Abstract
The outbreaks of viruses with wide spread and mortality in the world population have motivated the research for new therapeutic approaches. There are several viruses that cause a biochemical imbalance in the infected cell resulting in oxidative stress. These effects may be associated with the development of pathologies and worsening of symptoms. Therefore, this review is aimed at discussing natural compounds with both antioxidant and antiviral activities, specifically against coronavirus infection, in an attempt to contribute to global researches for discovering effective therapeutic agents in the treatment of coronavirus infection and its severe clinical complications. The contribution of the possible action of these compounds on metabolic modulation associated with antiviral properties, in addition to other mechanisms of action, is presented.
1. Introduction
Coronaviruses (CoVs) belong to a family of enveloped viruses with a positive sense, single-stranded RNA genome. CoVs cause illness ranging from upper respiratory tract infections (URTIs) resembling the common cold to lower respiratory tract infections (LRTIs) such as bronchitis, pneumonia, and even severe acute respiratory syndrome (SARS) with most serious disease outcomes in the elderly, immunocompromised patients, and infants [1, 2]. HCoV-OC43 (OC43), HCoV-229E (229E), HCoV-NL63 (NL63), and HCoV-HKU1 (HKU1) were the first documented human CoVs (HCoVs), which usually cause URTIs and less frequently are associated with LTRI diseases [3]. In the last decades, two human coronaviruses created great concern for the world medical community due to significant disease and mortality [4, 5]. In 2003, severe acute respiratory syndrome-coronavirus (SARS-CoV) was characterized by acute atypical pneumonia and diffuse alveolar damage (DAD) in roughly 8000 patients and with almost 800 deaths, representing a nearly 10% mortality rate [6]. More recently, in 2012, a new human coronavirus, designated as Middle East respiratory syndrome-coronavirus (MERS-CoV), was identified, and the global ongoing outbreak of MERS with over 2519 official cases and 866 deaths represented approximately 34% case fatality rate to date in humans [7].
Over the last few months, a new strain of human coronavirus, SARS-CoV-2 (also known as 2019-nCoV), has caught the world's seven continents' attention with its rapid global spread, affecting at least 200 countries and territories, infecting more than 3,000,000 and claiming more than 202,597 lives worldwide [8]. The coronavirus pandemic has promoted isolation and uncertainly fear and panic worldwide. In addition, it will likely lead to changes in political and economic power in ways that can be determined only later [9].
It is important to note that there are many similarities among different coronavirus species, but not in all aspects. Depending on the molecular mechanism of viral inhibition promoted by an antiviral agent, the analysis of the data and comparison between animal and human CoVs must be done very carefully. In fact, it is important to note that there are differences between human and animal CoV receptors, which will likely result in different affinities, or unlikely interactions, of an antiviral agent with the different CoV receptors. However, if the antiviral agent interferes with the replication and/or assembly of the CoVs, there is a higher probability of obtaining similar antiviral activity results in human CoV tests [1, 2, 10, 11]. Following this line, our search in specialized literature was focused, mainly, on studies that investigated the anticoronavirus effects of natural antioxidants by inhibiting proteases for viral replication.
2. Materials and Methods
The present study was carried out based on a search of the literature of natural antioxidants and coronavirus. The search, performed in the PubMed database, included studies published until March 2020 and used the following keywords: coronavirus, antioxidants, flavonoids, oxidative stress, MERS-CoV; SARS-CoV, 229E, NL63, OC43, HKU1, MERS-CoV virus infection; and Middle East Respiratory Syndrome Virus. The scientific publications were selected from studies published in the English language.
3. Pathogenic Mechanism of Coronavirus-Induced Cell Damage
The high mortality rate associated with the three pathogenic HCoVs has been mainly attributed to the development of digestive and respiratory tract injuries observed following infection. Acute atypical pneumonia and diffuse alveolar damage that progress to deposition of fibrous tissue, denuded airways, haemorrhage, and elevated macrophage infiltration are sometimes accompanied by watery diarrhoea, dehydration, and vomiting [2, 12, 13].
Despite the molecular mechanisms of coronavirus-induced intestine and lung pathogenesis not fully elucidated and still unclear, studies have suggested that late-term disease progression is unrelated to viremia. It is now believed more likely to be associated with the immunopathological mechanism [14, 15]. Viral clearance and subsequent recovery from infection require activation of an effective host immune response; however, many immune effector cells may also cause injury to host tissues [16]. Together with inflammatory and immune response signaling, the presence of oxidative compounds, such as reactive oxygen species (ROS), plays important roles in the pathogenic mechanism of cell damage induced by CoVs through oxidative stress [17].
Oxidative stress is defined as an interruption and/or deregulation of the signaling and redox system that can be caused by an imbalance in the production of oxidant and antioxidant species [18]. Among the main oxidant agents, ROS and reactive nitrogen species (RNS) stand out. In order to counterbalance the oxidant species, there is an antioxidant system formed by enzymes and nonenzymatic molecules [19, 20]. However, during pathological events, such as viral infections, there may be an increase in the production of oxidant species not neutralized by the antioxidant system, resulting in oxidative stress that promotes cellular damage through protein denaturation, changes in the functions of nucleic acids, lipid peroxidation, and cell death [21–23].
In addition, during viral infection, oxidative stress contributes to viral pathogenesis through stimulating inflammation, loss of immune function, and increased viral replication that may occur due to the activation of the nuclear factor kappa B (NF-κB) transcription pathway [24–26]. Current evidence suggests that cytokine dysregulation—also called cytokine storm—contributes to severe disease caused by the pathogenic CoVs [27, 28]. The exact mechanisms are not clear yet, but research on influenza A virus shows that infection causes a rapid influx of inflammatory cells. This is followed by an increase in reactive oxygen species production and cytokine expression and release, which ultimately leads to acute lung injury [29]. In general, RNA viruses promote changes in the body's antioxidant defense system, affecting enzymes such as superoxide dismutase (SOD) and catalase (CAT), in addition to reducing the levels of antioxidant molecules such as ascorbic acid, carotenoids, and reduced glutathione (GSH) [30–32]. Wu et al. reported that glucose-6-phosphate dehydrogenase- (an important antioxidant enzyme that produces NADPH) knockdown cells were more susceptible to infection by HCoV-229E than normal cells [33]. Interestingly, Ye and colleagues have reported that the inhibition of ROS production alleviates inflammation caused by influenza A virus infections [29].
In an experimental model of SARS-induced acute lung injury in mice, it was noted that phospholipid oxidation, due to oxidative stress, is one of the main triggering factors of acute lung injury. This happens through the activation of the innate immune response, culminating in the activation of pulmonary macrophages via TLR4-TRIF-TRAF6-NF-κB signaling [17]. Furthermore, hypoxia caused by acute lung injury can cause myocardial injury due to the production of ROS, aggravating infections caused by coronavirus disease 2019 (COVID-19) [34].
Mitochondria have an essential function in energy generation, and for this reason, their function and integrity are strictly regulated in order to respond to varying energy requirements and environmental conditions [35]. Mitochondria are known to function as the control point in apoptotic pathways, releasing proapoptotic factors, mainly ROS, which function as a signaling molecule that may result in cell death [36, 37]. Some studies have shown a relationship between coronavirus infection and dysfunctional or damaged mitochondria, leading to the release of ROS and other proapoptotic substances [38, 39]. In a recent study, Xu et al. reported that ROS and p53 play key roles in regulating many kinds of the cell process during coronavirus infection in Vero cells. According to the authors, coronavirus infection appears to induce a time-dependent ROS accumulation, which in turn is linked to regulatory mechanisms of p53 activation and apoptosis in infected cells [40].
Antioxidant substances promote improvement in cases of disease caused by coronaviruses, such as apolipoprotein D—a lipocalin that promoted a neuroprotective effect against encephalitis induced by human coronavirus OC43—in mice. This protective effect occurred through the reduction of oxidative stress, cerebral lipid peroxidation, and regulation of inflammation [41, 42]. Also, the treatment with antioxidants, such as pyrrolidine dithiocarbamate or N-acetylcysteine, significantly inhibits coronavirus-induced apoptosis [43]. Moreover, melatonin promotes downregulation of acute lung oxidative injury due to its anti-inflammatory and antioxidant actions, making it a possible compound in the treatment of COVID-19 [44]. Based on these studies, compounds that have antioxidant actions can be helpful in the treatment of infections promoted by coronavirus.
In general, antioxidant properties of polyphenolic compounds, such as some flavonoids, have been associated with the presence of aromatic phenolic rings that promote the electron donation and hydrogen atom transfer to free radicals, acting as free radical scavengers, reducing agents, and quenchers of single oxygen formation [45]. Thus, the aim of this study was to investigate the antioxidant capacity and antiviral activity of natural antioxidants against coronavirus. The compounds are illustrated in Figure 1.
Figure 1.
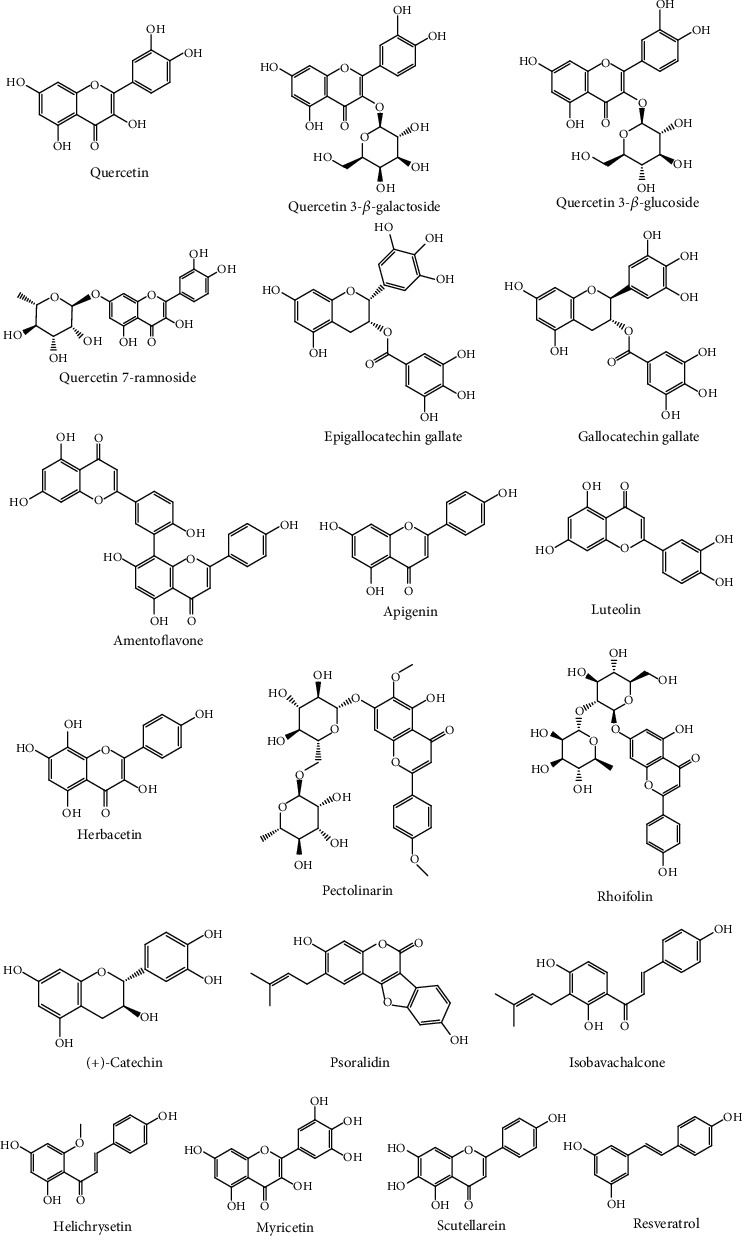
Chemical structures of bioactive antioxidants against coronavirus.
4. Occurrence and Antioxidant Properties of Anticoronavirus Compounds
Quercetin can be found in plants such as Rubus fruticosus L. and Lagerstroemia speciosa (L.) Pers. [46, 47]. Also, quercetin shows antioxidant activity at a concentration of 10 μmol/L in HepG2 cells, inhibiting oxidative stress promoted by H2O2 [48], promotes an increase in SOD, CAT, and glutathione peroxidase (GPx), and reduces lipid peroxidation in rats with chronic prostatitis/chronic pelvic pain syndrome [49]. Moreover, quercetin improves sepsis-induced acute lung injury in rats, by reducing lipid peroxidation and inflammation and increasing SOD and CAT levels [50].
In addition, quercetin glycosides with antioxidant activity, such as quercetin 3-β-glucoside, have already been isolated from plants such as Passiflora subpeltata Ortega and Chamomilla suaveolens (Pursh) Rydb. [51, 52]. The administration of quercetin 3-β-glucoside (40 mg/kg p.o.) in streptozotocin-induced diabetic rats promotes an increase in the levels of antioxidant enzymes (SOD, CAT, and GPx) and nonenzymatic antioxidants (vitamins C and E and GSH) and a reduction of lipid peroxidation [53]. Quercetin 3-β-galactoside (hyperoside) is found mainly in plants of the Hypericum genus such as Hypericum perforatum L. [54, 55]. Moreover, it showed cardioprotective activity in high glucose-induced injury of myocardial cells through decreased apoptosis and ROS production and increased SOD levels [56]. Quercetin 7-ramnoside is also found in plants of the Hypericum genus such as Hypericum japonicum Thunb. ex Murray. This flavonoid shows hepatoprotective activity against carbon tetrachloride in mice by decreasing lipid peroxidation and increasing CAT and GSH levels, in addition to presenting values of 118.75 μM and 128.47 μM in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assays, respectively [57].
Epigallocatechin gallate is present in Parkia roxburghii G. Don and is one of the main metabolites found in green tea and Liubao tea (Camellia sinensis LO Kuntze). Also, gallocatechin gallate can be found in this plant [58–61]. Literature data reveal that the administration (i.p.) of 2.5 mg/100 g of epigallocatechin gallate in rats, with streptozotocin-induced diabetes mellitus, promotes a reduction in oxidative stress through reductions in parameters such as indirect nitric oxide synthesis and status total oxidative, as well as an increase in levels of CAT and total antioxidant capacity of plasma [62]. Furthermore, it promotes cardioprotection by antioxidant mechanisms [63].
Green tea has a high antioxidant capacity, due to the high levels of catechins present [64]. He and collaborators compared the antioxidant activities of catechins and reported that epigallocatechin gallate has greater antioxidant activity via radical scavenging activity (400 μM) with values of 77.2 ± 4.3%, 90.2 ± 3.1%, and 100 ± 3.1% compared to its epimer, gallocatechin gallate, with values of 68.2 ± 3.4%, 82.2 ± 3.8%, and 95.5 ± 3.9% in the DPPH, ABTS, and ferric reducing antioxidant power (FRAP), respectively [65].
Amentoflavone is a biflavonoid present in leaves of Ginkgo biloba L., Garcinia brasiliensis L., and Nandina domestica L. [66–68]. This biflavonoid has a high antioxidant capacity (19.21–75.52%), demonstrated in scavenging tests of DPPH, ABTS, superoxide, and hydroxyl radicals [67]. Moreover, amentoflavone prevents acute lung injury induced by sepsis in rats by decreasing thiobarbituric acid reactive substance (TBARS) levels and by increasing levels of SOD and GSH [69].
Apigenin is mainly present in flowers and leaves, being abundantly found in Apium graveolens L., Petroselinum crispum (Mill.) Fuss, and Matricaria chamomilla L. [70]. Sánchez-Marzo and collaborators evaluated the antioxidant capacity of apigenin using the Trolox equivalent antioxidant capacity (TEAC), oxygen radical absorbance capacity (ORAC), and FRAP assays. The results show that apigenin has good antioxidant activity with values of 2022.2 ± 154.8 μmol TEa/mmol, 887.9 ± 5.8 μmol TEa/mmol, and 113.2 ± 12.2 μmol Fe2+/mmol, respectively [71]. In addition, oral administration of apigenin 25 mg/kg/day for 12 days in an experimental model of cardiotoxicity induced by doxorubicin in rats promoted cardioprotection by reducing levels of malondialdehyde (MDA), increasing SOD levels, and preventing cardiomyocyte apoptosis [72].
Luteolin is present in foods such as carrot, cabbage, tea, and apple and is found in Ugni molinae Turcz. [73, 74]. Data show that luteolin (50 μg/mL) increases the levels of GSH, the expression of GSH synthetase, and the activity of SOD and CAT in human colon cancer cells (HT-29) [75]. Furthermore, luteolin attenuates the sepsis-induced acute lung injury in mice by reducing lipid peroxidation and increasing SOD and CAT activity, in addition to suppressing the NF-κB pathway [76].
Herbacetin is ubiquitous in plants of the genus Rhodiola, such as Rhodiola rosea L. [77]. Herbacetin glycosides are also present in the roots of R. sachalinensis A. Bor and show antioxidant activity [78]. Veeramani et al. reported that the administration of herbacetin (40 mg/kg p.o.) in mice, with obesity-associated insulin resistance, promotes an increase in the activity of the enzyme glucose-6-phosphate dehydrogenase, which is directly related to the production of NADPH [79].
Pectolinarin is present in plants of the genus Cirsium such as Cirsium setidens Nakai and Cirsium japonicum DC. The administration of pectolinarin (10 and 20 mg/kg, p.o. for two weeks) in rats promotes antioxidant effects in hepatic injury induced by D-galactosamine by increasing levels of SOD, GSH, glutathione reductase, and glutathione S-transferase [80, 81].
Rhoifolin is found in citrus fruits, such as Citrus limetta Risso. Studies have indicated that its radical peroxyl scavenging capacity is higher than Trolox in ORAC assays (approximately 10 Trolox equivalents (μM)) [82, 83].
Meanwhile, the (+)-catechin is a flavonoid present in leaves of green tea, wine, and fruits [84, 85]. Grzesik et al. investigated the antioxidant action of catechin through the ABTS scavenging activity and FRAP tests. The results show values of 3.965 ± 0.067 (mol Trolox equivalents/mol) and 0.793 ± 0.004 (mol Trolox equivalents/mol), respectively. In addition, catechin shows greater protective properties in the dihydrorhodamine 123 oxidation assay (IC500.805 ± 0.072 μM) than GSH and ascorbic acid (14.1 and 13.9 μM, respectively) [86].
Psoralidin is a prenylated coumestan, which is found in plants of the Fabaceae family, such as Psoralea corylifolia L. Xiao and collaborators, investigating the antioxidant potential of compounds isolated from P. corylifolia, observed that psoralidin shows the best antioxidant activity by the method of electron spin resonance spectroscopy with an IC50 value of 44.7 μM [87].
The compound isobavachalcone has been isolated from plants of the Fabaceae and Moraceae families [88, 89]. Isobavachalcone shows a strong antioxidant activity in DPPH SC50, FRAP, and ABTS SC50 assays with values of 250.8 μM, 0.4 ± 0.05 mM equivalent to FeSO4·7H2O, and 510.1 mM, respectively. In addition, the compound has been reported to inhibit the NF-κB pathway in Sephadex-induced lung injury in rats [88, 90].
Helichrysetin is a chalcone that is found in plants of the Helichrysum genus such as Helichrysum odoratissimum L. [91]. In a study investigating the antioxidant activity of natural and prenylated chalcones, Vogel et al. found that helichrysetin is the substance that shows the highest antioxidant activity in the ORAC test with values of 4.4 ± 0.6 Trolox equivalents [92].
Myricetin is widely found in the plant families Myricaceae and Anacardiaceae and is widely used as health food supplement due to its antioxidant properties [93, 94]. Bennett et al. demonstrated that myricetin reacts with oxygen-centered galvinoxyl radicals more than 28 times higher than vitamin E (d-alpha-tocopherol). Furthermore, myricetin was able to scavenge 21% and 54% on the DPPH assay (5 μg/mL and 10 μg/mL, respectively) [95]. Interestingly, the compound prevents DNA damage, by lipid peroxidation and increasing the activity of SOD, CAT, and GPx in Chinese hamster lung fibroblast cells (V79-4) treated with H2O2 [96].
Scutellarein is found in Scutellaria barbata D. Don and Polygonum viscosum Buch-ham [97, 98]. Liu et al. investigated the antioxidant activity of scutellarein through the DPPH, ABTS, and superoxide scavenging assays. They noted that the compound shows good antioxidant activity with values of 18.7 ± 0.1 μM, 18.3 ± 1.2 μM, and 79.0 ± 0.5 μM, respectively, while the Trolox, a standard antioxidant compound, presented 20.2 ± 0.5 μM, 23.7 ± 0.4 μM, and 291.5 ± 40.6 μM, respectively [99].
Resveratrol is found in grapes, peanuts, and blueberries and can be isolated from Veratrum grandiflorum O. Loes [100]. Literature shows that resveratrol has good antioxidant activity with DPPH SC50/r2 values of 26.37/0.849 μmol/dm. Moreover, it is able to reduce the production of ROS by inhibiting the activity and expression of NADPH oxidases, by eliminating oxidant agents, including radical hydroxyl, superoxide, hydrogen peroxide, and peroxynitrite [101, 102]. The treatment of resveratrol (50 mg/kg p.o.) in rats reduces oxidative stress in obstructive lung disease by increasing SOD activity and reducing MDA levels, indicating a decrease in lipid peroxidation [103]. Table 1 shows the main actions of natural antioxidants discussed in this study, and Figure 2 illustrates these activities.
Table 1.
Antioxidant properties of natural inhibitors of coronavirus.
| Compound | Type of cells tested/assays/experimental models | Concentration / dose | Antioxidant effect | Reference |
|---|---|---|---|---|
| Quercetin | HepG2 cells | 10 μmol/L | Inhibiting oxidative stress promoted by H2O2 | [48] |
| Rats with chronic prostatitis/chronic pelvic pain syndrome | 50 mg/kg (p.o.) | Promoted an increase in SOD, CAT, and GPx and reduced lipid peroxidation | [49] | |
| Sepsis-induced acute lung injury in rats | 100 mg/kg (p.o.) | Reduces lipid peroxidation and increases SOD and CAT levels | [50] | |
| Quercetin 3-β-glucoside | Streptozotocin-induced diabetic rats | 40 mg/kg (p.o.) | Increases levels of SOD, CAT, GPx, vitamins C and E, and GSH and reduces lipid peroxidation | [53] |
| Quercetin 3-β-galactoside | High glucose-induced injury of myocardial cells | 20 nmol/L | Decreases apoptosis and ROS production and increases SOD levels | [56] |
| Quercetin 7-ramnoside | CCl4-induced liver damage model in mice DPPH ABTS |
20 mg/kg IC50 = 118.75 μM (DPPH) EC50 = 128.47 μM (ABTS) |
Decreases lipid peroxidation and increases CAT and GSH levels Scavenging of free radicals |
[57] |
| Epigallocatechin gallate | Rats with streptozotocin-induced diabetes mellitus | 2.5 mg/100 g (i.p.) | Reduces indirect nitric oxide synthesis and total oxidative status Increased levels of CAT and total antioxidant capacity of plasma |
[62] |
| Rats with streptozotocin-nicotinamide-induced diabetes mellitus | 2 mg/kg (p.o.) | Increased levels of CAT, SOD, and GSH Reduced levels of superoxide and protein carbonyl (PCO) and prevented DNA damage |
[63] | |
| Epigallocatechin gallate | DPPH ABTS FRAP |
400 μM | 77.2 ± 4.3%, 90.2 ± 3.1%, and 100 ± 3.1%, respectively 68.2 ± 3.4%, 82.2 ± 3.8%, and 95.5 ± 3.9%, respectively |
[65] |
| Gallocatechin gallate | Acute lung injury induced by sepsis in rats | 50 mg/kg | Decreases TBARS levels and increases levels of SOD and GSH | [69] |
| Amentoflavone | DPPH, ABTS, superoxide, and hydroxyl radicals | 50 μg/mL | Scavenging of free radicals (19.21-75.52%) | [67] |
| Apigenin | TEAC ORAC FRAP |
2022.2 ± 154.8 μmol TEa/mmol, 887.9 ± 5.8 μmol TEa/mmol, and 113.2 ± 12.2 μmol Fe2+/mmol, respectively | Scavenging of free radicals | [71] |
| Cardiotoxicity induced by doxorubicin in rats | 25 mg/kg (p.o.) | Reduces levels of MDA, increases SOD levels, and prevents cardiomyocyte apoptosis | [72] | |
| Luteolin | Human colon cancer cells (HT-29) | 50 μg/mL | Increases levels of GSH, expression of GSH synthetase, and the activity of SOD and CAT | [75] |
| Acute lung injury induced by sepsis in mice | 0.2 mg/kg (i.p.) | Reduces lipid peroxidation, increases the activity of SOD and CAT, and suppresses the NF-κB pathway | [76] | |
| Herbacetin | Mice with obesity-associated insulin resistance induction | 40 mg/kg (p.o.) | Increases the activity of glucose-6-phosphate dehydrogenase | [79] |
| Pectolinarin | Hepatic injury induced by D-galactosamine in rats | 10 and 20 mg/kg (p.o.) | Increases levels of SOD, GSH, glutathione reductase, and glutathione S-transferase | [80] |
| Rhoifolin | ORAC | Approximately 10 Trolox equivalents (μM) | Scavenging of free radicals | [83] |
| Catechin | ABTS FRAP Dihydrorhodamine 123 oxidation assay |
3.965 ± 0.067 (mol Trolox equivalents/mol), 0.793 ± 0.004 (mol Trolox equivalents/mol), and IC50 0.805 ± 0.072 μM, respectively | Scavenging of free radicals | [86] |
| Isobavachalcone | DPPH SC50 FRAP ABTS SC50 |
250.8 μM, 0.4 ± 0.05 mM equivalent to FeSO4·7H2O, and 510.1 mM, respectively | Scavenging of free radicals | [88] |
| Psoralidin | Electron spin resonance | IC50 = 4.7 μM | Scavenging of free radicals | [87] |
| Myricetin | DPPH Chinese hamster lung fibroblast cells (V79-4) treated with H2O2 |
5 μg/mL and 10 μg/mL 10 μg/mL |
Scavenging of free radicals (21% and 54%, respectively) Prevents DNA damage and lipid peroxidation Increases the activity of SOD, CAT, and GPx |
[96] |
| Helichrysetin | ORAC | 4.4 ± 0.6 Trolox equivalents | Scavenging of free radicals | [92] |
| Scutellarein | DPPH ABTS Superoxide radicals |
18.7 ± 0.1 μM, 18.3 ± 1.2 μM, and 79.0 ± 0.5 μM, respectively | Scavenging of free radicals | [99] |
| Resveratrol | DPPH SC50/r2 | 26.37/0.849 μmol/dm | Scavenging of free radicals | [101] |
| Rats with obstructive lung disease | 50 mg/kg | Increases SOD activity and reduces MDA levels | [103] | |
| Hypercholesterolemic ApoE-KO mouse | 100 mg/kg | Inhibits the activity and expression of NADPH oxidases Increases SOD, GPx, and CAT levels |
[102] |
Figure 2.
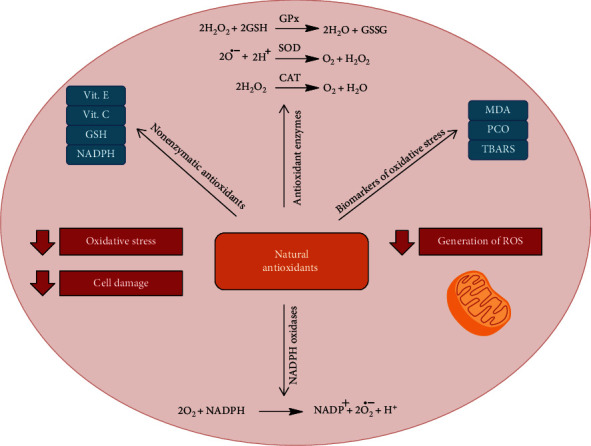
The main antioxidant mechanisms of natural compounds reported in this review. Dashed line: inhibition. Full line: activation.
5. Effect of Natural Antioxidants in Coronavirus Infections
This review focused on studies reporting on the anticoronavirus activity of natural antioxidants. Based on exclusion criteria, data from nineteen compounds were discussed.
The oxidative stress pathway could potentially be a key element in coronavirus-induced apoptosis and pathogenesis [104]. For this reason, it is interesting to investigate the use of antioxidants as potential therapeutic tools—either as an alternative or as an adjuvant to conventional therapies—in the treatment of coronavirus infections. Among the antioxidant compounds evaluated as for coronavirus infections are the flavonoids, which are compounds widely found in fruits, vegetables, and certain beverages. In fact, research groups have reported that antioxidant flavonoids, including (+)-catechin, luteolin, apigenin, quercetin, and quercetin 7-rhamnoside, inhibit ROS accumulation and apoptosis of cells infected with different coronavirus, including porcine epidemic diarrhoea coronavirus (PEDV) and transmissible gastroenteritis coronavirus (TGEV) [105–107].
As shown with the recent COVID-19 pandemic, the search for alternative or new antiviral therapies for the treatment of coronavirus diseases remains important. Based on the literature, antioxidant therapies offer an attractive option.
The high number of deaths and clinical complications observed in SARS- and MERS-CoV epidemics motivated the search for effective therapeutic agents. This was necessitated when many of the tested conventional drugs and antiviral therapies proved ineffective in treating SARS-CoV infections. For example, the initial treatment of SARS-CoV with antiviral agents such as ribavirin and corticosteroids did not achieve very satisfactory results, mainly because corticosteroids exert immunosuppressor effects on the humoral and cellular immune systems [108, 109]. Other drugs such as pentoxifylline were considered for the treatment of SARS due to its interesting therapeutic properties that include anti-inflammatory, antiviral, immunomodulatory, and bronchodilatory effects. However, it too was not successful in the clinical treatment of SARS-CoV infection [110].
Many antioxidant compounds show antiviral activity against SARS-CoV. The antiviral activity has been mainly attributed to the inhibition of the 3C-like protease (3CLpro) of SARS-CoV, a vital enzyme for SARS-CoV replication [111]. As an example, multiples studies have reported that quercetin and quercetin-derived compounds, such as quercetin 3-β-galactoside, display potent 3CLpro inhibitory e5ffect and consequent reduction of SARS-CoV replication [112]. Other antioxidants, such as epigallocatechin gallate, gallocatechin gallate, amentoflavone, apigenin, luteolin, herbacetin, rhoifolin, and pectolinarin, are also found to efficiently block the enzymatic activity of SARS-CoV 3CLpro [111, 113, 114].
Moreover, some natural antioxidants exhibit promising antiviral activity against SARS-CoV infection by interfering with different targets involved in SARS-CoV replication, in particular the SARS-CoV papain-like protease (PLpro) and SARS-CoV helicase protein. Kim et al. reported that isobavachalcone and psoralidin inhibit PLpro in a dose-dependent manner with IC50 ranging between 4.2 and 38.4 μM [115]. Previously, Yu et al. reported that myricetin and scutellarein potently inhibit the SARS-CoV helicase protein in vitro by affecting the ATPase activity [116].
MERS-CoV is another zoonotic coronavirus transmitted between animals and human beings that causes severe morbidity and mortality. No antiviral medicines with satisfactory efficacy for the treatment of MERS-CoV-infected patients have been identified to date. Similar to SARS-CoV, natural antioxidant libraries have been probed for potential inhibitory compounds against MERS-CoV 3C-like protease. Jo et al. showed that herbacetin, isobavachalcone, quercetin 3-β-d-glucoside, and helichrysetin, four compounds with recognized antioxidant activity, can block the enzymatic activity of MERS-CoV 3CLpro using a tryptophan-based fluorescence method. Furthermore, the experimental and computational studies show that flavonol and chalcone are favourite scaffolds to bind with the catalytic site of MERS-CoV 3CLpro [117].
In a study performed by Lin et al., the antiviral activities of resveratrol were investigated in MERS-infected Vero E6 cells. The authors reported a significant inhibition of MERS-CoV infection and prolonged host cell survival after virus infection, which they speculate was promoted by resveratrol. In addition, they also found that the expression of the nucleocapsid (N) protein, which is essential for MERS-CoV replication, is decreased after resveratrol treatment [118]. It is important to mention that in vitro models of coronavirus infection also show antiviral activity of flavonoids extracted from flowering cherry cultivars and black tea [119, 120]. Finally, antioxidants, such as resveratrol, also are able to block infection produced by herpesvirus [121, 122]. The discovery of antiviral compounds from a bioactive compound against other viruses is an interesting strategy for obtaining new antiviral drugs. Table 2 shows the main actions of the natural antioxidants against the coronavirus, and Figure 3 summarizes these activities.
Table 2.
Natural antioxidants tested in in vitro coronavirus infection models and their main results and mechanism of action.
| Antioxidant | Type of cells tested | Concentration (IC50) | Antiviral effect | Mechanism of action | Reference |
|---|---|---|---|---|---|
| (+)-Catechin | TGEV-infected ST cells | (+)-Catechin (20–80 μM) | Inhibition of TGEV-induced apoptosis | Suppression of the TGEV-induced Bcl-2 reduction, Bax redistribution, cytochrome c release, and caspase-3 activation | [107] |
| Resveratrol | MERS-infected Vero E6 cells. | Resveratrol (125-250 μM) | Inhibition of MERS-induced infection/apoptosis and prolonged cellular survival after virus infection | Reduction of the expression of nucleocapsid (N) protein essential for MERS-CoV replication | [118] |
| Quercetin Epigallocatechin gallate Gallocatechin gallate (GCG) |
Recombinant 3CLpro was expressed in Pichia pastoris GS115 | Quercetin (73 μM) Epigallocatechin gallate (73 μM) Gallocatechin gallate (47 μM) |
Inhibition of coronavirus replication | GCG displayed a binding energy of -14 kcal mol−1 to the active site of 3CLpro and the galloyl moiety at 3-OH position was required for 3CLpro inhibition activity | [114] |
| Quercetin 7-rhamnoside (Q7R) | PEDV-infected Vero cells | Q7R (10 μM) | Reduction of the formation of a visible cytopathic effect (CPE) without DNA fragmentation | Not specificity | [105, 106] |
| Amentoflavone Apigenin Luteolin Quercetin Quercetin 3-β-galactoside Herbacetin Rhoifolin Pectolinarin |
SARS-CoV 3CLpro inhibition using fluorescence resonance energy transfer analysis Molecular docking, SPR/FRET-based bioassays, and mutagenesis Tryptophan-based fluorescence method |
Amentoflavone (8.3 μM) Apigenin (208.8 μM) Luteolin (20.2 μM) Quercetin (23.8 μM) Quercetin 3-β-galactoside (5-200 μM) Herbacetin (33.17 μM) Rhoifolin (27.45 μM) Pectolinarin (37.78 μM) |
Inhibition of SARS-CoV replication | Flavonoids exhibited SARS-CoV 3CLpro inhibitory activity | [113, 112, 111] |
| Herbacetin, isobavachalcone, quercetin 3-β-d-glucoside, helichrysetin | Tryptophan-based fluorescence method | Herbacetin (40.59 μM) Isobavachalcone (35.85 μM) Quercetin 3-β-d-glucoside (37.03 μM) Helichrysetin (67.04 μM) |
Inhibition of MERS-CoV replication | Flavonoids exhibited MERS-CoV 3CLpro inhibitory activity | [117] |
| Isobavachalcone Psoralidin |
Lineweaver–Burk and Dixon plots | Isobavachalcone (7.30 μM) Psoralidin (4.02 μM) |
Inhibition of SARS-CoV replication | Isobavachalcone and psoralidin exhibited SARS-CoV papain-like protease inhibitory activity | [115] |
| Myricetin, scutellarein | SPR/FRET-based bioassays | Myricetin (2.71 μM) Scutellarein (0.86 μM) |
Inhibition of SARS-CoV replication | Myricetin and scutellarein potently inhibit the SARS-CoV helicase protein in vitro by affecting the ATPase activity | [116] |
Figure 3.
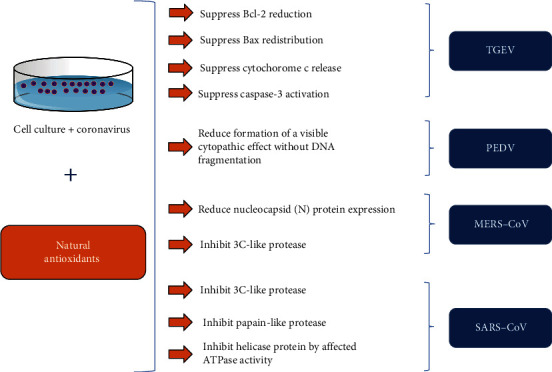
Inhibitory actions of natural antioxidants against coronavirus.
6. Conclusions
In conclusion, this review shows that antioxidant compounds, prominently flavonoids, exhibit antiviral action in models of coronavirus infections. In general, the antiviral activity might be attributed, at least in part, to the inhibitory effect on the enzymatic activity of targets involved in coronavirus replication, including SARS-CoV 3CLpro, SARS-CoV papain-like protease (PLpro), SARS-CoV helicase protein, and MERS-CoV 3CLpro. In addition, some studies provide evidence that the reduction of ROS accumulation retards the coronavirus-activated apoptotic signaling. Therefore, the mechanisms of oxidative stress could be the key element to be studied in coronavirus infections, including those related to inflammatory processes arising from the action of this virus. Obviously, further investigations are needed to elucidate other pharmacological mechanisms by which natural antioxidants play an antiviral effect. Despite the findings reported in this review, they cannot be generalized to COVID-19. However, the data provided support to the investigation of natural antioxidants as a potential therapeutic approach in the treatment for COVID-19 and its severe clinical complications, either as an alternative or as an adjuvant to conventional therapies, and contribute to the search for new prototypes in the development of drugs against coronavirus infections.
Acknowledgments
This research was supported by the National Council for Scientific and Technological Development (CNPq) and the Coordination for the Improvement of Higher Education Personnel (CAPES). Burtram C. Fielding receives funding from the National Research Foundation (NRF) (South Africa) and the University of the Western Cape Senate Research Fund.
Abbreviations
- 229E:
Human coronavirus-229E
- 3CLpro:
3C-like protease
- ABTS:
2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid
- CoVs:
Coronaviruses
- COVID-19:
Coronavirus disease 2019
- CAT:
Catalase
- DAD:
Diffuse alveolar damage
- DPPH:
2,2-Diphenyl-1-picrylhydrazyl
- FRAP:
Ferric reducing antioxidant power
- GSH:
Reduced glutathione
- GPx:
Glutathione peroxidase
- HCoVs:
Human coronaviruses
- HKU1:
Human coronavirus-HKU1
- LRTIs:
Lower respiratory tract infections
- MDA:
Malondialdehyde
- MERS-CoV:
Middle East respiratory syndrome-coronavirus
- NF-κB:
Nuclear factor kappa B
- NL63:
Human coronavirus-NL63
- OC43:
Human coronavirus-OC43
- ORAC:
Oxygen radical absorbance capacity
- PCO:
Protein carbonyl
- PEDV:
Porcine epidemic diarrhoea coronavirus
- PLpro:
Papain-like protease
- RNS:
Reactive nitrogen species
- ROS:
Reactive oxygenated species
- SARS:
Severe acute respiratory syndrome
- SARS-CoV:
Severe acute respiratory syndrome-coronavirus
- SOD:
Superoxide dismutase
- TBARS:
Thiobarbituric acid reactive substances
- TEAC:
Trolox equivalent antioxidant capacity
- TGEV:
Transmissible gastroenteritis coronavirus
- URTIs:
Upper respiratory tract infections.
Disclosure
Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in regard thereto.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- 1.Schoeman D., Fielding B. C. Coronavirus envelope protein: current knowledge. Virology Journal. 2019;16(1):p. 69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gralinski L. E., Baric R. S. Molecular pathology of emerging coronavirus infections. The Journal of Pathology. 2015;235(2):185–195. doi: 10.1002/path.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Hoek L. Human coronaviruses: what do they cause? Antiviral Therapy. 2007;12(4 Part B):651–658. [PubMed] [Google Scholar]
- 4.Arora P., Jafferany M., Lotti T., Sadoughifar R., Goldust M. Learning from history: coronavirus outbreaks in the past. Dermatologic Therapy. 2020;(article e13343) doi: 10.1111/dth.13343. [DOI] [PubMed] [Google Scholar]
- 5.Berry M., Gamieldien J., Fielding B. Identification of new respiratory viruses in the new millennium. Viruses. 2015;7(3):996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieto-Torres J. L., DeDiego M. L., Verdiá-Báguena C., et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathogens. 2014;10(5, article e1004077) doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Middle East respiratory syndrome (MERS) 2020. May 2020, https://www.emro.who.int/health-topics/mers-cov/mers-outbreaks.html.
- 8.World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) situation report – 99. 2020. May 2020, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200428-sitrep-99-covid-19.pdf?sfvrsn=119fc381_2.
- 9.Chawla S., Saxena S. K. Coronavirus Disease 2019 (COVID-19) Springer; 2020. Preparing for the perpetual challenges of pandemics of coronavirus infections with special focus on SARS-CoV-2; pp. 165–186. [DOI] [Google Scholar]
- 10.Berry M., Fielding B., Gamieldien J. Potential broad spectrum inhibitors of the coronavirus 3CLpro: a virtual screening and structure-based drug design study. Viruses. 2015;7(12):6642–6660. doi: 10.3390/v7122963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nature Reviews. Microbiology. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Zhao N., Shu Y., Han S., Chen B., Shu X. Effect of gastrointestinal symptoms in patients with COVID-19. Gastroenterology. 2020;158(8):2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo S., Zhang X., Xu H. Don’t overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clinical Gastroenterology and Hepatology. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skariyachan S., Challapilli S. B., Packirisamy S., Kumargowda S. T., Sridhar V. S. Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for Middle East respiratory syndrome coronavirus infections. Frontiers in Microbiology. 2019;10 doi: 10.3389/fmicb.2019.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris J. S. M., Chu C. M., Cheng V. C. C., et al. Clinical progression and viral load in a community outbreak of coronavirus- associated SARS pneumonia: a prospective study. The Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung T. S., Huang M., Liu D. X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus–host interactions. Virus Research. 2014;194:110–123. doi: 10.1016/j.virusres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai Y., Kuba K., Neely G. G., et al. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones D. P. Redefining oxidative stress. Antioxidants & Redox Signaling. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 19.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 20.Pisoschi A. M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. European Journal of Medicinal Chemistry. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravier-Hernández R., Gil-del Valle L., Valdes-Alonso L., et al. Oxidative stress in hepatitis C virus–human immunodeficiency virus co-infected patients. Annals of Hepatology. 2020;19(1):92–98. doi: 10.1016/j.aohep.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Rong L., Li Y.-P. Flaviviridae viruses and oxidative stress: implications for viral pathogenesis. Oxidative Medicine and Cellular Longevity. 2019;2019:17. doi: 10.1155/2019/1409582.1409582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camini F. C., da Silva Caetano C. C., Almeida L. T., de Brito Magalhaes C. L. Implications of oxidative stress on viral pathogenesis. Archives of Virology. 2017;162(4):907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz K. B. Oxidative stress during viral infection: a review. Free Radical Biology & Medicine. 1996;21(5):641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 26.Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. The Journal of Experimental Medicine. 1992;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D., Yang X. O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. Journal of Microbiology, Immunology, and Infection. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye S., Lowther S., Stambas J. Inhibition of reactive oxygen species production ameliorates inflammation induced by influenza A viruses via upregulation of SOCS1 and SOCS3. Journal of Virology. 2015;89(5):2672–2683. doi: 10.1128/JVI.03529-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogden J. D., Baker H., Frank O., et al. Micronutrient status and human immunodeficiency virus (HIV) infection. Annals of the New York Academy of Sciences. 1990;587(1):189–195. doi: 10.1111/j.1749-6632.1990.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 31.Chrobot A. M., Szaflarska-Szczepanik A., Drewa G. Antioxidant defense in children with chronic viral hepatitis B and C. Medical Science Monitor. 2000;6(4):713–718. [PubMed] [Google Scholar]
- 32.Reshi M. L., Su Y.-C., Hong J.-R. RNA viruses: ROS-mediated cell death. International Journal of Cell Biology. 2014;2014:16. doi: 10.1155/2014/467452.467452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y.-H., Tseng C.-P., Cheng M.-L., Ho H.-Y., Shih S.-R., Chiu D. T.-Y. Glucose-6-phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. The Journal of Infectious Diseases. 2008;197(6):812–816. doi: 10.1086/528377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. International Journal of Cardiology. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotiadis V. N., Duchen M. R., Osellame L. D. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840(4):1254–1265. doi: 10.1016/j.bbagen.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sena L. A., Chandel N. S. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L., Kepp O., Kroemer G. Mitochondria: master regulators of danger signalling. Nature Reviews Molecular Cell Biology. 2012;13(12):780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- 38.Chen C.-Y., Ping Y. H., Lee H. C., et al. Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis. The Journal of Infectious Diseases. 2007;196(3):405–415. doi: 10.1086/519166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favreau D. J., Meessen-Pinard M., Desforges M., Talbot P. J. Human coronavirus-induced neuronal programmed cell death is cyclophilin d dependent and potentially caspase dispensable. Journal of Virology. 2011;86(1):81–93. doi: 10.1128/JVI.06062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X., Xu Y., Zhang Q., et al. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/P53, but not P38 MAPK and SAPK/JNK signalling pathways. Veterinary Microbiology. 2019;232:1–12. doi: 10.1016/j.vetmic.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Do Carmo S., Jacomy H., Talbot P. J., Rassart E. Neuroprotective effect of apolipoprotein D against human coronavirus OC43-induced encephalitis in mice. The Journal of Neuroscience. 2008;28(41):10330–10338. doi: 10.1523/JNEUROSCI.2644-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganfornina M. D., Do Carmo S., Lora J. M., et al. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7(4):506–515. doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding L., Zhao X., Huang Y., et al. Regulation of ROS in transmissible gastroenteritis virus-activated apoptotic signaling. Biochemical and Biophysical Research Communications. 2013;442(1–2):33–37. doi: 10.1016/j.bbrc.2013.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang R., Wang X., Ni L., et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sciences. 2020;250, article 117583 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu S., Zhang Y., Ren F., et al. Structure–affinity relationship of the interaction between phenolic acids and their derivatives and β-lactoglobulin and effect on antioxidant activity. Food Chemistry. 2018;245:613–619. doi: 10.1016/j.foodchem.2017.10.122. [DOI] [PubMed] [Google Scholar]
- 46.Saraswathi V. S., Saravanan D., Santhakumar K. Isolation of quercetin from the methanolic extract of lagerstroemia speciosa by HPLC technique, its cytotoxicity against MCF-7 cells and photocatalytic activity. Journal of Photochemistry and Photobiology B: Biology. 2017;171:20–26. doi: 10.1016/j.jphotobiol.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 47.Zahoor M., Shah A. B., Naz S., Ullah R., Bari A., Mahmood H. M. Isolation of Quercetin from Rubus fruticosus, Their Concentration through NF/RO Membranes, and Recovery through Carbon Nanocomposite. A Pilot Plant Study. BioMed Research International. 2020;2020:7. doi: 10.1155/2020/8216435.8216435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim G. N., Jang H. D. Protective mechanism of quercetin and rutin using glutathione metabolism on H2O2-induced oxidative stress in HepG2 cells. Annals of the New York Academy of Sciences. 2009;1171(1):530–537. doi: 10.1111/j.1749-6632.2009.04690.x. [DOI] [PubMed] [Google Scholar]
- 49.Meng L. Q., Yang F. Y., Wang M. S., et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate. 2018;78(11):790–800. doi: 10.1002/pros.23536. [DOI] [PubMed] [Google Scholar]
- 50.Gerin F., Sener U., Erman H., et al. The effects of quercetin on acute lung injury and biomarkers of inflammation and oxidative stress in the rat model of sepsis. Inflammation. 2016;39(2):700–705. doi: 10.1007/s10753-015-0296-9. [DOI] [PubMed] [Google Scholar]
- 51.Raal A., Püssa T., Sepp J., Malmiste B., Arak E. Content of phenolic compounds in aerial parts ofChamomilla suaveolensfrom Estonia. Natural Product Communications. 2011;6(8) doi: 10.1177/1934578X1100600814. [DOI] [PubMed] [Google Scholar]
- 52.Shanmugam S., Thangaraj P., Lima B. . S., et al. Effects of luteolin and quercetin 3-β-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomedicine & Pharmacotherapy. 2016;83:1278–1285. doi: 10.1016/j.biopha.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Jayachandran M., Wu Z., Ganesan K., Khalid S., Chung S. M., Xu B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chemico-Biological Interactions. 2019;303:62–69. doi: 10.1016/j.cbi.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Butterweck V., Jürgenliemk G., Nahrstedt A., Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Medica. 2000;66(1):3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 55.Kucharíková A., Kusari S., Sezgin S., Spiteller M., Čellárová E. Occurrence and distribution of phytochemicals in the leaves of 17 in vitro cultured Hypericum spp. adapted to outdoor Conditions. Frontiers in Plant Science. 2016;7, article 1616 doi: 10.3389/fpls.2016.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang C., Li X., Liu Z., Han M. L., Hou Y. L., Guo C. L. The effect and mechanism of hyperoside on high glucose-induced oxidative stress injury of myocardial cells. Sichuan da xue xue bao. Yi xue ban = Journal of Sichuan University. Medical Science Edition. 2018;49(4):518–523. [PubMed] [Google Scholar]
- 57.Huang Z.-Q., Chen P., Su W.-W., et al. Antioxidant activity and hepatoprotective potential of quercetin 7-rhamnoside in vitro and in vivo. Molecules. 2018;23(5, article 1188) doi: 10.3390/molecules23051188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheikh Y., Maibam B. C., Talukdar N. C., Deka D. C., Borah J. C. In vitro and in vivo anti-diabetic and hepatoprotective effects of edible pods of Parkia roxburghii and quantification of the active constituent by HPLC-PDA. Journal of Ethnopharmacology. 2016;191:21–28. doi: 10.1016/j.jep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 59.Lou J., Wang W., Zhu L. Occurrence, formation, and oxidative stress of emerging disinfection byproducts, halobenzoquinones, in tea. Environmental Science & Technology. 2019;53(20):11860–11868. doi: 10.1021/acs.est.9b03163. [DOI] [PubMed] [Google Scholar]
- 60.Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2019;11(1):p. 39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pan Y., Long X., Yi R., Zhao X. Polyphenols in Liubao tea can prevent CCl4-induced hepatic damage in mice through its antioxidant capacities. Nutrients. 2018;10(9):p. 1280. doi: 10.3390/nu10091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bulboaca A. E., Boarescu P.-M., Porfire A. S., et al. The effect of nano-epigallocatechin-gallate on oxidative stress and matrix metalloproteinases in experimental diabetes mellitus. Antioxidants. 2020;9(2):p. 172. doi: 10.3390/antiox9020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Othman A. I., El-Sawi M. R., El-Missiry M. A., Abukhalil M. H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomedicine & Pharmacotherapy. 2017;94:362–373. doi: 10.1016/j.biopha.2017.07.129. [DOI] [PubMed] [Google Scholar]
- 64.Seeram N. P., Henning S. M., Niu Y., Lee R., Scheuller H. S., Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. Journal of Agricultural and Food Chemistry. 2006;54(5):1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 65.He J., Xu L., Yang L., Wang X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Medical Science Monitor. 2018;24:8198–8206. doi: 10.12659/MSM.911175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arwa P. S., Zeraik M. L., Ximenes V. F., da Fonseca L. M., da Silva Bolzani V., Silva D. H. S. Redox-active biflavonoids from Garcinia brasiliensis as inhibitors of neutrophil oxidative burst and human erythrocyte membrane damage. Journal of Ethnopharmacology. 2015;174:410–418. doi: 10.1016/j.jep.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 67.Bajpai V. K., Park I. W., Lee J. I., et al. Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chemistry. 2019;271:239–247. doi: 10.1016/j.foodchem.2018.07.159. [DOI] [PubMed] [Google Scholar]
- 68.Gan L., Ma J., You G., et al. Glucuronidation and its effect on the bioactivity of amentoflavone, a biflavonoid from Ginkgo biloba leaves. The Journal of Pharmacy and Pharmacology. 2020 doi: 10.1111/jphp.13247. [DOI] [PubMed] [Google Scholar]
- 69.Zong Y., Zhang H. Amentoflavone prevents sepsis-associated acute lung injury through Nrf2-GCLc-mediated upregulation of glutathione. Acta Biochimica Polonica. 2017;64(1):93–98. doi: 10.18388/abp.2016_1296. [DOI] [PubMed] [Google Scholar]
- 70.Tang D., Chen K., Huang L., Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opinion on Drug Metabolism & Toxicology. 2016;13(3):323–330. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 71.Sánchez-Marzo N., Pérez-Sánchez A., Ruiz-Torres V., et al. Antioxidant and photoprotective activity of apigenin and its potassium salt derivative in human keratinocytes and absorption in Caco-2 cell monolayers. International Journal of Molecular Sciences. 2019;20(9):p. 2148. doi: 10.3390/ijms20092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zare M. F. R., Rakhshan K., Aboutaleb N., et al. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sciences. 2019;232, article 116623 doi: 10.1016/j.lfs.2019.116623. [DOI] [PubMed] [Google Scholar]
- 73.Arwa P. S., Zeraik M. L., Ximenes V. F., da Fonseca L. M., da Silva Bolzani V., Silva D. H. S. Isolation and characterization of phenolic compounds and anthocyanins from murta (Ugni molinae Turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules. 2015;20(4):5698–5713. doi: 10.3390/molecules20045698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ou H.-C., Pandey S., Hung M.-Y., et al. Luteolin: a natural flavonoid enhances the survival of HUVECs against oxidative stress by modulating AMPK/PKC pathway. The American Journal of Chinese Medicine. 2019;47(3):541–557. doi: 10.1142/S0192415X19500289. [DOI] [PubMed] [Google Scholar]
- 75.Kang K. A., Piao M. J., Ryu Y. S., et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. International Journal of Oncology. 2017;51(4):1169–1178. doi: 10.3892/ijo.2017.4091. [DOI] [PubMed] [Google Scholar]
- 76.Rungsung S., Singh T. U., Rabha D. J., et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–343. doi: 10.1016/j.cyto.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 77.Péter Zomborszki Z., Kúsz N., Csupor D., Peschel W. Rhodiosin and herbacetin in Rhodiola rosea preparations: additional markers for quality control? Pharmaceutical Biology. 2019;57(1):295–305. doi: 10.1080/13880209.2019.1577460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choe K. I., Kwon J. H., Park K. H., et al. The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR. Molecules. 2012;17(10):11484–11494. doi: 10.3390/molecules171011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veeramani C., Alsaif M. A., Al-Numair K. S. Herbacetin, a flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes. Chemico-Biological Interactions. 2018;288:49–56. doi: 10.1016/j.cbi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Yoo Y.-M., Nam J.-H., Kim M.-Y., Choi J., Park H.-J. Pectolinarin and Pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biological & Pharmaceutical Bulletin. 2008;31(4):760–764. doi: 10.1248/bpb.31.760. [DOI] [PubMed] [Google Scholar]
- 81.Jang M., Kim K.-H., Kim G.-H. Antioxidant capacity of thistle (Cirsium japonicum) in various drying methods and their protection effect on neuronal PC12 cells and Caenorhabditis elegans. Antioxidants. 2020;9(3):p. 200. doi: 10.3390/antiox9030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barreca D., Bellocco E., Caristi C., Leuzzi U., Gattuso G. Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chemistry. 2011;129(2):417–422. doi: 10.1016/j.foodchem.2011.04.093. [DOI] [PubMed] [Google Scholar]
- 83.Van Kiem P., Mai N. T., Van Minh C., et al. Two new C-glucosyl benzoic acids and flavonoids from Mallotus nanus and their antioxidant activity. Archives of Pharmacal Research. 2010;33(2):203–208. doi: 10.1007/s12272-010-0203-8. [DOI] [PubMed] [Google Scholar]
- 84.Suo H., Tian R., Li J., et al. Compositional characterization study on high-molecular-mass polymeric polyphenols in red wines by chemical degradation. Food Research International. 2019;123:440–449. doi: 10.1016/j.foodres.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 85.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 86.Grzesik M., Naparło K., Bartosz G., Sadowska-Bartosz I. Antioxidant properties of catechins: comparison with other antioxidants. Food Chemistry. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 87.Xiao G., Li G., Chen L., et al. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. Journal of Chromatography. A. 2010;1217(34):5470–5476. doi: 10.1016/j.chroma.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 88.Abdullah S. A., Jamil S., Basar N., Abdul Lathiff S. M., Mohd Arriffin N. Flavonoids from the leaves and heartwoods of Artocarpus lowii King and their bioactivities. Natural Product Research. 2016;31(10):1113–1120. doi: 10.1080/14786419.2016.1222387. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Qin X., Li P., et al. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3β/β-catenin pathway in colorectal cancer cells. Drug Design, Development and Therapy. 2019;13, article 1449:1460. doi: 10.2147/DDDT.S192681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao D., Liu F., Li Z., Guan Y. Isobavachalcone attenuates Sephadex-induced lung injury via activation of A20 and NRF2/HO-1 in rats. European Journal of Pharmacology. 2019;848:49–54. doi: 10.1016/j.ejphar.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 91.Van Puyvelde L., De Kimpe N., Costa J., et al. Isolation of flavonoids and a chalcone from Helichrysum odoratissimum and synthesis of helichrysetin. Journal of Natural Products. 1989;52(3):629–633. doi: 10.1021/np50063a025. [DOI] [PubMed] [Google Scholar]
- 92.Vogel S., Ohmayer S., Brunner G., Heilmann J. Natural and non-natural prenylated chalcones: synthesis, cytotoxicity and anti-oxidative activity. Bioorganic & Medicinal Chemistry. 2008;16(8):4286–4293. doi: 10.1016/j.bmc.2008.02.079. [DOI] [PubMed] [Google Scholar]
- 93.Semwal D., Semwal R., Combrinck S., Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016;8(2):p. 90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki Y., Ichimura A., Sato S., et al. The natural flavonoid myricetin inhibits gastric H+, K+-ATPase. European Journal of Pharmacology. 2018;820:217–221. doi: 10.1016/j.ejphar.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 95.Bennett C. J., Caldwell S. T., McPhail D. B., Morrice P. C., Duthie G. G., Hartley R. C. Potential therapeutic antioxidants that combine the radical scavenging ability of myricetin and the lipophilic chain of vitamin E to effectively inhibit microsomal lipid peroxidation. Bioorganic & Medicinal Chemistry. 2004;12(9):2079–2098. doi: 10.1016/j.bmc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z. H., Ah Kang K., Zhang R., et al. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environmental Toxicology and Pharmacology. 2010;29(1):12–18. doi: 10.1016/j.etap.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Das S., Ganapaty S. Phytochemical evaluation of roots of Polygonum viscosum Buch-ham. Indian Journal of Pharmaceutical Sciences. 2015;77(3):352–356. doi: 10.4103/0250-474X.159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goh D., Lee Y. H., Ong E. S. Inhibitory effects of a chemically standardized extract from Scutellaria barbata in human colon cancer cell lines, LoVo. Journal of Agricultural and Food Chemistry. 2005;53(21):8197–8204. doi: 10.1021/jf051506+. [DOI] [PubMed] [Google Scholar]
- 99.Liu Q., Li X., Ouyang X., Chen D. Dual effect of glucuronidation of a pyrogallol-type phytophenol antioxidant: a comparison between scutellarein and scutellarin. Molecules. 2018;23(12):p. 3225. doi: 10.3390/molecules23123225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nunes S., Danesi F., Del Rio D., Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutrition Research Reviews. 2018;31(1):85–97. doi: 10.1017/S095442241700021X. [DOI] [PubMed] [Google Scholar]
- 101.Kotora P., Šeršeň F., Filo J., Loos D., Gregáň J., Gregáň F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules. 2016;21(1):p. 127. doi: 10.3390/molecules21010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xia N., Daiber A., Habermeier A., et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. The Journal of Pharmacology and Experimental Therapeutics. 2010;335(1):149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 103.Wang X.-L., Li T., Li J.-H., Miao S.-Y., Xiao X.-Z. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. 2017;22(9):p. 1529. doi: 10.3390/molecules22091529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delgado-Roche L., Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Archives of Medical Research. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi H.-J., Kim J.-H., Lee C.-H., et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Research. 2009;81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song J., Shim J., Choi H. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virology Journal. 2011;8(1):p. 460. doi: 10.1186/1743-422X-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang W., He L., Ning P., et al. (+)-Catechin inhibition of transmissible gastroenteritis coronavirus in swine testicular cells is involved its antioxidation. Research in Veterinary Science. 2015;103:28–33. doi: 10.1016/j.rvsc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Tawfiq J. A., Momattin H., Dib J., Memish Z. A. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. International Journal of Infectious Diseases. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ferron F., Subissi L., Silveira de Morais A. T., et al. Structural and molecular basis of mismatch correction and ribavirin excision from coronavirus RNA. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(2):E162–E171. doi: 10.1073/pnas.1718806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martín J. F. B., Jiménez J. L., MuEóz-Fernández A. Pentoxifylline and severe acute respiratory syndrome (SARS): a drug to be considered. Medical Science Monitor. 2003;9(6):SR29–SR34. [PubMed] [Google Scholar]
- 111.Jo S., Kim S., Shin D. H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen L., Li J., Luo C., et al. Binding interaction of quercetin-3-β-galactoside and its synthetic derivatives with SARS-CoV 3CLpro: structure–activity relationship studies reveal salient pharmacophore features. Bioorganic & Medicinal Chemistry. 2006;14(24):8295–8306. doi: 10.1016/j.bmc.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ryu Y. B., Jeong H. J., Kim J. H., et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorganic & Medicinal Chemistry. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen T. T. H., Woo H.-J., Kang H.-K., et al. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnology Letters. 2012;34(5):831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim D. W., Seo K. H., Curtis-Long M. J., et al. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds ofPsoralea Corylifolia. Journal of Enzyme Inhibition and Medicinal Chemistry. 2013;29(1):59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 116.Yu M.-S., Lee J., Lee J. M., et al. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, NsP13. Bioorganic & Medicinal Chemistry Letters. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jo S., Kim H., Kim S., Shin D. H., Kim M.‐. S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chemical Biology & Drug Design. 2019;94(6):2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin S.-C., Ho C.-T., Chuo W.-H., Li S., Wang T. T., Lin C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infectious Diseases. 2017;17(1):p. 144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clark K. J., Grant P. G., Sarr A. B., et al. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Veterinary Microbiology. 1998;63(2–4):147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Debiaggi M., Tateo F., Pagani L., Luini M., Romero E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica. 1990;13(3):207–213. [PubMed] [Google Scholar]
- 121.Docherty J. J., Fu M. M. H., Stiffler B. S., Limperos R. J., Pokabla C. M., DeLucia A. L. Resveratrol inhibition of herpes simplex virus replication. Antiviral Research. 1999;43(3):145–155. doi: 10.1016/S0166-3542(99)00042-X. [DOI] [PubMed] [Google Scholar]
- 122.Annunziata G., Maisto M., Schisano C., et al. Resveratrol as a novel anti-herpes simplex virus nutraceutical agent: an overview. Viruses. 2018;10(9):p. 473. doi: 10.3390/v10090473. [DOI] [PMC free article] [PubMed] [Google Scholar]


