Abstract
Objective
This study aimed to estimate the cost–utility of sofosbuvir/velpatasvir (SOF/VEL) compared with other direct-acting antivirals (DAAs) in Chinese patients with hepatitis C virus (HCV).
Design
A Markov model was developed to estimate the disease progression of patients with HCV over a lifetime horizon from the healthcare system perspective. Efficacy, clinical inputs and utilities were derived from the published literature. Drug costs were from the market price survey, and health costs for Markov health states were sourced from a Chinese study. Costs and utilities were discounted at an annual rate of 5%. One-way and probabilistic sensitivity analyses were conducted to test the impact of input parameters on the results.
Interventions
SOF/VEL was compared with sofosbuvir+ribavirin (SR), sofosbuvir+dasabuvir (SD), daclatasvir+asunaprevir (DCV/ASV), ombitasvir/paritaprevir/ritonavir+dasabuvir (3D) and elbasvir/grazoprevir (EBR/GZR).
Primary and secondary outcomes
Costs, quality-adjusted life years (QALYs) and incremental cost–utility ratios (ICURs).
Results
SOF/VEL was economically dominant over SR and SD. However, 3D was economically dominant compared with SOF/VEL. Compared with DCV/ASV, SOF/VEL was cost-effective with the ICUR of US$1522 per QALY. Compared with EBR/GZR, it was not cost-effective with the ICUR of US$369 627 per QALY. One-way sensitivity analysis demonstrated that reducing the cost of SOF/VEL to the lower value of CI resulted in dominance over EBR/GZR and 3D. Probabilistic sensitivity analysis demonstrated that 3D was cost-effective in 100% of iterations in patients with genotype (GT) 1b and SOF/VEL was not cost-effective.
Conclusions
Compared with other oral DAA agents, SOF/VEL treatment was not the most cost-effectiveness option for patients with chronic HCV GT1b in China. Lower the price of SOF/VEL will make it cost-effective while simplifying treatment and achieving the goal of HCV elimination.
Keywords: health economics, hepatology, public health
Strengths and limitations of this study.
To our knowledge, this is the first study including all available all-oral direct-acting antivirals (DAAs) for the treatment of patients with hepatitis C virus (HCV) GT1b and comparing the cost-effectiveness of sofosbuvir/velpatasvir with all other DAAs in the Chinese setting.
Some of the parameters were retrieved from the published literature due to the absence of the real-world data in China, which may result in some bias on our results.
Only the HCV GT1b was considered in this study, other genotypes were not included, which may restrict the generalibility of findings in this study.
Introduction
Chronic hepatitis C (CHC) is a major public health problem worldwide. It is estimated that there are around 71 million individuals chronically infected with hepatitis C virus (HCV), leading to approximately 399 000 deaths each year.1 2 In China, the number of patients infected with HCV was estimated to be approximate 10 million in 2006, and the most prevalent genotype (GT) is HCV GT1b (56.8%), followed by GT2 (15.8%), GT3 (8.7%) and GT6 (5.7%).3 4 The Chinese Center for Disease Control and Prevention reported that the incidence was showing an increasing trend, with an estimated 200 000 new cases annually from 2014 to 2018. The undiagnosed and untreated patients infected with chronic HCV are likely to develop serious liver-related complications such as decompensated cirrhosis (DC) and hepatocellular carcinoma (HCC), leading to substantial clinical and economic burden.5 6
The endpoint of treating HCV infection is achieving sustained virologic response (SVR), which can significantly reduce the risk of liver disease progression and avoid conversion to end-stage liver diseases.7 Patients achieving SVR are associated with lower costs and improved quality of life (QOL).8 Therefore, the treatment of HCV and achievement of SVR are of critical significance in reducing the health and economic burdens among patients with CHC.
For decades, the standard of care for patients infected with HCV in China has been based on pegylated interferon+ribavirin (PR) therapy, which is associated with low efficacy, long treatment durations, poor tolerability and much adverse event rates, especially in cirrhotic patients.9 The introduction of direct-acting antivirals (DAAs), with improved SVR and fewer side effects, has revolutionised HCV treatment. The latest Chinese guideline has suggested that DAA regimens should be applied if patients could afford medical expenses.9 In recent years, a range of drugs have been approved for HCV treatment by the Chinese State Food and Drug Administration. These all-oral regimens for patients infected with HCV, including sofosbuvir+ribavirin (SR), sofosbuvir+daclatasvir (SD), daclatasvir+asunaprevir (DCV/ASV), ombitasvir/paritaprevir/ritonavir+dasabuvir (3D), elbasvir/grazoprevir (EBR/GZR) and sofosbuvir/velpatasvir (SOF/VEL), have been currently available in China. All these interferon-free regimens resulted in higher efficacy and shorter duration, compared with interferon-based regimens.10 11 Specially, SOF/VEL was listed in the National Essential Medicines List of China as the only full-oral, direct anti-HCV drug in 2018. Unlike other DAAs, SOF/VEL is a pan-genotypic drug, which is the first fixed-dosage regimen able to achieve high rates of SVR, after only 12 weeks of treatment across all GTs, all fibrosis scores and typologies of patients.12
At the present, it is not clear whether SOF/VEL is cost-effective in Chinese patients with HCV GT1b. Therefore, the objective of this study was to estimate the cost-effectiveness of SOF/VEL compared with other available DAA regimens for treatment of patients with hepatitis HCV GT1b.
Methods
The Markov model can simulate the progression of patient with HCV through the natural history of HCV and treatment. We used a state transition Markov model to estimate the economic benefits of SOF/VEL regimen in Chinese patients with HCV GT1b, from the healthcare system perspective. The model simulated the disease progression of patients with HCV who received treatment with SOF/VEL or comparators. The model used an annual cycle length and a lifetime horizon. A discount rate of 5% was used for costs and utilities in this model, which was based on the recommendations in the China guideline for pharmacoeconomic evaluations.
Patients characteristics
The base-case population in the model represented treatment-naive patients infected with HCV GT1b, the major GT in China. According to a Chinese study, the mean age of Chinese patients infected with HCV were 44.5 years,4 so it was assumed that the patients entered the model at the age of 45 years old in this study. The baseline distribution was defined by the METAVIR (a fibrosis score system) fibrosis stages: no fibrosis (F0), portal fibrosis with no septa (F1), portal fibrosis with few septa (F2), numerous septa without cirrhosis (F3) and compensated cirrhosis (F4).13 Based on another Chinese investigation,14 the fibrosis distribution was as follows: F0 (0.8%), F1 (45.5%), F2 (41.3%), F3 (9.9%) and F4 (2.5%), respectively. Coinfection with HBV or HIV was not included. Due to the small proportion of treatment-experienced patients, only the treatment-naïve patients were considered.
Model structure and assumptions
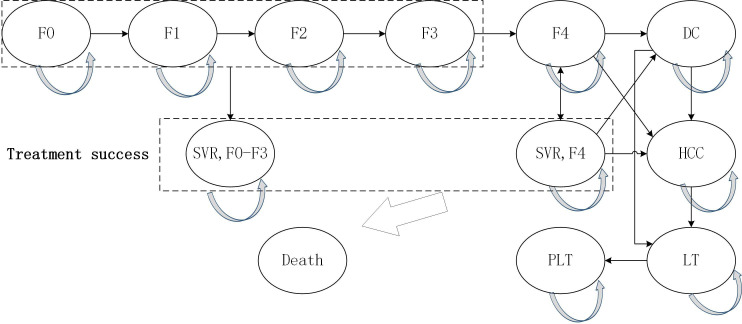
The structure of the model was based on other models of HCV disease, which have been published and validated in health economic analyses.15–17 The model consisted of 14 health states (figure 1). Fibrosis stage was defined by the METAVIR fibrosis scoring system and it was assumed that patients enter the model with a given fibrosis score: F0, F1, F2, F3 and F4. Patients may develop the more serious liver fibrosis, advanced liver disease (ie, DC, HCC and LT) or may keep that health state. If patients achieved SVR after the successful treatment, the disease progression was to halt. However, it allowed for the transitions from SVR to DC or HCC for patients with cirrhosis (F4) at a lower rate. Patients at the stage of compensated cirrhosis (F4) were at risk of developing DC or HCC. If a patient developed DC and/or HCC, then the patient may receive a liver transplant. Patients with advance liver disease had higher mortality rates than other patients. All the other patients had the same mortality rate as the general population.
Figure 1.
Model structure. DC, decompensated cirrhosis; F0–F4: METAVIR liver fibrosis scores, F0 (no fibrosis), F1 (portal fibrosis with no septa), F2 (portal fibrosis with fewsepta), F3 (numerous septa without cirrhosis), F4 (and compensated cirrhosis); HCC, hepatocellular carcinoma; LT, liver transplant (first year); PLT, post liver transplant (subsequent years); SVR, sustained virologic response.
It was assumed that there was no disease progression during treatment. Only cirrhotic patients (F4) could progress to DC and HCC and it still had risk of DC and HCC even if they achieved SVR. Adverse events were not considered due to the minimal rates in these interferon-free regimens.
Model comparators and clinical inputs
DCV+ASV, SOF/VEL, EBR/GZR, 3D and SOF-based regimens (SR: the combination of SOF and RBV; SD: the combination of SOF and dasabuvir) are all recommended for chronic GT1b infection in the Chinese setting. The duration of DCV+ASV and SR is 24 weeks, and for the other three regimens is 12 weeks. The treatment effectiveness was defined as SVR. The SVRs of DCV+ASV, SOF/VEL, EBR/GZR and 3D were derived from international multicenter clinical trials.18–22 The SVR of SD was derived from a systematic review.23 The SVR of SR was obtained from a clinical trial in the Chinese setting.24 The clinical inputs are shown in table 1.
Table 1.
Model inputs
| Parameter | Base case | Lower limit | Higher limit | Distribution | Parameter 1 | Parameter 2 | Reference |
| SVR rates | |||||||
| SOF/VEL, NC | 0.984 | 0.960 | 0.996 | Uniform | 0.960 | 0.996 | 18 |
| SOF/VEL, C | 0.958 | 0.789 | 0.999 | Uniform | 0.789 | 0.999 | 18 |
| EBR/GZR, NC | 0.980 | 0.937 | 1 | Uniform | 0.937 | 1 | 19 |
| EBR/GZR, C | 1 | 0.937 | 1 | Uniform | 0.937 | 1 | 19 |
| 3D, NC | 0.993 | 0.976 | 1 | Uniform | 0.976 | 1 | 20 |
| 3D, C | 0.942 | 0.892 | 0.991 | Uniform | 0.892 | 0.991 | 21 |
| DCV/ASV, NC | 0.889 | 0.85 | 0.94 | Uniform | 0.85 | 0.94 | 22 |
| DCV/ASV, C | 0.911 | 0.85 | 0.94 | Uniform | 0.85 | 0.94 | 22 |
| SD, NC | 0.98 | 0.95 | 1 | Uniform | 0.95 | 1 | 23 |
| SD, C | 0.98 | 0.95 | 1 | Uniform | 0.95 | 1 | 23 |
| SR, NC | 0.94 | 0.87 | 0.99 | Uniform | 0.87 | 0.99 | 24 |
| SR, C | 0.94 | 0.87 | 0.99 | Uniform | 0.87 | 0.99 | 24 |
| Annual transition probabilities | |||||||
| Fibrosis progression | |||||||
| F0–F1 | 0.117 | 0.104 | 0.130 | Beta | 274.98 | 2075.30 | 25 |
| F1–F2 | 0.085 | 0.075 | 0.096 | Beta | 210.06 | 2261.18 | 25 |
| F2–F3 | 0.120 | 0.109 | 0.133 | Beta | 288.05 | 2112.38 | 25 |
| F3–F4 | 0.116 | 0.104 | 0.129 | Beta | 270.61 | 2062.22 | 25 |
| Cirrhosis progression | |||||||
| F4-DC | 0.039 | 0.010 | 0.079 | Beta | 3.51 | 86.48 | 26 |
| F4-HCC | 0.014 | 0.010 | 0.079 | Beta | 0.18 | 12.38 | 26 |
| F4-SVR to DC | 0.003 | 0.002 | 0.004 | Beta | 96 | 31 821 | 27 |
| F4-SVR to DC | 0.006 | 0.005 | 0.007 | Beta | 95 | 15 814 | 27 |
| Liver disease progression | |||||||
| DC-HCC | 0.068 | 0.054 | 0.082 | Beta | 89 | 1226 | 28 |
| Receiving liver transplant | |||||||
| DC-LT | 0.0003 | 0.0002 | 0.0011 | Beta | 0 | 0.1 | 29 |
| HCC-LT | 0.0005 | 0.0 | 0.0024 | Beta | 4.1 | 8788.8 | 29 |
| Mortality rates | |||||||
| DC-death | 0.129 | 0.1032 | 0.5124 | Beta | 147.03 | 983.97 | 28 |
| HCC-death | 0.427 | 0.3416 | 0.5124 | Beta | 117.1 | 155.23 | 28 |
| LT-death | 0.116 | 0.060 | 0.420 | Beta | 1.3 | 9.9 | 30 |
| PLT-death | 0.044 | 0.060 | 0.420 | Beta | 4.7 | 101.6 | 30 |
| Drug costs | Local charge | ||||||
| SOF/VEL | 10 087 | 7565 | 12 608 | ||||
| EBR/GZR | 8548 | 6411 | 10 685 | ||||
| 3D | 8546 | 6409 | 10 682 | ||||
| DCV/ASV | 8378 | 6283 | 10 472 | ||||
| SD | 12 302 | 9226 | 15 377 | ||||
| SR | 17 096 | 12 822 | 21 370 | ||||
| Annual health state costs | |||||||
| F0–F3 | 992 | 671 | 1313 | Gamma | 6002 | 0.165 | 31 |
| F4 | 2823 | 1001 | 4646 | Gamma | 8570 | 0.329 | 31 |
| DC | 6287 | 3820 | 8755 | Gamma | 31 403 | 0.200 | 31 |
| HCC | 13 272 | 9544 | 17 000 | Gamma | 92 610 | 0.143 | 31 |
| LT (first year) | 56 719 | 40 983 | 81 956 | Gamma | 308 255 | 0.184 | 32 |
| LT (subsequent years) | 9016 | 8196 | 10 077 | Gamma | 170 113 | 0.053 | 32 |
| Utilities | |||||||
| F0–F3 | 0.790 | 0.632 | 0.948 | Beta | 19.4 | 5.2 | 33 35 |
| F4 | 0.748 | 0.598 | 0.898 | Beta | 23.5 | 7.9 | 33 35 |
| DC | 0.672 | 0.538 | 0.806 | Beta | 30.8 | 15.0 | 33 35 |
| HCC | 0.610 | 0.488 | 0.732 | Beta | 36.8 | 23.6 | 33 35 |
| LT (first year) | 0.560 | 0.520 | 0.780 | Beta | 33.0 | 17.8 | 33 35 |
| LT (subsequent years) | 0.709 | 0.567 | 0.851 | Beta | 27.2 | 11.2 | 33 35 |
| Post SVR | 0.87 | 0.65 | 1 | Uniform | 0.65 | 1 | 34 |
ASV, asunaprevir; C, cirrhotic; 3D, ombitasvir/paritaprevir/ritonavir+dasabuvir; DC, decompensated cirrhosis; DCV, daclatasvir; EBR, elbasvir; F0–F4, METAVIR liver fibrosis scores, F0 (no fibrosis), F1 (portal fibrosis with no septa), F2 (portal fibrosis with few septa), F3 (numerous septa without cirrhosis), and F4 (compensated cirrhosis); GZR, grazoprevir; HCC, hepatocellular carcinoma; LT, liver transplant; NC, non-cirrhotic; PLT, post liver transplant; SD, sofosbuvir+daclatasvir; SOF, sofosbuvir; SR, sofosbuvir+ribavirin; SVR, sustained virologic response; VEL, velpatasvir.
Transition probabilities
The transition probabilities are shown in table 1. The rates of fibrosis progression between F0 and F4 were derived from a meta-analysis.25 The probability from F4 to DC and HCC and from DC to HCC were estimated from the published literature.26 27 Patients achieving SVR were assumed to develop DC or HCC at a lower rate according to a prospective study.28 The probabilities of liver transplantation of DC or HCC were obtained from the published studies, in which the proportion of liver transplantation was derived from a previous study and was adjusted based on the donation rate ratio between Chinese (0.6 per million) and individuals of western countries (34.4 per million).29 The mortality rates associated with DC, HCC, liver transplantation in first year and liver transplantation in subsequent years, which were higher than general mortality, were sourced from the published literature.28 30 Age-specific all-cause mortality rates were obtained from the life tables of the WHO member states.
Direct medical costs
The Chinese healthcare perspective was adopted in this study. All costs were inflated to 2019 using the China Consumer Price Index and converted to US dollars using official exchange rates as of 2019 (US$1=¥6.90). The medical costs consisted of drug costs and liver-related health state costs (table 1). Drug costs were based on local charges without discounts because the majority of DAAs were not included in the national drug reimbursement list. The annual costs of F0–F4, DC and HCC were derived from a survey of patients infected with HCV in China, which included costs of liver-related care (eg, laboratory tests, procedures, medications and hospitalisations).31 The annual costs associated with liver transplant and post liver transplant were obtained from a study in the context of China.32 Patients after SVR were assumed to incur no medical costs. Future costs were discounted at 5% per year.
Health utilities
Utility weights for each health state of liver disease were mainly obtained from a published systematic review.33 The QOL of patients with SVR was based on a published study.34 Disutility was not considered during the therapy. The utilities are shown in table 1.
Model analysis
Costs and quality-adjusted life years (QALYs) were discounted at 5% per year. Incremental cost–utility ratios (ICURs) were reported to show cost–utility of SOF/VEL regimen relative to the comparator. We calculated ICURs by dividing the incremental costs by the incremental QALYs for the SOF/VEL regimen compared each of the comparators. In cases in which the SOF/VEL regimen was less costly and more effective than a comparator, it was concluded to be economically dominant. In other cases, ICURs were reported. US$28 106/QALY, the three times Chinese gross domestic product per capita, was used to be the willingness to pay threshold. In cases, the ICUR of SOF/VEL was lower than US$28 106/QALY, it was regarded as cost-effective. Otherwise, it was not cost-effective.
Sensitivity analysis
One-way sensitivity analyses were conducted to test the effect of varying input parameters on the ICUR of SOF/VEL treatment regimen compared with the comparator. The efficacy, costs, progression rates, utilities and discount rates were tested under the ranges defined in the inputs tables (table 1). The 95% CI of each parameter was used to be the varying range; in case the 95% CI was not available, the 25% of parameter would be used. In addition, discount rate varied ranging from 3% to 5%. The results were presented by tornado diagrams.
A probabilistic sensitivity analysis (PSA) was conducted in which all the input parameters were varied simultaneously. Inputs were sampled from predefined distributions with 1000 iterations (table 1). The key parameters of each specific distribution were calculated from the mean and SE. Beta distribution was applied to transition probabilities and utilities. Gamma distribution was applied to costs. Uniform distribution was applied in which the parameters were not available. The results of the PSA were presented using cost-effectiveness acceptability curves, which reflect the probability that the regimens will be cost-effective at various willingness-to-pay thresholds.
Patient and public involvement
The research study did not involve any direct patient and public involvement.
Results
Base-case analysis
The results of the base-case are presented in table 2. Compared with SD and SR, SOF/VEL was dominant with higher effectiveness and lower cost. The ICURs of SOF/VEL versus DCV/ASV was US$1522, which was lower than the threshold of US$28 106/QALY. The ICURs of SOF/VEL versus EBR/GZR was US$369 627, which exceeded the threshold. Compared with 3D, SOF/VEL was dominated with higher costs and fewer QALYs. All in all, 3D is the most effective strategy in patients with HCV GT1b, followed by the EBR/GZR, SOF/VEL strategies.
Table 2.
Base-case results
| Treatment regimen | Discounted costs (US$) | Discounted QALYs | Incremental costs (US$) | Incremental QALYs | ICUR, SOF/VEL (US$/QALY) |
| SR | 19 118 | 13.3342 | −7716 | 0.0921 | Dominant |
| SD | 13 727 | 13.4207 | −2325 | 0.0056 | Dominant |
| DCV/ASV | 11 155 | 13.2262 | 247 | 0.2001 | 1234 |
| 3D | 9792 | 13.4435 | 1510 | −0.0172 | dominated |
| EBR/GZR | 9966 | 13.4228 | 1436 | 0.0040 | 359 000 |
| SOF/VEL | 11 402 | 13.4263 | – | – | – |
SOF/VEL is considered as the reference treatment.
ASV, asunaprevir; 3D, ombitasvir/paritaprevir/ritonavir+dasabuvir; DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; ICUR, incremental cost–utility ratio; QALY, quality-adjusted life year; SD, sofosbuvir+daclatasvir; SOF, sofosbuvir; SR, sofosbuvir+ribavirin; VEL, velpatasvir.
The treatment costs among regimens ranged from US$9792 to US$19 118 (difference US$9326) and QALYs ranged from 13.2262 to 13.4435 (difference 0.2173). Costs were lowest for 3D and highest for SR; QALYs were highest for 3D and lowest for DCV/ASV. Compared with SOF-based regimens (SR and SD), the second-generation DAAs (3D, EBR/GZR, SOF/VEL and DCV/ASV) resulted in fewer costs and more QALYs.
Deterministic sensitivity analysis
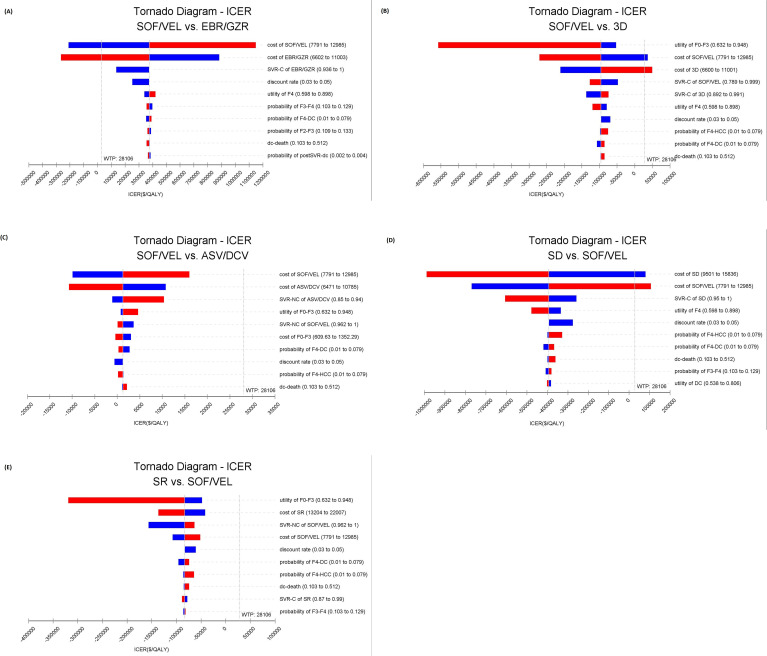
The 10 input parameters, to which ICURs were most sensitive, were presented in tornado diagrams (figure 2). ICURs were most sensitive to SVR rates and drug costs. However, only the cost of drugs can lead to changes of results. Reducing the cost of SOF/VEL to the lower bound of CI, US$8701, resulted in dominance over EBR/GZR. SOF/VEL was dominated by 3D in the base analysis, but reducing the cost of SOF/VEL to the lower value of CI of US$7945, resulted in SOF/VEL dominating the comparator of 3D. However, compared with DCV/ASV and SR, SOF/VEL was cost-effective no matter what parameter changes within the given range were.
Figure 2.
Tornado diagrams showed the impact of lower and upper values of each parameter in incremental cost-effectiveness ratio of SOF/VEL over other DAAs. (A) SOF/VEL versus EBR/GZR. (B) SOF/VEL versus 3D. (C) SOF/VEL versus ASV/DCV. (D) SD versus SOF/VEL. (E) SR versus SOF/VEL. The effect of 10 influential variables is shown. Each bar shows the variation in ICER, blue colour, low value; red colour, high value. ASV, asunaprevir; C, cirrhotic; 3D, ombitasvir/paritaprevir/ritonavir+dasabuvir; DAAs, direct-acting antivirals; DC, decompensated cirrhosis; DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; HCC, hepatocellular carcinoma; ICER: incremental cost-effectiveness ratio; NC, non-cirrhotic; QALY, quality-adjusted life year; SD, sofosbuvir+daclatasvir; SOF, sofosbuvir; SR, sofosbuvir+ribavirin; SVR, sustained virologic response; VEL, velpatasvir; WTP, willingness to pay.
Probabilistic sensitivity analysis
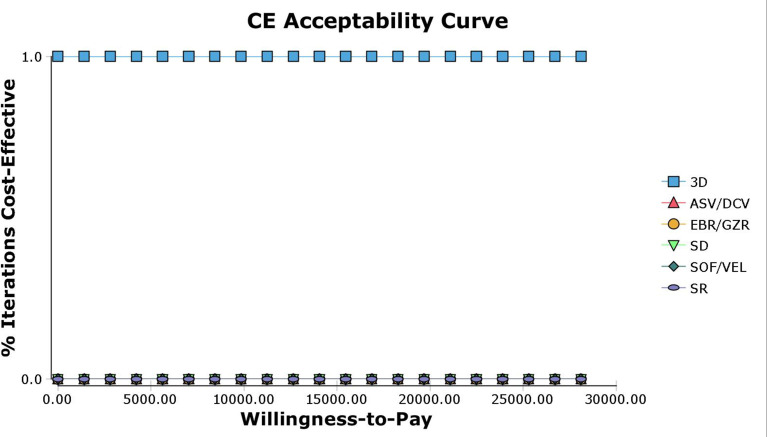
The result of probability sensitivity analysis was consistent with the base-case results. The cost-effectiveness acceptability curve showed that 3D was to be cost-effective in 100% of the 1000 PSA iterations run, at a willingness-to-pay threshold up to US$28 106/QALY (figure 3). The probabilities that SOF/VEL and other DAA regimens would be cost-effective were 0%.
Figure 3.
Acceptability curves comparing the cost-effectiveness of different competing strategies. ASV, asunaprevir; CE, cost-effectiveness; 3D, ombitasvir/paritaprevir/ritonavir+dasabuvir; DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; SD, sofosbuvir+daclatasvir; SOF, sofosbuvir; SR, sofosbuvir+ribavirin; VEL, velpatasvir.
Discussion
This study evaluated the cost–utility of all DAAs used among patients with HCV GT1b in China. The base-case results showed that SOF/VEL was economically dominant relative to SR and SD. Compared with DCV/ASV, SOF/VEL was also more cost-effective. However, relative to EBR/GZR, SOF/VEL was not cost-effectiveness in patients with HCV GT1b. 3D was dominant over SOF/VEL and all other DAAs regimens.
To our knowledge, this is the first study including all available all-oral DAAs for the treatment of patients with HCV GT1b and comparing the cost-effectiveness of SOF/VEL with all other DAAs in the Chinese setting. It is more comprehensive and practical than the previous study. Previous analyses in China have evaluated the cost-effectiveness of DAA regimen in patients with HCV GT1b. Chen H and Chen L35, and Chen GF et al31 compared DAAs with PR; however, the results of these two analyses may have potential deviation as the drug costs were from foreign countries because DAAs had not been approved in China when they did their studies. In another analysis, Liu et al conducted the cost-effectiveness of DCV/ASV with PR, and the result showed that DCV/ASV were cost-effective relative to PR-based treatment or general interferon treatment.29 Another study indicated that EBR/GZR was more cost-effectiveness than DCV/ASV.36 In addition, Wu et al evaluated cost-effectiveness of DAAs including DCV/ASV, 3D, SR and SD, which showed 3D was the most effective in Chinese patients with GT1b.32
There are several cost-effectiveness studies in other countries comparing the SOF/VEL with other all-oral DAA treatments. Corman et al compared SOF/VEL with EBR/GZR, 3D, LDV/SOF by subtype (GT1a or GT1b) and cirrhosis status, the results indicated that SOF/VEL was economically dominant relative to both 3D and LDV/SOF in GT1b treatment-naïve non-cirrhotic patients, whereas SOF/VEL was dominated by EBR/GZR.16 In our study, 3D was dominant compared with SOF/VEL, of which the reason was the cost of 3D was obviously lower than SOF/VEL, contrary to the situation in America. Another study conducted in India evaluated the cost-effectiveness of SOF/VEL versus GT-dependent treatments, the results showed the pan-genotypic SOF/VEL was cost-effective for HCV treatment compared with GT-dependent SD or LDV/SOF,37 which was similar to our analysis.
The sensitivity analysis demonstrated that drug costs could result in significant impacts on the ICURs of SOF/VEL, because the cost of SOF/VEL was 18% higher than the least expensive comparators, 3D and EBR/GZR, and yet the SVR rates difference between these regimens was small. Achieving better cost-effectiveness of SOF/VEL has significant health policy implications in China, where most of the patients with HCV are from rural areas, mainly in the low-income group, and prone to be impoverished due to disease. Because medical technologies and equipment in rural areas are relatively constrained, patients with HCV have to go to hospitals in big cities for diagnosis and treatment. Specifically, only large hospitals in big cities can perform genotyping test. The cost-effectiveness of SOF/VEL may be favourable if great inconvenience and extra direct non-medical expenses in the process of visiting a doctor and curing HCV are considered in the economic evaluation from a broader prospective. Thus, in order to achieve the goal of HCV elimination by 2030 within limited health resources in China, the SOF/VEL regimen, a pan-genotypic DAA treatment, has considerable significances. The pan-genotypic treatment provides ‘an opportunity to simplify the care pathway by removing the need for genotyping, and thus simplifying procurement and supply chains’.38 It does not need to test the GT and METAVIR fibrosis scores, and can be used in patients with all GT and all METAVIR fibrosis stages. Treatment simplification of SOF/VEL is of particular significance in achieving the goal of HCV elimination in China and other developing countries with limited resource. As a result, SOF/VEL was listed in the National Essential Medicines List of China in 2018.
Although the pan-genotypic treatment, SOF/VEL, could simply the process of HCV treatment, the results of this study indicated that it is not the most cost-effective therapy in treating Chinese patients with HCV GT1b from healthcare system. The conclusion is also driven by another cost parameter: the cost of genotyping test of only US$115 in China, which is trivial in comparison to DAA drug cost. If the price of SOF/VEL can be reduced to a reasonable level, more patients will afford this drug, which will make more patients be treated and cured. In addition, it will save much costs for medical insurance payer. In the resource-limited setting, a possible ideal policy option is to reduce the price of SOF/VEL by the negotiation between the government and drug manufacturers, which will make more underserved patients with HCV having access to the treatment. It will be a triple-win situation for medical insurance payer, drug companies and patients.
The analysis has some limitations. First, SVR rates were from several international multicenter clinical trials due to the absence of the effectiveness of real-world clinical setting in China. Although the DAAs have been available since 2017, we still need some time to get the real-world effectiveness data. The future studies will evaluate the real-world effectiveness when data are available. Second, the transition probabilities were also obtained from the international literature, in the absence of Chinese sources, which may result in some bias on our results. Third, the costs were estimated from market prices, and the results may differ from the final discounted prices after negotiated agreements. In addition, SOF/VEL may be the most cost-effective treatment in other GTs; however, our research did not include other GTs. In future studies, we will include other GTs to evaluate the cost-effective of SOF/VEL comprehensively. Finally, the lifetime model was built to simulate the progression of HCV, and the benefits of treatment in preventing transmission was not considered, which may have underestimated the value of HCV treatment.
Conclusion
This modelling study demonstrated SOF/VEL to be cost-effective compared with SR, SD and DCV/ASV, but not cost-effective versus EBR/GZR and 3D in patients with HCV GT1b. The government should negotiate with pharmaceutical companies to bring down the price of SOF/VEL, which will make it cost-effective while simplifying the treatment of HCV and achieving the goal of HCV elimination by 2030.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank our cooperation partners (Professor Qiang Li from Jinan Infectious Disease Hospital and Professor Lei Wang from the Second Hospital of Shandong University) for their help in data collection.
Footnotes
Contributors: HY and XS designed the study. HY, GZ and XS collected the data. HY performed the analyses and drafted the manuscript. XS and LS interpreted the results and edited the manuscript. All authors have read and approved the final manuscript.
Funding: This work was supported by Gilead Sciences Shanghai Pharmaceutical Technology Co., Ltd. The sponsor did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No additional data are available.
References
- 1.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–76. 10.1016/S2468-1253(16)30181-9 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Hepatitis C. fact sheet. Available: http://www.who.int/mediacentre/factsheets/fs164/en/ [Accessed 27 Jul 2019].
- 3.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013;28 Suppl 1:7–10. 10.1111/jgh.12220 [DOI] [PubMed] [Google Scholar]
- 4.Rao H, Wei L, Lopez-Talavera JC, et al. Distribution and clinical correlates of viral and host genotypes in Chinese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol 2014;29:545–53. 10.1111/jgh.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014;39:518–31. 10.1111/apt.12625 [DOI] [PubMed] [Google Scholar]
- 6.Razavi H, Elkhoury AC, Elbasha E, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013;57:2164–70. 10.1002/hep.26218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons B, Saleem J, Heath K, et al. Long-Term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis 2015;61:730–40. 10.1093/cid/civ396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-Palmer J, Cerri K, Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefits. BMC Infect Dis 2015;15:19. 10.1186/s12879-015-0748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinese Society of Hepatology The guidelines of prevention and treatment for chronic hepatitis C (2015 updated version). Chinese Hepatology 2015;33:192–4. [Google Scholar]
- 10.Asselah T, Boyer N, Saadoun D, et al. Direct-Acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int 2016;36 Suppl 1:47–57. 10.1111/liv.13027 [DOI] [PubMed] [Google Scholar]
- 11.Asselah T, Marcellin P. Optimal IFN-free therapy in treatment-naïve patients with HCV genotype 1 infection. Liver Int 2015;35 Suppl 1:56–64. 10.1111/liv.12745 [DOI] [PubMed] [Google Scholar]
- 12.Greig SL. Sofosbuvir/Velpatasvir: a review in chronic hepatitis C. Drugs 2016;76:1567–78. 10.1007/s40265-016-0648-2 [DOI] [PubMed] [Google Scholar]
- 13.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996;24:289–93. 10.1002/hep.510240201 [DOI] [PubMed] [Google Scholar]
- 14.Li J-F, Liu S, Ren F, et al. Fibrosis progression in interferon treatment-naive Chinese plasma donors with chronic hepatitis C for 20 years: a cohort study. Int J Infect Dis 2014;27:49–53. 10.1016/j.ijid.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 15.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015;162:397–406. 10.7326/M14-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-Effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015;162:407–19. 10.7326/M14-1152 [DOI] [PubMed] [Google Scholar]
- 17.Corman S, Elbasha EH, Michalopoulos SN, et al. Cost-Utility of Elbasvir/Grazoprevir in patients with chronic hepatitis C genotype 1 infection. Value Health 2017;20:1110–20. 10.1016/j.jval.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 18.Feld JJ, Jacobson IM, Hézode C, et al. Sofosbuvir and Velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599–607. 10.1056/NEJMoa1512610 [DOI] [PubMed] [Google Scholar]
- 19.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015;163:1–13. 10.7326/M15-0785 [DOI] [PubMed] [Google Scholar]
- 20.Ferenci P, Bernstein D, Lalezari J, et al. Abt-450/R-Ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014;370:1983–92. 10.1056/NEJMoa1402338 [DOI] [PubMed] [Google Scholar]
- 21.Dore GJ, Conway B, Luo Y, et al. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: the MALACHITE-I/II trials. J Hepatol 2016;64:19–28. 10.1016/j.jhep.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 22.Manns M, Pol S, Jacobson IM, et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1B: a multinational, phase 3, multicohort study. Lancet 2014;384:1597–605. 10.1016/S0140-6736(14)61059-X [DOI] [PubMed] [Google Scholar]
- 23.Zhu G-Q, Zou Z-L, Zheng J-N, et al. Systematic review and network meta-analysis of randomized controlled trials. Medicine 2016;95:e3004 10.1097/MD.0000000000003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei L, Xie Q, Hou JL, et al. Sofosbuvir plus ribavirin with or without peginterferon for the treatment of hepatitis C virus: results from a phase 3B study in China. J Gastroenterol Hepatol 2018;33:1168–76. 10.1111/jgh.14102 [DOI] [PubMed] [Google Scholar]
- 25.Thein H-H, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418–31. 10.1002/hep.22375 [DOI] [PubMed] [Google Scholar]
- 26.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463–72. 10.1053/gast.1997.v112.pm9024300 [DOI] [PubMed] [Google Scholar]
- 27.Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology 2011;54:396–405. 10.1002/hep.24370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Planas R, Ballesté B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol 2004;40:823–30. 10.1016/j.jhep.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Wang Z, Tobe RG, et al. Cost effectiveness of daclatasvir plus asunaprevir therapy for Chinese patients with chronic hepatitis C virus genotype 1B. Clin Drug Investig 2018;38:427–37. 10.1007/s40261-018-0621-9 [DOI] [PubMed] [Google Scholar]
- 30.Wolfe RA, Roys EC, Merion RM, et al. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant 2010;10:961–72. 10.1111/j.1600-6143.2010.03021.x [DOI] [PubMed] [Google Scholar]
- 31.Chen G-F, Wei L, Chen J, et al. Will sofosbuvir/ledipasvir (Harvoni) be cost-effective and affordable for Chinese patients infected with hepatitis C virus? an economic analysis using real-world data. PLoS One 2016;11:e0155934. 10.1371/journal.pone.0155934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Wang Z, Xie Q. Cost-effectiveness of novel regimens for Chinese patients with chronic hepatitis C. Curr Med Res Opin 2019;35:847–57. 10.1080/03007995.2018.1546678 [DOI] [PubMed] [Google Scholar]
- 33.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008;28:582–92. 10.1177/0272989X08315240 [DOI] [PubMed] [Google Scholar]
- 34.Thein H-H, Krahn M, Kaldor JM, et al. Estimation of utilities for chronic hepatitis C from SF-36 scores. Am J Gastroenterol 2005;100:643–51. 10.1111/j.1572-0241.2005.40976.x [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Chen L. Estimating cost-effectiveness associated with all-oral regimen for chronic hepatitis C in China. PLoS One 2017;12:e0175189. 10.1371/journal.pone.0175189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Ma A, Liu Q. Cost-Effectiveness of Elbasvir/Grazoprevir versus daclatasvir plus asunaprevir in patients with chronic hepatitis C virus genotype 1B infection in China. Clin Drug Investig 2018;38:1031–9. 10.1007/s40261-018-0702-9 [DOI] [PubMed] [Google Scholar]
- 37.Goel A, Chen Q, Chhatwal J, et al. Cost-effectiveness of generic pan-genotypic sofosbuvir/velpatasvir versus genotype-dependent direct-acting antivirals for hepatitis C treatment. J Gastroenterol Hepatol 2018;33:2029–36. 10.1111/jgh.14301 [DOI] [PubMed] [Google Scholar]
- 38.Organization WH Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. WHO, 2018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.