Abstract
Background
New evidence suggests that histidine triad nucleotide-binding protein 1 (Hint1) exerts a tumor suppressor effect in various human tumors, such as colorectal cancer and gastric cancer. However, it has not been reported whether Hint1 is involved in the occurrence and development of osteosarcoma (OS).
Materials and Methods
The present study investigated the role of Hint1 in human OS cells by using cell lines, including 143B, U2OS, KHOS-240S, Saos-2 and MG-63. Cell proliferation and apoptosis were detected by flow cytometry.
Results
The present result revealed that Hint1 is downregulated in these cell lines. The overexpression of Hint1 by adenovirus transfection in 143B and MG63 cell lines suppressed the proliferation and cell cycle, and increased the cell apoptosis. Mechanically, it was found that Hint1 downregulated the cyclin D1 expression via FOXO1 inhibition. Furthermore, FOXO1 overexpression in the 143B and MG63 cell lines significantly blurred the effects of Hint1 on cellular proliferation and apoptosis.
Conclusion
The present study indicates that Hint1 inhibits the development of OS by regulating FoxO1-cyclin D1, suggesting that Hint1 may be a new method for the treatment of OS.
Keywords: osteosarcoma, histidine triad nucleotide-binding protein 1, cell cycle, apoptosis, FOXO1
Introduction
Osteosarcoma (OS) is the most common malignant primary bone tumor that occurs in children and adolescents. It is characterized by early metastasis, rapid progression and poor prognosis.1 The 5-year survival rate of OS patients is merely 60%.2 The main therapeutic method of OS includes surgical resection combined with chemotherapy and radiotherapy.3 Although scientists have been trying to search for new ways to treat OS in the past few decades, its progress has been slow.4 More importantly, patients with advanced OS and relapsed OS have had a very poor prognosis.3 Thus, better understanding the mechanism underlying the progression of OS and finding new therapeutic targets are of great importance.
Histidine triad nucleotide-binding protein 1 (Hint1) is a haploinsufficient tumor suppressor gene that regulates cell proliferation and survival.5 Studies have revealed the effect of Hint1 on various cancer cells.5–7 The increased probability of colorectal cancer, breast cancer and ovarian tumors was observed in Hint1-deficient mice.8,9 In addition, Hint1 overexpression can reduce the proliferation rate of human SGC7901 gastric cancer cells and increase the sensitivity of cells to radiation.6 Hint1 was reported to suppress the migration and invasion of hepatocellular carcinoma cells.7 Hint1 was also found to associated with prostate cancer and melanoma.10,11 However, the expression pattern of Hint1 and the effect of Hint1 on OS remains unclear. The present study aims to explore the expression pattern and effect of Hint1 on OS cells.
Materials and Methods
Clinical Specimens
From January 2011 to December 2016, 10 surgically removed OS specimens and adjacent normal tissues in the First Hospital of Jingmen City were collected. All patients provided a signed informed consent. The Ethics Committee of the First Hospital of Jingmen City approved the use of human experimental specimens, and all experiments adhered to the Helsinki Declaration.
Cell Lines and Cell Culture
The Shanghai Institute of Cell Biology (Shanghai, China) supplied all the cell lines used in the present study, which included 143B, U2OS, KHOS-240S, Saos-2 and MG-63, and the normal cell line (hFOB1.19) RPMI-1640 medium supplemented by 10% fetal bovine serum (FBS) was used to culture cells in 5% CO2 at 37°C.
Cell Transfection
For the Hint1 overexpression, the coding sequence of human Hint1 and FOXO1 were constructed into an adenovirus vector (Ad) by Vigene Biosciences (Shangdong, China). Ad-Hint1 (6.12*108 VP/mL), or Ad-FOXO1 (5.45*108 VP/mL) or the negative control Ad-NC were transfected into 143B and MG63 cells (MOI=50).
Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8) assay kit was used to determine the cell proliferation. In brief, after cells reached 70% confluence, these cells were transfected with the adenovirus for 48 hours. Then, all cells were cultured for another 24, 48, 72 and 96 hours. Each well was added with 10 µL of CCK-8 solution. Then, a SUNRISE Microplate Reader (Tecan Group, Ltd., Mannedorf, Switzerland) was used to detect the absorbance at 490 nm.
Flow Cytometry Analysis of the Cell Cycle
After cells were transfected with either Ad-NC or Ad-Hint1, these were harvested and re-suspended in 1 mL of staining solution (50 µg/mL of propidium iodide and 20 µg/mL of RNase A). A fluorescence-activated cell sorting flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect these cells after incubating for 30 minutes. The ModFit LT software (version 3.2; Verity Software House, Topsham, ME, USA) was used to analyze the percentage of cells in the G0/G1, S and G2-M phases.
Flow Cytometry Analysis of Cell Apoptosis
After cells were transfected with either Ad-NC or Ad-Hint1, these were harvested and re-suspended. Then, annexin V and propidium iodide (30 μg/mL) were used to stain cells for 30 minutes with RNase (0.6 mg/mL) in phosphate-buffered saline plus 0.5% (v/v) Tween 20 and 2% fetal bovine serum. A FACSCalibur flow cytometer (BD Biosciences) was used for the analysis. The CellQuest software was used to analyze the percentage of apoptotic cells.
Western Blot Analysis and PCR
Radioimmunoprecipitation (RIPA) assay buffer (Wlaterson, Barcelona, Spain) was used to lyse cells. Then, 50 µg of protein in each group was loaded to 12% sodium dodecyl sulfate-polyacrylamide gels (sodium dodecyl sulfate polyacrylamide gel electrophoresis, SDS-PAGE). Afterwards, polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA) were used to transfer the protein. After blocking with 5% skim milk (w/v), these membranes were cultured with the primary antibodies, which included Cyclin D1, P27, FOXO1 and GAPDH (1:100; Cell Signaling Technology, Inc.). The secondary antibody was goat anti-rabbit IgG (LI-COR, 926–32211) or goat anti-mouse IgG (C11026-03; LI-COR). A two-color infrared imaging system (Odyssey, LICOR) was used for the scan. GAPDH protein was used as the reference.
The total RNA obtained from OS specimens and adjacent normal tissues were extracted using TRIzol™ (Roche Diagnostics, Germany). A SmartSpec Plus Spectrophotometer (Bio-Rad, USA) was used to detect the RNA purity. Then, 2 µg of each sample was reverse-transcribed into cDNA using oligo(DT) primers and the Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics). A LightCycler 480 SYBR® Green system was used for quantification. The GAPDH gene was used as a reference.
Coimmunoprecipitation Assays
The psicoR-HA-Hint1 and psicoRFlag-FOXO1 were transfected to the cultured 143B and MG-63 cell lines. Then, these cells were lysed in an immunoprecipitation buffer. For the immunoprecipitation procedure, 10 μL of protein A/G-agarose beads and 1 μg of antibody were incubated with each sample (50 μL) overnight at 4°C. After washing with the immunoprecipitation buffer, the eluted proteins were immunoblotted with the indicated primary antibodies.
Statistical Analysis
The data are expressed as mean ± standard deviation (SD). The differences among groups were analyzed by ANOVA, followed by Tukey’s post hoc test. Comparisons between two groups were analyzed by Student’s t-test. P<0.05 was considered statistically significant.
Results
The Expression Level of Hint1 in Human OS Tissues and OS Cell Lines
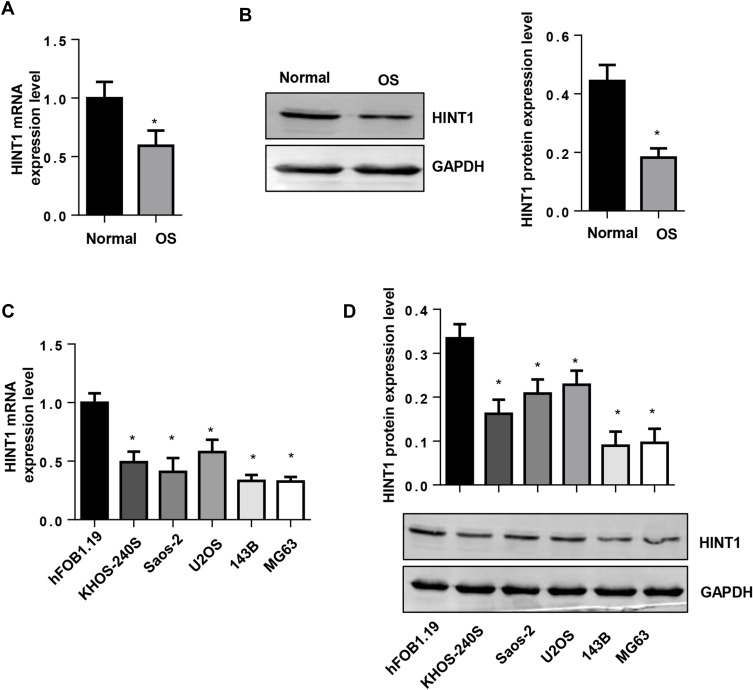
In order to explore the functional role of Hint1 on OS, the expression level of Hint1 was determined on human OS tissues. Interestingly, it was found that both the transcription and protein levels of Hint1 were reduced in human OS tissues (Figure 1A and B). Next, the expression pattern of Hint1 was explored on diverse OS cell lines, including 143B, U2OS, KHOS-240S, UMR-106, Saos-2 and MG-63. The hFOB1.19 cell line was used as the control. As expected, the RNA and protein expression level of Hint1 decreased in all OS cell lines, especially in the 143B and MG-63 cell lines, when compared to control cells (Figure 1C and D). Thus, the 143B and MG-63 cell lines were used to conduct further studies.
Figure 1.
The expression level of Hint1 in human OS tissues and OS cell lines.
Notes: (A) The mRNA expression level of Hint1 decreased in human OS tissues (n=10). (B) The protein expression level of Hint1 decreased in human OS tissues (n=10). *P<0.05 vs normal tissues. (C) The mRNA expression level of Hint1 decreased in the OS cell lines (n=6). (D) The protein expression level of Hint1 decreased in the OS cell lines (n=6). *P<0.05 vs hFOB1.19 cells.
The Overexpression of Hint1 Inhibits the Cell Cycle in OS Cell Lines
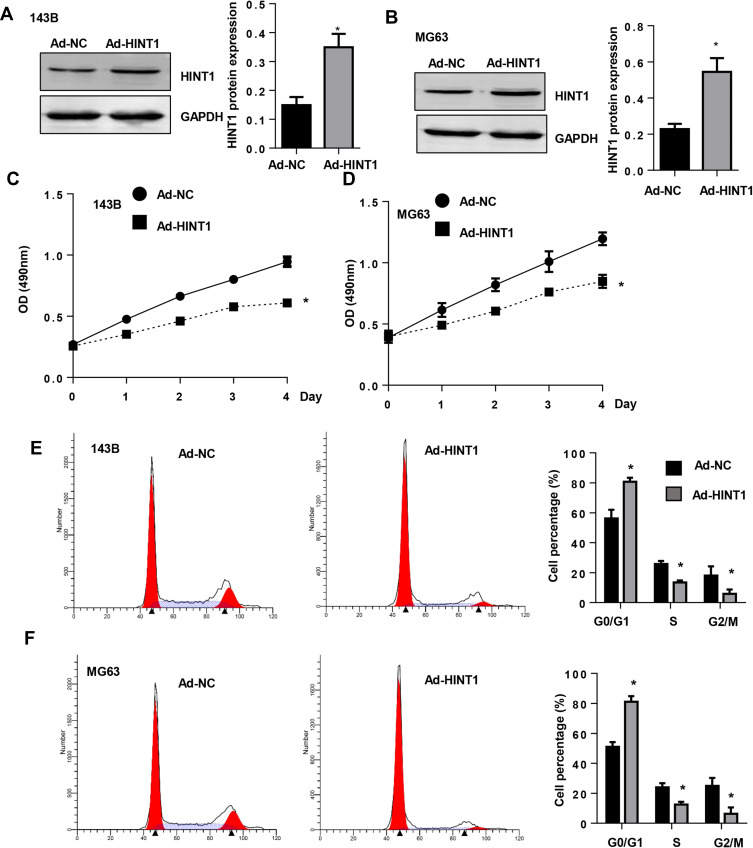
A Hint1 overexpressed adenovirus was constructed, and cells were transfected with Ad-Hint1 to overexpress Hint1 in the 143B and MG-63 cell lines (Figure 2A and B). The CKK-8 assay results revealed that the cell proliferation at 1, 2, 3 and 4 days in the Hint1 overexpression group sharply decreased, when compared to the negative control group (Ad-NC), in both the 143B and MG-63 cell lines (Figure 2C and D). The flow cytometry cell cycle experiment result revealed that cells in the G0/G1 stage increased, while cells in the S and G2/M stage decreased in the Hint1 overexpression group, when compared to the Ad-NC group, in both the 143B and MG-63 cell lines (Figure 2E and F). These results indicate that Hint1 inhibits the cell cycle of OS cells.
Figure 2.
The overexpression of Hint1 inhibits the cell cycle in OS cell lines.
Notes: (A and B) The protein expression level of Hint1 increased in 143B cells (A) and MG-63 cells (B) after transfection with Ad-Hint1 (n=6). (C and D) The cell proliferation decreased in the Ad-Hint1 group, as detected by CKK-8 assay (n=6) in 143B cells (C) and MG-63 cells (D). (E and F) The cell cycle was inhibited by Ad-Hint1, as detected by flow cytometry (n=6) in 143B cells (E) and MG-63 cells (F). *P<0.05 vs Ad-NC group.
The Overexpression of Hint1 Increases the Apoptosis in OS Cell Lines
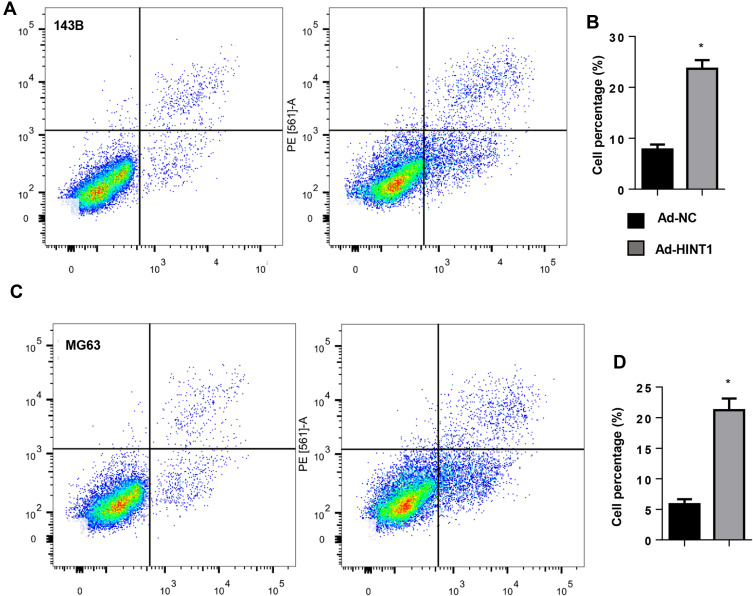
In order to determine whether Hint1 can affect the OS cell apoptosis, the apoptosis was detected by flow cytometry. The results revealed that the percentage of cell apoptosis significantly increased in Hint1 overexpressed cells, in both the 143B and MG-63 cell lines (Figure 3A–D). These suggests that Hin1 also affects the apoptosis of OS cells.
Figure 3.
The overexpression of Hint1 increased the apoptosis in OS cell lines.
Notes: (A and B) The cell apoptosis increased in Ad-Hint1: (A) the image, and (B) the quantitative result detected by flow cytometry (n=6) in 143B cells. (C and D) The cell apoptosis increased in Ad-Hint1: (C) the image, and (D) the quantitative result detected by flow cytometry (n=6) in MG-63 cells. *P<0.05 vs Ad-NC group.
The Overexpression of Hint1 Suppresses Cyclin D1 and FOXO1 in 143B and MG63 Cells
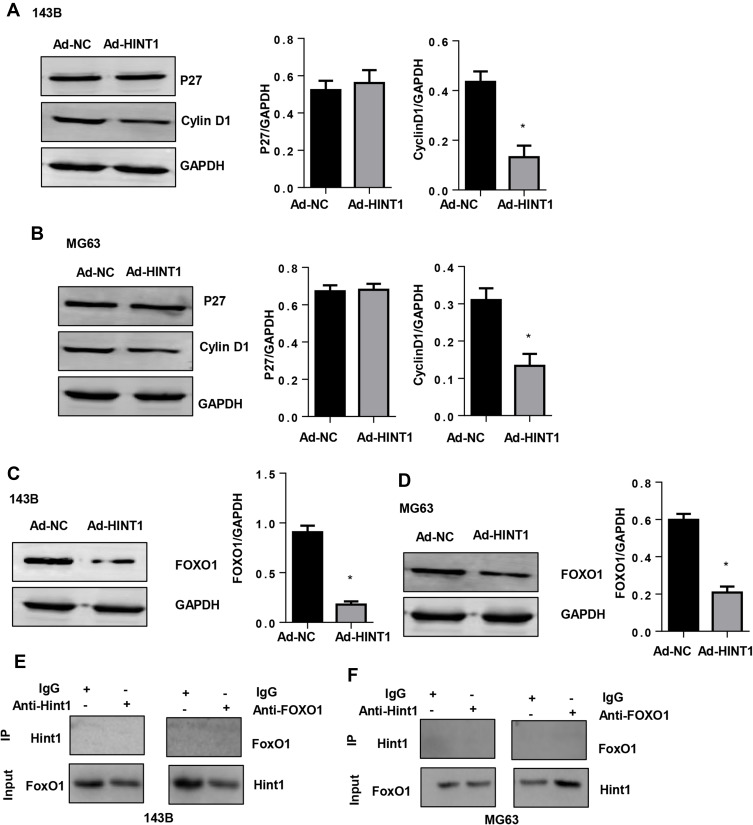
In order to elucidate the mechanism underlying Hint1 in affecting OS cells, the signaling pathway associated with the cell cycle and survival were screened. The results revealed that Hint1 overexpression reduced the expression of cyclinD1, but not the P27 protein level, in both the 143B and MG-63 cell lines, when compared to the Ad-NC group (Figure 4A and B). Furthermore, it was found that the expression level of pro-survival protein FOXO1 decreased in Hint1 overexpressed OS cells, in both the 143B and MG-63 cell lines (Figure 4C and D). These data suggests that FOXO1-cyclinD may be the target of Hint1 in OS cells. In order to determine whether Hint1 could directly bind to FOXO1, a Co-IP experiment was performed. As shown in Figure 4E and F, the direct binding effects of Hint1 and FOXO1 was not found in both the 143B and MG-63 cell lines.
Figure 4.
The overexpression of Hint1 suppresses cyclin D1 and FOXO1 in 143B and MG63 cells.
Notes: (A and B) The protein expression level of P27 and cyclinD1 in 143B cells (A) and MG-63 cells (B). (C and D) The protein expression level of FOXO1 decreased in the Ad-Hint1 group in 143B cells (C) and MG-63 cells (D). *P<0.05 vs the Ad-NC group. (E and F) The co-IP experiment of Hint1 and FOXO1 in 143B cells (E) and MG-63 cells (F).
FOXO1 Overexpression Counteracts the Effect on Hint1 on 143B and MG63 Cells
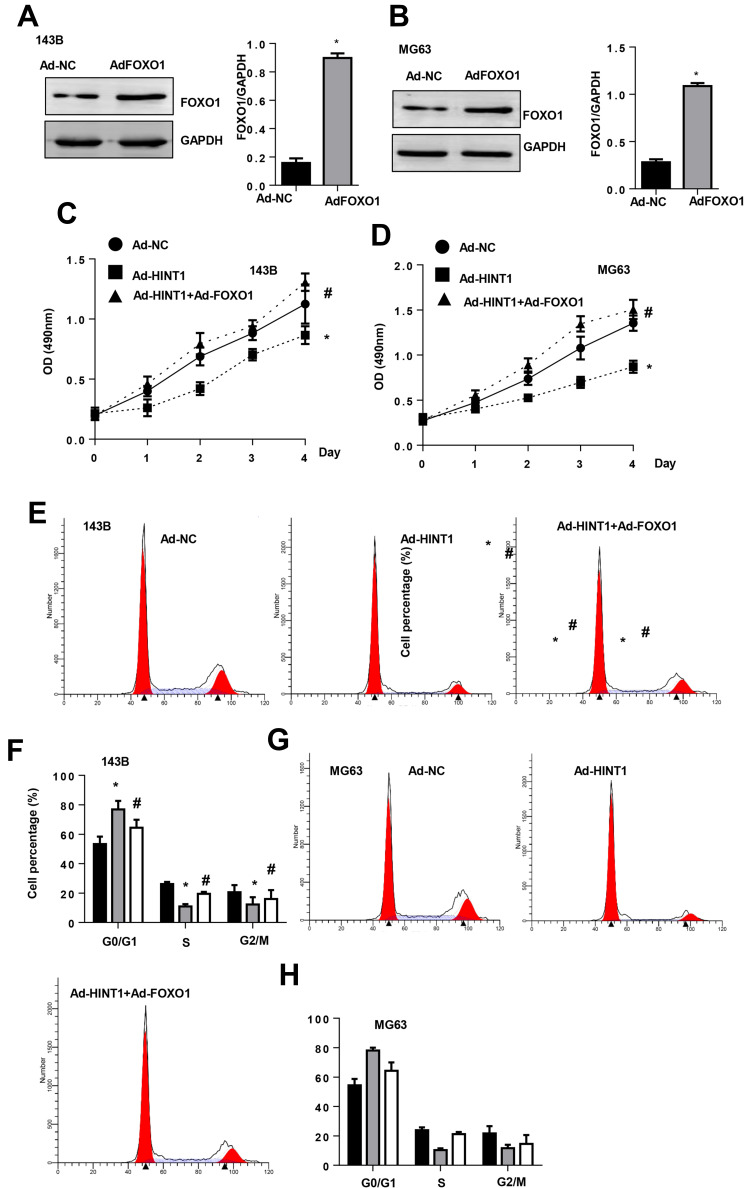
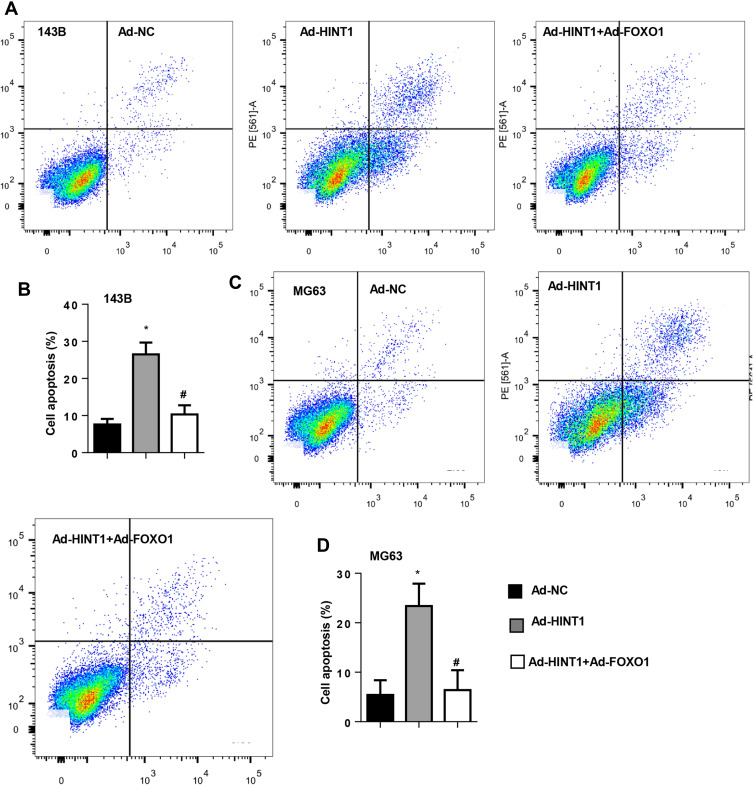
In order to confirm whether FOXO1-cyclinD is the only target of Hint1 in OS cells, 143B and MG63 cells were transfected with FOXO1 to overexpress FOXO1 (Figure 5A and B). The CKK-8 assay results revealed that the cell proliferation at 1, 2, 3 and 4 days after transfection decreased in the Hint1 overexpression group, but increased in the Hint1 and FOXO1 overexpression group, when compared to negative controls, in both 143B and MG63 cells (Figure 5C and D). Furthermore, the cell percentage in the G0/G1 stage increased in the Hint1 overexpression group, but decreased in the Hint1 and FOXO1 overexpression group, in both 143B and MG63 cells. In addition, the cell percentage in the S and G2/M stage decreased in the Hint1 overexpression group, but increased in the Hint1 and FOXO1 overexpression group, in both 143B and MG63 cells (Figure 5E–H). Cell apoptosis was also detected. As expected, the percentage of cell apoptosis increased in the Hint1 overexpression group, but decreased in the Hint1 and FOXO1 overexpression group, in both 143B and MG63 cells (Figure 6A–D). All these data indicate that Hint1 regulates the cell cycle and apoptosis of OS cells by targeting FOXO1-cycinD1.
Figure 5.
The FOXO1 overexpression counteracts the effect on Hint1 on 143B and MG63 cells.
Notes: (A and B) The protein expression level of FOXO1 increased in 143B cells (A) and MG-63 cells (B) after transfection with Ad-FOXO1 (n=6). (C and D) The cell proliferation detected by the CKK-8 assay (n=6) in 143B cells (C) and MG-63 cells (D). (E–H) The cell cycle detected by flow cytometry (n=6) in 143B cells (E and F) and MG-63 cells (G and H). *P<0.05 vs the Ad-NC group; #P<0.05 vs the Ad-Hint1 group.
Figure 6.
The FOXO1 overexpression counteracts the pro-apoptosis effect on Hint1 in 143B and MG63 cells.
Notes: (A–D) The cell apoptosis detected by flow cytometry (n=6) in 143B cells (A and B) and MG-63 cells (C and D). *P<0.05 vs the Ad-NC group; #P<0.05 vs the Ad-Hint1 group.
Discussion
OS is the most common primary bone tumor that occurs at the metaphysis of the long bone.3 In the past, the only standard treatment method was surgical resection of the tumor segment.4 In recent years, the application of multiagent chemotherapy regimens has significantly improved the prognosis for patients with non-metastatic OS.1 However, for patient with metastasized OS, the 5-year survival rate is less than 30%.2 Thus, finding new biomarkers and therapeutic targets is of great importance. Hint1 is a member of the histidine triad (HIT) enzyme superfamily, which was first described as a tumor suppressor.9 Recent studies have focused on the anti-tumor effects of Hint1 in various malignant tumors, including colorectal, mammary and ovarian tumors, hepatocellular carcinoma, prostate cancer and melanoma.5–7 In the present study, it was found that the expression level of Hint1 decreased in human OS tissues and OS cells lines. Hint1 overexpression inhibited the cell proliferation and cell cycle, but promoted cell apoptosis in OS cells lines. These indicate that Hint1 may be a potential treatment target for OS.
A new area of present cancer therapy is the regulation of the cell cycle by targeting regulatory cellular signaling. The targeted regulation of the cell cycle is a promising approach to present cancer therapy.12 The cell cycle is divided into five phases: G0 (gap 0), G1 (gap 1), S (DNA synthesis), G2 (gap 2) and M (mitosis).13 It was found that Hint1 overexpression enhanced the cell number in the G0/G1 phases, but reduced the cell number in S and G2/M phases. Furthermore, Hint1 induced the G1 phase arrest to inhibit cell proliferation, which is consistent with the study conducted by Wei Xs, in which Hind1 blocked the gastric cancer cell proliferation and inhibited the radiation damage in gastric cancer cell repair.6 Apoptosis is a programmed death mechanism that eliminates DNA-damaging cells. By targeting apoptosis, this can eliminate malignant cells, reduce tumor proliferation and inhibit tumor progression.14 Therefore, inducing the apoptosis of cancer cells is an important measure for treating tumors. In the present study, Hint1 overexpression increased the cell apoptosis in both the 143B and MG-63 cell lines, which indicates that Hint1 suppresses the cell cycle and induces cell apoptosis. Cell cycle progression is regulated by cyclins through the activation of cyclin-dependent kinase (CDKs). CDKs are positively regulated by cyclins (a, b, d and e), and negatively regulated by cyclin-dependent kinase inhibitors, such as p21 and p27.15 After being activated in the G1 phase, CyclinD1 can phosphorylate pRb, which in turn promotes cells to progress to the S phase.16 CyclinD1 plays an essential role in many cancers and cancer therapies.13,17 Bao et al reported that upregulating the Hint1 expression causes cyclinD1 inhibition, which suppresses liver cancer cell proliferation.18 In the present study, it was found that Hint1 downregulated the cyclinD1 expression level, but not the P27 level, suggesting that cyclinD1 may be the target of Hint1.
FOXO1 is an important transcription factor that belongs to the FOXOs family. The FOXOs family proteins act as tumor suppressors that induce cell proliferation inhibition and apoptosis progression in cancer cells.19 Pro-apoptotic genes, such as FasL and Bim, are the downstream of FoxO1 that mediate apoptosis.20 By directly targeting cyclinD1, FoxO1 promotes cell cycle arrest and apoptosis in cancer cells.21 A study reported that oncogenic transcription factors, such as MITF and β-catenin, were the target of Hint1.11 However, in the present study, it was found that the P27 level was not changed by Hint1, but rather, the FOXO1 expression was inhibited by Hint1. Furthermore, the FOXO1 overexpression counteracted the anti-tumor effects of Hint1 in OS cells. These indicates that FOXO1 is the only target of Hint1 in OS cells. However, the direct binding effects of Hint1 and FOXO1 was not found in both the 143B and MG-63 cell lines. Other factors or proteins may contribute to the association of Hint1 and FOXO1. Hence, further studies on the functional role of Hint1 on FOXO1 are needed. Since the number of human OS samples in the present study is only 10, a selective bias may occur due to the limited sample size. Thus, a large case-control study that also include samples from healthy donors or other non-malignant tumors is needed to confirm these present results.
In summary, these present results revealed that Hint1 is significantly downregulated in OS tissues. Hint1 serves as a tumor suppressor by targeting FOXO1-cyclinD1 in OS cells. Hint1 might be a therapeutic target for the treatment of patients with OS.
Funding Statement
This study was supported by the General Science and Technology Projects in Jingmen City (2018YFYB030).
Abbreviations
Ad, adenovirus vector; CKK-8, Cell Counting Kit 8; CDK, cyclin-dependent kinase; FOXO1, forkhead box O1; Hint1, histidine triad nucleotide-binding protein 1; NC, negative control; OS, osteosarcoma; RIPA, radio-immunoprecipitation.
Data Sharing Statement
All data are available upon request to the corresponding author.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang Y, Yang J, Zhao N, et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol Lett. 2018;16(5):6228–6237. doi: 10.3892/ol.2018.9434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asnafi AA, Behzad MM, Ghanavat M, Shahjahani M, Saki N. Singe nucleotide polymorphisms in osteosarcoma: pathogenic effect and prognostic significance. Exp Mol Pathol. 2019;106:63–77. doi: 10.1016/j.yexmp.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Otoukesh B, Boddouhi B, Moghtadaei M, Kaghazian P, Kaghazian M. Novel molecular insights and new therapeutic strategies in osteosarcoma. Cancer Cell Int. 2018;18:158. doi: 10.1186/s12935-018-0654-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Ren M, Zhao X, Wang A, Wang J. Emerging roles of circular RNAs in osteosarcoma. Med Sci Monit. 2018;24:7043–7050. doi: 10.12659/MSM.912092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zhang Y, Li H, et al. Hint1 inhibits growth and activator protein-1 activity in human colon cancer cells. Cancer Res. 2007;67(10):4700–4708. doi: 10.1158/0008-5472.CAN-06-4645 [DOI] [PubMed] [Google Scholar]
- 6.Wei X, Zhou J, Hong L, et al. Hint1 expression inhibits proliferation and promotes radiosensitivity of human SGC7901 gastric cancer cells. Oncol Lett. 2018;16(2):2135–2142. doi: 10.3892/ol.2018.8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XS, Bao TH, Ke Y, et al. Hint1 suppresses migration and invasion of hepatocellular carcinoma cells in vitro by modulating girdin activity. Tumour Biol. 2016;37(11):14711–14719. doi: 10.1007/s13277-016-5336-z [DOI] [PubMed] [Google Scholar]
- 8.Su T, Suzui M, Wang L, et al. Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci U S A. 2003;100(13):7824–7829. doi: 10.1073/pnas.1332160100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, zhang Y, Su T, Santella RM, Weinstein IB. Hint1 is a haplo-insufficient tumor suppressor in mice. Oncogene. 2006;25(5):713–721. doi: 10.1038/sj.onc.1209111 [DOI] [PubMed] [Google Scholar]
- 10.Symes AJ, Eilertsen M, Millar M, et al. Quantitative analysis of BTF3, Hint1, NDRG1 and ODC1 protein over-expression in human prostate cancer tissue. PLoS One. 2013;8(12):e84295. doi: 10.1371/journal.pone.0084295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese G, Ghosh P, Li H, et al. The tumor suppressor HINT1 regulates MITF and β-catenin transcriptional activity in melanoma cells. Cell Cycle. 2012;11(11):2206–2215. doi: 10.4161/cc.20765 [DOI] [PubMed] [Google Scholar]
- 12.Cheng CW, Tse E. PIN1 in cell cycle control and cancer. Front Pharmacol. 2018;9:1367. doi: 10.3389/fphar.2018.01367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batra A, Winquist E. Emerging cell cycle inhibitors for treating metastatic castration-resistant prostate cancer. Expert Opin Emerg Drugs. 2018;23(4):271–282. doi: 10.1080/14728214.2018.1547707 [DOI] [PubMed] [Google Scholar]
- 14.Mortezaee K, Salehi E, Mirtavoos-Mahyari H, et al. Mechanisms of apoptosis modulation by curcumin: implications for cancer therapy. J Cell Physiol. 2019;234(8):12537–12550. doi: 10.1002/jcp.28122 [DOI] [PubMed] [Google Scholar]
- 15.Chohan TA, Qayyum A, Rehman K, Tariq M, Akash MSH. An insight into the emerging role of cyclin-dependent kinase inhibitors as potential therapeutic agents for the treatment of advanced cancers. Biomed Pharmacother. 2018;107:1326–1341. doi: 10.1016/j.biopha.2018.08.116 [DOI] [PubMed] [Google Scholar]
- 16.Lin ZP, Zhu YL, Ratner ES. Targeting cyclin-dependent kinases for treatment of gynecologic cancers. Front Oncol. 2018;8:303. doi: 10.3389/fonc.2018.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong S, Zhu J, Li Y, et al. Butylene fipronil induces apoptosis in PC12 murine nervous cells via activation of p16-CDK4/6-cyclin D1 and mitochondrial apoptotic pathway. J Biochem Mol Toxicol. 2018:e22264. doi: 10.1002/jbt.22264 [DOI] [PubMed] [Google Scholar]
- 18.Bao T, Ke Y, Wang Y, et al. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J Mol Med. 2018;96(7):661–672. doi: 10.1007/s00109-018-1652-7 [DOI] [PubMed] [Google Scholar]
- 19.Coomans de Brachene A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73(6):1159–1172. doi: 10.1007/s00018-015-2112-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murtaza G, Khan AK, Rashid R, et al. FOXO transcriptional factors and long-term living. Oxid Med Cell Longev. 2017;2017:3494289. doi: 10.1155/2017/3494289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CG, Lee H, Gupta N, et al. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin Cancer Biol. 2018;50:142–151. doi: 10.1016/j.semcancer.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]