Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a neoteric virus belonging to the beta coronavirus class has created a global health concern, responsible for an outbreak of severe acute respiratory illness, the COVID-19 pandemic. Infected hosts exhibit diverse clinical features, ranging from asymptomatic to severe symptoms in their genital organs, respiratory, digestive, and circulatory systems. Considering the high transmissibility (R0: ≤6.0) compared to Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV, the quest for the clinical development of suitable antiviral nanotherapeutics (NTPs) is incessant. We are presenting a systematic review of the literature published between 2003 and 2020 to validate the hypothesis that the pharmacokinetics, collateral acute/chronic side effects of nano drugs and spike proteins arrangement of coronaviruses can revolutionize the therapeutic approach to cure COVID-19. Our aim is also to critically assess the slow release kinetics and specific target site chemical synthesis influenced competence of NTPs and nanotoxicity based antiviral actions, which are commonly exploited in the synthesis of modulated nanomedicines. The pathogenesis of novel virulent pathogens at the cellular and molecular levels are also considered, which is of utmost importance to characterize the emerging nano-drug agents as diagnostics or therapeutics or viral entry inhibitors. Such types of approaches trigger the scientists and policymakers in the development of a conceptual framework of nano-biotechnology by linking nanoscience and virology to present a smart molecular diagnosis/treatment for pandemic viral infections.
Keywords: SARS-CoV-2, COVID-19, Nanotherapeutics, Viral infection, Nanomedicine, Immunity
Graphical abstract
1. Introduction
The ongoing pandemic coronavirus disease outbreak (COVID-2019), stemming from the beta coronavirus class (SARS-CoV-2) causing severe acute respiratory syndrome (SARS), has created a global health emergency in >200 countries around the globe (Huang et al., 2020; Nalla et al., 2020). SARS-CoV-2, a novel coronavirus strain belonging to the Sarbecovirus subgenus (genus Betacoronavirus, family Coronaviridae) had first appeared in late 2019, in Wuhan, China infecting a large number of hosts (>3.1 million people) with a mortality rate of ≥3.6% (Neogi et al., 2020; Carter et al., 2020). Such putative etiopathogenic agents associated with the zoonotic viral transmission pathways are responsible for respiratory (viral pneumonia) and gastro-intestinal infections leading to multiple organ failure in infected people or patients having co-morbidities. Till date no therapeutic drug has been discovered for the treatment of SARS-CoV-2 infection, though the importance of monoclonal antibodies, protease and helicase inhibitors and interferons (IFNs-α) treatments have been highlighted in a handful of literature (Farzin et al., 2020; Torchilin, 2020; Palmieri and Papi, 2020). Development of drug-resistant strains, host cell toxicity/target specific actions, costs associated with the serological diagnosis and therapeutics limit the widespread application of synthetic drugs (nucleoside analogs) (Palmieri and Papi, 2020; Torchilin, 2020).
Harnessing the potential of nanobiotechnology in the biomedical science, development of engineered nanostructured materials/nanomedicines may lead to better drug delivery, advanced therapeutics and medical diagnostics at nanoscale. In recent years, with the advancement of clinical practices, the use of metal (gold, silver, zinc and copper) nanoparticles in magnetic immunoassay, viral diagnostics and microfluidic technology has driven the researchers to explore the potential of nanotherapeutics (Makvandi et al., 2020; Farzin et al., 2020). Letko et al. (2020) and Ahlawat and Narayan (2020) have investigated the multi-strain inhibition of SARS coronavirus (SARS-CoV), herpes simplex virus 1 (HSV-1) and human immunodeficiency virus (HIV of T-cells) by sulfonated nanoparticles binding. They have reported that small particle size, tunable surface charges, biomimetic properties, faster encapsulation have made the engineered nanoparticles smart and stable colloidal carriers for the delivery of genes and drugs. The mode of action of functionalized nanoparticles can be explained by their covalent linkages with biological substrates such as peptides, proteins, antibodies and nucleic acids. Some researchers have reported virus-nanoparticles electrostatic nonspecific interactions elsewhere (i.e. influenza A (H5N1)) strain inhibition) (Alizadeh and Khodavandi, 2020; Rothan and Byrareddy, 2020).
Nanoparticulate drug carriers through the cellular and mechanistic establishment crossed the membranes and with the help of capping agents such as sulfate polysaccharides/polymers undergo multivalent bond interactions with virus glycoproteins (i.e hemagglutinin (HA)) (Bachmaier et al., 2020; Balakrishna et al., 2020). Such surface proteins often act as a prime inducer of neutralizing antibodies as observed by Boulware et al. (2020) and Chaturvedi and Shrivastava (2005), where antigenic determinants enable nucleocapsid to take entry into the host cells as reported for in vitro SARS-CoV. Inhibition of virus replication and the fusion between the viral and host cell endosomal membrane are often facilitated by some emerging nano-based technologies i.e. silver nano-rod array surface enhanced Raman spectroscopy (SERS) substrate, interferometric biosensor immunoassay (Chauhan et al., 2020; Chhikara et al., 2020; Choudhary et al., 2020). The development of such biomolecular detectors enable the molecular binding of virus particles to target specific (antigen phase) antibodies (coated waveguide), which help in the fast and reliable detection of viral infections (Kirchdoerfer and Ward, 2019; Cojocaru et al., 2020; Das et al., 2020).
Considering the high transmissibility (R0: ≤6.0) and low to moderate pathogenicity of SARS-CoV-2 (<6%) compared to Middle East respiratory syndrome coronavirus (MERS-CoV) and SARS-CoV, the quest for clinical development of suitable antiviral nanotherapeutics is need of the hour (Rosenberg et al., 2019; Nalla et al., 2020; Dung et al., 2020). Though, it is noteworthy to mention that R0 is not data specific to the COVID-19 disease rather it depends on the place and the behavior of the population. It is associated with social isolation, hygienic habits, among others, and the variation must occur in different ways (Viceconte and Petrosillo, 2020; Li et al., 2020). The drug development for antiviral treatment (of SARS-CoV-2) depends on several factors such as pharmacokinetics (drug properties), collateral acute/chronic side effects and spike proteins (S) arrangement of coronaviruses (virus properties). These can act as therapeutic targets to prevent the fusion of the virus to host cell via binding with host cell receptors. Trimeric scaffolds such as NSP10 (a viral transcription factor) used along with secondary antigens in vaccines for lung and lower respiratory tract infections. These are essential to nucleate and stabilize pseudo-subdomains of protein and peptide antigens, which are also responsible for cell infection and polycistronic expression that may help in the nano-based therapeutic vaccine development (Dong et al., 2020; Grein et al., 2020; Etman et al., 2020).
Peginterferon α-2a/2b (Pegasys), IFN-α2b show excellent drug delivery actions against Hepatitis virus (c), whereas cationic/cross-linked nanoparticles (biodegradable polymers) such as m-polyethylene glycol (mPEG)–PLA, CLPM are found suitable for Hepatitis (B and C) virus infections. Synthetic and natural polymeric nanoparticles (PNPs) are widely used since the last decade for the effective control of pathogenic viruses based on their individual biochemical properties (immuno/biocompatibility, bio-distribution factors) (Table 1 ). Low to zero toxicity profile, high stability against proteinase degradation, improved safety/efficacy profiles and good internalization properties make such nanozymes effective against chronic infectious diseases and also help in exerting their cytoprotective actions against cytopathic effect. Polymeric nanoparticles in hybridized forms have found their way in biomedical sectors as therapeutics and diagnostics of human adenovirus, influenza virus and HIV. Therefore, the widespread application of hybridized nanoformulation systems have been documented by Kerry et al. (2019) and Kim et al. (2020), where functionalized DNAzyme along with cellular peptides encoding viral envelopes help in the knockdown of HSV-2, HVC and influenza A viruses. For the first time, the purpose of the present systematic review lays out the framework for: (a) developing a critical understanding of self-assembling metal nanoparticles targeting a variety of fusion proteins for vaccine development; (b) the spatial geometry (three fold symmetry axis) and radial distributions that drive the rapid antigen processing and render virucidal activity; (c) building up a deep insight for biomarkers research in both prophylactic and therapeutic approaches; (d) the critical assessment of nanoparticles as therapeutics and associated drug metabolism pathways (biodegradation).
Table 1.
A summary of biocompatible nanomaterials (and antiviral nanopharmaceuticals) commonly used for biomedical drug delivery action as virucidal agents (Souce of data: Weiss et al., 2020; Udugama et al., 2020; Letko et al., 2020; Jamshidi et al., 2020; Gao et al., 2020; Dong et al., 2020).
| Nanocarriers | Target specific cell | Virus types | Mode of antiviral activity | Virucidal action |
|---|---|---|---|---|
| AuNPs | BHK-21, HeLa-CD-LTR, Vero cells | HIN1, H3N2, HIV-1 | Binding with Peroxidase-mimic enzymes and viral gp120 | Immunization/viral detection |
| Immobilized AuNPs | C6/36, BALB/c mice | H3N2, Dengue virus, H5N1 | Antibody mediated inhibition | Viral detection |
| TiO2NPs | n.a.* | H3N2 | Viral capsid protein interaction | Inactivation of virus by photolysis |
| Modified TiO2NPs | MDCK | H5N1, H1N1 | Viral surface protein interaction | Virus inhibition/inactivation |
| AgNPs | Vero cells | H7N3 | Inhibit CD4-based binding | Viral entry inhibition |
| Engineered AgNPs | Human Rhabdomyosarcoma |
Feline Calicivirus, Influenza | Viral envelop rupture | Viral replication deformation |
| SiNPs | Vero cells | Papilloma Virus | Cell mediated immune/nucleic acid inhibition | Virus detection |
| Mesoporous Si | HEK293T | HSV-1, 2 | Hinder viral attachment | Viral entry inhibition |
| Fullerenes | n.a. | Bacteriophage λ | Viral capsid/envelop attachment and interaction | Virus destabilization |
| Modified Fullerenes | SupT1 | HIV-1 (wild and resistant type) | Impairing viral polyprotein/hinder Gag processing | Inhibition of virus entry |
| FeNPs | n.a. | Zika virus | Viral envelop/protein binding | Host pathogen interaction |
| Engineered FeNPs | n.a. | Bacteriophage MS2, H5N2 | Phosphatidylserine inhibit viral tropism | Viral detection/removal |
| Acid/basic Functionalized Nanotube | NCIH292 | H3N2 | Photoactivated mediated viral inhibition/destabilization | Virus inactivation |
| Metal functionalized Nanotube | Grass carp | Reovirus | VP7/DNA mediated inhibition | Immunization |
| Carbon dots | PK-15, MARC-145 | RSV, Pseudorabies virus | Type I interferon production inhibited | Viral inhibition |
| Graphene oxide (GO) | Vero cells | Porcine epidemic diarrhea virus | Negative single layered sharp edged particle interaction with virus | Viral entry hindrance |
| Polystyrene NPs | n.a. | HIV-1 | viral gp120 antigen binding and mannose/lectin specific inhibition action |
Mucosal vaccine development |
| Chitosan coated NPs | n.a. | Rabies virus | Immune system inhibition | Immunization |
| Peptide coated NPs | BALB/c strain | Influenza A virus | CD8+ T cells inhibition | Nanoparticulate vaccine |
| Protein coated NPs | BALB/c mice | Influenza A virus | antibodydependent cell-mediated phagocytosis/cytotoxicity |
Influenza vaccine |
| Amide coated NPs | neuro 2a cell lines |
HIV | Viral transcriptase inhibition | Antiviral therapy |
| Nano-liposomes | n.a. | n.a. | Gene silencing action | Drug delivery immunomodulator |
| Nanomicelle | APC49 Huh7.5 | Hepatitis C Virus | Viral cell entry inhibition | Antiviral activity and bioavailable vaccines |
| AuNPs-carbon nanotubes | n.a. | H3N2 | Peroxidase inhibition | Viral detection by colorimetric assay |
| Polymeric micelle | Male Wistar rats cell | HIV | Viral entry inhibition | Oral bioavailable drugs |
| AuNPs-AgNPs | MDCK | H1N1, H3N2 | Coagulation results from virus surface protein interaction | Viral inhibition and drug delivery action |
| Au/FeNPs-carbon nanotubes | n.a. | Norovirus, H1N1 | MagNB mediated enzymeatic signaling inhibition | Viral DNA detection |
| Nanolipid carriers | VK2/E6E7 | Papilloma virus | Cell cycle inhibition at G2/M phase | Nontoxic viral inhibition |
| Dendrimer | Vero cells, HELFs | HSV-1,2 | Glycosaminoglycan binding affinity and in vitro replication inhibition | m-RNA vaccine |
n.a*- Not applicable, AuNPs-Gold Nanoparticles, AgNPs-Silver Nanoparticles, FeNPs-Iron Nanoparticles, SiNPs-Silica Nanoparticles, TiO2- Titanium nanoparticles, GO-Graphene oxides.
This review also provides a guide map for the regulation of metabolic enzymes on selected nano-pharmaceuticals, through which the multigenerational effects can be evaluated. This publication is designed to provide vital information on biocompatible nanocarriers, active vs passive targeted drug delivery action of nanomedicines and critically analyze the possible hybrid nano-based therapy for SARS-CoV-2 inhibition. Our current review also highlights the state-of-art molecular fingerprinting techniques of virus identification through advanced biosensing methods, which critically explore integral surveillance and monitoring of novel viral genotypes.
2. Mode of action and biomedical applications of biocompatible nanocarriers
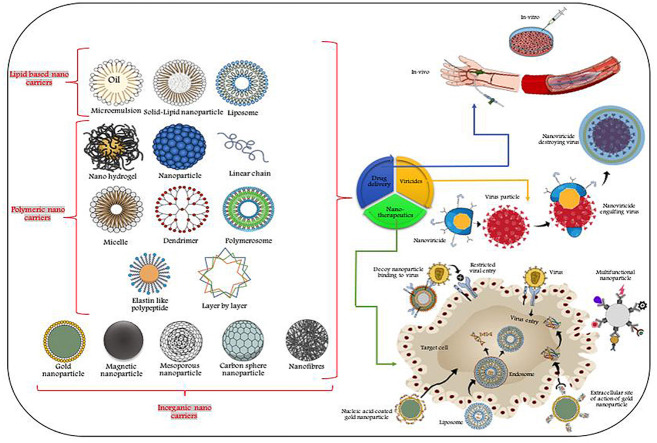
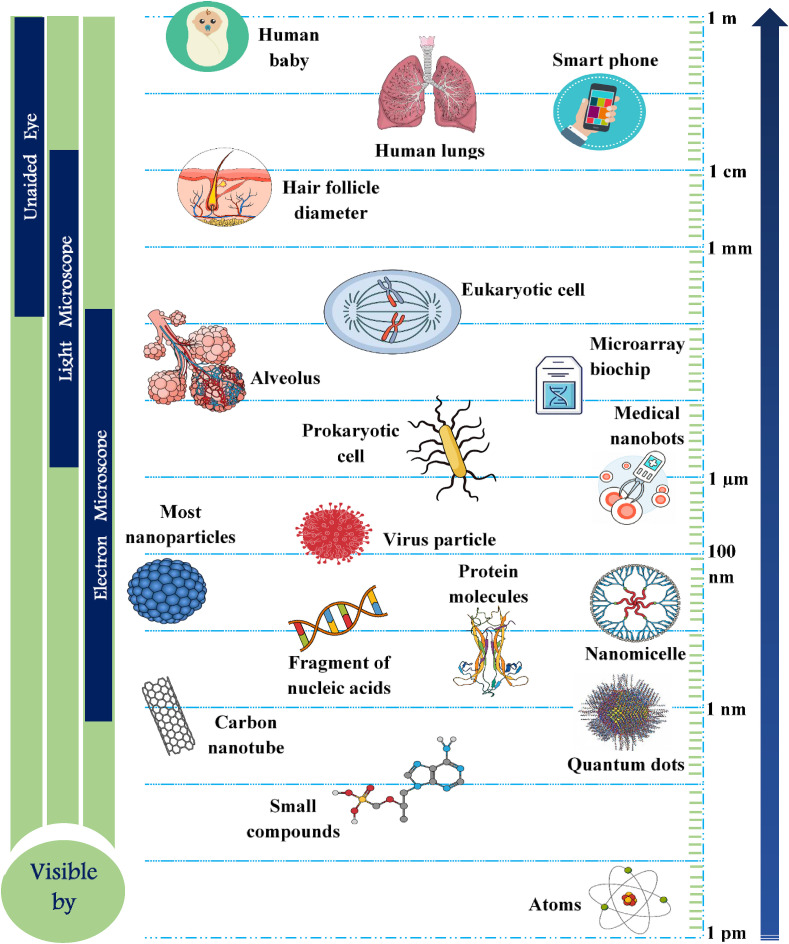
The different forms of nanomaterials that are mostly used as antiviral agents depend largely on the pathway of drug delivery system, which provide versatile forms of nano-based carriers starting from complex, organic hybrid nanosystems to simple inorganic metallic composites. Nanocapsules, nanocages and nanospheres are categorised under inorganic nanoparticle based systems, though nanocapsules can sometimes come under organic nanocarrier systems based on the their function as therapeutic agents (encapsulated, chemical attachment, adsorbed or dissolved) (Grein et al., 2020; Neogi et al., 2020; Gacem et al., 2020). Fig. 1 demonstrates the comparative size range of nanoparticles. Whereas, for hybrid nano-based systems, the molecular composition and target specific action of individual engineered nanoparticulate systems play a vital role in drug delivery actions (Table 1). The mode of action of such nano-based therapeutic agents can be categorised based on the permeability of vasculature (passive targeting for malignancy) or attachment of bioactive ligands to the selective nanotherapeutics (Kirchdoerfer and Ward, 2019; Gao et al., 2020; Gadade and Pekamwar, 2020).
Fig. 1.
Schematic of the size range of nanoparticles commonly applied in clinical practice as drug delivery agents. (for gene and drug delivery system).
2.1. Role of metallic nanoparticles as in vivo (and in vitro) drug discharging agents
The presence of multiple surface binding sites, in vivo clinical interaction with the targeted sites, composition based multiple interactions, shape, luminescence and large surface/volume ratio of inorganic nanoparticles render their multifarious biomedical applications (Ignatov et al., 2020; Dong et al., 2020). The virucidal activity of silver nanoparticles (AgNPs) allows its wider application in the annihilation or amelioration of several viral infections such as Poliovirus type-1, Coxsackievirus B3, influenza A virus, etc. The mode of action of such nanoparticles can be described either through inhibition of CD4-dependent cellular binding/pathogenesis or by covalent linking with sulfhydryl group (virion surface) (Hill, 2020; Neogi et al., 2020; El-Sheekh et al., 2020). The viral internalization can also be prevented by merging nanoparticles with viral genome/core protein that hinders viral replication/attachment, release of viral core into the cytoplasm and governing conformational changes in virus/co-receptor association (Letko et al., 2020; Hu et al., 2020). This type of viral replication inhibitions are quite effective in curing of dsRNA viruses, infectious HSV-2 and bursal disease viruses (Iravani, 2020; Jamshidi et al., 2020). On the other hand, gold nanoparticles (AuNPs) may also exert their antiviral efficacy through hindering of gp120 fusion to CD4 that are surrounded by capping agents (encapsulated AuNPs) (Table 1). Hybridized and charged AuNPs render virucidal mechanism after associating with nano-based formulation and mimicking peroxidase enzymatic reaction, where inhibited viral entry is facilitated by blocking of transcription within the host cell (Isida, 2019; Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020; Kang, 2020).
On the other hand, stabilization of magnetic nanoparticles is often carried out by biocompatible polymers, which helps in the translation of magneto responsive nanoparticulate systems in clinical diagnostics (bio-imaging, bio/resonance-imaging and cell separation). The use of superparamagnetic iron nanoparticles (γ-Fe2O3/Fe2O3/Fe3O4) is not directly involved in the therapeutics but their indirect application may inhibit viral multiplication even at post-entry cellular level as seen for Zika virus, H5N2 and HCV (Zhou et al., 2020a,b; Dong et al., 2020). Photothermal nanotherapy, high refractive index, photocatalytic activities and high solubility properties of titanium nanoparticles (TiNPs) enable them to find their wide antiviral application for bacteriophages and H3N2 viruses (Grein et al., 2020; Kar et al., 2019). The clinical application of functionalized silica nanoparticles (SiNPs) drives the diverse antiviral therapeutics or diagnostics application of nanocarriers in inhibition of HIV, Hepatitis B virus and other recombinant viruses (Kim et al., 2020). Nucleic acid hybridization and fluorescent based virus (viral protein) detection methods are getting popular based on the antiviral efficacy of SiNPs, though their meso to nanoporous biocompatible surfaces allow host/pathogen interaction. The emerging demand for novel antiviral nanotherapeutics triggers the researchers to ponder about immunomodulation and immunization of host cells. These are essential to regulate premature drug release or inhibition of viral entry through in vivo biodistribution (Kalantar-Zadeh et al., 2020; Kerry et al., 2019).
Quantum dots (QD), a type of nanosized crystals have excellent nano-based sensing, which allow them to be used as antiviral therapeutics or in vitro diagnosis of virulent pathogens, where strong chemical interactions/bonding render their biochemical conjugation with therapeutic molecules (Kostarelos, 2020). Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020 and Zhou et al. (2020a,b) have shown that different metallic composition (Pb, Cu, Ga, Zn, Hg) based QD showed target specific actions against HIV-1, human T-lymphotropic virus-1 after their binding with NH2- receptor/biotin acceptor. Some researchers have shown that nano-formulations based QDs crossed the BBB (in vitro model) along with target DNA and saquinavir antiviral agent, which have been widely utilized as highly active antiretroviral therapy (Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020; Lembo et al., 2018).
2.2. Evaluation of organic (and hybrid) nanoparticles as therapeutics and in drug delivery action
Organic nanoparticles/nanocarriers (ONP) play an important role in the drug delivery action if the therapeutic compounds are of large sized molecules (>10–1000 nm). Encapsulation of such nanoagents through specific design/structure render off-target toxicity which is required for target specific action (Lopez, 2020; Gacem et al., 2020). Slow release kinetics and specific target site chemical synthesis have direct influence on ONPs therapeutic competence (Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020; Li et al., 2020). Polymeric nanoparticles (PNPs) have the clinical potential to carry the target drugs to its core or can coordinate with target molecules on its planar surface ((Mainardes and Diedrich (2020); Nasrollahzadeh et al. (2020)). Bio-corona formation, ease of biodegradation, strong mechanical/thermal properties of carbon-based nanocarriers (nanorods, nanodots etc.) render their antiviral activities against Respiratory syncytial virus (RSV), HIV-1, Ebola virus etc. (Table 1). In spite of having broad spectrum activities against antiviral infections, the rate-limiting phase of these carbon materials limits their further biomedical applications.
Nikaeen et al. (2020) and Nguyen et al. (2020) have suggested that drug conjugated nanoparticles having excellent antiviral activities can help in the development of an influenza vaccine with Matrix protein 2 (M2e) and Hemagglutinin (HA)/amine functionalized gelatin surface coating. Though more research is required to validate the immunity and protection of cellular genes for such impeccable protein vaccines against infectious virulent pathogens like SARS-CoV-2. With the advancement of medical sciences, the emergence of multifunctional nano-based lipid nanocarriers opens a new door in the field of novel antiviral nanotherapeutics (Table 1). Read et al. (2019) and Risitano et al. (2020) have demonstrated that podophyllotoxin, stearic acid/glycol loaded lipid nano-carriers are useful in maintaining in vitro slow drug release, non-toxic viral inhibition and hemocompatibility of VK2/E6E7, HeLa cells. Such solid/lipid nanoparticles and other nanolipid carriers can become the exceptional antiviral drug discharging agents for infectious viral pathogens (i.e. HPV, HIV and Hepatitis C virus) (Núñez-Delgado, 2020; Prather et al., 2020).
Hyperbranched, monodispersed, easily biodegradable organic dendrimers have gained importance for more than a decade in the field of nanomedicine because they act as targeted carriers for biological systems. The antiviral activity of dendrimers is still under scientific investigations, which may be facilitated through conjugated drug delivery mechanisms (Patil et al. (2020); Palestino et al. (2020)). The efficacy of such nanoparticulated dendrimer system can be observed for the inhibition of HSV-1/influenza virus, where antibody mediated response/CD8+ T cell activation and regulation of gene expressions are achieved through small RNAs inactivation (Shang et al., 2020; Sportelli et al., 2020).
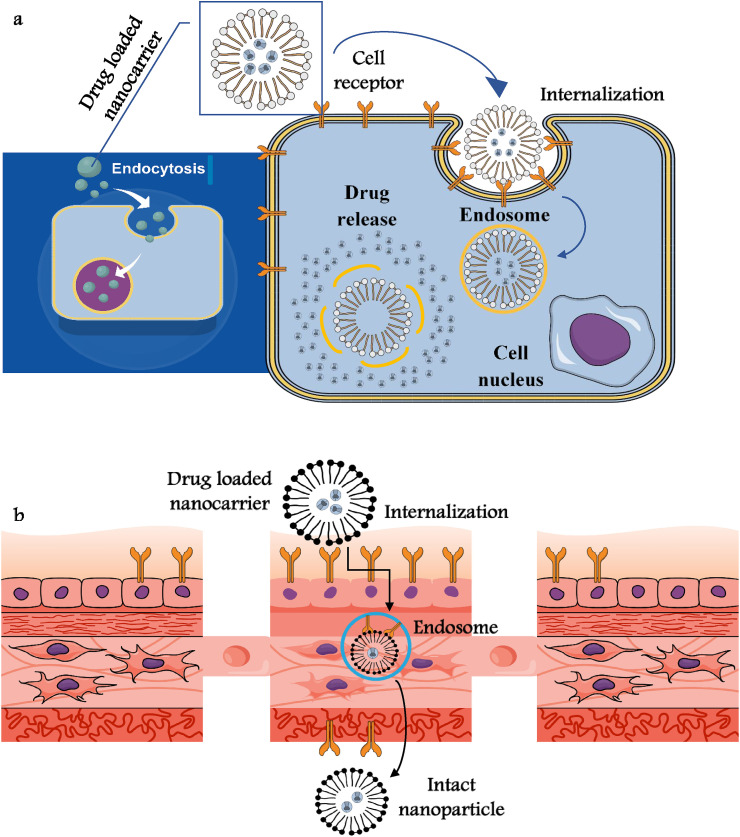
Niosomes, the non-ionic surfactant based liposome alike organic nanovesicle are getting popular in the advanced biomedical sciences considering their non-immunogenicity and stable optical properties, which make them a suitable carrier of both hydro/lipophilic drug molecules. Excellent bioavailability and controlled release of specific drugs at the targeted sites make such nanocarriers ideal antiviral agents (HSV-1 virus), where nano-niosome was loaded with suitable antiviral drug (acyclovir) (Sánchez-López et al., 2020). Improved drug delivery mechanism and suitable drug release kinetics may allow such nanocarriers to be used in infectious virus diseases (nanomicelles) (Fig. 2 ). Supramolecular globular micelles exhibit colloidal stability and super encapsulation potential, which help such polymeric micelles to show antiviral activity in vitro as observed from curcumin loaded bioavailable nanoformulations (hepatitis C virus) (Sivasankarapillai et al., 2020). Some researchers used graphene oxides conjugated AdNPs against infectious SARS and bursal viruses, where the drug resistance event after such antiviral efficacy was mediated by selenium nanoparticles (Se-NPs) and/or amantadine (AM) arrangement (Te Velthuis et al., 2010; Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020). Diagnosis or detection of such virulent pathogens have been documented by Viceconte and Petrosillo (2020) and Vazquez-Munoz and Lopez (2020) through thiol-stabilized gold cluster or enteroviruses labeling with cysteine molecule.
Fig. 2.
a and b: Schematic of the Internalization of nanodrugs through the plasma membrane and targeted drug release (a) and transcytosis of nanodrugs through cell barriers (b). Nanoparticulate drug carriers through the cellular and mechanistic establishment crossed the membranes (blood-brain barrier and blood-testis barrier) and with the help of capping agents such as sulfate polysaccharides/polymers undergo multivalent bond interactions with virus glycoproteins (i.e hemagglutinin (HA).
3. A brief overview about antiviral nanomedicines
Several nanomedicines (including nanovaccines) are under clinical trial or at least in the stage of commercialization for the cure of infectious viral diseases (Table 2 ). In general, the use of drugs for antiviral therapy is usually employed to target different life cycles of virulent pathogens (i.e. HIV, Ebola virus or HSV-1) (Valdiglesias and Laffon, 2020). ALN-RSV01 is a commonly used lipid nanoparticle drug for lower tract respiratory disease, which targets the nucleocapsid “N” gene of RSV virus (Tremiliosi et al., 2020; Udugama et al., 2020). The size and zeta potential of silver nanoparticles can exert inhibition effects on different Human parainfluenza three virus strains (or on their replication event). The nanocolloidal system of vivagel® is immensely used for the control of Zika virus infection. TKM-130803 is widely used in the treatment of Ebola virus utilizing the concept of RNAi-based therapy for the lipid-based nanosystem. It is well doccumented that for Human Norovirus treatment the employment of gold/copper sulfide core-shell capsid protein binding results into excellent virucidal activity (Ziaie et al., 2020). On the other hand, nanotrap particles are quite often used in the inhibition of infection of target cells by capturing viral RNA/viral proteins (i.e. influenza virus treatment) (Zhou et al., 2020a,b; Zhang et al., 2017).
Table 2.
Commercial nanomedicines (or under clinical trial) for the antiviral therapy/treatment (Souce of data: Neogi et al., 2020; Letko et al., 2020; Dong et al., 2020; Kalantar-Zadeh et al., 2020; Kang, 2020).
| Nanomedicines | Biomedical application | Year/stage of development | Mode of action | Disease indication |
|---|---|---|---|---|
| Influvac Plus | Virosome vaccine | 2005 | Presence of neuraminidase and hemagglutinin | Influenza |
| TKM-HBV | Solid/lipid nanoparticle | u.c.e | RNAi therapeutics | HBV |
| Cervisil | SiRNA therapeutic | Preclinical evaluation | Gene silencing | HPV |
| Doravirine | Nanoparticulate formulation | u.c.ea | Reverse trancriptase inhibitor (non-nucleoside) | HIV |
| DermaVir | Therapeutic vaccine | u.c.e | DNA immunogen with HIV specific T cell precursor | HIV |
| Inflexal V | Liposome vaccine | 1997 | Antigens specific on speherical carriers surface | Influenza |
| Epaxal | Liposome vaccine | 1999 | Natural process mimics peroxidases | HAV |
| Pegasys | PEGylated interferon | 2002 | PEGylation control stability of protein | HBV, HCV |
| Geovax | Antiviral therapy | p.e | Ankara—Virus alike drug therapy | SARS-Cov-2 |
| Novavax | Nanoparticulate therapeutics | p.e | Clinical stage antiviral nanobiotechnology | SARS-Cov-2 |
| Fluquit | SiRNA therapeutic | p.ea | Gene silencing | Influenza |
| Curevac | Infections virus vaccine | p.e | mRNA technique | SARS-Cov-2 |
| Peglntron | PEGylated interferon | 2001 | PEGylation control stability of protein | HCV |
| Vivagel | Dendrimer | u.c.e | Dendrimer with sulphonic acid group interaction | HSV, HIV |
p.e-preclinical evaluation, u.c.e-under clinical evaluation, HAV- hepatitis A virus, HIV- human immunodeficiency virus, HBV- hepatitis B virus, HPV- human papillomavirus, HCV- hepatitis C virus, HSV- herpes simplex virus.
Intrinsic in vivo instability, poor immunogenicity and toxicity, multiple therapeutic and prophylactic approaches can be overcome by nanovaccinology, where cellular and humoral immune response drive the faster uptake of mucosa/gut associated lymphoid tissue. Slow/controlled release of antigens is facilitated by surface modifications of nanovaccines with antibodies/carbohydrates, which results in the target specific immune response by different immune cells. Additionally, their small size and prolonged shelf life help in the faster recognition of the host/receptor immune system (i.e. hepatitis A virus (HAV) and influenza virus), where Epaxal/Exapal is used with immuno targeting agents (Yu et al., 2020; Yang and Wang, 2020). Non-responsive immune systems, high dose administration, cold-chain transport of parenteral vaccines limit their widespread application in drug therapy particularly for mucosally administered vaccines. The Chitosan/nanoparticle embedded system might be useful for therapeutic proteins or antigens having negative charges, which makes its wider application in vaccination against HBV virus through gene delivery systems (Table 2). The utilization of mouse model employing humoral and mucosal immune responses helped in the liposome-based vaccine development in case of HepaXen (used for hepatitis A, C, and E), which further utilized the recombinant surface antigen as a prophylactic vaccine (Waris et al., 2020; Wang et al., 2020, Wang et al., 2020). The usages of Inflexal V and Influvac as standard virosomal vaccine against influenza virus are getting quite popular considering its active biocompatible and immunogenicity (Wu et al., 2020; Weiss et al., 2020). These types of licensed subunit nanovaccines are found quite useful for older, infants and middle-aged group people in terms of nanosafety issue as they mimic natural infections (as seen in Table 2). A cysteine-guanine rich oligonucleotide combination with extracellular M2e-gold conjugates renders molecular protection for PR8-H1N1 influenza, which was further activated by thiol-gold interactions (Zhang et al. (2017); Zhou et al. (2020a,b)). From the discussion provided here, it is clear that most of the research has been done for influenza virus vaccination but some scanty literature also report vaccine research for Rota/Noro/Ebola virus, HPV, RSV and others (Dung et al., 2020; Das et al., 2020).
4. Metabolic pathway of nanotherapeutics and their limitations in clinical practice
Nanoparticles uptake (cellular) process (in nanotherapeutics) are governed by their physico-chemical properties along with cellular membrane characteristics, which may have direct influence on the rate of administered drug dosages and structure of engineered nanoparticles. It is hypothesized that nanoparticles with optimum diameter of ≤50 nm and high surface charge density are quite effective in crossing the cellular membranes for HIV-derived TAT cell penetrating peptides (Weiss et al., 2020; Waris et al., 2020). Immunoliposomes and other carbon based nanotubes/nanocarriers play an important role in the activation of the complement pathway of host immune systems to deregulate in vitro utilization of NPs (Neogi et al., 2020; Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020). Antibodies that are specifically targeted at polyethylene glycol (PEG)/macrogol polymers and PEG-like nanostructures can show independent therapeutic efficacy based on their individual immunotoxicology and risk assessment strategies (Letko et al., 2020; Read et al., 2019). Experimental findings (Wang et al., 2020, Wang et al., 2020; Hill, 2020) with nano-based therapeutic agents reveal the urgent requirement of more rigorous scientific investigations to prove their clinical efficiency in reversing the drug resistance event (i.e. H1N1 virus through Se-AM).
Biodegradation process of nanotherapeutics has gained special attention considering uniform biodistribution kinetics and sustained drug release, which are essential for improved drug design process. Distribution, metabolism, absorption, excretion are important pharmacokinetic features, which (rate of biochemical features) are directly governed by hydrophobic/hydrophilic profile and tacticity of the nano-based formulations at in vitro level (Patil et al., 2020). Exocytosis process plays a very important role in the clearance of the foreign nanoparticles out of the cell depending on (administered) nanocarriers. It was hypothesized that particles with <5 nm diameter can excrete through urine, whereas larger particles (>10 nm) may show their slow release behavior through colon, kidney or liver (Shang et al., 2020). Prather et al. (2020) has documented that PEGylated particles exhibit a faster degradation profile compared to cationic particles due to low (or no) agglomeration properties of the former than latter. The large sized nanoparticulates have the tendency to bioaccumulate inside the host cell (HIV, H1N1 infections) and can be a target of mononuclear phagocytic macrophages (Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020; Zhou et al., 2020a,b). Thus, removal of extraneous materials (and poor drug material selectivity) from the cellular surface can be overcome by the utilization of bioactive target specific ligands (Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020; Ziaie et al., 2020).
Cellular uptake of nanotherapeutics can be affected depending on the permeability factor of the biological membranes, which may further limit the endocytotic pathways and therefore restrict wider application of nanotherapeutics in biomedical sectors. Non-specific uptake of nano-based formulations are facilitated by the reticuloendothelial system and macrophages, which results in the prior removal of extraneous materials (and poor drug material selectivity) from the cellular surface (Bachmaier et al., 2020; Boulware et al., 2020). This can be overcome by the utilization of bioactive target specific ligands and PEG nanostructured materials as discussed in the usages of polymeric materials for the prevention of clustering of nanoparticles. These are quite useful in the reduction of aggregation properties of such particles in the aqueous phase (Chauhan et al., 2020). In general, the occurrence, fate, behavior and toxicity profile of nanoparticles largely depend on the steric stabilization factors, which may have direct correlation with the biochemical enzymatic degradation and specific receptors immune response (Dong et al., 2020; Grein et al., 2020). Much more scientific investigations are required in order to get more insights on nanotoxicity, where renal, pulmonary and hepatotoxicity studies are required to build a safety profile of such nanodelivery (therapeutic or diagnostic) agents.
4.1. Nano-based approach for SARS-CoV-2 infection inhibition
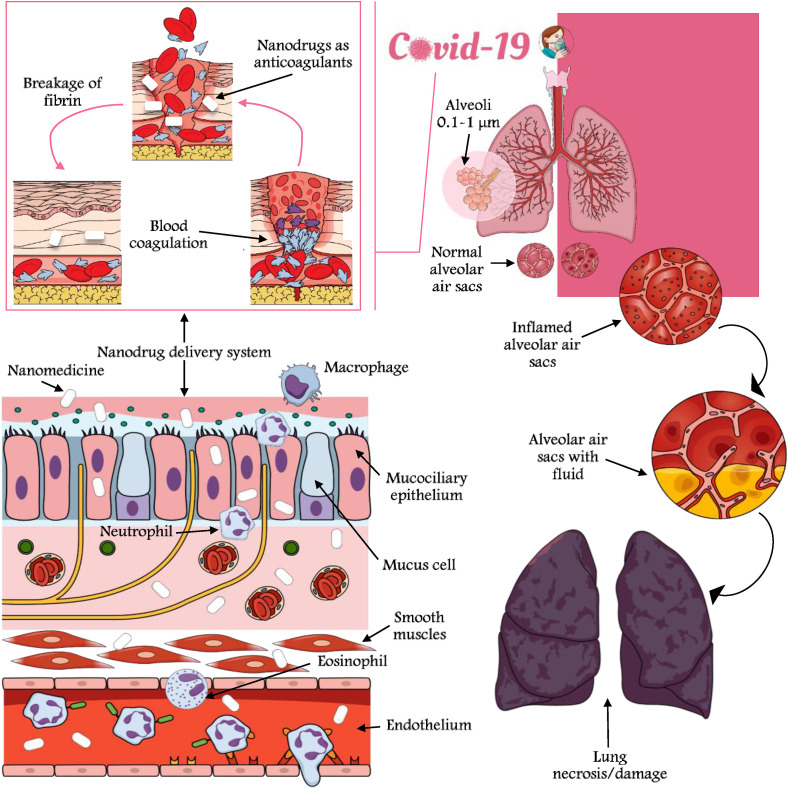
Nano-based formulations have already shown excellent therapeutic approaches for infectious virus detection as mentioned earlier under the section 2. The possible detection and treatment of SARS-CoV-2 have been documented elsewhere by Risitano et al. (2020) and Wang et al., 2020, Wang et al., 2020, where a molecular modeling approach was undertaken along with FMDV 3Dpol. The wider application of carbon based nanomaterials has been documented by Wang et al., 2020, Wang et al., 2020 and Letko et al. (2020) for the inactivation of H3N2, H5N1 and HBV. Oxidation of viral proteins that generate reactive oxygen species often function as an initiation inhibitor for the prevention of FMDV RNA synthesis that might promote mutations in RdRps through hydrophobic interactions (Table 3 ). Viral binding of gp120 and CD4 through host cell surface receptor and photothermal medium (regulation of cell pH) can be achieved in vitro (for HIV-1 virus with AuNPs) (Fig. 3 ). SARS-CoV-2 nsp12 (along with polymerase, interface and NiRAN domain) conserve the sequence variations in the NTP-binding motifs through N-terminal region of nsp12 (current CoV-2 strain) (Wang et al., 2020, Wang et al., 2020; Prather et al., 2020). Inhibition of virus entry can be mediated by virus surface glycoprotein interaction with nanomedicines (i.e. Nipah virus in vitro treatment) ((Dong et al. (2020) and Udugama et al. (2020)), where G and F surface proteins were targeted by lipid based nanoparticles. Conservative binding of viral genome with TiNPs, p53 phosphorylation, modulation of genetic engineering of viral vectors/transcription and selenium embedded nanotherapeutics can be effectively used for SARS-CoV-2 control (Fig. 3).
Table 3.
Diagnostic approaches adopted for the different species of genus Betacoronavirus by the developed and developing countries according to the preliminary laboratory clinical trials (Souce of data: Agostini et al., 2018; Kang, 2020; Wu et al., 2020; Weiss et al., 2020; Zhou et al., 2020a,b; Zhang et al., 2017).
| Species in the Betacoronavirus | Origin of clinical samples | Year/stage of development | Salient findings | Sensitivity/specificity |
|---|---|---|---|---|
| SARS-CoV | Real-time fluorescent PCR (Hong Kong) | 2003 | Enhanced real-time PCR method was effective | Threshold sensitivity |
| SARS-CoV | Blot assay with N195 protein (Singapore) | 2003 | >90% of the specificity observed | Threshold sensitivity |
| SARS-CoV | Biochemical assay (Taiwan) | 2004 | Neutralization test was found suitable in terms of sensitivity observed | Threshold sensitivity |
| MERS-CoV | RNA amplification kit (Japan) | 2014 | RT-PCR was able to detect even at lower detection range of viral RNA copies (~1.6–2.0) | Threshold sensitivity |
| MERS-CoV | RNA detection kits based on rRT-PCR (Korea) | 2016 | Kits were able to provide good specificity and sensitivity clinical specimens having high inhibition potential | Threshold sensitivity |
| MERS-CoV | upE and ORF1a gene based PCR (Korea) | 2017 | RT-iiPCR Assays and RT-qPCR assays were correlated |
Threshold sensitivity |
| SARS-CoV | RT-PCR biochemical assay (Canada) | 2004 | Natural process mimics peroxidases | Broad dynamic detection ranges |
| SARS-CoV | Real time qRT-PCR (Hong Kong) | 2005 | Immunocromatographic test and ELISA test was quite useful with specificity of 95% | Broad dynamic detection ranges |
| MERS-CoV | rRT-PCR based assay (Iran) | 2015 | non-nested RT-PCR assay with Cor-p-F2 and Cor-p-R1 was found suitable | Broad dynamic detection ranges |
| SARS-CoV-2 | rRT-PCR based assay (Germany) | 2020 | RdRp gene assays and E gene provided satisfactory results | Broad dynamic detection ranges |
| SARS-CoV-2 | RT-PCR test Kit based assay (U.K.) | 2020 | Broad dynamic detection range | Broad dynamic detection ranges |
| SARS-CoV-2 | RT-PCR test Kit based assay (China) | 2020 | Metagenomics sequencing kit provided good results | Broad dynamic detection ranges |
| SARS-CoV-2 | RT-PCR test Kit based assay (U.S.A.) | 2020 | Commercial process gave good results | Threshold sensitivity |
| SARS-CoV-2 | Enzyme-assisted nanocomplexes for nucleic acids detection (Singapore) | 2020 | High-throughput screening enabled monitoring of evolution | Broad dynamic detection ranges |
| SARS-CoV-2 | Convalescent plasma therapy (India) | 2020 | Under pre-clinical trial and not licensed for diagnostic procedures |
Threshold Sensitivity (more clinical trials/observations are required) |
Fig. 3.
Conceptual diagram of host/receptor cell infection after attachment with coronaviruses (SARS-CoV-2) and signaling pathways of virulent pathogens render overexpression of genetic and serological markers that help in the biochemical balance of cell survival and cell death after virulence. Nanodrugs can pass the physiological and anatomical barriers of the respiratory system and can act as anticoagulants.
On the other hand, oligonucleotides based nanocomposite binding, silencing of viral replication with niosomes/liposomes, polyadenylated and capped lineage B of β-CoVs sequencing etc. can be used to have more insights on nanoparticulate drug carriers against SARS-CoV-2 (Das et al., 2020). Much more clinical trials are needed to build strong hypotheses on nano-based antiviral therapy that can address subgenomic and individual RNAs translation into replication-transcription complexes (Table 3). Phylogenetic analysis of coronavirus-encoded papain-like proteases renders examples of antiviral targets, where some antiviral drugs such as 5-fluorouracil, remdesivir, ribavirin and other repurposed drugs can be conjugated to exert prophylactic/therapeutic action at the epithelial cells (Grein et al., 2020; Boulware et al., 2020). Blocking of the secretion of antiviral signaling molecules and the reduced expressions of the pattern recognition receptors may further help in the nanodrug therapy with known virus infection and histopathological features (Fig. 3). For the recent SARS-CoV-2 outbreak the utilization of genetic segments i.e Recombinant DNA (rDNA) will be helpful in the development of nanovaccines along with other subunit and virus like particles derived vaccines, etc. Angiotensin converting enzyme 2 (ACE2) is commonly found in the cellular matrices of small intestine and lung, which may hinder the action of nanotherapeutics considering the mode of action of SARS-CoV-2 (Dung et al., 2020; Chauhan et al., 2020).
Researchers (Choi et al., 2018; Iravani, 2020) have undergone several clinical trials for the real curative solution for SARS and MERS, but it was reported that social, economical and clinical limitations influenced the development of effective antiviral therapy in nanodrugs. Most of the clinical trials were in experimental stages (in vitro phase) and some of them have received green signals as a post-exposure prophylaxis (i.e.advanced drug treatment options for SARS-CoV) (Table 3). Many researchers (Agostini et al., 2018; Kang, 2020) have found that corticosteroids with interferon alfacon-1 may have effective antiviral actions for such group of coronaviruses having RNA-dependent RNA polymerases. Gao et al. (2020) and Gordon et al. (2020) have reported that niclosamide, novavax and geovax might have the potential to inhibit virus-cell entry and virus replication. They have documented that more clinical trials are needed to validate the clinical actions of new generation nanotherapeutics that might also have some immunomodulatory or immunostimulatory actions.
5. Role of antiviral nanotherapy in infectious virus control
Viruses as obligate intracellular infectious pathogens interact with host cells involving different types of receptor/ligand biochemical interactions. The prime requirement of drug design mainly depends on several biological factors such as replication dynamics, sub-cellular complex biochemical interaction, the chances of latent infection and development of drug resistance events (Chhikara et al., 2020; Chauhan et al., 2020). The use of nanotechnology has gained popularity in medical oncology though it has been reported that some chemotherapeutic agents do not possess target specific actions for malignant cells (Iravani, 2020; Ishida, 2019). The mode of action of such nano-based therapeutic agents can be categorised based on the permeability of vasculature or attachment of bioactive ligands to the selective nanotherapeutics (active targeting for virus infections) (Kalantar-Zadeh et al., 2020; Kang, 2020).
Specific subcellular regions or particular organelles trigger the mode of action of nanodrugs/nanocarriers based on the attachment potential of such agents with specific host/receptor cells with the nuclear localization signals (Fig. 2). Strand transfer reactions of the infectious virus are prevented by integrase inhibitors action, which on the other hand drive the disease drug development after traversing the inaccessible biological compartments (Jamshidi et al., 2020; Gao et al., 2020). The drug delivery actions (at all concentrations) are often controlled by BBB compartments as observed for HIV infections, where neurological disorders (due to replications) can result in latency in the brain in the absence of antiretroviral (ARV) drugs. ARV conjugated nanoparticles are quite useful in the control of viral replications due to their small size overcoming the barriers of targeted and specific cell site actions, which provides efficient delivery of the nanoformulations at high drug payload (Hu et al., 2020; Kumar et al., 2020, Kumar et al., 2020, Kumar et al., 2020). These types of molecular mechanisms will be useful in decreasing the probability of antiretroviral-drug resistance events for the therapeutic applications of nanoformulations in the clinical drug delivery and diagnostics of HIV/H5N1/HBV/HSV (Gao et al., 2020; Lembo et al., 2018).
6. Conclusions
In the present review, we have synthesized and critically analyzed the available literature data on nanobiotechnology aspects of biomedical research and highlighted the occurences, mode of action and limitations of a suite of nanotherapeutics commonly used in the treatment of viral infections. The pivotal conclusions and suggestions derived out of this review are summarized below:
-
a)
Over the past decades, a bunch of literature have been published on the conventional immune mediated antiviral drugs and vaccines, but little is known about the functionalized nanoparticle-based drug delivery systems for the treatment of SARS-CoV-2. Therefore, the present review provides deep insights on the feasibility of controlling of COVID-19 outbreaks by such nanotherapeutic agents.
-
b)
In vitro antiviral activity and optimized dosing of nano-based formulations have been found to be effective against hepatitis, HIV, Ebola and influenza viruses, where co-ordination with suitable targeting multivalent ligands enables such nanocarriers to bind with target host-receptor cells.
-
c)
The enhancement of drug efficiency (and gene delivery)/therapies are commonly applied to a spectrum of diseases (i.e. HPV, RSV, Noro and Ebola virus) that can be reliable and cost-effective methods for in vivo utilization of immunoliposomes and carbon nanotubes. These are useful for adults, children and middle aged people considering their non-toxic, good immunogenicity, stability and good biological profile.
-
d)
To understand the proper mode of action of nano-based strategies against SARS-CoV-2, pathogenesis of the novel coronavirus needs to be evaluated. The utilization of nanoparticles for nanomedicine/nano-based cell therapy will be useful in blocking the downstream signaling actions of pegylated α which will otherwise impart resistance to infected cells.
Author credit statement
Santanu Mukherjee: Conceptualization, Data curation, Writing - original draft. Payal Mazumder: Writing - review & editing, Visualization. Madhvi Joshi: Writing - review & editing, Visualization. Chaitanya Joshi: Writing - review & editing, Visualization. Sameer V. Dalvi: Visualization, Investigation. Manish Kumar: Conceptualization, Supervision, Validation, Writing - review & editing.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
Support from IIT-Gandhinagar is thankfully acknowledged. Any opinions, findings, and conclusions expressed in this material are those of the author's personal views. This work is funded by Science and Engineering Research Board National Post-doctoral (SERB-NPDF) Grant (File no : NPDF/2018/002751/EAS) awarded to Santanu Mukherjee, Earth Science, Indian Institute of Technology (IIT) Gandhinagar.
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S. 2018. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlawat J., Narayan M. Intelligent Nanomaterials for Drug Delivery Applications. Elsevier; 2020. Introduction to active, smart, and intelligent nanomaterials for biomedical application; pp. 1–16. [Google Scholar]
- Alizadeh F., Khodavandi A. Systematic review and meta-analysis of the efficacy of nanoscale materials against coronaviruses–possible potential antiviral agents for SARS-CoV-2. IEEE Trans. NanoBioscience. 2020;19(3):485–496. doi: 10.1109/TNB.2020.2997257. [DOI] [PubMed] [Google Scholar]
- Bachmaier K., Stuart A., Hong Z., Tsukasaki Y., Singh A., Chakraborty S., Mukhopadhyay A., Gao X., Maienschein-Cline M., Kanteti P., Rehman R. bioRxiv; 2020. Selective Nanotherapeutic Targeting of the Neutrophil Subset Mediating Inflammatory Injury. [Google Scholar]
- Balakrishna A., Sravya G., Surendra T.V., Reddy C.S., Zyryanov G.V., Reddy N.B. Combination Therapy against Multidrug Resistance. Academic Press; 2020. Multidrug resistance and the prospects of combination therapy; pp. 65–79. [Google Scholar]
- Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W. New England Journal of Medicine; 2020. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D.C., Wright B., Jerome W.G., Rose J.P., Wilson E. A unique protein self-assembling nanoparticle with significant advantages in vaccine development and production. J. Nanomater. 2020;2020:1–10. 4297937 2020. [Google Scholar]
- Chaturvedi U.C., Shrivastava R. Interaction of viral proteins with metal ions: role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005;43(2):105–114. doi: 10.1016/j.femsim.2004.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan G., Madou M.J., Kalra S., Chopra V., Ghosh D., Martinez-Chapa S.O. Nanotechnology for COVID-19: therapeutics and vaccine research. ACS Nano. 2020;14(7):7760–7782. doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- Chhikara B.S., Rathi B., Singh J., Poonam F.N.U. Corona virus SARS-CoV-2 disease COVID-19: infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chem. Biol. Lett. 2020;7(1):63–72. [Google Scholar]
- Choi J.H., Jeong K., Kim S.M., Ko M.K., You S.H., Lyoo Y.S., Kim B., Ku J.M., Park J.H. Synergistic effect of ribavirin and vaccine for protection during early infection stage of foot-and-mouth disease. J. Vet. Sci. 2018;19(6):788–797. doi: 10.4142/jvs.2018.19.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S., Kumar R., Dalal U., Tomar S., Reddy S.N. Materials Science and Engineering: C; 2020. Green Synthesis of Nanometal Impregnated Biomass–Antiviral Potential. [DOI] [PubMed] [Google Scholar]
- Cojocaru F.D., Botezat D., Gardikiotis I., Uritu C.M., Dodi G., Trandafir L., Rezus C., Rezus E., Tamba B.I., Mihai C.T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12(2):171. doi: 10.3390/pharmaceutics12020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Ahmed R., Akhtar S., Begum K., Banu S. 2020. An Overview of Basic Molecular Biology of SARS-CoV-2 and Current COVID-19 Prevention Strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Dung T.T.N., Nam V.N., Nhan T.T., Ngoc T.T.B., Minh L.Q., Nga B.T.T., Quang D.V. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express. 2020;6(12):1250g9. [Google Scholar]
- El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020:1–12. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- Etman S.M., Elnaggar Y.S., Abdallah O.Y. Fucoidan, a natural biopolymer in cancer combating: from edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020;147:799–808. doi: 10.1016/j.ijbiomac.2019.11.191. [DOI] [PubMed] [Google Scholar]
- Farzin L., Shamsipur M., Samandari L., Sheibani S. HIV biosensors for early diagnosis of infection: the intertwine of nanotechnology with sensing strategies. Talanta. 2020;206:120201. doi: 10.1016/j.talanta.2019.120201. [DOI] [PubMed] [Google Scholar]
- Gacem M.A., Gacem H., Ould-El-Hadj-Khelil A. Elsevier; 2020. Nanocarbons: antibacterial, antifungal, and antiviral activity and the underlying mechanism; pp. 505–533. (Carbon Nanomaterials for Agri-Food and Environmental Applications). [Google Scholar]
- Gadade D.D., Pekamwar S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharmaceut. Bull. 2020;10(2):166. doi: 10.34172/apb.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. Enhancing the development of therapeutics against SARS-CoV-2 by exploring the properties of therapeutic nano-structures. Precis. Nanomed. 2020;3(2):525–532. [Google Scholar]
- Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 2020;15(4):247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatov I. Global Congress on Infectious Diseases. Sci Tech Infectious Diseases; 2020. Antiviral effects of nano colloidal silver, water catholyte, oxidal with methylene blue. possible effects of influence over coronavirus SARS-CoV and SARS-CoV-2 with Disease COVID-19. 2020. [Google Scholar]
- Iravani S. Nanoparticles and Their Biomedical Applications. Springer; Singapore: 2020. Biomedical applications of lignin-based nanoparticles; pp. 217–224. [Google Scholar]
- Ishida T. Review on the role of Zn 2+ ions in viral pathogenesis and the effect of Zn 2+ ions for host cell-virus growth inhibition. Am. J. Biomed. Sci. Res. 2019;2 [Google Scholar]
- Jamshidi M., Lalbakhsh A., Talla J., Peroutka Z., Hadjilooei F., Lalbakhsh P., Jamshidi M., La Spada L., Mirmozafari M., Dehghani M., Sabet A. Artificial intelligence and COVID-19: deep learning approaches for diagnosis and treatment. IEEE Access. 2020;8:109581–109595. doi: 10.1109/ACCESS.2020.3001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar-Zadeh K., Ward S.A., Kalantar-Zadeh K., El-Omar E.M. Considering the effects of microbiome and diet on SARS-CoV-2 infection: nanotechnology roles. ACS Nano. 2020;14(5):5179–5182. doi: 10.1021/acsnano.0c03402. [DOI] [PubMed] [Google Scholar]
- Kang J.H. Multiscale biofluidic and nanobiotechnology approaches for treating sepsis in extracorporeal circuits. Biochip J. 2020:1–9. doi: 10.1007/s13206-020-4106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar M., Khan N.A., Panwar A., Bais S.S., Basak S., Goel R., Sopory S., Medigeshi G.R. Zinc chelation specifically inhibits early stages of dengue virus replication by activation of NF-kappaB and induction of antiviral response in epithelial cells. Front. Immunol. 2019;10:2347. doi: 10.3389/fimmu.2019.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry R.G., Malik S., Redda Y.T., Sahoo S., Patra J.K., Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yeom M., Lee T., Kim H.O., Na W., Kang A., Lim J.W., Park G., Park C., Song D., Haam S. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020;18(1):1–11. doi: 10.1186/s12951-020-00611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019;10(1):1–9. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarelos K. Nature Publishing Group; 2020. Nanoscale Nights of COVID-19 (Doctoral Dissertation. [DOI] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Dhangar K. Groundwater for Sustainable Development; 2020. The Most Eagerly Awaited Summer of the Anthropocene: A Perspective of SARS-CoV-2 Decay and Seasonal Change; p. 100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Dhangar K., Mazumder P., Sonne C., Rinklebe J., Kitajima M. Environmental Science & Technology; 2020. Potential Emergence of Antiviral-Resistant Pandemic Viruses via Environmental Drug Exposure of Animal Reservoirs. [DOI] [PubMed] [Google Scholar]
- Kumar M., Taki K., Gahlot R., Sharma A., Dhangar K. Science of The Total Environment; 2020. A Chronicle of SARS-CoV-2: Part-I-Epidemiology, Diagnosis, Prognosis, Transmission and Treatment; p. 139278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo D., Donalisio M., Civra A., Argenziano M., Cavalli R. Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expet Opin. Drug Deliv. 2018;15(1):93–114. doi: 10.1080/17425247.2017.1360863. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y., Xing X. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A.P. Nanotechnology in the era of covid-19. Nanomater. Nanotechnol. Res. 2020;1:1. [Google Scholar]
- Mainardes R.M., Diedrich C. 2020. The Potential Role of Nanomedicine on COVID-19 Therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi P., Wang C.Y., Zare E.N., Borzacchiello A., Niu L.N., Tay F.R. Advanced Functional Materials; 2020. Metal‐based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects; p. 1910021. [Google Scholar]
- Nalla A.K., Casto A.M., Huang M.L.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J. Clin. Microbiol. 2020;58(6):1–6. doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrollahzadeh M., Sajjadi M., Soufi G.J., Iravani S., Varma R.S. Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials. 2020;10(6):1072. doi: 10.3390/nano10061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogi U., Hill K.J., Ambikan A.T., Heng X., Quinn T.P., Byrareddy S.N., Sönnerborg A., Sarafianos S.G., Singh K. Feasibility of known RNA polymerase inhibitors as anti-SARS-CoV-2 drugs. Pathogens. 2020;9(5):320. doi: 10.3390/pathogens9050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Duong Bang D., Wolff A. 2019 novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2020;11(3):306. doi: 10.3390/mi11030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaeen G., Abbaszadeh S., Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine. 2020;15(15):1501–1512. doi: 10.2217/nnm-2020-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. Science of the Total Environment; 2020. What Do We Know about the SARS-CoV-2 Coronavirus in the Environment? p. 138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palestino G., García-Silva I., González-Ortega O., Rosales-Mendoza S. Can nanotechnology help in the fight against COVID-19? Expert Rev. Anti-infect. Ther. 2020:1–16. doi: 10.1080/14787210.2020.1776115. [DOI] [PubMed] [Google Scholar]
- Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020:100883. doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V.M., Singhal S., Masand N. Life Sciences; 2020. A Systematic Review on Use of Aminoquinolines for the Therapeutic Management of COVID-19: Efficacy, Safety and Clinical Trials; p. 117775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather K.A., Wang C.C., Schooley R.T. Reducing transmission of SARS-CoV-2. Science. 2020;368(6498):1422–1424. doi: 10.1126/science.abc6197. [DOI] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A.J., Rademaker A., Hochster H.S., Ryan T., Hensing T., Shankaran V., Baddi L., Mahalingam D., Mulcahy M.F., Benson A.B., III Docetaxel, oxaliplatin, and 5‐fluorouracil (DOF) in metastatic and unresectable gastric/gastroesophageal junction adenocarcinoma: a phase II study with long‐term follow‐up. Oncol. 2019;24(8):1039. doi: 10.1634/theoncologist.2019-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-López E., Paús A., Pérez-Pomeda I., Calpena A., Haro I., Gómara M.J. Lipid vesicles loaded with an HIV-1 fusion inhibitor peptide as a potential microbicide. Pharmaceutics. 2020;12(6):502. doi: 10.3390/pharmaceutics12060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W., Yang Y., Rao Y., Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ VACCINES. 2020;5(1):1–3. doi: 10.1038/s41541-020-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankarapillai V.S., Pillai A.M., Rahdar A., Sobha A.P., Das S.S., Mitropoulos A.C., Mokarrar M.H., Kyzas G.Z. On facing the SARS-CoV-2 (COVID-19) with combination of nanomaterials and medicine: possible strategies and first challenges. Nanomaterials. 2020;10(5):852. doi: 10.3390/nano10050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sportelli M.C., Izzi M., Kukushkina E.A., Hossain S.I., Picca R.A., Ditaranto N., Cioffi N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials. 2020;10(4):802. doi: 10.3390/nano10040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V., editor. Handbook of Materials for Nanomedicine: Lipid-Based and Inorganic Nanomaterials. CRC Press; 2020. [Google Scholar]
- Tremiliosi G.C., Simoes L.G.P., Minozzi D.T., Santos R.I., Vilela D.B., Durigon E.L., Machado R.R.G., Medina D.S., Ribeiro L.K., Rosa I.L.V., Assis M. BioRxiv; 2020. Ag Nanoparticles-Based Antimicrobial Polycotton Fabrics to Prevent the Transmission and Spread of SARS-CoV-2. [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y., Chen H., Mubareka S., Gubbay J.B., Chan W.C. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Valdiglesias V., Laffon B. Let’s not forget nanosafety!; Nanotoxicology: 2020. The Impact of Nanotechnology in the Current Universal COVID-19 Crisis; pp. 1–4. [DOI] [PubMed] [Google Scholar]
- Vazquez-Munoz R., Lopez-Ribot J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Preprints. 2020:2020060301. [Google Scholar]
- Viceconte G., Petrosillo N. COVID-19 R0: magic number or conundrum? Infect. Dis. Rep. 2020;12(1) doi: 10.4081/idr.2020.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Fu A., Hu B., Tong Y., Liu R., Gu J., Liu J., Jiang W., Shen G., Zhao W., Men D. medRxiv; 2020. Nanopore Target Sequencing for Accurate and Comprehensive Detection of SARS-CoV-2 and Other Respiratory Viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waris A., Ali M., Khan A.U., Ali A., Baset A. Role of nanotechnology in diagnosing and treating COVID-19 during the Pandemic. Int. J. Clin. Virol. 2020;4:65–70. [Google Scholar]
- Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., Scott J.A., Vitale F., Unal M.A., Mattevi C., Bedognetti D. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wang X. COVID-19: a new challenge for human beings. Cell. Mol. Immunol. 2020;17(5):555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Bu F., Zhou H., Wang Y., Cui J., Wang X., Nie G., Xiao H.H. Biosafety materials: an emerging new research direction of materials science from COVID-19 outbreak. Mater. Chem. Front. 2020;4:1930–1953. [Google Scholar]
- Zhang P., Liu G., Chen X. Nanobiotechnology: cell membrane-based delivery systems. Nano Today. 2017;13:7–9. doi: 10.1016/j.nantod.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Kroll A.V., Holay M., Fang R.H., Zhang L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020;32(13):1901255. doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaie S., Koucheck M., Miri M., Salarian S., Shojaei S., Haghighi M., Sistanizad M. Review of therapeutic agents for the treatment of COVID-19. J. Cell. Mol. Anesth. 2020;5(1):32–36. [Google Scholar]