Abstract
BACKGROUND
The influence of ventricular morphology on Fontan outcomes is controversial.
OBJECTIVES
The authors hypothesized that dysfunction of the single right ventricle (RV) and right atrioventricular valve regurgitation (AVVR) increases over time and adversely impacts late outcomes following a Fontan operation. A single-center retrospective study was performed.
METHODS
From 1985 through 2018, 1,162 patients underwent the Fontan procedure at our center and were included in this study. Transplant and takedown free survival, ventricular, and atrioventricular valve dysfunction after Fontan were analyzed. Death or heart transplantation information was obtained from the National Death Index and the Scientific Registry of Transplant Recipients.
RESULTS
The follow-up rate was 99%. Morphologic RV was present in 58% of patients. Transplant and takedown free survival were 91%, 75%, and 71% at 10 years, 20 years, and 25 years, respectively. Morphologic RV was an independent risk factor for transplant, takedown free survival (hazard ratio 2.4; p = 0.008). The AVVR, which preceded ventricular dysfunction in most cases, was associated with the development of ventricular dysfunction after Fontan (odds ratio 4.3; 95% confidence interval 2.7 to 6.7; p < 0.001). Furthermore, AVVR and ventricular dysfunction progressed over time after Fontan, especially in the RV (AVVR: p < 0.0001, ventricular dysfunction: p < 0.0001).
CONCLUSION
Morphologic RV is negatively associated with the long-term survival following the Fontan, possibly due to a tendency toward progressive AVVR and deterioration of the single ventricle function. Additional volume overload caused by AVVR may be one of the main factors accelerating the dysfunction of the single RV, implying that early valve intervention may be warranted.
Keywords: atrioventricular valve regurgitation, Fontan, heart transplant, long-term outcome, systemic right ventricle
Single ventricle heart disease comprises a wide variety of critical heart defects that result in one dominant single ventricle to provide systemic cardiac output and requires staged surgical palliation that culminates in the Fontan circulation (1). The single ventricle can be distinguished as having a left ventricle (LV) or right ventricle (RV) morphology, and in rare cases, the morphology is indeterminate (2). Morphologic left and right ventricles differ in their embryological origin, anatomy, and associated atrioventricular valves (3,4). Further, the RVs and LVs are generally subjected to different afterload conditions; the single RV being systemic and thereby pressure-overloaded (5,6). In congenitally corrected transposition of the great arteries, a defect of atrioventricular and ventriculoarterial discordance that places the RV in the systemic circulation, and the LV in the pulmonary circulation, the systemic RV and tricuspid valve are prone to failure (7).
With these considerations in mind, we hypothesized that patients with single RVs would have worse outcomes as compared with patients with single LVs due to early ventricular dysfunction. Existing clinical data regarding this has been conflicting. In a previous study of the outcomes following a Fontan procedure performed at our institution, ventricular morphology had no impact on survival with a mean follow-up of 50 months (8). More recent studies have also demonstrated that ventricular morphology does not influence 8 to 10 years of survival (9,10). However, Oster and colleagues (11) demonstrated that patients with a single RV had worse survival after Fontan, but could only speculate the cause of this was due to deteriorating function of the single RV. Further, insufficiency of the systemic tricuspid valve is also found in a substantial number of patients with hypoplastic left heart syndrome (HLHS) who have a single RV (12), and tricuspid valve regurgitation has been attributed to be secondary to annular dilation and poor function (13) or intrinsic anatomical abnormalities of the tricuspid valve (13–15).
To evaluate our hypothesis and to address these controversies, we performed a detailed analysis of the Fontan experience at our center. We specifically evaluated the effect of ventricular morphology on survival and Fontan takedown and its impact on the function of the atrioventricular valve and the single ventricle. This study also allowed us to perform a secondary analysis of the data to determine the overall long-term outcomes of the Fontan as well as perform an accompanying risk factor analysis.
METHODS
DATA COLLECTION AND PATIENT SELECTION.
A single-center retrospective cohort study of 1,162 patients who underwent Fontan completion at the University of Michigan, from January 1985 through March 2018 was performed. This cohort included the patients previously reported (8), albeit with a shorter follow-up and less detail. Baseline morphology (Table 1) and demographics as well as pre-, intra-, and post-operative data (Table 2) were collected by electronic and paper chart review for each patient. The most current vital status was obtained from our internal clinical database and electronic medical records, as well as the National Death Index (Centers for Disease Control and Prevention, National Center for Health Statistics). This study also used data from the Scientific Registry of Transplant Recipients (SRTR) (Minneapolis, Minnesota). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight to the activities of the Organ Procurement and Transplantation Network and SRTR contractors. Institutional review board approval was obtained before the initiation of this study, and the need for informed consent was waived.
TABLE 1.
Anatomic Diagnosis in Fontan Patients
| Diagnosis | n (%) | Ventricle Morphology | |
|---|---|---|---|
| RV | LV | ||
| HLHS | 484 (42) | 484 | 0 |
| TA | 181 (16) | 0 | 181 |
| DILV | 159 (14) | 0 | 159 |
| DORV/MA | 94 (8) | 94 | 0 |
| Heterotaxy | 84 (7) | 66 | 18 |
| PAIVS | 78 (7) | 0 | 78 |
| uAVSD | 41 (4) | 26 | 15 |
| Others | 41 (4) | 8 | 33 |
| Total | 1,162 | 678 | 484 |
The most common diagnosis was HLHS (n = 484, 42%). Fifty-five of 84 patients with heterotaxy had common atrioventricular valve morphology. A total of 96 patients with common atrioventricular valve morphology were detected, with 71 having a right dominant valve and 25 having a left dominant valve. Other diagnoses included variant HLHS, d-transposition of great arteries, truncus arteriosus, and Ebstein anomaly.
DILV = double inlet left ventricle; DORV = double outlet right ventricle; HLHS = hypoplastic left heart syndrome; MA = mitral atresia; PAIVS = pulmonary atresia with intact ventricular septum; TA = tricuspid atresia; uAVSD = unbalanced atrioventricular septal defect.
TABLE 2.
Baseline Cohort Characteristics With Morphologic Single Ventricle Comparison
| Total Cases | RV(%) | LV(%) | p Value | |
|---|---|---|---|---|
| Case number | 1,162 | 678(58) | 484(42) | |
| Female | 457 | 240(53) | 217(47) | 0.02 |
| Age at Fontan (y, IQR) | 2.2(1.8–3.1) | 2.1(1.7–2.8) | 2.3(1.8–3.7) | <0.001 |
| BW at Fontan (kg, IQR) | 11.8(10.3–13.8) | 11.3(10.1–13.0) | 12.4(10.6–14.9) | <0.001 |
| Stage I | 1015 | 631(62) | 384(38) | |
| Norwood | 582 | 512(88) | 70(12) | <0.001 |
| SP shunt | 330 | 80(24) | 250(76) | <0.001 |
| PAB | 103 | 39(38) | 64(62) | <0.001 |
| Stage II | 1007 | 644(64) | 363(36) | |
| HFP | 745 | 506(68) | 239(32) | <0.001 |
| BDG | 262 | 138(53) | 124(47) | 0.03 |
| Pre-Fontan evaluation | 1,125 | 667(59) | 458(41) | |
| SVEDP (mm Hg, IQR) | 8.0(6.0–10.0) | 8.0(6.0–10.0) | 8.0(6.0–10.0) | 0.75 |
| Mean PAP (mmHg, IQR) | 12.0(10.0–14.0) | 12.0(10.0–14.0) | 12.0(10.0–14.0) | 0.21 |
| PVR (Wood U × m2, IQR) | 1.8(1.3–2.4) | 1.9(1.4–2.4) | 1.7(1.1–2.3) | 0.006 |
| Dysfunction ≥ moderate | 24 | 19(79) | 5(21) | 0.04 |
| AVVR ≥ moderate | 144 | 114(79) | 30(21) | <0.001 |
| LT Fontan | 957 | 601(63) | 356(37) | <0.001 |
| ECC Fontan | 123 | 66(54) | 57(46) | 0.23 |
| APC/Bjork | 82 | 10(12) | 72(88) | <0.001 |
| CPB time (min, IQR) | 67(54–93) | 64(54–87) | 72(56–102) | <0.001 |
| Cross clamp time (min, IQR) | 29(22–40) | 29(23–37) | 30(22–47) | 0.20 |
| Fenestration | 929 | 668(74) | 452(36) | <0.001 |
| Concomitant procedures | ||||
| AVV repair/replacement | 89 | 75(84) | 14(16) | <0.001 |
| Fontan pathway | 77 | 45(58) | 32(42) | 1.0 |
| Rhythm surgery | 27 | 13(48) | 14(52) | 0.3 |
| Systemic obstruction | 21 | 7(33) | 14(67) | 0.02 |
| Others | 25 | 13(52) | 12(48) | 0.5 |
| Median follow-up (y, IQR) | 8.3(2.0–15.4) | 7.9(2.1–14.0) | 9.9(1.9–19.0) | 0.06 |
The normality of data was assessed with the Shapiro-Wilk test, and all variables were found to be non-normally distributed and were reported as median with interquartile range. Difference analysis was conducted by the chi-square test and Mann-Whitney U test. Concomitant procedures included 1) atrioventricular valve repair or replacement; 2) patch augmentation of pulmonary arteries, superior vena cava or inferior vena cava stenosis as “Fontan pathway”; 3) surgical ablation or pacemaker implantation as “Rhythm surgery”; 4) re-coarctation repair, bulboventricular foramen/ventricular septal defect enlargement or sub-aortic stenosis repair as “Systemic obstruction”; 5) atrial septectomy, pulmonary vein stenosis repair, collateral vessels ligation or diaphragm plication as “others.” The number of patients available of SVEDP, mean PAP, PVR was 1,123 (99%), 1,125 (99%), and 747 (66 %), respectively.
APC = atriopulmonary connection; AVVR = atrioventricular valve regurgitation; BDG, bidirectional Glenn; BW = bodyweight; CPB = cardiopulmonary bypass; ECC = extracardiac conduit; HFP = Hemi-Fontan procedure; IQR = interquartile range; LT = lateral tunnel; PAB = pulmonary artery banding; PAP = pulmonary artery pressure; PVR = pulmonary vascular resistance; SP = systemic pulmonary; SVEDP = systemic ventricle end-diastolic pressure.
Survival and risk factor analyses were performed. Primary endpoints for survival were Fontan takedown, heart transplant, and death. Covariates for risk factor analysis were selected based on clinical experience and previous reports (8,16–19) regarding survival outcomes after Fontan (Table 3). Survival and risk factor analyses were also performed in those who were discharged with an intact Fontan circulation to obtain conditional survival. Atrioventricular valve regurgitation (AVVR) and ventricular dysfunction analyses after Fontan procedure were obtained using data from postoperative echocardiogram studies, which were performed at the time of patient discharge as well as 3, 5, 10, 15, and 20 years after Fontan. Atrioventricular valve regurgitation and ventricular dysfunction data were graded and recorded as nominal with the following order: 1 = none or trivial dysfunction, 2 = mild dysfunction, 3 = moderate dysfunction, and 4 = severe dysfunction. Primary endpoints for AVVR and ventricular dysfunction analyses were at least moderate AVVR censored by atrioventricular valve replacement or repair and at least moderate ventricular dysfunction. In addition, competing risk analyses with cumulative incidence functions were performed for AVVR and ventricular dysfunction after Fontan and was stratified by ventricular morphology and atrioventricular valve morphology. Death, transplant, and Fontan takedown were defined as a competing risk.
TABLE 3.
Variables for Risk Factor Analysis
| Category | Specific Factors |
|---|---|
| Patient demographics | Age at Fontan, era |
| Morphologic factors | Hypoplastic left heart syndrome, heterotaxy, morphologic right ventricle |
| Interstage factors | Norwood procedure, primary Fontan |
| Pre-Fontan evaluation | Mean pulmonary artery pressure ≥15 mm Hg, systemic ventricle end-diastolic pressure ≥10 mm Hg, atrioventricular valve regurgitation ≥ moderate, ventricular dysfunction ≥ moderate |
| Intraoperative factors | Cardiopulmonary bypass time, cross clamp time, concomitant procedure at Fontan, type of Fontan (lateral tunnel and the others) |
| Post-operative factors | Chest tube drainage ≥ 2 weeks, length of hospital stay, atrioventricular valve regurgitation ≥ moderate, ventricular dysfunction ≥ moderate |
Risk factors were selected from clinical experience and previous reports.
SURGICAL TECHNIQUE/STRATEGY.
The Hemi-Fontan procedure (HFP) and the bidirectional Glenn (BDG) procedure were used for stage 2 palliation. Our surgical technique of HFP, followed by lateral tunnel Fontan, was described in the previous report (20). The HFP maintains continuity between the superior vena cava and right atrium, and the pulmonary arteries are augmented with a pulmonary homograft patch. In the BDG, the superior vena cava is transected and then directly connected to the right pulmonary artery. The HFP requires cardioplegic arrest as well as a more prolonged cardiopulmonary bypass (CPB) time compared with the BDG procedure. However, the HFP permits the creation of a lateral tunnel at the time of the Fontan (stage 3 palliation), which requires less dissection and CPB time than the extracardiac Fontan procedure that is typically performed after BDG. We also prefer the HFP because it results in widely patent pulmonary arteries and minimizes the development of restriction in the Fontan pathway after stage 3 palliation (20,21).
We usually perform the HFP at age 3 to 6 months, followed by a lateral tunnel Fontan procedure (8) at 2 to 3 years of age. In patients with systemic or pulmonary venous anomalies that preclude a lateral tunnel Fontan, the BDG procedure followed by an extracardiac conduit Fontan is used. The BDG procedure is also chosen for pulmonary atresia with intact ventricular septum with RV-dependent coronary circulation to avoid coronary ischemia by decompression of the RV that would potentially occur during the HFP. Contraindications for Fontan completion include severe diastolic or systolic dysfunction of the single ventricle and severe pulmonary hypertension that is nonresponsive to supplemental oxygen and nitric oxide.
DATA ANALYSIS.
Data analysis was performed using SPSS 26 software (SPSS, Inc, Chicago, Illinois) and SAS 9.4 (SAS Institute Inc., Cary, North Carolina). Non-normally distributed variables were reported as median with interquartile range (IQR). The Student’s t-test, chi-square test, and Mann-Whitney U test were used for characteristics comparison. Binary logistic regression was used for risk factor analysis of in-hospital death or takedown. Kaplan-Meier curves were created to estimate long-term survival. A Cox regression model was used for risk factor analysis of transplant and Fontan takedown free survival. For time-dependent covariates (at least moderate AVVR and single ventricular dysfunction after Fontan), time-varying covariates were created and analyzed. A nonparametric repeated measurement test (Cochran’s Q test) was used to conduct a comparative analysis for AVVR and ventricular dysfunction after Fontan and was performed for patients who had consistent data across each time point. The dichotomous variables are defined as AVVR < moderate versus AVVR ≥ moderate and ventricular dysfunction < moderate versus ventricular dysfunction ≥ moderate. A p value was calculated with Fine and Gray’s test for the cumulative incidence function of AVVR and ventricular dysfunction analysis. The ASSESS statement in PROC PHREG by SAS was used to examine Schoenfeld residuals for the assumption of proportional hazards. Nonviolation of assumptions was confirmed for the survival analyses as well as atrioventricular valve and ventricular function analyses. Statistical significance was considered as p < 0.05.
RESULTS
PATIENT CHARACTERISTICS.
The anatomic diagnoses and baseline cohort characteristics are shown in Table 1 and Table 2. The single ventricle morphology was RV in 678 (58%) and LV in 484 (42%), for a total of 1,162 patients. Hypoplastic left heart syndrome was the most common diagnosis (48%, n = 488). A Norwood procedure was performed in 57% (n = 582) and HFP was performed in 74% (n = 745). The median age and body weight at Fontan completion was 2.2 (IQR 1.6–3.1) years and 11.8 (IQR 10.3–13.8) kg. A lateral tunnel Fontan was performed in 82% (n = 957) and a fenestration in the lateral tunnel was created in 80% (n = 929).
IN-HOSPITAL DEATH OR TAKEDOWN RATE AND THE RISK FACTORS.
There were 45 (3.9%) in-hospital deaths and 33 (2.8%) in-hospital takedowns after Fontan. There have been no in-hospital deaths since 2008, and these 78 patients were excluded from the long-term survival analysis. The risk factors for in-hospital death or takedown were Fontan CPB time (odds ratio [OR]: 1.01 per min; 95% confidence interval (CI): 1.00 to 1.02; p = 0.001), a concomitant procedure at Fontan (OR: 2.1; 95% CI: 1.1 to 4.1; p = 0.03), and surgical era (1985–1997, OR: 6.1; 95% CI: 1.7 to 21.7; p = 0.005; 1998–2008, OR: 6.9; 95% CI 2.0 to 24.2; p = 0.003) by multivariable analysis (Table 4).
TABLE 4.
Risk Analysis of In-hospital Death and/or Takedown
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Heterotaxy | 2.9 (1.5–5.8) | 0.003 | 1.8 (0.7–4.3) | 0.21 |
| PAP ≥ 15 mm Hg | 2.1 (1.2–3.8) | 0.01 | 1.5 (0.7–3.0) | 0.28 |
| SVEDP ≥ 10 mm Hg | 2.1 (1.2–3.5) | 0.007 | 1.5 (0.8–2.9) | 0.20 |
| Dysfunction ≥ moderate | 6.0 (2.3–15.6) | <0.001 | 2.2 (0.6–8.2) | 0.25 |
| AVVR ≥ moderate | 2.6 (1.5–4.7) | 0.01 | 1.4 (0.7–3.1) | 0.36 |
| CPB time (min) | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.005–1.02) | 0.001 |
| Concomitant procedure | 2.5 (1.5–4.2) | 0.001 | 2.1 (1.07–4.1) | 0.03 |
| Era | ||||
| 1985–1997 | 9.9 (3.1–32.7) | <0.001 | 6.1 (1.7–21.7) | 0.005 |
| 1998–2007 | 6.3 (1.9–21.0) | <0.001 | 6.9 (2.0–24.2) | 0.003 |
| 2008–2018 | 1.0 | 1.0 | ||
| LT Fontan | 0.3 (0.2–0.5) | <0.001 | 0.5 (0.3–1.1) | 0.12 |
A binary logistic regression model was used for risk factor analysis. The era was divided into 3 periods. The earlier era was from 1985 to the end of 1997, the second era was 1998 to the end of 2007, and the latest era was from 2008 to March 2018.
AVVR = Atrioventricular valve regurgitation; CI = confidence interval; CPB = cardiopulmonary bypass; LT = lateral tunnel; OR = odds ratio; PAP = pulmonary artery pressure; SVEDP = systemic ventricle end-diastolic pressure.
DEATH, TAKEDOWN, AND HEART TRANSPLANT AFTER FONTAN.
After linking our Fontan clinical database to the National Death Index, we determined, as of December 31, 2018, the vital status of 1,161 patients (99.9%). This vital status determination allowed us to analyze the entire cohort of Fontan patients for long-term death, takedown, and transplant (Figure 1). A total of 123 (11 %) deaths occurred after post-Fontan discharge. A total of 40 takedowns occurred, and 33 of them were during the same hospitalization as the Fontan procedure, as noted previously (Supplemental Figure 1). Heart transplantation status was verified in 99% as of July 2019 by the SRTR. Seventy-five patients were listed for heart transplantation, and 43 patients underwent heart transplantation, including 16 at our center. Two of these 43 patients also underwent a second heart transplantation.
FIGURE 1. Fontan Cohort for Long-term Analysis.
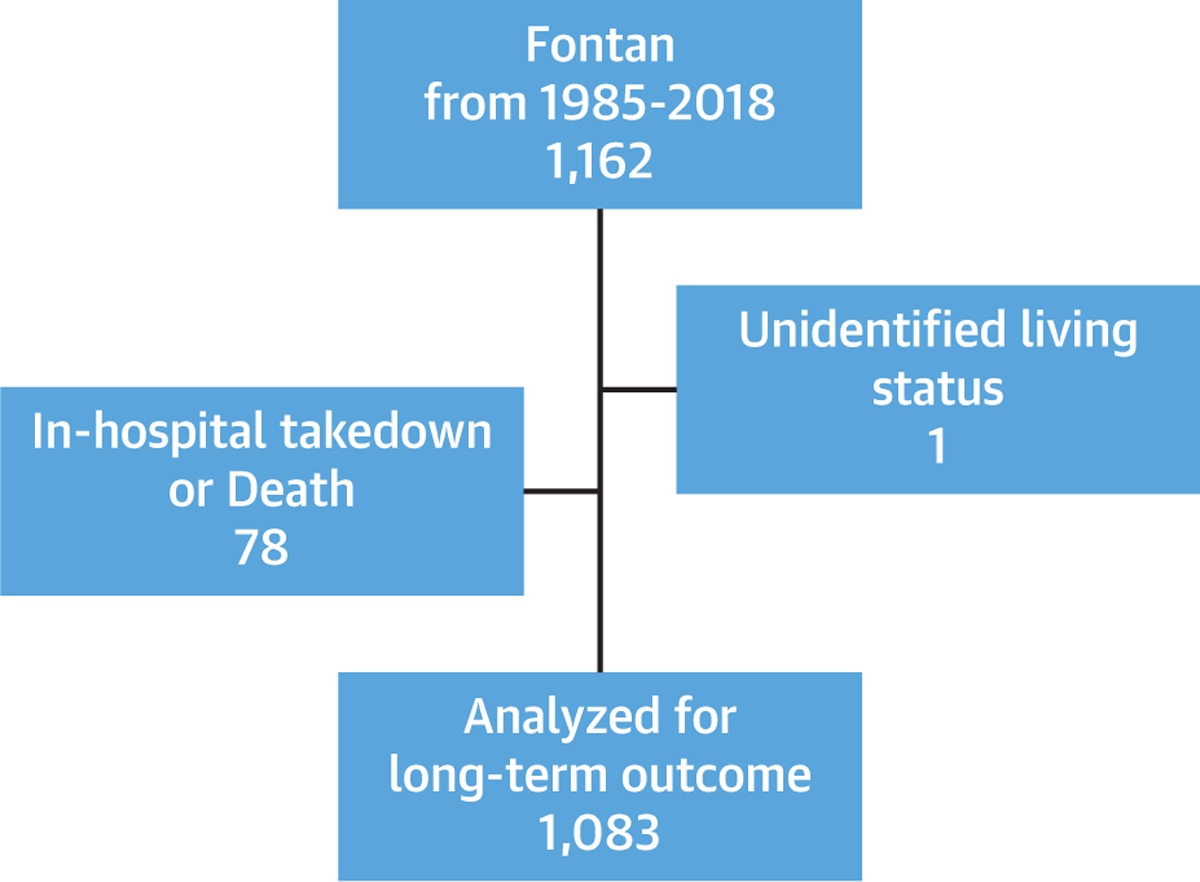
From January 1985 through 2018, a total of 1,162 Fontan patients were included in this study. Patients who had in-hospital death or takedown after Fontan, as well as unidentified vital status, were excluded from the long-term analysis. A total of 1,083 patients were included in our downstream analyses.
LONG-TERM SURVIVAL WITH INTACT FONTAN CIRCULATION AND RISK FACTORS FOR DEATH, TAKEDOWN, AND TRANSPLANT.
The median follow-up rate was 8.3 (IQR: 2.0–15.4) years, and at least one follow-up was obtained after hospital discharge in 90% of patients (n = 972). Transplantation and takedown free survival after Fontan was 91%, 75%, and 71% at 10, 20, and 25 years, respectively (Figure 2A). An analysis of the impact of single ventricle morphology found that transplantation and takedown free survival was significantly superior in the patients with a single LV as compared with that in patients with a single RV (LV: 92%, 82%, and 78% vs. RV: 90%, 68%, and 63% at 10, 20, and 25 years, respectively, p = 0.005, Figure 2B).
FIGURE 2. Transplant and Takedown Free Survival After Fontan.
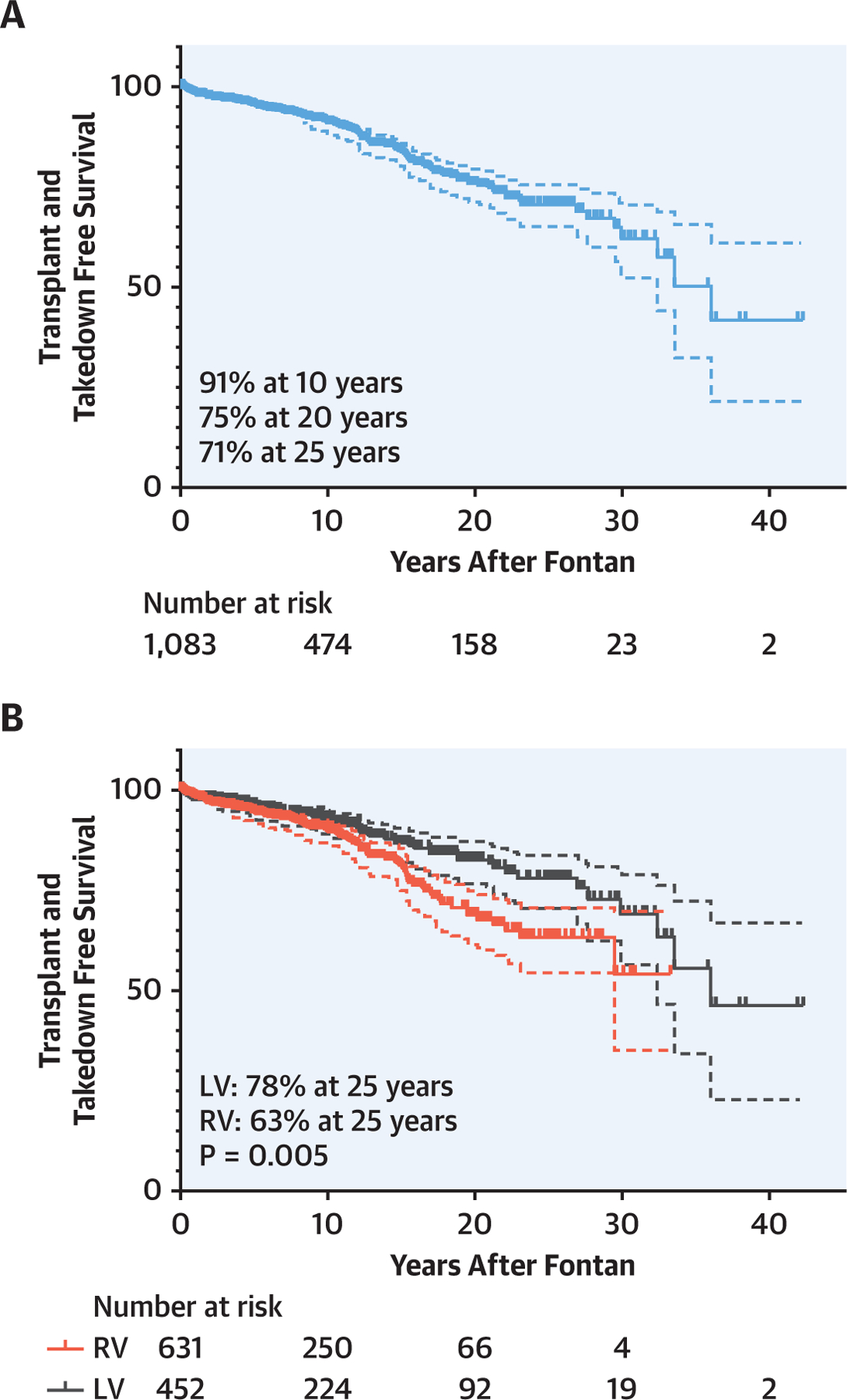
Transplant and takedown free survival after Fontan completion (A) and the comparison between single RV and LV (B). There is a significant survival difference between patients with a single RV and patients with a single LV patients (p = 0.005). LV = left ventricle; RV = right ventricle.
Risk factors for death, takedown, and transplantation are presented in Table 5. Using a Cox regression model, the independent risk factors for death, takedown, and transplant after Fontan that we identified were morphologic RV (HR: 2.4; 95% CI: 1.3 to 4.6; p = 0.008), greater than 15 mm Hg of mean pulmonary artery pressure before Fontan (HR: 1.6; 95% CI: 1.0 to 2.4; p = 0.03), concomitant procedures at Fontan (HR: 2.0; 95% CI: 1.3 to 3.0; p = 0.002), a requirement of chest tube greater than 2 weeks (HR: 2.1; 95% CI: 1.3 to 3.4; p = 0.006), length of hospital stay (HR 1.01 per day, 95% CI: 1.00 to 1.02; p = 0.001), other than lateral tunnel Fontan (HR: 2.0; 95% CI: 1.5 to 3.8; p < 0.001), and at least moderate AVVR after Fontan (HR: 2.4; 95% CI: 1.5 to 3.8; p < 0.001). Although the age at Fontan was a statistically significant risk factor with our analysis, it may be biased by the clinical factors that drove the decision for the timing of Fontan.
TABLE 5.
The Risk Analysis of Transplant and Takedown Free Survival
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| HLHS | 1.5 (1.1–2.1) | 0.02 | 1.2 (0.7–2.3) | 0.5 |
| Heterotaxy | 2.0 (1.2–3.3) | 0.008 | 1.3 (0.6–2.5) | 0.4 |
| Morphologic RV | 1.7 (1.2–2.4) | 0.005 | 2.4 (1.3–4.6) | 0.008 |
| Age at Fontan (Y) | 1.05 (1.03–1.08) | <0.0001 | 1.08 (1.03–1.1) | <0.001 |
| PAP ≥ 15 mm Hg | 1.7 (1.2–2.5) | 0.004 | 1.6 (1.0–2.4) | 0.03 |
| CPB time (min) | 1.01 (1.00–1.01) | 0.007 | 1.0 (1.0–1.004) | 0.6 |
| Concomitant procedure | 1.7 (1.2–2.4) | 0.006 | 2.0 (1.3–3.0) | 0.002 |
| CT drain ≥2 wk | 2.4 (1.7–3.5) | <0.0001 | 2.1 (1.3–3.4) | 0.006 |
| LOS (d) | 1.02 (1.01–1.03) | <0.0001 | 1.01 (1.00–1.02) | 0.001 |
| Other than LT Fontan | 2.3 (1.6–3.3) | <0.0001 | 2.0 (1.2–3.4) | 0.006 |
| AVVR ≥ moderate | 1.8 (1.2–2.8) | 0.006 | 2.4 (1.5–3.8) | <0.001 |
The risk analysis was performed using a Cox regression model with time-varying covariate for ventricular dysfunction and AVVR after Fontan. The reference groups of covariates are as follows: HLHS vs. non-HLHS, Heterotaxy vs. non-heterotaxy, morphologic RV vs. morphologic LV/2 ventricle morphology.
AVVR = atrioventricular valve regurgitation; BW = body weight; CI = confidence interval; CPB = cardiopulmonary bypass; CT = chest tube; HLHS = hypoplastic left heart syndrome; HR = hazard ratio; LOS = length of hospital stay; LT = lateral tunnel; PAP = pulmonary artery pressure; RV = right ventricle.
PROGRESSION OF AVVR AND VENTRICULAR DYSFUNCTION AFTER FONTAN.
A total of 5,140 (2,578 for ventricular dysfunction and 2,562 for AVVR) echocardiograms were reviewed for AVVR and ventricular dysfunction after the Fontan completion. The serial echocardiogram data revealed that atrioventricular valve and single ventricular dysfunction progressively increased after Fontan (Figures 3A and 3B). Morphological comparisons were then performed and demonstrated that progressive AVVR and ventricular dysfunction were more severe in patients with a single RV as compared with patients with a single LV (Figures 3C–3F). Further, we found a significant correlation between AVVR and ventricular dysfunction (Table 6). In patients with AVVR ≥ moderate, 18% developed subsequent ventricular dysfunction. On the other hand, only 4.9% of patients with AVVR< moderate developed ventricular dysfunction (OR: 4.3; 95% CI: 2.7 to 6.7; p < 0.001). Importantly, AVVR preceded ventricular dysfunction in most cases, suggesting preceding AVVR may be associated with the progression of systemic ventricular dysfunction.
FIGURE 3. Progression of Ventricular Dysfunction and AVVR After Fontan.
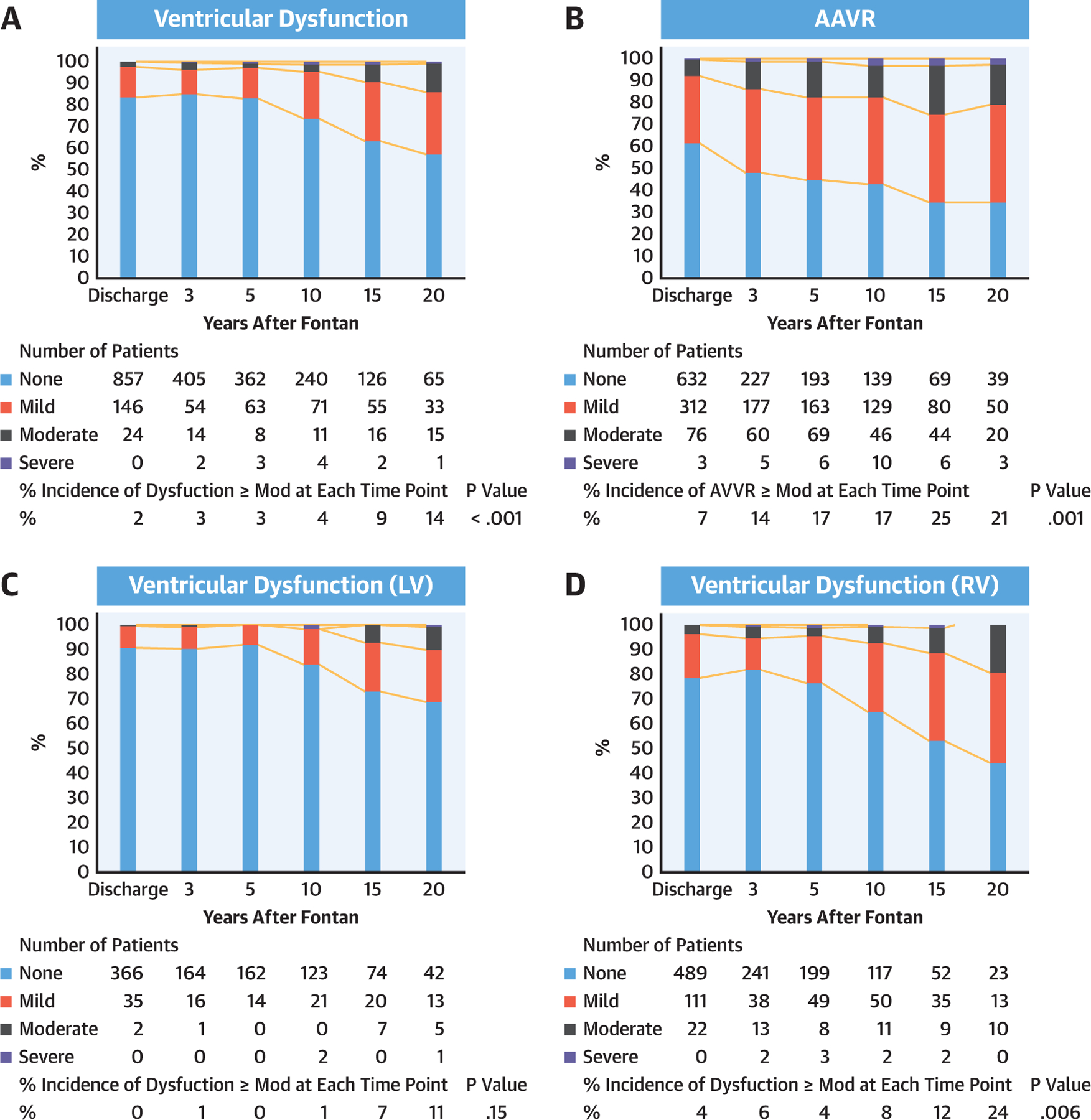
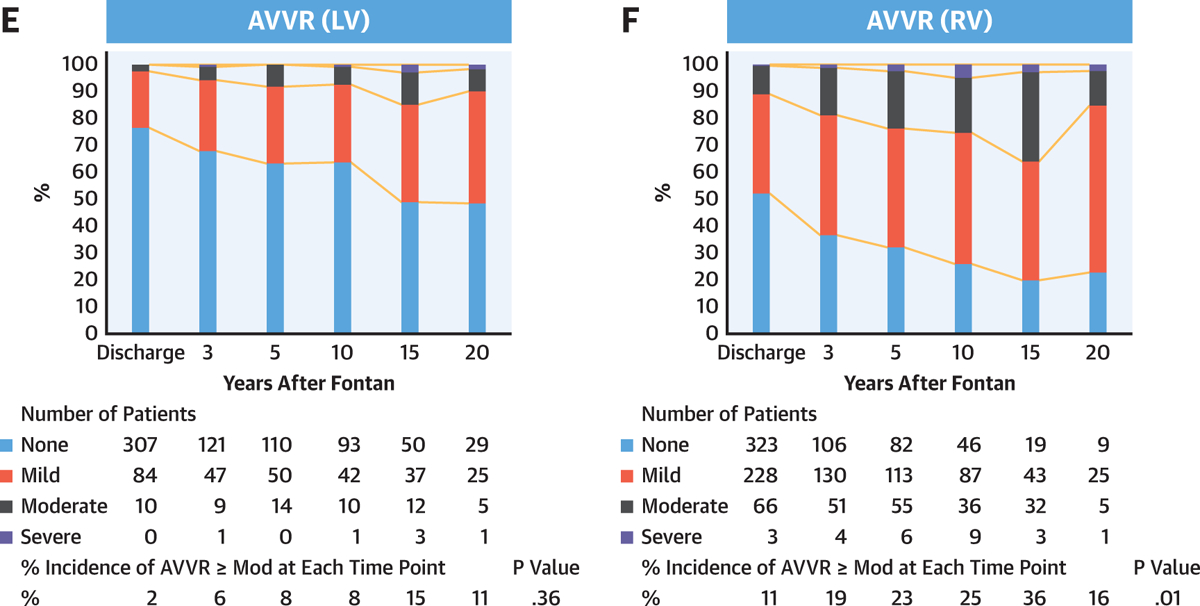
Progression of ventricular dysfunction (A) and AVVR (B) after Fontan by serial echocardiogram. Ventricular dysfunction (C and D) and AVVR (E and F) are worse in patients with a single RV (C–F). Percent incidence of ventricular dysfunction ≥ moderate and AVVR ≥ moderate at each time point was compared using Cochran’s Q test. AVVR = atrioventricular valve regurgitation; LV = left ventricle; Mod = moderate; RV = right ventricle.
TABLE 6.
The Correlation Between AVVR and Ventricular Dysfunction
| AVVR < Moderate | AVVR ≥ Moderate | |||||
|---|---|---|---|---|---|---|
| Total | RV | LV | Total | RV | LV | |
| Dysfunction < moderate | 843 | 442 | 401 | 198 | 125 | 37 |
| Dysfunction ≥ moderate | 42 | 31 | 11 | 36 | 31 | 5 |
| % incidence of dysfunction ≥ moderate (%) | 4.8* | 6.6 2.7 | 18* | 20 | 12 | |
The number and rate of developed ventricular dysfunction ≥ moderate are shown. The rate of ventricular dysfunction ≥ moderate was significantly higher in AVVR ≥ moderate group (18% vs. 4.8%, p < 0.001, odds ratio: 4.3; 95% confidence interval: 2.7–6.7).
p < 0.001.
AVVR = atrioventricular valve regurgitation; LV = left ventricle; RV = right ventricle.
Cumulative incidence rates were calculated for AVVR ≥ moderate and ventricular dysfunction ≥ moderate after Fontan with competing risk analysis (Figure 4). The cumulative incidence rate of AVVR ≥ moderate after Fontan was 36% in the single RV group versus 13% in the single LV group at 20 years, respectively (Figure 4A, HR: 3.4; 95% CI: 2.4 to 4.6; p < 0.0001). The cumulative incidence rate of ventricular dysfunction ≥ moderate after Fontan was 18% in the single RV group versus 5.8% in the single LV group at 20 years, respectively (Figure 4B, HR: 3.2; 95% CI: 1.9 to 5.2; p < 0.0001). Taken together, these results indicate that AVVR and ventricular dysfunction progressed more rapidly in patients with a single RV as compared with those with a single LV. Finally, the determined cumulative incidence rates stratified by the valve morphology demonstrated that the single ventricle and atrioventricular valve function rapidly deteriorated in the common atrioventricular valve and tricuspid valve as compared with the mitral valve (Figures 4C and 4D). However, when comparing the common atrioventricular valve with a single RV with the tricuspid valve (by definition, also with a single RV), there was no significant difference in the incidence rate of AVVR ≥ moderate (HR: 1.4; 95% CI: 0.9 to 2.0; p=0.1).
FIGURE 4. Cumulative Incidence Functions for AVVR ≥ Moderate and Ventricular Dysfunction ≥ Moderate.
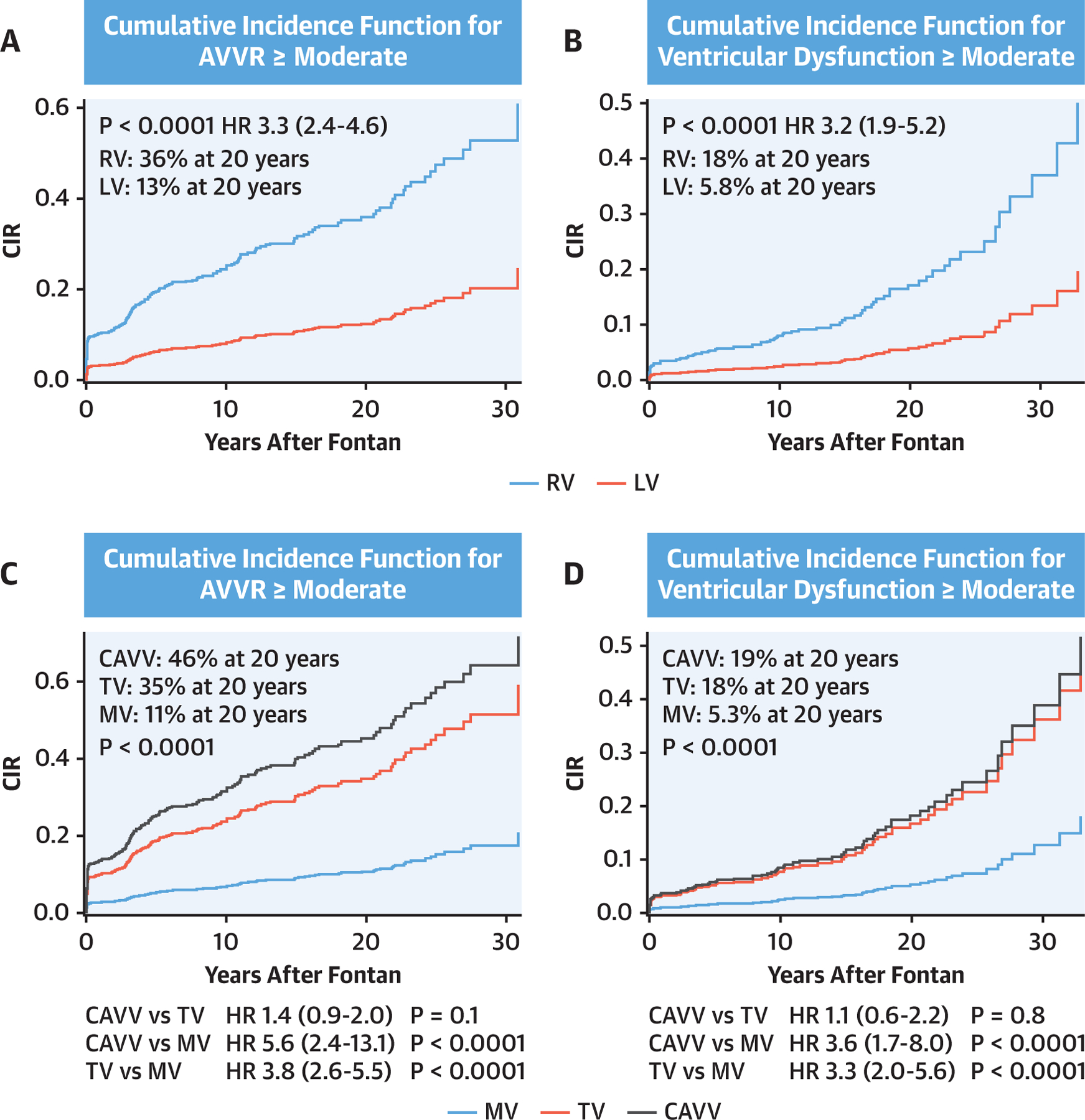
Death, transplant, and Fontan takedown were defined as a competing risk. The results were stratified by the single ventricle morphology (A and B), and the atrioventricular valve morphology (C and D). AVVR = atrioventricular valve regurgitation; CAVV = common atrioventricular valve; CIR = cumulative incidence rate; MV = mitral valve; TV = tricuspid valve.
DISCUSSION
In this study, we report the long-term survival outcome after the Fontan procedure at our center with 25 years of follow-up (Central Illustration). The uniqueness of our cohort is the large proportion of single morphologic RV (58%) with hypoplastic left heart syndrome (n = 488, 42%) and greater than 80% of lateral tunnel Fontan staged by the HFP. The HFP followed by the lateral tunnel Fontan completion has been our preferred stage 2 and stage 3 procedure for single ventricle palliation when anatomically feasible. The lateral tunnel Fontan had been primarily used since the mid-1990s; however, many institutions have begun to use the BDG and extracardiac Fontan since the late 1990s to the early 2000s (16,17,19,22). The consistent Fontan strategy over several decades in our institution provides the largest and longest follow-up of the lateral tunnel Fontan described to date. Any comparison between the lateral tunnel Fontan and the extracardiac Fontan in our study is inappropriate because there is a selection bias of the type of Fontan procedure based on anatomy. However, ease of Fontan completion, lower energy losses, more effective flow distribution to the pulmonary arteries (23), and growth potential of the Fontan pathway (24) provide practical and theoretical advantages of the HFP–lateral tunnel Fontan approach.
CENTRAL ILLUSTRATION. Takedown and Transplant-free Survival.
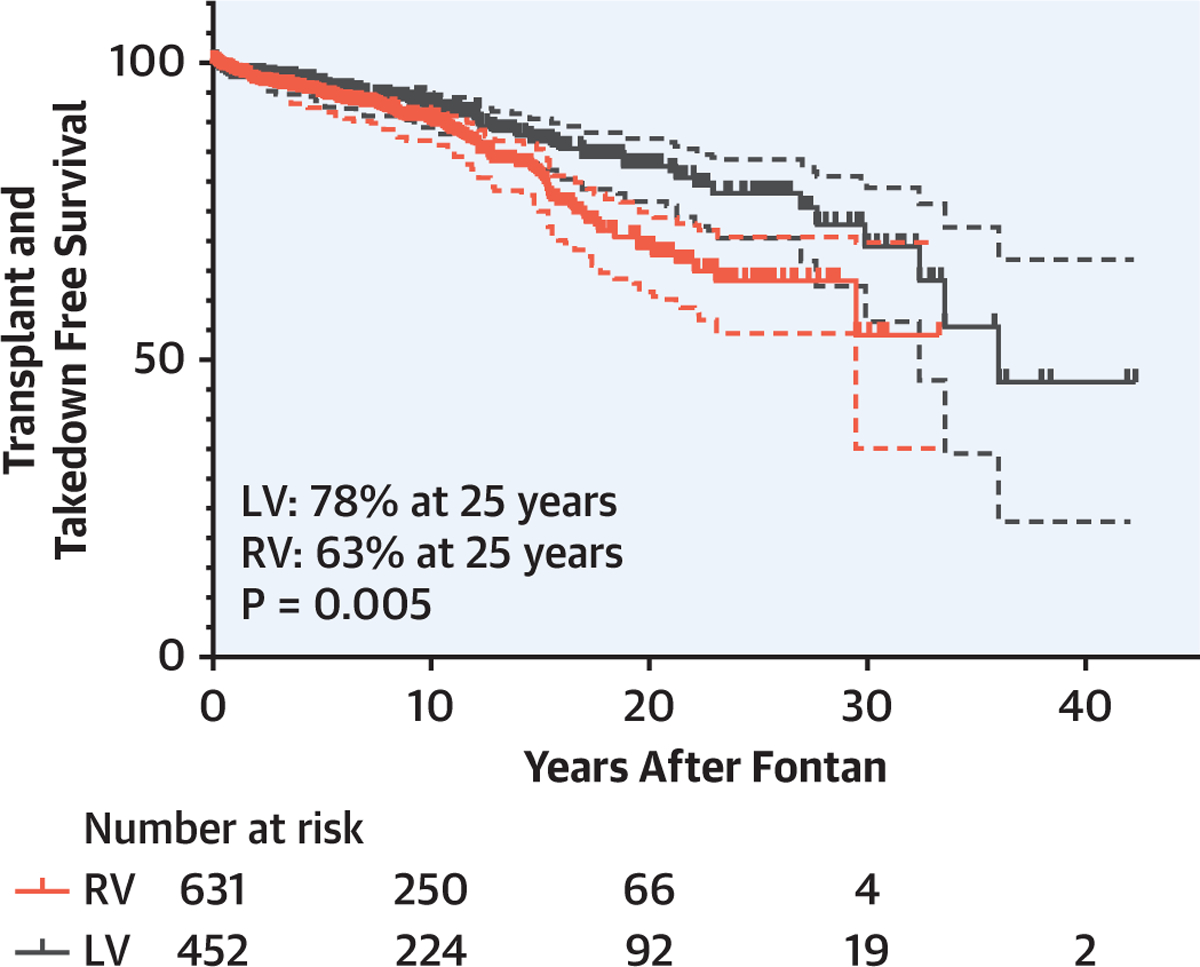
Survival rate was significantly better in the left ventricle (LV) than in the right ventricle (RV) (LV: 92%, 82%, and 78% vs. RV: 90%, 68%, and 63% at 10, 20, and 25 years, respectively, p = 0.005).
The early mortality rate of the Fontan has dramatically improved with staged surgical palliations as well as advances in perioperative and intraoperative management. Reported mortality rates for the Fontan have been less than 2% from modern series (16,25). A total of 45 in-hospital deaths (3.9%) were demonstrated in our study. However, most of these in-hospital deaths occurred before 1998, and after 2008 there has been no in-hospital mortality. As early mortality after Fontan now appears to be a historical challenge, increased attention is now being given toward the long-term outcomes. The number of living Fontan patients continues to increase with an estimated number of 50,000 to 70,000 worldwide in 2018, of whom 40% of these patients are older than 18 years (26). Several investigators have recently reported long-term overall survival rates after Fontan, with approximately 90% to 95% survival at 10 years (8,18,19,27) and 61% to 90% survival at 20 years (16–18). Our survival rates at 10 years and 20 years in this study were 91% and 75%, respectively, which were within the ranges reported in the aforementioned studies.
The various risk factors for long-term survival that we identified included the diagnosis of HLHS, preoperative pulmonary hypertension, preoperative AVVR, and prolonged postoperative chest tube drainage (8,17,18). Heterotaxy has been recognized as a risk factor for single ventricle palliation outcomes before and after the Fontan (17,28,29) and it was not found to be a significant risk factor for Fontan outcomes in our data. However, given the small numbers of patients with heterotaxy in our study, this result might have been because of the lack of statistical power. Yet another explanation for this result is that some patients with heterotaxy with a single ventricle died before Fontan and those who survived to Fontan at our center were a select group with favorable and yet-to-be-identified characteristics that led to non-inferior outcomes.
Although the poor late outcomes following an atrial switch operation for d-transposition of great arteries or after conventional repair of congenitally corrected transposition of great arteries are well known (30), the impact of single ventricle morphology has been controversial, with short- and mid-term studies demonstrating no effect (8–11), whereas a recent longer-term study demonstrated worse outcomes with a single RV (11). Given the potential developmental and anatomic differences as well as the pressure-overloaded state that make the single RV prone to failure (31), we hypothesized that dysfunction of the single RV increases over time and adversely affects the clinical outcomes of Fontan. Our analysis demonstrated a superior survival rate in patients with a single LV as compared with those with a single RV (Figure 2), and we found a morphologic RV to be a significant independent risk factor for late survival.
Regurgitation of the systemic tricuspid valve is relatively common in patients with HLHS (12), and it has also been attributed to worsening single RV function (13). However, recent studies have also indicated that these valves may have intrinsic anatomic abnormalities predisposing them to failure (13). To address this controversy, we ascertained the temporal relationship between systemic AVVR and single ventricular dysfunction to provide insight into possible causative relationships. We found that AVVR and single ventricular dysfunction progressively worsened with time, and these were significantly worse in patients with a single RV (Figures 3C–F). Interestingly, single ventricle function tends to be preserved for a substantial amount of time in the presence of AVVR. However, our assessment of single ventricle function in the presence of AVVR may underestimate the degree of ventricular dysfunction, as it has been well described in the estimation of LV function in the presence of mitral regurgitation (32). Nonetheless, we also demonstrated the cumulative incidence of 36% of AVVR ≥ moderate and 18% of ventricular dysfunction ≥ moderate at 20 years in patients with a single RV. In addition, when AVVR preceded the Fontan, this scenario was associated with worsening single ventricular dysfunction, suggesting that volume overload by AVVR will further impair the already pressure-overloaded single RV. The temporal relationship revealed by our analysis suggests that the development of AVVR in single RV may precede ventricular dysfunction, indicating that not only the annular dilation but also multiple factors may affect atrioventricular valve function, such as preexisting anatomic abnormalities of valve morphology, or abnormal ventricular septum mechanics due to a diminutive LV. Indeed, tricuspid valve abnormalities have been identified in patients with HLHS, including 12% with bicuspid, 33% with moderate dysplasia, and 2% with severe dysplasia of the tricuspid valve (15).
Historically, our surgical preference is bicuspidization of the systemic tricuspid valve, which yields a 73% early and 63% late success rate (33). However, a more selective approach tailored to address the underlying tricuspid valve abnormality may be warranted. The timing and indication of the atrioventricular valve intervention after Fontan are still controversial. Although there are some reports about atrioventricular valve repair or replacement following Fontan (34–36), perioperative risk and long-term outcomes remain unclear. Given the multiple risks, including redo sternotomy, significant collateral vessels, and limited CPB options, surgical atrioventricular valve intervention may have a high mortality and morbidity risk. Notwithstanding, our data revealed AVVR ≥ moderate after Fontan was a significant risk factor for long-term survival. Moreover, morphologic RV was associated with deteriorating atrioventricular valve and ventricular function. Therefore, particularly in patients with morphologic RV, early intervention for AVVR ≥ moderate might be beneficial for long-term Fontan outcomes if the risk for intervention is determined to be acceptable.
STUDY LIMITATIONS.
In this retrospective cohort study, one of the limitations was missing data, which included pre-Fontan echocardiogram and catheterization data. In particular, pulmonary vascular resistance data were missing in a substantial number of patients, and therefore, this parameter was excluded from the risk factor analysis. Because the definitions for indeterminant or 2-ventricle morphology are not standardized, we considered these few patients as having LV morphology. Another limitation was incomplete follow-up because a large number of patients live remotely from our center and receive their post-Fontan care elsewhere. However, survival and transplant follow-up data were obtained from our electronic medical records, the National Death Index, and SRTR, allowing us to achieve a99.9% follow-up rate. Further, we were able to analyze a total of 5,140 post-Fontan echocardiogram reports, which yielded sufficient statistical power. Because of the incomplete follow-up of our cases, we were not able to perform analysis of the effects of post-Fontan comorbidities on the Fontan outcomes. Considering 63% undetected transplant (n = 27) occurred outside of our institution, other comorbidities may be underestimated as well. Not all patients with a single ventricle have achieved Fontan completion. Given the incidence of interstage death and failing Glenn physiology, there might be a selection bias for this cohort.
CONCLUSIONS
The single morphologic RV is negatively associated with long-term survival following the Fontan procedure, possibly due to a tendency toward progressive AVVR and deterioration of ventricular function. Additional volume overload caused by AVVR is not well-tolerated in the pressure-overloaded, single RV, and accelerates ventricular dysfunction, implying that valve intervention before deteriorating ventricular function might be beneficial, provided that such intervention can be performed with acceptable risk.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Following the Fontan operation for patients with single ventricle congenital defects, progressive atrioventricular (AV) valve regurgitation can lead to deterioration of morphological right ventricular function with an adverse impact on long-term survival.
TRANSLATIONAL OUTLOOK:
Further studies are needed to determine whether early intervention to reduce AV valve regurgitation following the Fontan procedure could prevent or retard deterioration of ventricular function and improve long-term outcomes.
ACKNOWLEDGMENTS
The authors thank the support for data analysis provided by Consulting for Statistics, Computing, and Analytics Research of University of Michigan, as well as Jeremy Wolverton and Leslie Wacker for the database creation.
Dr. Si has received funding from the National Heart, Lung, and Blood Institute (NHLBI) (K08HL146351). Dr. Likosky has received funding from the Agency for Healthcare Research and Quality (AHRQ; R01HS026003) and NHLBI (HL146619-01A1). Opinions expressed in this manuscript do not represent those of NHLBI, AHRQ, or the U.S. Department of Health and Human Services. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. government.
ABBREVIATIONS AND ACRONYMS
- AVVR
atrioventricular valve regurgitation
- BDG
bidirectional Glenn
- CI
confidence interval
- CPB
cardiopulmonary bypass
- HFP
Hemi-Fontan procedure
- HLHS
hypoplastic left heart syndrome
- HR
hazard ratio
- IQR
interquartile range
- LV
left ventricle
- OR
odds ratio
- RV
right ventricle
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
APPENDIX For the supplemental figure, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
REFERENCES
- 1.van der Ven JPG, van den Bosch E, Bogers A, Helbing WA. State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Res 2018;7:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RH, Franklin RCG, Spicer DE. Anatomy of the functionally univentricular heart. World J Pediatr Congenit Heart Surg 2018;9:677–84. [DOI] [PubMed] [Google Scholar]
- 3.Günthel M, Barnett P, Christoffels VM. Development, proliferation, and growth of the mammalian heart. Mol Ther 2018;26:1599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spicer DE, Bridgeman JM, Brown NA, Mohun TJ, Anderson RH. The anatomy and development of the cardiac valves. Cardiol Young 2014;24: 1008–22. [DOI] [PubMed] [Google Scholar]
- 5.Tedford R. Determinants of right ventricular afterload (2013 Grover Conference series). Pulm Circ 2014;4:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmley WW, Tyberg JV, Glantz SA. Cardiac dynamics. Annu Rev Physiol 1977;39:277–99. [DOI] [PubMed] [Google Scholar]
- 7.Filippov AA, Del Nido PJ, Vasilyev NV. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation 2016;134: 1293–302. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg 2008;248: 402–10. [DOI] [PubMed] [Google Scholar]
- 9.Alsoufi B, Gillespie S, Kim D, et al. The impact of dominant ventricle morphology on palliation outcomes of single ventricle anomalies. Ann Thorac Surg 2016;102:593–601. [DOI] [PubMed] [Google Scholar]
- 10.Fauziah M, Lilyasari O, Liastuti LD, Rahmat B. Systemic ventricle morphology impact on ten-year survival after Fontan surgery. Asian Cardiovasc Thorac Ann 2018;26:677–84. [DOI] [PubMed] [Google Scholar]
- 11.Oster ME, Knight JH, Suthar D, Amin O, Kochilas LK. Long-term outcomes in single-ventricle congenital heart disease. Circulation 2018;138:2718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmi M, Hickey EJ, Williams WG, Van Arsdell G, Caldarone CA, McCrindle BW. Long-term tricuspid valve function after Norwood operation. J Thorac Cardiovasc Surg 2011;142:1341–7.e4. [DOI] [PubMed] [Google Scholar]
- 13.Kutty S, Colen T, Thompson RB, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging 2014;7:765–72. [DOI] [PubMed] [Google Scholar]
- 14.Bautista-Hernandez V, Brown DW, Loyola H, et al. Mechanisms of tricuspid regurgitation in patients with hypoplastic left heart syndrome undergoing tricuspid valvuloplasty. J Thorac Cardiovasc Surg 2014;148:832–8; discussion 838–40. [DOI] [PubMed] [Google Scholar]
- 15.Stamm C, Anderson RH, Ho SY. The morphologically tricuspid valve in hypoplastic left heart syndrome. Eur J Cardiothorac Surg 1997;12: 587–92. [DOI] [PubMed] [Google Scholar]
- 16.Downing TE, Allen KY, Glatz AC, et al. Long-term survival after the Fontan operation: twenty years of experience at a single center. J Thorac Cardiovasc Surg 2017;154:243–53.e2. [DOI] [PubMed] [Google Scholar]
- 17.d’Udekem Y, Iyengar AJ, Galati JC, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014;130:S32–8. [DOI] [PubMed] [Google Scholar]
- 18.Pundi KN, Johnson JN, Dearani JA, et al. 40-Year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol 2015;66:1700–10. [DOI] [PubMed] [Google Scholar]
- 19.Ono M, Kasnar-Samprec J, Hager A, et al. Clinical outcome following total cavopulmonary connection: a 20-year single-centre experience. Eur J Cardiothorac Surg 2016;50:632–41. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch-Romano JC, Bove EL, Si M-S, Ohye RG. Modified hemi-Fontan procedure. Oper Tech Thorac Cardiovasc Surg 2013;18:117–23. [Google Scholar]
- 21.Lee TM, Aiyagari R, Hirsch JC, Ohye RG, Bove EL, Devaney EJ. Risk factor analysis for second-stage palliation of single ventricle anatomy. Ann Thorac Surg 2012;93:614–8; discussion 619. [DOI] [PubMed] [Google Scholar]
- 22.Mery CM, De Leon LE, Trujillo-Diaz D, et al. Contemporary outcomes of the Fontan operation: a large single-institution cohort. Ann Thorac Surg 2019;108:1439–46. [DOI] [PubMed] [Google Scholar]
- 23.Bove EL, de Leval MR, Migliavacca F, Balossino R, Dubini G. Toward optimal hemodynamics: computer modeling of the Fontan circuit. Pediatr Cardiol 2007;28:477–81. [DOI] [PubMed] [Google Scholar]
- 24.Restrepo M, Mirabella L, Tang E, et al. Fontan pathway growth: a quantitative evaluation of lateral tunnel and extracardiac cavopulmonary connections using serial cardiac magnetic resonance. Ann Thorac Surg 2014;97:916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart RD, Pasquali SK, Jacobs JP, et al. Contemporary Fontan operation: association between early outcome and type of cavopulmonary connection. Ann Thorac Surg 2012;93:1254–60; discussion 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rychik J, Atz AM, Celermajer DS, et al. Evaluation and management of the child and adult with fontan circulation: a scientific statement from the American Heart Association. Circulation 2019. July 1 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T. Long-term results and re-intervention after the Fontan 0peration. Pediatr Cardiol Cardiac Surg 2017;33:362–70. [Google Scholar]
- 28.Alsoufi B, McCracken C, Schlosser B, et al. Outcomes of multistage palliation of infants with functional single ventricle and heterotaxy syndrome. J Thorac Cardiovasc Surg 2016;151: 1369–77.e2. [DOI] [PubMed] [Google Scholar]
- 29.Alsoufi B, McCracken C, Kanter K, Shashidharan S, Border W, Kogon B. Outcomes of multistage palliation of infants with single ventricle and atrioventricular septal defect. World J Pediatr Congenit Heart Surg 2020;11:39–48. [DOI] [PubMed] [Google Scholar]
- 30.Brida M, Diller GP, Gatzoulis MA. Systemic right ventricle in adults with congenital heart disease: anatomic and phenotypic spectrum and current approach to management. Circulation 2018;137:508–18. [DOI] [PubMed] [Google Scholar]
- 31.Si MS, Ohye RG. Stem cell therapy for the systemic right ventricle. Expert Rev Cardiovasc Ther 2017;15:813–23. [DOI] [PubMed] [Google Scholar]
- 32.Chen HY, Li J, Wang CS, Pan CZ, Shu XH. Left heart ejection fraction as a load-independent parameter for patients with mitral regurgitation. J Heart Valve Dis 2017;26:437–46. [PubMed] [Google Scholar]
- 33.Dinh D, Gurney J, Donohue J, et al. Tricuspid valve repair in hypoplastic left heart syndrome. Pediatr Cardiol 2011;32:599–606. [DOI] [PubMed] [Google Scholar]
- 34.King G, Ayer J, Celermajer D, et al. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol 2019;73:810–22. [DOI] [PubMed] [Google Scholar]
- 35.Mahle WT, Gaynor JW, Spray TL. Atrioventricular valve replacement in patients with a single ventricle. Ann Thorac Surg 2001;72:182–6. [DOI] [PubMed] [Google Scholar]
- 36.Alshami N, Sarvestani AL, Thomas AS, St. Louis J, Kochilas L, Raghuveer G. Valve replacement in children with single ventricle physiology. Pediatr Cardiol 2020;41:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


