Abstract
Purpose:
The presence of brain metastases (BM) in patients with non-seminomatous germ cell tumor (NSGCT) is associated with poor prognosis. While radiation therapy (RT) is an important treatment for patients with NSGCT BM, there is a paucity of data on the optimal regimen. We sought to investigate the impact of RT on clinical outcomes in patients with NSGCT BM.
Methods:
Patients with NSGCT BM who received RT at our institution from 2002–2017 were included. Sixty-three consecutive patients were identified. Clinical factors associated with intracranial control (ICC) and overall survival (OS) were evaluated using Cox regression analysis and Kaplan Meier method.
Results:
Median age was 31 years and number of BM was 3. Fifteen patients presented with BM at diagnosis, while 48 developed BM at a median time of 8.4 months from diagnosis. At a median follow-up of 3.6 years, ICC and OS were 39.7% and 30.1%. On multivariate analysis, ICC (hazard ratio [HR]=0.93, p=0.03) and OS (HR=0.93, p=0.005) were both significantly associated with biologically effective dose (BED) of RT. The 4-year OS of patients who received BED <39Gy, 39Gy, 40–50 Gy, and ≥50 Gy were 0%, 14.7%, 34.1%, and 70.0%, respectively. Patients who achieved intracranial control after RT were able to achieve long-term survival (4-year OS 68.1% vs. 0%, p<0.0001).
Conclusions:
Our data supports that a higher BED is required for durable ICC, and that ICC is needed for patients with NSGCT to achieve long-term survival. Prospective studies evaluating radiation dose-escalation for the treatment of NSGCT BM should be considered.
Keywords: non-seminomatous germ cell tumor, brain metastases, whole brain radiation therapy, stereotactic radiosurgery, dose-response relationship
INTRODUCTION
Although most patients with advanced non-seminomatous germ cell tumors (NSGCT) can achieve long-term survival, patients with NSGCT and brain metastases (BM) have been considered to have poor prognosis [1, 2]. Due to the rarity of developing BM from NSGCT (1% at diagnosis and 0.4–4% subsequently) [2–4], there is a paucity of data defining the optimal treatment regimen in this population. While some patients are treated with multi-modality therapy, others are treated with systemic therapy alone or with local therapy alone [5–12].
Despite BM being a defining feature of poor prognosis in NSGCT, retrospective studies have suggested the possibility of long-term cure in select patients, especially in patients who received radiation therapy (RT) for BM [1, 4, 8]. For example, in a small series from Indiana University Medical Center, 6 of 14 patients with NSGCT BM treated with curative intent became long-term survivors, all of whom had received high doses of whole brain RT [8]. Nevertheless, despite evidence that RT plays a critical role for patients with NSGCT BM [4, 8], little is known regarding the optimal technique and dose of RT needed to maximize intracranial control and survival; various modalities and doses of RT have been employed ranging from purely palliative approaches to curative attempts. Given the possibility of cure in this young patient population, it is essential to identify those patients with NSGCT BM that may benefit most from an aggressive approach, and what exactly this approach should entail. As such, we sought to evaluate the impact of RT on intracranial control and survival in patients with NSGCT BM, with the goal of identifying the necessary dose and technique of RT needed for long-term cure.
METHODS
Study population
This is a retrospective study of all patients diagnosed with NSGCT and BM at our institution from 2/2002–8/2017. Institutional Review Board approval was obtained for this study. Sixty-three consecutive patients were identified. Patient and tumor characteristics were collected, including date of diagnosis, histology, primary tumor site, first-line chemotherapy received, date of development of BM, extent of systemic disease at time of BM, number of BM, size of largest BM, surgical resection for BM, chemotherapy at time of BM, tumor markers at the time of BM (including AFP, beta hCG, and LDH), RT fields (whole brain radiation therapy [WBRT], stereotactic radiosurgery [SRS]), dose and fractionation of RT, the use of steroids during RT, and acute and late toxicity from RT.
Statistical Analysis
The primary endpoints were intracranial control (ICC) and overall survival (OS). ICC was calculated as the time from RT to intracranial relapse. Imaging at the time of intracranial relapse was compared with baseline imaging at the time of RT to determine the location of the recurrence in relation to prior intracranial involvement. OS was calculated as the time from RT to death. The Kaplan-Meier method was used to estimate ICC and OS following RT. Univariate and multivariate cox proportional hazard modeling was utilized to assess the impact of clinical factors including histology (mixed vs. embryonal vs. yolk sac vs. choriocarcinoma), primary tumor site (testicular vs. mediastinal), chemotherapy (before RT vs. concurrent vs. after RT), surgery, number of BM, extent of systemic disease at time of brain RT (brain only vs. lung vs. liver vs. bone), RT fields (WBRT +/−SRS), biologically effective dose (BED) of RT, and use of steroids (yes vs. no) on ICC and OS. For the BED analysis, α/β ratio of 10 was used for calculation, and only patients who received WBRT as a part of their treatment were included. Variables with a p value ≤0.05 on univariate analysis were included in the multivariate analysis for ICC and OS.
RESULTS
Patient Population
Table 1 details the baseline patient and tumor characteristics. The median age at diagnosis of BM was 31 years (range=14–62 years) and median KPS was 80 (range=60–100); only 5 patients had a KPS <80. Fifteen patients (24%) presented with BM synchronous at initial diagnosis while 48 (76%) developed BM metachronous at relapse. For patients in the latter group, the median time to development of brain metastases from initial diagnosis was 8.4 months (range=3.7–222.9). Other systemic disease at the time of BM included lung (n=39), liver (n=20), and bone (n=8). Median number of BM at the time of RT was 3; specifically, 14 patients (22%) had 1 BM at time of RT; 33 (52%) had 2–5; and 16 (25%) had >5. Median size of BM was 1.8cm (range=0.4–6.3cm). Of the 15 patients with BM >3.0cm, 9 (60%) underwent surgical resection prior to RT. Mean AFP, beta hCG, and LDH at time of BM were 1800 ng/ml, 47873 miU/ml, and 400 U/L, respectively. Thirty-eight patients (60%) received steroids during their course of RT.
Table 1.
Patient and tumor characteristics
| N (%) | |
|---|---|
| Age (years) | |
| Median (range) | 31 (14–62) |
| Primary site of origin | |
| Testicular | 52 (83) |
| Mediastinal | 11 (17) |
| IGCCG Risk Group | |
| 1 | 5 (8) |
| 2 | 13 (21) |
| 3 | 45 (71) |
| Metastatic to brain at diagnosis | |
| Yes | 15 (24) |
| No | 48 (76) |
| Metastatic involvement at time of brain relapse | |
| Lung | 41 (63) |
| Liver | 21 (33) |
| Bone | 8 (13) |
| Brain | 63 (100) |
| Number of brain metastases | |
| 1 | 14 (22) |
| 2–5 | 33 (52) |
| >5 | 16 (25) |
| Size of largest brain metastases (cm) | |
| Median (range) | 1.8 (0.4–6.3) |
| Surgery for brain metastases | |
| Yes | 16 (25) |
| No | 47 (75) |
| Chemotherapy for treatment of brain metastases | |
| Concurrent | 11 (17) |
| Before RT | 14 (22) |
| After RT | 10 (16) |
| Radiation field | |
| WBRT | 45 (71) |
| SRS | 7 (11) |
| WBRT + SRS / Cone-down | 9 (14) |
| Partial brain | 1 (2) |
| No RT | 1 (2) |
Treatment of Brain Metastases
All patients but one who presented with BM ultimately received RT. RT fields consisted of WBRT alone in 45 patients (71%), SRS alone in 7 (11%), WBRT+SRS or a boost in 9 (14%), and partial brain RT in 1 (2%). WBRT doses varied but included most commonly 30 Gy in 10 fractions (n=22) and 50 Gy in 25–28 fractions (n=10). Of the 48 patients who developed BM at the time of relapse, 26 (54%) received chemotherapy: 6 before RT, 15 after the completion of RT, and 5 concurrently with RT. Of the 15 patients who presented at diagnosis with BM, all received chemotherapy: 9 before RT, and 6 concurrently. Chemotherapy regimens varied at the time of BM diagnosis and included paclitaxel, ifosfamide, and cisplatin (n=17); bleomycin, etoposide and cisplatin (n=9); paclitaxel, ifosfamide, carboplatin, and etoposide (n=4); etoposide, ifosfamide and cisplatin (n=4); and temozolomide (n=1). Sixteen patients (25%) underwent surgical resection of BM prior to RT, of which half were gross total resections and half subtotal resections.
Intracranial Control
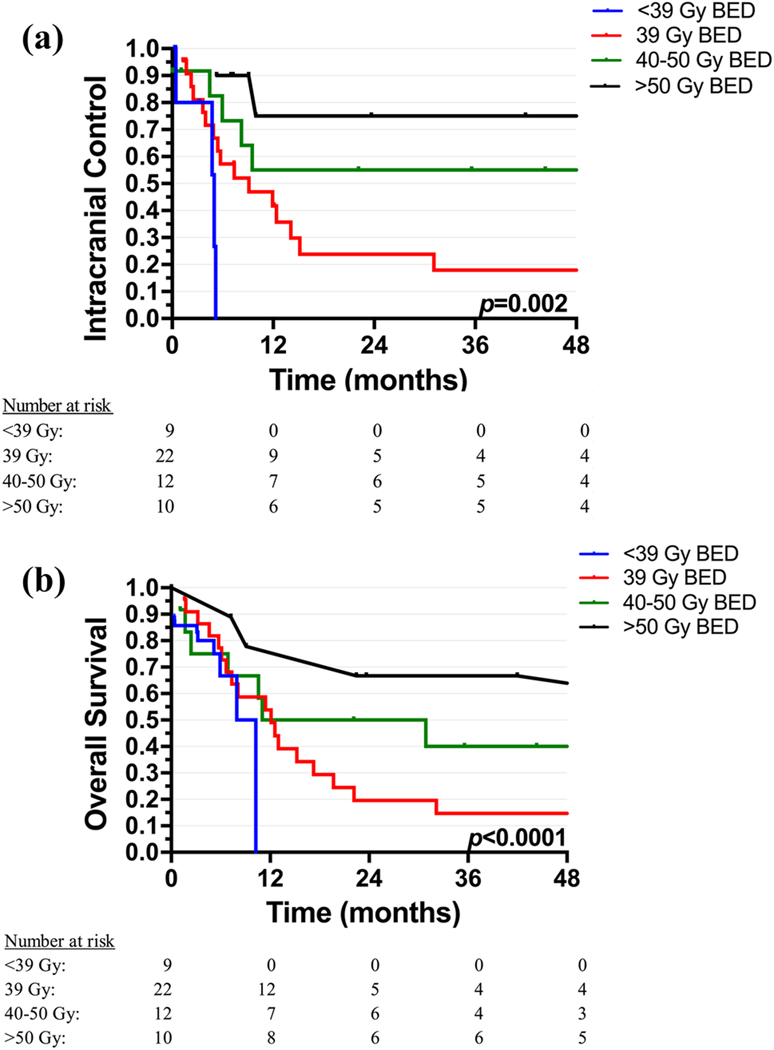
The median follow up was 3.6 years (range=1.5 months-15.8 years). The 4-year ICC rate among the entire cohort was 39.7% (95% confidence interval [CI], 26.1–53.3%). On univariate analysis, ICC was significantly associated with primary tumor site, number of BM, RT fields, BED of RT, and receipt of steroids during RT; but not with histology, time of diagnosis of BM, systemic extent of disease at time of BM, receipt of surgery for BM, and receipt or timing of chemotherapy (before, concurrent, or after RT) (Table 2). Specifically, ICC was improved in patients who received a combination of WBRT with a focal SRS boost or cone-down compared to patients who received SRS or WBRT alone (4-year ICC 74.1% vs. 32.8%). There was also a dose-response relationship seen with respect to ICC: the 4-year ICC was 0% in those who received a BED of <39 Gy; 17.9% in those who received a BED of 39 Gy (equivalent to 30 Gy in 10 fractions); 55.0% in those who received a BED of 40–50 Gy; and 75% in those who received a BED ≥ 50 Gy (Figure 1a). On MVA, only higher BED remained significantly associated with ICC (hazard ratio [HR] = 0.93, p=0.03).
Table 2.
Univariate analysis of intracranial control and overall survival by patient and tumor characteristics
| Intracranial Control | Overall Survival | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value |
| Histology | ||||
| Mixed vs Embryonal | 1.04 (0.24–4.44) | 0.96 | 0.65 (0.16–2.72) | 0.55 |
| Mixed vs Choriocarcinoma | 0.65 (0.22–1.89) | 0.43 | 0.57 (0.21–1.50) | 0.25 |
| Mixed vs Yolk Sac | 0.83 (0.11–6.23) | 0.85 | 0.49 (0.07–3.60) | 0.48 |
| Primary tumor site | ||||
| Testicular vs Mediastinal | 2.23 (1.00–4.98) | 0.05 | 1.85 (0.91–3.77) | 0.09 |
| Timing of brain metastases | ||||
| At relapse vs Diagnosis | 0.8 (0.37–1.72) | 0.57 | 0.71 (0.35–1.44) | 0.34 |
| Number of brain metastases | ||||
| 1 vs 2–5 | 2.47 (0.83–7.41) | 0.11 | 2.36 (0.96–5.80) | 0.06 |
| 1 vs >5 | 5.09 (1.65–15.67) | 0.005 | 5.19 (1.98–13.62) | 0.001 |
| Systemic disease in the lungs | ||||
| No vs Yes | 1.29 (0.60–2.80) | 0.52 | 1.93 (0.99–3.77) | 0.05 |
| Systemic disease in the liver | ||||
| No vs Yes | 1.06 (0.50–2.23) | 0.89 | 1.39 (0.73–2.64) | 0.32 |
| Systemic disease in the bone | ||||
| No vs Yes | 1.26 (0.30–5.31) | 0.76 | 5.38 (2.41–12.01) | <0.0001 |
| Surgery of brain metastases | ||||
| No vs Yes | 0.97 (0.44–2.16) | 0.95 | 0.92 (0.47–1.82) | 0.81 |
| Chemotherapy at time of brain metastases | ||||
| No vs Yes | 1.04 (0.48–2.27) | 0.91 | 0.84 (0.45–1.58) | 0.59 |
| Chemotherapy timing | ||||
| Before RT vs concurrent with RT | 0.95 (0.34–2.62) | 0.92 | 0.70 (0.31–1.59) | 0.39 |
| Before RT vs after RT | 0.90 (0.31–2.61) | 0.85 | 0.87 (0.40–1.88) | 0.72 |
| Radiation fields | ||||
| WBRT or SRS vs Combination | 0.24 (0.06–1.00) | 0.05 | 0.38 (0.14–1.08) | 0.07 |
| Radiation dose (BED, continuous) | 0.90 (0.86–0.96) | <0.0001 | 0.91 (0.88–0.95) | <0.0001 |
| Steroids during radiation | ||||
| No vs Yes | 5.30 (1.50–16.90) | 0.009 | 3.03 (1.25–7.33) | 0.01 |
Figure 1.
a) Intracranial control and b) overall survival by the biologically effective dose (BED) of radiation therapy
Of the 26 patients who developed intracranial recurrence after WBRT alone, 54% recurred at previously treated sites; 25% developed new brain metastases; and 17% recurred at previously treated sites as well as developed new brain metastases. Of the 4 patients who developed intracranial recurrence after SRS, 75% (3 of 4) recurred at previous sites, and 1 patient developed new brain metastases. Of the 2 patients who failed intracranially after receiving WBRT and a SRS boost, 1 developed new brain metastases, and 1 recurred at the previously treated site as well as developed new brain metastases.
Overall Survival
Among the entire cohort, the 4-year OS rate was 30.1% (95% CI, 18.2–42.0%). On univariate analysis, OS was significantly associated with systemic extent of disease at the time of BM (lungs and/or bone), number of BM, BED of RT, and receipt of steroids during RT; but not with histology, primary tumor site, time of diagnosis of BM, receipt of surgery for BM, receipt or timing of chemotherapy for BM, or RT fields (Table 2). On MVA, patients with systemic disease in bone continued to have significantly worse OS, while patients who received higher BED of RT continued to have significantly better OS (HR=3.01, p=0.03 and HR=0.93, p=0.005, respectively, Table 3). There was a strong dose-response relationship with respect to OS: the 4-year OS was 0% in those who received a biologically effective dose (BED) of <39 Gy; 14.7% in those who received a BED of 39 Gy; 40.0% in those who received a BED of 40–50 Gy; and 66.7% in those who received a BED ≥ 50 Gy (Figure 1b). Importantly, patients with intracranial control after RT were able to achieve long-term survival (4-year OS 68.1% vs. 0% in those who relapsed in the brain, p<0.0001).
Table 3.
Multivariate analysis of overall survival
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Number of brain metastases | ||
| 1 vs 2–5 | 0.91 (0.19–4.40) | 0.90 |
| 1 vs >5 | 1.36 (0.26–7.08) | 0.71 |
| Systemic disease in the lungs | ||
| No vs Yes | 1.17 (0.47–2.92) | 0.73 |
| Systemic disease in the bone | ||
| No vs Yes | 3.01 (1.13–8.02) | 0.03 |
| Radiation dose (BED, continuous) | 0.93 (0.88–0.98) | 0.005 |
| Steroids during radiation | ||
| No vs Yes | 1.49 (0.57–3.93) | 0.42 |
Toxicity
Acute toxicity (defined as toxicity developed during and within 30 days of RT) from RT was minimal. Five patients experienced grade ≥2 acute toxicity, including 2 patients with grade 2 fatigue; 1 with grade 2 nausea; 1 with grade 3 dermatitis after 50 Gy in 25 fractions; and 1 with grade 4 hemorrhage from the tumor after 30.5 Gy in 15 fractions. Documented late toxicity in the 19 long-term survivors consisted of short-term memory loss (n=3) and RT necrosis (n=2).
DISCUSSION
BM in NSGCT remain a clinically challenging entity. Although potentially curable, there is a paucity of data evaluating the necessary dose and role of RT for patients with NSGCT BM. In addition, given the heterogeneity in treatment of NSGCT BM, it is difficult to decipher the optimal treatment regimen for these patients from various retrospective series. As such, we sought to investigate the specific role of RT in NSGCT BM. To our knowledge, we present the largest series to date evaluating the impact of RT on intracranial control and survival in patients with NSGCT BM.
Our data suggests that the BED of RT is the most important factor affecting both ICC and OS. In addition, we show that a BED of 39 Gy (equivalent to 30 Gy in 10 fractions, the typical dose used for WBRT) does not seem to provide durable ICC or long-term survival in patients with NSGCT BM. This is in concordance with results from Indiana University Medical Center including 24 patients with NSGCT BM that suggested a WBRT dose of 40–50 Gy in fractions of 2 Gy (BED 48–60 Gy using α/β ratio of 10) may be necessary for long-term control [8]. The lack of response to a BED of 39 Gy is likely due to NSGCT being more radioresistant in comparison to other diseases such as seminoma. Importantly, we also found that patients with brain relapse in our cohort had a 0% long-term cure, compared to a 4-year survival of 68% in patients with intracranial control. Unlike other diseases like breast and lung cancer in which patients typically receive WBRT with a palliative intent, patients with NSGCT are potentially curable, further emphasizing the need to use a RT dose that achieves durable disease control. From our results, we concluded that a BED of 50 Gy or higher is needed to achieve durable ICC and OS in patients with NSGCT BM.
Regarding RT fields, patients who received WBRT with a boost to radiographically evident tumors either in the form of a cone-down or SRS had improved ICC on univariate analysis but not on MVA. As BED was the only significant factor on MVA, we concluded that the total dose to the tumor, rather than RT fields, is the most significant contributing factor to intracranial control. Of the patients who developed intracranial recurrence after RT, only 25% developed new brain metastases without failure at previously treated sites. We therefore speculate that while a high BED is necessary to achieve disease control of radiographically evident BM, a lower BED dose is needed to prevent development of new brain metastases. As such, a lower dose WBRT followed by a SRS boost to known metastases may be a feasible strategy to improve the therapeutic ratio by ensuring tumor control while limiting high dose RT to normal brain tissue, therefore reducing the risk of RT toxicities in this young patient population. We believe this approach should be evaluated in a prospective manner to determine the optimal dose of WBRT needed when given in combination with SRS. Lastly, as we only had 7 patients in our cohort treated with SRS alone, we were not able to evaluate whether SRS alone is adequate to achieve durable ICC. Although SRS alone is the favored approach in many disease sites for patients with limited number of brain metastases, there is little data evaluating this approach in NSGCT; further studies evaluating the role of SRS alone in NSGCT are needed. However, given the high recurrence rates in the brain even after WBRT, it is likely that patients who receive SRS will need multiple courses over their lifetime.
Only 16 patients in our cohort underwent surgical resection; although we did not see improved ICC with such an approach, surgery is both appropriate and necessary for patients with large or symptomatic BM. Similar to surgery, the receipt of chemotherapy did not affect ICC or survival in our cohort. It is important to note that the majority of our cohort included patients who developed BM at the time of relapse rather than at diagnosis. As such, the benefit of cisplatin-based chemotherapy, which plays a critical role in newly diagnosed metastatic NSGCT, could not be evaluated. In addition, given the limited number of patients in our cohort who presented with BM at diagnosis, we were unable to observe a difference in outcomes between patients with BM at diagnosis compared to those with BM at relapse, as has been shown in other series [1, 4]. However, it is likely that patients who develop BM after the exposure to frontline chemotherapy represent a more unfavorable group with chemo-resistant cell clones. Therefore, for patients who present with BM at relapse, we agree that a multi-modality approach including RT is critical [1, 4]. Furthermore, we observed 8 long-term survivors in our cohort who had systemic disease in the lung, liver and/or bones at the time of brain relapse and received brain RT. Therefore, unlike what has been concluded in previous series [4, 8], we believe durable disease control is still achievable in some patients with systemic and intracranial relapse; not all patients with extracranial disease at the time of brain relapse should be treated with a purely palliative approach.
Although toxicity in our cohort was minimal, our study was not designed to address late morbidity from RT and only captured documented late effects. Five patients from the Indiana series who received a total of 40–50 Gy in 18–28 fractions along with concurrent cisplatin-based chemotherapy developed symptoms consistent with progressive multifocal leukoencephalopathy at a median time of 72 months from WBRT [13]. Although we did not see this among our survivors treated with 40–50 Gy, we did see RT necrosis and short-term memory loss in patients that received high doses of WBRT. Consideration of late morbidity further supports the use of a lower dose of WBRT followed by a SRS boost to balance disease control with neurologic toxicity. In addition, hippocampal sparing WBRT can be considered given the potential to spare short memory loss as was seen on RTOG 0933 [14].
There are several limitations of our study. Given the retrospective nature, patients were not randomized to receive one dose of RT or the other, and thus there may be confounding clinical factors affecting choice of RT dosing and therefore our results. However, as only 5 patients in our cohort had a KPS <80, performance status was not likely to be a significant factor affecting the RT dose utilized. In addition, on multivariate analysis, BED remained significant after accounting for baseline patient and tumor characteristics.
In summary, we found that RT plays an important role in achieving durable ICC and improving long-term survival in patients with BM from NSGCT. Furthermore, an aggressive approach with a higher BED of RT (≥ 50 Gy) is needed for durable disease control in the brain. Prospective studies evaluating WBRT with a SRS boost for the treatment of NSGCT BM should be considered.
Acknowledgments
Funding: NIH grant P30 CA 008748
Footnotes
Conflicts of Interest: No relevant conflicts of interest. K.B. reports serving as a shareholder of MMT, outside of the submitted work.
REFERENCES
- 1.Feldman DR, Lorch A, Kramar A, Albany C, Einhorn LH, Giannatempo P, Necchi A, Flechon A, Boyle H, Chung P et al. : Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol 2016, 34(4):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997, 15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 3.Raina V, Singh SP, Kamble N, Tanwar R, Rao K, Dawar R, Rath GK: Brain metastasis as the site of relapse in germ cell tumor of testis. Cancer 1993, 72(7):2182–2185. [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD, Bokemeyer C, Gerl A, Culine S, Jones WG, Mead GM, Germa-Luch JR, Pont J, Schmoll HJ, Tjulandin S: Treatment outcome of patients with brain metastases from malignant germ cell tumors. Cancer 1999, 85(4):988–997. [DOI] [PubMed] [Google Scholar]
- 5.Boyle HJ, Jouanneau E, Droz JP, Flechon A: Management of brain metastases from germ cell tumors: a single center experience. Oncology 2013, 85(1):21–26. [DOI] [PubMed] [Google Scholar]
- 6.Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca JG, Kellie S, Maher P, Vlamis V, Walker RW, Leibel S et al. : Chemotherapy without irradiation--a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 1996, 14(11):2908–2915. [DOI] [PubMed] [Google Scholar]
- 7.Hardt A, Krell J, Wilson PD, Harding V, Chowdhury S, Mazhar D, Berney D, Stebbing J, Shamash J: Brain metastases associated with germ cell tumors may be treated with chemotherapy alone. Cancer 2014, 120(11):1639–1646. [DOI] [PubMed] [Google Scholar]
- 8.Spears WT, Morphis JG, 2nd, Lester SG, Williams SD, Einhorn LH: Brain metastases and testicular tumors: long-term survival. Int J Radiat Oncol Biol Phys 1992, 22(1):17–22. [DOI] [PubMed] [Google Scholar]
- 9.Girones R, Aparicio J, Roure P, Germa-Lluch JR, Garcia Del Muro X, Vazquez-Estevez S, Saenz A, Sastre J, Arranz Arija J, Gallardo E et al. : Synchronous versus metachronous brain metastasis from testicular germ cell tumors (TGCT): an analysis from the Spanish Germ Cell Cancer Group data base. Clin Transl Oncol 2014, 16(11):959–965. [DOI] [PubMed] [Google Scholar]
- 10.Mahalati K, Bilen CY, Ozen H, Aki FT, Kendi S: The management of brain metastasis in nonseminomatous germ cell tumours. BJU Int 1999, 83(4):457–461. [DOI] [PubMed] [Google Scholar]
- 11.Nonomura N, Nagahara A, Oka D, Mukai M, Nakai Y, Nakayama M, Nishimura K, Kakimoto K, Nakamura T, Usami M et al. : Brain metastases from testicular germ cell tumors: a retrospective analysis. Int J Urol 2009, 16(11):887–893. [DOI] [PubMed] [Google Scholar]
- 12.Kollmannsberger C, Nichols C, Bamberg M, Hartmann JT, Schleucher N, Beyer J, Schofski P, Derigs G, Ruther U, Bohlke I et al. : First-line high-dose chemotherapy +/−radiation therapy in patients with metastatic germ-cell cancer and brain metastases. Ann Oncol 2000, 11(5):553–559. [DOI] [PubMed] [Google Scholar]
- 13.Doyle DM, Einhorn LH: Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys 2008, 70(5):1361–1364. [DOI] [PubMed] [Google Scholar]
- 14.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN et al. : Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014, 32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]