparata, Paeoniae Radix Alba, Magnoliae Officinalis Cortex, Herba Patriniae, Ginseng Radix et Rhizoma, Poria, Cervi Cornu | Tai Ping Sheng Hui Fang ⟪太平圣惠方⟫ Volume 61 (Wang, 1958) |
| Qianliexian Tang (前列腺汤) |
Decoction | Salviae Miltiorrhizae Radix et Rhizoma, Lycopi Herba, Paeoniae Radix Rubra, Persicae Semen, Carthami Flos, Olibanum, Myrrha, Vaccariae Semen, Citri Reticulatae Pericarpium, Toosendan Fructus, Foeniculi Fructus, Angelicae Dahuricae Radix, Herba Patriniae, Taraxaci Herba | Surgery of Chinese medicine ⟪中医外科学⟫ (Beijing Traditional Chinese Medicine Hospital, 1982) |
| Qumai Wan (瞿麦丸) |
Pills | Dianthi Herba, Realgar, Vaccariae Semen, Rehmanniae Radix, Ephedrae Herba, Imperatae Rhizoma, Herba Patriniae, Saposhnikoviae Radix, Achyranthis Bidentatae Radix, Rhei Radix et Rhizoma | Qian Jin Yi Fang ⟪千金翼方⟫ Volume 20 (Sun, 1955) |
| Yinqiao Hongjiang Jiedu Tang (银翘红酱解毒汤) |
Decoction | Lonicerae Japonicae Flos, Forsythiae Fructus, Sargentodoxae Caulis, Herba Patriniae, Moutan Cortex, Gardeniae Fructus, Paeoniae Radix Rubra, Persicae Semen, Coicis Semen, Corydalis Rhizoma, Toosendan Fructus, Olibanum, Myrrha | Obstetrics and Gynecology ⟪妇产科学⟫ (Shanghai College of Traditional Chinese Medicine, 1973) |
| Danhuang Quyu Jiaonang (丹黄祛瘀胶囊) |
Capsules | Astragali Radix, Salviae Miltiorrhizae Radix et Rhizoma, Dioscoreae Rhizoma, Smilacis Glabrae Rhizoma, Angelicae Sinensis Radix, Spatholobi Caulis, Euryales Semen, Houttuyniae Herba, Sparganii Rhizoma, Curcumae Rhizoma, Scorpio, Herba Patriniae, Cinnamomi Cortex, Atractylodis Macrocephalae Rhizoma, Zingiberis Rhizoma Praepatum, Eupolyphaga Steleophaga, Corydalis Rhizoma, Toosendan Fructus, Sophorae Flavescentis Radix |
https://www.yaozh.com/ NMPA |
| Qianlieping Jiaonang (前列平胶囊) |
Capsules | Herba Patriniae, Salviae Miltiorrhizae Radix et Rhizoma, Paeoniae Radix Rubra, Persicae Semen, Carthami Flos, Lycopi Herba, Pyrrosiae Folium, Olibanum, Myrrha |
https://www.yaozh.com/ NMPA |
| Fuping Jiaonang (妇平胶囊) |
Capsules | Fagopyri Dibotryis Rhizoma, Violae Herba, Curcumae Rhizoma, Herba Patriniae, Polygoni Perfoliati Herba, Solidaginis Herba |
https://www.yaozh.com/ NMPA |
| Fuyan Kangfu Jujue Pian (妇炎康复咀嚼片) |
Tablets | Herba Patriniae, Coicis Semen, Toosendan Fructus, Bupleuri Radix, Scutellariae Radix, Paeoniae Radix Rubra, Citri Reticulatae Pericarpium |
https://www.yaozh.com/ NMPA |
| Fuyan Kangfu Pian (妇炎康复片) |
Tablets | Herba Patriniae, Coicis Semen, Toosendan Fructus, Bupleuri Radix, Scutellariae Radix, Citri Reticulatae Pericarpium |
https://www.yaozh.com/ NMPA |
| Fuyan Kangfu Jiaonang (妇炎康复胶囊) |
Capsules | Herba Patriniae, Coicis Semen, Toosendan Fructus, Bupleuri Radix, Scutellariae Radix, Paeoniae Radix Rubra, Citri Reticulatae Pericarpium |
https://www.yaozh.com/ NMPA |
| Fuyan Kangfu Keli (妇炎康复颗粒) |
Granules | Herba Patriniae, Coicis Semen, Toosendan Fructus, Bupleuri Radix, Scutellariae Radix, Paeoniae Radix Rubra, Citri Reticulatae Pericarpium |
https://www.yaozh.com/ NMPA |
| Fuyanxiao Jiaonang (妇炎消胶囊) |
Capsules | Herba Patriniae, Trichosanthis Radix, Rhei Radix et Rhizoma, Moutan Cortex, Atractylodis Rhizoma, Linderae Radix |
https://www.yaozh.com/ NMPA |
| Fuyanqing Xiji (妇炎清洗剂) |
Lotion | Taraxaci Herba, Herba Patriniae, Coicis Semen, Paeoniae Radix Rubra, Atractylodis Rhizoma, Angelicae Sinensis Radix, Chuanxiong Rhizoma, Cyperi Rhizoma, Corydalis Rhizoma, Alismatis Rhizoma, |
https://www.yaozh.com/ NMPA |
| Xiaoer Reke Koufuye (小儿热咳口服液) |
Oral liquid | Ephedrae Herba, Armeniacae Semen Amarum, Forsythiae Fructus, Rhei Radix et Rhizoma, Trichosanthis Fructus, Mori Cortex, Herba Patriniae, Carthami Flos, Glycyrrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Kangfu Xiaoyan Shuan (康复消炎栓) |
suppository | Sophorae Flavescentis Radix, Violae Herba, Herba Patriniae, Andrographis Herba, Suis Fellis Pulvis, Taraxaci Herba, Arnebiae Radix, Aloe | (State Pharmacopoeia Commission of P. R. China, 2015) https://www.yaozh.com/ |
| Kangfu Xiaoyan Jiaonang (康妇炎胶囊) |
Capsules | Taraxaci Herba, Herba Patriniae, Paeoniae Radix Rubra, Coicis Semen, Angelicae Sinensis Radix, Atractylodis Rhizoma, Chuanxiong Rhizoma, Cyperi Rhizoma, Alismatis Rhizoma, Corydalis Rhizoma |
https://www.yaozh.com/ NMPA |
| Manshenning Heji (慢肾宁合剂) |
Decoction | Astragali Radix, Cinnamomi Ramulus, Epimedii Folium, Rehmanniae Radix, Asini Corii Colla, Poria, Alismatis Rhizoma, Scutellariae Radix, Herba Patriniae, Moutan Cortex, Leonuri Herba |
https://www.yaozh.com/ NMPA |
| Nankang Pian (男康片) |
Tablets | Paeoniae Radix Rubra, Rehmanniae Radix Praeparata, Cistanches Herba, Glycyrrhizae Radix et Rhizoma, Taraxaci Herba, Pyrolae Herba, Phellodendri Chinensis Cortex, Carthami Flos, Houttuyniae Herba, Epimedii Folium, Fubi Fructus, Atractylodis Macrocephalae Rhizoma, Astragali Radix, Cuscutae Semen, Violae Herba, Herba Patriniae, Chrysanthemi Indici Flos, Angelicae Sinensis Radix | (State Pharmacopoeia Commission of P. R. China, 2015) https://www.yaozh.com/ NMPA |
| Zhishushi Xiye (痔舒适洗液) |
Lotion | Sophorae Fructus, Notoginseng Radix et Rhizoma, Sophorae Flavescentis Radix, Bletillae Rhizoma, Cnidii Fructus, Artemisiae Argyi Folium, Herba Patriniae, Lonicerae Japonicae Flos, Portulacae Herba, Saposhnikoviae Radix, Alumen, Borneolum Syntheticum, Glycyrrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Baijiang Ganmao Keli (白酱感冒颗粒) |
Granules | PV, Sucrose, dextrin |
https://www.yaozh.com/ NMPA |
| Yifei Qinghua Gao (益肺清化膏) |
Ointment | Astragali Radix, Codonopsis Radix, Glehniae Radix, Liriopes Radix, Agrimoniae Herba, Bistortae Rhizoma, Fritillariae Cirrhosae Bulbus, Asteris Radix et Rhizoma, Platycodonis Radix, Armeniacae Semen Amarum, Herba Patriniae, Glycyrrhizae Radix et Rhizoma | (State Pharmacopoeia Commission of P. R. China, 2015) https://www.yaozh.com/ NMPA |
| Ganfule Pian (肝复乐片) |
Tablets | Codonopsis Radix, Trionycis Carapax, Paridis Rhizoma, Atractylodis Macrocephalae Rhizoma, Astragali Radix, Citri Reticulatae Pericarpium, Eupolyphaga Steleophaga, Rhei Radix et Rhizoma, Persicae Semen, Scutellariae Barbatae Herba, Herba Patriniae, Poria, Coicis Semen, Curcumae Radix, Sappan Lignum, Ostreae Concha, Artemisiae Scopariae Herba, Cyperi Rhizoma |
https://www.yaozh.com/ NMPA |
| Gandujing Keli (肝毒净颗粒) |
Granules | Polygoni Cuspidati Rhizoma et Radix, Ardisiae Japonicae Herba, Sedi Herba, Scutellariae Barbatae Herba, Magnoliae Officinalis Cortex, Herba Patriniae, Arnebiae Radix, Wenyujin Rhizoma Concisum |
https://www.yaozh.com/ NMPA |
| Lanwei Xiaoyan Wan (阑尾消炎丸) |
Pills | Lonicerae Japonicae Flos, Isatidis Folium, Herba Patriniae, Taraxaci Herba, Spatholobi Caulis, Toosendan Fructus, Rhei Radix et Rhizoma, Aucklandiae Radix, Persicae Semen, Paeoniae Radix Rubra, Scutellariae Radix |
https://www.yaozh.com/ NMPA |
| Lanwei Xiaoyan Pian (阑尾消炎片) |
Tablets | Lonicerae Japonicae Flos, Isatidis Folium, Herba Patriniae, Taraxaci Herba, Sargentodoxae Caulis, Toosendan Fructus, Rhei Radix et Rhizoma, Aucklandiae Radix, Persicae Semen, Paeoniae Radix Rubra, Scutellariae Radix |
https://www.yaozh.com/ NMPA |
| Lanweiling Keli (阑尾灵颗粒) |
Granules | Lonicerae Japonicae Flos, Herba Patriniae, Taraxaci Herba, Moutan Cortex, Toosendan Fructus, Paeoniae Radix Rubra, Rhei Radix et Rhizoma, Persicae Semen, Aucklandiae Radix |
https://www.yaozh.com/ NMPA |
| Shuangshi Tongli Jiaonang (双石通淋胶囊) |
Capsules | Atractylodis Rhizoma, Poria, Acori Tatarinowii Rhizoma, Plantaginis Semen, Indigo Naturalis, Phellodendri Amurensis Cortex, Talcum, Herba Patriniae, Salviae Miltiorrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Yigong Keli (益宫颗粒) |
Granules | Herba Patriniae, Salviae Miltiorrhizae Radix et Rhizoma, Leonuri Herba, Codonopsis Radix, Dipsaci Radix, Angelicae Sinensis Radix, Scutellariae Radix, Cyperi Rhizoma |
https://www.yaozh.com/ NMPA |
| Ganfule Jiaonang (肝复乐胶囊) |
Capsules | Aquilariae Lignum Resinatum, Cyperi Rhizoma, Akebiae Caulis, Artemisiae Scopariae Herba, Ostreae Concha, Sappan Lignum, Curcumae Radix, Coicis Semen, Poria, Herba Patriniae, Scutellariae Barbatae Herba, Rhei Radix et Rhizoma, Persicae Semen, Eupolyphaga Steleophaga, Citri Reticulatae Pericarpium, Astragali Radix, Atractylodis Macrocephalae Rhizoma, Paridis Rhizoma, Trionycis Carapax, Codonopsis Radix, Bupleuri Radix |
https://www.yaozh.com/ NMPA |
| Lianqi Jiaonang (莲芪胶囊) |
Capsules | Ligustri Lucidi Fructus, Ginseng Radix et Rhizoma, Angelicae Sinensis Radix, Astragali Radix, Hirudo, Coicis Semen, Atractylodis Macrocephalae Rhizoma, Fritillariae Thunbergii Bulbus, Sparganii Rhizoma, Curcumae Rhizoma, Herba Patriniae, Scutellariae Barbatae Herba, Glycyrrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Niaosaitong Jiaonang (尿塞通胶囊) |
Capsules | Foeniculi Fructus, Toosendan Fructus, Vaccariae Semen, Alismatis Rhizoma, Citri Reticulatae Pericarpium, Paeoniae Radix Rubra, Carthami Flos, Persicae Semen, Herba Patriniae, Lycopi Herba, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Dahuricae Radix |
https://www.yaozh.com/ NMPA |
| Niaosaitong Pian (尿塞通片) |
Tablets | Foeniculi Fructus, Toosendan Fructus, Vaccariae Semen, Alismatis Rhizoma, Citri Reticulatae Pericarpium, Paeoniae Radix Rubra, Carthami Flos, Persicae Semen, Herba Patriniae, Lycopi Herba, Salviae Miltiorrhizae Radix et Rhizoma, Angelicae Dahuricae Radix | (State Pharmacopoeia Commission of P. R. China, 2015) |
| Jinma Gantai Keli (金马肝泰颗粒) |
Granules | Verbenae Herba, Stephaniae Tetrandrae Radix, Herba Patriniae, Epimedii Folium, Astragali Radix, Paeoniae Radix Rubra, Salviae Miltiorrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Shufeng Jiedu Jiaonang (疏风解毒胶囊) |
Capsules | Polygoni Cuspidati Rhizoma et Radix, Forsythiae Fructus, Isatidis Radix, Bupleuri Radix, Herba Patriniae, Verbenae Herba, Phragmitis Rhizoma, Glycyrrhizae Radix et Rhizoma |
https://www.yaozh.com/ NMPA |
| Longqing Pian (癃清片) |
Tablets | Alismatis Rhizoma, Plantaginis Semen, Herba Patriniae, Lonicerae Japonicae Flos, Moutan Cortex, Paeoniae Radix Rubra, Agrimoniae Herba, Coptidis Rhizoma, Phellodendri Chinensis Cortex | (State Pharmacopoeia Commission of P. R. China, 2015) |
| Qianliexin Jiaonang (前列欣胶囊) |
Capsules | Persicae Semen, Salviae Miltiorrhizae Radix et Rhizoma, Carthami Flos, Vaccariae Semen, Herba Patriniae, Toosendan Fructus, Pyrrosiae Folium, Myrrha, Paeoniae Radix Rubra, Lycopi Herba, Angelicae Dahuricae Radix, Taraxaci Herba, Gleditsiae Spina, Lycii Fructus | (State Pharmacopoeia Commission of P. R. China, 2015) |
PV: Patrinia villosa Juss.; Herba Patriniae: not indicate species.
NMPA was cited from the website: http://www.nmpa.gov.cn/WS04/CL2042/.
The tender stems and leaves of Herba Patriniae are rich in nutrients, fresh in taste, and grow in the mountains without environmental pollution. It is a high-quality vegetable that urban and rural residents like to eat. PV tea is also abundant in Hubei Province and Fujian Province (Jiang, 2019a; Xu et al., 2018). Herba Patriniae is not only used in human health, but also in agriculture, fishery, and animal husbandry. Interplanting Herba Patriniae in the newly reclaimed tea garden can increase the natural vegetation and reduce soil erosion and surface runoff caused by rainstorm erosion in the rainy season (Chen, 2001). The combination of Herba Patriniae and other medicinal plants can be used to treat poisoned wound of cattle by Agkistrodon acutus bitting, crawling bee disease, liver and skin diseases of turtle and fish, and postpartum abdominal pain in cattle (Chen, 2006; Li, 2002; Shi, 2010; Zhao, 2003).
3. Botany
Patrinia villosa Juss often grows in roadsides, grassy areas, thickets, forest margins or forests, with an altitude of 100–2000 m. It is a perennial herb with a height of 50–120 cm. Its rhizomes are long and laterally, rarely stoloniferous. The stems are yellowish green with anatropous white coarse hairs, rarely uniformly glabrous or glabrescent. The basal leaves are rosulate, long petiolate, ovate, broadly ovate, or oblong-lanceolate to ovate-lanceolate and with an area of 4–25 (l.) × 2–18 (w.) cm; the base of its leaf is decurrent and margin serrate or pinnatifid, with 1 or 2(-4) pairs of segments, apex acuminate. The coarsely serrate cauline leaves are opposite, petiole 1–3 cm, upper leaves subsessile; it is bright green or dark green on the upper surface, white-green on the underside, and the blades are similar to basal leaves or rhombic-ovate, hispidulous or glabrescent, base decurrent, apex caudate-acuminate or acuminate. White terminal panicles or umbellifers are composed of cymes; lateral branches are usually 5 or 6 pairs, densely hirsute. The corolla is campanulate; the tube is 1.5–2.6 (l.) × 1.7–2.3 (w.) mm, deeply 5-lobed; its lobes are dissimilar in shape, ovate, ovate-oblong, or ovate-elliptic, 0.7–2 (l.) × 1.1–1.8 (w.) mm. The 4 stamens of the plant are usually exserted from the corolla. Achenes are obovoid; the flowering of PV occurs from August to October whereas fruiting occurs from September to November (Fig. 1A) (Flora of China Editorial Committee, 1986).
Patrinia scabiosaefolia Fisch generally grows in roadsides, grassy areas, thickets, forest margins, and forests at an altitude of (50-) 400–2100 (−2600) m. It is a perennial herb with a height of 30–100 (−200) cm. The rhizomes can be horizontal or oblique. It has yellow-green to yellow-brown erect stems, sometimes pale purplish, glabrate basally, hispidulous apically. Its basal leaves are rosulate, ovate, elliptic, or elliptic-lanceolate, simple to pinnatifid or pinnatisect, and up to 1.8–10.5 cm long and 1.2–3 cm wide, wilted at anthesis; petiole 3–12 cm; the apex of its blade is obtuse or acute with the upper surface appears dark green whereas the underside appears pale green and glabrate or hispidulous on veins, and margins having ciliate, entire to coarsely serrate, base cuneate. The cauline leaves are in pinnatifid or pinnatisect shape with 5–15 cm long, lateral segments in 2–5 pairs, both surfaces hispidulous to glabrescent. Its blade is sessile, broadly ovate to lanceolate and margin have coarsely serrate, apex acuminate. The terminal inflorescence is a large corymb composed of cymes with 5–7 pairs of lateral branches; the abaxial surface of the peduncle is densely covered with white hirsute. The campanulate corolla appears yellow and its tube is 1.5 × 1.5 mm. Flowering from July to September, fruiting from September to October (Fig. 1B) (Flora of China Editorial Committee, 1986).
4. Phytochemistry
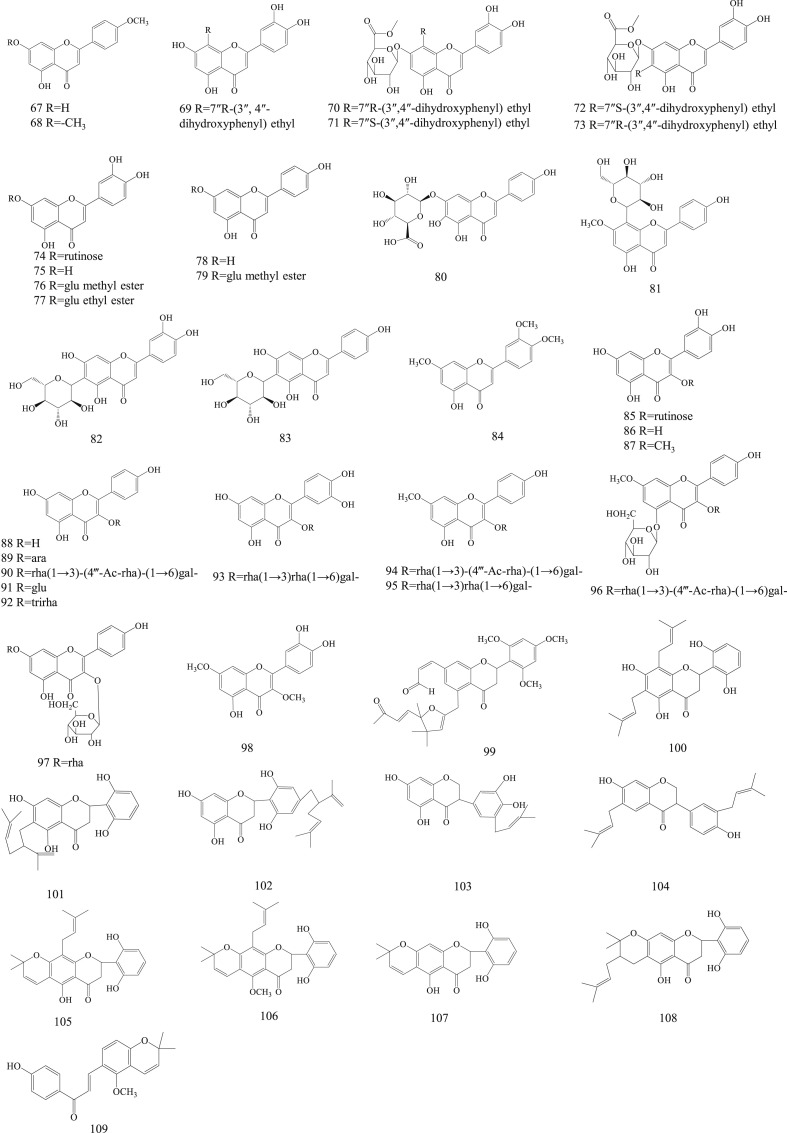
Up to now, 233 compounds have been reported from Herba Patriniae, including 66 triterpenoid saponins, 43 flavonoids, 13 organic acids, 21 iridoids, 69 volatile oils, and 21 miscellaneous compounds. Among them, 153 components are discovered in PS, with triterpenoid saponins and volatile components as the main components, while 102 components, mainly including flavonoids, are from PV. The detailed information for these compounds is summarized in Table 3 .
Table 3.
The compounds in the Herba Patriniae.
| NO. | Compound Name | Molecular Formula | Species | Reference | |
|---|---|---|---|---|---|
| Triterpenoid aglycones and triterpenoid saponins | |||||
| 1 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid | C46H74O15 | PS | Li and Lou (2007) | |
| 2 | Giganteaside D | C41H66O11 | PS | Li and Lou (2007) | |
| 3 | 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid | C47H76O16 | PS | Choi and Woo (1987) | |
| 4 | Patrinia-glycosides B-II | C47H76O16 | PS | Ren et al. (2013) | |
| 5 | Conformer of Patrinia-glycoside B-II | C47H76O16 | PS | Ren et al. (2013) | |
| 6 | 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-arabinopyranosyl oleanolic acid | C41H66O12 | PS | Jiang et al. (2003) | |
| 7 | 3-O-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid | C41H66O11 | PS | Nakanishi et al. (1993) | |
| 8 | Oleanolic acid | C30H48O3 | PS, PV | (Li et al., 2002; Xu et al., 1985) | |
| 9 | Scabiosides B | C41H66O12 | PS | Bukharov et al. (1970) | |
| 10 | 3-O-β-D-xylopyranosyl oleanolic acid | C35H56O7 | PS | Li et al. (2002) | |
| 11 | 3-O-α-L-rhamnopyanosyl-(1 → 2)-β-D-xylopyranosyl oleanolic acid | C41H66O11 | PS | Gao et al. (2011b) | |
| 12 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester | C52H84O20 | PS | Li and Lou (2007) | |
| 13 | 3-O-β-D-glucopyranosyl-(1 → 4)-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl-oleanolic acid 28-O-β-D-glucopyranoside | C58H94O25 | PS | Gao et al. (2011a) | |
| 14 | 3-O-β-D-xylopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl ester | C41H66O12 | PS | Gao et al. (2011b) | |
| 15 | 3-O-α-L-rhamnopanosyl-(1 → 2)-β-D-xylopyanosyl oleanolic acid 28-O-glucopyanosyl ester | C47H76O16 | PS | Gao et al. (2011b) | |
| 16 | 28-O-β-D-glucopyranosyl oleanolic acid | C36H58O8 | PV | Yang et al. (2000) | |
| 17 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl oleanolic acid 28-O-β-D-glucopyranoside | C52H84O20 | PS | Gao et al. (2012) | |
| 18 | 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside | C59H96O26 | PS | Choi and Woo (1987) | |
| 19 | Oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl ester | C42H68O13 | PS | Gao et al. (2011b) | |
| 20 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside | C58H94O25 | PS | Gao et al. (2011a) | |
| 21 | 3-O-β-D-glucopyranosyl-(1 → 4)-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside | C64H104O30 | PS | Gao et al. (2011a) | |
| 22 | 3-O-β-D-glucopyranosyl-(1 → 4)-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl oleanolic acid 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside | C64H104O30 | PS | Gao et al. (2011a) | |
| 23 | Scabiosides F | C57H92O24 | PS | Bukharov and Karlin (1970a) | |
| 24 | Scabiosides G | C63H102O29 | PS | Bukharov and Karlin (1970b) | |
| 25 | Sulfapatrinosides II | C42H67NaO17S | PS, PV | (Inada et al., 1988; Zou, 1994) | |
| 26 | Patrinovilosides A | C46H74O17 | PV | Lee et al. (2013) | |
| 27 | 2α-hydroxyoleanolic acid | C30H48O4 | PS | Li et al. (2002) | |
| 28 | 2α, 3β-23-trihydroxyolean-12-en-28-oic acid | C30H48O5 | PS | Xia et al. (2010) | |
| 29 | 2α, 3β, 19α, 23-tetrahydroxyolean-12-en-28-oic acid | C30H48O6 | PS | Xia et al. (2010) | |
| 30 | 3β, 12α-dihydroxy-oleanan-13β, 28-olide | C30H48O4 | PS | Gao et al. (2011b) | |
| 31 | 3-O-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl-12β, 30-dihydroxy-olean-28, 13β-olide | C41H66O13 | PS | Gao et al. (2011a) | |
| 32 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl-12β, 30-dihydroxy-olean-28, 13β-olide | C46H74O17 | PS | Gao et al. (2011a) | |
| 33 | 3-O-β-D-xylopyranosyl-(1 → 2)-β-D-glucopyranosyl-12β,30-dihydroxy-olean-28, 13β-olide | C41H66O14 | PS | Gao et al. (2011a) | |
| 34 | Patrinolide A | C30H46O5 | PS | Yang et al. (2001) | |
| 35 | 11α, 12α-epoxy-3-O-β-D-xylopyranosyl-olean-28, 13β-olide | C35H54O8 | PS | Gao et al. (2012) | |
| 36 | 11α, 12α-epoxy-3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-β-D-xylopyranosyl-olean-28, 13β-olide | C46H72O16 | PS | Gao et al. (2012) | |
| 37 | 3-Oxooleanolic acid | C30H46O3 | PS | Choi and Woo (1984) | |
| 38 | 3-oxo-29-hydroxy-olean-12-en-28-oic acid | C30H46O4 | PS | Gao et al. (2011b) | |
| 39 | 3, 11-dioxoolean-12-en-28-oic acid | C30H44O4 | PS | Gao et al. (2011b) | |
| 40 | 3-hydroxyolean-11-oxo-12-en-28-oic acid | C30H46O4 | PS | Gao et al. (2011b) | |
| 41 | Prosapogenin CP3 | C64H104O30 | PS | Li and Lou (2007) | |
| 42 | Kalopanaxsaponin B | C59H96O26 | PS | Kim (1997) | |
| 43 | Patrinia saponin H3 | C65H106O31 | PS | Kang et al. (1997) | |
| 44 | Hederagenin | C30H48O4 | PS | Kim (1997) | |
| 45 | Sapindoside A | C41H66O12 | PS | Li and Lou (2007) | |
| 46 | 3-Ο-(2′-O-acetyl)-β-arabinopyranosyl hederagenin | C37H58O9 | PS | Yang et al. (2000) | |
| 47 | 3-Ο-α-L-arabinopyranosyl-(1 → 3)-β-D-xylopyranosyl hederagenin | C40H64O12 | PS | Yang et al. (2000) | |
| 48 | Scabiosides A | C35H5608 | PS | Bukharov et al. (1970) | |
| 49 | Scabiosides C | C41H66013 | PS | Bukharov et al. (1970) | |
| 50 | α-hederin | C41H66O12 | PS | Choi and Woo (1984) | |
| 51 | Hederagenin 3-O-β-D-glucopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranoside | C47H76O17 | PS | Kang et al. (1997) | |
| 52 | Hederagenin 3-O-β-D-glucopyranosyl-(1 → 3′)-(2′-O-acetyl)-α-L-arabinopyranoside | C43H68O14 | PS | Yang et al. (2002) | |
| 53 | 3-O-(2′-O-acetyl)-α-L-arabinopyranosyl hederagenin 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl estsr | C49H78O19 | PS | Choi and Woo (1987) | |
| 54 | 3-O-α-L-arabinopyranosyl hederagenin 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosid | C47H76O18 | PS | Choi and Woo (1987) | |
| 55 | 28-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosyl-hederagenin ester | C42H68O14 | PS | Yang et al. (1998) | |
| 56 | 3-O-α-L-rahmnopyanosyl-(1 → 2)-α-L-arabinopyanosyl hederagenin 28-O-α-L-arabinopyanosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosid | C58H94O26 | PS | Gao et al. (2011a) | |
| 57 | 3-O-β-D-xylopyranosyl-(1 → 3)-α-L-rahmnopyanosyl-(1 → 2)-α-L-arabinopyanosyl hederagenin 28-O-α-L-arabinopyanosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranosid | C63H102O30 | PS | Gao et al. (2011a) | |
| 58 | 2α-hydroxyursolic acid | C30H48O4 | PS | Li et al. (2002) | |
| 59 | Patrinia-glycosides B–I | C47H76O16 | PS | Nakanishi et al. (1993) | |
| 60 | Patrinia-glycosides A-I | C41H66O11 | PS | Nakanishi et al. (1993) | |
| 61 | 3-O-[β-D-glucopyranosyl-(1 → 3)-α-L-arabinopyranosyl] ursolic acid | C41H66O12 | PS | Nakanishi et al. (1993) | |
| 62 | Ursolic acid | C30H48O3 | PV | Li et al. (2008) | |
| 63 | Sulfapatrinosides I | C42H67NaO17S | PS, PV | (Inada et al., 1988; Zou, 1994) | |
| 64 | 23-hydroxyursolic acid | C30H48O4 | PS | Inada et al. (1988) | |
| 65 | Patrinovilosides B | C60H96O27 | PV | Lee et al. (2013) | |
| 66 | 3α-ursoloic acid | C30H48O3 | PS | Gao et al. (2011b) | |
| Flavonoids | |||||
| 67 | Acacetin | C16H12O5 | PV | Han et al. (2020) | |
| 68 | 5-hydroxyl-7, 4′-dimethoxy flavone | C17H14O5 | PV | Peng et al. (2006a) | |
| 69 | 8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5, 7-tetrahydroxyflavone | C23H18O8 | PV | Feng et al. (2018) | |
| 70 | 7-O-β-D-glucuronide methyl ester-8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone | C30H28O14 | PV | Feng et al. (2018) | |
| 71 | 7-O-β-D-glucuronide methyl ester-8-(7″S-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone | C30H28O14 | PV | Feng et al. (2018) | |
| 72 | 7-O-β-D-glucuronide methyl ester-6-(7″S-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone | C30H28O14 | PV | Feng et al. (2018) | |
| 73 | 7-O-β-D-glucuronide methyl ester-6-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone | C30H28O14 | PV | Feng et al. (2018) | |
| 74 | Luteolin-7-O-rutinoside | C27H30O16 | PV | Feng et al. (2018) | |
| 75 | Luteolin | C15H10O6 | PS, PV | (Jang, 2017; J. Y. Peng et al., 2006b) | |
| 76 | Luteolin-7-O-β-D-glucuronide methyl ester | C22H20O12 | PV | Feng et al. (2018) | |
| 77 | Luteolin-7-O-β-D-glucuronide ethyl ester | C23H22O12 | PV | Feng et al. (2018) | |
| 78 | Apigenin | C15H10O5 | PV | Feng et al. (2018) | |
| 79 | Apigenin 7-O-β-D-glucuronide methyl ester | C22H20O11 | PV | Feng et al. (2018) | |
| 80 | Scutellarin | C21H18O12 | PV | Han et al. (2020) | |
| 81 | 8-C glucosylprunetin | C22H22O10 | PV | Peng et al. (2006a) | |
| 82 | Isoorientin | C21H20O11 | PV | Peng et al. (2006a) | |
| 83 | Isovitexin | C21H20O10 | PV | Peng et al. (2006a) | |
| 84 | 5-hydroxyl-7, 3′, 4′-trimethoxy flavone | C18H16O6 | PV | Peng et al. (2006a) | |
| 85 | Rutin | C27H30O16 | PS, PV | (Kim, 1997; Li et al., 2008) | |
| 86 | Quercetin | C15H10O7 | PS, PV | (Jang, 2017; Peng et al., 2006a) | |
| 87 | 3-O-methylquercetin | C16H12O7 | PV | Song et al. (2016) | |
| 88 | Kaempferol | C15H10O6 | PS, PV | (Han et al., 2020; Jang, 2017) | |
| 89 | Kaempferol-3-O-arabinoside | C20H18O10 | PV | Song et al. (2016) | |
| 90 | Kaempferol-3-O-α-L-rhamnopyranosyl-(1 → 3)-(4-O-acetyl)-O-α-L-rhamnopyranosyl-(1 → 6)-O-β-D-galactopyranoside | C35H42O20 | PV | Lee et al. (2013) | |
| 91 | Kaempferol-3-O-β-D-glucopyranoside | C21H20O11 | PV | Huang et al. (2007) | |
| 92 | Kaempferol-3-O-β-D-trirhamninoside | C33H40O18 | PV | Zou (1994) | |
| 93 | Flavovilloside | C33H40O20 | PV | Zou (1994) | |
| 94 | Patrivilosides 1 | C36H44O20 | PV | Lee et al. (2013) | |
| 95 | Catharticin | C34H42O19 | PV | Lee et al. (2013) | |
| 96 | Patrivilosides 2 | C42H54O25 | PV | Lee et al. (2013) | |
| 97 | Kaempferol-3-O-β-D-glucopyranoside-7-O-α-L-rhamnoside | C27H30O15 | PV | Huang et al. (2007) | |
| 98 | 3, 7-dimethoxy-5, 3′, 4′-trihydroxyflavanon | C17H14O8 | PV | Wu et al. (2019) | |
| 99 | Patriniaflavanone A | C33H36O8 | PV | Xiang et al. (2016) | |
| 100 | (2S)-5, 7, 2′, 6′-tetrahydroxy-6, 8-di (γ, γ-dimethylallyl) flavanone | C25H28O6 | PV | Peng et al. (2005c) | |
| 101 | (2S)-5, 7, 2′, 6′-tetrahydroxy-6-lavandulylated flavanone | C25H28O6 | PV | Peng et al. (2006c) | |
| 102 | (2S)-5, 7, 2′, 6′-tetrahydroxy-4′-lavandulylated flavanone | C25H28O6 | PV | Peng et al. (2006c) | |
| 103 | Bolusanthol B | C20H20O6 | PV | Peng et al. (2005c) | |
| 104 | Tetrapterol I | C25H28O4 | PV | Peng et al. (2005c) | |
| 105 | Orotinin | C25H26O6 | PV | Peng et al. (2006b) | |
| 106 | Orotinin-5-methyl ether | C26H28O6 | PV | Peng et al. (2006d) | |
| 107 | (2S)-5, 2′, 6′-trihydroxy-2″, 2″-dimethylpyrano [5″, 6″: 6, 7] flavanone | C20H18O6 | PV | Peng et al. (2006c) | |
| 108 | (2S, 3″S)-5, 2′, 6′-trihydroxy-3″-γ, γ-dimethylallyl-2″,2″-dimethyl-3″, 4″-dihydropyrano [5″, 6″: 6, 7] flavanone | C25H28O6 | PV | Peng et al. (2006c) | |
| 109 | Licoagrochalcone B | C21H20O4 | PV | Peng et al. (2006c) | |
| Organic acids | |||||
| 110 | Chlorogenic acid | C16H18O9 | PS, PV | (Han et al., 2020; Liu et al., 2013a) | |
| 111 | Caffeic acid | C9H8O4 | PS, PV | (Han et al., 2020; Xia et al., 2010) | |
| 112 | Iso-chlorogenic acid A | C25H24O12 | PS, PV | Liu and Lei (2019) | |
| 113 | Iso-chlorogenic acid C | C25H24O12 | PS, PV | Liu and Lei (2019) | |
| 114 | Ferulic acid | C10H10O4 | PS, PV | (Li et al., 2008; Zhao and Yang, 2016) | |
| 115 | Protocatechuic acid | C7H6O4 | PS | Zhao and Yang (2016) | |
| 116 | Gallic acid | C7H6O5 | PS | Zhao and Yang (2016) | |
| 117 | trans-caffeic acid | C9H8O4 | PV | Xiang et al. (2016) | |
| 118 | Hydrocaffeate | C9H10O4 | PV | Han et al. (2020) | |
| 119 | Palmic acid | C16H32O2 | PS, PV | (Liu et al., 2016a; Xu et al., 1985) | |
| 120 | Linoleic acid | C18H32O2 | PV | Tian et al. (2005) | |
| 121 | n-dotriacontanoic acid | C32H64O2 | PV | Peng et al. (2005a) | |
| 122 | Cryptochlorogenic acid | C16H18O9 | PS | Zhong et al. (2017) | |
| Iridoids | |||||
| 123 | Valerosidate | C21H34O11 | PV | Lee et al. (2013) | |
| 124 | Patrinoside | C21H34O11 | PS | Liu et al. (2019c) | |
| 125 | Patrinoside A | C21H32O11 | PS | Liu et al. (2019c) | |
| 126 | Scabroside J | C16H26O9 | PS | Zu (2013) | |
| 127 | Villosol | C10H16O4 | PV | Xu et al. (1985) | |
| 128 | Villosolside | C16H26O9 | PV | Xu et al. (1985) | |
| 129 | Patriscabrol | C10H16O4 | PS | Zu (2013) | |
| 130 | PatriscabrosideⅠ | C16H26O9 | PS | Zu (2013) | |
| 131 | Loganin | C17H26O10 | PS, PV | (Lee et al., 2013; Zu, 2013) | |
| 132 | Loganic acid | C16H24O10 | PV | Lee et al. (2013) | |
| 133 | Isopatriscabrol | C10H16O4 | PS | Zu (2013) | |
| 134 | IsopatriscabrosideⅠ | C16H26O9 | PS | Zu (2013) | |
| 135 | Scabroside L | C16H26O9 | PS | Zu (2013) | |
| 136 | Morroniside | C17H26O11 | PV | Xu et al. (1985) | |
| 137 | Villoside | C16H26O8 | PV | Taguchi et al. (1973) | |
| 138 | Patrinovalerosidate | C21H36O10 | PV | Lee et al. (2013) | |
| 139 | Jatamanin J | C10H18O4 | PS | Zu (2013) | |
| 140 | Scabroside K | C14H19O7 | PS | Zu (2013) | |
| 141 | Jatamanin A | C10H14O4 | PS | Zu (2013) | |
| 142 | 8-Epideoxyloganic acid | C16H24O9 | PS | Zhong et al. (2017) | |
| 143 | 7-Deoxyloganic acid | C16H24O9 | PS | Zhong et al. (2017) | |
| Volatiles | |||||
| 144 | (R)-methyl 2-(benzyloxy)propanoate | C11H14O3 | PS | Xue et al. (2016) | |
| 145 | β-Ionone | C13H20O | PV | Liu et al. (2016a) | |
| 146 | Methyl hexadecanoate | C17H34O2 | PS | Liu et al. (2016a) | |
| 147 | Diisobutyl phthalate | C16H22O4 | PS | Liu et al. (2016a) | |
| 148 | cis-Anethol | C10H12O | PS | Liu et al. (2016a) | |
| 149 | Methyl isovalerate | C6H12O2 | PS | Liu et al. (2016a) | |
| 150 | o-Cymene | C10H14 | PS | Xue et al. (2016) | |
| 151 | Camphogen | C10H14 | PV | Liu et al. (2016a) | |
| 152 | Isodurene | C10H14 | PV | Liu et al. (2016a) | |
| 153 | 4-Methyldecane | C11H24 | PV | Liu et al. (2016a) | |
| 154 | n-Heptadecane | C17H36 | PS | Liu et al. (2016a) | |
| 155 | n-Tetradecane | C14H30 | PS | Liu et al. (2016a) | |
| 156 | Toluene | C7H8 | PS | Liu et al. (2016a) | |
| 157 | α-Terpineol | C10H18O | PS | Xue et al. (2016) | |
| 158 | cis-β-Terpineol | C10H18O | PS | Xue et al. (2016) | |
| 159 | 4-Terpinenol | C10H18O | PS | Xue et al. (2016) | |
| 160 | Borneol | C10H18O | PS | Xue et al. (2016) | |
| 161 | trans-piperitol | C10H18O | PS | Xue et al. (2016) | |
| 162 | α-cadinol | C15H26O | PS | Xue et al. (2016) | |
| 163 | Spathulenol | C15H24O | PS | Xue et al. (2016) | |
| 164 | γ-eudesmol | C15H26O | PS | Xue et al. (2016) | |
| 165 | Lanceol | C15H24O | PS | Xue et al. (2016) | |
| 166 | Phytol | C20H40O | PS | Xue et al. (2016) | |
| 167 | Cedrol | C15H26O | PS | Liu et al. (2016a) | |
| 168 | Linolool oxide | C10H18O2 | PS | Liu et al. (2016a) | |
| 169 | Artemisia ketone | C10H16O | PS | Xue et al. (2016) | |
| 170 | Thujone | C10H16O | PS | Xue et al. (2016) | |
| 171 | 2-Camphanone | C10H16O | PS | Xue et al. (2016) | |
| 172 | β-Damascenone | C13H18O | PS, PV | Liu et al. (2016a) | |
| 173 | Nerylacetone | C13H22O | PV | Liu et al. (2016a) | |
| 174 | Geranylacetone | C13H22O | PS | Liu et al. (2016a) | |
| 175 | γ-Terpinene | C10H16 | PS | Xue et al. (2016) | |
| 176 | α-Copaene | C15H24 | PS | Xue et al. (2016) | |
| 177 | Caryophyllene | C15H24 | PS | Yang et al. (2007) | |
| 178 | α-Caryophyllene | C15H24 | PS | Yang et al. (2007) | |
| 179 | δ-elemene | C15H24 | PS | Tian and Cao (2004) | |
| 180 | β-gurjunene | C15H24 | PS | Tian and Cao (2004) | |
| 181 | Aristolene | C15H24 | PS | Tian and Cao (2004) | |
| 182 | D-Limonene | C10H16 | PS | Liu et al. (2016a) | |
| 183 | (1S)-(1)-beta-Pinene | C10H16 | PS | Liu et al. (2016a) | |
| 184 | Germacrene B | C15H24 | PS | Xue et al. (2016) | |
| 185 | β-Copaene | C15H24 | PS | Xue et al. (2016) | |
| 186 | Aromadendrene | C15H24 | PS | Xue et al. (2016) | |
| 187 | β-Farnesene | C15H24 | PS | Xue et al. (2016) | |
| 188 | delta-Cadinene | C15H24 | PS | Xue et al. (2016) | |
| 189 | β-Cadinene | C15H24 | PS | Tian and Cao (2004) | |
| 190 | Caryophyllene oxide | C15H24O | PS | Xue et al. (2016) | |
| 191 | Hexanal | C6H12O | PS, PV | Liu et al. (2016a) | |
| 192 | Benzeneacetaldehyde | C8H8O | PS, PV | Liu et al. (2016a) | |
| 193 | 1-Nonanal | C9H18O | PV | Liu et al. (2016a) | |
| 194 | β-cyclocitral | C10H16O | PV | Liu et al. (2016a) | |
| 195 | 2-Hexenal | C6H10O | PV | Liu et al. (2016a) | |
| 196 | Octadecanal | C18H36O | PS | Liu et al. (2016a) | |
| 197 | Hexadecanal | C16H32O | PS | Liu et al. (2016a) | |
| 198 | Benzaldehyde | C7H6O | PS | Liu et al. (2016a) | |
| 199 | Furfural | C5H4O2 | PS | Liu et al. (2016a) | |
| 200 | Tetradecanoic acid | C14H28O2 | PS | Liu et al. (2016a) | |
| 201 | Nonanoic acid | C9H18O2 | PS | Liu et al. (2016a) | |
| 202 | 3-Methylpentanoic acid | C6H12O2 | PS | Liu et al. (2016a) | |
| 203 | Hexanoic acid | C6H12O2 | PS | Liu et al. (2016a) | |
| 204 | 5-Methyl-2-acetylfuran | C7H8O2 | PS | Xue et al. (2016) | |
| 205 | 2-Pentylfuran | C9H14O | PS, PV | Liu et al. (2016a) | |
| 206 | Anethofuran | C10H16O | PV | Liu et al. (2016a) | |
| 207 | 1, 8-Cineole | C10H18O | PS | Liu et al. (2016a) | |
| 208 | 2H-chromene | C9H8O | PV | Liu et al. (2016a) | |
| 209 | Dehydro-ar-ionene | C13H16 | PV | Liu et al. (2016a) | |
| 210 | α-Ionene | C13H18 | PS, PV | Liu et al. (2016a) | |
| 211 | Propofol | C12H18O | PV | Liu et al. (2016a) | |
| 212 | Phenanthrene | C14H10 | PS | Liu et al. (2016a) | |
| Other Compounds | |||||
| 213 | β-sitosterol | C29H50O | PS, PV | (Li et al., 2008; Zu, 2013) | |
| 214 | β-daucosterol | C35H60O6 | PV | Li et al. (2008) | |
| 215 | 7β-hydroxysitosterol | C29H50O2 | PS, PV | (Peng et al., 2005a; Zhao and Yang, 2016) | |
| 216 | Stigmasterol | C29H48O | PV | Peng et al. (2005a) | |
| 217 | 2, 6, 2′, 6′-tetramethoxy-4, 4′-bis (1, 2-trans-2, 3-epoxy-1-hydroxypropyl) biphenyl | C22H26O8 | PV | Yan et al. (2016) | |
| 218 | 2, 6, 2′, 6′-tetramethoxy-4, 4′-bis (2, 3-epoxy-1-hydroxypropyl) biphenyl | C22H26O8 | PV | Yan et al. (2016) | |
| 219 | (7S, 8R)-3′, 4, 9′-trihydroxy-4-methoxy-9-O-shikkyl-acyl-7, 8-dihydrobenzofuran-1′-propyl ligana | C20H26O6 | PS | Jang (2017) | |
| 220 | Laricircsinol | C20H24O6 | PS | Jang (2017) | |
| 221 | 1β-O-β-D-glucopyranosyl-15-O-(p-hydroxylphenylacetyl)-5α, 6βH-eudesma-3-en-12, 6α-olide | C29H38O11 | PV | Yang et al. (2016) | |
| 222 | Aurentiamide acetate | C27H28N2O4 | PV | Peng et al. (2005b) | |
| 223 | Patriscabratine | C27H28N2O4 | PS | Zhong et al. (2017) | |
| 224 | trans-caffeic acid methylate | C10H10O4 | PV | Xiang et al. (2016) | |
| 225 | Chlorogenic acid n-butyl ester | C20H26O9 | PV | Yang et al. (2016) | |
| 226 | Caffeic acid ethyl ester | C11H12O4 | PV | Liu et al. (2019a) | |
| 227 | Caffeic acid n-butyl ester | C13H16O4 | PV | Liu et al. (2019a) | |
| 228 | p-hydroxyphenylacetic acid methyl ester | C9H10O3 | PV | Xiang et al. (2016) | |
| 229 | 3, 4, 5-tri-O-p-hydroxylphenylacetylquinic acid methyl ester | C32H32O12 | PV | Yang et al. (2016) | |
| 230 | 3, 4-di-O-caffeoylquinic acid methyl ester | C26H26O12 | PV | Yang et al. (2016) | |
| 231 | Aesculetin | C9H6O4 | PS | Xiang et al. (2016) | |
| 232 | Inositol | C6H12O6 | aHerba Patriniae | Wang et al. (2002) | |
| 233 | Impecylone A | C14H14O5 | PV | Liu et al. (2019b) | |
Patrinia villosa Juss. (PV); Patrinia scabiosafolia Fisch. (PS).
Not indicate species.
4.1. Triterpenoid aglycones and triterpenoid saponins
Triterpenoid aglycones and triterpenoid saponins are one of the main active constituents in Herba Patriniae. To date, more than 66 compounds (1–66) have been isolated from Herba Patriniae. It's worth noting that 62 compounds are in PS, 7 compounds are in PV, and only 3 compounds are in both PS and PV. According to the aglycone, all of them were divided into oleanane type (1–57) and ursane type (58–66). Further, the oleanane type is divided into 5 different types, including oleanolic acid type (1–29), 13, 28-epoxy-oleanolic acid type (30–34), 11, 12 epoxy-13, 28-cyclooxy-oleanolic type (35–36), 3-carbonyl oleanolic acid type (37–40), and hederagenin type (41–57) according to their structures. Their chemical structures were draw by ChemBioDraw Ultra 14.0 and described in Fig. 2 .
Fig. 2.
Chemical structures of triterpenoid aglycones and triterpenoid saponins in Herba Patriniae.
Oleanolic acid (8), hederagenin (44) and ursolic acid (62) are typical representatives of triterpenoid aglycones in Herba Patriniae. An increasing number of studies have proved that oleanolic acid (8) possess antitumor (Mbaveng et al., 2020), antimicrobial (Zhou et al., 2020), and antiviral (Meng et al., 2019) activities. Hederagenin (44) gave anti-inflammatory and anti-apoptotic activities to alleviate ethanol-induced liver damage (Kim et al., 2017). Ursolic acid (62) has been reported with antioxidant and antiproliferative activities (Zhang et al., 2020), as well as protective effects against cisplatin-induced ototoxicity (Di et al., 2020) and alleviates hypercholesterolemia (Hao et al., 2020). In addition, giganteaside D (2) was found with the induction effect on ROS-mediated apoptosis (Liu et al., 2016b). Alpha-hederin (50) showed acute anti-inflammatory activities in carrageenan-induced rat paw edema (Gepdiremen et al., 2005). But the biological activities of most triterpenoid aglycones and triterpenoid saponin were still unclear.
4.2. Flavonoids
Flavonoids is a class of famous natural products with widely biological activities, such as anti-oxidation, anti-inflammation and anti-tumor. There are 43 flavonoids were identified in Herba Patriniae. They are mainly classified into five groups according to the structure of aglycone, including flavones (67–84), flavonols (85–98), flavonones (99–102), isoflavanones (103–104), and other types (105–109). Luteolin (75), apigenin (78) and scutellarin (80) were blong to flavones. All of them have been widely studied in cardiovascular and tumor fields (Park et al., 2020; Xu et al., 2020; Bao et al., 2020). Isoorientin (82) and isovitexin (83) were two specific compounds in Herba Patriniae due to C-6 forming a C–C bond. Quercetin (86) and kaempferol (88) are two of the most common flavonols in dicotyledons. Compounds 85, 87 and 93 were derived from quercetin, while compounds 89, 90, 91, 92, 94, 95, 96, 97 and 98 were derived from kaempferol. It is interesting to note that more than 43 flavonoids (67–109) have been identified from PV, but are rarely found in PS (75, 85, 86, 88). Their chemical structures are prescribed in Fig. 3 .
Fig. 3.
Chemical structures of flavonoids in Herba Patriniae.
4.3. Organic acids
To date, only 13 organic acids (110–122) have been identified from Herba Patriniae. The contents of chlorogenic acid (110), caffeic acid (111) and protocatechuic acid (115) are higer. These phenolic compounds possess many health-promoting properties, including antioxidant, antiinflammatory, antidiabetic, and antihypertensive activities (Al-Megrin et al., 2020; Paciello et al., 2020; El-Sonbaty et al., 2019; Li et al., 2020). For example, protocatechuic acid administration for twelve-week could improves insulin-induced vasorelaxation in aging spontaneously hypertensive rats. Of these, 10 organic acids (110–114 and 117–121) are in PV, 9 organic acids (110–116, 119 and 122) are in PS. Among them, 6 compounds are in both PS and PV. Their chemical structures are shown in Fig. 4 .
Fig. 4.
Chemical structures of organic acids in Herba Patriniae.
4.4. Iridoids
Iridoids are a kind of very important natural products in plant kingdom, structurally characterized with bicyclic cis-fused cyclopentane-pyrans or cleavage of a bond in the cyclopentane ring (secoiridoid). Clinically, iridoids extract or traditional Chinese medicine rich in iridoids have been proved with anti-inflammatory, antiviral and antitumor effects, as well as protection of cardiovascular and immunomodulatory activities (Wang et al., 2020; Jie et al., 2015; Ji et al., 2019; Luan et al., 2019). To date, 21 iridoids (123–143) have been isolated in Herba Patriniae.
Of these, loganin (131), loganic acid (132) and morroniside (136) are the compounds with more reported biological activities. Loganin has been proved with inhabitation effect on inflammatory response (Wen et al., 2020; Wei et al., 2013a) and mitigative effect on osteoarthritis (Hu et al., 2020). Morroniside has shown protective effect against H2O2-induced damage in human neuroblastoma cells (Zhong et al., 2017). It is worth pointing out that 8 iridoids were obtained from PV, and 14 iridoids were isolated from PS. Only 1 compound (131) was a common constituent of the two plants. Their chemical structures were prescribed in Fig. 5 .
Fig. 5.
Chemical structures of iridoids in Herba Patriniae.
4.5. Volatiles
The volatile constituent is one of the most abundant compounds in Herba Patriniae with many biological activities, such as antioxidant, antiinflammatory and anti-tumor effects, and it also proved with sedative and hypnotic effects (Lin et al., 2018; Luo et al., 1986). Approximately 69 volatile compounds (144–212) have been isolated from Herba Patriniae. All the volatiles were obtained by GC-MS method and list here for reference. Of these, 57 volatiles were isolated from PS, and 17 volatiles were separated from PV. Their chemical structures were prescribed in Fig. 6 . These two sources of plants have 5 common volatile constituents.
Fig. 6.
Chemical structures of volatile in Herba Patriniae.
4.6. Other compounds
In addition to the above compounds, Herba Patriniae also contains steroids (213–216), lignans (217–220), sesquiterpene lactone glycosides (221), amides (222–223), and other compounds (224–233). Of these, 17 compounds were isolated from PV, and 7 compounds were obtained from PS. Compounds 213 and 215 are common components of the two plants. Their chemical structures were prescribed in Fig. 7 .
Fig. 7.
Chemical structures of other compounds in Herba Patriniae.
5. Pharmacology
“Heat” is a conception in traditional Chinese medicine, which is the synonym of “fire” and is the predominant pathogenic factor of summer. Excessive “heat” consumes Yin fluid and results in imbalance between Yin and Yang, which can produce endogenous toxins lurking in the human body (Tu et al., 2016). With activities of heat-clearing and detoxifying, Herba Patriniae exerted anti-cancer, anti-inflammatory, antioxidant, antimicrobial effects, as well as a sedative, hypnotic, proangiogenic, anti-diabetic, antipruritic, and anti-diarrheal effects in vitro and in vivo. There are different pharmacological effects between PV and PS. We have enlisted an overview of the modern pharmacological studies in the following sections (Table 4 ).
Table 4.
Pharmacological effects of Herba Patriniae.
| Model | Experimental subject and administration | Results | Species | Reference |
|---|---|---|---|---|
| Anti-cancer effect | ||||
| CRC cell line SW480 (negative control: culture solution) | total saponin extract (PV was crushed and immersed in 1000 mL 70% ethanol for 12 h, refluxed and extracted at 40 °C, and concentrated under reduced pressure to remove ethanol and purified to obtain the total saponin extract.); 1/16, 1/32, 1/64 and 1/128 mL/mL for 48 h | The total saponin of PV significantly inhibited TGF-β-induced EMT, and up-regulated the expression of E-cadherin and down-regulated the expression of N-cadherin and NF-κBp65 in CRC SW480 cell. | PV | Xia et al. (2018) |
| CRC mouse xenograft model (negative control: saline), HT-29 and HUVECs cells (negative control: culture solution) | ethanol extract (500 g PS was extracted by refluxing with 5000 mL 85% ethanol and filtered. The ethanol solvent was concentrated by rotary evaporation to a relative density of 1.05. The dry powder was obtained by spray drying. mouse: the powder was dissolved in saline with a working concentration of 250 mg/mL; cells: the powder was dissolved in 50% DMSO with a stock concentration of 250 mg/mL); mouse: intragastric administration, 1.93 g/kg/day, 5 days a week for 21 days; cells: 0.5, 1, 2 mg/mL for 24 h | After treatment with the ethanol extract of PS, the tumor volume of CRC xenograft mice (0.65 ± 0.15 cm3) was significantly suppressed compared with the control group (1.20 ± 0.31 cm3), the tumor angiogenesis of CRC xenograft mice and HUVECs were suppressed in a dose-dependent manner (0.5–2 mg/mL), and the VEGF-A expression of CRC xenograft mice and HT-29 cells were obviously decreased. | PS | Chen et al. (2013) |
| CRC mouse xenograft model (negative control: saline), HT-29 cells (negative control: culture solution) | ethanol extract (500 g PS was extracted by refluxing with 5000 mL 85% ethanol and filtered. The ethanol solvent was concentrated by rotary evaporation to a relative density of 1.05. The dry powder was obtained by spray drying. mouse: the powder was dissolved in saline with a working concentration of 250 mg/mL; cells: the powder was dissolved in 50% DMSO with a stock concentration of 250 mg/mL); mouse: intragastric administration, 1.93 g/kg/day, 5 days a week for 21 days; cells: 0.5, 1, 2 mg/mL for 24 h | The ethanol extract from PS treatment increased apoptosis and the ratio of pro-apoptotic Bax/Bcl-2 in HT-29 cells and CRC tumor tissues. It also induced a decrease in mitochondrial membrane potential in HT-29 cells and activated caspase-9 and -3. | PS | Liu et al. (2013b) |
| SMMC-7721 cells (negative control: 0.1% (v/v) DMSO culture solution; positive control: 5-Fluorouracil) | Patriniaflavanone A (The air-dried leaves of PV (15 kg) were extracted with 70% ethanol reflux for 3 times. After purification, 16.5 mg of Patriniaflavanone A was obtained.) | Patriniaflavanone A exhibited a moderate cytotoxic effect on SMMC-7721 cells with an IC50 value of 61.27 μM. | PV | Xiang et al. (2016) |
| A375-S2, A549, HeLa; HepG2, HT1080, K562, HL-60 and U937 cells (negative control: culture solution; positive control: 5-Fluorouracil) | Patrinia-glycosides B-II (Patrinia-glycosides B-II was synthesized by linear 11-step sequence 11 with an overall yield of 9.4%.); administration 48 h | Patrinia-glycosides B-II showed powerful inhibitory activity against eight tumor cell lines at micromolar concentrations (3.4–28.7 μM). | PS | Ren et al. (2013) |
| AGS, SGC-7901, BV-2, 5-FU/HCT-8, HepG2, HT-29, HeLa and MDA-MB-231cells (negative control: culture solution) | essential oil extract (The dried whole plant of PS (500 g) was distilled with double distilled water of 5000 mL for 4 h, and the yellow essential oil of 0.2 mg/g (w/w) was obtained.); 50–200 μg/mL for 24 h | The essential oil of PS exhibited remarkable dose-dependent growth inhibition in the dilution range of 50–200 μg/mL. | PS | Lin et al. (2018) |
| A498, A549, BEL-7402, HT-29, MCF-7, K562 and SGC-7901 cell lines | six flavonoids isolated from PV (A 75% aqueous ethanol crude extract (400 mg) of the leaves of PV was separated in one single isolation procedure to obtain 44.9 mg of (2S)-5, 7, 2′, 6′-tetrahydroxy-6, 8-di (γ, γ-dimethylallyl) flavanone with 99.1% purity, 35.5 mg of (2S)-5, 7, 2′, 6′-tetrahydroxy-6-lavandulylated flavanone with 98.8% purity, 79.8 mg of (2S)-5, 7, 2′, 6′-tetrahydroxy-4′-lavandulylated flavanone with 99.3% purity, and 45.8 mg of (2S)-5, 2′, 6′-trihydroxy-2″, 2″-dimethylpyrano [5″, 6′′: 6, 7] flavanone with purity 98.8%, 39.8 mg of (2S, 3″S)-5, 2′, 6′-trihydroxy-3″-γ, γ-dimethylallyl-2″, 2″-dimethyl-3″, 4″-dihydropyrano [5″,6′′: 6, 7] flavanone with 98.6% purity, 9.6 mg of licoagrochalcone B with 97.5% purity.); administration 3 day | (2S)-5, 7, 2′, 6′-tetrahydroxy-6, 8-di (γ, γ-dimethylallyl) flavanone, (2S)-5, 7, 2′, 6′-tetrahydroxy-6-lavandulylated flavanone and (2S)-5, 7, 2′, 6′-tetrahydroxy-4′-lavandulylated flavanone exhibited high anticancer activities (IC50 < 7 μg/mL) in a dose-dependent manner, and when the concentration of these compounds exceeded 15 μg/mL, the proliferation of cancer cells were completely inhibited, especially for K562 cancer cells (IC50 < 3.1 μg/mL). | PV | Peng et al. (2006c) |
| U14 mice of cervical cancer (negative control: distilled water; positive control: cyclophosphamide) | ethanol extract (100 g PV was refluxed and extracted with 1000 mL 70% ethanol for 2 h, extracted twice, and the solid crude extract was obtained by vacuum drying.); intragastric administration, 10, 15 mg/kg/day, 14 days | The ethanol extract of PV increased the tumor inhibition rate of U14-bearing mice with the inhibitory rate of 49.19% and 54.23% at the dosage of 10 g/kg and 15 g/kg, respectively. | PV | Chen et al. (2019c) |
| SMMC-7721 cell lines (negative control: culture solution) | total flavonoids extract (50 g PS was refluxed and extracted with 750 mL 60% ethanol for 1 h, extracted for 3 times, then evaporated and purified to obtain total flavonoids with a purity of 91.32%.); 0.125–2 mg/mL | The total flavonoids extract suppressed the growth of SMMC-7721 cells in a dose-dependent manner (0.125–2 mg/mL). | PS | Zhao et al. (2019) |
| SMMC-7721 cell lines (negative control: culture solution) | total flavonoids extract (10 g PV was refluxed and extracted with 100 mL70% ethanol for 1.5 h, extracted for 3 times, then combined and dried to obtain 0.094 g extract. The extraction method of PS was the same as PV, the extract was 0.115g); 1–10 mg/mL for 24 h | The total flavonoids extract PS and PV inhibited the growth of SMMC-7721 cells in a concentration-dependent manner (1–10 mg/mL), with IC50 values of 7.631 and 5.63 mg/mL, respectively. | PS, PV | Wei et al. (2013b) |
| A375, A549, MCF-7, HepG2 and PC-3 cell lines (negative control: 0.5% (v/v) DMSO culture solution) | ethyl acetate extract (100 g PS was immersed in 1000 mL methanol for 3 days at room temperature, immersed twice. The combined methanol was rotary evaporated for 2 h, dried in a dryer for 1 day (0.5 g), then dissolved in 10 mL distilled water, extracted with ethyl acetate and dried (0.05 g), and the dry powder was dissolved in DMSO (100 mg/mL), filtered and blown dry.); 0.8–800 μg/mL for 48 h | The extract has the most significant growth inhibitory effect on MCF-7 cells (IC50 = 112.3 μg/mL), which is through activation of caspase-independent mitochondrial cell death pathway. | PS | Chiu et al. (2006) |
| U266 cells (negative control: 0.5% (v/v) DMSO culture solution) | ethanol extract (500 g PS was extracted by refluxing with 5000 mL 85% ethanol and filtered. The ethanol solvent was concentrated by rotary evaporation to a relative density of 1.05. The dry powder was obtained by spray drying and dissolved with DMSO to 200 mg/mL); 0.25–1 mg/mL for 24 h | The ethanol extract dose-dependently reduced proliferation and promoted the apoptosis of cancer cells via inhibition of the STAT3 pathway at the dosage of 0.25–1 mg/mL. | PS | Peng et al. (2011) |
| U14 mice of cervical cancer (negative control: distilled water; positive control: cyclophosphamide) | saponin extract (4 kg PV was refluxed and extracted with 10 volumes 70% ethanol for 4 h, extracted for 3 times, and evaporated under reduced pressure to obtain an ethanol extract. By separating the ethanol extract, 25.2 g of dry saponin extract was obtained.); intragastric administration, 50, 100 mg/kg/day, 15 days | The saponin extract remarkably inhibited the tumor growth of mice bearing the U14 cervical cancer cells in a dose-dependent manner (50, 100 mg/kg). Which induced the apoptosis of tumor cells and reduced the ratio of tumor cells in the G0/G1 phase, and decreased the expression of PCNA and Bcl-2, mutant p53 protein. | PV | Zhang et al. (2008) |
| HepG2, A549 and A2780 cell lines (negative control: culture solution) | Impecylone A (The air-dried leaves of PV (15 kg) were refluxed and extracted with 70% ethanol for 2 h, extracted for three times, and evaporated under reduced pressure to obtain a concentrated extract (2.8 kg). After purification, 23.2 mg of Impecylone A was obtained.); A549 cells: 12.5–50 μM; HepG2 cells: 20–80 μM; A2780 cells: 6.25–100 μM; 24 h | Impecylone A remarkably inhibited the proliferation of A549 and HepG2 cells with the IC50 value of 29.22 μM and 38.37 μM, respectively, but had no remarkable inhibitory effect on A2780 cells. Which induced apoptosis and cell cycle arrest at the G2/M phase in both two cells in a dose-dependent manner (12.5–50 μM and 20–80 μM, respectively). | PV | Liu et al. (2019b) |
| HepG2 and MCF-7 cells (negative control: culture solution; positive control: cisplatin) | Patrinia monoterpene iridoid ether esters extract (After immersed Herba Patriniae with 95% ethanol for 48 h, it was extracted with dichloromethane three times and separated, the yield was 1.24%.); HepG2 cells: 12.5–50 μg/mL; MCF-7 cells: 1.75–7 μg/mL, 24 h | The Patrinia monoterpene iridoid ether esters extract inhibited proliferation and induced apoptosis, down-regulated the expression of Bcl-2, Cdc2 and Cyclin B1, and up-regulated the expression of Bax and caspase3 in HepG2 and MCF7 cells. | aHerba Patriniae | Ji et al. (2019) |
| HCT-8 and 5-FU/HCT-8 cells (negative control: culture solution) | ethanol extract (500 g PS was extracted by refluxing with 5000 mL 85% ethanol and filtered. The ethanol solvent was concentrated by rotary evaporation to a relative density of 1.05. The dry powder was obtained by spray drying. The powder was dissolved in 50% DMSO with a stock concentration of 250 mg/mL); 0.5–2 mg/mL for 24 h | 1 and 2 mg/mL of the ethanol extract remarkably inhibited the growth of 5-FU/HCT-8 cells by suppressing cellular proliferation and promoting apoptosis, and significantly reduced the level of phosphorylated AKT and the ratio of Bcl-2/Bax by inhibiting AKT pathway. | PS | Huang et al. (2019a) |
| 786-O and HK-2 cells (negative control: culture solution) | ethanol extract (1 kg PS was immersed in 10 L 95% ethanol for 3 days and filtered. The filtrate was freeze-dried after the relative density was 1.05 in vacuum evaporator. And dissolved in DMSO to form a stock solution with a concentration of 300 mg/mL); 0.2–1 mg/mL for 24 h; 0.6 mg/mL for 0–24 h | The ethanol extract of PS inhibited the growth and promoted the death of 786-O cells in both dose- (0.2–1 mg/mL) and time-dependent (0–24 h) manner. At the dose of 0.6 or 1 mg/mL, it markedly increased the levels of intracellular ROS and Ca2+, and significantly down-regulated the expression of SIRT-1 and reduced the ratio of pmTOR/mTOR. | PS | Li et al. (2018) |
| CRC mouse xenograft model (negative control: saline), HT-29 (negative control: culture solution) | ethanol extract (500 g PS was extracted by refluxing with 5000 mL 85% ethanol and filtered. The ethanol solvent was concentrated by rotary evaporation to a relative density of 1.05. The dry powder was obtained by spray drying. mouse: the powder was dissolved in saline with a working concentration of 250 mg/mL; cells: the powder was dissolved in 50% DMSO with a stock concentration of 250 mg/mL); mouse: intragastric administration, 1.93 g/kg/day, 5 days a week for 3 weeks; cells: 0.5–2 mg/mL for 24 h | The ethanol extract of PS (1.93 g/kg) markedly inhibited the tumor volume and the expression of PCNA in CRC mice, and dose-dependently (0.5–2 mg/mL) decreased the proliferation of HT-29 cells by G1/S cell cycle arrest. It also down-regulated the expression levels of CyclinD1 and CDK4 both in vivo and in vitro at the level of mRNA and protein. | PS | Zhang et al. (2015) |
| Anti-inflammatory effect | ||||
| Female ICR mice, Female SD rats, PID rat model (negative control: saline; positive control: dexamethasone) | ethanol extract (30g PV was extracted with 300 mL 70% ethanol under reflux for 1h, extracted twice, filtered and concentrated to 1 g/mL concentration, and then dried.); female ICR mice: intragastric administration, 0.08 g/kg for 0, 0.25, 0.5, 1 and 2 h to measure the ear edema thickness, or continuously observe and record the number of writhes in the 15min; SD rats: intragastric administration, 0.55 g/kg for 1 h to measure the paw volume or 0.08 g/kg for 7 days to measure the cotton pellet weight; PID rats: intragastric administration, 0.55 g/kg for 28 days | The 70% ethanol extract of PV (0.08 g/kg) was significantly reduced the ear edema thickness induced by arachidonic acid and the writhing response induced by acetic acid in ICR mice (80% and 48%, respectively), and (0.55 g/kg and 0.08 g/kg) inhibited the carrageenin-induced paw edema and the granuloma formation induced by a cotton pellet in SD rats (23% and 44%, respectively), as well as markedly (0.55 g/kg) suppressed the serum levels of IL-8, TNF-α, and IL-6 protein in PID rats. | PV | Zheng et al. (2012) |
| AP rat model (negative control: saline) | water extract (Decocting PS with boiling distilled water for 3 h); intragastric administration, 100 mg/kg/day for 5 days | The water extract of PS (100 mg/kg) reduced the ratio of pancreatic weight/body weight, the levels of serum lipase and amylase, and suppressed the secretion of IL-1β, IL-6, and TNF-α in AP rats, as well as increasing the levels of HSP60 and HSP72 in pancreas. | PS | Seo et al. (2006) |
| PID rat model (negative control: saline) | ethanol extract (500 g PS was extracted with 5 L of hot ethanol under reflux for 1h, extracted twice, filtered and dried to yield 63.9 g); intragastric administration, 600 mg/kg/day for 21 days | After treatment with ethanol extract of PS (600 mg/kg), the infiltration of inflammatory cells and the expression of cytokines in the upper genital tract of PID rats were significantly decreased. | PS | Zou et al. (2015) |
| RAW 264.7 cells (negative control: culture solution); BALB/c mice (negative control: saline) | ethyl acetate extract (15 kg PS was extracted with hot ethanol under reflux for 4h, extracted for four times, and 220 g total extract was obtained by vacuum evaporation. This extract was then dissolved in distilled water, extracted and separated with ethyl acetate, and evaporated in vacuo to yield 5.9 g); cells: 10–100 μg/mL for 24 h; mice: intragastric administration, 300 mg/kg for 24 h | The ethyl acetate extract of PS treatment on RAW 264.7 cells, inhibited the production of NO and IL-6 induced by LPS and the expression of iNOS and COX-2 at the protein and mRNA levels in a concentration-dependent manner (10–100 μg/mL), in which the inhibition was operated by suppressed the level of NF-κB activity. In addition, the ethyl acetate extract of PS inhibited the production of TNF-α and IL-6 in splenocytes of BALB/c mice stimulated by LPS. | PS | Lee et al. (2012) |
| UC mice model (negative control: saline; positive control: 5-ASA) | methanol extract (206.65 g PS was refluxed and extracted twice with 70% methanol, and evaporated under reduced pressure to obtain a solid extract 22.21 g); intragastric administration, 10, 30, 50 mg/kg for 7 days | The administration of 10, 30 and 50 mg/kg of the methanol extract of PS for 7 days in UC mouse model considerably reduced ulcerative colitis DAI scores and tissue MPO accumulation, prevented enlargement of spleen and shortening of colon length in a dose-dependent manner, and also inhibited the mRNA expression of IL-1β, IL-6 and TNF-α. | PS | Cho et al. (2011) |
| BV-2 cells (negative control: culture solution; positive control: indometacin) | essential oil extract (The dried whole plant of PS (500 g) was distilled with double distilled water of 5000 mL for 4 h, and the yellow essential oil of 0.2 mg/g (w/w) was obtained.); 100, 150, 200 μg/mL for 24 h | The secretion of IL-1β and IL-6 induced by LPS in BV-2 cells was remarkably inhibited by the essential oil extract of PS treatment in a dose-dependent manner (100–200 μg/mL). Therefore, the essential oil extract of PS has a significant anti-neuroinflammatory activity. | PS | Lin et al. (2018) |
| Rat models of focal cerebral ischemia-reperfusion (negative control: saline; positive control: nimodipine and Naoluotong) | total flavonoids (The content of total flavonoids in Herba Patriniae is 51.76%.); intragastric administration, 50, 100, 200 mg/kg/day for 7 days | The total flavonoids (50, 100, 200 mg/kg) significantly reduced the levels of IL-1β, ICAM-1, IL-6, Caspase-3, Bax and enhanced the levels of Bcl-2 and NGF in the brain tissue of rats. Moreover, the total flavonoids (100, 200 mg/kg) significantly suppressed the percentage of cerebral infarction area, the production of NF-κB p65 and TNF-α, which showed significant neuroprotective effect. | aHerba Patriniae | Wei et al. (2019) |
| Antioxidant effect | ||||
| Caco2 cells (negative control: culture solution) | eleven phenylethyl flavones (PV was extracted with ethanol under reflux for 2h, extracted twice, and 8.2 g crude extract was separated and purified to yield 8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5, 7-tetrahydroxyflavone (2.7 mg), 7-O-β-D-glucuronide methyl ester-8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone (9.5 mg), 7-O-β-D-glucuronide methyl ester-8-(7″S-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone (21.3 mg), 7-O-β-D-glucuronide methyl ester-6-(7″S-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone (7.7 mg), 7-O-β-D-glucuronide methyl ester-6-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone (10.5 mg), Luteolin-7-O-rutinoside (13.9 mg), Luteolin (11 mg), Luteolin-7-O-β-D-glucuronide methyl ester (12.4 mg), Luteolin-7-O-β-D-glucuronide ethyl ester (7.2 mg), Apigenin (6.3 mg), Apigenin 7-O-β-D-glucuronide methyl ester (11.6 mg).); 25 μM for 24 h |
8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5, 7-tetrahydroxyflavone, 7-O-β-D-glucuronide methyl ester-8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone and 7-O-β-D-glucuronide methyl ester-6-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone isolated from PV (25 μM) reduced the generation of ROS in Caco2 cells induced by H2O2, 8-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5, 7-tetrahydroxyflavone and 7-O-β-D-glucuronide methyl ester-6-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone increased the mRNA levels of NQO-1 and HO-1, and 7-O-β-D-glucuronide methyl ester-6-(7″R-(3″, 4″-dihydroxyphenyl) ethyl)-3′, 4′, 5-trihydroxyflavone decreased the expression of mir-144-3p, thus increasing the level of Nrf2 protein in Caco2 cells, which was helpful to resist oxidative stress in Caco2 cells. | PV | Feng et al. (2018) |
| DPPH and ABTS+ | volatiles extract (The volatiles extract of 200 g PV was extracted by supercritical CO2 fluid extraction for 2 h, and the total volatile was composed of hydrocarbon (49.65%), fatty acid (22.38%), aldehyde (16.66%), terpene (9.04%) and little alcoholic.); 10–150 μg/mL | The EC50 values against DPPH and ABTS+ of the volatiles from PV were 32.01 and 50.90 μg/mL, respectively. | PV | Xie et al. (2008) |
| DPPH | essential oil extract (The dried whole plant of PS (500 g) was distilled with double distilled water of 5000 mL for 4 h, and the yellow essential oil of 0.2 mg/g (w/w) was obtained.); 0.5–2 mg/mL | The volatiles extract of PS has a dose-dependent scavenging effect on DPPH radical, with IC50 of 1.455 mg/mL. | PS | Lin et al. (2018) |
| mice with acute liver injury (negative control: saline) | 60% ethanol extract (20 g PV powder was refluxed and extracted with 400 mL petroleum ether for 5 h, then dissolved with 400 mL 60% ethanol, refluxed and extracted for 2 h at 70 °C, extracted twice. The extract was centrifuged at 4000 r/min for 10 min, collected the supernatant and dried to obtain crude extract.); intragastric administration, 60, 120, 240 mg/kg/day for 10 days | The 60% ethanol extract of PV had obvious antioxidant activity in mice with acute liver injury by decreasing the activities of ALT and AST in serum, reducing the content of MDA and activity of LDH in liver, and enhancing the activities of T-SOD, T-AOC and GSH in liver. | PV | Huang et al. (2019b) |
| OH, DPPH and O2- | ethanol and water extract (The ethanol extract: 100 g PS or PV was immersed in 1.5 L 70% ethanol for 30 min, refluxed and extracted for 2 h, filtered, repeated 3 times, combined filtrate, concentrated and freeze-dried. The contents of chlorogenic acid, caffeic acid and total flavonoids in the ethanol extract of PV were 64.37 ± 2.43, 21.19 ± 1.24, and 293.00 ± 2.65 mg/g, respectively, and in the ethanol extract of PS were 83.80 ± 1.15, 1.12 ± 0.09, and 318.00 ± 2.65 mg/g, respectively. The water extract: 100 g PS or PV was immersed in 1.5 L distilled water for 30 min, refluxed and extracted for 2 h, filtered, repeated 3 times, combined filtrate, concentrated and freeze-dried. The contents of chlorogenic acid, caffeic acid and total flavonoids in the water extract of PV were 94.18 ± 1.94, 19.05 ± 0.75, and 334.00 ± 5.20 mg/g, respectively, and in the water extract of PS were 117.29 ± 0.85, 1.52 ± 0.09, and 383.00 ± 3.61 mg/g, respectively.); 5, 10, 15 mg/mL for scavenging OH and DPPH; 0.5, 1, 1.5 mg/mL for scavenging O2- | The water extract exhibited a stronger clearance rate for scavenging DPPH and OH than ethanol extract both in PS and PV. Moreover, the contents of chlorogenic acid and total flavonoids in the extracts are positively correlated with free radical scavenging ability. | PS, PV | Sun et al. (2018) |
| Antimicrobial effects | ||||
| HeLa cells (negative control: culture solution; positive control: ribavirin) | Polysaccharide mixture (AP3) (1 kg of Herba patriniae was immersed in 7000 mL distilled water at room temperature for 12 h, filtered, the drug residue was repeatedly extracted with 5000 mL of distilled water, the two extracted filtrates were combined, concentrated under reduced pressure to 1000 mL, and AP3 was obtained after purification.); 0.02–2 mg/mL for 0, 2, 4, 6, 8 h | The polysaccharide mixture (AP3) exerted an obvious dose-dependent anti-RSV effect with TC50 and EC50 values of 11.45 and 0.0986 mg/mL, respectively. Moreover, the therapeutic index (TI = TC50/EC50) was 116.12. | aHerba Patriniae | Li et al. (2004) |
| λ-Lysogen (negative control: without water extract and irradiation; positive control: without water extract but irradiated) | water extract (Herba Patriniae was immersed in water at room temperature for 30 min, then boiled slowly for 30 min, and the volume was adjusted to 50 mL, and 750 mg/mL water extract was obtained after filtration.); 93.75, 187.5, 375, 750 mg/mL | The inhibitory rates of the water extract from Herba Patriniae anti-SARS virus reached 45.0% at the concentration of 750.0 mg/mL. | aHerba Patriniae | Li et al. (2006) |
| Staphylococcus aureus, Streptococcus, Pasteurella, Salmonella, Escherichia coli | ethanol and water extract (The crude drug content in ethanol and water extract are both 1 g/mL); 0.5 g/mL for 20 h | The water extract of PS (0.5 g/mL) has a strong inhibitory effect against Staphylococcus aureus, Streptococcus and Escherichia coli, and it has a weak antibacterial effect on Pasteurella and Salmonella, while the ethanol extract (0.5 g/mL) has a relatively weak antibacterial effect on these five kinds of bacteria. | PS | Tan et al. (2003) |
| Staphylococcus aureus, Escherichia coli, Proteus spp., Bacillus subtilis (negative control: without bacteria but have 70% ethanol extract; positive control: without 70% ethanol extract but have bacteria) | 70% ethanol extract (200 g of PV was immersed in 70% ethanol at 60 °C for 2.5 h, repeatedly extracted twice, and the two ethanol extracts were combined, filtered and concentrated to 200 mL by rotary evaporation, and obtained ethanol extract with a crude drug concentration of 1 g/mL); 125–250 mg/mL | The minimum inhibitory concentration of the extract was between 125-250 mg/mL. When the temperature was 23–160 °C, and the UV irradiation time was 10–50 min, the extract maintains high antimicrobial activity. | PV | Dai and Lin (2011) |
| Pseudomonas aeruginosa (negative control: deionized water) | water extract (Herba Patriniae was crushed and immersed in deionized water for 2 h, then boiled for 2 h (w/v was 1/5), filtered and evaporated in vacuum, and then freeze-dried to obtain solid extract.); 1.6 mg/mL for 24 h | The water extract of Herba Patriniae dramatically suppressed the expression of biofilm-associated key genes (algA, algU, bdlA, pelA, ppyR and pslM), thus decreased the biofilm formation and changed the structure of the biofilm of Pseudomonas aeruginosa.It also reduced exopolysaccharide production and increased swarming motility. | aHerba Patriniae | Fu et al. (2017) |
| Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Salmonella typhimurium, Shigella dysenteriae, Aspergillus niger, Aspergillus flavus, Beer yeast (positive control: potassium sorbate) | tannin extract (1 g of PV powder was added to 60 mL 50% acetone and extracted under 80 W ultrasonic power for 60 min, the extraction rate was 4.648%.); 1.0 mg/mL for 48 h | The inhibition rate of tannin extract with 1.0 mg/mL concentration on Escherichia coli was 50.78%, which was significantly different from that of potassium sorbate in the control group (30.45%), and it also had a significant inhibition rate on other four kinds of bacteria (23.45 %–49.89%). However, the inhibition rate on three kinds of fungi was poor, and the inhibition rate was 8.78 %–28.03%. | PV | (Fan, 2014) |
| Sedative and hypnotic effects | ||||
| Normal mice (negative control: ethanol and water; positive control: pentobarbital) | ethanol and water extract (The crude drug content in ethanol and water extract are both 1 g/mL); intraperitoneal injection, 0.5 g/mL/10 g | The ethanol extract of PS (0.5 g/mL/10 g) had an obvious sedative effect on mice, its sedative time was longer than water extract and its intensity was similar to that of pentobarbital, while without hypnotic effect. | PS | Tan et al. (2003) |
| Male ICR mice (negative control: physiological saline solution of 20% 1, 2-propanediol) | petroleum ether, chloroform, ethyl acetate and n-butanol extract from 95% ethanol extract and 95% ethanol extract (4 kg PS was crushed and refluxed with 95% ethanol for 3 times. The extracts were combined and distilled under reduced pressure to obtain 331.2 g. Take part of it to reduce pressure and dry. Another 330 g was diluted with water and extracted with petroleum ether, chloroform, ethyl acetate and n-butanol. After vacuum drying, the extract was 24.5 g, 42.1 g, 63.6 g, 74.3 g, respectively.); petroleum ether, chloroform, ethyl acetate and n-butanol extract from 95% ethanol extract: intragastric administration, 0.12 g/kg, 5 h after administration, the number of mouse activity in 10 min was recorded; 95% ethanol extract: intragastric administration, 0.45 g/kg, 5 h after administration, the number of mouse activity in 10 min was recorded | 95% ethanol extract of PS had an obvious sedative effect on mice. Ethyl acetate extract and n-butanol extract significantly reduced the spontaneous activity of mice, while n-butanol extract significantly prolonged the sleep time of mice induced by threshold dose of pentobarbital sodium. | PS | Xu et al. (2007) |
| Mice (negative control: saline), patients | 60% ethanol extract, volatile oil and dried ethanol extract (60% ethanol extract: the root of PS was crushed and immersed in 60% ethanol, then distilled under reduced pressure until 20% extract contains 10–15% ethanol.); 60% ethanol extract: (mice: intragastric administration, 30 g/kg); volatile oil (mice: intragastric administration, 0.2 g/kg; patients: 20 mg/capsule, 1–2 capsules/day, 1 times/day, 10–14 days); dried ethanol extract (mice: intragastric administration, 7.5 g/kg; patients: 1 g/tablet, 2–4 tablets/day, 2–3 times/day, 10–14 days) | The 60% ethanol, volatile oil, and dried ethanol extracts from PS possessed sedative and hypnotic effect on mice induced by pentobarbital sodium and patients suffered from neurasthenia or neurasthenic syndromes with insomnia. | PS | Luo et al. (1986) |
| Mice (negative control: saline) | water extract (The water extract concentration was 2 g/L); intraperitoneal injection, 20 and 40 mg/kg | The water extract of PV inhibited the spontaneous activity, shortened the time of falling asleep and prolonged the sleeping time induced by pentobarbital sodium in a dose-dependent manner (20 and 40 mg/kg). | PV | Chen et al. (2005) |
| Mice (negative control: saline) | water extract (The crude drug content in water extract was 4.69 g/g); intraperitoneal injection, 40 and 60 mg/kg | The water extract of PV inhibited the spontaneous activity, shortened the time of falling asleep and prolonged the sleeping time induced by pentobarbital sodium in a dose-dependent manner (40 and 60 mg/kg). | PV | Zhong et al. (2004) |
| Proangiogenic effect | ||||
| HUVECs cells (negative control: culture solution; positive control: VEGF), mouse hindlimb ischemia model (negative control: PBS; positive control: VEGF) | water extract (600 g PV was boiled in distilled water for 2 h, filtered and evaporated in vacuum, and then freeze-dried to obtain solid extract 52.8 g); cells: 10 and 100 mg/L for 72 h; mice: intramuscular injection, 2.5 g/L, 20 μL/day, 3 day | The water extract of PV (10 and 100 mg/L) significantly increased HUVEC cell proliferation and migration as well as the formation of capillary-like structures, induced phosphorylation of FAK and Akt in a time-dependent manner. Furthermore, intramuscular injection of the extract (20 μL of 2.5 g/L) significantly decreased the necrosis probability of ischemic limbs in vivo. | PV | Jeon et al. (2010) |
| Antipruritic effect | ||||
| Male ICR mice | methanol extract (50 g PV was refluxed and extracted with 300 mL methanol for 3 h, and evaporated under reduced pressure and freeze-dried.); intragastric administration, 200 mg/kg administrated before the test | The methanol extract of PV suppressed substance P-induced itch-scratch response at a dosage of 200 mg/kg without affecting locomotor. | PV | Tohda et al. (2000) |
| Anti-diabetic | ||||
| Mouse 3T3-L1 preadipocytes (negative control: culture solution; positive control: Sodium Orthovanadate) | Patrinoside and patrinoside A (5 kg PS powder was extracted in 95% ethanol at room temperature, then concentrated under reduced pressure and purified to obtain Patrinoside and patrinoside A.); 6.25–200 μM for 48 h | Patrinoside and patrinoside A significantly elevated expression levels of p-IRS-1, p-Akt and GLUT4 at the dose of 50, 25 μM, respectively. The mechanisms of improving insulin resistance may be exerted via the activation of PI3K/Akt signaling pathway. | PS | Liu et al. (2019c) |
| Anti-diarrheal effect | ||||
| isolated intestine cramps model, Castor oil-induced diarrhea rats (negative control: distilled water; positive control: Changshu tablet) | 60% ethanol, dichloromethane layer, ethyl acetate layer, N-butanol layer and water layer extract (The 60% ethanol extract: 50 g PV was crushed and refluxed with 500 mL 60% ethanol at 85 °C for 2 h, extracted twice, filtered and concentrated by rotary evaporation under reduced pressure to obtain a solid powder 4.75 g; extracts of different polar parts: 500 g PV was crushed and refluxed with 5000 mL 60% ethanol at 85 °C for 2 h, extracted twice, filtered and concentrated by rotary evaporation under reduced pressure to no alcohol taste, and then diluted with 2000 mL water and extracted with dichloromethane, ethyl acetate and N-butanol, extracted twice. After vacuum drying, the extract was 1.77, 2.28 and 10.61 g, respectively, the water layer extract was 23.34 g); isolated intestine cramps model: 0.02–1.28 mg/mL; in vivo: intragastric administration, 100, 200, 400 mg/kg/day, 7 days | All the five extracts of PV exhibited an anti-diarrheal effect in a dose-dependent manner (100–400 mg/kg), in which the effect of dichloromethane layer is stronger than that of other polar extracts. | PV | Zhang et al. (2019a) |
Patrinia villosa Juss. (PV); Patrinia scabiosafolia Fisch. (PS).
Not indicate species.
5.1. Anti-cancer effect
Cancer is one of the main diseases that endanger human health, and recent studies have shown that Herba Patriniae plays an important role in anti-cancer. Some extracts of Herba Patriniae have been used to study the anti-cancer effect in several human cancer cell lines. Among them, the ethanol extract of PS and PV showed remarkable anti-cancer effects both in vitro and in vivo. Many studies showed the anti-cancer effect of the ethanol extract of PS. Chen et al. (2013) discovered that the ethanol extract of PS suppressed the proliferation, migration, and tube formation of HUVECs (human umbilical vein endothelial cells) at the dose of 0.25–2 mg/mL. According to the studies of Liu et al. (2013b) and Zhang et al. (2015), it also inhibited the angiogenesis and the expression of CyclinD1 and CDK4 in HT-29 (human colon cancer cells), increased the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2, induced the activation of caspase-3 and -9 in HT-29 cells via the mitochondrial-dependent pathway, and decreased the proliferation of HT-29 cells by G1/S cell cycle arrest. When the ethanol extract of PS was intragastrically administrated to colorectal cancer mice at the dose of 1.93 g/kg, the tumor volume was significantly decreased, and the intratumoral microvessel density and the expression of PCNA were reduced in tumor tissues. Huang et al. (2019a) found that the ethanol extract of PS decreased the drug resistance of HCT-8/5-FU (human colorectal cancer cells) by inhibiting AKT pathway and inducing apoptosis. Moreover, in human multiple myeloma U266 cells, it dramatically suppressed proliferation, decreased Cyclin D1 and Bcl-2 mRNA levels, and eventually induced apoptosis in a dose-dependent manner (0.25–1 mg/mL), with a mechanism of suppressing STAT3 activation (Peng et al., 2011). When the concentration of the ethanol extract of PS was 1.0 mg/mL, the necrotic and apoptosis rate of 786-O cells (human renal cell carcinoma) were markedly increased, and the intracellular ROS and Ca2+ levels were significantly elevated. In addition, it significantly inhibited SIRT-1 and mTOR signaling. Therefore, Li et al. (2018) believed that the ethanol extract of PS induces the death of 786-O cells through metabolic disorders mediated by SIRT-1 and mTOR signaling. On the other hand, the studies on anti-cancer effect of ethanol extract of PV were relatively less. For example, Chen et al. (2019c) found that the ethanol extract of PV increased the tumor inhibition rate of U14-bearing mice with the inhibitory rate 49.19% and 54.23% at the dosage of 10 g/kg and 15 g/kg, respectively. However, the dose level of 10 g/kg and 15 g/kg is very high, and the extraction process of the extract needs to be further optimized.
The other extracts of Herba Patriniae also showed potential anti-cancer effects, though these effects may have some differences between them. The essential oil extract of PS showed a significant dose-dependent inhibition effect on 8 kinds of tumor cells at dose of 50–200 μg/mL (Lin et al., 2018). Ethyl acetate extract from PS and Patrinia monoterpene iridoid ether esters extract from PS or PV were evaluated on HepG2 (human liver hepatocellular carcinoma) or MCF7 (human breast adenocarcinoma cells) for the inhibition of proliferation and induction of apoptosis. Results showed that the expression levels of Bcl-2/Bcl-xL, Cdc2, and Cyclin B1 proteins were down-regulated, while the expression levels of Bax and caspase-3 proteins were up-regulated. These results suggested that the anti-hepatoma and anti-breast cancer effects of Herba Patriniae can also be achieved by caspase-independent mitochondrial cell death pathway (Chiu et al., 2006; Ji et al., 2019).
There are many kinds of compounds within Herba Patriniae, which may support the anti-cancer effect of Herba Patriniae, and hence, scholars wish to clarify what kinds of compounds exert this pharmacological effect. After treatment with the saponins extract of PV, the EMT and NF-κB signaling pathways were down-regulated in vitro, which subsequently inhibited the invasion and metastasis of human colorectal cancer cells (Xia et al., 2018). Moreover, at the dose of 50 mg/kg and 100 mg/kg, it also effectively reduced the weight of U14 cervical tumor in vivo (35.1% and 57.1%, respectively), which was closely related to the increase of apoptosis and G0/G1 tumor cells and the decrease of mutant P53 and Bcl-2 protein expression (Zhang et al., 2008). Patrinia-glycoside B-II from PS showed strong cytotoxic activities against HeLa (human cervical carcinoma cells), HepG2, HT1080 (human fibrosarcoma cells), A549 (human lung adenocarcinoma cells), A375-S2 (human melanoma cells), K562 (human erythroleukemic cells), HL60 (human leukemia cells), and U937 (Human leukemic monocyte lymphoma cells) with an IC50 of 5.4, 4.2, 18.0, 27.9, 15.8, 6.2, 6.6, and 5.5 μM, respectively (Ren et al., 2013). Therefore, terpenoids in Herba Patriniae can be used as an anti-cancer potential component for in-depth research in vivo and in vitro. Flavonoids, as another kind of active compounds rich in Herba Patriniae, have also been proved to have considerable anti-cancer effects. Peng et al. (2006c) carried out the growth inhibition experiments on A498 (human kidney cancer cells), A549, BEL-7402 (human hepatocellular carcinoma cells), HT-29, MCF-7, SGC-7901 (human gastric adenocarcinoma cells), and K562 with 6 flavonoids isolated from PV. The results showed that (2S)-5, 7, 22S 62S)-5, 7, showed 8-di (γ, γ-dimethylallyl) flavanone, (2S)-5, 7, 28- 68- tetrahydroxy-6-lavandulylated flavanone and (2S)-5, 7, 2te 6tetrahydroxy-6-lavandulylated fl flavanone exhibited high anticancer activities (IC50 < 7 μg/mL) in a dose-dependent manner, and when the concentration of these compounds exceeded 15 μg/mL, the proliferation of cancer cells was completely inhibited, especially for K562 cells (IC50 < 3.1 μg/mL). Patriniaflavanone A isolated from PV and the total flavonoid extracts of PS and PV showed obvious dose-dependent inhibitory effects on SMMC7721 hepatocarcinoma cells (0.125–10 mg/mL). Meanwhile, the total flavonoids extract of PV has a better inhibitory effect on the growth of SMMC-7721 cells than PS (Wei et al., 2013b; Xiang et al., 2016; Zhao et al., 2019). Besides, impecylone A isolated from PV showed an inhibition effect on 2 kinds of tumor cells at the doses of 12.5–50 μM (Liu et al., 2019b).
5.2. Anti-inflammatory effect
Herba Patriniae has been widely used in traditional medicine to treat pelvic inflammatory disease (PID), pancreatitis, colitis, and other inflammatory diseases (Ji, 2006; Qiu, 2005; Zhang, 1997a). Modern pharmacological studies have demonstrated that various effective extracts of Herba Patriniae showed anti-inflammatory effects in different inflammatory-related diseases. In vitro, the ethyl acetate extract (10–100 μg/mL) and essential oil extract (100–200 μg/mL) of PS inhibited the secretion of pro-inflammatory cytokines in RAW 264.7 (murine macrophage cells) or BV-2 (mouse microglia cells) in a dose-dependent manner, respectively (Lee et al., 2012; Lin et al., 2018). In vivo, both the ethanol extract of PS (600 mg/kg) and the 70% ethanol extract of PV (0.55 g/kg) showed strong anti-inflammatory effects in rats with PID, which inhibited the infiltration of inflammatory cells and the production of cytokines in the upper genital tract of PID rats, as well as the levels of pro-inflammatory cytokines in serum (Zheng et al., 2012; Zou et al., 2015). On the other hand, the water extract and methanol extract of PS and the total flavonoids of Herba Patriniae exhibited obvious anti-inflammatory effects in animal models of acute pancreatitis, ulcerative colitis, and focal cerebral ischemia-reperfusion, respectively. All of these extracts inhibited the secretion of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Cho et al., 2011; Seo et al., 2006; Wei et al., 2019). Although these nonclinical pharmacological studies on Herba Patriniae extracts have indicated the good pharmacological effects of Herba Patriniae in the anti-inflammatory field, further clinical studies are more needed to extend its clinical use.
5.3. Antioxidant effect
Recently, the antioxidant effect of Herba Patriniae has been evaluated in vivo and in vitro, providing information on the pharmacological activity of both mixtures and single compounds. The phenylethyl flavones isolated from PV (25 μM) showed a significant antioxidant effect, and this effect was achieved through the modulation of the mir-144-3p/Nrf2 pathway, thereby eliminating intracellular ROS in vitro (Feng et al., 2018). The water, ethanol, and volatiles extract from PV and PS possessed strong antioxidant effect by DPPH assays, and especially the chlorogenic acid and total flavonoids in the water extracts of PS exhibited stronger free radical scavenging ability (Lin et al., 2018; Sun et al., 2018; Xie et al., 2008). Chlorogenic acid and flavonoids of Herba Patriniae are be regarded as bioactive constituents for antioxidant activity. In vivo, it's worth noting that the 60% ethanol extract of PV had a remarkable antioxidant effect on mice with acute liver injury, which is reflected by the reductions of ALT and AST activities in the serum, MDA content and LDH activity in the liver, and the enhanced liver T-SOD, T-AOC and GSH activities (Huang et al., 2019b). These results provide ideas for further research on its traditional medicine.
5.4. Anti-microbial, anti-viral, and anti-fungi effects
Herba Patriniae is widely used in traditional medicine to treat respiratory diseases caused by viruses. At present, the researches on the antiviral effect of Herba Patriniae were mainly focused on the respiratory syncytial virus (RSV) and severe acute respiratory syndrome (SARS) virus. Li et al. (2006) investigated the antiviral effects of water extract of PS or PV against the SARS virus in vitro. The extract possessed a strong inhibition rate of 45.0% at a concentration of 750.0 mg/mL, and could effectively quench the free radicals that occurred in the process of UV irradiation on λ-lysogenic cells. Li et al. (2004) reported that polysaccharide mixture of PS or PV has an effective inhibitory effect on RSV in HeLa cells, with a therapeutic index of 116.12 and an EC50 = 0.0986 mg/mL. These results provide a potential for using animal models to further explore the anti-virus infection mechanism of Herba Patriniae.
In vitro, the antibacterial effect of water extract and ethanol extract from PS were tested on Staphylococcus aureus, Streptococcus, Pasteurella, Salmonella, and Escherichia coli. Among them, the water extract showed a strong inhibitory effect against Staphylococcus aureus, Streptococcus, and Escherichia coli at a concentration of 0.5 g/mL (Tan et al., 2003). However, this paper have not showed the reason for the different antibacterial effects between water extract and ethanol extract, and further study should be carried out to illustrate this issue. In the past researches, saponins, volatile oils, organic acids and flavonoids were considered as the main chemical components within PS. These compounds may have played a key role in the antibacterial process. Among them, the content of total flavonoids and organic acids in the water extract of PS is higher than that of ethanol extract (Sun et al., 2018), and this may be the reason for the results of Tan et al. (2003). The 70% ethanol extract of PV showed dose dependent growth inhibition in Staphylococcus aureus, Escherichia coli, Proteus spp and Bacillus subtilis at 12.5–100 mg/mL, and has high UV radiation stability and thermostability (Dai and Lin, 2011). The tannin extract of PV not only had a significant inhibitory effect on Escherichia coli, Bacillus subtilis and Staphylococcus aureus, but also on Shigella dysenteriae and Salmonella typhimurium, with an inhibition rate of 23.45 %–50.78% (Fan, 2014). Besides, Fu et al. (2017) discovered that the water extract of Herba Patriniae showed an obvious inhibitory effect on key genes related to biofilm, and could decrease biofilm formation and change the structure of the biofilms of Pseudomonas aeruginosa. Nevertheless, this study is lack of dose-dependent results, and a test on the drug exposure time to kill bacteria may also be meaningful for the antibacterial study. Besides, its further study is necessary to associate the antibacterial activity with the corresponding diseases. Taken together, as a natural antimicrobial plant, Herba Patriniae is worthwhile to further study the active components, and besides, it also could be put into the industrial production of food and feed.
Diseases caused by fungal infection include superficial, subcutaneous and deep mycoses, which have a high incidence and easily cause systemic infectious diseases (Liu and He, 2014; Ran et al., 2017). Wu (2017a) studied the anti-fungal effect of Herba Patriniae on Cryptococcus neoformans, Candida albicans, Trichophyton rubrum and Aspergillus fumigatus, but it showed no inhibitory effect on these four fungi. Fan (2014) investigated the inhibitory effect of tannin extract of PV on Aspergillus niger, Aspergillus flavus, and Saccharomyces cerevisiae, and found that its inhibitory effect on these three fungi was poor, and the inhibition rate was less than 28.03%. At present, there are few studies on the anti-fungal effect of Herba Patriniae, and the anti-fungal effect of Herba Patriniae is worthy of further study.
5.5. Sedative and hypnotic effects
At present, neurological diseases have become a major problem affecting people's quality of life. In traditional medicine, Herba Patriniae has a good curative effect on insomnia and neurasthenia (Qiu, 2005; Yang, 2002). A study conducted by Luo et al. (1986) demonstrated that 60% ethanol, and volatile oil extract from PS have sedative and hypnotic effects. In addition, the ethanol extract of PS at a dose of 1 g/mL showed an observable sedative effect on mice. Its sedative effect was stronger than that of the water extract and its intensity was similar to that of pentobarbital. The 95% ethanol extract of PS was further extracted with petroleum ether, chloroform, ethyl acetate, and n-butanol respectively for sedation and hypnosis experiment. The sedative effect of the n-butanol extract was the most significant among the four different polarity extracts (Tan et al., 2003; Xu et al., 2007). Therefore, the n-butanol extraction part of PS is considered to be an effective sedative extract. Moreover, the water extract of PV also has obvious sedative and central nervous system inhibitory effect, which can shorten the falling asleep time and prolong the sleep time induced by pentobarbital sodium, and the effect was enhanced with the increase of dose (20–60 mg/kg) (Chen et al., 2005; Zhong et al., 2004). Although these studies support the traditional medication experience of Herba Patriniae, systematic studies are needed to determine the bioactive compounds of this effect.
5.6. Others
The proangiogenic effect of PV has been tested on HUVECs, in an ex vivo mouse aortic ring assay, and in an in vivo murine hindlimb ischemia model. In vitro and in vivo pharmacological studies have indicated that the water extract of PV significantly promoted cell proliferation and migration, and induced angiogenesis via activating the FAK signaling pathway (Jeon et al., 2010). In traditional medicine, Herba Patriniae is widely used in the treatment of various skin diseases, such as psoriasis vulgaris, itching (Wang and Wang, 2002; Yan et al., 2015; Zhu and Jiang, 2015). Tohda et al. (2000) reported that the methanol extracts of PV exhibited a significant inhibitory on substance P-induced itch-scratch response. Although the mechanism of antipruritic action is not clear, these results demonstrated that PV could be developed as an antipruritic for the treatment of cutaneous diseases. In addition, decoction containing Herba Patriniae is reported to be used for diarrhea (Qiu, 2005). A study conducted by Zhang et al. (2019a) demonstrated that the 60% ethanol, dichloromethane layer, ethyl acetate layer, N-butanol layer, and water layer extracts of PV exhibited anti-diarrheal effects in a dose-dependent manner (100–400 mg/kg), and the dichloromethane layer showed the strongest anti-diarrheal effect in vivo and in vitro. Furthermore, Patrinoside and patrinoside A isolated from PS were shown to exert a significant effect on improving insulin resistance in 3T3-L1 adipocytes via activation PI3K/Akt signaling pathway, which plays an important role in type 2 diabetes (Liu et al., 2019c). Although both PV and PS contain iridoids, there is no report on the insulin resistance of PV. Moreover, there is no research on the proangiogenic, antipruritic, and anti-diarrheal effects of PS. Therefore, there are some differences in pharmacological effects between these two species of Herba Patriniae. It is suggested that further study should be conducted to determine the difference of pharmacological activities between the two plants, so as to provide scientific reference for safe and effective medication.
6. Toxicity
In traditional use, Herba Patriniae is almost non-toxic. However, mild side effects can be produced when large dosage is used. Excessive use of PV water extract can cause temporary leukopenia, dizziness, nausea and other symptoms (Deng, 2001). According to the reported toxicity of PS, the methanol extract from the root of PS increased the serum aminotransferase and caused histopathological changes in mice (Wang, 2009). In addition, when the oral dose of PS up to 200 mg/kg, it will lead to polyuria (Wang, 2009). Gavage of 30 g/kg of PS alcohol extract in mice can cause mild diarrhea and mild respiratory depression, but there is no adverse reaction at the dose of 24 g/kg (Wang and Sun, 1997). The volatile oil of PS was given 400, 750 and 1500 times as much as that of human (0.8 mg/kg) to mice for 7 days, that showed no abnormal performance (Luo et al., 1986). In clinical application, individual patients suffered from dry mouth and stomach discomfort after taking PS, and it will disappear after stopping the medicine. Its volatile oil products have no side effects on liver and kidney function and leukocyte count (Wang and Sun, 1997). Above all, Herba Patriniae is quite safe for animals and people at a reasonable dosage (see Table 5).
Table 5.
Toxicity of herba patriniae.
| Extract | Dosage | Subject | Adverse reaction | Reference |
|---|---|---|---|---|
|
PS, root, methanol extract |
– | mice | increased the serum aminotransferase and caused histopathological changes | Wang (2009) |
| PS | 200 mg/kg | patient | polyuria | Wang (2009) |
| PS | 200 mg/kg | mice | polyuria | Wang (2009) |
|
PS, ethanol extract |
30 g crude drug/kg | mice | mild diarrhea and mild respiratory depression | Wang and Sun (1997) |
|
PS, rhizome and root, 60% ethanol extract |
2-4 tablets (1 g crude drug/tablet), 2–3 times daily, for 10 days | patient | dryness in the mouth and stomach discomfort | Wang et al. (1983) |
|
PS, root, ethanol extract |
5–10 mL (1 g crude drug/mL), 2–3 times daily, for 10–14 days | patient | temporary stomach discomfort or blushing in the face | Luo et al. (1986) |
|
PS, root, 60% ethanol extract |
2-4 tablets (1 g crude drug/tablet), 2–3 times daily, for 10–14 days | patient | dryness in the mouth and stomach discomfort | Luo et al. (1986) |
|
PS, root, volatile oil extract |
20 mg/capsule, 1–2 capsules daily, for 10–14 days | patient | Nauseous or sleepy the next day | Luo et al. (1986) |
Patrinia scabiosafolia Fisch. (PS).
7. Quality control
An effective quality control method plays a key role in drug safety and effectiveness. With development of technology, UV, HPLC, and GC-MS have been used for monitor the variety of components in Herba Patriniae (Table 6 ). The content of total flavonoids has been quantified by UV method. It is worth concerning that the contents of total flavonoids were great difference in different parts, for example, 47.9–57.4 mg/g in leaves, while 18.1–29.2 mg/g in stems (Xu and Zhou, 2004). The extraction solvent also has great influence on the content of total flavonoids. Sun et al. found that the content of total flavonoids in PV extract by water was higher than that by 70% ethanol (334.00 ± 5.20 mg/g v.s. 293.00 ± 2.65 mg/g) (Sun et al., 2018). This phenomenon also occurs in the extraction of organic acids, such as chlorogenic acid and caffeic acid (Sun et al., 2018). These results indicate that flavonoids and organic acids are more easily extracted by aqueous solution.
Table 6.
Quantitative analysis for the quality control of Herba Patriniae.
| Analytes | Extraction method | Determination method | Results | Species | Reference |
|---|---|---|---|---|---|
| Flavonoids | The water boiled extraction method: 2.5 g PV powder was boiled in 50 mL water for three times, 2 h, 1.5 h, 1 h, respectively, the extract was filtered and water was added to 250 mL for constant volume. The ethanol refluent extraction method: 2.5 g PV powder was refluxed and extracted with 50 mL methanol for three times, 2 h, 1.5 h, 1 h, respectively, the extract was filtered and water was added to 250 mL for constant volume. The suoshi extraction method: 2.5 g PV powder was extracted in a cable extractor with 50 mL methanol for 4 h with 50 mL methanol, the extract was filtered and water was added to 250 mL for constant volume. The supersonic extraction method: 2.5 g PV powder was subjected to ultrasonic extracted with 50 mL methanol for 45 min, three times, the extract was filtered and water was added to 250 mL for constant volume. |
UV | The contents of flavonoids have a close relationship with the extraction method and the parts of PV. To exact flavonoids in roots, stems, leaves and whole grasses of PV by the water boiled extraction method, the contents of flavonoids were 24.4, 18.1, 47.9, 34.2 mg/g, respectively; by the ethanol refluent extraction method, the contents of flavonoids were 30.3, 24.1, 51.6, 39.8 mg/g, respectively; by the suoshi extraction method, the contents of flavonoids were 34.9, 29.2, 57.4, 43.3 mg/g, respectively; by the supersonic extraction method, the contents of flavonoids were 32.8, 27.5, 54.1, 41.2 mg/g, respectively. | PV | Xu and Zhou (2004) |
| Total flavonoids Chlorogenic acid Caffeic acid |
The ethanol extract: 100 g PS or PV was immersed in 1.5 L 70% ethanol for 30 min, refluxed and extracted for 2 h, filtered, repeated 3 times, combined filtrate, concentrated and freeze-dried. The water extract: 100 g PS or PV was immersed in 1.5 L distilled water for 0.5 h, refluxed and extracted for 2 h, filtered, repeated 3 times, combined filtrate, concentrated and freeze-dried. |
HPLC and UV | In the water extract of PV, the contents of total flavonoids, chlorogenic acid and caffeic acid were 334.00 ± 5.20, 94.18 ± 1.94 and 19.05 ± 0.75 mg/g, respectively. In the ethanol extract of PV, the contents of them were 293.00 ± 2.65, 64.37 ± 2.43 and 21.19 ± 1.24 mg/g, respectively. Moreover, in the water extract of PS, the contents of them were 383.00 ± 3.61, 117.29 ± 0.85 and 1.52 ± 0.09 mg/g, respectively. In the ethanol extract of PS, the contents of them were 318.00 ± 2.65, 83.80 ± 1.15 and 1.12 ± 0.09, mg/g, respectively. | PS, PV | Sun et al. (2018) |
| Inositol | 100 g of Herba patriniae was immersed in 300 mL 90% ethanol for 12 h, filtered, the drug residue was extracted with 200 mL of 90% ethanol, refluxed and extracted for 2 h, then filtered and volatilized residual ethanol on a water bath. | UV | The contents of inositol of 3 batches were from 28.9-30.1 mg/kg. | aHerba Patriniae | Wang et al. (2002) |
| Oleanolic acid | 100 g of PV was immersed in 1.5 L water for 0.5 h, then boiled for 0.5 h, filtered, the drug residue was repeatedly boiled with 1 L of water, the two extracted filtrates were combined, the 50 mL extract was concentrated in a water bath to a thick paste. | UV | The contents of oleanolic acid from 3 batches of PV were from 2.29% to 2.41%. | PV | Wang et al. (2012) |
| Quercetin Kaempferol |
1 g PS or PV powder was extracted with 20 mL of hydrochloric acid-methanol (1:20, v/v) mixed solution under reflux for 1 h, filtered and methanol was added to 25 mL for constant volume. | HPLC | In PV, twelve batches have been determined with the contents of 0.061–1.046 mg/g for quercetin and 0.082–0.701 mg/g for kaempferol. In PS, fourteen batches have been determined with the contents of 0.062–0.938 mg/g for quercetin and 0.045–0.542 mg/g for kaempferol, indicating that the quercetin and kaempferol content in the samples from different sources were significantly different. | PS, PV | Liu et al. (2015) |
| Ursolic acid Oleanolic acid |
1 g Herba Patriniae powder was subjected to ultrasonic extracted with 30 mL methanol for 45 min, after the weight loss is made up, filtered and the extract of 1 mL was extracted and fixed volume to 10 mL with methanol. | HPLC | The contents of ursolic acid and oleanolic acid for 6 batches were 0.218%–0.498% and 0.158%–0.473%, respectively. | aHerba Patriniae | Zhou (2014) |
| Isovitexin Isoorientin |
1 g PV powder was subjected to ultrasonic extracted with 20 mL methanol for 30 min, extracted twice, the two extracted filtrates were combined. | HPLC | Four batches have been determined with the contents of 0.78–1.68 mg/g for isovitexin and 1.87–2.42 mg/g for isoorientin, indicating that the isovitexin and isoorientin content in the samples from different sources were significantly different. | PV | Chen (2008) |
| Hederagenin Oleanolic acid Ursolic acid |
2 g Herba Patriniae powder was subjected to ultrasonic extracted with 50 mL methanol for 60 min, filtered, the drug residue was washed with proper amount of methanol, combined the filtrate and the washing solution, evaporated, and dissolved in 10 mL water, then extracted with 20 mL water-saturated n-butanol, extracted three times, combined the extracts and evaporated to dryness. The residue was heated and hydrolyzed with 20 mL methanol and 4 mL hydrochloric acid for 4 h, then added with 10 mL water and shaken and extracted with 20 mL chloroform, extracted twice, combined the extracts and evaporated to dryness. The residue was dissolved and fixed volume to 10 mL with methanol. | HPLC | The contents of hederagenin, oleanolic acid and ursolic acid have been determined in ten batches. The result was that the contents of the compounds were significantly different among the different samples. The concentration ranges were 0.002%–0.556%, 0.019%–0.592% and 0.022%–0.630% for hederagenin, oleanolic acid and ursolic acid. | aHerba Patriniae | Mao et al. (2012) |
| Protocatechuic acid Chlorogenic acid Caffeic acid |
0.5 g Herba Patriniae powder was subjected to ultrasonic extracted with 20 mL 60% methanol with 5% formic acid for 30 min, and methanol was added to 25 mL for constant volume. | HPLC | Twenty batches have been determined with the contents of 0.176–0.547, 0.950–7.26 and 0.046–0.340 mg/g for protocatechuic acid, chlorogenic acid and caffeic acid, indicating that the contents for these compounds in the samples from different sources were significantly different. | aHerba Patriniae | Liu et al. (2013a) |
| Protocatechuic acid Chlorogenic acid Caffeic acid Iso-chlorogenic acid A Iso-chlorogenic acid C |
1 g Herba Patrinia powder was extracted with 25 mL of 80% methanol under reflux for 0.5 h, filtered and methanol was added to 25 mL for constant volume. | HPLC | Five compounds from 10 batches of Herba Patriniae have been quantified with contents of 0.108–1.198, 0.143–0.805, 0.054–0.384, 0.026–0.114 and 0.056–0.203 mg/g for chlorogenic acid, iso-chlorogenic acid A, protocatechuic acid, caffeic acid and iso-chlorogenic acid C, respectively, indicating that the contents for these compounds were quite different from different regions. | aHerba Patriniae | Liu and Lei (2019) |
| Palmic acid Hexanoic acid cis-Anethol Camphogen β-Ionone β-Damascenone |
30 g PS or PV powder was immersed in 300 mL water for 0.5 h, then extracted under reflux for 3 h and collected volatile oil. | GC-MS | In PS, the contents of palmic acid, hexanoic acid and cis-anethol were 9.54–12.14%, 8.27–10.23%, 6.68–8.34%, respectively. In PV, the contents of camphogen, β-ionone and β-damascenone were 9.55–11.89%, 5.98–6.54%, 5.94–7.56%, respectively. | PS, PV | Liu et al. (2016a) |
Patrinia villosa Juss. (PV); Patrinia scabiosafolia Fisch. (PS).
Not indicate species.
Up to now, nineteen compounds, including quercetin, kaempferol, isovitexin, isoorientin, ursolic acid, oleanolic acid, hederagenin, chlorogenic acid, caffeic acid, iso-chlorogenic acid A, iso-chlorogenic acid C, protocatechuic acid, inositol, palmic acid, hexanoic acid, cis-anethol, camphogen, β-ionone, and β-damascenone, have been quantified in Herba Patriniae. The content of compouds showed significantly different in Herba Patriniae from different sources (Chen, 2008; Liu et al., 2013a; Liu and Lei, 2019; Mao et al., 2012). In addition, the same compounds showed significant different content in two species. For instance, the content of kaempferol was 0.082–0.701 mg/g in PV, while that was 0.045–0.542 mg/g in PS (Liu et al., 2015). The chemical composition in herb is closely related to its pharmacological action. Therefore, the herb species, sources, and processing method should be fully considered in Herba Patriniae clinical use (Liu et al., 2016c; Zhang et al., 2011).
8. Pharmacokinetics
Pharmacokinetics is conducive to understanding the process of drug absorption, distribution, metabolism and excretion in the body. Unfortunately, there are few investigations on the pharmacokinetics of Herba Patriniae. Han et al. (2020) determind the serum pharmacochemistry after intragastric administration of total flavonoids extract from PV in rats by UPLC-Q-TOF-MS method. As result, 7 prototypical components (chlorogenic acid, caffeic acid, scutellarin, isoorientin, isovitexin, luteolin, and apigenin) and 7 metabolic components (Hydrocaffeate, scutellarein, Sulfated apigenin, sulfated luteolin, sulfated kaempferol, methylated kaempferol and one unknown compound) were detected in plasma, which might be the pharmacodynamic basis of its antitumor effect (Han et al., 2020).
9. Patents containing Herba Patrininae in China
To date, researchers have already filed more than 3000 patents related to the compositions containing Herba Patriniae in China. The patents for compositions containing both PS and PV in the last year and those containing either of them in the last decade are listed in Table 7 . Most of the patents are human health products (88), 13 patents for animal husbandry, 6 patents for agriculture and 1 patent for fisheries.
Table 7.
Patents containing Herba Patrniae in the last year, patents contain PS or PV in the past decade.
| Category | Formulations | Application | Reference |
|---|---|---|---|
| aHerba Patrniae | |||
| Gynecological diseases | Syrup/wine | Endometriosis (regulates qi, activates blood circulation, removes blood stasis, relieves pain) | (Chen et al., 2019b, Chen et al., 2019) |
| Pill/powder/tablet/capsule/suppository/granule/syrup/decoction/enema/oral liquid | Chronic PID (clears heat, detoxifies, regulates qi, relieves pain) | (Dai et al., 2019; Deng, 2019; He et al., 2019a, 2019c; Hou, 2019; Lu, 2019; Luo, 2019a; Lv et al., 2019; Zou et al., 2019) | |
| Decoction/lotion/tablet | Vaginitis (kills bacteria, relieves itching, improves abnormal symptoms of leucorrhea) | (Jiang, 2019b; Li and Li, 2019a; Long, 2019; Luo, 2019b; Nong et al., 2019) | |
| Lotions/health food | Cervicitis and cervical erosion (clears heat, promotes diuresis, removes decaying tissue, promotes new tissue formation) | (Li, 2019a; Zhang and Jiang, 2019) | |
| Capsule | Endometritis | (Gao, 2019a, 2019b) | |
| Pill/tablet/capsule/granule/decoction | Dysmenorrhea (warms channels, dispels cold, improves blood circulation, regulates menstrual cycle) | (Yang, 2019a; Xie and Xie, 2019a) | |
| Decoction/capsule/wine | Breast disease (increases immunity, removes inflammation, promotes blood circulation, removes blood stasis, regulates qi, detumescence, alleviates pain) | (Gao, 2019c; Wu, 2019; Yan, 2019a; Zhang. 2019a) | |
| Granule | Uterine fibroids | He and Hong (2019) | |
| Granule | Tubal blockage | Li (2019b) | |
| Urinary system disease | Decoction/patch | Prostatitis and prostate hyperplasia (clears heat, detoxifies, diuresis, detumescence, removes blood stasis, relieves pain) | (Li, 2019c; Long and Yin, 2019) |
| Decoction | Orchitis | Yuan (2019) | |
| Respiratory diseases | Capsule | Chronic obstructive pulmonary disease | Fei et al. (2019) |
| Decoction | Chronic laryngitis (improves immunity) | Gao (2019d) | |
| Spray | Rhinitis (antibacterial) | Gong (2019) | |
| Oral liquid/granule | Cough caused by tuberculosis, pneumonia, chronic pharyngitis and lung heat (clears lung heat, relieves cough, eliminates phlegm) | (Peng et al., 2019a; Yang, 2019b, 2019c, 2019d) | |
| Decoction | Lung cancer (promotes blood circulation, removes blood stasis, moistens lung, removes phlegm) | (Wang, 2019a; Wang et al., 2019b) | |
| Decoction | Nasopharyngeal carcinoma (improves immunity) | Xie and Xie (2019b) | |
| Tablet/capsule/granule/syrup/oral liquid | Lung pain | Zhang (2019b) | |
| Decoction | Bronchitis (clears heat, detoxifies, dispels cold, relieves asthma) | Zhao (2019a) | |
| Digestive system disease | Capsule/powder | Gastric ulcers (relieves stomachache) | (Gao, 2019e; Liu and Liu, 2019) |
| Tablet/pill/decoction | Gastritis (promotes blood circulation, relieves pain, hemostasis) | (Li, 2019d; Xiong, 2020) | |
| Tablet/decoction/granule/syrup/oral liquid/powder | Hepatitis (detoxification, diuresis, protects liver and kidney, replenishes qi, clears heat, eliminates dampness, removes virus, enhance immunity) | (Feng, 2019; Jiang et al., 2019; Liu, 2019b; Ru, 2019; Zhao 2019b) | |
| Patch/granule/pill/tablet/capsule/oral liquid/decoction/injection | Pancreatitis (clears heat, detoxifies, removes blood stasis, relieves pain) | (Dang et al., 2019; Li, 2019e) | |
| Decoction/powder/granule/pill | Appendicitis (clears heat, detoxifies, benefits qi, promotes blood circulation, relieves pain, reduces inflammation) | (Zeng, 2019; Zhang, 2019c) | |
| Decoction | Colitis and proctitis | Chen et al. (2019d) | |
| Metabolic disease | Tablet/capsule/granule | Diabetes | Zhang (2019d) |
| Leukemia | Decoction/pill/powder/granule/tablet/capsule | Leukemia (enhances human immunity, improves blood circulation and metabolic function) | (Ding, 2019; Jin, 2019) |
| Infectious disease | Decoction | Acquired immune deficiency syndrome | Xu (2019) |
| Skin diseases | Decoction/pill | Shingles | Dong (2019) |
| Ointment | Wound infection (clears heat, detoxifies, promotes wound healing) | (Jiang, 2019c; Tan, 2019; Yu, 2019) | |
| Effervescent tablet | Tinea pedis and itchy skin (clears heat, relieves itching, detoxifies) | (Gao, 2019f) | |
| Health care | Tea | Nourishes intestines, moistens intestines, detoxifies | (Zhang, 2019e) |
| Beverage | Prevents hepatobiliary disease | Li and Li (2019b) | |
| Hygiene | Oral care liquid | Anti-bacterial, sterilizes, clears heat, detoxifies, detumescence, relieves pain | (Peng et al., 2019b; Ye et al., 2019) |
| Soap | Skin care, whitening | Shi et al. (2019) | |
| Laundry liquid | Antibacterial, environmental Protection | Xie (2019) | |
| Toilet paper | Anti-inflammatory, sterilizes | (Zhang, 2019f) | |
| Agriculture | Insecticide | Insecticidal (easy to decompose, no residue, harmless to human beings and animals, green environmental protection) | (Li, 2019f; Li, 2019g; Yan, 2019b, 2019c) |
| Herbicide | Eupatorium adenophorum (environment friendly, harmless to human beings) | Bao (2019) | |
| Fertilizer | Promotes crops growth (environment friendly) | (Zhang, 2019g) | |
| Fisheries | Veterinary drug | Crayfish tail rot | Liu and Chi (2019b) |
| Animal husbandry | Animal feed | Enhances immunity, promotes growth | (Gai, 2019; Liu et al., 2019d; Sun et al., 2019; Wang, 2019b) |
| Veterinary drug | Sow metritis | Ding et al. (2019) | |
| Veterinary drug | Chronic eczema of pig | Gao et al. (2019) | |
| Veterinary drug | Swine fever | (Zhang, 2019h) | |
| Veterinary drug | Blue ear disease of pig | Zhu et al. (2019) | |
| Veterinary drug | Infectious bronchitis and fallopian tube injury in chickens | Zhang et al. (2019b) | |
| Veterinary drug | Pigeon diarrhea | (Zhang, 2019i) | |
| Veterinary drug | Pigeon trichomoniasis | Yang et al. (2019) | |
| Veterinary drug | Suppurative sinus of horse | Liu and Chi (2019a) | |
| Veterinary drug | Animal mastitis | Zhou et al. (2019) | |
| PS | |||
| Nervous system disorders | Decoction/tablet/capsule/granule/pill/oral liquid | Aeurasthenia (improves insomnia, calms) | (Gao, 2017; Liu and Hu, 2011) |
| Skin diseases | Decoction | Acne (clears heat, detoxifies, diuresis, invigorates spleen, lower internal heat, dehumidification, nourishes yin, promotes fluid) | Wang (2012) |
| Health care | Health food | Anti-inflammatory, antiviral, antitumor | (Liu et al., 2011, 2012) |
| Hygiene | Mask | Antioxidation | Wu (2017b) |
| PV | |||
| Metabolic disease | Pill/tablet/capsule/oral liquid | Postmenopausal osteoporosis | Zhang et al. (2012) |
| Skin diseases | Patch/decoction | Eczema (clears heat, detoxifies, anti-inflammation, antibacterial, reduces swelling and pain, relieves itching) | Mo (2017) |
| Health care | Tea | Improves human immunity, relieves constipation, anti-hypertension, delays ageing, promotes blood circulation, anti-cancer, anti-tumor, detoxifies | Qiu (2019) |
| Health food | Antioxidation | Gao et al. (2013) | |
| Hygiene | Cream | Anti-aging (reduces melanin deposition) | (Chen et al.,2018) |
| Essence | Acne (promotes blood circulation, enhances metabolism, anti-bacterial) | Ou (2018) | |
| Lotion/toner/mask | Whitens (preserves moisture, reduces skin pigmentation) and inhibiting melanin production | (Geng et al., 2012; Li, 2018a, 2018b) | |
| Cream | Dark circles | Wang et al. (2018) | |
Patrinia villosa Juss. (PV); Patrinia scabiosafolia Fisch. (PS).
Not indicate species.
Comprehensive consideration of the pharmacological activities, the prescription containing Herba Patrniae in Chinese patent is mainly used to treat gynecological diseases, such as chronic PID (Dai et al., 2019; Deng, 2019; He et al., 2019a, 2019c; Hou, 2019; Lu, 2019; Luo, 2019a; Lv et al., 2019; Zou et al., 2019), vaginitis (Jiang, 2019b; Li and Li, 2019a; Long, 2019; Luo, 2019b; Nong et al., 2019), cervicitis (Li, 2019a; Zhang and Jiang, 2019), and breast disease (Gao, 2019c; Wu, 2019; Yan, 2019a; Zhang, 2019a); urinary system diseases, such as prostatitis (Li, 2019c; Long and Yin, 2019) and orchitis (Yuan, 2019); respiratory diseases, such as chronic laryngitis (Gao, 2019d), rhinitis (Gong, 2019), and bronchitis (Zhao, 2019); digestive diseases, such as gastritis (Li, 2019d; Xiong, 2020), hepatitis (Feng, 2019; Jiang et al, 2019; Liu, 2019b; Ru, 2019; Zhao 2019b), pancreatitis (Dang et al., 2019; Li, 2019e), appendicitis (Zeng, 2019; Zhang, 2019c), colitis and proctitis (Chen et al., 2019d); skin diseases, such as shingles (Dong, 2019), wound infection (Jiang, 2019c; Tan, 2019; Yu, 2019), and tinea pedis (Gao, 2019f); and animal inflammatory diseases, such as infectious bronchitis and fallopian tube injury in chickens (Zhang et al., 2019b), and animal mastitis (Zhou et al., 2019). Besides, in hygiene, it can be used to prepare oral care liquid (Peng et al., 2019b; Ye et al., 2019), laundry liquid (Xie, 2019), and toilet paper (Zhang, 2019f). Other patented products containing Herba Patriniae include an anti-cancer decoction for lung cancer (Wang, 2019a; Wang et al., 2019b) and nasopharyngeal carcinoma (Xie and Xie, 2019b), a cosmetic for antioxidation (Wu, 2017b), anti-aging (Chen et al., 2018), acne (Ou, 2018), whitens (Geng et al., 2012; Li, 2018a, 2018b), and dark circles (Wang et al., 2018).
10. Conclusion and perspectives
Herba Patriniae can be used in clinical treatment in the form of a single herb or compound formulae of TCM to treat diseases for more than 2000 years in China. So far, a total of 233 compounds have been identified from the two species of Herba Patriniae, including triterpenoid saponins, flavonoids, organic acids, iridoids, and volatiles. However, the two species applied as Herba Patriniae gave obviously different in phytochemistry. PS is rich in triterpenoid saponins and volatiles, while PV contains more flavonoids. Meanwhile, it's easy for us to see that the content of the same compounds (chlorogenic acid, caffeic acid, quercetin, kaempferol, palmic acid, hexanoic acid, cis-anethol, camphogen, β-ionone and β-damascenone) were different in two plants. Even within the same plant, the content of the compounds (protocatechuic acid, chlorogenic acid, caffeic acid, iso-chlorogenic acid A, iso-chlorogenic acid C, ursolic acid, oleanolic acid, isovitexin, isoorientin, hederagenin) were variable in different sources, indicating that the herb species, sources and processing method should be took into full consideration in its clinical use. And it is urgent to seek a feasible and reliable standardized sample processing method for Herba Patriniae. The pharmacological studies of Herba Patriniae in vivo, in vitro, and in clinic were summarized. Two source plants of Herba Patriniae gave similar pharmacological effects, including anti-cancer, anti-inflammatory, antioxidant, antimicrobial, sedative, and hypnotic effects. These functions are closely related to the SIRT-1, mTOR, AKT, mir-144-3p/Nrf2, FAK, PI3K/Akt, EMT and NF-κB signaling pathways. But there are no reports on antipruritic, proangiogenic, and anti-diarrheal effects for PS, and there are no studies on anti-diabetic effects for PV. This difference in pharmacological activities possibly related to the differences in the phytochemistry. So the interrelationship between compounds and pharmacological activities should be further studied. As the dose increases up to 20 mg/kg in mice, mild side effects were found. While the dose that causes the side effects in human was up to 4–12 g/day, and there are individual differences. Pharmacokinetics can provide scientific explanations for pharmacological and toxicological findings. Unfortunately, the pharmacokinetics of Herba Patriniae was lack, thus a range of pharmacokinetic studies on its active compounds are needed to provide comprehensive data for clinical application. Based on this review, the research on the dosage form of Herba Patriniae is not in-depth, so strengthening the work in this area will help to promote the development and utilization of Herba Patriniae. Altogether, this review extensively summarized the traditional uses, botanical description, phytochemistry, pharmacology, and quality control of Herba Patriniae, and provided information on the similarities and differences between the two plants for their further research and clinical applications.
Author contribution
Keyang Zheng and Birui Shi collected and analyzed documentations. Linna Gong drafted the manuscript. Menghua Liu and Wei Zou designed this review and critically revised the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the NHC Key Laboratory of Birth Defects Research and Prevention (grant number KF2019002), the Hunan Provincial Science and Technology Department (grant number 2019JJ30013), the Hunan Administration of Traditional Chinese Medicine (grant number 202020), and the College Students’ Innovation and Entrepreneurship Training Program (grant number 201912121034) of Southern Medical University.
References
- Affiliated Hospital of Guangzhou College of Traditional Chinese Medicine . Affiliated Hospital of Guangzhou College of Traditional Chinese Medicine; Guangzhou: 1978. Selected Data of Acute Abdomen Treated by Integrated Traditional Chinese and Western Medicine. [Google Scholar]
- Al-Megrin W.A., Metwally D.M., Habotta O.A., Amin H.K., Abdel Moneim A.E., El-Khadragy M. Nephroprotective effects of chlorogenic acid against sodium arsenite-induced oxidative stress, inflammation, and apoptosis. J. Sci. Food Agric. 2020 doi: 10.1002/jsfa.10565. [DOI] [PubMed] [Google Scholar]
- Bao J., Xia L., Zhao Y.C., Xia R.X. Scutellarin exerts anticancer effects on human leukemia cells via induction of Sub-G1 cell cycle arrest, apoptosis and also inhibits migration and invasion by targeting Raf/MEK/ERK signalling pathway. J BUON. 2020;25(2):1050–1055. [PubMed] [Google Scholar]
- Bao Q. 2019. A Biological Herbicide for Killing Eupatorium Adenophorum. CN110583712A. [Google Scholar]
- Beijing Municipal Bureau of Health . Printing Office of Beijing Medicinal Materials Company; Beijing: 1974. Beijing Chinese Traditional Patent Medicine Specification. [Google Scholar]
- Beijing Traditional Chinese Medicine Hospital . People's Medical Publishing House; Beijing: 1982. Surgery of Chinese Medicine. [Google Scholar]
- Biological Products Identification Institute of the Ministry of Health . Vol. 2. People's Medical Publishing House; Beijing: 1990. (Chinese National Medicine Annals). [Google Scholar]
- Bukharov V.G., Karlin V.V. Structure of scabioside F. Chem. Nat. Compd. 1970;6(3):372–373. doi: 10.1007/BF00567336. [DOI] [Google Scholar]
- Bukharov V.G., Karlin V.V. Structure of scabioside G. Chem. Nat. Compd. 1970;6(3):373–374. doi: 10.1007/BF00567337. [DOI] [Google Scholar]
- Bukharov V.G., Karlin V.V., Sidorovich T.N. Triterpene glycosides of Patrinia scabiosofolia I. Chem. Nat. Compd. 1970;6(1):69–74. doi: 10.1007/BF00564160. [DOI] [Google Scholar]
- Cao C.L. People's Medical Publishing House; Beijing: 1983. Compilation of Traditional Chinese Medicine Preparations. [Google Scholar]
- Chen B. Five key points of improving fertility rate of cows. Rural science and technology. 2006;6:69. doi: 10.19777/j.cnki.issn1002-6193.2006.06.064. [DOI] [Google Scholar]
- Chen G.Q., W F., Chen H.B. 2019. Syrup for Preventing and Treating Endometriosis of Qi Stagnation and Blood Stasis Type. [Google Scholar]
- Chen G.Q., W F., Chen H.B. May 14, 2019. Wine for Preventing and Treating Endometriosis of Qi Stagnation and Blood Stasis Type. [Google Scholar]
- Chen H.J., Ge X.H., Wang Y.f., Wang X.Y. 2018. A composition for preventing skin aging and a skin care product containing the composition. [Google Scholar]
- Chen K.J. Zhonghua Book Company; Beijing: 1981. Selection of Medical Prescriptions of Cixi and Guangxu. [Google Scholar]
- Chen L., Chen P., Zhang T., Tian L.M. The study on anti-tumor effect and its mechanism on sarcoma U14 mice of cervical cancer by the alcohol extract of Patrina villosa Juss. Heilongjiang Medicine and Pharmacy. 2019;42(3):63–65+68. [Google Scholar]
- Chen L., Liu L., Ye L., Shen A., Chen Y., Sferra T.J., Peng J. Patrinia scabiosaefolia inhibits colorectal cancer growth through suppression of tumor angiogenesis. Oncol. Rep. 2013;30(3):1439–1443. doi: 10.3892/or.2013.2582. [DOI] [PubMed] [Google Scholar]
- Chen S.L., Han L. Modern research progress on Patriniae. J. Guangdong Pharma. Univ. 2017;33(6):816–821. doi: 10.16809/j.cnki.2096-3653.2017091402. [DOI] [Google Scholar]
- Chen X.H. The benefit of interplanting bitter vegetables in newly planted tea garden is good. Tea in Fujian. 2001;1:25. doi: 10.3969/j.issn.1005-2291.2001.01.013. [DOI] [Google Scholar]
- Chen X.H., Yu T., Gao P.F., Xie M.N., Meng Y.F. October 15, 2019. Traditional Chinese Medicine Composition for Treating Colitis and Proctitis and Application Thereof. CN110327435A. [Google Scholar]
- Chen Y. Determination of isovitexin and isoorientin from Patrinia villosa Juss by HPLC. China Pharmaceuticals. 2008;16:24–25. [Google Scholar]
- Chen Y.P., Zeng J., Ye H.Y. Inhibitory effect of aqueous extract of Patrinia villosa Juss on central nerve system. Chin. Remedies Clin. 2005;6:439–440. [Google Scholar]
- Chiu L.C., Ho T.S., Wong E.Y., Ooi V.E. Ethyl acetate extract of Patrinia scabiosaefolia downregulates anti-apoptotic Bcl-2/Bcl-X(L) expression, and induces apoptosis in human breast carcinoma MCF-7 cells independent of caspase-9 activation. J. Ethnopharmacol. 2006;105(1–2):263–268. doi: 10.1016/j.jep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Cho E.J., Shin J.S., Noh Y.S., Cho Y.W., Hong S.J., Park J.H., Lee J.Y., Lee J.Y., Lee K.T. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J. Ethnopharmacol. 2011;136(3):428–435. doi: 10.1016/j.jep.2010.04.047. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Woo W.S. Coumarins and triterpenoid glycosides from the roots of Patrinia scabiosaefolia. Arch Pharm. Res. (Seoul) 1984;7(2):121–126. doi: 10.1007/BF02856624. [DOI] [Google Scholar]
- Choi J.S., Woo W.S. Triterpenoid glycosides from the roots of Patrinia scabiosaefolia. Planta Med. 1987;53(1):62–65. doi: 10.1055/s-2006-962622. [DOI] [PubMed] [Google Scholar]
- Dai C.J., Lin P.Q. Study on antimicrobial actions and stability of Patrinia villosa charantia extractive. Food & Machinery. 2011;27(6):157–159. [Google Scholar]
- Dai L.B., Li J.Y., Wang Q.L., Wen R.Y., Zhu Z.Q., Wang Q.F., Zhou K., Liu Y.B., Kang G.J., Xie B. August 23, 2019. Removing Blood Stasis and Relieving Pain Prescription for the Treatment of Pelvic Inflammation. CN110151946A. [Google Scholar]
- Dang Z.Q., Li M.G., Dang Z.B., Qian P.G., Wang Y.L., Xu X.Q. 2019. A Traditional Chinese Medicine Patch for the Treatment of Acute Pancreatitis. CN109876116A. [Google Scholar]
- Deng L.Y. Herba Patriniae for Eliminating abscess and expelling pus. Family & Tradition. Chin. Med. 2001;10:55. [Google Scholar]
- Deng Z.T. January 29, 2019. Oral Chinese Medicine Composition for Treating Pelvic Inflammatory Disease. CN109276674A. [Google Scholar]
- Di Y., Xu T., Tian Y., Ma T.T., Qu D.H., Wang Y., Lin Y.H., Bao D.Y., Yu L., Liu S.Y., Wang A.M. Ursolic acid protects against cisplatin-induced ototoxicity by inhibiting oxidative stress and TRPV1-mediated Ca2+-signaling. Int. J. Mol. Med. 2020;46(2):806–816. doi: 10.3892/ijmm.2020.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q. November 19, 2019. Traditional Chinese Medicine Composition for Treating Leukemia and Preparation Method Thereof. CN110464784A. [Google Scholar]
- Ding Y.Z., Chen X.Y., Tan X.Z., Liang S.R., Wu Y.L. November 1, 2019. A Combination of Traditional Chinese Medicine Infusion Drug for Anti-inflammation of Sow Uterus and its Preparation Method and Application. CN110393768A. [Google Scholar]
- Dong P.J. August 13, 2019. Oral Medicine for Treating Shingles. CN110115736A. [Google Scholar]
- Drug Control Institute of Yunnan Chuxiong Health Bureau . Sichuan nationalities Publishing House; Chengdu: 1983. Yi Medicine Annals. [Google Scholar]
- El-Sonbaty Y.A., Suddek G.M., Megahed N., Gameil N.M. Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie. 2019;167:119–134. doi: 10.1016/j.biochi.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Fan X.M. Zhejiang University; Zhejiang: 2014. Extraction, Separation and Activity Study of Tannin of Patrinia Villosa Juss[D] 2014. [Google Scholar]
- Fei G.H., Ji S., Zhang D.W., Wu X., Wei Y.Y., Guo Y. November 22, 2019. New Use of Traditional Chinese and Western Medicine Composition. CN110478440A. [Google Scholar]
- Feng X.F. June 7, 2019. A Traditional Chinese Medicine Preparation for the Treatment of Hepatitis. CN109846966A. [Google Scholar]
- Feng Y., Li N., Ma H.M., Bei B., Han Y.Q., Chen G. Undescribed phenylethyl flavones isolated from Patrinia villosa show cytoprotective properties via the modulation of the mir-144-3p/Nrf2 pathway. Phytochemistry. 2018;153:28–35. doi: 10.1016/j.phytochem.2018.05.016. [DOI] [PubMed] [Google Scholar]
- Flora of China Editorial Committee, The Chinese Academy of Sciences . Beijing Science Press; Beijing: 1986. Flora of China. [Google Scholar]
- Fu B., Wu Q., Dang M., Bai D., Guo Q., Shen L., Duan K. 2017. Inhibition of Pseudomonas aeruginosa Biofilm Formation by Traditional Chinese Medicinal Herb Herba Patriniae; p. 9584703. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai S.Y. March 5, 2019. Fattening Pig Feed. CN109418580A. [Google Scholar]
- Gao L., Zhang L., Li N., Liu J.Y., Cai P.L., Yang S.L. New triterpenoid saponins from Patrinia scabiosaefolia. Carbohydr. Res. 2011;346(18):2881–2885. doi: 10.1016/j.carres.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Gao L., Zhang L., Liu J.Y., Cai P.L., Yang S.L. Chemical constituents of Patrinia scabiosaefolia. Chin. Tradit. Herb. Drugs. 2011;42(8):1477–1480. [Google Scholar]
- Gao L., Zhang L., Wang L.M., Liu J.Y., Cai P.L., Yang S.L. New triterpenoid saponins from Patrinia scabiosifolia. J. Asian Nat. Prod. Res. 2012;14(4):333–341. doi: 10.1080/10286020.2011.653685. [DOI] [PubMed] [Google Scholar]
- Gao L.Y. January 25, 2019. A Traditional Chinese Medicine for the Treatment of Endometritis. CN109260323A. [Google Scholar]
- Gao L.Y. April 23, 2019. Medicine for Treating Mastitis. CN109663025A. [Google Scholar]
- Gao L.Y. July 2, 2019. An Effervescent Tablet Containing Pseudolaricis Cortex and Dictamni Cortex for Dispelling Pathogenic Wind and Relieving Itching. CN109954050A. [Google Scholar]
- Gao Q. August 18, 2017. Chinese Medicine Formula for Treating Neurasthenia. CN107050386A. [Google Scholar]
- Gao W. January 4, 2019. A Traditional Chinese Medicine for the Treatment of Endometritis. CN109125509A. [Google Scholar]
- Gao W. April 23, 2019. Traditional Chinese Medicine for Treating Chronic Laryngitis and Improving Immunity. CN109663075A. [Google Scholar]
- Gao W. September 6, 2019. A Traditional Chinese Medicine Composition for Invigorating Spleen, Clearing Heat and Removing Blood Stasis for Treating Gastric Ulcer. CN110201129A. [Google Scholar]
- Gao X., Chen G., Kong S.Z. November 27, 2013. Preparation Method of Antioxidant Preparation for Root of Patrinia Villosa. CN103405484A. [Google Scholar]
- Gao Z.W., Zhang X.N., Guo C.C., Su L.H., Liu S.M., Li Z.C., Wang M.J., Bian W. September 13, 2019. Composition for Pig Chronic Eczema and Preparation Method Thereof. CN110227109A. [Google Scholar]
- Geng F., Liu H.Y., Zhao X., Ren Y.D., Zhou Q., Qi Y.H., Zhang N. February 29, 2012. Preparation and Application of Compound Extract of Traditional Chinese Medicine for Whitening and and Freckle Removing. CN102362842A. [Google Scholar]
- Gepdiremen A., Mshvildadze V., Süleyman H., Elias R. Acute anti-inflammatory activity of four saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine. 2005;12(6–7):440–444. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gong L. May 24, 2019. A Bacteriostatic Solution for Rhinitis. [Google Scholar]
- Guizhou Medical Products Administration . Guizhou Science & Technology Press; Guiyang: 2003. Quality Standard for Chinese Herbal Medicine and Ethnic Herbal Medicine in Guizhou Province (2003 Edition) [Google Scholar]
- Han X., Wang S., Yang X.X., Li T.J., Zhao H.J., Zhou L.P., Zhao L., Bao Y.R., Meng X.S. Analysis of plasma migration components in Patrinia villosa (Thunb.) Juss. effective parts by UPLC-Q-TOF-MS. Biomed. Chromatogr. 2020;34(1) doi: 10.1002/bmc.4701. [DOI] [PubMed] [Google Scholar]
- Hao W., Kwek E., He Z., Zhu H., Liu J., Zhao Y., Ma K.Y., He W.S., Chen Z.Y. Ursolic acid alleviates hypercholesterolemia and modulates the gut microbiota in hamsters. Food Funct. 2020 doi: 10.1039/d0fo00829j. [DOI] [PubMed] [Google Scholar]
- He B. Treatment of infantile diarrhea with fresh Herba patriniae and Anisodamine. Chinese Commun. Doctors. 1991;7:25–26. [Google Scholar]
- He H.X., Yang W., Luo H.L., Zhang K.Y., Xu L.J. August 6, 2019. Vaginal Suppository for Treating Chronic Pelvic Inflammatory Disease. [Google Scholar]
- He J.Y., Ren Y.Y., Ma R., Wu J.Y. Experience of Dachengqi decoction with Coicis Semen and Herba patriniae in the treatment of acute pancreatitis. Chin. Med. Modern Distance Educ. China. 2019;17(21):63–64. [Google Scholar]
- He X.R., Luan F., Zhao Z.F., Ning N., Li M.X., Jin L., Chang Y., Zhang Q., Wu N., Huang L.H. The Genus Patrinia: a review of traditional uses, phytochemical and pharmacological studies. Am. J. Chin. Med. 2017;45(4):637–666. doi: 10.1142/s0192415x17500379. [DOI] [PubMed] [Google Scholar]
- He Y.C., Mo Q.Y., Huang R.S. August 30, 2019. Shiwei Shengui Enema and its Preparation Process. CN110179893A. [Google Scholar]
- He Y.X., Hong Z. November 26, 2019. Medicine for Treating Uterine Fibroids and Preparation Method Thereof. CN110496197A. [Google Scholar]
- Heilongjiang Medical Products Administration . 2001 edition. Heilongjiang Science & Technology Press; Haerbin: 2001. Standard of Chinese Herbal Medicine in Heilongjiang Province. [Google Scholar]
- Hou F.X. June 21, 2019. Enema Drug for Treating Pelvic Inflammatory Disease. CN109908299A. [Google Scholar]
- Hu J.M., Zhou J.Y., Wu J.T., Chen Q., Du W.B., Fu F.D., Yu H., Yao S., Jin H.T., Tong P.J., Chen D., Wu C.L., Ruan H.F. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J. Ethnopharmacol. 2020;247:112261. doi: 10.1016/j.jep.2019.112261. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhang R.S., Wang C.F., Zhang S.F. Studies on chemical constituents of Patrinia villosa. J. Chin. Med. Mater. 2007;30(4):415–417. [PubMed] [Google Scholar]
- Huang S.Z., Liu W.Y., Huang Y., Shen A.L., Liu L.Y., Peng J. Patrinia scabiosaefolia inhibits growth of 5-FU-resistant colorectal carcinoma cells via induction of apoptosis and suppression of AKT pathway. Chin. J. Integr. Med. 2019;25(2):116–121. doi: 10.1007/s11655-018-3002-6. [DOI] [PubMed] [Google Scholar]
- Huang S.H., Chen T., Lin F., Hong Y.J., Hong Y.N., Qiu L.X. Protective effects of alcohol extract of Patrinia villosa Juss on acute liver injury in mice induced by carbon tetrachloride. J. Northwest For. Univ. 2019;47(7):47–53+61. doi: 10.13207/j.cnki.jnwafu.2019.07.007. [DOI] [Google Scholar]
- Hunan Food and Drug Administration . Hunan Science & Technology Press; Changsha: 2010. Standard of Chinese Herbal Medicine in Hunan Province (2009 Edition) [Google Scholar]
- Inada A., Yamada M., Murata H., Kobayashi M., Toya H., Kato Y., Nakanishi T. Phytochemical studies of seeds of medicinal plants. I. Two sulfated triterpenoid glycosides, sulfapatrinosides I and II, from seeds of Patrinia scabiosaefolia FISCHER. Chem. Pharm. Bull. (Tokyo) 1988;36(11):4269–4274. doi: 10.1248/cpb.36.4269. [DOI] [PubMed] [Google Scholar]
- Jang J. Anhui University; Anhui: 2017. Studies on Chemical Constituents of Ethyl Acetate Extraction Phase from Patrinia Scabiosaefolia Fisch[D] 2017. [Google Scholar]
- Jeon J., Lee J., Kim C., An Y., Choi C. Aqueous extract of the medicinal plant Patrinia villosa Juss. induces angiogenesis via activation of focal adhesion kinase. Microvasc. Res. 2010;80(3):303–309. doi: 10.1016/j.mvr.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Ji J.H. Ji Guang's clinical experience in the application of Coicis Semen-radix Aconiti Lateralis Preparata-Herba patriniae powder. Jiangsu J. Tradition. Chin. Med. 2006;12:10–11. [Google Scholar]
- Ji L.Q., Wu J.M., Min W., Chen Y.F., Xie M.H. Effects of PMIEE on proliferation and apoptosis of HepG2 and MCF7 cells. Chin J Mod Appl Pharm. 2019;36(6):692–697. doi: 10.13748/j.cnki.issn1007-7693.2019.06.009. [DOI] [Google Scholar]
- Jia C.D. Determination of paeoniflorin in Herba patriniae tablets by HPLC. Clin. J. Tradition. Chin. Med. 2010;22(4):342–344. doi: 10.16448/j.cjtcm.2010.04.033. [DOI] [Google Scholar]
- Jiang H., Chu Z.Y., Wang H.X., Li L., Liu Y.F. Chemical constituents of patrinia scabiosaefolia fisch. Chin. Tradit. Herb. Drugs. 2003;11:21–23. [Google Scholar]
- Jiang M. January 22, 2019. Traditional Chinese Medicine for Treating Gynecological Inflammation and Preparation Method Thereof. CN109248244A. [Google Scholar]
- Jiang M.Y. March 8, 2019. Preparation for External Use of Danhuang and its Preparation Process. CN109432238A. [Google Scholar]
- Jiang P.L., Jiang Y.K., Ye X.D., Peng L.Z. September 6, 2019. A Traditional Chinese Medicine Composition for Treating Impaired Liver Function. [Google Scholar]
- Jiang Y.L. Herba Patriniae: durian in wild vegetables. Food and life. 2019;7:66–67. doi: 10.3969/j.issn.1004-5473.2019.07.034. [DOI] [Google Scholar]
- Jie X.X., Geng C.A., Huang X.Y., Ma Y.B., Zhang X.M., Zhang R.P., Chen J.J. Five new secoiridoid glycosides and one unusual lactonic enol ketone with anti-HBV activity from Swertia cincta. Fitoterapia. 2015;102:96–101. doi: 10.1016/j.fitote.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Jin H.P. December 17, 2019. A Traditional Chinese Medicine Formula for Treating Leukemia and its Application Method. CN110575531A. [Google Scholar]
- Kang S.S., Kim J.S., Kim Y.H., Choi J.S. A triterpenoid saponin from Patrinia scabiosaefolia. J. Nat. Prod. 1997;60(10):1060–1062. doi: 10.1021/np970175v. [DOI] [PubMed] [Google Scholar]
- Kim G.J., Song D.H., Yoo H.S., Chung K.H., Lee K.J. Hederagenin Supplementation alleviates the pro-inflammatory and apoptotic response to alcohol in rats. Nutrients. 2017;9(1) doi: 10.3390/nu9010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.H. Studies on components of Patrinia scabiosaefolia. Pharma. 1997;28(2):93–98. [Google Scholar]
- Kong L.B., Zhao J. Progress in clinical application of Herba patriniae Chinese. J. Modern Drug Appl. 2008;8:113–114. [Google Scholar]
- Lee E.J., Kim C., Kim J.Y., Kim S.M., Nam D., Jang H.J., Kim S.H., Shim B.S., Ahn K.S., Choi S.H., Jung S.H., Ahn K.S. Inhibition of LPS-induced inflammatory biomarkers by ethyl acetate fraction of Patrinia scabiosaefolia through suppression of NF-kappaB activation in RAW 264.7 cells. Immunopharmacol. Immunotoxicol. 2012;34(2):282–291. doi: 10.3109/08923973.2011.602412. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Kim J.S., Kim Y.S., Kang S.S. Glycosides from the aerial parts of Patrinia villosa. Chem. Pharm. Bull. (Tokyo) 2013;61(9):971–978. doi: 10.1248/cpb.c13-00306. [DOI] [PubMed] [Google Scholar]
- Li H. May 11, 2018. Whitening Skin Perfecting Lotion and Preparation Method Thereof. CN108014059A. [Google Scholar]
- Li P.Y. February 9, 2018. Preparation Method of Whitening Toner. CN107669533A. [Google Scholar]
- Li N., Zhao B., Yu Y.F., Dong X.P. Studies on anti-inflammation chemical constitutes of Patrinia villosa. J. Chin. Med. Mater. 2008;31(1):51–53. [PubMed] [Google Scholar]
- Li P.S., Peng Y.Y., Ma Q., Li Z.Y., Zhang X.H. Study on the formation of antihypertensive Twin drugs by caffeic acid and Ferulic acid with Telmisartan. Drug Des. Dev. Ther. 2020;14:977–992. doi: 10.2147/DDDT.S225705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.S., Li H.Y., Pu Y.A., Liu D.L., Tian W.J., Dong Y.P. The anti-respiratory syncytial virus effect of an active compound (AP3) from a Chiese medicinal herb-Herba patriniae in vitro. Chin. J. Epidemiol. 2004;2:63–66. [PubMed] [Google Scholar]
- Li X.J., Li H.S. June 4, 2019. Health Beverage with the Function of Preventing Hepatobiliary Disease and Preparation Method Thereof. CN109832527A. [Google Scholar]
- Li Y., Li J., Fang C. Inhibitory effects of anti-SARS traditional Chinese medicines on the UV irradiation of lambda-lysogen. Am. J. Chin. Med. 2006;34(1):147–155. doi: 10.1142/s0192415x06003710. [DOI] [PubMed] [Google Scholar]
- Li Y.F., Li M.H., Lou F.C., Tang Y.P. Studies on the constituents of patrinia scabiosaefolia fisch. J. China Pharm. Univ. 2002;2:23–25. [Google Scholar]
- Li Y.F., Lou F.C. Isolation and identification of triterpenoid saponins from Patrinia scabiosaefolia Fisch. West China J. Pharm. Sci. 2007;5:483–486. [Google Scholar]
- Li Y.H., Li X.R. August 23, 2019. A Traditional Chinese Medicine Lotion for Treating Vulvar Inflammation and its Application Method. CN110151873A. [Google Scholar]
- Li Y.L. Prevention and treatment of "climbing beedisease" with Herba Patriniae. J. Bee. 2002;8:20. doi: 10.3969/j.issn.1003-9139.2002.08.016. [DOI] [Google Scholar]
- Li W.S. September 24, 2019. A Health Product for Cervicitis. CN110269900A. [Google Scholar]
- Li M.J. December 17, 2019. Traditional Chinese Medicine Composition for Treating Gynecological Tubal Blockage and Preparation Method Thereof. CN110575482A. [Google Scholar]
- Li H. June 21, 2019. A Traditional Chinese Medicine Prescription for the Treatment of Chronic Prostatitis. CN109908255A. [Google Scholar]
- Li L. January 15, 2019. Medicine for Treating Gastritis and Preparation Method Thereof. CN109200177A. [Google Scholar]
- Li Z.X. February 5, 2019. A Traditional Chinese Medicine Composition for Treating Pancreatitis. CN109303829A. [Google Scholar]
- Li C.X. January 18, 2019. Low-toxicity Cotton Bactericide. CN109221264A. [Google Scholar]
- Li Z.B. June 14, 2019. A Preparation Method for Extracting Non-residual Insecticides from Plants. CN109874784A. [Google Scholar]
- Li Z.Y., Tang Y.Q., Zhu S.Q., Li D.W., Han X., Gu G.L., Xing N.D., Ren J.C., Guo Z.X., Jiao W., Yan L., Xu Z.H., Zhang W.H. Ethanol extract of Patrinia scabiosaefolia induces the death of hum, an renal cell carcinoma 786-O cells via SIRT-1 and mTOR signaling-mediated metabolic disruptions. Oncol. Rep. 2018;39(2):764–772. doi: 10.3892/or.2017.6139. [DOI] [PubMed] [Google Scholar]
- Liaoning Food and Drug Administration . Liaoning Science & Technology Publishing House; Shenyang: 2009. Standard of Chinese Herbal Medicine in Liaoning Province (2009 Edition) [Google Scholar]
- Lin J., Cai Q.Y., Xu W., Lin J.M., Peng J. Chemical composition, anticancer, anti-neuroinflammatory, and antioxidant activities of the essential oil of Patrinia scabiosaefolia. Chin. J. Integr. Med. 2018;24(3):207–212. doi: 10.1007/s11655-016-2459-4. [DOI] [PubMed] [Google Scholar]
- Liu F., Wang D., Liu X.S. July 2, 2019. Composite Pig Feed Containing Rhubarb and Preparation Method Thereof. CN109953196A. [Google Scholar]
- Liu H., He X.L. Current situation and laboratory research progress of invasive aspergillus infection. J. Clin. Res. 2014;11:2262–2264. doi: 10.3969/j.issn.1671-7171.2014.11.070. [DOI] [Google Scholar]
- Liu H.T., Lei P. Simultaneous determination of five organic acids in patriniae Herba by dual- wavelength Reversed Phase high performance liquid chromatography. Tradit. Chin. Drug Res. Clin. Pharmacol. 2019;30(3):349–353. doi: 10.19378/j.issn.1003-9783.2019.03.013. [DOI] [Google Scholar]
- Liu H.T., Lei P., Liu H.Y., Zou Y., Zhang C.H. Simultaneous determination of protocatechuic acid, chlorogenic acid and caffeic acid in Herba Patriniae by dual-wavelength HPLC. Chin J Pharm Anal. 2013;33(4):611–615. [Google Scholar]
- Liu H.T., Zhu L., Lei P., Lan S.N., Huang H.F. Simultaneous determination of quercetin and kaempferol in Herba Patriniae by RP-HPLC. Central South Pharmacy. 2015;13(3):291–294. [Google Scholar]
- Liu J., Hu S.M. May 18, 2011. A Method for Preparing the Extract of Patrinia Scabiosaefolia. CN102058637A. [Google Scholar]
- Liu J.F. Clinical application progress of Patriniae in gynecological diseases. Chin. Med. Modern Dist. Edu. China. 2019;17(21):132–134. [Google Scholar]
- Liu J.S., Wei X.D., Wu Y.F., Wang Y.N., Qiu Y.W., Shi J.M., Zhou H.L., Lu Z.B., Shao M., Yu L.Z., Tong L.Z. Giganteaside D induces ROS-mediated apoptosis in human hepatocellular carcinoma cells through the MAPK pathway. Cell. Oncol. 2016;39(4):333–342. doi: 10.1007/s13402-016-0273-9. [DOI] [PubMed] [Google Scholar]
- Liu J.Y., Gao L., Cai P.L., Yang S.L. December 21, 2011. Oleanolic Acid Derivative and Preparation Method Thereof. CN102286056A. [Google Scholar]
- Liu J.Y., Yang S.L., Li X.R., Xu Q.M., Liu Y.L., Gao L. August 8, 2012. A Triterpenoid Saponin with Antitumor Effect. CN102627683A. [Google Scholar]
- Liu L.Y., Shen A.L., Chen Y.Q., Wei L.H., Lin J.M., Sferra T.J., Hong Z.F., Peng J. Patrinia scabiosaefolia induces mitochondrial-dependent apoptosis in a mouse model of colorectal cancer. Oncol. Rep. 2013;30(2):897–903. doi: 10.3892/or.2013.2528. [DOI] [PubMed] [Google Scholar]
- Liu M.F. The nursing experience of the treatment of ulcerative colitis with the preserved enema of Herba Patriniae decoction. Modern J. Integrated Tradition. Chin. Western Med. 2011;20(1):108–109. [Google Scholar]
- Liu S.J., Liu Y.X. December 24, 2019. A Traditional Chinese Medicine Formula for the Treatment of Stomach Disease. [Google Scholar]
- Liu S.X., Liu Y., Bai X., Yu Y.M., Li X.Y., Bi S., Zhang T.J. Studies on fingerprint of patriniae Herba. Chin. Tradit. Herb. Drugs. 2016;47(12):2074–2077. [Google Scholar]
- Liu W., Jia S.H., Xiang Z. Determination of volatile component from patrinia villosa (Thunb.) Juss. And patrinia scabiosaefolia fisch by GC- MS. J. Harbin Univ. Commerce (Nat. Sci. Ed.) 2016;32(1):6–10. doi: 10.19492/j.cnki.1672-0946.2016.01.002. [DOI] [Google Scholar]
- Liu Y. June 25, 2019. A Traditional Chinese Medicine Composition for Treating Non-alcoholic Fatty Liver of Liver Stagnation and Spleen Deficiency Type and its Application. CN109925469A. [Google Scholar]
- Liu Y., Chi Z.W. 2019. Traditional Chinese Medicine Composition for Treating Horse Sinus Abscess and Preparation Method Thereof. CN109303875A. [Google Scholar]
- Liu Y., Chi Z.W. 2019. Traditional Chinese Medicine Composition for Treating Tail Rot of Crayfish and Preparation Method Thereof. CN109303874A. [Google Scholar]
- Liu Y.C., Liu W., Chen C.L., Xiang Z., Liu H.W. A cytotoxic natural product from Patrinia villosa Juss. Anticancer Agents Med Chem. 2019;19(11):1399–1404. doi: 10.2174/1871520619666190416101014. [DOI] [PubMed] [Google Scholar]
- Liu Y.C., Liu W., Chen G., Xiang Z., Chen C.L. Chemical constituents from patrinia villosa Juss. J. Harbin Univ. Commerce (Nat. Sci. Ed.) 2019;35(2):138–140+170. doi: 10.19492/j.cnki.1672-0946.2019.02.003. [DOI] [Google Scholar]
- Liu Z.H., Xu L.T., Xu X.Q., Niu Y., Saadeldeen F.A., Kang W.Y. Effects and mechanisms of iridoid glycosides from Patrinia scabiosaefolia on improving insulin resistance in 3T3-L1 adipocytes. Food Chem. Toxicol. 2019;134:110806. doi: 10.1016/j.fct.2019.110806. [DOI] [PubMed] [Google Scholar]
- Long R. December 3, 2019. Medicine for Treating Leucorrhea Abnormality and Preparation Method Thereof. CN110522836A. [Google Scholar]
- Long X.P., Yin Y.F. March 8, 2019. Patch for Regulating Prostate Hyperplasia and its Conditioning Method. CN109432375A. [Google Scholar]
- Lu H.L. November 26, 2019. Oral Medicine for Treating Chronic Pelvic Inflammatory Disease. CN110496198A. [Google Scholar]
- Lu K.H. Guizhou Science and Technology Publishing House; Guiyang: 1992. Dong Medicine. [Google Scholar]
- Lu Y.X. Research on Materia Medica of Herba patriniae. Lishizhen Med. Materia Medica Res. 1996;3:4–5. [Google Scholar]
- Luan F., Han K.Q., Li M.X., Zhang T., Liu D.H., Yu L.H., Lv H.Z. Ethnomedicinal Uses, phytochemistry, pharmacology, and toxicology of species from the Genus Ajuga L.: a systematic review. Am. J. Chin. Med. 2019;47(5):959–1003. doi: 10.1142/S0192415X19500502. [DOI] [PubMed] [Google Scholar]
- Luo H.C., Cui Y.H., Shen Y.C., Lou Z.Q. Clinical observation and pharmacological investigation of the sedative and hypnotic effects of the Chinese drug rhizome and root of Patrinia Scabiosaefolia Fisch. J. Tradit. Chin. Med. 1986;6(2):89–94. [PubMed] [Google Scholar]
- Luo Y.H. January 4, 2019. Medicine Combination for Treating Vaginitis and Preparation Method Thereof. [Google Scholar]
- Luo Y.H. January 18, 2019. Traditional Chinese Medicine for Promoting the Rehabilitation of Pelvic Inflammation and its Preparation Method. CN109224058A. [Google Scholar]
- Lv M., Zhu X.D., Zuo C., Wang L., Li X., Wang Z., Wang H., Lv N. March 1, 2019. Application of Traditional Chinese Medicine in the Preparation of Drugs for the Prevention And/or Treatment of Chronic Pelvic Inflammation. CN109394922A. [Google Scholar]
- Mao H.M., Ping Y.H., Zong X.X., Liu F. Simaltanous determination of hederagenina, oleanolic acid and ursolic acid in Herba Patriniae by HPLC. Chin. J. Exp. Tradition. Med. Formulae. 2012;18(15):89–92. doi: 10.13422/j.cnki.syfjx.2012.15.037. [DOI] [Google Scholar]
- Mbaveng A.T., Chi G.F., Bonsou I.N., Abdelfatah S., Tamfu A.N., Yeboah E.M.O., Kuete V., Efferth T. N-acetylglycoside of oleanolic acid (aridanin) displays promising cytotoxicity towards human and animal cancer cells, inducing apoptotic, ferroptotic and necroptotic cell death. Phytomedicine. 2020;76:153261. doi: 10.1016/j.phymed.2020.153261. [DOI] [PubMed] [Google Scholar]
- Meng L.K., Su Y.Q., Yang F., Xiao S.L., Yin Z.L., Liu J.X., Zhong J.D., Zhou D.M., Yu F. Design, synthesis and biological evaluation of amino acids-oleanolic acid conjugates as influenza virus inhibitors. Bioorg. Med. Chem. 2019;27(23):115147. doi: 10.1016/j.bmc.2019.115147. [DOI] [PubMed] [Google Scholar]
- Mo K.T. February 1, 2017. Drug for Internal and External Application in the Treatment of Chronic Eczema and Preparation Method Thereof. CN106361899A. [Google Scholar]
- Nakanishi T., Tanaka K., Murata H., Somekawa M., Inada A. Phytochemical studies of seeds of medicinal plants. III. Ursolic acid and oleanolic acid glycosides from seeds of Patrinia scabiosaefolia Fischer. Chem. Pharm. Bull. (Tokyo) 1993;41(1):183–186. doi: 10.1248/cpb.41.183. [DOI] [PubMed] [Google Scholar]
- Nong C.D., Huang Y.N., Mao J.L. October 1, 2019. Gynecological Anti-itching Tablet and its Preparation Method. CN110292604A. [Google Scholar]
- Ou Y.X. August 14, 2018. Natural Acne Composition and Application Thereof. CN108392450A. [Google Scholar]
- Paciello F., Di Pino A., Rolesi R., Troiani D., Paludetti G., Grassi C., Fetoni A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: in vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020;111555 doi: 10.1016/j.fct.2020.111555. [DOI] [PubMed] [Google Scholar]
- Park H.S., Lee K., Kim S.H., Hong M.J., Jeong N.J., Kim M.S. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J. Food Biochem. 2020 doi: 10.1111/jfbc.13358. [DOI] [PubMed] [Google Scholar]
- Peng E.G., Wang A.J., Xiao X.Z., Bian J.J., Chen Y.L. October 8, 2019. Preparation Method and Application Method of Breath Freshening Oral Care Liquid. CN110302292A. [Google Scholar]
- Peng J., Chen Y.Q., Lin J.M., Zhuang Q.C., Xu W., Hong Z.F., Sferra T.J. Patrinia scabiosaefolia extract suppresses proliferation and promotes apoptosis by inhibiting the STAT3 pathway in human multiple myeloma cells. Mol. Med. Rep. 2011;4(2):313–318. doi: 10.3892/mmr.2011.413. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Chai Y.T., Wu Y.T. Efficient new method for extraction and isolation of three flavonoids from Patrinia villosa Juss. by supercritical fluid extraction and high-speed counter-current chromatography. J. Chromatogr. A. 2006;1102(1–2):44–50. doi: 10.1016/j.chroma.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Wu Y.T. Studies on chemical constituents of Patrinia villosa Juss. J. Chin. Med. Mater. 2005;28(10):883–884. doi: 10.13863/j.issn1001-4454.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Wu Y.T. Supercritical fluid extraction of aurentiamide acetate from Patrinia villosa Juss and subsequent isolation by silica gel and high-speed counter-current chromatography. J. Chromatogr. A. 2005;1083(1–2):52–57. doi: 10.1016/j.chroma.2005.05.097. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Wu Y.T. Studies on chemical constituents of Patrinia villosa. China J. Chin. Mater. Med. 2006;31(2):128–130. [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Wu Y.T. Isolation and structure identification of chemical constituents from Patrinia villosa. Acta Pharm. Sin. 2006;41(3):236–240. doi: 10.16438/j.0513-4870.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Fan G.R., Wu Y.T. Preparative isolation of four new and two known flavonoids from the leaf of Patrinia villosa Juss. by counter-current chromatography and evaluation of their anticancer activities in vitro. J. Chromatogr. A. 2006;1115(1–2):103–111. doi: 10.1016/j.chroma.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Peng J.Y., Yang G.J., Fan G.R., Wu Y.T. Preparative isolation and separation of a novel and two known flavonoids from Patrinia villosa Juss by high-speed counter-current chromatography. J. Chromatogr. A. 2005;1092(2):235–240. doi: 10.1016/j.chroma.2005.07.073. [DOI] [PubMed] [Google Scholar]
- Peng Y.H., Guan X.Z. Guizhou nationalities Publishing House; Guiyang: 1994. Tujia Medicine. [Google Scholar]
- Peng Q.H., Peng C.C., Wang P.Y., Zhao Y.H., Wen W.J. November 12, 2019. A Preparation Method of Qingre Xuanfei Xiaoer Reke Oral Liquid. CN110433225A. [Google Scholar]
- Pharmacopoeia Committee of the Ministry of Health of P. R. China . 1977 edition. vol. 1. People’s Medical Publishing House; Beijing: 1978. (Pharmacopoeia of the People's Republic of China). [Google Scholar]
- Qin Y.F., Diao B.S. Efficacy of Baijiangcao San on immune function and intestinal function in children with recurrent upper respiratory tract infection. Clin. J. Chinese Med. 2018;10(10):8–11. doi: 10.3969/j.issn.1674-7860.2018.10.003. [DOI] [Google Scholar]
- Qiu D.W. Guizhou Science and Technology Publishing House; Guiyang: 2005. Miao Medicine Colume of Chinese Materia Medica. [Google Scholar]
- Qiu H.Y. March 12, 2019. Herbal Health Tea. CN109452429A. [Google Scholar]
- Ran Y.P., Yang Q., Zhuang K.W., Tang J.Q., Xu X.Q., You Z.M., Xiao H., Ran X., Dai Y.L. Clinical and fungal morphological examination of common dermatomycosis. Chin. J. Clin. Lab. Sci. 2017;35(10):750–757. doi: 10.13602/j.cnki.jcls.2017.10.08. [DOI] [Google Scholar]
- Ren L., Liu Y.X., Lv D., Yan M.C., Nie H., Liu Y., Cheng M.S. Facile synthesis of the naturally cytotoxic triterpenoid saponin Patrinia-glycoside B-II and its conformer. Molecules. 2013;18(12):15193–15206. doi: 10.3390/molecules181215193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru S.M. January 18, 2019. A Traditional Chinese Medicine for the Treatment of Liver Disease. [Google Scholar]
- Scientific Research Cooperation Group of Herba Patriniae in Yan'an City for the Prevention and Control of Keshan Disease Observation on the treatment of 154 cases of keshan disease by Herba Patriniae tablets. Shaanxi Medical Journal. 1979;12:13–14. [Google Scholar]
- Seo S.W., Park C.S., Hong S.H., Kwon K.B., Moon H.C., Song B.K., Kim K.Y., Park Y.M., Song H.J., Kim H.M., Park S.J. Inhibitory effect of Patrinia scabiosaefolia on acute pancreatitis. World J. Gastroenterol. 2006;12(7):1110–1114. doi: 10.3748/wjg.v12.i7.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandong Medical Products Administration . Shandong Friendship Publishing House; Jinan: 2002. Standard of Chinese Herbal Medicine in Shandong Province (2002 Edition) [Google Scholar]
- Shanghai College of Traditional Chinese Medicine . Shanghai; 1973. Obstetrics and Gynecology. [Google Scholar]
- Shi J.F., Wu T.L., Wu X. February 4, 2019. Whitening and Dispelling Yellow Soap and Preparation Method Thereof. CN109135965A. [Google Scholar]
- Shi W.L. Yunnan Institute for Drug Control; Kunming: 1983. List of Yunnan Ethnic Medicine. [Google Scholar]
- Shi X.Y. Herba Patriniae decoction enema in the treatment of anal cryptitis: a report of 367 cases. J. Pract. Tradition. Chin. Internal Med. 2012;26(11):84–85. [Google Scholar]
- Shi Y.L. Experiment of Chinese herbal medicine in the treatment of venomous snake bite of Xiangxi yellow cattle. Chin. J. Tradition. Vet. Sci. 2010;3:47–48. doi: 10.3969/j.issn.1003-8655.2010.03.026. [DOI] [Google Scholar]
- 2010 edition. Sichuan Science & Technology Press; Chengdu: 2011. Standard of Chinese Herbal Medicine in Sichuan Province. [Google Scholar]
- Song J.L., Yuan L., Li S., Wang Z.L. Chemical constituents from Patrinia villosa. J. Chin. Med. Mater. 2016;39(5):1038–1040. doi: 10.13863/j.issn1001-4454.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Song Z.H. Treatment of 52 cases of acute hepatitis with Artemisiae Scopariae Herba-Herba patriniae decoction. Shaanxi J. Tradit. Chin. Med. 1987;5:226. [Google Scholar]
- State Pharmacopoeia Commission of P. R. China . vol. 1. China Medico-Pharmaceutical Science & Technology Publishing House; Beijing: 2015. (Pharmacopoeia of the People's Republic of China (2015 Edition)). [Google Scholar]
- Su Y.Z., Zheng Y.X., Zhao J.Q., Chen X.J. Artificial cultivation technology of edible wild vegetable Herba Patriniae. Fujian Agri. Sci. Technol. 1999;S1:172. doi: 10.13651/j.cnki.fjnykj.1999.s1.139. [DOI] [Google Scholar]
- Sun C.J., Yao G.J., Dong J.W., Shen Y.M. January 18, 2019. Weaning Piglet Feed Additive and Preparation Method Thereof. CN109221675A. [Google Scholar]
- Sun S.M. People's Medical Publishing House; Beijing: 1955. Qian Jin Yi Fang. [Google Scholar]
- Sun S.M. 1998. Qian Jin Fang. China Traditional Chinese Medicine Press, Beijing. [Google Scholar]
- Sun X.C., Lei L.Y., Huang W.J., Shi X.B., Song Z.X., Tang Z.S. Correlation between chemical components and antioxidant activity of Patriniae Herba extracts. China Pharmacist. 2018;21(12) 2111-2113+2132. [Google Scholar]
- Sun X.F. Observation on the efficacy of self-made Herba Patriniae decoction in the treatment of typhoid and paratyphoid fever. Chin. J. Inform. Tradition. Chin. Med. 2000;8:52. [Google Scholar]
- Sun Y.M. Henan Science and Technology Publishing House; Zhengzhou: 1981. Lin Zheng Yi an Yi Fang. [Google Scholar]
- Taguchi H., Yokokawa Y., Endo T. Studies on the constituents of patrinia villosa Juss. Yakugaku Zasshi. 1973;93(5):607–611. doi: 10.1248/yakushi1947.93.5_607. [DOI] [PubMed] [Google Scholar]
- Tan C., Sun Z.L., Zhou K.Y., Xu J.P. Studies on chemical constituents and sedation and antibacterial effect of Patrinia scabiosaefolia Fisch. J. Tradit. Chin. Vet. Med. 2003;4:3–5. doi: 10.13823/j.cnki.jtcvm.2003.04.001. [DOI] [Google Scholar]
- Tan G.C. March 15, 2019. Ointment for Treating Skin Wound and Infection and Preparation Method Thereof. [Google Scholar]
- Teaching and Research Group of Traditional Chinese Medicine, the First Affiliated Hospital of Xi'an Medical College . Shaanxi People's Publishing House; Xi'an: 1975. Study on the Treatment of Common Diseases with Traditional Chinese Medicine. [Google Scholar]
- Tian C.X., Tian S. The Proceedings of the National Symposium on Traditional Chinese Medicine Research. Nanchang; China: 2003. Examples of clinical application of Herba Patriniae mandshurica[C] pp. 93–94. [Google Scholar]
- Tian Z.Y., Cao J.H. Studies on chemical components of volatile oils of Patrinia scabiosaefolia Fisch and P. heterphylla Bunge. J. Henan Univ. 2004;23(1):35–36+39. [Google Scholar]
- Tian Z.Y., Cao J.H., Dong C.M., Chen S.Q. Studies on chemical constitutents of volatile oils of Patriniae monandra C. B. Clerke and Patrinia sinensis Koidz. Lishizhen Med. Materia Medica Res. 2005;16(8):738–739. [Google Scholar]
- Tohda C., Kakihara Y., Komatsu K., Kuraishi Y. Inhibitory effects of methanol extracts of herbal medicines on substance P-induced itch-scratch response. Biol. Pharm. Bull. 2000;23(5):599–601. doi: 10.1248/bpb.23.599. [DOI] [PubMed] [Google Scholar]
- Tu X., Deng Y.P., Chen J., Hu Q., He C.S., Jordan J.B., Zhong S. Screening study on the anti-angiogenic effects of Traditional Chinese Medicine-Part I: heat-clearing and detoxicating TCM. J. Ethnopharmacol. 2016;194:280–287. doi: 10.1016/j.jep.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Wang C.C., Gong X., Bo A., Zhang L., Zhang M.X., Zang E.H., Zhang C.H., Li M.H. Iridoids: research Advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules. 2020;25(2) doi: 10.3390/molecules25020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yan K.K., Wang D. Medication rules for prescriptions containing Herba Patriniae based on data mining. Chin. J. Basic Med. Tradition. Chin. Med. 2019;25(11):1590–1593. [Google Scholar]
- Wang E.H., Chen W.L., Chen H.K. April 3, 2018. Manufacturing Process and Quality Control Method of Pharmaceutical Composition with Effect of Treating Dark Circles. CN107865927A. [Google Scholar]
- Wang G.Q. February 1, 2019. Chinese Herbal Medicine Preparation for Treating Cancer and Preparation Method Thereof. CN109288985A. [Google Scholar]
- Wang H.Y., Hou L.L., Xiao L.L., Li Y. March 19, 2019. Traditional Chinese Medicine Composition for Treating Lung Cancer with Phlegm and Blood Stasis Syndrome and Preparation Method Thereof. CN109481609A. [Google Scholar]
- Wang H.Y. People's Medical Publishing House; Beijing: 1958. Tai Ping Sheng Hui Fang. [Google Scholar]
- Wang J. November 11, 2012. A Traditional Chinese Medicine for the Treatment of Acne. CN102764395A. [Google Scholar]
- Wang L.L., Zhou Y.Z., Chen X.Q. Study on quality standard of Herba Patriniae distributed in Henan province. Acta Chinese Medicine. 2012;27(9):1150–1152. [Google Scholar]
- Wang R.J., Sun B.M. Pharmacological study and clinical application of Patrinia scabiosaefolia. J. Changchun Univ. Chin. Med. 1997;2:47. doi: 10.13463/j.cnki.cczyy.1997.02.049. [DOI] [Google Scholar]
- Wang S.Y., Wang P. Treatment of cutaneous pruritus mainly by Herba Patriniae. J. Tradit. Chin. Med. 2002;12:894. doi: 10.13288/j.11-2166/r.2002.12.011. [DOI] [Google Scholar]
- Wang W.M. November 19, 2019. Livestock Feed Formula. CN110463826A. [Google Scholar]
- Wang X.L., Li Y.Y., Zhou H. Determination of inositol in Herba patriniae. J. Anhui Univ. Chin. Med. 2002;1:52–54. [Google Scholar]
- Wang Y. Guizhou Science and Technology Publishing House; Guizhou: 2002. Color Atlas of Miao Medicine in China. [Google Scholar]
- Wang Y. Study on development of chemical composition and pharmacological action of Patrinia scabiosaefolia Fisch. J. Pharmaceut. Res. 2009;28(4):222–225. [Google Scholar]
- Wang Y.S., Xue C.S., Deng W.L. People's Medical Publishing House; Beijing: 1983. Pharmacology and Application of Traditional Chinese Medicine. [Google Scholar]
- Wei S.H., Chi H.D., Kodama H., Chen G. Anti-inflammatory effect of three iridoids in human neutrophils. Nat. Prod. Res. 2013;27(10):911–915. doi: 10.1080/14786419.2012.668687. [DOI] [PubMed] [Google Scholar]
- Wei Y.M., Wang S., Meng X.S., Bao Y.R. Medicine effect compare and extraction process study on two kinds of patrinia based on inhibition rate of SMMC-7721 hepatocarcinoma cells. China Med. Pharm. 2013;3(11):35–37. [Google Scholar]
- Wei Z.Z., Fang X.Y., Wang C., Miao M.S., Jia J.J. Effects of total flavonoids from Patriniae on neuroprotective effect and inflammatory factors of focal cerebral ischemia reperfusion injured rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 2019;30(4):396–402. doi: 10.19378/j.issn.1003-9783.2019.04.002. [DOI] [Google Scholar]
- Wen H.L., Xing L., Sun K., Xiao C.S., Meng X.X., Yang J.Z. Loganin attenuates intestinal injury in severely burned rats by regulating the toll-like receptor 4/NF-κB signaling pathway. Exp Ther Med. 2020;20(1):591–598. doi: 10.3892/etm.2020.8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang G.K., Wu J.L., Han J., Liu J.S. Chemical components of the petroleum ether extract of Patrinia Villosa. J. AnHui Univ. Chin. Med. 2019;38(6):76–78. [Google Scholar]
- Wu Y.R. October 20, 2017. A Facial Mask to Enhance the Ability of Antioxidation. CN107260614A. [Google Scholar]
- Wu J. The Second Military Medical University; Shanghai: 2017. Study on Antifungal Activity Screening and Mechanism of Traditional Chinese Medicines[D] 2017. [Google Scholar]
- Wu Z.X. July 19, 2019. A Medicinal Wine for Preventing Breast Disease. CN110025757A. [Google Scholar]
- Xia L., Zhang B., Yan Q., Ruan S. Effects of saponins of patrinia villosa against invasion and metastasis in colorectal cancer cell through NF-kappaB signaling pathway and EMT. Biochem. Biophys. Res. Commun. 2018;503(3):2152–2159. doi: 10.1016/j.bbrc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Xia M.W., Tan Q.Q., Yang L., Shang Z.P., Zhao Q.C., Shi G.B. Studies on chemcial constituents in Patrinia scabiosaefolia. Chin. Tradit. Herb. Drugs. 2010;10:38–41. [Google Scholar]
- Xiang Z., Chen N., Xu Y., Wu J., Liu Y.J., Tan C., Ji Y.B., Li W.L. New flavonoid from Patrinia villosa. Pharm. Biol. 2016;54(7):1219–1222. doi: 10.3109/13880209.2015.1064449. [DOI] [PubMed] [Google Scholar]
- Xiao K. Clinical application of Herba patriniae. Yunnan J. Tradition. Chin. Med. Materia Medica. 1995;2:31–32. [Google Scholar]
- Xie H.Q., Xie J.Y. February 19, 2019. Traditional Chinese Medicine Composition for Treating Dysmenorrhea and Preparation Method Thereof. CN109350676A. [Google Scholar]
- Xie H.Q., Xie J.Y. March 12, 2019. A Traditional Chinese Medicine Composition for Treating Cancer. CN109453344A. [Google Scholar]
- Xie H.Z. November 29, 2019. Antibacterial and Environment-Friendly Laundry Detergent and Preparation Method Thereof. CN110511832A. [Google Scholar]
- Xie Y., Peng J., Fan G., Wu Y. Chemical composition and antioxidant activity of volatiles from Patrinia Villosa Juss obtained by optimized supercritical fluid extraction. J. Pharmaceut. Biomed. Anal. 2008;48(3):796–801. doi: 10.1016/j.jpba.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Xiong X.P. January 10, 2020. Medicine for Treating Stomach Disease and Preparation Method Thereof. CN110664956A. [Google Scholar]
- Xu C.L. June 4, 2019. Formula for Treating AIDS. CN109833449A. [Google Scholar]
- Xu C.J., Zeng X.Y., Yu D.Q. Studies on the constituents of patrinia villosa. Acta Pharm. Sin. 1985;9:652–657. [PubMed] [Google Scholar]
- Xu J.X., Zhou F.Q. Flavonoids extracting study on different parts of Patrinia Villosa Juss by four methods. Guangzhou Food Sci. Technol. 2004;4:42–43. [Google Scholar]
- Xu L., Zaky M.Y., Yousuf W., Ullah A., Abdelbaset G.R., Zhang Y., Ahmed O.M., Liu S., Liu H. The anticancer potential of apigenin via Immunoregulation. Curr. Pharmaceut. Des. 2020 doi: 10.2174/1381612826666200713171137. [DOI] [PubMed] [Google Scholar]
- Xu S.W., Yu A.A., Zheng L., Xia W.J., Yang S., Shi S.S. Processing technology of tea or food applied production made of Patrinia villosa. Hubei Agric. Sci. 2018;57(21):91–92. doi: 10.14088/j.cnki.issn0439-8114.2018.21.022. [DOI] [Google Scholar]
- Xu Z.M., Huang Z.H., Zhu B., Zhang Y.H. Study on the sedative active parts of Herba Patriniae. Zhejiang J. Integrated. Tradition. Chin. Western Med. 2007;6:347–348. [Google Scholar]
- Xue X.L., Zhang X.H., Sun P., Cui Y., Wang X.C., Kong L.Y. GC-MS analysis of volatile oil from six medicinal plants in Changbai mountain. J. Chin. Med. Mater. 2016;39(5):1062–1066. doi: 10.13863/j.issn1001-4454.2016.05.027. [DOI] [Google Scholar]
- Yan S.Q., Qiu F.Z. Clinical observation on the therapeutic effect of Herba Patriniae decoction in the treatment of pelvic endometriosis. Hubei J. Tradition. Chinese Med. 2013;35(8):38. [Google Scholar]
- Yan W.H., Song X., Wang Q.L., Tang S.W., Shen F., Wang F., Xie S.Q. Clinical observation of compound Herba Patriniae tablets in the treatment of 62 cases of psoriasis vulgaris. Chinese. J. Dermatovenereol. Integrat. Tradition. Western Med. 2015;14(5):290–292. [Google Scholar]
- Yan X.J. March 29, 2019. Traditional Chinese Medicine Formula for Treating Breast Diseases and Preparation Method Thereof. CN109528870A. [Google Scholar]
- Yan X.J., Liu W., Zhao Y., Chen N., Xu Y., Wu J., Wang Tan, Li Yue, Xiang Z. A new biphenyl neolignan from leaves of Patrinia villosa (Thunb.) Juss. Phcog. Mag. 2016;12(45):1–3. doi: 10.4103/0973-1296.175988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X.Y. February 22, 2019. A Kind of Botanical Insecticide. CN109362803A. [Google Scholar]
- Yan X.Y. February 26, 2019. An Agricultural Insecticide. CN109380333A. [Google Scholar]
- Yan Y.X., Liu C.R. Henan Science and Technology Press; Zhengzhou: 1983. Gu Jin Ming Fang. [Google Scholar]
- Yang B., Sheng D.F., Ding L.X., Chen Y.J. Isolation and identification of new acetylated saponin from radix and rhizome of Patrinia scabiosaefolia. Chin. Tradit. Herb. Drugs. 2002;33(8):685–687. [Google Scholar]
- Yang B., Sheng D.F., Zhao P., Li L.J. Study on the chemical components from Patrinia scabiosaefolia Fish. ex Link by supercritical fluid extraction. Lishizhen Med. Materia Medica Res. 2007;11:2706–2707. [Google Scholar]
- Yang B., Tong L., Jin M., Zhao W., Chen Y. Isolation and identification of triterpenoide compound from Patrinia scabiosaefolia. J. Chin. Med. Mater. 1998;21(10):513–514. [PubMed] [Google Scholar]
- Yang C. January 4, 2019. Medicine for Treating Dysmenorrhea. CN109125510A. [Google Scholar]
- Yang D.H., Wei L.X., Cai S.Q., Wang X. Studies on the chemical constituents of Patrinia scabiasaefolia fisch. China J. Chin. Mater. Med. 2000;25(1):39–41. [PubMed] [Google Scholar]
- Yang D.M. Herba Patriniae is good at treating neurasthenia. J. Tradit. Chin. Med. 2002;43(12):892. doi: 10.3321/j.issn:1001-1668.2002.12.004. [DOI] [Google Scholar]
- Yang H., Tang Q.H., Wang F.Y., Yang C., He L.F., Tang X., Tang J.Y., Liu H.J. October 8, 2019. Chinese Herbal Medicine Compound Preparation for Preventing and Treating Pigeon Trichomoniasis and Preparation Method Thereof. CN110302326A. [Google Scholar]
- Yang M.Y., Choi Y.H., Yeo H., Kim J. A new triterpene lactone from the roots of Patrinia scabiosaefolia. Arch Pharm. Res. (Seoul) 2001;24(5):416–417. doi: 10.1007/bf02975186. [DOI] [PubMed] [Google Scholar]
- Yang X.W. December 10, 2019. A Traditional Chinese Medicine Granule for Treating Acute Cough Caused by Pulmonary Tuberculosis. CN110548097A. [Google Scholar]
- Yang X.W. December 10, 2019. A Traditional Chinese Medicine Granule for Treating Cough Caused by Pneumonia. CN110548106A. [Google Scholar]
- Yang X.W. December 10, 2019. A Traditional Chinese Medicine Granule for Treating Cough Caused by Chronic Pharyngitis. CN110548112A. [Google Scholar]
- Yang Y.F., Ma H.M., Chen G., Wang H.F., Xiang Z., Feng Q.M., Hua H.M., Pei Y.H. A new sesquiterpene lactone glycoside and a new quinic acid methyl ester from Patrinia villosa. J. Asian Nat. Prod. Res. 2016;18(10):945–951. doi: 10.1080/10286020.2016.1173678. [DOI] [PubMed] [Google Scholar]
- Ye B.D., Sheng Y.P., Lin W.B., Yu Q.H., Gao Y.T., Zhang Y.Y., Yu F.F., Chen T., June 7 . 2019. Preparation Method and Application of Traditional Chinese Medicine Mouthwash. CN109846786A. [Google Scholar]
- Yu F.J. January 18, 2019. A Traditional Chinese Medicine External Cleaning Agent for Treating Infectious Wounds Caused by Multiple Drug-Resistant Bacteria. CN109223933A. [Google Scholar]
- Yuan R. January 22, 2019. Medicine for Treating Orchitis and Preparation Method Thereof. CN109248247A. [Google Scholar]
- Zeng X.L., Wang D., Zou J.J., Yang J., Shi Y.M., Zheng L., Zhong J.Q., Chen H.G. The comparison and analysis of the Patrinia villosa tea quality by different processing technologies. Modern Food Science and Technology. 2019;35(1):157–164+232. doi: 10.13982/j.mfst.1673-9078.2019.1.023. [DOI] [Google Scholar]
- Zeng X.Q. January 1, 2019. Preparation Method for Preventing and Treating Appendicitis. CN109106833A. [Google Scholar]
- Zhang B. September 6, 2019. Bacteriostatic Toilet Paper and Preparation Method Thereof. CN110205863A. [Google Scholar]
- Zhang D.D., Pu C.Y., Ren Y.D., Liu H.Y., Zhang C.L., Zhang N., Wang F.S. March 7, 2012. Compound Extract of Traditional Chinese Medicine for the Treatment of Postmenopausal Osteoporosis and its Preparation Method. CN102366493A. [Google Scholar]
- Zhang F.F. March 29, 2019. Composite Preparation for Preventing and Treating Pigeon Diarrhea. CN109528869A. [Google Scholar]
- Zhang G.F. May 7, 2019. Traditional Chinese Medicine Preparation for Controlling Swine Fever. CN109718328A. [Google Scholar]
- Zhang J. March 19, 2019. A Chinese Medicinal Composition for Treating Diabetes. CN109481606A. [Google Scholar]
- Zhang J. December 31, 2019. Medicine for Treating Appendicitis. CN110624064A. [Google Scholar]
- Zhang J.Z., Jiang H.L. January 18, 2019. Traditional Chinese Medicine Composition for Treating Cervical Erosion of Women. CN109223943A. [Google Scholar]
- Zhang L.S. May 21, 2019. A Traditional Chinese Medicine Composition for Treating Lung Pain. CN109771520A. [Google Scholar]
- Zhang M.Y., Sun G.D., Shen A.L., Liu L.Y., Ding J.Z., Peng J. Patrinia scabiosaefolia inhibits the proliferation of colorectal cancer in vitro and in vivo via G1/S cell cycle arrest. Oncol. Rep. 2015;33(2):856–860. doi: 10.3892/or.2014.3663. [DOI] [PubMed] [Google Scholar]
- Zhang Q.H. March 22, 2019. Compound Chinese Herbal Medicine Fertilizer for Promoting Growth. CN109503252A. [Google Scholar]
- Zhang S.S., Fu S., Zhao Y.M., Fu Y.M., He L.S., Liu D.L. Anti-diarrheal effect of extracts from Patrinia villosa Juss. J. Log. Univ. PAP (Med. Sci.) 2019;28(2):12–16. doi: 10.16548/j.2095-3720.2019.02.003. [DOI] [Google Scholar]
- Zhang T., Li Q.W., Li K., Li Y.R., Li J., Wang G.J., Zhou S.B. Antitumor effects of saponin extract from Patrinia villosa (Thunb.) Juss on mice bearing U14 cervical cancer. Phytother Res. 2008;22(5):640–645. doi: 10.1002/ptr.2354. [DOI] [PubMed] [Google Scholar]
- Zhang X.D., Liu Y.M., Zhang F.Q., Gao C.B., Me H.B., Liu S.Y., Zheng C.L., Wang G.Q., Zhao J.F., Zahng H.J., Fu Y. January 11, 2019. Traditional Chinese Medicine Preparation for Treating Chicken Infectious Bronchitis and Anti-fallopian Tube Injury and Preparation Method Thereof. CN109172759A. [Google Scholar]
- Zhang X., Li T., Gong E.S., Liu R.H. Antiproliferative activity of ursolic acid in MDA-MB-231 human breast cancer cells through Nrf2 pathway regulation. J. Agric. Food Chem. 2020 doi: 10.1021/acs.jafc.0c03202. [DOI] [PubMed] [Google Scholar]
- Zhang X.T. Treatment of 287 cases of pelvic inflammation with self-made Smilacis Glabrae Rhizoma-Herba Patriniae decoction. Shaanxi J. Tradit. Chin. Med. 1997;2:79. [Google Scholar]
- Zhang Y.D. 2019. A traditional Chinese medicine composition for anti-breast cancer. CN110269916A. [Google Scholar]
- Zhang Y.F., Zhou S.Q., Wu Z.G. Study on HPLC fingerprints of Patrinia villosa. China Pharmacy. 2011;22(3):242–243. [Google Scholar]
- Zhang Y.L. February 26, 2019. A Preparation Method of Detoxification and Moistening Intestines Peony Scented Tea. CN109380571A. [Google Scholar]
- Zhang Z.J. Classics Press; Beijing: 1997. Jin Gui Yao Lüe. Traditional Chinese Medicine. [Google Scholar]
- Zhao C.G. vol. 38. 2003. (Wild Grass Is a Good Feed for Disease Prevention of Soft-Shelled Turtle and Fish (2). Fishery Guide to Be Rich). [Google Scholar]
- Zhao J. People's Medical Publishing House; Beijing: 1982. Sheng Ji Zong Lu. [Google Scholar]
- Zhao L., Zhao H.J., Bao Y.R., Wang S., Li T.J., Meng X.S., Xiang Z. The process optimization of extraction and purification for total flavonoids and comparative study on the efficacy of anti-liver tumors in vitro before and after purification. Lishizhen Med. Materia Medica Res. 2019;30(3):546–550. [Google Scholar]
- Zhao X.D. 2019. Traditional Chinese Medicine for Treating Cirrhotic Ascites and Preparation Method Thereof. July 16, CN110013515A. [Google Scholar]
- Zhao Y.M. July 2, 2019. A Traditional Chinese Medicine Formula for the Treatment of Bronchitis. CN109954099A. [Google Scholar]
- Zhao Z.Y., Yang J. Analysis of chemical constituents of Patrinia scabiosaefolia Fisch. J Liaoning Med. Univ. 2016;37(2):16–17+115. doi: 10.13847/j.cnki.lnmu.2016.02.005. [DOI] [Google Scholar]
- Zheng Y., Jin Y., Zhu H.B., Xu S.T., Xia Y.X., Huang Y. The anti-inflammatory and anti-nociceptive activities of Patrinia villosa and its mechanism on the proinflammatory cytokines of rats with pelvic inflammation. Afr. J. Tradit., Complementary Altern. Med. 2012;9(3):295–302. doi: 10.4314/ajtcam.v9i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S.M., Li G.Y., Lin H.P., Jin S.H., Qian X.B. An analysis of nutrient constituents of wild Patrinaia villosa. Chinese Wild Plant Resources. 2001;1:45–46. [Google Scholar]
- Zhong X.M., Jiang S.Z., Huang Y.S., Zeng J. Effect of Patrinia villosa Juss extract on the sleep function and spontaneous activity of mice. Chin. J. Clin. Rehabil. 2004;30:6688–6689. [Google Scholar]
- Zhong Y.M., Zhong X.L., Wang J.H., Han L. Rapid analysis and identification of the main constituents in Patrinia scabiosaefolia Fisch. by UPLC/Q-TOF-MS/MS. Acta Chromatogr. 2017;29(2):267–277. doi: 10.1556/1326.2017.29.2.10. [DOI] [Google Scholar]
- Zhou S.Z., Xie Z.H., Jiao W.L., Yuan X.Q., Li Y.C. March 19, 2019. Composition for Preventing and Treating Animal Mastitis and Preparation Method and Application Thereof. CN109481540A. [Google Scholar]
- Zhou X.C. Determination of oleanolic acid and ursolic acid in Herba Patriniae from different habitats. Asia-Pacific. Tradition. Med. 2014;10(12):32–33. [Google Scholar]
- Zhou Y.L., Guo Y., Sun X.D., Ding R., Wang Y.L., Niu X.D., Wang J.F., Deng X.M. Application of oleanolic acid and its analogues in combating pathogenic bacteria in vitro/vivo by a two-pronged strategy of β-lactamases and hemolysins. ACS Omega. 2020;26(5):11424–11438. doi: 10.1021/acsomega.0c00460. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S. People's Medical Publishing House; Beijing: 1959. Pu Ji Fang (Volumes 351) [Google Scholar]
- Zhu S.Y., Hong W.M., Qing F., Liu Y., Wu S., Guo C.M., Wu Z., Wang A.P., Wang Y.J., Feng Q. December 17, 2019. Application of Herba Patriniae and Traditional Chinese Medicine Composition for Treating Pig Blue Ear Disease. CN110575479A. [Google Scholar]
- Zhu T., Jiang P.C. The ancient and modern use of Herba Patriniae. Guangming. J. Chinese Med. 2015;30(3):638–640. doi: 10.3969/j.issn.1003-8914.2015.03.108. [DOI] [Google Scholar]
- Zou J.H. Phytochemical studies on the seeds of medicinal plants (4): flavonoids and triterpenoids in Patrinia Villosa. Foreign Med. (Fascicles. Tradition. Chin. Med.) 1994;4:32. [Google Scholar]
- Zou W., Wen X.K., Ou Y.B., Xiao Z.Q. June 14, 2019. Traditional Chinese Medicine Composition and Traditional Chinese Medicine Preparation. CN109876104A. [Google Scholar]
- Zou W., Wen X.K., Zheng Y., Xiao Z.Q., Luo J.Y., Chen S.Q., Wang Y.C., Cheng Z.Z., Xiang D.X., Nie Y.C. Metabolomic study on the preventive effect of Patrinia scabiosaefolia Fisch on multipathogen induced pelvic inflammatory disease in rats. Evid Based Complement Alternat Med. 2015:1–9. doi: 10.1155/2015/170792. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu L.B. Anhui University; Anhui: 2013. The Chemical Constitutes from Patrinia Scabiosaefolia Fisch[D] 2013. [Google Scholar]