Abstract
All-solid-state electrolytes have received extensive attention due to their excellent safety and good electrochemical performance. However, due to the harsh conditions of the preparation process, the commercial production of all-solid-state electrolytes remains a challenge. The outbreak of the novel coronavirus pneumonia (COVID-19) has caused great inconvenience to people, while also allowing soft, lightweight and mass-producible non-woven fabrics in masks come into sight. Here, a polymer/polymer solid composite electrolyte is obtained by introducing the polyamide 6 (PA6) microfiber non-woven fabric into PEO polymer through the hot-pressing method. The addition of the PA6 non-woven fabric with lithium-philic properties can not only reduce the crystallinity of the polymer, but also provide more functional transmission sites and then promote the migration of lithium ions at the molecular level. Moreover, due to the sufficient mechanical strength and flexibility of the PA6 non-woven fabric, the composite electrolyte shows excellent inhibition ability of lithium dendrite growth and high electrochemical stability. The novel design concept of introducing low-cost and large-scale production of non-woven fabrics into all-solid-state composite electrolytes to develop high-performance lithium metal batteries is attractive, and can also be broadened to the combination of different types of polymers to meet the needs of various batteries.
Keywords: PA6 microfiber non-woven fabric, Large-scale production, All-solid-state electrolyte, Lithium metal battery
Highlights
-
•
An composite electrolyte based on the PA6 non-woven fabric is successfully prepared.
-
•
Composite electrolyte exhibits superior mechanical strength and interface stability.
-
•
The preparation process can promote the commercialization of lithium batteries.
1. Introduction
With the rapid development of portable electronic equipment and electric vehicle technology, people have put forward higher requirements for the energy density of rechargeable lithium batteries [1,2]. Among the different battery systems that have been developed, all-solid-state lithium metal batteries have attracted strong interest from researchers due to their excellent safety performance and high energy density [3,4]. Using non-flammable all-solid-state electrolyte to replace flammable organic liquid electrolyte can not only completely solve the problem of electrolyte leakage and greatly improve the safety performance of the battery, but also can match the lithium metal anode and the high-voltage cathode to make the battery have a higher energy density [5]. However, using lithium metal with high reactivity as the anode inevitably generates lithium dendrites during the charge and discharge process, which in turn causes the battery to exhibit poor cycle life, low Coulombic efficiency and serious safety hazards [6,7]. In addition, the development of all-solid-state electrolytes with high ionic conductivity, wide electrochemical window, low interfacial impedance and mass production is still the core challenge for achieving high specific energy solid-state batteries [8,9].
In order to solve core issues mentioned above about lithium dendrite growth and poor ion transport characteristics of electrolytes, many kinds of electrolyte materials have been developed [10]. In general, all-solid-state electrolytes can be divided into three aspects including inorganic solid electrolyte, organic solid polymer electrolyte (SPE) and inorganic/polymer solid composite electrolyte (SCE) [11]. Among the above-mentioned different types of solid electrolytes, inorganic solid electrolytes have the most excellent ionic conductivity, and some of the sulfide solid electrolytes can have an ionic conductivity of 10−3 S/cm at room temperature [12,13]. However, the inorganic solid electrolytes that have been developed so far are usually thick, and the problems of poor interface stability with the electrodes and large interface impedance still exists [14]. In addition, the brittleness of the inorganic particles themselves makes even small fluctuations may lead to the destruction of their structures, which greatly hinder the commercialization of all-solid-state batteries [15]. In comparison, SPE exhibits excellent interface stability with the electrode and good processability due to the flexibility of the polymer [16,17]. In the most widely studied polyethylene oxide (PEO) polymer electrolyte, lithium ions can be transported along the PEO molecular chain through the complexation-decomplexation with the ether oxygen bond [18,19]. Nonetheless, because PEO is a semi-crystalline substance, the ionic conductivity of this type of polymer electrolyte at room temperature is poor, making it difficult to meet the requirements of high-performance all-solid-state batteries [20]. Inorganic/polymer SCE as an organic and inorganic composite electrolyte can combine the high ionic conductivity of inorganic solid electrolyte and the excellent interface compatibility of SPE [21]. However, the presence of inorganic ceramics in the two-phase composite SCE may cause lithium ions to be deposited directly inside the electrolyte, which ultimately leads to battery short-circuiting [22]. At the same time, the brittleness problem of the electrolyte as a whole has not been effectively solved, and the high-temperature sintering of the inorganic substances in the preparation process also greatly limits their large-scale preparation for the batteries [23].
The outbreak of the novel coronavirus pneumonia (COVID-19) in 2019 has had a great impact on people all over the world [24,25]. At the same time, it has also brought non-woven fabric into everyone's perspective. As the base fabric of masks, melt blown and spunbonded non-woven fabrics have become the items that major companies are competing to purchase to complete the industrial transformation [26,27]. With the increase in the production capacity of masks, the advantages of non-woven fabrics that can be prepared in large quantities are clearly reflected [28,29]. Compared with ordinary ultrafine fiber production technology such as electrospinning technology, spunbonded technology has the characteristics of wide source of raw materials, simple process, fast production speed, and controllable fiber structure [30]. At the same time, compared with traditional woven fabrics, non-woven fabrics are lighter and softer due to the absence of warp and weft yarns [31,32]. Therefore, combining the excellent flexibility, the controllable fiber structure and the advantages of easy scale production of non-woven fabrics, we considered whether it can be applied to all-solid-state lithium metal batteries to promote the battery industrialization process.
Here, for the first time, we introduce polyamide 6 (PA6) non-woven fabric into PEO polymer to prepare all-solid-state polymer/polymer SCE for lithium metal batteries. The SCE is prepared by combining the bicomponent spunbond-spunlace method and the hot-pressing process, which can promote the commercial production process of all-solid-state batteries. In addition, the introduction of flexible PA6 non-woven fabric as a nanofiller into the polymer electrolyte can not only significantly reduce the crystallinity of the PEO polymer, but also enhance the interfacial wetting ability of the composite electrolyte and improve the interface compatibility between the solid electrolyte and the electrodes. The polar groups (C =O and N–H bonds) on the PA6 molecular chain can also provide additional transmission channels for lithium ions, thereby accelerating the migration of lithium ions inside the electrolyte. Moreover, the increase in the mechanical strength of the solid electrolyte derived from the non-woven fabric can effectively suppress the growth of lithium dendrites, which in turn improves the long-cycle ability of the Li/Li symmetric battery and LiFePO4/Li battery. Benefiting from the controllability of non-woven fabric production technology and the wide range of raw materials, the method of introducing non-woven fabric into the all-solid-state electrolyte to improve the performance of lithium metal batteries can be broadened to other energy fields to promote the large-scale production of batteries.
2. Experiment section
2.1. Fabrication of PA6 microfiber non-woven fabric
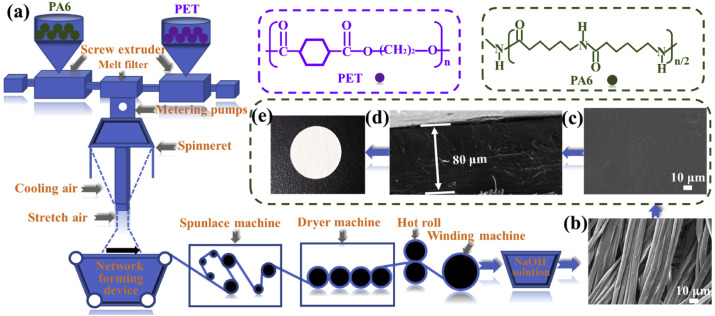
The PA6 microfiber non-woven fabric was made by bicomponent spunbond-spunlace method and the specific preparation process was shown in Fig. 1 a. Firstly, the PA6 and polyethylene terephthalate (PET) polymer chips were placed in vacuum drums at 120 °C and 60 °C, respectively, and dried for 5 h. Secondly, the dried PET and PA6 chips were sent to the screw extruder to make them squeeze and melt, and then the molten slurry was sent to the melt filter and the metering pump in sequence. The functions of the melt filter and the metering pump were to remove impurities in the melt slurry and deliver the melt to the spinneret accurately and evenly. Subsequently, the PET and PA6 polymer melts were transported to the spinning equipment, which includes the spinning box and the spinneret. The function of the spinning box was to maintain the temperature of the spinneret, and the spinneret was used to ensure that the melt was evenly distributed to each spinning hole in preparation for the subsequent spinning process. After that, a fine stream of polymer melt was ejected from the spinneret holes of the spinneret and cooled under the side blowing of a certain temperature to form a web. Finally, the fiber web was sent to the spunlace area after pre-humidification, and the fiber was opened under the action of high-pressure water flow and entangled to form a denser non-woven fabric. After drying the wet web and evaporating most of the water, the final PET/PA6 superfine fiber spunbond-spunlace non-woven fabric was obtained. In order to obtain pure PA6 non-woven fabric, the PET/PA6 bicomponent spunbond-spunlace non-woven fabric was further soaked in NaOH solution to completely remove the PET component by alkali reduction method, in which the PET was dissolved in NaOH solution and the insoluble PA6 component can be left behind.
Fig. 1.
(a) Schematic illustration for the preparation of the PA6 microfiber non-woven fabric. SEM images of the (b) PA6 microfiber non-woven fabric, (c) Top-view and (d) cross-section of the PEO/PA6 30% SCE. (e) Optical picture of the PEO/PA6 30% SCE.
2.2. Fabrication of PEO/PA6 solid composite electrolyte
The PEO/PA6 solid composite electrolytes were obtained by combining the solution casting and hot-pressing technology. Specifically, the PEO polymer electrolyte membrane with a thickness of around 40–60 μm was firstly prepared by casting a mixed solution containing PEO (MW = 600000 g mol−1, Sigma-Aldrich) and bistrifluoromethanesulfonimide lithium salt (LiTFSI, 99.99%, Sigma-Aldrich) on a Teflon plate, where the solvent used was anhydrous acetonitrile (CH3CN, AR grade, Sigma-Aldrich) and the ratio of ethylene oxide [EO] to [LI] was set as 10:1. The stirring time of the mixed solution of PEO and LiTFSI before casting was one whole day, and the drying condition of the PEO polymer electrolyte membrane after casting was set at 60 °C for 24 h. Subsequently, PA6 microfiber non-woven fabric was used as a carrier and PEO films were composited on the top and bottom of the non-woven fabric respectively to obtain the electrolyte membrane with a PEO/PA6/PEO sandwich structure. Then, the PEO/PA6/PEO electrolyte membrane was pressurized at 40 °C and a pressure of 20 MPa for 25 min to prepare a dense PA6 based all-solid-state composite polymer electrolyte with a thickness of about 80–100 μm. In order to better distinguish each electrolyte, the prepared composite electrolyte membrane was named as PEO/PA6 x (x refers to the mass fraction of PA6 microfiber non-woven fabric in the composite electrolyte: 10%, 20%, 30% and 40%).
2.3. Materials characterization
The Quanta 200 scanning electron microscope (SEM) of FEI Corporation was used to take pictures with different magnifications at an acceleration voltage of 10 kV to observe the microtopography of the PA6 non-woven fabric and PEO/PA6 electrolyte membranes. The thermal stability of the composite electrolyte was characterized by a 200 F3 differential scanning calorimeter (DSC), in which the temperature range was from 20 °C to 120 °C and the heating rate was 10 °C/min. The STA409PC comprehensive thermal analyzer of the German NETZSCH company was applied to perform thermogravimetric analysis of electrolytes under N2 environment. The heating range and heating rate were set to 20–800 °C and 10 °C/min respectively. The crystallization performance of the solid electrolyte membranes was measured on the D8 type X-ray diffractometer produced by the German BRUKER company with a scanning angle of 10–50°. The tensile breaking strength of the electrolyte membrane was obtained under the condition of 20 °C and 20% humidity using a YG004 A/N electronic single fiber strength meter at a tensile speed of 20 mm/min.
2.4. Electrochemical characterization
The stainless steel (SS)/electrolyte/SS sandwich batteries were assembled to investigate the ionic conductivities of the solid electrolyte membranes through the electrochemical impedance spectroscopy (EIS) method, where the range of frequency and temperature were set as 106-10−1 Hz and 30–70 °C, respectively. After the EIS test, the specific ionic conductivity value (σ) of the electrolyte can be calculated by the impedance value (R) according to formula (1),
| (1) |
in which L was the thickness of the solid composite electrolyte and S was the area of the SS. The lithium ion transfer number (tLi +) of the composite electrolyte membrane was obtained by applying a 10 mV direct current (DC) polarization voltage to the Li/electrolyte/Li symmetric battery until its current reaches a steady state. The formula used for the calculation of tLi + was as follows
| (2) |
where ΔV was 10 mV, I 0 and Iss were the initial and steady-state current, respectively. R 0 and R ss were the resistance value of the Li/electrolyte/Li symmetric battery measured by EIS method before and after polarization, respectively. The solid electrolyte membrane was sandwiched between SS and lithium foil to characterize its electrochemical stability between 2.5 V and 6 V by linear sweep voltammetry (LSV) test with the scanning speed of 0.001 v/s, where SS was used as the working electrode and lithium foil was used as the reference electrode. All the above measurements were conducted on the CHI 660D electrochemical workstation.
The LAND-BT2013C battery test system was applied to characterize the electrochemical performance of the Li/electrolyte/Li symmetric battery under 0.3 mA cm−2 and the Li/LiFePO4 battery at different rates with the voltage range of 2.5 V–4.2 V. For the preparation of the cathode material used in the Li/LiFePO4 battery, a mixture slurry of the LiFePO4, carbon black, PEO and LiTFSI were dissolved in anhydrous acetonitrile with a mass ratio of 6:1:2:1 in sequence and then cast on the aluminum foil with the doctor blade. After that, the aluminum foil coated with the slurry was dried in a vacuum oven at 60 °C for 36 h to eliminate the influence of the solvent as much as possible. The diameter of the cathode material was fixed as 14 mm through the cutting machine.
3. Results and discussion
The schematic illustration for the preparation of the PA6 microfiber non-woven fabric is shown in Fig. 1a. Firstly, the PET/PA6 non-woven fabric is obtained by the bicomponent spunbond-spunlace method, and then the non-woven fabric is soaked in NaOH solution to remove the PET component by the alkali reduction method, thereby obtaining pure PA6 non-woven fabric. The surface morphology of the prepared PA6 ultra-fine fiber non-woven fabric is shown in Fig. 1b. As can be seen, the fiber diameter distribution in the PA6 non-woven fabric is uniform and most of the fiber are concentrated around 5 μm. After that, the PEO polymer and PA6 non-woven fabric are compounded by hot pressing technology, and the surface morphologies of the PEO/PA6 SCEs with different mass fractions of the PA6 non-woven fabric are presented in Fig. S1. It can be clearly seen that when the content of the PA6 non-woven fabric is 10%, 20% and 30%, the surface of the obtained PEO/PA6 SCEs is relatively smooth, indicating that the PEO polymer is uniformly filled on the surface and inside of the non-woven fabric. However, when the content of the PA6 non-woven fabric is continuously increased to 40%, a pore structure appears on the PEO/PA6 SCE surface, which may be because relatively few PEO polymer cannot completely fill PA6 non-woven fabric. The top-view and cross-section SEM image of the prepared PEO/PA6 30% composite electrolyte is further presented in Fig. 1c and d, respectively. It can be concluded that the composite electrolyte is relatively dense with the thickness of about 80 μm. Since the pores between the fibers in the PA6 non-woven fabric are completely filled, the PEO/PA6 30% composite electrolyte can effectively avoid direct contact between the cathode and lithium anode, thereby preventing the occurrence of short circuits and greatly improving the safety performance of the lithium metal battery. Fig. 1e further shows the optical picture of PEO/PA6 30% SCE. It can be seen that the final prepared all-solid-state composite electrolyte has a regular morphological structure, which confirms the feasibility of the large-scale preparation process.
Ionic conductivity is the primary parameter for evaluating the performance of all-solid-state electrolytes [33]. Since the prepared polymer/polymer SCE is composed of a combination of PEO polymer and PA6 non-woven fabric, the effect of the mass ratio of the two polymers on the ionic conductivity is firstly investigated and shown in Fig. 2 a. The results show that in the temperature range of 30 °C–70 °C, the ionic conductivities of all SCEs are significantly higher than that of pure PEO electrolyte containing only the PEO polymer and lithium salt, indicating that the introduction of PA6 non-woven fabric is conducive to the transport of lithium ions inside the electrolyte. The phenomenon may be due to the fact that the prepared PA6 non-woven fabric is beneficial to promote the local molecular chain movement of PEO polymer, and thus accelerate the complexation-decomplexation of lithium ion and ether oxygen bond on the PEO main chain [34]. Thus, it can be concluded that the ionic conductivity of SCE increases firstly and then decreases with the addition of PA6 non-woven fabric content, and the maximum value among these prepared samples is obtained when the mass fraction of PA6 non-woven fabric is 30%. The reason for the initial increase in lithium ion conductivity may be attributed to the following points: First of all, with the increase of PA6 non-woven fabric content, the number of molecular chains in PEO polymer that can move freely increases, which greatly reduces the barriers to lithium ion transmission. Secondly, the polar functional groups on the PA6 molecular chain including C O and NH2 can provide additional transmission channels for lithium ions, thereby promoting the migration of lithium ions [35]. The subsequent reduce in ionic conductivity of SCE can be explained by the fact that when the content of PA6 non-woven fabric is too high, the relative decrease in the content of PEO polymer mainly transporting lithium ions hinders the further increase of the conductivity of SCE. Fig. 2b is used to visually observe the impedance values of the solid electrolytes at 60 °C. It can be clearly seen that the specific impedance value of PEO/PA6 30% SCE is only 10.8 Ω, which is obviously lower than the 51.5 Ω of pure PEO electrolyte. This result is consistent with the above discussion, indicating that the addition of 30% mass fraction of PA6 non-woven fabric can make the ionic conductivity of the SCE reach the optimal value.
Fig. 2.
Characterization of ionic conductivities and crystallization behavior of all-solid-state electrolytes. (a) Arrhenius plots, (b) impedance spectra under 60 °C, (c) XRD results, and (d) DSC profiles of the PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric.
The XRD patterns are presented in Fig. 2c to characterize the crystallize behavior of the PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric. It shows that the pure PEO electrolyte presents a significant characteristic diffraction peak at both 19.1° and 23.2° [36]. Then, with the addition of PA6 non-woven fabric, the peak intensity of the PEO polymer gradually decreases, and reaches the weakest value when the PA6 non-woven fabric mass fraction is 30% among these prepared samples. Therefore, it can be concluded that the introduction of PA6 non-woven can effectively reduce the crystallinity of PEO polymer, which in turn contributes to the rapid migration of lithium ions. However, when the mass fraction of the non-woven fabric is too high reaching 40%, the signal of the PEO crystallization peak is instead enhanced. This may be because excess PA6 hinders the movement of the local molecular chains of the PEO polymer to a certain extent, thereby reducing the number of freely moving molecular chains in the SCE. The DSC test results in Fig. 2d are consistent with XRD. It can be clearly seen that the pure PEO electrolyte shows a significant endothermic peak at 57.9 °C, while the value and the area of the endothermic peak of SCE containing PA6 non-woven fabric both are reduced [37]. When the mass fraction of PA6 non-woven fabric is 30%, the SCE shows the lowest endothermic peak among the prepared samples at 38.6 °C. This phenomenon well demonstrates the positive effect of the PA6 non-woven fabric on the reduction of the crystallinity of PEO polymer.
The thermal stability of the PA6 non-woven fabric, pure PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric are obtained by TG-DSC measurements. As shown in Fig. 3 a and Fig. S2, all the PEO/PA6 SCEs exhibit similar thermal stability and a significant quality drop appears in the heating range of 320 °C–500 °C, which can be attributed to the thermal decomposition of PEO polymer and PA6 polymer [38]. Therefore, it can be concluded that the introduction of PA6 non-woven fabric did not have a significant impact on the thermal stability of the solid electrolyte, which can still meet the needs of the commercial application of lithium metal batteries [39]. Since the causes of lithium metal battery safety problems mainly include the growth of lithium dendrites and the volume expansion of anodes, it is required that the prepared all-solid-state electrolytes should have sufficient mechanical strength and certain flexibility [40]. In order to evaluate the above related properties, the PA6 non-woven fabric, PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric are subjected to the tensile test. As can be seen in Fig. 3b, the tensile strength of the PA6 non-woven fabric and pure PEO electrolyte is only 2.55 MPa and 1.62 MPa, respectively, while the corresponding value of PEO/PA6 SCEs are higher. Especially, the mechanical strength of PEO/PA6 30% is up to 8.3 MPa with the elongation-at-break of 60.3%. The reason for this phenomenon may be that the PA6 non-woven fabric in the composite electrolyte can provide basic skeleton support, and the PEO polymer filled in the non-woven fabric can enhance the bonding force between the fibers and improve the flexibility of the electrolyte [41]. At the same time, the good combination of the PEO polymer and the non-woven fabric through hot pressing technology can further promote the strength of the overall electrolyte. The superior mechanical stability and elongation mean that the SCE with the PA6 non-woven fabric can not only prevent from being pierced by lithium dendrites, thereby avoiding the occurrence of thermal runaway of the battery, but also can effectively adapt to the expansion of the electrode and always maintain good interface contact between the lithium metal and the electrolyte [42].
Fig. 3.
(a)TG profiles, (b) Stress–strain curves and (C) LSV curves of the PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric. (d) Impedance plots of Li/PEO/Li and Li/PEO/PA6 30%/Li symmetric batteries taken after different storage times at 60 °C.
The electrochemical stability is an important parameter to evaluate the actual performance of the all-solid-state electrolyte [39]. The wide electrochemical window generally means that electrolyte can be matched with high-voltage cathodes [43]. According to the test results in Fig. 3c, the current value of pure PEO electrolyte increases sharply after 4.23 V versus Li+/Li, indicating that the electrolyte begins to decompose under this voltage condition [37]. However, the decomposition voltage of all SCEs composed of PA6 non-woven fabric and PEO polymer is as high as 4.94 V versus Li+/Li. The enhanced electrochemical stability of the SCE can broaden the application range of the battery. In addition to the electrochemical stability, the interface stability between electrolyte and the lithium anode is also a vital indicator that affects the electrochemical performance of the battery [44,45]. EIS method is applied to measure the impedance plots of Li/PEO/Li and Li/PEO/PA6 30%/Li symmetric batteries taken after different storage times at 60 °C. It can be seen from Fig. 3d that the resistance value of the Li/PEO/Li symmetric battery increases continuously from the initial value of 84.5 Ω–167.8 Ω after 15 days, indicating the poor interface stability between the PEO electrolyte and the lithium metal anode. In contrast, the impedance value of the Li/PEO/PA6 30%/Li symmetric battery just experience slightly change from 44.8 Ω to 55.3 Ω. The small change and low value in the aspect of impedance of the PEO/PA6 30% electrolyte can be explained by the following reasons: The first reason is that as shown in Fig. 3b above, the addition of PA6 non-woven fabric can enhance the flexibility of the PEO/PA6 30% SCE, which allows the solid electrolyte always maintain close contact with the lithium metal. Another reason is that the high lithium ion conductivity of the PEO/PA6 30% SCE can ensure the rapid transmission of lithium ions at the interface, thereby effectively reducing the impedance value of the Li/Li symmetric battery. All in all, the presence of PA6 non-woven fabric can give the SCE excellent electrochemical stability and ensure its good contact with lithium metal, which will lay the foundation for the realization of high energy density lithium metal batteries.
Lithium ion transference number (tLi +) is an important index for evaluating the lithium ion migration capacity of electrolyte [46]. The low tLi + may cause serious concentration polarization of the electrolyte during charging and discharging process, resulting in uneven deposition of lithium ions and ultimately affecting the cycle rate performance of the battery [47,48]. According to the Bruce-Vincent formula and the combination of the DC polarization and AC impedance test methods, the tLi + value of the pure PEO electrolyte and PEO/PA6 30% SCE can be obtained [47,49]. Specifically, for the PEO/PA6 30% SCE (Fig. 4 b), the current gradually decreases from the initial 0.10 mA to a stable value of 0.06 mA after 1000 s, and the interface resistance accordingly changes from 47.6 Ω before polarization to 47.9 Ω after polarization. The value of the calculated tLi + of the PEO/PA6 30% SCE is 0.44, which is significantly higher than 0.13 of the pure PEO electrolyte (Fig. 4a). This result well confirms that the addition of PA6 non-woven fabric is beneficial to improve lithium ion migration ability of the solid electrolyte. For one reason, the introduction of PA6 non-woven fabric as a filler into the SCE can effectively suppress the crystallization of PEO polymer, which in turn allows lithium ions to be transported along more amorphous regions [50,51]. For another reason, the polar functional groups (C O and NH2) on the PA6 molecular chain and the two-phase interface between the non-woven fabric and the polymer can provide additional functional sites and fast transmission interfaces for lithium ions [35,52]. Overall, the high tLi + due to the addition of the PA6 non-woven fabric may help to enhance the electrochemical performance of the solid electrolyte.
Fig. 4.
Chronoamperometry profile of Li/Li symmetric batteries assembled with (a) pure PEO electrolyte and (b) PEO/PA6 30% SCE under 60 °C, inset: the impedance plots of the batteries before and after polarization.
As a major issue affecting the safety performance of lithium metal batteries, the continuous growth of lithium dendrites can cause battery short circuits and thermal runaway [53]. In order to evaluate the ability of the all-solid-state electrolyte to hinder the lithium dendrites growth, a constant current charge and discharge test at 0.3 mA cm−2 and 60 °C is performed on the Li/PEO/Li and Li/PEO/PA6 30%/Li symmetric batteries. As can be clearly seen in Fig. 5 a, the Li/PEO/PA6 30%/Li symmetric battery exhibits excellent cycle stability, in which the voltage can be maintained between −0.11 V and 0.11 V for about 1000 h without significant changes. On the contrary, the voltage value of the Li/PEO/Li battery increases with time before 170 h, and then violently oscillates around 200 h (Fig. 5b). The excellent cycle stability of the Li/PEO/PA6 30%/Li battery can be attributed to the high ionic conductivity and sufficient mechanical strength of the PEO/PA6 30% SCE, which can promote the rapid transmission of lithium ions and effectively suppress the lithium dendrites growth. At the same time, the excellent flexibility of PA6 non-woven fabric can also ensure that the solid electrolyte and the lithium metal anode are always maintained in good contact during the cycle as presented in Fig. 5c. It can be seen that the initial impedance of the battery is 82.4 Ω, and the impedance only increases to 88.4 Ω after 1000 h of cycling. The rather small change in interface impedance means excellent interface compatibility can be obtained between the electrolyte and the lithium anode.
Fig. 5.
The lithium dendrites growth inhibition ability of the all-solid-state electrolyte. Galvanostatic plating/stripping profiles of the Li/Li symmetric cells armed with (a) PEO/PA6 30% SCE and (b) pure PEO electrolyte at 0.3 mA cm−2 and 60 °C. (c) Impedance change curve of Li/PEO/PA6 30%/Li battery after different cycling times. The SEM images of lithium anode surface after cycling obtained from the (d) Li/PEO/Li battery and (c) Li/PEO/PA6 30%/Li battery.
To more intuitively investigate the influence of solid electrolyte on the growth of lithium dendrites, the surface morphologies of lithium metal anode obtained from the Li/PEO/Li and Li/PEO/PA6 30%/Li batteries after the galvanostatic plating/stripping are observed and presented in Fig. 5d and e. It can be concluded that the lithium metal surface corresponding to the pure PEO electrolyte has a large number of irregular dendrite growth, while the lithium metal surface obtained from the PEO/PA6 30% SCE is relatively smooth and no dendrites appear. The uniform deposition of lithium ions in the Li/PEO/PA6 30%/Li battery can be explained by the high tLi + and mechanical stability of the PEO/PA6 30% electrolyte. Specifically, the addition of PA6 non-woven fabric can not only suppress the crystallization behavior of the PEO polymer and then allow lithium ions to travel along more amorphous regions, but also provide more transmission channels for lithium ions through the interaction of the polar groups on the PA6 molecular chain [35]. Moreover, the integral three-dimensional frame structure formed by the entanglement between ultrafine fibers in PA6 non-woven fabric helps to inhibit the continuous growth of lithium dendrites and prevent the solid electrolyte from being pierced [50]. In conclusion, all these results demonstrate that the introduction of PA6 non-woven fabric can effectively improve the interfacial compatibility of the PEO/PA6 30% SCE against the lithium metal, thereby promoting the uniform and rapid migration of lithium ions inside the all-solid-state battery.
The cycle and rate performance of the all-solid-state electrolytes are measured to assess their values in practical applications. Fig. 6 a shows the rate performance of LiFePO4/Li batteries assembled with pure PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric under 60 °C. It is noted that the discharge capacity of the LiFePO4/PEO/PA6 30%/Li cell at any rate (from 0.2C to 2 C) is significantly higher than these of the other four batteries. At the same time, the LiFePO4/PEO/PA6 30%/Li battery also shows excellent rate recovery, and its discharge capacity is still as high as 145.7 mA h/g when the rate is restored to 0.2C, which is only slightly lower than the initial value of 158.9 mA h/g. The superior rate performance of the PEO/PA6 30% may be mainly due to its high ionic conductivities and fast lithium ion migration ability, which allows lithium ions to be quickly transported inside the battery to reduce the concentration difference about polarization phenomenon [54]. The typical charge and discharge profiles of LiFePO4/PEO/PA6 30%/Li battery at different rates are further investigated as presented in Fig. 6b. As the rates increases from 0.2C, 0.5C, 1 C–2 C, the corresponding discharge capacities of LiFePO4/PEO/PA6 30%/Li battery gradually changes from 153.8 mA h/g, 149.2 mA h/g, 139.8 mA h/g to 121.2 mA h/g, respectively. Moreover, the smooth charge-discharge platform and small voltage polarization from the figure can also be observed, indicating that the PEO/PA6 30% SCE has excellent electrochemical stability. All of the above results prove that the introduction of PA6 non-woven fabric is conducive to improving the electrochemical performance of the all-solid-state electrolyte.
Fig. 6.
The cycle and rate performance of the all-solid-state LiFePO4/Li batteries assembled with pure PEO electrolyte and PEO/PA6 SCEs with different mass fractions of PA6 non-woven fabric under 60 °C and the voltage range of 2.5–4.2 V. (a) Rate performance of LiFePO4/Li batteries. (b) The typical charge and discharge profiles of LiFePO4/PEO/PA6 30%/Li battery at 0.2C, 0.5C, 1 C and 2 C. (c) Cycling performance, and corresponding impedance curves of LiFePO4/Li batteries (d) before and (e) after cycling at 0.5C. (f) Long-term cycling performance of LiFePO4/Li batteries at 1 C.
Fig. 6c represents the cycling performance of the LiFePO4/Li batteries at 0.5C and 60 °C. It can be clearly seen that the initial discharge capacity of the LiFePO4/PEO/PA6 30%/Li battery is 154.3 mA h/g, and then the discharge capacity and Coulombic efficiency can still be maintained at 130.6 mA h/g and 99.6% over 200 cycles, respectively, which means the excellent interface compatibility between the PEO/PA6 30% SCE and the electrode. In contrast, the LiFePO4/Li batteries assembled with pure PEO electrolyte and other proportions of SCE exhibit low discharge capacity and unstable Coulombic efficiency, all of which are worse than these of the PEO/PA6 30% SCE. The reason for this phenomenon can be attributed to the addition of the PA6 non-woven fabric, which endows the electrolyte high lithium ion migration number and promotes the uniform and rapid deposition of lithium ions during the cycling process. More importantly, the excellent flexibility of the PEO/PA6 30% SCE itself can also always ensure its good interface contact with the electrode during the battery cycle, thereby greatly reducing the interface impedance of the battery, which can be proved by Fig. 6d and e. As can be seen in Fig. 6c, the initial resistance of the LiFePO4/Li battery assembled from pure PEO, PEO/PA6 10%, PEO/PA6 20% and PEO/PA6 40% solid electrolyte is 88.2, 80.4, 64.9 and 48.0 Ω, which all are significantly higher than the 33.74 Ω of PEO/PA6 30% SCE. Similarly, the impedance values of the LiFePO4/PEO/PA6 30%/Li battery after cycling are 51.72 Ω, which is still lower than the 154.8, 135.9, 87.64 and 69.8 Ω of pure PEO, PEO/PA6 10%, PEO/PA6 20% and PEO/PA6 40% solid electrolyte. The smallest impedance value and the lowest impedance change of the PEO/PA6 30% electrolyte among them are good proof of its excellent interface wetting ability provided by the addition of a suitable amount of the PA6 non-woven fabric.
The long-term cycling performance of all-solid-state batteries at a large current density of 1 C are further investigated and shown in Fig. 6f. It can be concluded that the LiFePO4/Li batteries armed with pure PEO electrolyte and PEO/PA6 10% SCE all show low discharge capacity, and their Coulombic efficiency dropped sharply after 93 and 243 cycles, respectively, indicating the poor cycle stability of these two solid electrolytes. In addition, the discharge capacity of SCE with a mass fraction of 10% and 20% for PA6 non-woven fabric is significantly lower than that for the PEO/PA6 30% SCE. Moreover, the Coulombic efficiency of the PEO/PA6 30% SCE is still as high as 99.8% even after 300 cycles under 1 C. All these results show that when the mass fraction of PA6 non-woven fabric is 30% among them, the prepared SCE has the most excellent long-cycle performance. This phenomenon may be mainly due to the highest mechanical strength of the PEO/PA6 30% SCE, which helps to provide a strong framework support for the solid electrolyte to effectively hinder the lithium dendrites growth and avoid being pierced by the dendrites. At the same time, the wide electrochemical stability window above 4.94 V can also prevent the PEO/PA6 30% SCE from decomposing within the set voltage of 2.5–4.2 V and reduce the occurrence of adverse reactions between the electrode and the SCE [54].
4. Conclusion
In summary, a polymer/polymer all-solid-state composite electrolyte are obtained by combining the PA6 non-woven fabric and PEO polymer through the bicomponent spunbond-spunlace method and the hot-pressing process. As the base fabric of masks, non-woven fabrics can not only reduce the risk of people being infected with COVID-19, but also have potential application value in the commercial production of all-solid-state batteries. Compared with the pure PEO electrolyte, the addition of the PA6 non-woven fabric endows the SCE superior ionic conductivities, high lithium ion transference number and sufficient mechanical strength with appropriate flexibility, which promote the rapid migration of the lithium ions and effectively suppress the lithium dendrite growth. Furthermore, the enhanced electrochemical stability and the interface compatibility between the SCE and electrode is also conducive to improving the electrochemical performance of the all-solid-state battery. The Li/Li symmetric battery assembled with the PEO/PA6 30% SCE exhibits excellent lithium plating/stripping performance, and can be stably cycled for 1000 h without voltage fluctuation. Moreover, the PEO/PA6 30% SCE also shows good rate recovery performance and long cycle stability even at high rates when it is applied to the integrated LiFePO4/Li battery. The design idea of the proposed all-solid-state composite electrolyte is an effective solution for preparing high-performance and commercially available lithium metal batteries.
CRediT authorship contribution statement
Lu Gao: Conceptualization, Methodology, Validation, Investigation, Data curation, Writing - review & editing. Bushra Sarmad: Writing - review & editing, Visualization. Jianxin Li: Data curation, Writing - review & editing, Visualization. Bowen Cheng: Visualization, Investigation, Formal analysis. Weimin Kang: Methodology, Supervision. Nanping Deng: Formal analysis, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The author would like to thank the National Natural Science Foundation of China (51973157, 51673148 and 51678411), the China Postdoctoral Science Foundation Grant (2019M651047), and the Science and Technology Plans of Tianjin (No. 17PTSYJC00040 and 18PTSYJC00180) for their financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpowsour.2020.228663.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Park K.-H., Kaup K., Assoud A., Zhang Q., Wu X., Nazar L.F. Acs Energy Lett. 2020;5:533–539. [Google Scholar]
- 2.Deng S., Li X., Ren Z., Li W., Luo J., Liang J., Liang J., Banis M.N., Li M., Zhao Y., Li X., Wang C., Sun Y., Sun Q., Li R., Hu Y., Huang H., Zhang L., Lu S., Luo J., Sun X. Energy Storage Mater. 2020;27:117–123. [Google Scholar]
- 3.Fan R., Liu C., He K., Cheng S.H.-S., Chen D., Liao C., Li R.K.Y., Tang J., Lu Z. ACS Appl. Mater. Interfaces. 2020;12:7222–7231. doi: 10.1021/acsami.9b20104. [DOI] [PubMed] [Google Scholar]
- 4.Bielefeld A., Weber D.A., Janek J. ACS Appl. Mater. Interfaces. 2020;12:12821–12833. doi: 10.1021/acsami.9b22788. [DOI] [PubMed] [Google Scholar]
- 5.Gao B., Jalem R., Tateyama Y. ACS Appl. Mater. Interfaces. 2020;12:16350–16358. doi: 10.1021/acsami.9b23019. [DOI] [PubMed] [Google Scholar]
- 6.Hatzell K.B., Chen X.C., Cobb C.L., Dasgupta N.P., Dixit M.B., Marbella L.E., McDowell M.T., Mukherjee P.P., Verma A., Viswanathan V., Westover A.S., Zeier W.G. Acs Energy Lett. 2020;5:922–934. [Google Scholar]
- 7.Shinzo S., Higuchi E., Chiku M., Hayashi A., Inoue H. ACS Appl. Mater. Interfaces. 2020 doi: 10.1021/acsami.0c01759. [DOI] [PubMed] [Google Scholar]
- 8.Zheng B., Liu X., Zhu J., Zhao J., Zhong G., Xiang Y., Wang H., Zhao W., Umeshbabu E., Wu Q.-H., Huang J., Yang Y. Nano Energy. 2020;67:104252. [Google Scholar]
- 9.Johari N.S.M., Adnan S.B.R.S., Mohamed N.S., Ahmad N. Ceram. Int. 2020;46:8039–8046. [Google Scholar]
- 10.Zhang X., Wang S., Xue C., Xin C., Lin Y., Shen Y., Li L., Nan C.W. Adv. Mater. 2019;31:1806082. doi: 10.1002/adma.201806082. [DOI] [PubMed] [Google Scholar]
- 11.Fergus J.W. J. Power Sources. 2010;195:4554–4569. [Google Scholar]
- 12.Samson A.J., Hofstetter K., Bag S., Thangadurai V. Energy Environ. Sci. 2019;12:2957–2975. [Google Scholar]
- 13.Wang C., Fu K., Kammampata S.P., McOwen D.W., Samson A.J., Zhang L., Hitz G.T., Nolan A.M., Wachsman E.D., Mo Y., Thangadurai V., Hu L. Chem. Rev. 2020 doi: 10.1021/acs.chemrev.9b00427. [DOI] [PubMed] [Google Scholar]
- 14.Wu N., Chien P.-H., Li Y., Dolocan A., Xu H., Xu B., Grundish N.S., Jin H., Hu Y.-Y., Goodenough J.B. J. Am. Chem. Soc. 2020;142:2497–2505. doi: 10.1021/jacs.9b12233. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z., Shao Y., Lotsch B., Hu Y.-S., Li H., Janek J., Nazar L.F., Nan C.-W., Maier J., Armand M., Chen L. Energy Environ. Sci. 2018;11:1945–1976. [Google Scholar]
- 16.Lua F., Li G., Yu Y., Gao X., Zheng L., Chen Z. Chem. Eng. J. 2020;384:123237. [Google Scholar]
- 17.Zhao Y., Bai Y., Liu A., Li W., An M., Bai Y., Chen G. J. Power Sources. 2020;450:227614. [Google Scholar]
- 18.Liu L., Lyu J., Mo J., Yan H., Xu L., Peng P., Li J., Jiang B., Chu L., Li M. Nano Energy. 2020;69:104398. [Google Scholar]
- 19.Hsu S.-T., Tran B.T., Subramani R., Nguyen H.T.T., Rajamani A., Lee M.-Y., Hou S.-S., Lee Y.-L., Teng H. J. Power Sources. 2020;449:227518. [Google Scholar]
- 20.Hu J., Wang W., Zhou B., Feng Y., Xie X., Xue Z. J. Membr. Sci. 2019;575:200–208. [Google Scholar]
- 21.Cha J.H., Didwal P.N., Kim J.M., Chang D.R., Park C.-J. J. Membr. Sci. 2020;595:117538. [Google Scholar]
- 22.Han Q., Wang S., Jiang Z., Hu X., Wang H. ACS Appl. Mater. Interfaces. 2020 doi: 10.1021/acsami.0c03430. [DOI] [PubMed] [Google Scholar]
- 23.Wang S., Zhang X., Liu S., Xin C., Xue C., Richter F., Li L., Fan L., Lin Y., Shen Y., Janek J., Nan C.-W. J. Materiomics. 2020;6:70–76. [Google Scholar]
- 24.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., Tang C., Zhang N., Zhong N., Li S. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui X.P. Adv. Mater. Res. 2013;634–638:307–310. [Google Scholar]
- 27.Krucińska I., Surma B., Chrzanowski M., Skrzetuska E., Puchalski M. Textil. Res. J. 2012;83:859–870. [Google Scholar]
- 28.Ozen M.S., Sancak E., Akalin M. Textil. Res. J. 2014;85:804–815. [Google Scholar]
- 29.S O.M., I U., M Y., E S., N S. Fibres Text. East. Eur. 2018;26:94–100. [Google Scholar]
- 30.Kothari V.K., Das A. J. Textil. Inst. 1993;84:16–30. [Google Scholar]
- 31.J F.D., R J.F., J L., J M. Hernia. 2000;4:228–233. [Google Scholar]
- 32.Parikh D.V., Nam S., He Q. J. Fire Sci. 2012;30:187–200. [Google Scholar]
- 33.Park K.H., Bai Q., Kim D.H., Oh D.Y., Zhu Y., Mo Y., Jung Y.S. Adv. Energy Mater. 2018;8:1800035. [Google Scholar]
- 34.Li D., Chen L., Wang T., Fan L.Z. ACS Appl. Mater. Interfaces. 2018;10:7069–7078. doi: 10.1021/acsami.7b18123. [DOI] [PubMed] [Google Scholar]
- 35.Li C., Liu S., Shi C., Liang G., Lu Z., Fu R., Wu D. Nat. Commun. 2019;10:1363. doi: 10.1038/s41467-019-09211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Wang Z., Sun J., Zheng F., Kotobuki M., Wu T., Zeng K., Lu L. J. Power Sources. 2020;454:227949. [Google Scholar]
- 37.Zhu L., Zhu P., Fang Q., Jing M., Shen X., Yang L. Electrochim. Acta. 2018;292:718–726. [Google Scholar]
- 38.Wang Q., Liu X., Cui Z., Shangguan X., Zhang H., Zhang J., Tang K., Li L., Zhou X., Cui G. Electrochim. Acta. 2020;337:135843. [Google Scholar]
- 39.Zhang H., Oteo U., Zhu H., Judez X., Martinez-Ibanez M., Aldalur I., Sanchez-Diez E., Li C., Carrasco J., Forsyth M., Armand M. Angew. 2019;58:7829–7834. doi: 10.1002/anie.201813700. [DOI] [PubMed] [Google Scholar]
- 40.Xin S., You Y., Wang S., Gao H.-C., Yin Y.-X., Guo Y.-G. Acs Energy Lett. 2017;2:1385–1394. [Google Scholar]
- 41.Watanabe T., Inafune Y., Tanaka M., Mochizuki Y., Matsumoto F., Kawakami H. J. Power Sources. 2019;423:255–262. [Google Scholar]
- 42.Yang C., Fu K., Zhang Y., Hitz E., Hu L. Adv. Mater. 2017;29:1701169. doi: 10.1002/adma.201701169. [DOI] [PubMed] [Google Scholar]
- 43.Li Y., Zhang W., Dou Q., Wong K.W., Ng K.M. J. Mater. Chem. A. 2019;7:3391–3398. [Google Scholar]
- 44.Wang Q., Cui Z., Zhou Q., Shangguan X., Du X., Dong S., Qiao L., Huang S., Liu X., Tang K., Zhou X., Cui G. Energy Storage Mater. 2020;25:756–763. [Google Scholar]
- 45.Dixit M.B., Zaman W., Hortance N., Vujic S., Harkey B., Shen F., Tsai W.-Y., De Andrade V., Chen X.C., Balke N., Hatzell K.B. Joule. 2020;4:207–221. [Google Scholar]
- 46.Gao B., Jalem R., Ma Y., Tateyama Y. Chem. Mater. 2020;32:85–96. [Google Scholar]
- 47.Zhang W., Nie J., Li F., Wang Z.L., Sun C. Nano Energy. 2018;45:413–419. [Google Scholar]
- 48.Cao C., Li Y., Feng Y., Peng C., Li Z., Feng W. Energy Storage Mater. 2019;19:401–407. [Google Scholar]
- 49.Pareek T., Dwivedi S., Ahmad S.A., Badole M., Kumar S. J. Alloys Compd. 2020;824:153991. [Google Scholar]
- 50.Chen L., Fan L.Z. Energy Storage Mater. 2018;15:37–45. [Google Scholar]
- 51.Chen L., Li Y., Li S.-P., Fan L.-Z., Nan C.-W., Goodenough J.B. Nano Energy. 2018;46:176–184. [Google Scholar]
- 52.Zou Z., Li Y., Lu Z., Wang D., Cui Y., Guo B., Li Y., Liang X., Feng J., Li H., Nan C.W., Armand M., Chen L., Xu K., Shi S. Chem. Rev. 2020 doi: 10.1021/acs.chemrev.9b00760. [DOI] [PubMed] [Google Scholar]
- 53.Cheng X.B., Hou T.Z., Zhang R., Peng H.J., Zhao C.Z., Huang J.Q., Zhang Q. Adv. Mater. 2016;28:2888–2895. doi: 10.1002/adma.201506124. [DOI] [PubMed] [Google Scholar]
- 54.Wan Z., Lei D., Yang W., Liu C., Shi K., Hao X., Shen L., Lv W., Li B., Yang Q.-H., Kang F., He Y.-B. Adv. Funct. Mater. 2019;29:1805301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.